Abstract
The aim of this study was to determine if there were differences between the types of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria associated with particulate material and planktonic samples obtained from the northwestern Mediterranean Sea. A nested PCR procedure performed with ammonia oxidizer-selective primers was used to amplify 16S rRNA genes from extracted DNA. The results of partial and full-length sequence analyses of 16S rRNA genes suggested that different groups of ammonia-oxidizing bacteria were associated with the two sample types. The particle-associated sequences were predominantly related to Nitrosomonas eutropha, while the sequences obtained from the planktonic samples were related to a novel marine Nitrosospira group (cluster 1) for which there is no cultured representative yet. A number of oligonucleotide probes specific for different groups of ammonia oxidizers were used to estimate the relative abundance of sequence types in samples of clone libraries. The planktonic libraries contained lower proportions of ammonia oxidizer clones (0 to 26%) than the particulate material libraries (9 to 83%). Samples of the planktonic and particle-associated libraries showed that there were depth-related differences in the ammonia oxidizer populations, with the highest number of positive clones in the particle-associated sample occurring at a depth of 700 m. The greatest difference between planktonic and particle-associated populations occurred at a depth of 400 m, where only 4% of the clones in the planktonic library were identified as Nitrosomonas clones, while 96% of these clones were identified as clones that were related to the marine Nitrosospira species. Conversely, all ammonia oxidizer-positive clones obtained from the particle-associated library were members of the Nitrosomonas group. This is the first indication that Nitrosomonas species and Nitrosospira species may occupy at least two distinct environmental niches in marine environments. The occurrence of these groups in different niches may result from differences in physiological properties and, coupled with the different environmental conditions associated with these niches, may lead to significant differences in the nature and rates of nitrogen cycling in these environments.
Nitrification is carried out by autotrophic ammonia- and nitrite-oxidizing bacteria, which sequentially oxidize ammonia to nitrate. This process is central to the cycling of nitrogen in marine ecosystems, where it is responsible for production of nitrate, the largest inorganic N pool, which frequently limits primary production (7). Nitrifying bacteria can alleviate eutrophic conditions by competing with phytoplankton for available ammonia (4), and nitrification is associated with production of greenhouse gases through generation of nitrous oxide by ammonia oxidizers and through provision of nitrate for denitrification (21). Ammonia oxidation rather than nitrite oxidation usually limits the rate of nitrification, which is greatest immediately below the photic zone, where competition for ammonia by phytoplankton and light inhibition are reduced (19). Nitrification activity decreases with depth as the levels of organic material decrease, which reduces the supply of ammonia through decomposition of organic nitrogen. Although techniques for performing process studies of nitrification in natural environments are available, investigations of the community structure and species diversity of natural populations have been severely limited by technical difficulties associated with enrichment, purification, and identification of ammonia oxidizers due to their low growth rates and low biomass yields in laboratory cultures and the limited number of distinguishing characteristics.
Our ability to characterize and analyze natural microbial communities has been greatly enhanced by the use of 16S ribosomal DNA (rDNA)-based techniques (6, 9, 11–13, 31–33), which have proved to be particularly appropriate for the study of ammonia-oxidizing bacteria (14, 16, 17, 24, 27–29, 39, 40, 46, 47). The ammonia oxidizers that have been cultured form two monophyletic groups, one within the γ subdivision of the class Proteobacteria (γ-proteobacteria), containing strains of Nitrosococcus oceanus (54) and Nitrosococcus halophila (23), and the other within the β-proteobacteria, containing members of two genera, the genera Nitrosomonas and Nitrosospira (15, 42, 53, 54). PCR primers specific for the β-proteobacterial ammonia oxidizers have been used to amplify 16S rDNA sequences from enrichment cultures and from DNA extracted directly from marine sediments and soils (28, 39, 46). These studies have demonstrated that there are at least seven sequence clusters within the β-proteobacteria, four sequence clusters within the Nitrosospira group and three sequence clusters within the Nitrosomonas group. Phylogenetic analysis of 16S rDNA sequences, denaturing gradient gel electrophoresis (24), and probing (16, 27, 40) have revealed that 16S rDNA-based techniques can detect environment-associated differences in ammonia oxidizer community structure.
Organic matter-rich particulate material plays an important role in regulation of biogeochemical cycling in marine systems and is produced mainly in the surface layers of the water column at the seasonal thermocline (2, 25). This material provides an environment that is enriched with nutrients compared to the surrounding water, which leads to greater bacterial concentrations and activity (44, 45). In addition, several workers (1, 3, 6) have demonstrated that there are differences in the microbial communities associated with planktonic material and particle-associated material, suggesting that there are functional and metabolic differences between the two environments. Increased activity leads to the establishment of nutrient and oxygen concentration gradients within particles, which allow interactions which would not be possible in the water column (52), but planktonic cells are traditionally considered to be responsible for the majority of the activities measured (22, 36).
Colonization of particulate material may provide significant benefits to nitrifying bacteria because of a regular supply and higher localized concentrations of ammonia from decomposing organic material. Sedimentation of organic particles may lead to nitrification at greater depths and may provide protection from photoinhibition. High ammonia concentrations are believed to favor growth of Nitrosomonas species, while analysis of DNA from low-ammonia environments has revealed a greater abundance of Nitrosospira species in these environments (16). In addition, biofilm populations of Nitrosomonas species produce extracellular polymeric material which may enhance aggregate formation (5), as suggested previously for transparent exopolymeric material in large marine snow aggregates (18). The aim of this study was to test the hypothesis that conditions within particulate material lead to selection for Nitrosomonas spp., while in planktonic populations there may be a greater abundance of Nitrosospira species, which are commonly believed to prefer lower ammonia concentrations. This hypothesis was tested by performing a 16S rDNA sequence analysis of planktonic and particle-associated samples obtained from the Mediterranean Sea.
MATERIALS AND METHODS
Sampling of particle-associated and planktonic cells.
