Abstract
Study Design:
Systematic review.
Objectives:
Adult spinal deformity (ASD) can be a debilitating condition with a profound impact on patients’ health-related quality of life (HRQoL). Many reports have suggested that the frailty status of a patient can have a significant impact on the outcome of the surgery. The present review aims to identify all pre-operative patient-specific frailty markers that are associated with postoperative outcomes following corrective surgery for ASD of the lumbar and thoracic spine.
Methods:
A systematic review of the literature was performed to identify findings regarding pre-operative markers of frailty and their association with postoperative outcomes in patients undergoing ASD surgery of the lumbar and thoracic spine. The search was performed in the following databases: PubMed, Embase, Cochrane and CINAHL.
Results:
An association between poorer performance on frailty scales and worse postoperative outcomes. Comorbidity indices were even more frequently employed with similar patterns of association between increased comorbidity burden and postoperative outcomes. Regarding the assessment of HRQoL, worse pre-operative ODI, SF-36, SRS-22 and NRS were shown to be predictors of post-operative complications, while ODI, SF-36 and SRS-22 were found to improve post-operatively.
Conclusions:
The findings of this review highlight the true breadth of the concept of “frailty” in ASD surgical correction. These parameters, which include frailty scales and various comorbidity and HRQoL indices, highlight the importance of identifying these factors preoperatively to ensure appropriate patient selection while helping to limit poor postoperative outcomes.
Keywords: adult spinal deformity, frailty, index, spine, surgery, health-related quality of life, HRQoL, degenerative, outcomes, comorbidity
Introduction
Adult spinal deformity (ASD) can be a debilitating condition with a profound impact on patients’ health-related quality of life (HRQoL). 1 While ASD can arise from multiple etiologies, age-related degeneration of the spine seems to be the main reason of deformity. With an aging population, the incidence of ASD and surgical correction has doubled between the 2000 and 2010. 2 Although ASD corrective surgery has been shown to be beneficial, operating in an older population frequently means dealing with patients who have multiple comorbidities.3,4 Surgical outcomes are not solely depend on the success of the operation itself, but also on the risk factors defined pre-operatively.5-8 To address this issue, several studies have been carried out to evaluate the role of frailty and develop robust tools for predicting risk profile and outcomes in ASD correction. 9 , 6
As per Xue et al., frailty is defined as a clinically recognizable state of increased vulnerability, resulting from age-associated decline in reserve and function across multiple physiologic systems, such that the ability to cope with every day or acute stressors is compromised. 10 Multiple tools have been developed over the years to quantify this important condition and guide clinical management, such as the Frailty phenotype 11 and Frailty Index. 12 For example, the authors of the Canadian Study of Health and Aging used the Clinical Frailty Scale and found that pre-admission frailty was independently associated with adverse discharge destination in geriatric trauma patients. 13
Regarding ASD, frailty scales such as the modified Frail Index (mFI) and the FRAIL scale have been applied to help predict surgical outcomes. 7 , 14 Moreover, Miller et al. developed the Adult Spinal Deformity–Frailty Index (ASD-FI), which targets frailty in ASD patients specifically and has been shown to be effective in identifying patients at risk of major complications, proximal junctional kyphosis (PJK) and reoperation, among other various adverse effects. 6 Furthermore, a variety of other metrics have been used as surrogates for frailty in the ASD literature, such as HRQoL scores and the American Society of Anesthesiologist (ASA) physical status classification system.8,14,15 Thus, in order to have a better global understanding of how frailty can be assessed to improve risk stratification and prognostication, we have conducted a comprehensive systematic review to identify all pre-operative patient-specific frailty markers that are associated with postoperative outcomes following corrective surgery for ASD of the lumbar and thoracic spine.
Methods
Protocol and Registration
We performed a systematic review of the literature regarding pre-operative markers of frailty and their association to postoperative outcomes in patients undergoing ASD surgery for the lumbar and thoracic spine, according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 16
Eligibility Criteria
Frailty was defined as “a patient phenotype of multi-system diminished physical reserve or capacity predisposing to worse outcomes.” 5 , 17 We included patients who were subjected to ASD surgery of the lumbar and thoracic spine. The number of relevant studies concerning the cervical spine was too small to present a meaningful analysis and these patients were not included. The following parameters were chosen as exclusion criteria: pediatric patients, patients with ASD surgery for cervical spine, language other than English, date of publication before 1999, conference abstracts, review articles and meta-analyses. In addition, all duplicate reports were identified manually.
