Abstract
Objectives
Chronic exposure to arsenic has been reported as a risk factor for nonmelanoma skin cancer, notably squamous cell carcinoma. However, current knowledge is limited about the association between arsenic exposure and melanoma. Our objectives were to (1) measure the association between total urinary arsenic levels and melanoma compared with nonmelanoma skin cancer and no cancer and (2) analyze the association between water source and melanoma and nonmelanoma skin cancer.
Methods
We collected cross-sectional data from the 2003-2016 cycles of the National Health and Nutrition Examination Survey. We conducted univariate and multivariate logistic regressions. To evaluate the possible association of skin cancer with source of tap water, we calculated odds ratios for participants with melanoma and nonmelanoma skin cancer, compared with participants with no cancer.
Results
White race, higher education, higher socioeconomic status, and smoking history were associated with melanoma and nonmelanoma skin cancer in the full study population. After adjusting for age and race/ethnicity, the adjusted odds ratio of participants with >50 μg/L of total urinary arsenic for melanoma or nonmelanoma skin cancer was 1.87 (95% CI, 0.58-6.05) and 2.23 (95% CI, 1.12-4.45) times higher compared with no cancer, respectively. Participants with nonmelanoma skin cancer had 2.06 increased odds of reporting a nonmunicipal water source compared with participants without cancer.
Conclusions
We did not find a relationship between the incidence of melanoma and exposure to arsenic among US adults. Nonmunicipal water sources were associated with nonmelanoma skin cancer and should be further investigated.
Keywords: arsenic, melanoma, NHANES, nonmelanoma skin cancer, skin cancer
Skin cancer, notably melanoma and nonmelanoma skin cancer, is one of the most common cancer diagnoses in the United States. 1,2 An estimated 1 in 5 people in the United States will develop skin cancer by age 70 years. 3 Nonmelanoma skin cancers are the most common forms of skin cancer, are highly treatable, and have low rates of metastasis and mortality, 1 whereas melanoma is less common (1% of skin cancer diagnoses) but far more dangerous because of its ability to metastasize if not managed early. 4 Melanoma is a serious public health concern, with the 2017 age-adjusted rate of melanoma at 22.7 per 100 000 population in the United States and the number of new melanoma cases expected to increase by 5.8% in 2021. 3,5
Arsenic, consisting of 2 chemical forms (organic and inorganic), is considered one of the most toxic metals. Exposure to arsenic contributes to several health problems, including an increased risk of cardiovascular disease, diabetes, and pulmonary disease. Inorganic arsenic is classified as a human carcinogen and is associated with cancer of the bladder, kidney, prostate, and liver, as well as nonmelanoma skin cancer. 6 -9 More than 140 million people in 50 countries consume water that contains arsenic at a level >10 μg/L. 8 The US Environmental Protection Agency lowered the maximum contaminant level of arsenic allowable in public water systems from 50 to 10 parts per billion in 2001. 10 However, private wells are not monitored by this agency, and approximately 10% of people in the United States drink water from an unregulated source. 10,11
Studies that analyzed possible links between melanoma and arsenic exposure had conflicting results. A case-control study in Iowa investigated the association between the concentration of arsenic in participants’ toenails and the incidence of melanoma compared with people in a control group who had a diagnosis of colorectal cancer. In that study, participants with the highest arsenic levels in their toenails (≥0.084 μg/g) were 2 times more likely than participants with the lowest levels (≤0.020 μg/g) to develop melanoma. 12 In contrast, a retrospective study (adjusted for ultraviolet radiation) conducted in New Mexico in a non-Hispanic White population found no association between the concentration of arsenic in toenails and urine and the incidence of melanoma (0.09 ± 0.06 μg/g and 5.0 ± 3.3 μg/L, respectively) compared with controls (0.11 ± 0.06 μg/g and 6.0 ± 3.2 μg/L, respectively). 13
Although studies have provided evidence of the occurrence of nonmelanoma skin cancer with increasing arsenic exposure, the possible association between melanoma and arsenic exposure is not fully understood. Hence, the primary objective of this study was to measure the association between melanoma and total urinary arsenic levels. A secondary objective was to compare this association with the association between nonmelanoma skin cancer and total urinary arsenic levels. We also analyzed the association between water source and melanoma and nonmelanoma skin cancer.
