Abstract
Objective:
Saliva specimens collected in school populations may offer a more feasible, noninvasive alternative to nasal swabs for large-scale COVID-19 testing efforts in kindergarten through 12th grade (K-12) schools. We investigated acceptance of saliva-based COVID-19 testing among quarantined K-12 students and their parents, teachers, and staff members who recently experienced a SARS-CoV-2 exposure in school.
Methods:
We surveyed 719 participants, in person or by telephone, who agreed to or declined a free saliva-based COVID-19 reverse-transcription polymerase chain reaction test as part of a surveillance investigation about whether they would have consented to testing if offered a nasal swab instead. We conducted this investigation in 6 school districts in Greene County (n = 3) and St. Louis County (n = 3), Missouri, from January 25 through March 23, 2021.
Results:
More than one-third (160 of 446) of K-12 students (or their parents or guardians), teachers, and staff members who agreed to a saliva-based COVID-19 test indicated they would have declined testing if specimen collection were by nasal swab. When stratified by school level, 51% (67 of 132) of elementary school students or their parents or guardians would not have agreed to testing if a nasal swab was offered.
Conclusions:
Some students, especially those in elementary school, preferred saliva-based COVID-19 testing to nasal swab testing. Use of saliva-based testing might increase voluntary participation in screening efforts in K-12 schools to help prevent the spread of SARS-CoV-2.
Keywords: COVID-19, saliva-based testing, screening, school health, K-12 schools
An estimated one-third of COVID-19 cases are asymptomatic, and symptom-based screening alone may be insufficient to contain outbreaks in schools.1,2 The Centers for Disease Control and Prevention (CDC) recommends a tiered approach to testing in school environments as part of a comprehensive prevention strategy. 3 Screening testing, when used in combination with other preventive measures such as wearing face masks, physical distancing, handwashing, contact tracing, instituting quarantine policies, and vaccinating, may help reduce secondary transmission of COVID-19 in kindergarten through 12th grade (K-12) schools. 3 However, this testing effort depends on voluntary participation in SARS-CoV-2 testing by school populations to identify and isolate infected people.
Willingness of students (including their parents or guardians) and school staff members to participate in testing for SARS-CoV-2 may be closely associated with the type of specimen collected, as perceptions may exist about discomfort or pain associated with certain types of collection. 4 Compared with an administered nasal swab, self-collected saliva samples offer several advantages, such as lower risk of exposure to health professionals or school staff members collecting specimens, decreased need for personal protective equipment, reduced discomfort during collection, and room temperature storage of specimens for nucleic acid amplification testing (NAAT). Moreover, studies have shown the sensitivity and specificity of saliva NAAT to be comparable to that of the highly sensitive nasopharyngeal NAAT for diagnosis of COVID-19.5,6
Saliva specimens used in school populations may offer a more feasible, noninvasive alternative to nasal swabs for large-scale testing in K-12 schools. We investigated the perceived influence of the type of specimen collection—saliva versus nasal swab—on agreement to test among quarantined people who recently experienced a K-12 school-based COVID-19 exposure.
Methods
A total of 843 participants, including 765 students and 78 teachers and staff members, voluntarily participated in an investigation to evaluate prevention strategies and quarantine policies to prevent transmission of SARS-CoV-2 in K-12 schools. CDC, Washington University in St. Louis, state and local health departments, and local school officials in 6 school districts in Greene County (n = 3) and St. Louis County (n = 3), Missouri, conducted this investigation from January 25 through March 23, 2021. School officials conducted contact tracing to identify school-based close contacts of students, teachers, or staff members with laboratory-confirmed COVID-19. CDC defines a close contact as “someone who was within 6 feet of an infected person (laboratory-confirmed or a clinical diagnosis) for a cumulative total of 15 minutes or more over a 24-hour period.” 7 As part of this larger surveillance investigation, investigators offered real-time reverse transcription polymerase chain reaction (RT-PCR) testing (performed at Washington University in St. Louis, School of Medicine) using saliva as the specimen type at no cost to all participants, those who previously received a positive test result for SARS-CoV-2, and their identified close contacts during their isolation and quarantine periods, at a centralized testing location or during a home visit by investigators. People who were fully vaccinated or had known COVID-19 infection within the last 3 months were exempted from quarantine.
