Abstract
Study Design:
A retrospective study.
Objective:
To investigate the effects of percutaneous transforaminal endoscopic decompression (PTED) for lumbar stenosis associated with adult degenerative scoliosis and to analyze the correlation between preoperative radiological parameters and postoperative surgical outcomes.
Methods:
Two years of retrospective data was collected from 46 patients with lumbar stenosis associated with adult degenerative scoliosis who underwent PTED. The visual analog scale (VAS), Oswestry Disability Index, and modified MacNab criteria were used to evaluate the clinical outcomes. Multiple linear regression analysis was used to analyze the correlation between radiological parameters and surgical outcomes.
Results:
The mean age of the 33 female and 13 male patients was 73.5 ± 8.1 years. The mean follow-up was 27.6 ± 3.5 months (range from 24 to 36). The average coronal Cobb angle was 24.5 ± 8.2°. There were better outcomes of the VAS for leg pain and Oswestry Disability Index after surgery. Based on the MacNab criteria, excellent or good outcomes were noted in 84.78% of patients. Multiple linear regression analysis showed that Cobb angle and lateral olisthy may be the predictors for low back pain.
Conclusion:
Transforaminal endoscopic surgery may be an effective and safe method for geriatric patients with lumbar stenosis associated with degenerative scoliosis. The predictive factors of clinical outcomes were severe Cobb angle and high degree lateral subluxation. Transforaminal endoscopic surgery may not be recommended for patients with Cobb angle larger than 30° combined with lateral subluxation.
Keywords: percutaneous endoscopic, adult degenerative scoliosis, stenosis, decompression alone
Introduction
Adult degenerative scoliosis (ADS) is a spinal deformity in a skeletally mature patient with coronal Cobb >10°, 1 and it is undeniably becoming a clinical matter in the aging population.2-5 Due to the unbalanced stress of spine, the progression of scoliosis is always combined with disc herniation, ligament hypertrophy, and facet ossification. These pathological changes resulted in unilateral stenosis (lateral recess, foraminal and extraforaminal stenosis), which was prevalent in symptomatic ADS patients. 6 Although operative treatment might bring patients more effective results than nonsurgical treatment, 7 there has been a debate about surgical strategies for stenosis associated with ADS: decompression alone or decompression with fusion.1,8,9 With development of radiology technology, unilateral stenosis can be clearly diagnosed before surgery according to the results of physical examination, computed tomography (CT), and magnetic resonance imaging (MRI). 10 Precise localization provides a feasible basis for focal decompression, which is considered to be a safe and effective method for geriatric patients with ADS. 11
Decompression alone was considered as a less invasively surgery with comparative outcomes than the fusion surgery for elder patients, 12 and it also had the lowest complication rate compared with full correction surgery. 11 However, defects in the posterior structure of spine, such as facet joint and lamina, may result in segmental instability, iatrogenic low back pain, and exacerbating deformity. 13 In recent years, several authors demonstrated that successful decompression alone surgery was based on the preservation of the posterior spine elements.10,14,15 Percutaneous transforaminal endoscopic discectomy (PTED) has been confirmed as an ultra-minimally invasive option to treat lumbar disc herniation in the past years. 16 This method may be an efficient alternative progression compared to traditional open decompression surgery for lumbar stenosis. 17 There are several advantages in PTED, for example, operation under local anesthesia, rapid recovery, shorter stay in hospital, and preservation of spinal biomechanical structure. 18 A previous study has shown that PTED may be an effective and safe method for patients with mild ADS. 19 However, the authors did not elaborate on the key points of surgical procedure for these patients, and they also did not put forward a guideline to define ideal surgical patients. 19 The purpose of this study was to evaluate the feasibility of PTED for unilateral stenosis with ADS in a 2-year follow-up, share our surgical experience, and investigate the correlation between preoperative radiological parameters and postoperative surgical outcomes and provide some available experiences.
Methods
Patient Selection
Forty-six patients were retrospectively included between January 2015 and February 2018. All patients provided written consent. Our hospital institutional review board approved this study. All operations were performed by the same surgeon. The surgical pathology as verified by MRI, CT, and full-length spine digital radiographs, and flexion-extension radiographic views were used for determining spinal segmental instability. All patients required at least 2 months invalid nonsurgical treatment including physical therapy, nonsteroidal anti-inflammatory drugs, or epidural steroid blocking. Only patients over 60 years old with unilateral radiculopathy induced by lateral recess, foraminal, and/or extraforaminal stenosis and coexisting ADS were included in this study cohort. And the exclusion criteria were severe low back pain, segmental instability, and coexisting pathological conditions such as acute inflammation, infection, and tumor.
