Abstract
We examined whether the dorsolateral pontine cholinergic cells project to the paramedian reticular nucleus (PRN) of the caudal medulla. In 3 cats, wheat germ agglutinin-conjugated horseradish peroxidase (WGA-HRP) was injected into the PRN and we noted cells in the dorsolateral pons that contained the HRP reaction product, cells that were immunolabelled for choline acetyltransferase (ChAT), and cells that contained the HRP reaction product and were ChAT positive. We found cholinergic projections from the pedunculopontine tegmental and laterodorsal tegmental nuclei to the PRN. This finding is consistent with studies indicating a cholinoceptive region in the medial medulla mediating suppression of muscle tone. Our results demonstrate that this medullary region has monosynaptic input from pontine neurons implicated in generating the atonia of rapid eye movement sleep.
Keywords: Acetylcholine, Rapid eye movement sleep, Muscle control, Brainstem, Immunocytochemistry
INTRODUCTION
Magoun and Rhines first noted that electrical stimulation of the medial medulla produced a suppression of electromyographic activity in decerebrate cats13. Subsequently, it was found that such stimulation produced inhibitory postsynaptic potentials in both flexor and extensor motoneurons11,12. A projection from dorsolateral pontine regions to the nucleus magnocellularis (NMC) of the rostral medial medulla has been described and it has been suggested that this pathway may be responsible for the loss of muscle tone which normally occurs during rapid eye movement (REM) sleep. The NMC has been shown to project directly to the spinal cord32.
Recently, Lai and Siegel9 described another, more caudal region in the medulla, the paramedian reticular nucleus (PRN), which when electrically stimulated, produced loss of muscle activity in the decerebrate cat. They also found that microinfusion of acetylcholine into this region readily suppressed muscle tone. In this study we examined the brainstem source of cholinergic input into the PRN. Aside from cranial motor nuclei, the only other large concentrations of cholinergic cells in the brainstem are located in the dorsolateral pontine tegmentum5,29,34. In this study we placed wheat germ agglutinin conjugated horseradish peroxidase (WGA-HRP) into the PRN and determined whether the cholinergic neurons from the dorsolateral pontine tegmentum innervated the PRN.
MATERIALS AND METHODS
Three cats were deeply anesthetized with sodium pentobarbital (35 mg/kg) and bilateral (one cat) or unilateral (two cats) stereotaxic microinjections of WGA-HRP (0.5%, Sigma) dissolved in 0.9% saline were made into the PRN using a 1.0 μl Hamilton microsyringe. 0.05 μl of the retrograde tracer was injected at each site at a rate of 0.01 μl/min. The microsyringe was left in place for 15 min after the injection. After a 2-day period of survival, the cats were given an overdose of sodium pentobarbital and 15 min later given 3000 units of heparin intravenously. The brains were fixed by cardiac perfusion with 300 ml of 0.9% saline, 2 liters of 3% paraformaldehyde-0.1% glutaraldehyde in 0.1 M phosphate buffer (PB), and 2 liters of 10% sucrose in 0.1 M PB. The brains were blocked in the stereotaxic apparatus at the precollicular and pontomedullary levels, and then placed in 30% sucrose in 0.1 M PB solution at 4 °C until equilibrium was reached. Sections were cut at a thickness of 50 μm on an American Optical freezing microtome and immediately processed for visualization of the retrograde tracer.
All tissue sections were initially processed for HRP detection using tetramethylbenzidine (TMB) as the chromagen. The TMB was stabilized using the diaminobenzidine-cobalt chloride-hydrogen peroxide solution according to the method described by Rye et al.20. For the medullary region, alternate sections were mounted on gelatine-coated slides and counterstained with Neutral red for histological verification of the WGA-HRP injection site. The remaining series of medullary sections were mounted on gelatine-coated slides and after dehydration through a graded sequence of alcohol-xylene solutions, coverslipped with Permount. The pontine sections were processed as follows. One in four series of sections were mounted on gelatine-coated slides, and after dehydration, examined under the light microscope for HRP-labelled cells. The remaining sections in the series were immunolabelled with polyclonal antibodies against choline acetyltransferase (Chemicon), the acetylcholine synthesizing enzyme, using the avidin-biotin immunocytochemical labelling procedure29. Subsequently, the immunolabelled sections were mounted on gelatine-coated slides and after dehydration through a graded series of alcohol-xylene solutions, the slides were coverslipped with Permount.