Seawater samples (approximately 10 liters) were collected in April 1995 in Niskin bottles from two sites (sites 1 and 2) in the Mediterranean Sea; site 1 (depths, 100 and 400 m) and site 2 (depth, 700 m) were 28 and 5.5 miles, respectively, from the coast of Nice, France (43°25N, 7°52E and 43°39N, 7°27E, respectively). The concentrations of bacteria were determined by epifluorescence microscopy of DAPI (4′,6-diamidino-2-phenylindole)-stained material (43, 44). Nitrite and nitrate concentrations were measured with an Autoanalyser II system (Technicon Instruments Corp., Tarrytown, N.Y.), and chlorophyll a concentrations were measured by fluorometry (55). Data were obtained as part of the European Commission’s Marine Science and Technology Programme 2 (Mediterranean Targeted Project, European Microbiology of Particulate Systems) (Table 1). Planktonic cells were obtained by first filtering samples through 0.8-μm-pore-size cellulose nitrate filters (diameter, 4.5 cm) to remove debris and particulate material. The resulting filtrate was then passed through 0.45- and 0.2-μm-pore-size cellulose nitrate filters, which were frozen immediately at −70°C prior to extraction of total DNA. Particulate material was obtained by filtering a known volume of seawater (ca. 500 liters) with an in situ pump (Challenger Oceanic) equipped with a 10-cm-diameter, 10-μm-pore-size Nuclepore filter. The particulate material was detached by washing the filter with 35 ml of filter-sterilized (pore size, 0.2 μm) seawater obtained from the same depth. Aliquots of suspensions were frozen immediately at −70°C prior to DNA extraction.
TABLE 1.
Sampling details, bacterial cell concentrations, and nitrite, nitrate, and chlorophyll a concentrations at the three sampling sites in the northwestern Mediterranean Seaa
| Station | Sampling depth (m) | Sampling date (day/mo/yr) | Bacterial cell concn (105 cells ml of unfiltered seawater−1) | Nitrite concn (μg of NO2− N ml−1) | Nitrate concn (μg of NO3− N ml−1) | Chlorophyll a concn (μg liter−1) | Vol of water sampled to obtain for aggregate material (liters) |
|---|---|---|---|---|---|---|---|
| 1 | 700 | 7/4/95 | 1.02 | 0.09 | 8.05 | NDb | 515 |
| 2 | 100 | 8/4/95 | 2.20 | 0.01 | 0.44 | 0.022 | 520 |
| 2 | 400 | 8/4/95 | 2.23 | 0.17 | 6.62 | 0.045 | 566 |
Data were obtained from the European Commission’s Marine Science and Technology Programme 2 (Mediterranean Targeted Project, European Microbiology of Particulate Systems).
ND, not determined.
Enumeration of particle-associated and planktonic populations.
The concentrations of viable ammonia-oxidizing bacterial cells were determined by using the most-probable-number (MPN) method. Samples (1 ml) of suspensions of planktonic and particle-associated cells (prepared as described above) were added to five 25-ml Universal bottles containing 10 ml of mineral salts medium supplemented with 5 μg of NH4+-N ml−1 prepared in artificial seawater (51). Tenfold serial dilutions of each suspension were inoculated into four Universal bottles containing mineral salts medium in order to obtain five replicates consisting of five 10-fold dilutions. The cultures were incubated in the dark at 30°C for 6 months. Growth was assessed on the basis of acid production, as measured by color change, and the appearance of nitrite, as determined by spot tests performed with Griess Ilosvay’s reagents I and II (BDH, Poole, United Kingdom).
DNA extraction and purification.
DNA was extracted from particle-associated and planktonic samples by using a modification of the method of Somerville et al. (37). Stored suspensions (500 μl) or filters were thawed and incubated on ice for 15 min after 3.6 ml of STE extraction buffer (10 mM Tris, 1 mM EDTA, 100 mM NaCl; pH 8) and 124 μl of lysozyme (5 mg ml−1) were added. Then 80 μl of a 10% (wt/vol) sodium dodecyl sulfate (SDS) solution was added to each sample, and the samples were incubated on a tube roller for 1 h at room temperature. The samples were then incubated with 100 μl of proteinase K (20 mg ml−1) at room temperature for 4 h. The supernatants were decanted into sterile Corex tubes, 1 ml of STE extraction buffer was added to each sample, and the samples were incubated at room temperature for an additional 30 min. The two washes of each sample were pooled, and the protein was precipitated by incubation with 2 ml of 10.5 M sodium acetate at room temperature for 15 min prior to centrifugation at 4°C and 14,500 × g for 10 min. The supernatant was decanted, and the DNA was precipitated by overnight incubation at −20°C with 11 ml of ice-cold ethanol. The suspension was centrifuged (20 min, 12,000 × g, room temperature), after which the pellet was dried in a vacuum desiccator and resuspended in 300 μl of TE buffer (10 M Tris, 1 M EDTA; pH 7). The DNA was concentrated by using a Microcentricon 100 spin dialysis unit (Amicon, Stonehouse, Glos., United Kingdom) and purified further by standard gel electrophoresis through a 1% (wt/vol) agarose gel. The DNA band (>8 kb) was excised from the gel and subsequently cleaned by using a Spin Bind DNA recovery system (Flowgen Instruments Ltd., Kent, United Kingdom).
PCR amplification.
The initial amplification was carried out by using the universal eubacterial primers BF and 1541r, which bind at positions 24 to 42 and 1541 to 1525, respectively, of the Escherichia coli 16S rRNA and amplify a product that is approximately 1.5 kb long (8). The secondary amplification was carried out by using the internal primers βAMOf and βAMOr (28), which are selective but not completely specific for β-proteobacterial ammonia oxidizers. These primers bind at positions 141 to 161 and 1301 to 1320, respectively, of the E. coli 16S rRNA and amplify an ∼1.2-kb product. The primers were synthesized commercially (Oswell DNA Service, University of Edinburgh, Edinburgh, United Kingdom).
A nested PCR was performed with both planktonic and particulate samples. The protocol used for PCR amplification with the universal eubacterial primers was as follows: initial denaturation at 95°C for 5 min prior to addition of the Taq polymerase; 30 cycles consisting of 94°C for 40 s, 50°C for 30 s, and 72°C for 2 min; and 72°C for 5 min. The same conditions were used for the primers βAMOf and βAMOr, but the annealing temperature was increased to 55°C. Five universal eubacterial primer-positive PCR products were pooled in order to minimize the effects of stochastic bias in single reactions (48) and were purified by electrophoresis on a 1% (wt/vol) agarose gel. The PCR bands were excised, and the DNA was purified further with Qiaex resin (Qiagen Ltd., Surrey, United Kingdom) and was eluted in a final volume of 20 μl, and 1 μl was used for amplification with primers βAMOf and βAMOr. Negative control reaction mixtures containing no template DNA were included in all amplifications. The PCR products were resolved by electrophoresing 5 μl of the reaction mixture in a 1% (wt/vol) agarose minigel run in 1× TAE buffer.
Cloning and sequencing of rDNA.