Patients’ data including demographics, operation time, clinical outcomes, and preoperative imaging measurements was recorded. Clinical outcomes were measured by using visual analogue scale (VAS) and Oswestry Disability Index (ODI) scores at preoperative, postoperative, 6 weeks, 3 months, 6 months, 1 year, and final follow-up. Also, the modified MacNab criteria were used to assess the outcomes of these surgical treatment at the final follow-up. The preoperative imaging parameters included (1) lumbar coronal Cobb angle, (2) pelvic tilt (PT), (3) pelvic incidence (PI), (4) sacral slope (SS), (5) lumbar lordosis (LL), (6) max vertebral rotation, (7) sagittal vertebral axis (SVA), and (8) maximal lateral vertebral subluxation. The measurement methods of these radiographic parameters referred to previous studies,1,8 and 3 doctors did the measurements of radiographic parameters. Two of them respectively measured these data, and the third one evaluated the data obtained. The patients were divided into 3 group according to the coronal Cobb angle, A (10° ≤ Cobb < 20°), B (20° ≤ Cobb < 30°), and C (Cobb ≥30°). MRI and CT were used to determine the foraminal stenosis and/or lateral stenosis. If it was difficult to judge the responsive nerve root according to the imaging, we usually used nerve block to confirm the affected root. Dynamic radiographic films were used to exclude patients with radiographic instability.
Surgical Method
According to physical examination and compression degree in image, the responsible levels were determined for surgery. The patients were in prone position on a radiolucent bed. Some patients were in lateral position because of poor cardiopulmonary function. All surgical procedures were under local anesthesia. The location and inserting working cannula procedures were similar with our previous study. 20 However, several tips should be concerned during the location procedures. Because of the rotation and lateral subluxation of vertebral body in scoliosis patients, the symmetrical position of bilateral vertebral pedicle should be adjusted under the anteroposterior position of imaging views, and posterior walls of vertebral body should be overlapping in a line under the lateral radiographs. By this method, the influence of rotation for puncturing needle and inserting working cannula can be removed.
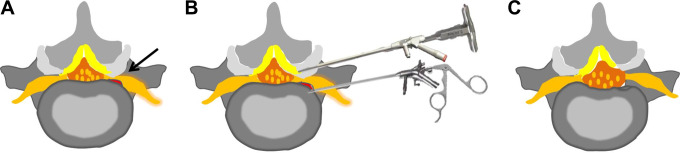
A dorsal and ventral endoscopic decompression of nerve root was necessary for degenerative conditions of scoliosis spine (Figure 1). In order to get an enlarged foraminal window for surgical manipulation, the hypertrophic superior articular process was removed by endoscopic chisel or trephine via the dorsal side. Through this manipulating window, the punch or nucleus forceps was used to remove the hypertrophic ligament flavum. At the ventral side, degenerative conditions, such as hypertrophic posterior longitudinal ligament and extruded disc, were excised by nucleus forceps. Subsequently, osteophytes of the posterior margin of vertebral body were removed bit by bit using an endoscopic chisel. After the perineural scars of nerve root were removed by forceps, the free nerve root could be identified, and it always could be visualized that the nerve root was pulsed with the heart rate. After adequate hemostasis with a bipolar coagulator, the endoscope was withdrawn, and a sterile dressing was applied with a 1-point subcutaneous suture. Patients should be observed for 2 hours lying in the bed and then can be allowed to ambulate with a flexible back brace if the patients did not have any discomfort.
Figure 1.
Schematic illustrations of the endoscopic decompression procedure in axial views. (A) Degenerative conditions before surgery: the nerve root was compressed by osteophytes (red part adjacent to nerve root), extruded disc, hypertrophic ligament flavum, and facet joint osteophyte (black arrow). (B) Sequential decompression: ventral nerve decompression was performed by removing the extruded disc, hypertrophic posterior longitudinal ligament, and osteophytes with large duckbilled forceps or endoscopic burr; dorsal nerve decompression was performed by foraminal unroofing using an endoscopic chisel and removing the hypertrophic ligament flavum. (C) Decompression conditions after surgery: a freedom nerveroot can be seen after the decompression.
Statistical Analysis
All statistical analyses were performed using SPSS 24.0 for windows (SPSS, IBM Inc). Continuous data was expressed as the mean ± SD and compared by t test. Enumeration data was compared by χ2 test. Repeated-measures analysis of variance and a paired t test were used to analyze preoperative and postoperative clinical outcomes in pain and functional status. One-way repeated-measures ANOVA was used to analyze data between 3 groups. To assess predictors of degree of final follow-up VAS for back scores, multiple linear regression analysis was performed to analyze the imaging parameters. P values <.05 were considered significantly.
Results
Demographics and Surgical Data
As shown in Table 1, there were 33 women and 13 men (46 patients) with a mean age of 73.5 ± 8.1 years (range from 60 to 99 years). The operated-on levels were totally 66 (26 for single, and 20 for 2 levels). Most of the stenotic foramens were located at the concave side (n = 41, 68.33%), which was higher than those in the convex side. None of the patients had a history of lumbar surgery. The mean follow-up time was 27.6 ± 3.5 months. The mean operative time was 66.9 ± 14.3 minutes, and the intraoperative blood loss was almost negligible. All patients were discharged on the second day after surgery. In Table 2, the overall mean Cobb angle was 24.5 ± 8.2°, the PT was 18.4 ± 11.7°, SS was 27.6 ± 11.4°, PI was 46 ± 11.7°, LL was 31.6 ± 16.1°, and SVA was 46.4 ± 40.7 mm. Data of each group can be seen in Tables 1 and 2.
Table 1.