Under the light microscope we could identify cells that contained the HRP reaction product only, cells that were choline acetyltransferase (ChAT) positive, or those cells that contained both HRP and were ChAT positive. The location of the HRP-positive, and the double-labelled HRP + ChAT-positive cells was plotted using a drawing tube attached to a Zeiss microscope. The drawings were then transferred to an IBM-PC computer using a computer-assisted design program (Prodesign, American Small Business Computers, Pryor, OK) and final plots were made on a plotter (Hewlett-Packard 7475A). The location and counts of HRP- and HRP + ChAT-positive cells were determined from all sections that were immunolabelled with the ChAT antibody. Sections that were processed for visualization of the HRP reaction product were used to confirm the presence of retrogadely labelled cells.
RESULTS
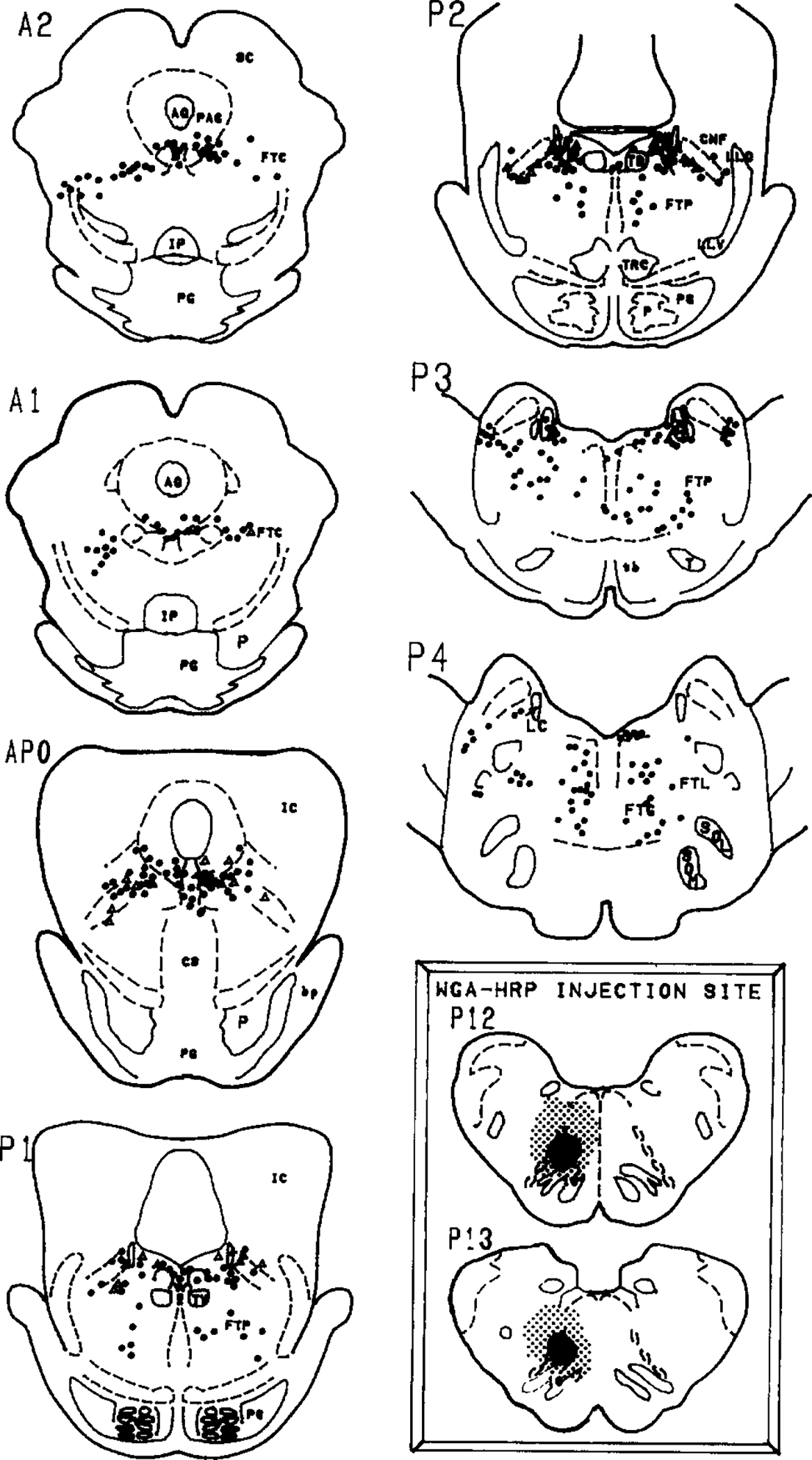
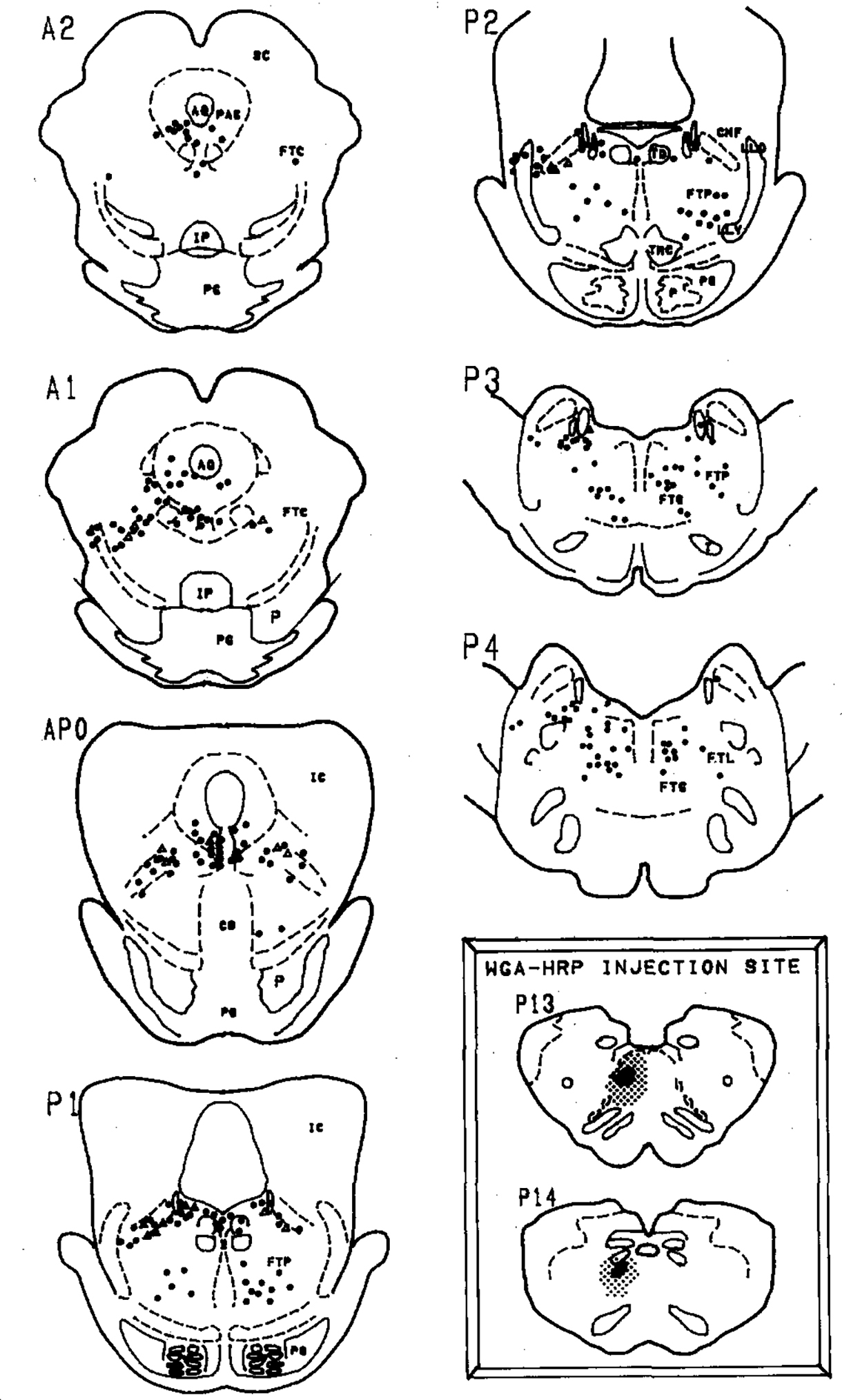
Figs. 1–3 summarize the distribution of retrogradely labelled cells in the dorsolateral pontine tegmentum. Bilateral injection of the retrograde tracer into the medial portions of the caudal medulla (Fig. 1, inset) or unilateral injections into the dorsal (Fig. 2, inset) or ventral (Fig. 3, inset) portions of the PRN produced large numbers of retrogradely labelled cells within the lateral dorsal tegmental (LDT) and pedunculopontine tegmental (PPT) cholinergic nuclei bilaterally. These two cell groups were defined as cholinergic by the presence of ChAT-immunopositive somata in accordance with previous studies by us and others5,29,34. HRP-labelled cells were noted within the gigantocellular tegmental field, the paralemniscal tegmental field (FTP), the periaqueductal gray and within the ventral portions of the locus ceruleus.