Information Sources
The search was performed in the following database: PubMed, Embase, Cochrane and CINAHL, from 1999 to August of 2019.
Search
Search terms included subject headings (MeSH terms) specific to the respective databases, please see appendix for complete search strategy (Supplemental Digital Content: Appendix A).
Study Selection
The studies were initially screened through the abstract by 2 independents reviewers (CL and PA). A full text review was then performed on the remaining eligible studies by the independent reviews based on the selection criteria. All accepted studies were included in the systematic review. In cases of disagreement, consensus was reached via open discussion and detailed review of the full text.
Data Collection Process
After finalizing the review, the data from the selected articles was extracted independently and then compared by the 2 reviewers. In cases of disagreement on the inclusion of data, a senior author (MW) was consulted. The included studies were stratified according to level of evidence based on the Oxford Centre for Evidence-Based Medicine 2011.
Data Items
The following variables were investigated: frailty indices (mFI, ASD-FI and FRAIL), comorbidity burden (Charlson Comorbidity Index [CCI], ASA, individual diseases), patient demographics (age, gender, weight, smoking), HRQoL indices (Oswestry Disability Index [ODI], Scoliosis Research Society questionnaire [SRS-22], Short Form survey [SF-36], Numeric Rating Scale [NRS], Post-operative Quality of Recovery Scale [PQRS], Lumbar Stiffness Disability Index [LSDI]) in relation to complications, hospital stay, cost and postoperative function.
Risk of Bias in Individual Studies
The risk of bias was formally assessed at the study level while performing the systematic review of the manuscripts using the Newcastle-Ottawa scale. 18
Summary Measures
The principal measures used were: Odds Ratio (OR) and Incidence Risk Ratio (IRR).
Synthesis of Results
Due to the heterogeneity of the reported parameters and the variability in study methods, a meta-analysis was not feasible.
Risk of Bias Across Studies
Careful comparison of the findings with the study protocol was done to identify any selective reporting bias in the study included in this review.
Additional Analyses
None were performed.
Results
Literature Search
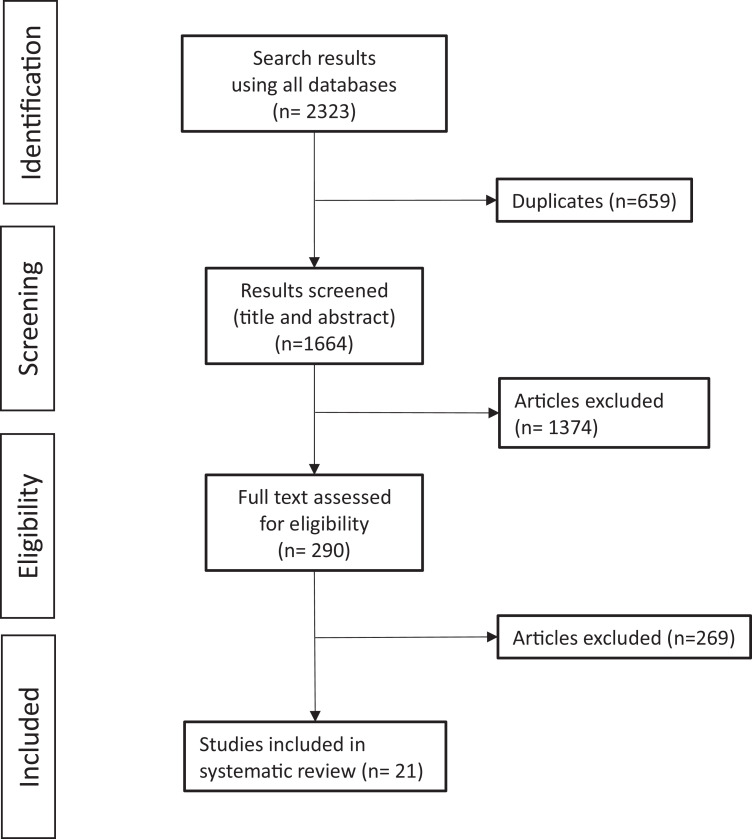
Our initial literature search led to 1,664 results after removal of any duplicates. Afterward, we excluded 1,374 articles based on their title and/or abstract and 269 after studying the full text. Finally, 21 studies were found to be eligible according to the aforementioned criteria (Figure 1, Table 1). The quality of the studies included were screened using the Newcastle-Ottawa scale (Table 2).
Figure 1.
Literature search.
Table 1.