General Characteristics of Patients Before Surgery.
| Variable | Overall (mean ± SD) | Group A | Group B | Group C | P value |
|---|---|---|---|---|---|
| Gender (total) | 46 | 16 | 15 | 15 | — |
| Female | 33 | 12 | 10 | 11 | .62 |
| Male | 13 | 4 | 5 | 4 | |
| Mean age (years) | 73.5 ± 8.1 | 74.9 ± 9.3 | 73.7 ± 8.3 | 71.9 ± 6.9 | .611 |
| Level of discectomy | 66 | 22 | 21 | 23 | — |
| L1/2 | 3 | 1 | 1 | 1 | — |
| L2/3 | 3 | 1 | 1 | 1 | — |
| L3/4 | 10 | 3 | 4 | 3 | — |
| L4/5 | 37 | 15 | 10 | 12 | — |
| L5/S1 | 13 | 2 | 5 | 6 | — |
| Single level | 26 | 12 | 9 | 5 | — |
| Two levels | 20 | 5 | 6 | 9 | — |
| Follow-up (months) | 27.6 ± 3.5 | 28.1 ± 3.8 | 28 ± 3.6 | 26.7 ± 3.2 | .474 |
| Time of surgery (minutes) | 66.9 ± 14.3 | 59 ± 7.2 | 66.9 ± 14.4 | 75.2 ± 16 | .005a |
| Hospital stay (days) | 1.5 ± 0.6 | 1.5 ± 0.9 | 1.3 ± 0.4 | 1.6 ± 0.5 | .423 |
a The time of surgery in group C was higher than in group A or B, and boldfaced values indicate statistical significance at P < 0.01.
Table 2.
Preoperation Radiographic Data by Subgroup.
| Overall | Group A | Group B | Group C | P value | |
|---|---|---|---|---|---|
| Mean coronal Cobb angle (° ±SD) | 24.5 ± 8.2 | 15.8 ± 2.1 | 23.8 ± 2.4 | 34.5 ± 3.5 | <.01a |
| LL (° ±SD) | 31.6 ± 16.1 | 33.8 ± 10.6 | 32.8 ± 21.8 | 28.1 ± 14.6 | .592 |
| PI (° ±SD) | 46 ± 11.7 | 46.6 ± 9.6 | 47.3 ± 13 | 44.1 ± 12.9 | .751 |
| PT (° ±SD) | 18.4 ± 11.7 | 17.5 ± 10.2 | 19.6 ± 11.2 | 18.3 ± 14 | .890 |
| SS (° ±SD) | 27.6 ± 11.4 | 29.1 ± 11.1 | 27.7 ± 13.9 | 25.9 ± 9.1 | .746 |
| SVA (mm) | 46.4 ± 40.7 | 41.3 ± 47.1 | 46.2 ± 33 | 52.1 ± 42.3 | .768 |
| Max-vertebral rotation (Nash-Moe) | |||||
| 0° | 14 | 8 | 5 | 1 | <.01b |
| I° | 21 | 8 | 8 | 5 | |
| II° | 10 | 0 | 2 | 8 | |
| III° | 1 | 0 | 0 | 1 | |
| IV° | 0 | 0 | 0 | 0 | |
| Max lateral Olisthy (mm ± SD) | 2.6 ± 1.8 | 1.4 ± 1.1 | 2.3 ± 1.5 | 4.3 ± 1.6 | <.01a |
Abbreviations: LL, lumbar lordosis; PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; SVA, sagittal vertical axis.
aThe mean Cobb angle and mean lateral olisthy in group C were larger than in group A or B, and boldfaced values indicate statistical significance at P < 0.01.
bThere were differences in distribution between groups using χ2 test, and boldfaced value indicates statistical significance at P < 0.01.