Investigators asked participants, including students of all 3 school levels or their consenting parents or guardians, teachers, and staff members, who agreed to saliva-based testing at the time of specimen collection, “If we had been offering a nasal swab instead of a saliva sample, would you have agreed to the testing?” Investigators asked participants who declined saliva-based testing, “If we had been offering a nasal swab, would you have consented to this testing?” If a parent’s or guardian’s response differed from that of the student (for students aged <18 years), we recorded only the parent’s response. We defined the generic term “nasal swab” as collection of any nasal secretion sample by swab for diagnostic testing of COVID-19. We did not distinguish among nasopharyngeal, anterior nares, or mid-turbinate swabs during administration of survey questions; as such, we did not individually evaluate preferences for these specimen collection methods as part of this study.
The RT-PCR test performed as part of this work was developed for diagnostic and surveillance purposes and has been authorized for emergency use by the US Food and Drug Administration under an Emergency Use Authorization (Fluidigm). 8 The turnaround time for test results was 1 or 2 days. We required parent or guardian verbal consent for all students aged <18 years and student assent for students aged 12-17 years. We based stratification by school level on the grade; elementary, middle, and high school students were enrolled in grades K-5, 6-8, and 9-12, respectively. We performed a Pearson χ2 test of independence to examine the relationship between school level and nasal swab acceptance, with P < .05 considered significant. As part of the larger investigation, during the initial telephone interview, we used a standardized questionnaire to collect data on the following demographic characteristics of participants: sex (female, male, unknown), race (White, other, unknown/prefer not to answer), ethnicity (Hispanic/Latino, non-Hispanic/Latino, unknown/prefer not to answer), vaccination status (received ≥1 dose of a COVID-19 vaccine), student grade level (K-5, 6-8, 9-12), and staff member age (18-24, 25-44, 45-64, ≥65 years). The Washington University in St. Louis Institutional Review Board and CDC reviewed and approved this project, which was conducted consistent with applicable federal law and CDC policy (45 CFR part 46, 21 CFR part 56; 42 USC Sect. 241(d); 5 USC Sect. 552a; 44 USC Sect. 3501 et seq).
Results
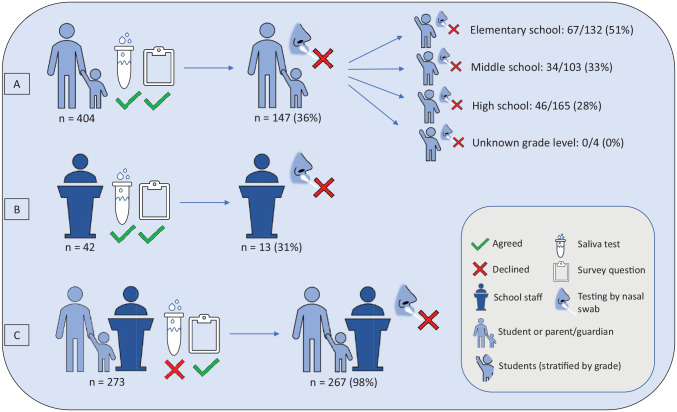
Of 657 students surveyed, 328 (50%) were male, 605 (92%) were non-Hispanic/Latino, and 1 had received ≥1 dose of the COVID-19 vaccine. Of 62 staff members surveyed, 44 (71%) were teachers, 52 (84%) were female, 56 (90%) were non-Hispanic/Latino, and 3 (5%) had received ≥1 dose of the COVID-19 vaccine (Table 1). Among the 843 participants, 446 (53%) provided a saliva specimen and answered our survey question; 404 of these participants were students (Figure). Among the 404 students, 147 (36%) students or their parents/guardians indicated they would have declined testing if specimens had been collected by nasal swab (Table 2). Of the 147 student participants who would have declined testing, 85 (58%) indicated this test was their first COVID-19 test. When stratified by school level, 51% (67 of 132) of elementary school students, 33% (34 of 103) of middle school students, and 28% (46 of 165) of high school students (or their parents/guardians) would not have agreed to testing if a nasal swab had been offered. None of the 4 students who had an unknown grade level indicated they would have declined testing by nasal swab.