Review of Association of Postoperative Outcomes With Frailty Measures and Their Surrogates in ASD Patients.
| First author |
Type of study | Level of evidence | Frailty index | Frailty and outcomes | Comorbidity index | Comorbidity and outcomes | HRQoL score |
|---|---|---|---|---|---|---|---|
| Leven 5 | Retrospective cohort study | 3 | mFI | Any complications (OR 1.6) Reoperation (OR 2.3) Wound complications (OR 2.2) |
--- | --- | --- |
| Passias 22 | Retrospective cohort study | 3 | mFI | --- | CCI | --- | --- |
| Yagi 14 | Retrospective cohort study | 3 | mFI | --- | CCI | --- | ODI / SRS-22 |
| Yagi 19 | Retrospective cohort study | 3 | mFI | Lower mFI score complications–Perioperative (OR 0.23), 2 yr (OR 0.51) | CCI | --- | SRS-22 |
| Yagi 21 | Retrospective cohort study | 3 | mFI | Adverse effect (RR 2.2) | CCI | --- | --- |
| Yagi 20 | Case-control study | 3 | mFI | Major complications (OR 5.4) | CCI |
Major complications Diabetes mellitus (OR 1.4) |
ODI / SRS-22 |
| Miller 6 | Retrospective cohort study | 3 | ASD-FI | Major complications (F OR 2.8, SF OR 4.1) Any complications (F OR 2.1, SF OR 2.1) PJK (F OR 2.8, SF OR 3.1) Reoperation (F OR 1.7, SF OR 2.1) Wound dehiscence (SF OR 13.4) Pseudoarthrosis (SF OR 13) Deep wound infection (SF OR 8.0) |
CCI / ASA | --- | --- |
| Miller 7 | Retrospective cohort study | 3 | ASD-FI | Major complications (SF OR 4.5) Longer hospital stay (F OR 1.4, SF OR 2.1) PJK (SF OR 7.0) Reoperation (SF OR 3.9) Wound infection (SF OR 9.7) |
CCI | --- | --- |
| Miller 23 | Retrospective cohort study | 3 | ASD-FI | Major complications (SF OR 3.7) | --- | --- | --- |
| Passias 1 | Retrospective cohort study | 3 | ASD-FI | Rapid functional decline (HR 1.4) | CCI |
Postoperative functional
decline Lung disease (HR 4.3) |
ODI / SF36 |
| Reid 25 | Retrospective cohort study | 3 | ASD-FI | --- | CCI | --- | ODI / SF36 / NRS |
| Rothrock 24 | Prospective cohort study | 2 | FRAIL Scale | Lower odds of functional recovery at 3 days and 3
months: Patients with higher baseline ADL (OR 0.65, 0.55, respectively) Lower odds of cognitive recovery at 3 days: Patients with lower baseline PQRS C3 test score (OR 0.53) |
ASA | --- | PQRS |
| Scheer 26 | Retrospective cohort study | 3 | --- | --- | CCI | --- | ODI / SF36 / SRS-22 |
| Scheer 27 | Retrospective cohort study | 3 | --- | --- | CCI / ASA | --- | ODI / PCS / SRS-22 / NRS |
| Sciubba 28 | Retrospective cohort study | 3 | --- | --- | CCI / ASA | --- | LSDI |
| Somani 8 | Retrospective cohort study | 3 | --- | --- | ASA |
ASA III Any complications (OR 1.89) RBC transfusion (OR 1.69) ASA IV Any complications (OR 3.58) Length of stay >5 days (OR 3.34) Pulmonary complications (OR 8.81) RBC transfusion (OR 2.52) Reoperation (OR 2.52) |
--- |
| Soroceanu 15 | Retrospective cohort study | 3 | --- | --- | ASA |
Intra / Postoperative medical
complications Smoking (IRR 2.38) Hypertension (IRR 2.42) Longer duration of symptoms (IRR 2.23) |
ODI / SF36 |
| Soroceanu 31 | Retrospective cohort study | 3 | --- | --- | ASA | Medical complications (IRR 1.33) Radiographical / implant related complications (1.75-fold increase for every 1 ASA score increase) |
ODI / SF-36 / SRS-22 |
| Theologis 29 | Retrospective cohort study | 3 | --- | --- | CCI / ASA | --- | ODI / SF36 / SRS22 |
| Whitmore 30 | Prospective cohort study | 2 | --- | --- | CCI / ASA | --- | --- |
| Zou 32 | Retrospective cohort study | 3 | --- | --- | --- | --- | ODI |
Table 2.