Five PCR products resulting from amplification with primers βAMOf and βAMOr were purified as described above, and 20 ng of products was ligated into the pGEM T-vector system (Promega Ltd., Southampton, United Kingdom) and transformed into XL1-Blue MRF Kan supercompetent E. coli cells (Stratagene Inc., Cambridge, United Kingdom). The transformed cells were plated onto agar supplemented with the reporter chemicals X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and IPTG (isopropyl-β-d-thiogalactopyranoside) and the antibiotics kanamycin, ampicillin, and methicillin as recommended by the manufacturer (Stratagene Inc.). White colonies were picked and checked for inserts of the correct size by PCR amplification with vector primers T7 and SP6 (Promega Ltd.). The PCR products were purified with a chloroform-isoamyl alcohol (1:1) mixture and then manually sequenced by using a Thermosequenase cycle sequencing kit (Amersham International plc, Little Chalfont, Buckinghamshire, United Kingdom). Partial sequencing of the V1 and V2 regions of the 16S rDNA sequence was performed by using the reverse sequencing primer 537r (8).
Full-length sequencing of the entire 1.1-kb insert was performed with four clones associated with the particle-associated and planktonic samples. This sequencing was carried out by using vector primers SP6 and T7 (Promega Ltd.) and internal 16S rDNA primers in addition to primer 537r described above. A phylogenetic analysis was carried out by aligning the partial 16S rRNA sequences of the clones with the sequences of ammonia oxidizers and other β-proteobacteria available from the Ribosomal Database Project (26). All data manipulations were performed with Genetic Data Environment software, version 2.2, distributed by the Ribosomal Database Project. The partial alignments comprised 250 bases corresponding to E. coli positions 122 to 372. The full-length sequences comprised 1,114 bases corresponding to E. coli positions 162 to 1276. Phylogenetic trees were generated by using the Jukes-Cantor (20) correction and neighbor joining (34) performed with PHYLIP software (version 3.1) (10). Most of the sequences were deposited in the GenBank database; the only exception was the sequence of clone 400 FREE (Z6), which was found to be chimeric after the full-length sequence analysis.
Colony hybridization and probing.
Library clones were checked for inserts of the correct size by PCR by using vector primers SP6 and T7 (see above). Dot blots were prepared by denaturing 4 μl of PCR product at 95°C with an equal volume of 20× SSC (1× SSC is 0.15 M sodium citrate plus 0.015 M sodium chloride). The product was spotted onto Hybond N+ nylon membrane paper (Amersham International plc), and the DNA was denatured, neutralized, and fixed with 0.4 M NaOH as recommended by the manufacturer. For each of the six libraries examined, 90 clones were spotted onto a membrane along with six controls representing members of the Nitrosomonas and Nitrosospira clusters. Probes β-AO233, Nsp436, and Nmo254 were used; these probes recognize all β-proteobacterial ammonia oxidizer sequences, all Nitrosospira sequences, and all Nitrosomonas sequences, respectively (Table 2) (40). Each probe (20 pmol) was end labelled by using T4 polynucleotide kinase (Promega Ltd.) and 20 μCi of [γ32P]ATP (3,000 Ci mmol−1; Amersham International plc) in a 10-μl (final volume) reaction mixture.
TABLE 2.
Oligonucleotide probe sequences and hybridization temperatures used for the probe analysis of clone libraries obtained from particle-associated and planktonic samples from the northwestern Mediterranean Seaa
| Probe | Sequence (5′-3′) | Target group | Temp (°C) |
|---|---|---|---|
| β-AO233 | AGCTAATCAGRCATCGG | All β-subgroup ammonia oxidizers | 44 |
| Nsp436 | TTTCGTTCCGGCTGAAAG | All Nitrosospira spp. | 45 |
| Nmo254 | GTAGGCCSTTACCCYACC | All Nitrosomonas spp. | 43 |
The probes were designed and optimized by Stephen et al. (40).
Prehybridization of the membranes in Quickhyb solution (Stratagene Inc.) was carried out at 42°C for 30 min before the radiolabelled probe was added. Hybridization was carried out for 4 h or overnight in a Hybaid hybridization oven. Unbound probe was removed by washing with 2× SSC–0.1% SDS (Sigma, Poole, Dorset, United Kingdom) for 10 min at room temperature and then with 0.1× SSC–0.1% SDS at 42°C for 30 min. The membranes were exposed to X-ray film overnight. Before being reprobed, the membranes were stripped by washing them twice in a large volume of boiling 0.1× SSC–0.1% SDS. The membranes were checked for complete removal of bound probe by ensuring that radioactive counts had returned to background levels. The membranes were rinsed in distilled water, air dried, and stored at 4°C until they were reprobed. Samples of clones that hybridized to each of the probes used were partially sequenced as described above to confirm the identities based on probing data.
Nucleotide sequence accession numbers.
The nucleotide sequences determined in this study were deposited in the GenBank database under accession numbers AF063619 to AFO63638.
RESULTS
DNA extraction and purity.
The DNA yield was low due to the low concentration of bacterial cells (1 × 108 to 2.2 × 108 cells per liter of unfiltered seawater) (Table 1). Particulate material and planktonic samples produced ca. 20 ng of DNA per liter of seawater when they were filtered through 10- and 0.2-μm-pore-size filters, respectively, as estimated by comparing band intensities on ethidium bromide-stained agarose gels with lambda mass ladder standards (Promega Ltd.).
Enumeration of ammonia-oxidizing bacteria.
No growth of ammonia-oxidizing bacteria was detected in any of the cell suspensions used for MPN counts for particle-associated or planktonic samples after incubation for 6 months. The viable cell concentrations, as assessed by this method, were therefore below the level of detection, which was 103 cells liter−1.
Phylogenetic analysis.
PCR amplification performed with primers βAMOf and βAMOr alone did not yield detectable products, perhaps because of the low DNA yields and low ammonia oxidizer cell concentrations. Nested PCR amplification performed with eubacterial primers and then with primers βAMOf and βAMOr did, however, yield amplification products of the expected size. We obtained partial sequences comprising 252 bases of the 5′ region of these products for 6 to 10 clones belonging to each of the six clone libraries obtained from particle-associated samples (AGG clones) and planktonic samples (FREE clones) from each of three depths (100, 400, and 700 m).
Site 1, 100-m depth.