Methods
Data Source
We conducted a cross-sectional study using National Health and Nutrition Examination Survey (NHANES) data from 2003-2016. Details on NHANES are available elsewhere. 14 Briefly, NHANES is a continuous, annual national mobile health survey conducted in cycles and designed to assess adults’ and children’s health and nutritional status in the United States. The NHANES database is representative of the US general noninstitutionalized population. NHANES consists of interview questionnaires and examination components. The survey uses a complex, multistage, and stratified design with oversampling. Its protocols were approved by the National Center for Health Statistics Institutional Review Board, and consent was obtained from all participants. 14
Study Sample, Inclusion and Exclusion Criteria, and Data Collection
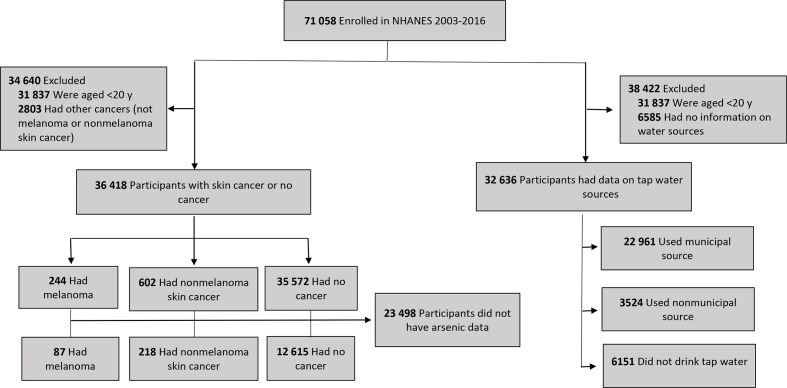
The NHANES cycles from 2003 through 2016 had a total of 71 058 participants. We excluded NHANES participants if they were aged <20 years (n = 31 837) or had a history of cancer other than skin cancer (n = 2803) (Figure). We divided this subsample of 36 418 participants into 3 groups (no cancer, n = 35 572; melanoma, n = 244; and nonmelanoma skin cancer, n = 602) and examined their characteristics. We then excluded participants missing data on total urinary arsenic levels (n = 23 498), resulting in a sample of 12 920 participants, whom we further classified into the same 3 groups (no cancer, n = 12 615; melanoma, n = 87; and nonmelanoma skin cancer, n = 218). For the analysis on water sources, we excluded participants with no information on tap water source (n = 6585). The population with data on tap water source was 32 636 participants.
Figure.
Flow diagram of participants in study on arsenic exposure and melanoma among US adults aged ≥20, National Health and Nutrition Examination Survey (NHANES), 2003-2016. Data source: Centers for Disease Control and Prevention. 15
Cancer data in NHANES are self-reported in the medical conditions questionnaire (MCQ), which is administered only to participants aged ≥20. We identified participants with skin cancer by using codes MCQ230A, MCQ230B, MCQ230C, and MCQ230D from the MCQ. NHANES measured total urinary arsenic levels on a subsample (approximately one-third) of the NHANES population by using inductively coupled plasma dynamic reaction cell mass spectrometry. 16 We extracted laboratory data on total urinary arsenic levels for the first 5 NHANES cycles (2003-2012) by using these data: “Arsenic—Total & Speciated—Urine” or “Arsenic—Total & Speciated—Urine—Special Sample.” For the last 2 cycles (2013-2016), we extracted these data by using “Arsenic—Total—Urine” or “Arsenic—Total—Urine—Special Sample.” 15 We categorized data on total urinary arsenic levels into 2 groups (≤50 μg/L or ˃50 μg/L) based on previous data on arsenic toxicity in urine from the Minnesota Department of Health. 17
Covariates
We examined the following demographic variables from NHANES 2003-2016: age included in years and as groups (20-34, 35-49, 50-64, and ≥65), sex (male or female), and race/ethnicity. We categorized race/ethnicity as White or non-White; the latter category included Mexican American, other Hispanic, non-Hispanic Black, and other races. We also examined data on 2 indicators of socioeconomic status (SES): income and education. For income, we used ratio of family income to poverty, defined in NHANES as total family income divided by the federal poverty level. On the basis of the Supplemental Nutrition Assistance Program (SNAP) eligibility criteria cited in NHANES analytic guidelines 1999-2010, we used the following income categories: 0-1.30 (hereinafter, lowest income), 1.31-3.50 (hereinafter, middle income), or 3.51-5.00 (hereinafter, highest income). 18 We used the following categories of education: ≤high school graduate, some college or associate’s degree, or ≥college graduate. 18
We extracted additional data, as available, on the number of sunburns. These data are collected by NHANES only for adults aged 20-59; we categorized these data into “never” and “ever” as a measure of ultraviolet light exposure. Smoking status was also categorized into 2 groups (never or ever) based on the following questions: “Have you smoked at least 100 cigarettes in your entire life?” and “Do you now smoke cigarettes?” We collected data on tap water source from the dietary survey and categorized these data as municipal (community supplies), nonmunicipal (well/rain cistern and springs), and does not drink tap water.
Statistical Methods
NHANES is designed to produce national estimates representative of the total noninstitutionalized US population. We followed NHANES guidelines for combining survey cycles, generating sample weights for subsamples, and using the strata and primary sampling unit information (variance) provided on public-use files for variance estimation. This process estimates the statistical results as though the entire sampling frame were surveyed. We divided the subsample weight variables that are included with arsenic data in NHANES by 7 to ensure national representation for the 7 cycles.