Table 1.
Demographic characteristics of students and staff members (N = 719) in an analysis of acceptance of saliva-based COVID-19 testing at kindergarten through 12th grade schools in St. Louis, Missouri, January 25 through March 23, 2021 a
| Characteristic | No. (%) |
|---|---|
| Students | |
| Total no. | 657 |
| Grade level | |
| Elementary (K-5) | 213 (32) |
| Middle (6-8) | 171 (26) |
| High (9-12) | 268 (41) |
| Unknown b | 5 (1) |
| Sex | |
| Female | 326 (50) |
| Male | 328 (50) |
| Unknown | 3 (<1) |
| Race | |
| White | 499 (76) |
| Other c | 138 (21) |
| Prefer not to answer | 6 (1) |
| Unknown | 14 (2) |
| Ethnicity | |
| Hispanic/Latino | 45 (7) |
| Non-Hispanic/Latino | 605 (92) |
| Prefer not to answer | 0 |
| Unknown | 7 (1) |
| Vaccination status d | |
| Received ≥1 dose of COVID-19 vaccine | 1 (<1) |
| Staff members | |
| Total no. | 62 |
| Staff position | |
| Teacher | 44 (71) |
| Other | 17 (27) |
| Unknown b | 1 (2) |
| Age, y | |
| 18-24 | 6 (10) |
| 25-44 | 34 (55) |
| 45-64 | 19 (31) |
| Unknown | 3 (5) |
| Sex | |
| Female | 52 (84) |
| Male | 9 (15) |
| Unknown | 1 (2) |
| Race | |
| White | 51 (82) |
| Other c | 10 (16) |
| Unknown | 1 (2) |
| Ethnicity | |
| Hispanic/Latino | 5 (8) |
| Non-Hispanic/Latino | 56 (90) |
| Unknown/prefer not to answer | 1 (2) |
| Vaccination status d | |
| Received ≥1 dose of a COVID-19 vaccine | 3 (5) |
Included in this table are students and staff members who received a positive test result for SARS-CoV-2 and their close contacts who agreed to or declined saliva-based testing and answered the survey question.
Unknown denotes a blank value for that characteristic.
Other includes Asian, Black/African American, multiracial, and Native Hawaiian/Other Pacific Islander.
Vaccination status is available only for people who agreed to testing.
Figure.
Flow diagram depicting survey results of participants (N = 719) who agreed to (n = 446) or declined (n = 273) saliva-based COVID-19 testing in kindergarten through 12th grade schools, Missouri, January 25 through March 23, 2021. Participants included students and staff members who previously received a positive test result for SARS-CoV-2 and their identified school-based close contacts during their isolation and quarantine periods. (A) 36% of student participants who agreed to a saliva test, or their parent or guardian, indicated they would have declined testing if specimens had been collected by nasal swab. (B) For participating teachers and staff, 31% indicated they would have refused a nasal swab test. (C) 98% of participants who declined a saliva test but answered our survey reported they would have refused a nasal swab test. In addition, 118 participants initially agreed to testing but were lost to follow-up before specimen collection and not administered the survey question, and 6 participants were tested but not asked the survey question.
Table 2.