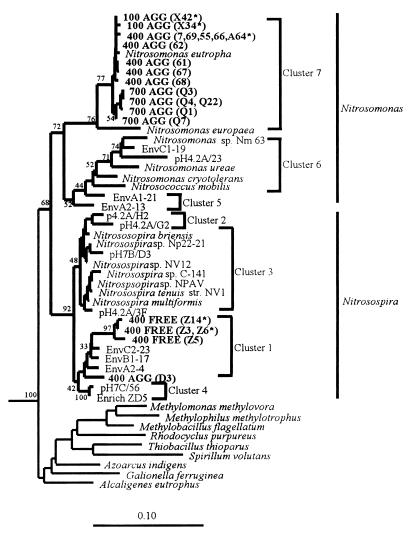
Most clones (18 of the 20 clones from both planktonic and particle-associated libraries) obtained from a depth of 100 m at site 1 did not belong to the ammonia oxidizer clade and were more closely related to other members of the β-proteobacteria, particularly Comamonas testosteroni, Methylococcus capsulatum, and Thiobacillus thioparus (data not shown). This may reflect the low numbers and low relative abundance of ammonia-oxidizing β-proteobacteria, as indicated by MPN counts. Two of the 10 clones from the particle-associated clone library [clones 100 AGG (X34) and 100 AGG (X42)] fell in the ammonia oxidizer clade, clustering with Nitrosomonas eutropha (Fig. 1). None of the 10 clones from the planktonic clone library (100 FREE clones) grouped with the ammonia oxidizers.
FIG. 1.
Phylogenetic tree showing the positions, within the β-proteobacterial ammonia oxidizers, of partial sequences obtained from DNA extracted from planktonic samples and particulate material obtained from site 1 at depths of 100 and 400 m and from site 2 at a depth of 700 m. Clones were obtained by using primers designed to amplify sequences from the β-proteobacterial ammonia oxidizers (27). The tree was generated by using a 252-bp region of the 5′ region of the 16S rDNA and neighbor joining (34) with the Jukes-Cantor (20) correction in ARB. The cluster designations are the cluster designations described by Stephen et al. (39, 40). Asterisks indicate clones whose full-length sequences were used to construct the tree shown in Fig. 2. Environmental clones whose designations begin with Env and pH were obtained from the ammonia oxidizer database of Stephen et al. (39). Clone pH4.2A/23 is a representative of cluster 6a. The other members of cluster 6 are cluster 6b organisms (40). Scale bar = 0.1 estimated change per nucleotide position.
Site 1, 400-m depth.
The sequences of all 10 clones obtained from the particle-associated clone library derived from site 1 at a depth of 400 m (400 AGG clones) fell in the β-proteobacterial ammonia oxidizer clade (Fig. 1), which suggested that the relative abundance of these organisms was greater at 400 μm than at 100 m. Nine of the 10 clones clustered with N. eutropha and formed a tight cluster. Partial sequences of five of these clones [clones 400 AGG (7), 400 AGG (69), 400 AGG (A64), 400 AGG (66), and 400 AGG (55)] were identical over a 260-base region. A single clone [clone 400 AGG (D3)] from the library obtained from the particle-associated 400-m-depth samples clustered with Nitrosospira cluster 1 sequences (Fig. 1). Four of the 10 clones from the planktonic samples obtained at a depth of 400 m (400 FREE clones) clustered with Nitrosospira cluster 1 [clones 400 FREE (Z14), 400 FREE (Z3), 400 FREE (Z6), and 400 FREE (Z5)] (Fig. 1), and the sequences of two of these clones [clones 400 FREE (Z3) and 400 FREE (Z6)] were identical. The remaining six clones grouped with non-ammonia-oxidizing β-proteobacteria.
Site 2, 700-m depth.
Five of the six clones obtained from the particle-associated clone library derived from site 2 at a depth of 700 m (700 AGG clones) clustered with N. eutropha, and two of these clones [clones 700 AGG(Q4) and 700 AGG(Q22)] were identical for the stretch of sequence compared. None of the six clones obtained from the planktonic library (700 FREE clones) clustered with the ammonia oxidizers; these clones were closely related to Thiobacillus thioparus, Azoarcus indigens, and Alcaligenes eutrophus.
Full-length sequence analysis.
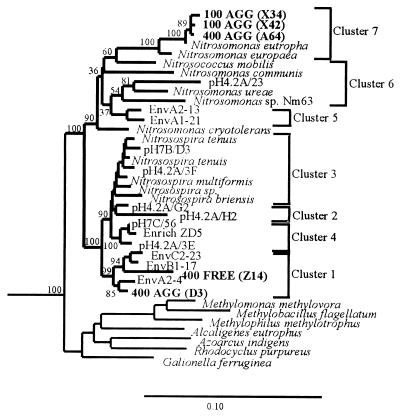
A full-length sequence analysis of six clones from the planktonic library (two 400 FREE clones) and the particle-associated library (two 100 AGG clones and two 400 AGG clones) confirmed that these clones were closely related to Nitrosospira cluster 1 and N. eutropha, respectively (Fig. 2). Clone sequences obtained from 100- and 400-m samples formed a unique cluster within Nitrosomonas cluster 7 in 89% of the bootstrap replicates; the closest cultured relative was N. eutropha. Clones 400 FREE (Z14) and 400 AGG (D3) were closely related to cloned Nitrosospira cluster 1 sequences obtained from direct DNA extraction of sediment samples from the west coast of Scotland (85 and 94% of bootstrap replicates, respectively). Clone 400 FREE (Z6) was probably chimeric; the 5′ end of the sequence exhibited similarity to Coxiella burnetti, and the 3′ related end exhibited similarity to Nitrosospira cluster 1 clone sequences.
FIG. 2.
Phylogenetic tree showing the positions, within the ammonia oxidizer group of the β-proteobacteria, of almost full-length sequences obtained from DNA extracted from planktonic samples and particulate material obtained from site 1 at depths of 100 and 400 m and from site 2 at a depth of 700 m. The tree was generated by using a 1,011-bp region of the 16S rDNA and neighbor joining (34) with the Jukes-Cantor (20) correction in ARB. The cluster designations are the cluster designations described by Stephen et al. (39, 40). For other details see the legend to Fig. 1.
Colony hybridization.
Colony hybridization with ammonia oxidizer-specific oligonucleotide probes allowed us to more rapidly analyze a large number of clones from each of the libraries obtained from particle-associated and planktonic populations. Of the 87 clones obtained from the particle-associated library derived from site 2 at a depth of 700 m, 72 (83%) hybridized with the β-AO233 probe, which was designed to detect all β-proteobacterial ammonia oxidizers (Table 3 and Fig. 3A). This suggests that the relative abundance of ammonia oxidizer sequences was higher in this library than in the planktonic library derived from this depth, since only 6 (7%) of the planktonic clones hybridized with β-AO233. Fewer clones in the libraries obtained from shallower depths (400 and 100 m), hybridized with β-AO233; only 15 (17%), 23 (26%), and 8 (9%) of the 400 AGG, 400 FREE, and 100 AGG clones hybridized, and none of the 100 FREE clones hybridized. These results supported evidence obtained from the sequence analysis which showed that there were differences between particle-associated and planktonic populations and differences with depth in the water column.