Fig. 1.
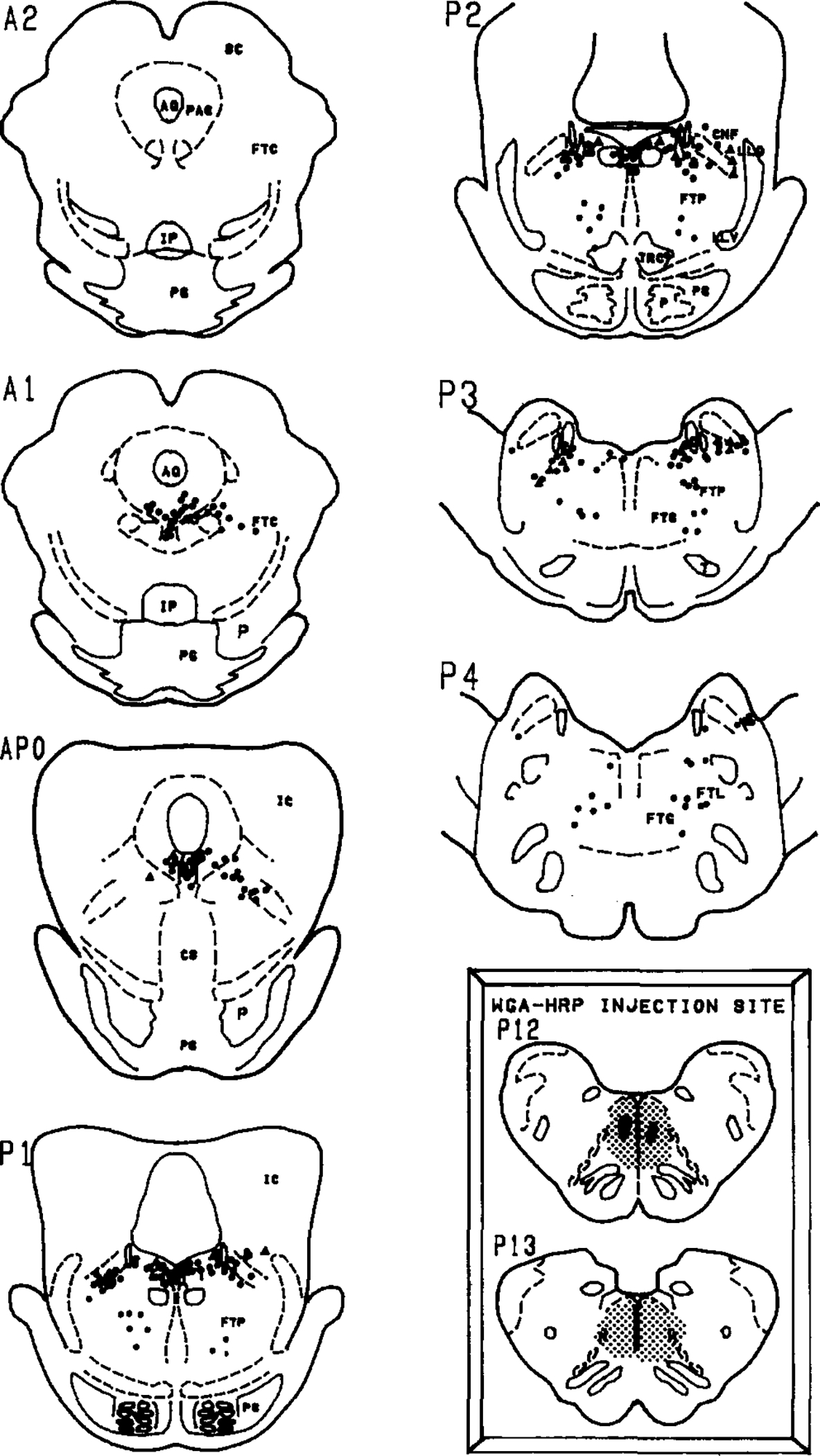
Distribution of retrogradely labelled cells in the pons following injection of WGA-HRP into the paramedial reticular nucleus of the caudal medulla (inset). Solid circles represent neurons that were singly labelled with the HRP reaction product while open triangles represent neurons that were retrogradely labelled and were ChAT immunopositive. Insets: the WGA-HRP injection site is depicted by a heavy black area while the stipling shows the extent of the diffusion. The anterior–posterior planes are from Berman’s atlas of the cat brainstem2. Selected abbreviations: AQ, aqueduct; CNF, nucleus cuneiformis; FTC, central tegmental field; FTG, gigantocellular tegmental field; FTL, lateral tegmental field; FTP, perilemniscal tegmental field; IC, inferior colliculus; IP, interpeduncular nucleus; LC, locus ceruleus; PAG, periaqueductal area; SC, superior colliculus; PG, pontine gray.