Clinical Outcomes
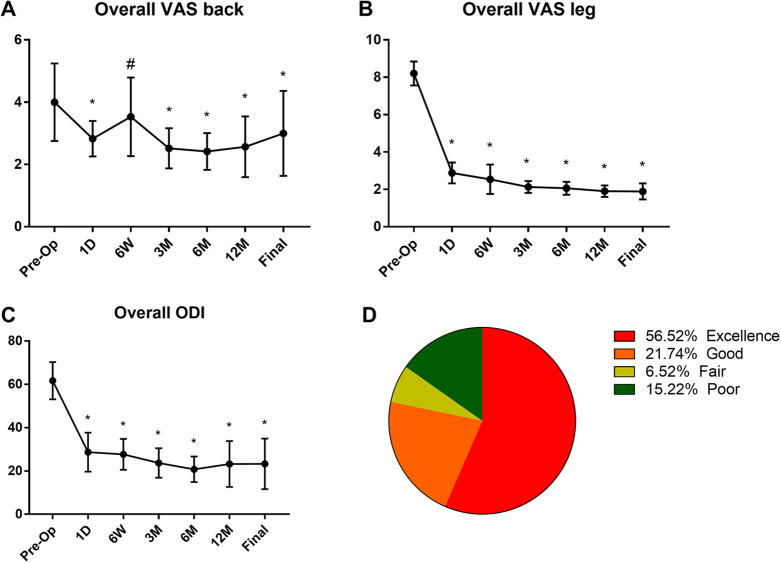
The overall mean preoperative VAS score for back pain was 4 ± 1.2, and for leg pain it was 8.2 ± 0.6. This postoperative VAS for back pain score was improved to 2.8 ± 0.6, 3.0 ± 1.3, 2.5 ± 0.6, 2.4 ± 0.6, 2.6 ± 1, and 3 ± 1.4 at postoperative 1 day, 6 weeks, 3 months, 1 year, and final follow-up, respectively (P < .05; Figure 2). The mean postoperative VAS for leg pain was 2.9 ± 0.6 (at postoperative 1 day), 2.5 ± 0.8 (6 weeks), 2.1 ± 0.3 (3 months), 2.1 ± 0.3 (6 months), 1.9 ± 0.3 (1 year), and 1.9 ± 0.4 (final follow-up; P < .01). The overall mean preoperative ODI score was 62 ± 8.6. The mean postoperative ODI was 29 ± 9, 28 ± 7.1, 24 ± 6.8, 21 ± 5.9, 23 ± 10.6, and 23 ± 11.7 at postoperative 1 day, 6 weeks, 3 months, 6 months, 1 year, and final follow-up, respectively (P < .001). At the final follow-up review, the modified MacNab criteria were rated as follows: excellent in 26 patients (56.52%), good in 10 patients (21.74%), fair in 3 patients (6.52%), and poor in 7 patients (15.22%). Therefore, excellent and good results were obtained in 78.26% of the patients.
Figure 2.
Overall visual analogue scale (VAS) for low back pain (A), leg pain (B), and Oswestry Disability Index (ODI) scores (C) preoperatively (Pre-OP) and at 1 day (1D), 6 weeks (6W), 3 months (3M), 6 months (6M), 1 year (12M), and final follow-up. The results of modified MacNab evaluation criteria at final follow-up (D). #P < .05 versus preoperation group, *P < .05 compared with preoperation.
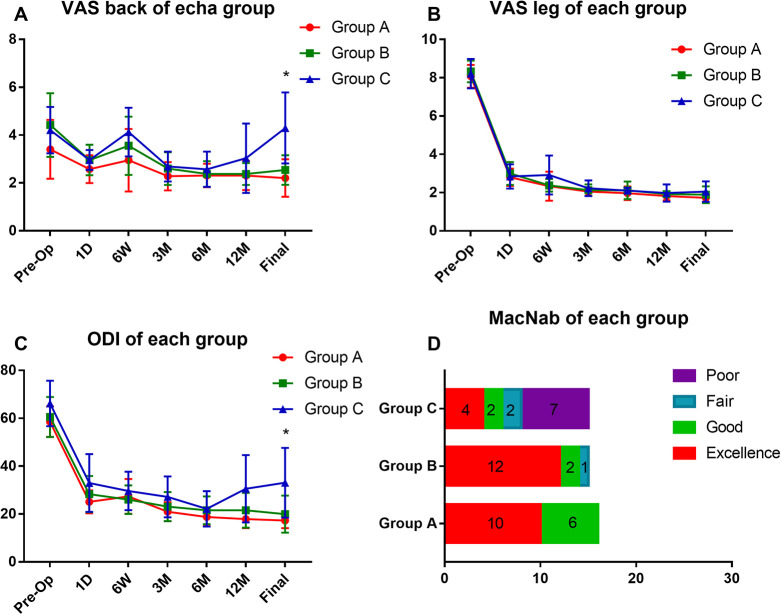
The data of VAS for back and leg pain in each group is shown in Figure 3. At the final follow-up, the VAS for back pain of group A was 2.2 ± 0.8, group B 2.5 ± 0.6, and group C 4.3 ± 1.5. The patients in group C reported higher back pain compared with group A or B (P < .01). While there was no difference between these groups in the data of VAS for leg pain. Similarly, the ODI score of each group at final follow-up was 17 ± 3.2 (group A), 20 ± 7.7 (group B), and 33 ± 14.6 (group C). The patients in group C reported a lower quality of life (P < .01; Figure 3C).
Figure 3.
Each group’s VAS for low back pain (A), leg pain (B), and ODI (C) scores preoperatively (Pre-OP) and at 1 day (1D), 6 weeks (6W), 3 months (3M), 6 months (6M), 1 year (12M), and final follow-up. (D) The results of modified MacNab evaluation criteria at final follow-up in each group. *P < .05, group C compared with group A or B.
As shown in Table 3, the results of multiple linear regression analysis showed that Cobb angle and olisthy may be the predictors for low back pain of patients combined with unilateral stenosis associated with ADS in a long-term follow-up. Unstandardized coefficient B of Cobb was 0.065, standardized coefficient beta was 0.388, P = .03, confidence interval was [0.006, 0.123]. Unstandardized coefficient B of olisthy was 0.276, standardized coefficient beta was 0.373, P = .009, confidence interval was [0.072, 0.48].
Table 3.