TABLE 3.
Percentages of clones in clone libraries derived from particle-associated and planktonic material from the northwestern Mediterranean Sea that hybridized with probes β-AO233, Nsp436, and Nmo254
| Clone library | No. of clones | Clones that hybridized witha:
|
|||||
|---|---|---|---|---|---|---|---|
| β-AO233
|
Nsp436
|
Nmo254
|
|||||
| No. | % | No. | % | No. | % | ||
| 100 FREE | 90 | 0 | 0 | 0 | 0 | 0 | 0 |
| 100 AGG | 90 | 8 | 9 | 0 | 0 | 8 | 100 |
| 400 FREE | 89 | 23 | 26 | 22 | 96 | 1 | 4 |
| 400 AGG | 90 | 15 | 17 | 0 | 0 | 15 | 100 |
| 700 FREE | 91 | 6 | 7 | 1 | 17 | 5 | 83 |
| 700 AGG | 87 | 72 | 83 | 3 | 4 | 69 | 96 |
The results were corrected for clones which gave unexpected probe profiles and which after sequence analysis proved to be closely related to non-ammonia-oxidizing species. The values for Nsp436 and Nmo254 were calculated as percentages of the number of β-AO233-positive clones.
FIG. 3.
Hybridization of members of the clone library obtained from aggregate samples collected at site 2 at a depth of 700 m (700 AGG clones) with ammonia oxidizer-specific probes. (A, B, and C) Autoradiographs showing the results of hybridizations with probes β-AO233, Nsp436, and Nmo254, respectively. Controls 1 through 5 were representatives of ammonia oxidizer cultures (control 1, Nitrosospira sp. strain Np 22-21 [cluster 3]; control 2, Nitrosospira sp. strain NPAV [cluster 3]; control 3, Nitrosospira multiformis [cluster 3]; control 4, Nitrosospira sp. strain C-141 [cluster 3]; control 5, Nitrosomonas europaea [cluster 7]). Partial sequences of the clones enclosed in boxes were determined to check the validity of the results.
β-AO233-positive clones were also hybridized with probes specific for Nitrosomonas clusters 5 to 7 (probe Nmo254) and Nitrosospira clusters 1 to 4 (probe Nsp436) (Fig. 3B and C). A total of 69 (96%) of the β-AO233-positive clones from libraries derived from particle-associated samples obtained at a depth of 700 m hybridized with probe Nmo254, indicating that these libraries were dominated by Nitrosomonas species, while only 3 (4%) hybridized with the Nsp436 probe. Similarly, only one (17%) of the clones from the planktonic clone library obtained at 700 m hybridized with Nsp436, while five clones (83%) hybridized with Nmo254. This result demonstrates the benefits associated with probing, which allows screening of a larger number of clones, as no ammonia oxidizer sequences were detected in the original sequence data. The hybridization results obtained with Nmo254 were fainter than the hybridization results obtained with the β-AO233 and Nsp436 probes. This was probably due to a mismatch in Nmo254, which was revised (40) in order to take account of new sequence information. However, the mismatch did not affect the ability of the probe to differentiate between Nitrosomonas-like and Nitrosospira-like sequences, as Nmo254 did not hybridize with any Nitrosospira sequence.
In contrast to the findings obtained with the 700-m samples, 22 (96%) of the β-AO233-positive clones obtained from planktonic site 1 samples from a depth of 400 m hybridized with Nsp436, while only one (4%) hybridized with Nmo254, suggesting that this library was dominated by Nitrosospira-like sequences. None of the 90 clones from the particle-associated library obtained at 400 m hybridized with Nsp436, but they all hybridized with Nmo254, which supported the findings of the sequence analysis. All eight β-AO233-positive clones from the particle-associated clone library obtained at 100 m hybridized with Nmo254, suggesting that this library was dominated by Nitrosomonas-like sequences. No β-AO233-positive clones were among the 90 clones selected for probe analysis from the planktonic library obtained at this site.
Identification of some of the clones was complicated by hybridization patterns which were not characteristic of β-proteobacterial ammonia oxidizers. This was due to the use of the primers of McCaig et al. (28), which are not completely specific. For example, Fig. 3 shows the autoradiographs produced when we probed clones from the 700-m particle-associated library with probes β-AO233, Nmo254, and Nsp436. Some clones hybridized with Nmo254 but not with β-AO233 (Fig. 3, reactions A5 and D10), some hybridized with β-AO233 but not with Nmo254 or Nsp436 (Fig. 3, reactions A1, B9, and D7), and some hybridized with all three probes. Unexpected hybridization profiles were more evident in planktonic libraries, in which there was a higher incidence of clones which hybridized with Nmo254 or Nsp436 but not with β-AO233, which was designed to detect all ammonia oxidizers. Partial sequencing was carried out with representatives of such clones and of clones that produced expected hybridization patterns to check the fidelity of the probes. Clones that produced unexpected hybridization patterns were closely related to non-ammonia-oxidizing species belonging to the β-proteobacteria (for example, M. capsulatum, C. testosteroni, and Oxaligenese formigenes) (data not shown). Clones that hybridized with the β-AO233 and Nmo254 probes were closely related to N. eutropha (cluster 7), and clones that hybridized with β-AO233 and Nsp436 grouped with Nitrosospira cluster 1. The results presented above and in Table 3 were adjusted for clones that produced non-ammonia-oxidizer hybridization profiles.
DISCUSSION
In this study we compared the community structures of particle-associated and planktonic populations of ammonia-oxidizing bacteria by performing PCR amplification of rDNA genes with primers selective for β-proteobacterial ammonia oxidizers, followed by cloning, sequence analysis, and colony hybridization with specific probes. PCR amplification of ammonia oxidizer sequences required the use of a nested PCR performed with primers βAMOf and βAMOr after initial amplification with eubacterial primers. Similar strategies have been necessary in other studies of aquatic systems (16, 47), and the requirement for such strategies may reflect the low in situ concentrations of ammonia oxidizers. This contrasts with studies performed with sediments, soil, and seawater enrichment cultures (28, 39), in which the concentrations and relative abundance of ammonia oxidizers are likely to be greater. PCR amplification and sequence analysis provided evidence that ammonia oxidizers were present, even though the MPN counts and nitrification rates were below the levels of detection. This indicates the sensitivity of PCR amplification for detection and the lack of suitable laboratory media and incubation conditions for viable cell enumeration of natural populations.