Survey responses of students and staff members (N = 719) in an analysis of acceptance of saliva-based COVID-19 testing at kindergarten through 12th grade schools in Missouri, January 25 through March 23, 2021 a
| Characteristic | Total participants, no. | Participants who responded no to the question, “Would you have agreed/consented to testing if a nasal swab was offered instead?”, no. (%) |
|---|---|---|
| Total participants who answered the survey | 719 | 427 (59) |
| Participants who agreed to saliva-based testing | 446 | 160 (36) |
| Students (parents or guardians) | 404 | 147 (36) |
| Elementary school grade | 132 | 67 (51) |
| Middle school grade | 103 | 34 (33) |
| High school grade | 165 | 46 (28) |
| Unknown grade level | 4 | 0 |
| Staff members | 42 | 13 (31) |
| Participants who declined saliva-based testing | 273 | 267 (98) |
| Students | 253 | 248 (98) |
| Staff members | 20 | 19 (95) |
Participants who agreed to saliva-based testing were asked in person by investigators at the time of specimen collection, “If we had been offering a nasal swab instead of a saliva sample, would you have agreed to the testing?” Participants who declined saliva-based testing were asked by telephone, “If we had been offering a nasal swab, would you have consented to this testing?”
Parents and guardians of elementary school students were more likely than students or parents and guardians of students in higher grade levels to answer that they would not have agreed to testing if a nasal swab had been offered (χ2 = 17.4; P < .001). Among the 7 student contacts who received a positive test result for SARS-CoV-2 as part of this investigation, 4 indicated they would not have agreed to a nasal swab. Of 42 participating teachers and staff members, 13 (31%) indicated they would have refused testing by a nasal swab.
Of the 278 participants who declined a saliva-based test as part of this investigation, 75 (27%) reported they had already been tested elsewhere because of their exposure and 273 (98%) answered our survey question. Of those who answered, 267 (98%) indicated that they would have refused the test if offered a nasal swab (Figure). A total of 118 participants in this investigation initially agreed to testing but were lost to follow-up and not administered the survey question, and 6 participants were tested but not asked the question. We observed no notable differences in participation between sites or schools implementing different quarantine policies. For all participants in the school-based investigation in Missouri who either agreed to or declined free saliva-based testing and answered our survey question, 59% (427 of 719) would not have agreed to testing if the specimen type had been a nasal swab instead of saliva.
Among students who provided a saliva specimen, we observed no notable differences in the percentage of participants who would have declined a nasal swab for any characteristic (Table 3). For students who agreed to a saliva test, 35% of Hispanic/Latino, 37% of non-Hispanic/Latino, 40% of females, and 33% of males indicated they would have declined testing if offered a nasal swab instead. Compared with other school staff members, teachers who agreed to saliva testing were less likely to decline testing by nasal swab if offered (43% vs 25%).
Table 3.