The dependent variables were melanoma and nonmelanoma skin cancer. The primary independent variable was total urinary arsenic level. We also explored tap water source as an independent variable. We used the Pearson χ2 test or Fisher exact test for categorical variables and t test for continuous variables to compare participants with melanoma and participants with nonmelanoma skin cancer with participants with no cancer. We considered P < .05 to be significant. We conducted univariate and multivariate logistic regression analyses to detect variables that had a significant effect on melanoma and nonmelanoma skin cancer. We reported crude odds ratios (ORs), adjusted ORs (aORs), and 95% CIs for the univariate and multivariate analyses. We used the Breslow-Day test (at the .10 significance level) to identify effect modifiers with total urinary arsenic levels. If we found no interaction, we tested for confounders on the basis of a 10% difference between the OR and the aOR.
We built 2 models: one for melanoma and another for nonmelanoma skin cancer. These models comprised all available participants after adjusting for age and race/ethnicity. We did not add the sunburn variable to the multivariate analysis because of low statistical power. We used SAS version 9.4 (SAS Institute, Inc) for all analyses.
Results
Study Population
In our sample of 36 418 participants, the mean (SE) age for melanoma and nonmelanoma skin cancer was similar at 62.7 (0.8) and 63.5 (0.7) years, respectively. Participants without cancer were significantly younger (mean [SE] age, 45.4 [0.2] years; P < .001). We found more men than women in both skin cancer groups; however, the association between skin cancers and sex was not significant (Table 1).
Table 1.
Characteristics of study participants aged ≥20, by type of skin cancer (N = 36 418), National Health and Nutrition Examination Survey (NHANES), 2003-2016a,b
| Characteristic | No cancer, no. (column %
c
) (n = 35 572) |
Melanoma, no. (column %
c
) (n = 244) |
P value d | Nonmelanoma skin cancer, no. (column % c ) (n = 602) | P value d |
|---|---|---|---|---|---|
| Age, mean (SE), y | 45.4 (0.2) | 62.8 (0.8) | <.001 | 63.5 (0.7) | <.001 |
| Age group, y | <.001 | <.001 | |||
| 20-34 | 10 108 (28.4) | 8 (3.3) | 10 (1.7) | ||
| 35-49 | 9455 (26.6) | 25 (10.2) | 48 (8.0) | ||
| 50-64 | 8553 (24.0) | 59 (24.2) | 137 (22.8) | ||
| ≥65 | 7456 (21.0) | 152 (62.3) | 407 (67.6) | ||
| Sex | .34 | .06 | |||
| Male | 17 256 (48.5) | 135 (55.3) | 341 (56.6) | ||
| Female | 18 316 (51.5) | 109 (44.7) | 261 (43.4) | ||
| Race/ethnicity | <.001 | <.001 | |||
| White | 14 750 (41.5) | 221 (90.6) | 570 (94.7) | ||
| Non-White e | 20 822 (58.5) | 23 (9.4) | 32 (5.3) | ||
| Education level | <.001 | <.001 | |||
| ≤High school graduate | 17 817 (50.2) | 90 (36.9) | 192 (31.9) | ||
| Some college or associate’s degree | 10 103 (28.4) | 81 (33.2) | 184 (30.6) | ||
| ≥College graduate | 7594 (21.4) | 73 (29.9) | 226 (37.5) | ||
| Missing | 58 | 0 | 0 | ||
| Ratio of family income to poverty f | <.001 | <.001 | |||
| 0-1.30 | 10 669 (32.8) | 38 (17.1) | 78 (13.9) | ||
| 1.31-3.50 | 12 150 (37.3) | 93 (41.9) | 206 (36.8) | ||
| 3.51-5.00 | 9723 (29.9) | 91 (41.0) | 276 (49.3) | ||
| Missing | 3030 | 22 | 42 | ||
| Had a sunburn | .61 | .08 | |||
| Never | 13 668 (64.1) | 31 (51.7) | 60 (48.4) | ||
| Ever | 7646 (35.9) | 29 (48.3) | 64 (51.6) | ||
| Missing | 14 258 | 184 | 478 | ||
| Smoking status | <.001 | <.001 | |||
| Never smoker | 19 774 (55.6) | 102 (41.8) | 262 (43.5) | ||
| Ever smoker | 15 764 (44.4) | 142 (58.2) | 340 (56.5) | ||
| Missing | 34 | 0 | 0 | ||
aData source: Centers for Disease Control and Prevention. 15
bOf 71 058 participants in NHANES in 2003-2016, participants aged <20 years (n = 31 837) or who had a history of cancer other than skin cancer (n = 2803) were excluded.
cPercentages may not total to 100 because of rounding.
d P values for continuous variables determined by t test; for categorical values, Pearson χ2 test or Fisher exact test; P < .05 was considered significant.
eIncludes Mexican American, other Hispanic, non-Hispanic Black, and other races.
fOn the basis of the Supplemental Nutrition Assistance Program eligibility criteria cited in NHANES analytic guidelines 1999-2010, 0-1.30 indicates lowest income, 1.31-3.50 indicates middle income, and 3.51-5.00 indicates highest income. 18
Participants with melanoma or nonmelanoma skin cancer were significantly more likely to be White than non-White (P < .001). Participants with at least a college degree or associate’s degree were more likely to report melanoma or nonmelanoma skin cancer than no skin cancer (P < .001). The percentage of participants with melanoma and nonmelanoma skin cancer increased significantly as income increased (P < .001). We found no difference in history of sunburn between participants with melanoma and participants with nonmelanoma skin cancer. Ever smokers were significantly more likely than never smokers to report melanoma (P < .001) or nonmelanoma skin cancer (P < .001) (Table 1).