Although the bacterial cell concentrations determined by DAPI staining and epifluorescence microscopy were similar at the 100- and 400-m depths at site 1, fewer ammonia oxidizer sequences were detected in the library obtained at 100 m. This may have been due to photoinhibition, although any analysis of sequence abundance data must consider possible biases associated with cell extraction, cell lysis, and PCR amplification. In addition, the nested PCR protocol, which was necessary in this study, may increase the probability of PCR bias, although such bias was minimized by using the same extraction procedures and cloning techniques for all samples and by analyzing pooled replicate PCR products.
The PCR primers used in this study were deliberately designed with a degree of degeneracy to reduce the risk of missing closely related sequences of previously uncharacterized ammonia oxidizers. This led, particularly in planktonic samples, to amplification of sequences of non-ammonia oxidizers similar to the sequences found in other studies (39). The ammonia oxidizer sequences fell into the groups defined by Stephen et al. (39), and the planktonic population at 400 m was dominated by marine Nitrosospira cluster 1, which was first detected by Stephen et al. (39) in sediments beneath Atlantic salmon cages on the west coast of Scotland. This group is phylogenetically distinct from the cultured representatives of the Nitrosospira group, all of which were isolated from terrestrial environments. No cultured representative of Nitrosospira cluster 1 has been obtained, which has prevented reliable assessment of the physiological properties of this cluster and of its environmental significance, but this study provides further evidence that it is present in marine environments. Sequences typical of terrestrial Nitrosospira ammonia oxidizers were not found in either planktonic or particle-associated samples.
Particle-associated samples were dominated by sequences related to the sequence of N. eutropha, which is reportedly favored by saline environments (38). This contrasts with the results of other studies of marine sediments and enrichment cultures, in which clone libraries were dominated by sequences of Nitrosospira species and Nitrosomonas strains that were not specifically related to N. eutropha (27, 39). The sequences at sites 1 and 2 were similar despite the fact that the sites were separated by 28 miles, although the site 1 sequences form a separate group within cluster 7 (bootstrap value, 54%). Differences at the depths examined are not likely to be due to terrestrial or freshwater input and may result from the Lignau Provencal current, which occurs at depths of approximately 400 to 600 m and introduces warmer and more saline water. Restriction fragment length polymorphism analysis of planktonic and particle-associated populations in the Mediterranean Sea has also revealed depth-associated differences in community structure and has indicated that the diversity within planktonic populations is greater than the diversity within particle-associated populations (1).
The principal aim of this study was to determine whether planktonic and particle-associated populations were dominated by Nitrosospira-like organisms and Nitrosomonas-like organisms, respectively. Only one Nitrosospira-like sequence was found in clones obtained from particulate material, and no planktonic clones contained Nitrosomonas-like sequences. Our analysis of clones by the colony hybridization method supported these findings; the majority of the particle-associated clones hybridized with Nmo254, and the planktonic clones hybridized with Nsp436. This difference may result from higher ammonia concentrations in the particulate material, which are due to decomposition of organic material. Hovanec and DeLong (17), using 16S rRNA probes, also found that Nitrosomonas spp. are responsible for nitrification in filters of marine aquaria containing high amounts of organic material. Other factors may also have led to selection, including production of extracellular polymeric material, photoinhibition, and an ability to compete for ammonia. The last two factors should be most significant in surface waters, which may explain the lack of planktonic ammonia oxidizers at 100 m. Ammonia oxidizers were, however, detected in particulate material at this depth, providing the potential for nitrification in the photic zone and explaining the imbalance in nitrate production (4). Methodological factors may also have influenced the results. Planktonic cells may have been trapped within membrane filters, and differences in size between Nitrosomonas and Nitrosospira cells may have affected differential filtration, although this would have led to detection of greater relative abundance of Nitrosospira sequences in aggregate material. Other workers have observed differences in species composition in particulate and planktonic environments (1, 3, 6, 30), although these differences could not be related to differences in physiological properties. Immunofluorescence detection performed with antisera against laboratory pure cultures of members of two genera, the genera Nitrosococcus and Nitrosomonas, indicated that Nitrosomonas cells are generally more abundant in seawater, but the relative importance of these organisms and uncultured organisms is not known (49, 50). The high incidence of γ-proteobacteria in studies of the community diversity of water column systems (6, 12, 32, 35, 41, and 52) suggests that more research is needed in order to assess the importance of γ-proteobacterial ammonia oxidizers, which have been cultured only from marine environments but appear to be rare.
In conclusion, this study demonstrated that particle-associated and planktonic material may be dominated by two phylogenetically distinct populations of β-proteobacterial ammonia-oxidizers and that the differences observed may be related to the different physiological characteristics of cultured representatives of these groups. The lack of detailed physiological studies means that the environmental significance of these differences cannot be assessed in detail, but the data available suggest that nitrification in particulate material may result from activities of organisms that are distinct from the members of the planktonic population.
ACKNOWLEDGMENTS
This research was undertaken in the framework of the Mediterranean Targeted Project (MTP)-EMPS project. We acknowledge the support of the European Commission’s Marine Science and Technology (MAST) Programme under contract MAS2-CT94-0090.
We thank Allison McCaig, John Stephen (University of Aberdeen), and Richard Christen (CNRS and Université Paris 6, Paris, France).