Demographic characteristics of students and staff members (N = 719) at kindergarten through 12th grade schools included in the SARS-CoV-2 saliva acceptance analysis, stratified by testing participation, St. Louis, Missouri, January 25 through March 23, 2021
| Characteristic | Agreed to saliva test | Did not agree to saliva test | Overall | |||
|---|---|---|---|---|---|---|
| Total no. | Would have declined nasal swab, no. (%) | Total no. | Would have declined nasal swab, no. (%) | Total no. | Would have declined nasal swab, no. (%) | |
| Students | ||||||
| Total | 404 | 147 (36) | 253 | 248 (98) | 657 | 395 (60) |
| Sex | ||||||
| Female | 200 | 80 (40) | 126 | 123 (98) | 326 | 203 (62) |
| Male | 203 | 67 (33) | 125 | 123 (98) | 328 | 190 (58) |
| Unknown/prefer not to answer | 1 | 0 | 2 | 2 (100) | 3 | 2 (67) |
| Race | ||||||
| White | 307 | 109 (36) | 192 | 187 (97) | 499 | 296 (59) |
| Other a | 85 | 33 (39) | 53 | 53 (100) | 138 | 86 (62) |
| Unknown/prefer not to answer | 12 | 5 (42) | 8 | 8 (100) | 20 | 13 (65) |
| Ethnicity | ||||||
| Hispanic/Latino | 26 | 9 (35) | 19 | 19 (100) | 45 | 28 (62) |
| Non-Hispanic/Latino | 374 | 138 (37) | 231 | 226 (98) | 605 | 364 (60) |
| Unknown/prefer not to answer | 4 | 0 | 3 | 3 (100) | 7 | 3 (43) |
| Vaccination status | ||||||
| Received ≥1 dose of a COVID-19 vaccinea,b | 1 | 0 | 0 | 0 | 1 | 0 |
| Staff members | ||||||
| Total | 42 | 13 (31) | 20 | 19 (95) | 62 | 32 (52) |
| Staff position | ||||||
| Teacher | 28 | 7 (25) | 16 | 15 (94) | 44 | 22 (50) |
| Other | 14 | 6 (43) | 3 | 3 (100) | 17 | 9 (53) |
| Unknown/prefer not to answer | 0 | 0 | 1 | 1 (100) | 1 | 1 (100) |
| Age, y | ||||||
| 18-24 | 3 | 0 | 3 | 3 (100) | 6 | 3 (50) |
| 25-44 | 25 | 9 (36) | 9 | 8 (89) | 34 | 17 (50) |
| 45-64 | 13 | 4 (31) | 6 | 6 (100) | 19 | 10 (53) |
| Unknown/prefer not to answer | 1 | 0 | 2 | 2 (100) | 3 | 2 (67) |
| Sex | ||||||
| Female | 34 | 11 (32) | 18 | 17 (94) | 52 | 28 (54) |
| Male | 8 | 2 (25) | 1 | 1 (100) | 9 | 3 (33) |
| Unknown/prefer not to answer | 0 | 0 | 1 | 1 (100) | 1 | 1 (100) |
| Race | ||||||
| White | 33 | 8 (24) | 18 | 17 (94) | 51 | 25 (49) |
| Other a | 9 | 5 (56) | 1 | 1 (100) | 10 | 6 (60) |
| Unknown/prefer not to answer | 0 | 0 | 1 | 1 (100) | 1 | 1 (100) |
| Ethnicity | ||||||
| Hispanic/Latino | 2 | 0 | 3 | 2 (67) | 5 | 2 (40) |
| Non-Hispanic/Latino | 40 | 13 (33) | 16 | 16 (100) | 56 | 29 (52) |
| Unknown/prefer not to answer | 0 | 0 | 1 | 1 (100) | 1 | 1 (100) |
| Vaccination status b | ||||||
| Received ≥1 dose of a COVID-19 vaccine | 3 | 0 | 0 | 0 | 3 | 0 |
Other includes Asian, Black/African American, multiracial, and Native Hawaiian/Other Pacific Islander.
Vaccination status is available only for people who agreed to testing.