Study Population With Arsenic Data
Of the 12 920 participants with arsenic data, 7.3% had total urinary arsenic levels >50 μg/L. Skin cancer was significantly more common among White participants (90.8%) than among non-White participants (9.2%) (P < .001). Education and income levels were not significant for the melanoma group, but these SES factors were significant for the nonmelanoma skin cancer group (P = .01 for education; P = .002 for income) when compared with participants with no cancer. The association between history of sunburn and melanoma (P = .07) or nonmelanoma skin cancer (P = .43) was not significant. The association between smoking status and nonmelanoma skin cancer, but not melanoma, was significant (P = .01) (Table 2).
Table 2.
Characteristics of study participants aged ≥20 with information on total urinary arsenic levels (n = 12 920), by type of skin cancer, National Health and Nutrition Examination Survey (NHANES), 2003-2016a,b
| Characteristic | No cancer, no. (column %
c
) (n = 12 615) |
Melanoma, no. (column %
c
) (n = 87) |
P value d | Nonmelanoma skin cancer, no.
(column %
c
) (n = 218) |
P value d |
|---|---|---|---|---|---|
| Total urinary arsenic levels | .41 | .09 | |||
| ≤50 μg/L | 11 698 (92.7) | 82 (94.2) | 199 (91.3) | ||
| >50 μg/L | 917 (7.3) | 5 (5.8) | 19 (8.7) | ||
| Age, mean (SE), y | 45.4 (0.3) | 64.6 (0.7) | <.001 | 63.3 (1.0) | <.001 |
| Age group, y | <.001 | <.001 | |||
| 20-34 | 3598 (28.5) | 1 (1.2) | 4 (1.8) | ||
| 35-49 | 3446 (27.3) | 10 (11.5) | 20 (9.2) | ||
| 50-64 | 3163 (25.1) | 23 (26.4) | 59 (27.1) | ||
| ≥65 | 2408 (19.1) | 53 (60.9) | 135 (61.9) | ||
| Sex | .59 | .19 | |||
| Male | 6314 (50.0) | 45 (51.7) | 124 (56.9) | ||
| Female | 6301 (50.0) | 42 (48.3) | 94 (43.1) | ||
| Race/ethnicity | <.001 | <.001 | |||
| White | 5283 (41.9) | 79 (90.8) | 205 (94.0) | ||
| Non-White e | 7332 (58.1) | 8 (9.2) | 13 (6.0) | ||
| Education level | .25 | .01 | |||
| ≤High school graduate | 6435 (51.0) | 33 (37.9) | 69 (31.6) | ||
| Some college or associate’s degree | 3495 (29.3) | 31 (35.6) | 79 (36.2) | ||
| ≥College graduate | 2477 (19.6) | 23 (26.4) | 70 (32.1) | ||
| Missing | 8 | 0 | 0 | ||
| Ratio of family income to poverty f | .07 | .002 | |||
| 0-1.30 | 4000 (33.4) | 13 (15.7) | 37 (18.1) | ||
| 1.31-3.50 | 4367 (37.6) | 40 (48.2) | 79 (38.5) | ||
| 3.51-5.00 | 3248 (28.0) | 30 (36.1) | 89 (43.4) | ||
| Missing | 1000 | 4 | 13 | ||
| Had a sunburn | .07 | .43 | |||
| Never | 5059 (64.0) | 7 (29.2) | 27 (54.0) | ||
| Ever | 2846 (36.0) | 17 (70.8) | 23 (46.0) | ||
| Missing | 4710 | 63 | 168 | ||
| Smoking status | .08 | .01 | |||
| Never smoker | 6200 (49.2) | 31 (35.6) | 83 (38.1) | ||
| Ever smoker | 6411 (50.8) | 56 (64.4) | 135 (61.9) | ||
| Missing | 4 | 0 | 0 | ||
aData source: Centers for Disease Control and Prevention. 15
bOf 71 058 participants in NHANES in 2003-2016, participants who were aged <20 years (n = 31 837), had a history of cancer other than skin cancer (n = 2803), or had no information on total urinary arsenic levels (n = 23 498) were excluded.
cPercentages may not total to 100 because of rounding.
d P values for continuous variables determined by t test; for categorical values, Pearson χ2 test or Fisher exact test; P < .05 was considered significant.
eIncludes Mexican American, other Hispanic, non-Hispanic Black, and other races.
fOn the basis of the Supplemental Nutrition Assistance Program eligibility criteria cited in NHANES analytic guidelines 1999-2010, 0-1.30 indicates lowest income, 1.31-3.50 indicates middle income, and 3.51-5.00 indicates highest income. 18
We found no significant association between melanoma and total urinary arsenic levels (OR = 1.45; 95% CI, 0.46-4.62) or between nonmelanoma skin cancer and total urinary arsenic levels (OR = 1.69; 95% CI, 0.91-3.16) (Table 3). We found a significant association between skin cancer and age. For each year increase in age, the OR increased by 1.08 (95% CI, 1.07-1.10) for melanoma and 1.07 (95% CI, 1.06-1.08) for nonmelanoma skin cancer.