REFERENCES
- 1.Acinas S G, Rodriguez-Valera F, Pedrós-Alió C. Spatial and temporal variation in marine bacterioplankton diversity as shown by RFLP fingerprinting of PCR amplified 16S rDNA. FEMS Microbiol Ecol. 1997;24:27–40. [Google Scholar]
- 2.Alldredge A L, Passow U, Logan B E. The abundance and significance of a class of large transparent organic particles in the ocean. Deep Sea Res Part I. 1993;40:1131–1140. [Google Scholar]
- 3.Bidle K D, Fletcher M. Comparison of free-living and particle-associated bacterial communities in the Chesapeake Bay by stable low-molecular weight RNA analysis. Appl Environ Microbiol. 1995;61:944–952. doi: 10.1128/aem.61.3.944-952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capone D G. Methane, nitrogen oxides and halomethanes. In: Rodgers J E, Whitman W B, editors. Microbial consumption of greenhouse gases. Washington, D.C: American Society for Microbiology; 1991. pp. 225–275. [Google Scholar]
- 5.Cox D J, Bazin M J, Gull K. Distribution of bacteria in a continuous-flow nitrification column. Soil Biol Biochem. 1980;12:241–246. [Google Scholar]
- 6.De Long E F, Franks D G, Alldredge A L. Phylogenetic diversity of aggregate-attached vs. free-living bacterial assemblages. Limnol Oceanogr. 1993;38:924–934. [Google Scholar]
- 7.Dugdale R C. Nutrient limitation in the sea: dynamics, identification and significance. Limnol Oceanogr. 1967;12:685–695. [Google Scholar]
- 8.Embley T M. The linear PCR reaction: a simple and robust method for sequencing amplified rRNA genes. Lett Appl Microbiol. 1991;13:171–174. doi: 10.1111/j.1472-765x.1991.tb00600.x. [DOI] [PubMed] [Google Scholar]
- 9.Embley T M, Stackebrandt E. The use of 16S ribosomal RNA sequences in microbial ecology. In: Pickup R W, Saunders J R, editors. Molecular approaches in environmental microbiology. London, United Kingdom: Prentice-Hall and Ellis-Horwood; 1996. pp. 39–62. [Google Scholar]
- 10.Felsenstein J. PHYLIP: phylogeny inference package. Seattle: University of Washington; 1993. [Google Scholar]
- 11.Fuhrman J A, Campbell L. Microbial microdiversity. Nature. 1998;393:410–411. [Google Scholar]
- 12.Fuhrman J A, McCallum K, Davis A A. Phylogenetic diversity of subsurface marine microbial communities from the Atlantic and Pacific oceans. Appl Environ Microbiol. 1993;59:1294–1302. doi: 10.1128/aem.59.5.1294-1302.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giovannoni S J, Britschgi T B, Moyer C L, Field K G. Genetic diversity in Sargasso Sea bacterioplankton. Nature. 1990;345:60–63. doi: 10.1038/345060a0. [DOI] [PubMed] [Google Scholar]
- 14.Hastings R C, Ceccherini M T, Miclaus N, Saunders J R, Bazzicalupo M, McCarthy A J. Direct and biological analysis of ammonia oxidising bacteria populations in cultivated soil plots treated with swine manure. FEMS Microbiol Ecol. 1997;23:45–54. [Google Scholar]
- 15.Head I M, Hiorns W D, Embley T M, McCarthy A J, Saunders J R. The phylogeny of autotrophic ammonia-oxidising bacteria as determined by analysis of 16S ribosomal gene sequences. J Gen Microbiol. 1993;139:1147–1153. doi: 10.1099/00221287-139-6-1147. [DOI] [PubMed] [Google Scholar]
- 16.Hiorns W D, Hastings R C, Head I M, McCarthy A J, Saunders J R, Pickup R W, Hall G H. Amplification of 16S ribosomal RNA genes of autotrophic ammonia-oxidising bacteria. Microbiology. 1995;141:2793–2800. doi: 10.1099/13500872-141-11-2793. [DOI] [PubMed] [Google Scholar]
- 17.Hovanec T A, DeLong E F. Comparative analysis of nitrifying bacteria associated with freshwater and marine aquaria. Appl Environ Microbiol. 1996;62:2888–2896. doi: 10.1128/aem.62.8.2888-2896.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson G A. TEP and coagulation during a mesocosm experiment. Deep Sea Res Part II. 1995;42:215–222. [Google Scholar]
- 19.Johnstone B H, Jones R D. Effects of light and CO on the survival of a marine ammonia-oxidizing bacterium during energy source deprivation. Appl Environ Microbiol. 1988;54:2890–2893. doi: 10.1128/aem.54.12.2890-2893.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 21.Kemp W M, Sampou P, Caffrey J, Mayer M, Henriksen K, Boynton W R. Ammonium recycling versus denitrification in Chesapeake Bay sediments. Limnol Oceanogr. 1990;35:1545–1563. [Google Scholar]
- 22.Kirchman D, Mitchell R. Contribution of particle-bound bacteria to the total microheterotrophic activity in five ponds and two marshes. Appl Environ Microbiol. 1982;43:200–209. doi: 10.1128/aem.43.1.200-209.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koops H-P, Bottcher B, Moller U C, Pommerening-Roser A, Stehr G. Description of a new species of Nitrosococcus. Arch Microbiol. 1990;154:244–248. [Google Scholar]
- 24.Kowalchuk G A, Stephen J R, De Boer W, Prosser J I, Embley T M, Woldendorp J W. Analysis of ammonia-oxidizing bacteria of the beta subdivision of the class Proteobacteria in coastal sand dunes by denaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbiol. 1997;63:1489–1497. doi: 10.1128/aem.63.4.1489-1497.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lampitt R S, Wishner K F, Turley C M, Angel M V. Marine snow studies in the North East Atlantic Ocean. Distribution, composition and role as food source for migrating plankton. Mar Biol. 1993;116:689–702. [Google Scholar]
- 26.Maidak B L, Larsen N, McCasughey J, Overbeek R, Olsen G J, Fogel K, Blandy J, Woese C R. The Ribosomal Database Project. Nucleic Acids Res. 1994;22:3485–3487. doi: 10.1093/nar/22.17.3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCaig A E, Phillips C J, Stephen J R, Kowalchuk G A, Harvey S M, Herbert R A, Embley T M, Prosser J I. Nitrogen cycling and community structure of proteobacterial β-subgroup ammonia-oxidizing bacteria within polluted marine fish farm sediments. Appl Environ Microbiol. 1999;65:213–220. doi: 10.1128/aem.65.1.213-220.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McCaig A E, Prosser J I, Embley T M. Molecular analysis of enrichment cultures of marine ammonia-oxidisers. FEMS Microbiol Lett. 1994;120:363–368. doi: 10.1111/j.1574-6968.1994.tb07059.x. [DOI] [PubMed] [Google Scholar]