Discussion
In K-12 schools with in-person learning, rapid detection of people with positive test results for SARS-CoV-2, subsequent contact tracing and case investigation to identify exposed people within the school, and vaccination of teachers, staff members, and eligible students are critical aspects of preventing secondary transmission. Although diagnostic testing is intended to identify symptomatic cases and close contacts, screening testing can identify infectious asymptomatic people to prevent further transmission. While testing performed during this investigation was for diagnostic and surveillance purposes, saliva as a method of specimen collection may be applicable for other school-based testing programs. Previous studies in K-12 schools identified low rates of secondary transmission among students, and many of those secondary cases were reportedly asymptomatic people.9,10 As of January 2022, CDC recommended that people who are up-to-date on COVID-19 vaccinations and have come into close contact with someone with COVID-19 be tested at least 5 days after exposure. 11 Screening testing should be offered to teachers and staff members who are not fully vaccinated in all schools at least once per week and for students who are not fully vaccinated when community transmission is moderate to high. 3 Screening testing can also provide added protection for those who are not fully vaccinated but are participating in sports and extracurricular activities that have an increased risk of spreading COVID-19. 3
When schools are deciding which assay to use for testing, in addition to sensitivity, cost, turnaround time, and feasibility, acceptability should also be considered. In this investigation, acceptability of SARS-CoV-2 testing was influenced by the type of specimen collection offered. Our results indicate the use of nasal swabs instead of saliva could have led to a reduction in participation by more than one-third, as 160 of 446 K-12 students, or their parents or guardians, teachers, and staff members, indicated they would not have agreed to testing that required nasal swab specimens. About half of elementary school students’ parents or guardians would have declined COVID-19 testing for the student if offered a nasal swab instead of saliva collection, and more than one-quarter of participating high school students or their parents or guardians and staff members would have refused. Because more than half of people who were tested for the first time would have declined a nasal swab, offering saliva testing could increase acceptance for first-time testers.
Although this RT-PCR test and testing strategy were used for diagnostic and surveillance purposes, saliva in general may be used as a specimen type for some screening assays. 12 Offering saliva-based tests may increase participation in voluntary screening testing in schools, particularly among elementary school students. Of the 7 student contacts who received a positive test result for SARS-CoV-2 as part of this investigation, 5 were asymptomatic and 4 indicated they would have declined testing by nasal swab. If saliva testing had not been offered, these infected students might not have been identified.
Limitations
This study had several limitations. First, we had a low participation rate in testing during the investigation, which was lower than in similar studies where in-school testing was offered to participants. 13 We speculate that this low participation rate could have been a result of COVID-19 fatigue, staff and students exposed in school receiving testing outside this investigation, or limited incentive for testing, as some school districts’ quarantine policies did not allow people to return to school early based on a negative RT-PCR test result. Second, a nasal swab was not offered as an alternative specimen collection method to saliva, and acceptance preferences do not reflect how participants would have responded if only a nasal swab had been offered. The results reported herein are based on answers to a hypothetical question, and future investigations may expand on this work to evaluate other factors that could influence K-12 students and staff members to participate in testing, such as convenience, test accuracy, and turnaround time to results.
Conclusions
Overall, nearly 60% of K-12 students or their parents or guardians, and teachers and staff members indicated by survey that they would not have agreed to free testing if a nasal swab had been used instead of saliva. This investigation provided evidence that a saliva-based COVID-19 test was preferred to a nasal swab test for some K-12 students and their parents or guardians, most notably in elementary grades. Given the ease and safety of use, similar accurate diagnostic performance, and increased participation rate of K-12 students, teachers, and staff members to agree to testing, collection of saliva specimens could play a role in maximizing RT-PCR screening testing efforts to help prevent the spread of SARS-CoV-2 in schools. Implementation of a layered mitigation approach that includes screening testing with increased participation rates may help ensure the safety of students, teachers, and staff members.
Acknowledgments
The authors acknowledge Clay Goddard, Jon Mooney, Machelle Petit, and Catherine Rains from the Springfield–Greene County Health Department; students, families, educators, nurses, administrators, and staff members from participating schools and school districts in Springfield, Greene County, and St. Louis County, Missouri; and Ryan Lash from CDC. The authors also acknowledge the SARS-CoV-2 Specimen Collection Field Team: Victoria Foltz, BS; Shaniece C. Theodore, PhD; Elaine Stevens-Emilien, MS; Michelle O’Hegarty, PhD; Thu-Ha Dinh, MD; and Jonathan Steinberg, MPH (CDC); and Brock K. Montgomery, BS; Alex S. Plattner, MD; Suong T. Nguyen, MD, PhD; Sarah E. Greene, MD, PhD; and Jaimee Hall, DO (Washington University in St. Louis).