Table 3.
Univariate logistic regression of study participants aged ≥20 with information on total urinary arsenic levels (n = 12 920), by type of skin cancer, National Health and Nutrition Examination Survey (NHANES), 2003-2016a,b
| Characteristic | Melanoma, odds ratio (95% CI) | Nonmelanoma skin cancer, odds ratio (95% CI) |
|---|---|---|
| Total urinary arsenic levels | ||
| ≤50 μg/L | 1.00 [Reference] | 1.00 [Reference] |
| >50 μg/L | 1.45 (0.46-4.62) | 1.69 (0.91-3.16) |
| Age, y | 1.08 (1.07-1.10) | 1.07 (1.06-1.08) |
| Sex | ||
| Male | 1.15 (0.68-1.94) | 1.26 (0.89-1.79) |
| Female | 1.00 [Reference] | 1.00 [Reference] |
| Race/ethnicity | ||
| White | 18.13 (8.24-39.90) | 27.71 (12.74-60.28) |
| Non-White c | 1.00 [Reference] | 1.00 [Reference] |
| Education level | ||
| ≤High school graduate | 1.00 [Reference] | 1.00 [Reference] |
| Some college or associate’s degree | 0.96 (0.54-1.73) | 1.70 (1.13-2.56) |
| ≥College graduate | 1.53 (0.81-2.89) | 2.06 (1.34-3.18) |
| Ratio of family income to poverty d | ||
| 0-1.30 | 1.00 [Reference] | 1.00 [Reference] |
| 1.31-3.50 | 1.91 (1.01-3.62) | 2.23 (1.29-3.86) |
| 3.51-5.00 | 2.37 (1.11-5.04) | 2.74 (1.55-4.85) |
| Had a sunburn | ||
| Never | 1.00 [Reference] | 1.00 [Reference] |
| Ever | 2.93 (0.84-10.21) | 1.29 (0.84-10.21) |
| Smoking status | ||
| Never smoker | 1.00 [Reference] | 1.00 [Reference] |
| Ever smoker | 1.57 (0.94-2.63) | 1.57 (1.14-2.15) |
aData source: Centers for Disease Control and Prevention. 15
bOf 71 058 participants in NHANES in 2003-2016, participants who were aged <20 (n = 31 837), had a history of cancer other than skin cancer (n = 2803), or had no information on total urinary arsenic levels (n = 23 498) were excluded. Reference group for analysis is participants with no cancer.
cIncludes Mexican American, other Hispanic, non-Hispanic Black, and other races.
dOn the basis of the Supplemental Nutrition Assistance Program eligibility criteria cited in NHANES analytic guidelines 1999-2010, 0-1.30 indicates lowest income, 1.31-3.50 indicates middle income, and 3.51-5.00 indicates highest income. 18
Participants with melanoma were more likely to be White than non-White (OR = 18.13; 95% CI, 8.24-39.90) and be in the highest income group than in the lowest income group (OR = 2.37; 95% CI, 1.11-5.04). Other covariates measured showed no association with melanoma (Table 3). Participants with nonmelanoma skin cancer were more likely to be White than non-White (OR = 27.71; 95% CI, 12.74-60.28). Higher levels of education and income were significantly associated with nonmelanoma skin cancer. Ever smokers were 1.57 (95% CI, 1.14-2.15) times more likely than never smokers to have nonmelanoma skin cancer (Table 3).
Multivariate Models for Skin Cancer and Total Urinary Arsenic Levels
Among participants with melanoma, compared with no cancer, after adjusting for age and race/ethnicity (n = 87), the aOR comparing total urinary arsenic levels >50 μg/L with total urinary arsenic levels ≤50 μg/L was 1.87 (95% CI, 0.58-6.05; P = .29). However, among participants with nonmelanoma skin cancer, compared with no cancer, after adjusting for age and race/ethnicity (n = 218), the aOR comparing total urinary arsenic levels was significant at 2.23 (95% CI, 1.12-4.45) (P = .02).
Tap Water Source, Skin Cancer, and Socioeconomic Status
Among the 32 636 participants with information on tap water sources, participants with nonmelanoma skin cancer had 2.06 (95% CI, 1.25-3.41) increased odds of reporting a nonmunicipal water source compared with participants without cancer. We found no significant difference in tap water source between participants with melanoma and participants with no cancer (Table 4).
Table 4.