Fig. 3.
Distribution of retrogradely labelled cells in the pons following injection of WGA-HRP into the paramedial reticular nucleus of the caudal medulla (inset). Solid circles represent neurons that were singly labelled with the HRP reaction product while open triangles represent neurons that were retrogradely labelled and were ChAT immunopositive. Insets: the WGA-HRP injection site is depicted by a heavy black area while the stipling shows the extent of the diffusion. The anterior–posterior planes are from Berman’s atlas of the cat brainstem2. Selected abbreviations: AQ, aqueduct; CNF, nucleus cuneiformis; FTC, central tegmental field; FTG, gigantocellular tegmental field; FTL, lateral tegmental field; FTP, perilemniscal tegmental field; IC, inferior colliculus; IP, interpeduncular nucleus; LC, locus ceruleus; PAG, periaqueductal area; SC, superior colliculus; PG, pontine gray.
Fig. 2.
Distribution of retrogradely labelled cells in the pons following injection of WGA-HRP into the paramedial reticular nucleus of the caudal medulla (inset). Solid circles represent neurons that were singly labelled with the HRP reaction product while open triangles represent neurons that were retrogradely labelled and were ChAT immunopositive. Insets: the WGA-HRP injection site is depicted by a heavy black area while the stipling shows the extent of the diffusion. The anterior–posterior planes are from Berman’s atlas of the cat brainstem2. Selected abbreviations: AQ, aqueduct; CNF, nucleus cuneiformis; FTC, central tegmental field; FTG, gigantocellular tegmental field; FTL, lateral tegmental field; FTP, perilemniscal tegmental field; IC, inferior colliculus; IP, interpeduncular nucleus; LC, locus ceruleus; PAG, periaqueductal area; SC, superior colliculus; PG, pontine gray.
Some of the retrogradely labelled cells in the PPT and LDT were found to be ChAT positive. Within the PPT, we noted that the retrogradely labelled cholinergic cells were located mostly within the ventral and lateral portions of the PPT. Within the LDT, we found double-labelled cells to lie close to the locus ceruleus. However, the ventral portion of the locus ceruleus which Sakai et al.22 labels the locus ceruleus alpha region, was relatively devoid of double-labelled cells even though there were numerous HRP-labelled cells and some ChAT-positive cells in this region.
Table I summarizes the numbers of retrogradely labelled and double-labelled cells. We noted that 9.8–25.6% of retrogradely labelled cells in the PPT were ChAT positive, while in the LDT, 4.9–21.4% of the retrogradely labelled cells were ChAT positive. There were more retrogradely labelled cells in the PPT ipsilateral to the injection site. However, we noted that the percentage of HRP + ChAT-positive cells was about evenly distributed to the ipsilateral and contralateral sides. Thus, the cholinergic cells in the LDT and PPT project uniformly to both the ipsilateral and contralateral PRN.
TABLE I. Number of cells that were retrogradely labelled in the lateral dorsal tegmental (LDT) and pedunculopontine tegmental (PPT) nuclei.