Newcastle-Ottawa Scale for Assessing Quality of Nonrandomized Studies.
| First author | Type of study | Level of evidence | Selection | Comparability | Outcome |
|---|---|---|---|---|---|
| Leven 5 | Retrospective cohort study | 3 | *** | ** | ** |
| Passias 22 | Retrospective cohort study | 3 | *** | ** | ** |
| Yagi 14 | Retrospective cohort study | 3 | **** | ** | *** |
| Yagi 19 | Retrospective cohort study | 3 | *** | ** | *** |
| Yagi 21 | Retrospective cohort study | 3 | **** | ** | *** |
| Yagi 20 | Case-control study | 3 | **** | ** | *** |
| Miller 6 | Retrospective cohort study | 3 | ** | ** | *** |
| Miller 7 | Retrospective cohort study | 3 | **** | * | *** |
| Miller 23 | Retrospective cohort study | 3 | *** | * | *** |
| Passias 1 | Retrospective cohort study | 3 | *** | ** | *** |
| Reid 25 | Retrospective cohort study | 3 | *** | ** | *** |
| Rothrock 24 | Prospective cohort study | 2 | *** | * | ** |
| Scheer 26 | Retrospective cohort study | 3 | *** | * | *** |
| Scheer 27 | Retrospective cohort study | 3 | *** | * | ** |
| Sciubba 28 | Retrospective cohort study | 3 | **** | ** | *** |
| Somani 8 | Retrospective cohort study | 3 | *** | ** | ** |
| Soroceanu 15 | Retrospective cohort study | 3 | **** | ** | *** |
| Soroceanu 31 | Retrospective cohort study | 3 | *** | * | *** |
| Theologis 29 | Retrospective cohort study | 3 | *** | * | *** |
| Whitmore 30 | Prospective cohort study | 2 | *** | ** | ** |
| Zou 32 | Retrospective cohort study | 3 | ** | * | ** |
Characteristics of Patients and Studies
A combined total of 13,942 patients were studied by the eligible reports with 5,318 males (38%) and 8,624 females (62%) and a mean age of 57 years. Average number of patients per cohort was 273. The patient cohorts were located in North America (63%), Asia (25%) and Europe (8%), while a multi-center study recruited patients from all these 3 different continents (4%). In terms of study design, we found 19 (90.5%) retrospective cohort studies and only 2 (9.5%) prospective cohort studies. Regarding the level of evidence, 2 studies were classified as level II, and the majority of the body of evidence (n = 19, 90.5%) as level III (Table 1).
Correlation of Frailty Indices With Postoperative Outcomes
Correlation between frailty status and postoperative outcome was assessed by 12 (57%) studies. The mFI was the most frequently used frailty index (n = 6, 50%), followed by ASD-FI (n = 5, 42%) and the FRAIL Scale (n = 1, 8%).
Modified frailty index
Of the six (6) studies utilizing the mFI, 5 (83%) found frailty status to positively correlate with postoperative complications. As an unadjusted risk factor, a higher mFI score was shown to increase the risk of blood transfusion, deep vein thrombosis / pulmonary embolism, any postoperative complication and mortality, PJK, proximal junction failure, and higher C7 sagittal vertical axis (SVA).5,14,19 Concerning less frail patients, one study found decreased rates of complications both perioperatively and at 2 years in univariate (OR: 0.23-0.36) and multivariate (OR 0.45-0.51) analysis. 19 Applying univariate analysis, increased frailty was found to be a significant risk factor for major complications (OR 5.4). 20 This was also confirmed by multivariate analysis, in which a score of ≥2 of the mFI 5-item index was associated with an increased risk of complications (Relative Risk [RR 2.2]) 21 by 4 times; and an increased mFI 11-item score with any complication (OR 1.6), wound complications (OR 2.2), and any reoperation (OR 2.3). 5 Additionally, the mFI 11-item index was found to be a superior predictor of reoperation after multivariate analysis when compared to age >60 and Body Mass Index (BMI) >40. 5 Only 1 (17%) study found the mFI to negatively correlate with complications after an analysis of the cohort trends over a 4-year period. 22
Adult spinal deformity frailty index
The ASD-FI was the second most used frailty index and all five (5) studies which used it, suggested that frailty was predictive of postoperative complications. Both univariate and multivariate analysis showed that frail patients (ASD-FI score 0.3-0.5) had similarly increased odds of any complication (OR 1.9-2.1), 6 major complications (OR 1.8-2.9),6,7 and longer hospital stay (OR 1.2-1.4). 7 Also, similar figures were seen after univariate analysis for significantly frail patients (ASD-FI score >0.5) with regard to any complication (OR 2.1), 6 major complications (OR 2.6-3.7),6,7,23 and longer hospital stay (OR 2.0). 7 Nonetheless, their status was also predictive of wound dehiscence (OR 11), 6 deep wound infection (OR 4.3), 6 PJK (OR 2.4) 6 and reoperation (OR 2.0). 6