Characteristics of study participants aged ≥20, by tap water sources (N = 32 636), National Health and Nutrition Examination Survey (NHANES), 2003-2016a,b
| Characteristic | Municipal tap water source, no. (column %) (n = 22 961) | Nonmunicipal tap water source, no. (column %) (n = 3524) | Crude odds ratio (95% CI) | Does not drink tap water, no. (column %) (n = 6151) | Crude odds ratio (95% CI) |
|---|---|---|---|---|---|
| Skin cancer type | |||||
| Melanoma | 149 (0.7) | 49 (1.5) | 1.87 (0.87-4.04) | 16 (0.3) | 0.50 (0.20-1.22) |
| Nonmelanoma skin cancer | 399 (1.9) | 102 (3.2) | 2.06 (1.25-3.41) | 36 (0.6) | 0.59 (0.30-1.15) |
| No cancer | 20 746 (97.4) | 3029 (95.3) | 1.00 [Reference] | 5727 (99.1) | 1.00 [Reference] |
| Missing | 1667 | 344 | — | 372 | — |
| Education level | |||||
| ≤High school graduate | 10 317 (45.0) | 1837 (52.2) | 1.00 [Reference] | 3688 (60.0) | 1.00 [Reference] |
| Some college or associate’s degree | 6675 (29.1) | 1040 (29.5) | 0.84 (0.71-1.00) | 1693 (27.6) | 0.71 (0.62-0.81) |
| ≥College graduate | 5947 (25.9) | 643 (18.3) | 0.57 (0.43-0.75) | 765 (12.4) | 0.33 (0.27-0.40) |
| Missing | 22 | 4 | — | 5 | — |
| Ratio of family income to poverty c | |||||
| 0-1.30 | 6208 (29.1) | 876 (26.5) | 1.00 [Reference] | 2286 (41.2) | 1.00 [Reference] |
| 1.31-3.50 | 7936 (37.3) | 1323 (40.0) | 1.32 (1.03-1.69) | 2114 (38.1) | 0.68 (0.58-0.80) |
| 3.51-5.00 | 7153 (33.6) | 1108 (33.5) | 1.15 (0.85-1.54) | 1147 (20.7) | 0.38 (0.32-0.46) |
| Missing | 1664 | 217 | — | 604 | — |
Abbreviation: —, does not apply.
aData source: Centers for Disease Control and Prevention. 15
bOf 71 058 participants in NHANES in 2003-2016, participants who were aged <20 (n = 31 837) or had no information on water sources (n = 6585) were excluded. Reference group for analysis is participants who reported using tap water from a municipal source.
cOn the basis of the Supplemental Nutrition Assistance Program eligibility criteria cited in NHANES analytic guidelines 1999-2010, 0-1.30 indicates lowest income, 1.31-3.50 indicates middle income, and 3.51-5.00 indicates highest income. 18
We found associations between tap water sources and SES variables. Participants with the highest education level (≥college graduate) were less likely than participants with the lowest education level (≤high school graduate) to report drinking from nonmunicipal sources (OR = 0.57; 95% CI, 0.43-0.75) and not report drinking tap water (OR = 0.33; 95% CI, 0.27-0.40). Participants in the middle income group were 1.32 (95% CI, 1.03-1.69) times more likely than participants in the lowest income group to report drinking from a nonmunicipal water source, and the OR decreased with highest income group (OR = 1.15; 95% CI, 0.85-1.54). In addition, we found that participants in the middle (OR = 0.68; 95% CI, 0.58-0.80) and highest (OR = 0.38; 95% CI, 0.32-0.46) income groups were less likely to report not drinking tap water than to report drinking from a municipal water source (Table 4).
Discussion
A positive association between melanoma and exposure to arsenic was reported in 2 previous studies that measured arsenic levels in toenails and drinking water systems, in Iowa 12 and Denmark. 19 However, these studies measured arsenic differently, and the Denmark study was an ecological study, and ecological studies are prone to ecological fallacy. A case-control study (n = 67) conducted in New Mexico measured arsenic exposure by analyzing the mean difference in urinary arsenic levels between cases and controls. Similar to our study, that study found no association between melanoma and arsenic exposure. 13
Our results agree with the results of previous studies that found an association between nonmelanoma skin cancer and arsenic exposure. 20 -24 A study in Wisconsin found higher adjusted odds (aOR = 1.81; 95% CI, 1.10-3.14) of nonmelanoma skin cancer with chronic exposure to arsenic concentrations in water sources from 1.0 μg/L to 9.9 μg/L, after adjusting for age, sex, and tobacco use. In addition, after adjusting for these covariates, the study found the highest aOR among cases with arsenic concentrations ≥10 μg/L (aOR = 1.92; 95% CI, 1.01-3.68). 20 Several other studies measured arsenic, particularly inorganic arsenic, in urine and toenails and found that a higher concentration of arsenic increased the association of being diagnosed with basal cell carcinoma and squamous cell carcinoma. 22 -24 A prospective study from 2004-2015 conducted in Bangladesh analyzed the association between nonmelanoma skin cancer and the ingestion of arsenic-contaminated groundwater. The authors found a positive association between higher arsenic levels in groundwater and the prevalence of basal cell carcinoma and squamous cell carcinoma compared with other locations with lower levels of arsenic in groundwater. 24
Our study found a significant association between nonmelanoma skin cancer and drinking from nonmunicipal water sources. We found no relationship between total urinary arsenic levels and water sources. Some ecological studies noted an increased OR between the level of arsenic in drinking water and the incidence of melanoma and nonmelanoma skin cancer. 19,21 Nonmunicipal water sources can be contaminated naturally or by human activities, by heavy metals, nitrate, and other hazards. 25 Other components, in addition to arsenic, in nonmunicipal water sources may have driven the association with nonmelanoma skin cancer in our study.