Ipsi and contra refer to ipsilateral and contralateral. For AT4, where the WGA-HRP was injected bilaterally into the medial caudal medulla, ipsi and contra represent left and right sides of the pons (see Fig. 1). The distribution of retrogradely labelled cell for subjects AT7 and AT5 are shown in Figs. 2 and 3, respectively. Numbers in parentheses represent percentage of retrogradely labelled cells that were also ChAT positive.
| Subject | Pedunculopontine tegmental nucleus | Lateral dorsal tegmental nucleus | ||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Retrogradely labelled cells | ChAT + HRP cells | Retrogradely labeled cells | ChAT + HRP cell | |||||
|
|
|
|
|
|||||
| Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | Ipsi | Contra | |
|
| ||||||||
| AT4 | 61 | 136 | 6 (9.8) | 14 (10.3) | 61 | 131 | 3 (4.9) | 11 (8.4) |
| AT7 | 86 | 21 | 22 (25.6) | 5 (23.8) | 27 | 12 | 4 (14.8) | 1 (8.3) |
| AT5 | 142 | 112 | 19 (13.4) | 15 (13.4) | 38 | 42 | 6 (15.8) | 9 (21.4) |
As for the percentage of ChAT-positive cells that project to the PRN, we found that the medial portions of the PRN (Fig. 1, inset) receives projections from 0.9% of cholinergic PPT neurons while the dorsal and ventral PRN receives projections from 1.2% and 1.5% of PPT cholinergic neurons, respectively. As for the LDT, we noted that the medial portion of the PRN receives cholinergic input from 1.3% of LDT cells while the dorsal and ventral PRN received 0.5% and 1.4%, respectively. Thus, only about 0.5–1.5% of dorsolateral pontine cholinergic cells project to the PRN. Previously it has been shown that the major portion of the projection of the LDT and PPT is ascending3,4,14,17,23–25,31,35,36.
DISCUSSION
This study demonstrated that the cholinergic cells from the LDT and PPT project to the area of PRN in the caudal medulla from where electrical stimulation and microinfusion of acetylcholine and cholinergic agonists readily produce loss of muscle tone in decerebrate cats. Therefore, these findings provide anatomical support for the study by Lai and Siegel9. These results are also in agreement with the most recent findings of projections from the PPT to the caudal medulla by Rye et al. in the rat19. Thus, the cholinergic projection from the dorsolateral pons to the PRN may be part of the pathway producing the suppression of muscle tone seen in the decerebrate cat after systemic as well as local administration of cholinergic agonists. This pathway may also be involved in mediating the sudden loss of muscle tone (cataplexy) which occurs in narcolepsy. Besides cataplectic attacks, narcolepsy is characterized by excessive daytime sleepiness, direct transitions from waking to REM sleep and hypnagogic hallucinations. It has been suggested that some of the systems of narcolepsy, such as cataplexy and sleep-onset REM sleep, may be due to hyperactive brainstem cholinergic mechanisms26.
Support for the role of cholinergic mechanisms in atonia is derived from studies where microinjection of cholinergic agonists into the dorsolateral and medial pons produces a profound suppression of muscle tone in cats1,6,16,27,28. Electrical and cholinergic stimulation of the midline pons produces loss of muscle tone in cats18. Recently, we have determined that the cholinergic-induced loss of muscle tone in the pons may be mediated by the M2 muscarinic receptor33. It has been postulated for some time that the cholinergic-induced inhibition of muscle tone may be mediated by cholinergic as well as cholinoceptive pontine cells21,26. In this study we found in the midline pons (raphe pontis) and medial and dorsolateral pons, numerous retrogradely labelled cells which were not ChAT immunopositive. These cells were in areas where infusion of cholinergic agonists readily triggers atonia and REM sleep, and therefore, these cells might be hypothesized to be cholinoceptive. The medial pontine cholinoceptive cells receive input from the LDT-PPT cholinergic neurons10,15,30, and therefore, atonia may be mediated by a polysynaptic pathway involving the LDT/PPT, medial pons and PRN. Some of the axons may descend within the medial longitudinal fasciculus18.
Besides cholinergic agonists, glutamate has also been found to evoke atonia when injected into the regions ventral to the locus ceruleus9. Lai and Siegel suggest that the glutamate-sensitive/glutamatergic neurons induce atonia by impinging on cells in the NMC9. Electrical stimulation and microinjections of glutamate into the NMC readily evoke atonia in decerebrate cats and this region corresponds to the Magoun and Rhines inhibitory center9.
Thus, we hypothesize that loss of muscle tone may be due to both poly- and mono-synaptic pathways which involve activation of glutamate-sensitive and cholinergic/cholinoceptive cells in the ventral and lateral portions of the dorsolateral and medial pons. The pontine glutaminergic neurons act via the NMC and the cholinergic/cholinoceptive neurons exert their influence via pontine cholinoceptive neurons and via the PRN. Both the NMC and PRN have been shown to have massive projections to the spinal cord7,8,32.