Moreover, on multivariate analysis frail patients presented increased odds of PJK (OR 2.8), 6 reoperation (OR 1.7), 6 and rapid functional decline (Hazard Ratio [HR] 1.4). 1 In addition, the multivariate method revealed that significantly frail patients presented similarly high odds of any complication (OR 2.1) 6 and longer hospital stay (OR 2.1), 7 and more increased odds of major complications (OR 4.1-4.5),6,7 wound infection (OR 8.0-9.7)6,7 or wound dehiscence (OR 13.4), 6 PJK (OR 3.1-7.0),6,7 reoperation (OR 2.1-3.9),6,7 and pseudoarthrosis (OR 13). 6
FRAIL scale
The only study which applied the FRAIL scale suggested that this scale status was associated with decreased odds of postoperative cognitive recovery at 3 days and 3 months (univariate analysis), but the results did not reach statistical significance. 24
Correlation of Comorbidity Burden With Postoperative Outcomes
Patient comorbidity was assessed using indices by 18 (86%) studies, which either applied the Charlson Comorbidity Index (CCI) (n = 14, 78%),6,7,1,14,19-22,25-30 or the ASA Physical Status Classification System (n = 9, 50%),6,8,15,24,27-31 or both (n = 4, 22%).6,28-30
Charlson comorbidity index
Of the 14 studies utilizing the CCI, 8 (57%) reported the preoperative CCI scores without any correlation analysis to postoperative outcomes.6,7,19,21,25,26,28,29 Of the 6 (43%) studies which investigated the association of CCI scores with postoperative outcomes, 5 found a positive correlation of higher CCI scores1,14,22,27,30 with postoperative complications while 1 publication did not find a significant correlation. 20 Furthermore, higher CCI scores were an unadjusted risk factor for high-grade Clavien grade complications, 22 lower HRQoL, 14 major complications 27 and any complications. 30 Also, the univariate analysis showed that patients with postoperative functional decline had higher preoperative CCI scores. 1
American Society of Anesthesiologist Physical Status Classification System
Nine studies applied the ASA grading, but 4 (44%) of them only reported the preoperative ASA scores without any correlation analysis to postoperative outcomes.6,24,27-29 The remaining 5 (56%) studies saw a positive correlation between higher ASA grades and postoperative complications8,15,27,31,30 and in one of them the ASA grade was also found to be an independent predictor. 27
Higher ASA scores were associated with increased medical complications (IRR 1.33) 15 and radiographical and implant related complications, after univariate analysis. 31 In addition, bivariate analysis showed that compared to ASA grade II, ASA grade III patients had higher percentages of 30-day mortality, any complications, hospital stay over 5 days, wound, pulmonary, cardiac or renal complications, transfusion of red blood cells (RBC), sepsis, peripheral nerve injury, reoperation and unplanned readmission. 8
According to multivariate analysis, ASA grade III and IV were independent risk factors for any complication (OR 1.89 and 3.58 respectively) and RBC transfusion (OR 1.69 and 2.52 respectively), and ASA grade IV was also an independent risk factor for hospital stay over 5 days (OR 3.34), pulmonary complications (OR 8.81), and reoperation (OR 2.52). 8 Moreover, multivariate analysis revealed that ASA scores were positively correlated with radiographical or implant-related complications with a 1.75 fold increased risk for each added point in ASA score, 31 and higher medical costs. 30
Individual diseases
The relationship between comorbidity outside of a collective index with postoperative outcomes was assessed by 4 articles (22%) out of the 18 that recorded comorbidities.1,15,20,22,27,31 Various non-adjusted risk factors for major complications have been reported, i.e. diabetes mellitus, chronic obstructive pulmonary disease, central nervous system tumors, disseminated cancer, use of steroids for chronic conditions, and osteoporosis.22,27 Furthermore, univariate analysis identified significant risk factors for major complications (diabetes mellitus OR 1.4), 20 or complications in general (lung disease, 1 low bone mineral density [OR 3.2], preoperative anemia [IRR 1.61], heart disease [IRR 2.07], hypertension [IRR 2.42], and depression [IRR 1.6]). 15 According to multivariate analysis, lung disease was an independent risk factor for functional decline (HR 4.3) and hypertension for intra- and perioperative complications (IRR 2.42).1,15
Correlation of Individual Factors With Postoperative Outcomes
Patient demographics
It has been suggested that increased patient age correlated with loss of function and was a risk factor for increased rates of complications.1,27 Also, female patients accounted for a greater proportion of patients with low-grade Clavien grade complications. 22 Regarding weight, a higher BMI was associated with increased rates of complications, 27 whereas a lower BMI with an increased proportion of low-grade Clavien complications instead of major. 22