In our total population, but not in the population with arsenic data, we found an association between increases in SES and increases in melanoma, consistent with previously reported results. A study conducted in Canada during 1992-2006 showed an increased risk of melanoma as SES increased; the research team hypothesized that the association might have resulted from improved access to cancer screening in addition to economic security allowing leisure time spent in sunny areas. 26 Disparities in water systems resulting from disparities in SES may increase the risk of exposure to toxic chemicals or pathogens from unregulated sources among people with low SES in the United States and other countries. 27 -29 Also, our study showed consistent results on SES and tap water sources.
Limitations
Our study had several limitations. First, the number of melanoma patients was relatively small, which may have led to bias and affected the reliability of results. Second, because of the cross-sectional study design, total urinary arsenic levels corresponded to a 3-day exposure history. We were unable to determine whether acute exposure to arsenic indicated chronic exposure. We could not establish a temporal relationship between arsenic exposure and outcomes of melanoma and nonmelanoma skin cancer. Third, urinary arsenic acid levels were the only measurement of inorganic arsenic, and we could not run an analysis on inorganic arsenic because of missing data. Almost 18% of our total study population and 21% of participants with melanoma did not have data on inorganic arsenic, and no participants with melanoma exceeded 0.71 μg/L of arsenic acid. Fourth, age at exposure and length of exposure to arsenic may be important factors in the relationship with cancer, but we were unable to measure these factors. Fifth, because of missing data, our study did not have adequate information on ultraviolet light exposure (history of sunburn) for participants with melanoma. Thus, we did not use this information in our multivariate models. Although the association between multiple sunburns and the development of melanoma is well established, we did not find this association in our study. 13,24 Finally, the association between SES and melanoma incidence may indicate a bias in how NHANES selects participants for arsenic testing. Further research with improved study design and complete information is warranted to determine any associations between arsenic and melanoma.
Conclusions
We did not find a relationship between the incidence of melanoma and exposure to total urinary arsenic levels among US adults participating in NHANES during 2003-2016. After adjusting for age and race/ethnicity, the aOR of participants with >50 μg/L arsenic in their urine and nonmelanoma skin cancer was 2.23 (95% CI, 1.12-4.45) compared with participants with ≤50 μg/L. Nonmunicipal water sources were associated with nonmelanoma skin cancer and may play a role in melanoma. This possible association warrants further investigation.
Acknowledgments
The authors thank Ms Elizabeth Lyden for her time and assistance with our statistical analysis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Ahmed Bedaiwi, MPH https://orcid.org/0000-0003-3897-1112
References
- 1. American Cancer Society . What are basal and squamous cell skin cancers? Updated July 26, 2019. Accessed January 16, 2021. https://www.cancer.org/cancer/skin-cancer/prevention-and-early-detection/what-is-skin-cancer.html
- 2. Skin Cancer Foundation . Melanoma overview: a dangerous skin cancer. Updated May 2020. Accessed January 16, 2021. https://www.skincancer.org/skin-cancer-information/melanoma
- 3. Skin Cancer Foundation . Skin cancer facts & statistics: what you need to know. Updated January 2021. Accessed January 16, 2021. https://www.skincancer.org/skin-cancer-information/skin-cancer-facts
- 4. American Cancer Society . What is melanoma skin cancer? Updated August 14, 2019. Accessed January 16, 2021. https://www.cancer.org/cancer/melanoma-skin-cancer/about/what-is-melanoma.html
- 5. Centers for Disease Control and Prevention . United States cancer statistics: data visualizations. Updated June 2020. Accessed January 16, 2021. https://gis.cdc.gov/Cancer/USCS/DataViz.html
- 6. American Cancer Society . Arsenic and cancer risk. Updated August 5, 2020. Accessed January 18, 2021. https://www.cancer.org/cancer/cancer-causes/arsenic.html
- 7. Pichler G., Grau-Perez M., Tellez-Plaza M. et al. Association of arsenic exposure with cardiac geometry and left ventricular function in young adults: evidence from the Strong Heart Family Study. Circ Cardiovasc Imaging. 2019;12(5): 10.1161/CIRCIMAGING.119.009018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Arsenic. February 15, 2018. Accessed January 18, 2021. https://www.who.int/news-room/fact-sheets/detail/arsenic