Predictive Factors to Degree of VAS for Back Pain at the Final Follow-up.
| Model | Unstandardized coefficients | Standardized coefficients | t | P value | 95% Confidence interval for B | ||
|---|---|---|---|---|---|---|---|
| B | SE | Beta | Lower bound | Upper bound | |||
| Constant | −0.022 | 0.635 | −0.350 | .972 | −1.307 | 1.263 | |
| Cobb | 0.065 | 0.29 | 0.388 | 2.248 | a .030 | 0.006 | 0.123 |
| SS | 0.014 | 0.018 | 0.113 | 0.754 | .456 | −0.023 | 0.050 |
| PT | 0.002 | 0.014 | 0.015 | 0.127 | .899 | −0.026 | 0.029 |
| LL | −0.008 | 0.011 | −0.092 | −0.706 | .484 | −0.030 | 0.015 |
| SVA | 0.001 | 0.003 | 0.018 | 0.172 | .864 | −0.006 | 0.008 |
| Olisthy | 0.276 | 0.101 | 0.373 | 2.734 | a .009 | 0.072 | 0.480 |
| Max vertebral rotation | 0.260 | 0.224 | 0.160 | 1.159 | .254 | −0.194 | 0.713 |
Abbreviations: SS, sacral slope; PT, pelvic tilt; LL, lumbar lordosis; SVA, sagittal vertical axis.
aBoldfaced values indicate statistical significance at P < 0.05.
Discussion
Our study provides an assessment and experiences of PTED for unilateral stenosis in a consecutive series of geriatric ADS patients. All patients underwent PTED with coronal average Cobb angle of 24.5 ± 8.2° (the proportion of Cobb angle ≥30° was 32.6%). The goal of our treatment was to alleviate the leg pain, reduce the incidence of perioperative complications, improve the quality of patients’ life, and evaluate the correlation between preoperative radiological parameters and postoperative surgical outcomes. The VAS score of leg and functional status (ODI score) has significant improvement at the postoperative evaluation, and comfortable results were reported during the 2-year follow-up period. PTED surgery did not cause postoperative low back pain in most of the patients at the final follow-up. The ODI score decreased by 62.9% at the final follow-up. The surgical successful rate was 84.78% according to the MacNab criteria. Collectively, PTED, which we carefully tried, significantly alleviated the pain and improve the quality of patients’ life.
Multiple factors, such as disc degeneration, asymmetrical stress, poor nutrition, and so on, contributed to the disc extrusion, hypertrophy of flavum ligamentum and facet joint, lateral slip of vertebral body and superior facet, pedicular kinking, and ossification. 21 As the space around the nerve root decreases, 3 areas may result in neurological symptoms: lateral recess, foraminal and extraforaminal zone. 22 To make a complete decompression, traditional surgery algorithm is decompression with corrective fusion. Direct and indirect decompression can be achieved by this way. Although the correction surgery was demonstrated with relative good outcomes, 23 perioperative medical complications in the elderly are getting much more attention due to rapid growth of aging population. 24 Therefore, surgical methods should be chosen vividly based on patients’ deformity, presentation, and medical condition, and focal decompression was gradually accepted to treat ADS patients associated with stenosis rather than deformity correction surgery, and several studies have reported decompression with limited fusion, 25 laminectomy decompression alone,8,23 and lamina fenestration decompression generated good clinical outcome. 10 However, the complications, such as adjacent segment disease, iatrogenic instability, and low back pain, cannot be completely avoided.8,23 Recent developments in PTED surgery has several advantages compared to traditional methods, such as less soft tissue resected, local anesthesia, less blood loss, short in-hospital days, and rapid recovery. 18 PTED was reported to treat disc herniation, lateral recess stenosis, 26 foraminal stenosis, 27 and extraforaminal stenosis successfully. 28 In PTED procedure, both ventral and dorsal decompression are necessary to treat degenerative stenosis. Endoscopic chisel or trephine was used to remove the hypertrophied superior articular process, then dorsal decompression can be performed under an enlarged foraminal window. The hypertrophied ligament flavum could be resected via this approach. Immediately after dorsal decompression, working cannula should be reposition at ventral side to remove the herniated disc, hypertrophied ligament. Aggressive manipulation should be avoided for dural tears or injury of nerve root during this decompression procedure. In our present study, significant improvements were found in VAS pain according to the data of reviews obtained at 1 day, 6 weeks, 3 months, 1 year, and 2 years. At the final review, the mean decrease in VAS score was 1.9 ± 0.4 for leg pain and 3 ± 1.4 for back pain (P < .01, respectively).