While univariate analysis showed that age (OR 1.5), 20 obesity (BMI>30, IRR 1.61) 15 and male gender (IRR 1.54, 15 OR 1.4) 20 were significant risk factors for postoperative complications, multivariate analysis identified smoking (IRR 2.49) and longer duration of symptoms (IRR 2.23) as independent predictors. 15 Also, according to multivariate analysis higher BMI was correlated with lower odds of recovery (OR 0.88) 24 and higher percentages of radiological / implant-related complications; the latter being also associated with history of previous spine surgery. 31
Surgical risk grading tool
A study by Yagi et al. 20 developed a surgical risk grading tool using a combination of factors such as frailty (mFI score), age, male gender, diabetes mellitus, bone mineral density, C7 SVA, pedicle subtraction osteotomy, lowest instrumented level at pelvis, and Cobb angle. Their tool showed an excellent correlation between risk grading and development of postoperative complications (r2 = 0.969) or a Clavien grade of complications greater than 2 (r2 = 0.949); while the authors observed an exponential increase in complications with increasing risk grade. 20 Additionally, their findings were validated on separate patient cohorts at 2 other hospitals. 20
Health Related Quality of Life Measures and Postoperative Outcomes
Out of the 14 (67%) studies that assessed HRQoL, 9 (43%) used more than 1 index. The indices used were the following: ODI (n = 10, 71%),1,14,15,20,25-27,29,31,32 SRS-22 (n = 7, 50%),14,19,20,26,27,29,31 SF-36 (n = 7, 50%),1,15,25-27,29,31 NRS (n = 2, 14%),25,27 PQRS (n 1, 7%), 24 and LSDI (n = 1, 7%), 28 while 1 study (7%) evaluated patients based on level of reliance on others for completing daily activities. 22 Also, ODI was shown to be significantly correlated on linear regression with SF-36 at baseline, 1 year, 2 years and 3 years postoperatively. 1
Concerning HRQoL population trends, one study found that over the period of 2010-2014 the overall preoperative functional status of patients undergoing ASD surgery had increased along with reduced rates of complications and a bigger number of anterior approaches. 22 Multiple studies observed significant improvement in HRQoL scores postoperatively compared to baseline (ODI, SRS-22, SF-36).15,20,25,31,32 However, upon stratification, the rate of improvement has been reported as slower in patients with complications (ODI and SRS-22), 20 faster in frail patients (ODI, SF-36 physical component score [PCS] and leg pain), 25 or without any significant difference in 1 study regardless of postoperative complication status (ODI, SF-36). 15 Also, increased frailty has been also associated with worsening ODI and SRS-22 scores 14 and better rates of patients reaching substantial clinical benefit postoperatively for ODI and SF-36 (PCS and leg pain) scores. 25
Two studies have suggested an association between baseline HRQoL status and postoperative outcomes using multivariate analysis.24,27 Scheer et al have found that ODI, SF-36 (PCS), SRS-22 and NRS scores were significant predictors of postoperative complications. 27 Furthermore, patients with higher baseline ADLs had decreased odds of presenting measurable functional recovery at 3 days and 3 months (OR 0.65 and 0.55, respectively). 24 Also, lower postoperative cognitive recovery at 3 days were found in patients with lower baseline performance on the PQRS C3 (numbers backward) test (OR 0.53). 24
Discussion
Patients with adult spinal deformity can suffer from significant disability, with the overall impact on patients’ health being comparable to that of severe chronic diseases such as diabetes mellitus. 33 Operative ASD treatment has been shown to provide significant relief of pain and disability when nonoperative treatment fails.34,35 A recent prospective study by Smith et al. showed that surgery for ASD significantly improves the HRQoL at the 2-year follow-up point, whereas conservative treatment does little to reduce pain and disability. 36 Nevertheless, it has been shown that the postoperative outcomes is dependent not only on the success of the surgical intervention itself but also on the health status of the patient. 5 -8 Furthermore, the varying possibility of complications (some of which are major), the fact that ASD surgery is elective and the undetermined durability of satisfactory clinical outcomes following ASD-corrective surgery are crucial factors regarding the decision-making and patient selection.37,38 The purpose of this review was to determine the association between frailty and its surrogate markers with postoperative outcomes following ASD correction of the lumbar and thoracic spine in order to improve decision-making by healthcare professionals treating ASD and the relevant policies so that the cost-benefit ratio is maximized.