Acknowledgements.
We thank Ms. Cheryl Floyd for providing assistance with histology. This research was supported by grants from the American Narcolepsy Association (P.J.S, J.S.), NIH-NS25212 (P.J.S.), HL41370, MH43811 (J.S.).
REFERENCES
- 1.Baghdoyan HA, Rodrigo-Angula ML, McCarley RW and Hobson JA, Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem sites, Brain Research, 306 (1984) 39–52. [DOI] [PubMed] [Google Scholar]
- 2.Berman AL, In The Brainstem of the Cat, The University of Wisconsin Press, Madison, 1968. [Google Scholar]
- 3.Hoover DB and Baisden RH, Localization of putative cholinergic neurons innervating the anteroventral thalamus, Brain Res. Bull, 5 (1980) 519–524. [DOI] [PubMed] [Google Scholar]
- 4.Hoover DB and Jacobowitz DM, Neurochemical and histochemical studies of the effect of a lesion of the nucleus cuneiformis on the cholinergic innervation of discrete areas of the rat brain, Brain Research, 170 (1979) 113–122. [DOI] [PubMed] [Google Scholar]
- 5.Jones BE and Beaudet A, Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyltransferase and tyrosine hydroxylase immunohistochemical study, J. Comp. Neurol, 261 (1987) 15–32. [DOI] [PubMed] [Google Scholar]
- 6.Katayama Y, DeWitt DS, Becker DP and Hayes RL, Behavioral evidence for cholinoceptive pontine inhibitory area: descending control of spinal motor output and sensory input, Brain Research, 296 (1984) 241–262. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers HGJM and Maisky VA, Retrograde axonal transport of horseradish peroxidase from spinal cord to brainstem cell group in the cat, Neurosci. Lett, 1 (1975) 9–14. [DOI] [PubMed] [Google Scholar]
- 8.Kuypers HGJM and Maisky VA, Funicular trajectories of the descending brainstem pathway in cat, Brain Research, 136 (1977) 159–165. [DOI] [PubMed] [Google Scholar]
- 9.Lai YY and Siegel J, Medullary regions mediating atonia, J. Neurosci, 8 (1988) 4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leichnetz G, Carlton S, Katayama Y, Gonzalo-Ruiz A, Holstege G, DeSalles A and Hayes RL, Afferent and efferent connections of the cholinoceptive medial pontine reticular formation (region of the ventral tegmental nucleus) in the cat, Brain Res. Bull, 22 (1989) 665–688. [DOI] [PubMed] [Google Scholar]
- 11.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons, J. Neurophysiol, 27 (1964) 579–591. [DOI] [PubMed] [Google Scholar]
- 12.Llinas R and Terzuolo CA, Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms upon flexor motoneurons, J. Neurophysiol, 28 (1965) 413–422. [DOI] [PubMed] [Google Scholar]
- 13.Magoun HW and Rhines R, An inhibitory mechanism in the bulbar reticular formation, J. Neurophysiol, 9 (1946) 165–171. [DOI] [PubMed] [Google Scholar]
- 14.Mesulam MM, Mufson EJ, Wainer BH and Levey AI, Central cholinergic pathways in the rat: an overview based on an alternative nomenclature, Neuroscience, 10 (1983) 1185–1201. [DOI] [PubMed] [Google Scholar]
- 15.Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K and McCarley RW, Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat, Brain Research, 451 (1988) 397–402. [DOI] [PubMed] [Google Scholar]
- 16.Mitler M and Dement WC, Cataplectic-like behavior in cats after microinjection of carbachol in pontine reticular formation, Brain Research, 468 (1974) 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nomura T, Mizuno N and Sugimoto T, Direct projections from the pedunculopontine tegmental nucleus to the subthalamic nucleus in the cat, Brain Research, 196 (1980) 223–227. [DOI] [PubMed] [Google Scholar]
- 18.Ohta Y, Mori S and Kimura H, Neuronal structures of the brainstem participating in postural suppression in cats, Neurosci. Res, 5 (1988) 181–202. [DOI] [PubMed] [Google Scholar]
- 19.Rye DB, Lee HJ, Saper CB and Wainer BH, Medullary and spinal efferents of the pedunculopontine tegmental nucleus and adjacent mesopontine tegmental in the rat, J. Comp. Neurol, 269 (1988) 315–341. [DOI] [PubMed] [Google Scholar]