There are several pathologic factors that cause human low back pain. First, degenerative changes in spinal elements always induce mild or moderate low back pain. In this position, the nerve roots were stimulated by the protruded disc, ligamentum flavum hypertrophy, facet joint hypertrophy, and inflammation cytokines. During the PTED procedure, facet debridement, foraminal unroofing, and resection of hypertrophied soft tissue could be performed and this process may avoid low back pain caused by above compression factors. 29 Second, mechanical low back pain was usually caused by spinal imbalance and muscle weak, which were induced by scoliosis itself. Decompression alone open surgery is very invasive because of the destruction of the posterior structure of spine. This process always results in iatrogenic spinal instability and causes severe postoperative back pain. As an ultra-minimal invasive decompression surgery, PTED achieves good decompression without destructing essential elements of spine via the paraspinal approach. 18 It can minimize the possibility of iatrogenic instability and low back pain. Our present work showed that PTED was an effective method in treating foraminal stenosis associated with ADS, and the effectiveness of PTED decompression for stenosis have been examined in several previous studies,18,27,30 and Madhavan et al 19 suggested PTED was an effective method to decompress the symptomatic pain of patients with mild scoliosis. All patients, who underwent PTED in this study, had good outcomes in 36 of 46 patients in a 2-year follow-up term. The mean operation time was 66.9 ± 14.3 minutes in our study with negligible blood loss. The ODI score is a self-report outcome measurement in clinical practice, 31 and it was based on patients’ disability, anxiety, and depression. 32 The mean decrease of ODI was 39 ± 8.3 (decreased by 62.9%, P < .01). The clinic relevant can be considered in ODI score if the reduction is more than 20%. 33 It is generally believed that ADS was associated with unpopular outcome after decompression alone using traditional open methods in spinal stenosis. 34 However, with the development of minimally invasive technology in recent decades, minimal invasive surgery (MIS) or PTED led to new spinal surgical approaches with less tissue damage, postoperative pain, and shorted hospital stays compared with traditional surgical approaches. 35 Kelleher et al 36 reported a series of 25 patients who underwent MIS tubular micro-endoscopic decompression for unilateral stenosis concurrent with ADS. They reported that clinical outcome evaluated by ODI has a significant reduction and their result was similar with Daubs and colleagues’ work, 37 which reported a series of patients underwent traditional fusion surgery. Furthermore, Hasan et al 38 reported that PTED and minimally invasive surgery tubular micro-endoscope resulted in similar functional outcomes for lumbar stenosis with mild to moderate deformity for the ODI improvements between the 2 groups were similar (62% vs 52%) at the final follow-up. Our data of ODI improvements was similar with Hasan and colleagues’ work. Collectively, these data suggest that PTED may be an efficient method to treat such selected patients.
Low back pain at long-term follow-up may be correlated with the curve progression in ADS patients. In the present study, the VAS for back pain of each group at the final follow-up showed that patients of group C reported higher scores compared with group A or B (Figure 3A). Similarly, the ODI score of group C was also higher than other groups (Figure 3C). According to MacNab criteria, 7 patients, who all belonged to group C, reported poor outcomes (Figure 3D). Pritchett and Bortel 39 reported that continued curve progression happened in patients with a Cobb angle >30°, and lateral subluxation of more than 3 mm also contributed to progressive increases. 40 Furthermore, lateral subluxation combined with vertebral rotation was thought to be a 3-dimension deformity and a trigger of curve progression in ADS patients. 41 Large Cobb angle combined with apical rotation and lateral subluxation mainly contributed to instability and progression of scoliosis and finally caused low back pain.1,8,24,42 The results, in current study, of multiple linear regression analysis showed that larger Cobb angle and/or higher degree of lateral olisthy were good predictors of low back pain in a long-term follow-up (Table 3), indicating that the large coronal Cobb angle (>30°) was an imbalance factor to cause curve progression and then induced mechanical low back pain.39,43 Although PTED surgery could successfully alleviate the radicular pain, it could not prevent long-term low back pain associated with large Cobb scoliosis. Therefore, according to our experiences, PTED should not be recommended in patients with coronal Cobb angle larger than 30° combined with lateral slip and vertebral rotation, because these factors mean that scoliosis is unstable and high likely to progress.
Several limitations were present in our study. First, the subjects are not randomized patients but rather patients specifically selected. Second, there may be a learning curve for junior surgeons to perform a successful decompression using PTED in such patients. Third, the small sample sizes of patients resulted in limited statistical power to detect changes in clinical outcomes pre-surgically and post-surgically.
Conclusion
Because of the increase in the proportion of the elderly population, the number of patients is undeniably increasing to seek treatment for degenerative scoliosis. It is important to choose appropriate surgical strategies for these patients. This study shows that PTED may be an effective and safe method for radicular pain in some ADS geriatric patients. Satisfactory clinical outcomes can be achieved using PTED if the patients have mild Cobb angle without severe rotation or lateral-olisthy. Otherwise, transforaminal endoscopic surgery may not be recommended for patients with Cobb angle larger than 30° combined with lateral-olisthy.
Acknowledgments
We thank Dr Cheng-Cheng Liu from the Office of Cancer Screening, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China, for supervising and improving the confidence of statistical analysis.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (81 772 292, 81 270 027) and the Medico-Engineering Cooperation Fund of Shanghai Jiao Tong University (No. YG2016MS54). These funding agencies played no roles in the investigation.