Footnotes
Authors’ Note: Heathery P. McLaughlin and Mary Claire Worrell contributed equally to this work.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclaimer: The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by CDC.
Funding: The authors received the following financial support for the research, authorship, and/or publication of this article: Work activities were funded by Missouri Department of Health and Senior Services.
ORCID iD: Heather P. McLaughlin, PhD  https://orcid.org/0000-0003-4155-5347
https://orcid.org/0000-0003-4155-5347
References
- 1. Oran DP, Topol EJ. The proportion of SARS-CoV-2 infections that are asymptomatic: a systematic review. Ann Intern Med. 2021;174(5):655-662. doi: 10.7326/M20-6976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palitel AD, Zheng A, Walensky RP. Assessment of SARS-CoV-2 screening strategies to permit safe reopening of college campuses in the United States. JAMA Netw Open. 2020;3(7):e2016818. doi: 10.1001/jamanetworkopen.2020.16818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Centers for Disease Control and Prevention. Guidance for COVID-19 prevention in K-12 schools. Updated August 5, 2021. Accessed September 7, 2021. https://www.cdc.gov/coronavirus/2019-ncov/community/schools-childcare/k-12-guidance.html#contact-tracing
- 4. Zimba R, Kulkarni S, Berry A, et al. SARS-CoV-2 testing service preferences of adults in the United States: discrete choice experiment. JMIR Public Health Surveill. 2020;6(4):e25546. doi: 10.2196/25546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Butler-Laporte G, Lawandi A, Schiller I, et al. Comparison of saliva and nasopharyngeal swab nucleic acid amplification testing for detection of SARS-CoV-2: a systematic review and meta-analysis. JAMA Intern Med. 2021;181(3):353-360. doi: 10.1001/jamainternmed.2020.8876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuo Jing Teo A, Choudhury Y, Beehaut Tan I, et al. Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID‑19 infection. Sci Rep. 2021;11(1):3134. doi: 10.1038/s41598-021-82787-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. COVID-19: appendices. Updated January 4, 2022. Accessed January 26, 2022. https://www.cdc.gov/coronavirus/2019-ncov/php/contact-tracing/contact-tracing-plan/appendix.html#contact
- 8. Fluidigm. Advanta Dx SARS-CoV-2 RT-PCR assay. Accessed June 1, 2021. https://www.fda.gov/media/141541/download
- 9. Dawson P, Worrell MC, Malone S, et al. Pilot investigation of SARS-CoV-2 secondary transmission in kindergarten through grade 12 schools implementing mitigation strategies—St. Louis County and City of Springfield, Missouri, December 2020. MMWR Morb Mortal Wkly Rep. 2021;70(12):449-455. doi: 10.15585/mmwr.mm7012e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hershow RB, Wu K, Lewis NM, et al. Low SARS-CoV-2 transmission in elementary schools—Salt Lake County, Utah, December 3, 2020–January 31, 2021. MMWR Morb Mortal Wkly Rep. 2021;70(12):442-448. doi: 10.15585/mmwr.mm7012e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention. Stay up to date with your vaccines. Updated January 16, 2022. Accessed January 26, 2022. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/stay-up-to-date.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fvaccines%2Ffully-vaccinated-guidance.html
- 12. Senok A, Alsuwaidi H, Atrah Y, et al. Saliva as an alternative specimen for molecular COVID-19 testing in community settings and population-based screening. Infect Drug Resist. 2020;13:3393-3399. doi: 10.2147/IDR.S275152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lewis NM, Hershow RB, Chu VT, et al. Factors associated with participation in elementary school–based SARS-CoV-2 testing—Salt Lake County, Utah, December 2020–January 2021. MMWR Morb Mortal Wkly Rep. 2021;70(15):557-559. doi: 10.15585/mmwr.mm7015e1 [DOI] [PMC free article] [PubMed] [Google Scholar]