- 9. Abdul KSM., Jayasinghe SS., Chandana EPS., Jayasumana C., De Silva PMCS. Arsenic and human health effects: a review. Environ Toxicol Pharmacol. 2015;40(3):828-846. 10.1016/j.etap.2015.09.016 [DOI] [PubMed] [Google Scholar]
- 10. US Environmental Protection Agency . Basic information about your drinking water. Updated December 19, 2018. Accessed January 18, 2021. https://www.epa.gov/ground-water-and-drinking-water/basic-information-about-your-drinking-water
- 11. US Environmental Protection Agency . Drinking water requirements for states and public water systems: chemical contaminant rules. Accessed January 18, 2021. https://www.epa.gov/dwreginfo/chemical-contaminant-rules
- 12. Beane Freeman LE., Dennis LK., Lynch CF., Thorne PS., Just CL. Toenail arsenic content and cutaneous melanoma in Iowa. Am J Epidemiol. 2004;160(7):679-687. 10.1093/aje/kwh267 [DOI] [PubMed] [Google Scholar]
- 13. Yager JW., Erdei E., Myers O., Siegel M., Berwick M. Arsenic and ultraviolet radiation exposure: melanoma in a New Mexico non-Hispanic White population. Environ Geochem Health. 2016;38(3):897-910. 10.1007/s10653-015-9770-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention . About the National Health and Nutrition Examination Survey. Updated September 15, 2017. Accessed January 18, 2021. https://www.cdc.gov/nchs/nhanes/about_nhanes.htm
- 15. Centers for Disease Control and Prevention . NHANES questionnaires, datasets, and related documentation. Updated August 4, 2020. Accessed January 18, 2021. https://wwwn.cdc.gov/nchs/nhanes
- 16. Centers for Disease Control and Prevention . National Health and Nutrition Examination Survey. 2015-2016 Data documentation, codebook, and frequencies. Arsenic—Total—Urine (UTAS_I). Accessed January 18, 2021. https://wwwn.cdc.gov/Nchs/Nhanes/2015-2016/UTAS_I.htm
- 17. Minnesota Department of Health . Biomonitoring and arsenic. Accessed January 19, 2021. https://data.web.health.state.mn.us/biomonitoring_arsenic#
- 18. Johnson CL., Paulose-Ram R., Ogden CL. et al. National Health and Nutrition Examination Survey: analytic guidelines, 1999-2010. September 2013. Accessed January 19, 2021. https://stacks.cdc.gov/view/cdc/21305 [PubMed]
- 19. Baastrup R., Sørensen M., Balstrøm T. et al. Arsenic in drinking-water and risk for cancer in Denmark. Environ Health Perspect. 2008;116(2):231-237. 10.1289/ehp.10623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Knobeloch LM., Zierold KM., Anderson HA. Association of arsenic-contaminated drinking-water with prevalence of skin cancer in Wisconsin’s Fox River Valley. J Health Popul Nutr. 2006;24(2):206-213. [PubMed] [Google Scholar]
- 21. Applebaum KM., Karagas MR., Hunter DJ. et al. Polymorphisms in nucleotide excision repair genes, arsenic exposure, and non-melanoma skin cancer in New Hampshire. Environ Health Perspect. 2007;115(8):1231-1236. 10.1289/ehp.10096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilbert-Diamond D., Li Z., Perry AE., Spencer SK., Gandolfi AJ., Karagas MR. A population-based case-control study of urinary arsenic species and squamous cell carcinoma in New Hampshire, USA. Environ Health Perspect. 2013;121(10):1154-1160. 10.1289/ehp.1206178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Karagas MR., Stukel TA., Morris JS. et al. Skin cancer risk in relation to toenail arsenic concentrations in a US population-based case-control study. Am J Epidemiol. 2001;153(6):559-565. 10.1093/aje/153.6.559 [DOI] [PubMed] [Google Scholar]
- 24. Choudhury MIM., Shabnam N., Ahsan T. et al. Cutaneous malignancy due to arsenicosis in Bangladesh: 12-year study in tertiary level hospital. Biomed Res Int. 2018;2018:4678362. 10.1155/2018/4678362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Environmental Protection Agency . Potential well water contaminants and their impacts. Updated January 7, 2021. Accessed January 19, 2021. https://www.epa.gov/privatewells/potential-well-water-contaminants-and-their-impacts
- 26. Johnson-Obaseki SE., Labajian V., Corsten MJ., McDonald JT. Incidence of cutaneous malignant melanoma by socioeconomic status in Canada: 1992-2006. J Otolaryngol Head Neck Surg. 2015;44(1):53. 10.1186/s40463-015-0107-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Flanagan SV., Spayd SE., Procopio NA. et al. Arsenic in private well water part 3 of 3: socioeconomic vulnerability to exposure in Maine and New Jersey. Sci Total Environ. 2016;562:1019-1030. 10.1016/j.scitotenv.2016.03.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dungumaro EW. Socioeconomic differentials and availability of domestic water in South Africa. Physics Chem Earth, Parts A/B/C. 2007;32(15-18):1141-1147. 10.1016/j.pce.2007.07.006 [DOI] [Google Scholar]
- 29. Mbulaiteye SM., Biggar RJ., Pfeiffer RM. et al. Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr. 2005;38(4):474-479. 10.1097/01.qai.0000132495.89162.c0 [DOI] [PubMed] [Google Scholar]