ORCID iD: Xin-Feng Li, PhD  https://orcid.org/0000-0003-0095-3138
https://orcid.org/0000-0003-0095-3138
References
- 1.Silva FE, Lenke LG. Adult degenerative scoliosis: evaluation and management. Neurosurg Focus. 2010;28:E1. [DOI] [PubMed] [Google Scholar]
- 2.Kebaish KM, Neubauer PR, Voros GD, Khoshnevisan MA, Skolasky RL. Scoliosis in adults aged forty years and older: prevalence and relationship to age, race, and gender. Spine (Phila Pa 1976). 2011;36:731–736. [DOI] [PubMed] [Google Scholar]
- 3.Hong JY, Suh SW, Modi HN, Hur CY, Song HR, Park JH. The prevalence and radiological findings in 1347 elderly patients with scoliosis. J Bone Joint Surg Br. 2010;92:980–983. [DOI] [PubMed] [Google Scholar]
- 4.Koerner JD, Reitman CA, Arnold PM, Rihn J. Degenerative lumbar scoliosis. JBJS Rev. 2015;3(4). [DOI] [PubMed] [Google Scholar]
- 5.Urrutia J, Diaz-Ledezma C, Espinosa J, Berven SH. Lumbar scoliosis in postmenopausal women: prevalence and relationship with bone density, age, and body mass index. Spine (Phila Pa 1976). 2011;36:737–740. [DOI] [PubMed] [Google Scholar]
- 6.Fu K, Rhagavan P, Shaffrey C, Chernavvsky D, Smith J. Prevalence, severity, and impact of foraminal and canal stenosis among adults with degenerative scoliosis. Neurosurgery. 2011;69:1181–1187. [DOI] [PubMed] [Google Scholar]
- 7.Smith J, Shaffrey C, Berven S, et al. Operative versus nonoperative treatment of leg pain in adults with scoliosis: a retrospective review of a prospective multicenter database with two-year follow-up. Spine (Phila Pa 1976). 2009;34:1693–1698. [DOI] [PubMed] [Google Scholar]
- 8.Schwab F, Ungar B, Blondel B, et al. Scoliosis Research Society-Schwab adult spinal deformity classification: a validation study. Spine (Phila Pa 1976). 2012;37:1077–1082. [DOI] [PubMed] [Google Scholar]
- 9.Aebi M. The adult scoliosis. Eur Spine J. 2005;14:925–948. [DOI] [PubMed] [Google Scholar]
- 10.Masuda K, Higashi T, Yamada K, Sekiya T, Saito T. The surgical outcome of decompression alone versus decompression with limited fusion for degenerative lumbar scoliosis. J Neurosurg Spine. 2018;29:259–264. [DOI] [PubMed] [Google Scholar]
- 11.Transfeldt EE, Topp R, Mehbod AA, Winter RB. Surgical outcomes of decompression, decompression with limited fusion, and decompression with full curve fusion for degenerative scoliosis with radiculopathy. Spine (Phila Pa 1976). 2010;35:1872–1875. [DOI] [PubMed] [Google Scholar]
- 12.Hansraj KK, Cammisa FP, Jr, O’Leary PF, et al. Decompressive surgery for typical lumbar spinal stenosis. Clin Orthop Relat Res. 2001;(384):10–17. [DOI] [PubMed] [Google Scholar]
- 13.Houten JK, Nasser R. Symptomatic progression of degenerative scoliosis after decompression and limited fusion surgery for lumbar spinal stenosis. J Clin Neurosci. 2013;20:613–615. [DOI] [PubMed] [Google Scholar]
- 14.Bresnahan L, Ogden AT, Natarajan RN, Fessler RG. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976). 2009;34:17–23. [DOI] [PubMed] [Google Scholar]
- 15.Tsutsui S, Kagotani R, Yamada H, et al. Can decompression surgery relieve low back pain in patients with lumbar spinal stenosis combined with degenerative lumbar scoliosis? Eur Spine J. 2013;22:2010–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen RT, Garfin SR. The economics of minimally invasive spine surgery: the value perspective. Spine (Phila Pa 1976). 2010;35(26 suppl):S375–S382. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Yuan S, Tian Y, et al. Comparison of percutaneous endoscopic transforaminal discectomy, microendoscopic discectomy, and microdiscectomy for symptomatic lumbar disc herniation: minimum 2-year follow-up results. J Neurosurg. 2018;28:317–325. [DOI] [PubMed] [Google Scholar]
- 18.Ahn Y. Percutaneous endoscopic decompression for lumbar spinal stenosis. Expert Rev Med Devices. 2014;11:605–616. [DOI] [PubMed] [Google Scholar]
- 19.Madhavan K, Chieng LO, McGrath L, Hofstetter CP, Wang MY. Early experience with endoscopic foraminotomy in patients with moderate degenerative deformity. Neurosurg Focus. 2016;40:E6. [DOI] [PubMed] [Google Scholar]
- 20.Li XF, Jin LY, Lv ZD, et al. Endoscopic ventral decompression for spinal stenosis with degenerative spondylolisthesis by partially removing posterosuperior margin underneath the slipping vertebral body: technical note and outcome evaluation. World Neurosurg. 2019;126:e517–e525. [DOI] [PubMed] [Google Scholar]
- 21.Liu H, Ishihara H, Kanamori M, Kawaguchi Y, Ohmori K, Kimura T. Characteristics of nerve root compression caused by degenerative lumbar spinal stenosis with scoliosis. Spine J. 2003;3:524–529. [PubMed] [Google Scholar]