Prognostication of Complications
While statistical methods, such as multivariate analysis and logistic regression, have identified various independent risk factors for complications and other outcomes, we cannot yet give a very specific prediction to any given patient. 39 Nevertheless, several predictive tools for outcomes in ASD surgery have been presented in the literature, which unfortunately are not without limitations. The Adult Deformity Surgery Complexity Index is a valid tool for quantifying the complexity of ASD surgery and predicting surgical blood loss and time, and postoperative complications. 40 However, it was created based on expert consensus and included only surgical parameters without any frailty factors. The Seattle Spine Score is a predictive score for the 30-day complication risk after ASD surgery. 41 Although this scoring system used clinical parameters indicative of frailty (age, BMI, smoking, hypertension, anemia, diabetes), it also included a wide range of ages and lacked external validation of the predictive model. 41 Yagi et al. have also developed a model to predict complications up to 2 years postoperatively. 42 Their investigation demonstrated the importance of detecting long term complications, because those patients may require revision surgery at a percentage reaching 73%. 42 In addition, their model has a highly accurate predictive capacity and has undergone internal and external validation. Nonetheless, the dataset is based only on an East Asian population, which may limit generalizability.
Since the occurrence of complications is a multifactorial phenomenon, it is difficult to build a robust predictive model based on numerous potential risk variables that could be applicable worldwide. Therefore, an approach similar to the retrospective review of a multi-center ASD database by Sheer et al. might be worth to investigate. 27 The authors identified 20 variables, including demographic, radiographic and surgical factors, which were associated with major complications with a high degree of accuracy (87%) and some of them have been related to frailty (age, CCI, ASA, ODI, SF-36, SRS). Although the researchers did not focus on frailty only, a similar approach might be feasible to create a tool for prognosticating outcomes in frail patients undergoing ASD correction.
Frailty Indices
It should be emphasized that frailty is difficult to define with a single clinically relevant score, because it is a multidimensional condition characterized by a reduction in the physiologic reserve in multiple organ systems and loss in the ability to maintain cellular/organ/system homeostasis. 43 To reflect this state, however, 3 distinct tools have been already applied in the relevant literature analyzed in this review: 1) the mFI, 2) the ASD-FI and 3) the FRAIL scale. The number of variables included in each of these tools range from 5 to 42. Thus, the real challenge in the clinical setting is to have a tool that guides the surgeon in surgical decision-making accurately, while being sufficiently user-friendly. Yagi et al. compared both mFI-5 and mFI-11 indices and suggested that they were equally effective in the prediction of major complications. 21 However, these tools are not specifically designed for patients undergoing ASD surgery. On the other hand, the ASD-FI was created for the patient population in question and since its validation it appears to have become more popular in the ASD literature.6,7,23 Nonetheless, a major limitation is that this index consists of 42 independent parameters, which is obviously a large number. The large number of parameters required by this tool, which afford a higher degree of granularity, ultimately may serve as a barrier to wide scale adoption and may actually encourage heterogeneity in the frailty indices used to assess ASD patients.
Prediction of Postoperative HRQoL
As described previously, multiple tools have been used in the literature to quantify the effect of surgery on the function and quality of life of patients, namely ODI, SRS-22, SF36, NRS, PQRS and LSDI. While the majority of studies investigated the change of HRQoL after surgery, the association between baseline HRQoL status and other postoperative outcomes was only investigated by 2 manuscripts.24,27 Reid et al. 25 showed that frail patient may benefit the most from ASD surgery, even if they are inherently more at risk for medical complications. While some studies saw a decrease in HRQoL index after complications, another study saw no difference in the postoperative HRQoL recovery rate regardless of occurrence of medical complications. 15 The wide variety of HRQoL tools not only highlights the challenges with quantifying quality of life, but also introduces a great deal of heterogeneity. Thus, it was difficult to provide clear guidelines in terms of which HRQoL indices are best suited to assess frail patients. As aforementioned, more specific but practical tools aimed at evaluating and predicting functional outcome in these patients might be more appropriate.