- 20.Rye DB, Saper CB and Wainer BH, Stabilization of the tetramethylbenzidine (TMB) reaction product: application for retrograde and anterograde tracing, and combination with immunohistochemistry, J. Histochem. Cytochem, 32 (1984) 1145–1153. [DOI] [PubMed] [Google Scholar]
- 21.Sakai K, Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In Hobson JA and Brazier MB (Eds.), The Reticular Formation Revisited, Raven Press, New York, 1980, pp. 427–447. [Google Scholar]
- 22.Sakai K, Sastre JP, Salvert D, Touret M, Tohyama M and Jouvet M, Tegmentoreticular projections with special reference to the muscular atonia during paradoxical sleep in the cat: an HRP study, Brain Research, 176 (1979) 233–254. [DOI] [PubMed] [Google Scholar]
- 23.Saper CB, Reciprocal parabrachial–cortical connections in the rat, Brain Research, 242 (1982) 33–40. [DOI] [PubMed] [Google Scholar]
- 24.Saper CB and Loewy AD, Efferent connections of the parabrachial nucleus in the rat, Brain Research, 197 (1980) 291–317. [DOI] [PubMed] [Google Scholar]
- 25.Saper CB and Loewy AD, Projections of the pedunculopontine tegmental nucleus in the rat: evidence for additional extrapyramidal circuitry, Brain Research, 252 (1982) 367–372. [DOI] [PubMed] [Google Scholar]
- 26.Shiromani P, Gillin JC and Henriksen SJ, Acetylcholine and the regulation of REM sleep: basic mechanisms and clinical implication for affective illness and narcolepsy, Annu. Rev. Pharmacol. Toxicol, 27 (1987) 137–156. [DOI] [PubMed] [Google Scholar]
- 27.Shiromani P and McGinty DJ, Pontine neuronal response to local cholinergic microinfusion: relation to REM sleep, Brain Research, 386 (1986) 20–31. [DOI] [PubMed] [Google Scholar]
- 28.Shiromani P, Siegel JM, Tomaszewski K and McGinty DJ, Alterations in blood pressure and REM sleep after pontine carbachol microinfusion, Exp. Neurol, 91 (1986) 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiromani PJ, Armstrong DM, Berkowitz A, Jeste DV and Gillin JC, Distribution of choline acetyltransferase immunoreactive somata in the feline brainstem: implications for REM sleep generation, Sleep, 11 (1988) 1–16. [DOI] [PubMed] [Google Scholar]
- 30.Shiromani PJ, Armstrong DM and Gillin JC, Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study, Neurosci. Lett, 95 (1988) 19–23. [DOI] [PubMed] [Google Scholar]
- 31.Sofroniew MV, Priestley JV, Consolazione A, Eckenstein E and Cuello AC, Cholinergic projections from the midbrain and pons to the thalamus in the rat, identified by combined retrograde tracing and choline acetyltransferase immunohistochemistry, Brain Research, 329 (1985) 213–223. [DOI] [PubMed] [Google Scholar]
- 32.Tohyama M, Sakai K, Salvert D, Touret M and Jouvet M, Spinal projections from the lower brainstem in the cat as demonstrated by the horseradish peroxidase technique. I. Origins of the reticulospinal tracts and their funicular trajectories, Brain Research, 173 (1979) 383–403. [DOI] [PubMed] [Google Scholar]
- 33.Velazquez-Moctezuma J, Gillin JC and Shiromani PJ, Effect of specific M1,M2 muscarinic receptor agonists on REM sleep generation, Brain Research, 503 (1989) 128–131. [DOI] [PubMed] [Google Scholar]
- 34.Vincent SR and Reiner PB, The immunohistochemical localization of choline acetyltransferase in the cat brain, Brain Res. Bull, 18 (1987) 371–415. [DOI] [PubMed] [Google Scholar]
- 35.Wilson PM, A photographic perspective on the origins, form, course and relations of the acetylcholinesterase containing fibres of the dorsal tegmental pathway in the rat brain, Brain Res. Rev, 10 (1985) 85–118. [DOI] [PubMed] [Google Scholar]
- 36.Woolf NJ and Butcher LL, Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain, Brain Res. Bull, 16 (1986) 603–637. [DOI] [PubMed] [Google Scholar]