- 22.Jenis LG, An HS. Spine update. Lumbar foraminal stenosis. Spine (Phila Pa 1976). 2000;25:389–394. [DOI] [PubMed] [Google Scholar]
- 23.Wong E, Altaf F, Oh LJ, Gray RJ. Adult degenerative lumbar scoliosis. Orthopedics. 2017;40:e930–e939. [DOI] [PubMed] [Google Scholar]
- 24.Ploumis A, Transfledt E, Denis F. Degenerative lumbar scoliosis associated with spinal stenosis. Spine J. 2007;7:428–436. [DOI] [PubMed] [Google Scholar]
- 25.Liang Y, Zhao Y, Wang T, Zhu Z, Liu H, Mao K. Precision treatment of adult lumbar degenerative scoliosis complicated by lumbar stenosis with the use of selective nerve root block. World Neurosurg. 2018;120:e970–e975. [DOI] [PubMed] [Google Scholar]
- 26.Kambin P, Casey K, O’Brien E, Zhou L. Transforaminal arthroscopic decompression of lateral recess stenosis. J Neurosurg. 1996;84:462–467. [DOI] [PubMed] [Google Scholar]
- 27.Ahn Y, Oh H, Kim H, Lee S, Lee H. Percutaneous endoscopic lumbar foraminotomy: an advanced surgical technique and clinical outcomes. Neurosurgery. 2014;75:124–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn Y, Lee SH, Park WM, Lee HY, Shin SW, Kang HY. Percutaneous endoscopic lumbar discectomy for recurrent disc herniation: surgical technique, outcome, and prognostic factors of 43 consecutive cases. Spine (Phila Pa 1976). 2004;29:E326–E332. [DOI] [PubMed] [Google Scholar]
- 29.Pan F, Shen B, Chy SK, et al. Transforaminal endoscopic system technique for discogenic low back pain: a prospective cohort study. Int J Surg. 2016;35:134–138. [DOI] [PubMed] [Google Scholar]
- 30.Wen B, Zhang X, Zhang L, Huang P, Zheng G. Percutaneous endoscopic transforaminal lumbar spinal canal decompression for lumbar spinal stenosis. Medicine (Baltimore). 2016;95:e5186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain disability questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 32.Saltychev M, Mattie R, McCormick Z, Bärlund E, Laimi K. Psychometric properties of the Oswestry disability index. Int J Rehabil Res. 2017;40:202–208. [DOI] [PubMed] [Google Scholar]
- 33.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33:90–94. [DOI] [PubMed] [Google Scholar]
- 34.Frazier DD, Lipson SJ, Fossel AH, Katz JN. Associations between spinal deformity and outcomes after decompression for spinal stenosis. Spine (Phila Pa 1976). 1997;22:2025–2029. [DOI] [PubMed] [Google Scholar]
- 35.Minamide A, Yoshida M, Iwahashi H, et al. Minimally invasive decompression surgery for lumbar spinal stenosis with degenerative scoliosis: predictive factors of radiographic and clinical outcomes. J Orthop Sci. 2017;22:377–383. [DOI] [PubMed] [Google Scholar]
- 36.Kelleher MO, Timlin M, Persaud O, Rampersaud YR. Success and failure of minimally invasive decompression for focal lumbar spinal stenosis in patients with and without deformity. Spine (Phila Pa 1976). 2010;35:E981–E987. [DOI] [PubMed] [Google Scholar]
- 37.Daubs MD, Lenke LG, Cheh G, Stobbs G, Bridwell KH. Adult spinal deformity surgery: complications and outcomes in patients over age 60. Spine (Phila Pa 1976). 2007;32:2238–2244. [DOI] [PubMed] [Google Scholar]
- 38.Hasan S, McGrath LB, Sen RD, Barber JK, Hofstetter CP. Comparison of full-endoscopic and minimally invasive decompression for lumbar spinal stenosis in the setting of degenerative scoliosis and spondylolisthesis. Neurosurg Focus. 2019;46:E16. [DOI] [PubMed] [Google Scholar]
- 39.Pritchett JW, Bortel DT. Degenerative symptomatic lumbar scoliosis. Spine (Phila Pa 1976). 1993;18:700–703. [DOI] [PubMed] [Google Scholar]
- 40.Watanuki A, Yamada H, Tsutsui S, En-yo Y, Yoshida M, Yoshimura N. Radiographic features and risk of curve progression of de-novo degenerative lumbar scoliosis in the elderly: a 15-year follow-up study in a community-based cohort. J Orthop Sci. 2012;17:526–531. [DOI] [PubMed] [Google Scholar]
- 41.Trammell TR, Schroeder RD, Reed DB. Rotatory olisthesis in idiopathic scoliosis. Spine (Phila Pa 1976). 1988;13:1378–1382. [DOI] [PubMed] [Google Scholar]
- 42.Ploumis A, Transfeldt EE, Gilbert TJ, Jr, Mehbod AA, Dykes DC, Perra JE. Degenerative lumbar scoliosis: radiographic correlation of lateral rotatory olisthesis with neural canal dimensions. Spine (Phila Pa 1976). 2006;31:2353–2358. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein SL, Ponseti IV. Curve progression in idiopathic scoliosis. J Bone Joint Surg Am. 1983;65:447–455. [PubMed] [Google Scholar]