- 29.Mobarry B K, Wagner M, Urbain V, Rittman B E, Stahl D A. Phylogenetic probes for analyzing abundance and spatial organization of nitrifying bacteria. Appl Environ Microbiol. 1996;62:2156–2162. doi: 10.1128/aem.62.6.2156-2162.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mullins T D, Britschgi T B, Krest R L, Giovannoni S J. Genetic comparisons reveal the same unknown bacterial lineages in Atlantic and Pacific bacterioplankton communities. Limnol Oceanogr. 1995;40:148–158. [Google Scholar]
- 31.Murray A E, Preston C M, Massana R, Taylor L T, Blakis A, Wu K, DeLong E F. Seasonal and spatial variability of bacterial and archaeal assemblages in the coastal waters near Anvers Island, Antarctica. Appl Environ Microbiol. 1998;64:2585–2595. doi: 10.1128/aem.64.7.2585-2595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rappé M S, Kemp P F, Giovannoni S J. Phylogenetic diversity of marine coastal picoplankton 16S rRNA genes cloned from the continental shelf off Cape Hatteras, North Carolina. Limnol Oceanogr. 1997;42:811–826. [Google Scholar]
- 33.Rath J, Wu K, Herndl G, DeLong E F. High phylogenetic diversity in a marine-snow-associated bacterial assemblage. Aquat Microb Ecol. 1998;14:261–269. [Google Scholar]
- 34.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 35.Schmidt T M, DeLong E F, Pace N R. Analysis of a marine picoplankton community by 16S rRNA gene cloning and sequencing. J Bacteriol. 1991;173:4371–4378. doi: 10.1128/jb.173.14.4371-4378.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simon M, Alldredge A L, Azam F. Bacterial carbon dynamics on marine snow. Mar Ecol Prog Ser. 1990;65:205–211. [Google Scholar]
- 37.Somerville C C, Knight I T, Straube W L, Colwell R R. Simple, rapid method for direct isolation of nucleic acids from aquatic environments. Appl Environ Microbiol. 1989;55:548–554. doi: 10.1128/aem.55.3.548-554.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stehr G, Bottcher B, Dittberner P, Rath G, Koops H-P. The ammonia-oxidising nitrifying population of the River Elbe estuary. FEMS Microbiol Ecol. 1995;17:177–186. [Google Scholar]
- 39.Stephen J R, McCaig A E, Smith Z, Prosser J I, Embley T M. Molecular diversity of soil and marine 16S rRNA gene sequences related to beta-subgroup ammonia-oxidizing bacteria. Appl Environ Microbiol. 1996;62:4147–4154. doi: 10.1128/aem.62.11.4147-4154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephen J R, Kowalchuk G A, Bruns M-A V, McCaig A E, Phillips C J, Embley T M, Prosser J I. Analysis of β-subgroup proteobacterial ammonia oxidizer populations in soil by denaturing gradient gel electrophoresis analysis and hierarchical phylogenetic probing. Appl Environ Microbiol. 1998;64:2958–2965. doi: 10.1128/aem.64.8.2958-2965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki M T, Rappé M S, Haimberger Z W, Winfield H, Adair N, Ströbel J, Giovannoni S J. Bacterial diversity among small-subunit rRNA gene clones and cellular isolates from the same seawater sample. Appl Environ Microbiol. 1997;63:983–989. doi: 10.1128/aem.63.3.983-989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Teske A, Alm E, Regan J M, Rittman B E, Stahl D A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J Bacteriol. 1994;176:6623–6630. doi: 10.1128/jb.176.21.6623-6630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turley C M, Borsheim K, Iriberri J, Prosser J I. The estimation of bacterial biomass in the Mediterranean. In: Turley C M, editor. The handbook of method protocols for the quality assurance pilot study of selected methods used in the Mediterranean Targetted Project, version 2. EC MAST Programme SOMG 7/84. 1996. pp. 27–34. [Google Scholar]
- 44.Turley C M, Mackie P J. Biogeochemical significance of attached and free-living bacteria and flux of particles in the NE Atlantic Ocean. Mar Ecol Prog Ser. 1994;115:191–203. [Google Scholar]
- 45.Turley C M, Lochte K, Lampitt R S. Transformations of biogenic particles during sedimentation in the North Eastern Atlantic. Phil Trans R Soc Lond B Biol Sci. 1995;348:179–189. [Google Scholar]
- 46.Utåker J B, Bakken L, Jiang Q Q, Nes I F. Phylogenetic analysis of seven new isolates of ammonia-oxidising bacteria based on 16S rRNA sequences. Syst Appl Microbiol. 1996;18:549–559. [Google Scholar]
- 47.Voytek M A, Ward B B. Detection of ammonia-oxidizing bacteria in the beta-subclass of the class Proteobacteria in aquatic samples with the PCR. Appl Environ Microbiol. 1995;61:1444–1450. doi: 10.1128/aem.61.4.1444-1450.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wagner A N, Blackstone N, Cartwright P, Dick M, Mishof B, Snow P, Wagner G P, Bartels J, Murtha M, Pendleton J. Surveys of genetic shift using polymerase chain reaction: PCR selection and PCR drift. Syst Biol. 1994;43:250–261. [Google Scholar]
- 49.Ward B B. Oceanic distribution of an ammonia-oxidizing bacteria determined by immunofluorescent assay. J Mar Res. 1982;40:1155–1172. [Google Scholar]
- 50.Ward B B, Carlucci A F. Marine ammonia- and nitrite-oxidizing bacteria: a serological diversity determined by immunofluorescence in culture and in the environment. Appl Environ Microbiol. 1985;50:194–201. doi: 10.1128/aem.50.2.194-201.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watson S W, Bock E, Harms H, Koops H-P, Hooper A B. Nitrifying bacteria. In: Staley J T, Bryant M P, Pfennig N, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 3. Baltimore, Md: Williams and Wilkins Co.; 1989. pp. 1808–1834. [Google Scholar]
- 52.Weiss P, Schweitzer B, Amann R, Simon M. Identification in situ and dynamics on limnetic organic aggregates (lake snow) Appl Environ Microbiol. 1996;62:1998–2005. doi: 10.1128/aem.62.6.1998-2005.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woese C R, Weisburg W G, Paster B J, Hahn C M, Tanner R S, Kreig N R, Koops H-P, Harms H, Stackebrandt E. The phylogeny of the purple bacteria: the beta subdivision. Syst Appl Microbiol. 1984;5:327–336. doi: 10.1016/s0723-2020(84)80034-x. [DOI] [PubMed] [Google Scholar]
- 54.Woese C R, Weisburg W G, Hahn C M, Paster B J, Zablen L B, Lewis B J, Mackie T J, Ludwig W, Stackebrandt E. The phylogeny of the purple bacteria: the gamma subdivision. Syst Appl Microbiol. 1985;6:25–33. [Google Scholar]
- 55.Yentsch C S, Menzel D W. A method for the determination of phytoplankton chlorophyll and pheophytin by fluorescence. Deep Sea Res. 1963;10:221–231. [Google Scholar]