Radiographic Parameters and Frailty
It is known that some radiographic parameters have been associated with a higher rate of complications or a lower proportion of functional recovery.20,27,31 For example, C7 SVA had a weak but significant correlation with ODI scores pre- and postoperatively. 32 Moreover, greater baseline C7 SVA (OR 2.4), T1 pelvic angle, Cobb angle >70 (OR 1.7), pelvic incidence minus lumbar lordosis (OR 1.6), pelvic tilt and SRS-Schwab coronal curve type L have all been associated with higher rates of complications.20,27,31 Therefore, it would be worth investigating whether such radiographic parameters could be used as surrogates of physical frailty or are only indicative of the complexity and duration of the indicated operation and the severity of postoperative stress and need for adaptation of the tissues or the result of a combination of the aforementioned factors. However, it seems that in some cases there may be a causal relationship, e.g. chronic restrictive lung disease due to restricted lung expansion or frequent falls with sequelae, both of which can be caused by severe spinal deformity.
Advances in Personalized Therapies/Algorithm for Adult Spinal Deformity
Standard classification tools are usually based on linear or logical regression models to identify preoperative factors associated with postoperative outcomes. While these tools can be applied to a large part of the population, they lack specificity to individual patients. The use of artificial intelligence via machine learning has led to recent advances in predictive analyses and can provide useful hindsight in a massive amount of data through the detection of non-linear relationships previously too intricate to manage with standard regression techniques.44,45 Thus, the application of these novel computational techniques may leverage the ability of a spine surgeon to make far more accurate and individualized treatment plans in the future. Applications include corroboration of preoperative decision-making by providing a risk-benefit grid, prediction of cost, HRQoL measures and reoperation rates, and the risk of major complications, blood transfusion, and hospital readmission.27,42,46-49, Furthermore, AI was used to create a novel classification system for ASD patients, which could potentially help spine surgeons to treat patients with unique risk-benefit profiles. 50
Limitations of the Present Review
The major strength of this review is its scope as our findings highlight the wide variety of parameters or tools used in the clinical practice to evaluate frailty and its impact on surgical outcomes. Not only were specific frailty indices such as the mFI and ASD-FI assessed, but also other surrogates of frailty were included in order to provide a more thorough analysis of how frailty affects outcomes in ASD correction. Nonetheless, it should be noted that the broad spectrum of parameters used by the various studies eventually precluded quantitative comparisons and a meta-analysis, which is a significant limitation of this review. In addition, only 2 studies (9.5%) reached a level of evidence 2, while no randomized control trial could be found in the literature. Thus, the outcomes and conclusions presented in this review should be evaluated with caution. Also, given that only ASD-FI has been designed for ASD, it could not be compared with any other index targeting this population in order to assess which is the most suitable and for which reasons.
Moreover, it is important to note that studies concerning cervical deformity surgery were excluded, because their number was limited. As a result, our analysis cannot be applied to this patient population. Lastly, the majority of the patients investigated from the included articles are located in North America (63%) and Asia (25%), which limits generalizability worldwide.
Conclusions
This systematic review summarizes the literature regarding surrogate markers of frailty and their ability to predict outcome following surgery for ASD. Various studies have suggested an association between worse post-operative outcomes and poorer scores regarding frailty or comorbidity indices.1,7,14,15,27,31 Concerning HRQoL worse pre-operative ODI, SF-36, SRS-22 and NRS were shown to be predictors of post-operative complications, while ODI, SF-36 and SRS-22 were found to improve post-operatively.15,20,25,31,32 Finally, demographic data frequently associated with frailty, including BMI and smoking, were also found to correlate with worse post-operative outcomes.
All of the aforementioned parameters, which include but are not limited to frailty indices, highlight the importance of identifying these factors preoperatively to ensure appropriate patient selection while helping to limit poor postoperative outcomes.
Supplemental Material
Supplemental Material, sj-docx-1-gsj-10.1177_21925682211004250 for Adult Spinal Deformity Surgery and Frailty: A Systematic Review by Carl Laverdière, Miltiadis Georgiopoulos, Christopher P. Ames, Jason Corban, Pouyan Ahangar, Khaled Awadhi and Michael H. Weber in Global Spine Journal
List of Abbreviations
- ASA
American Society of Anesthesiologist
- ASD
adult spinal deformity
- ASD-FI
Adult Spinal Deformity–Frailty Index
- BMI
body mass index
- CCI
Charlson comorbidity index
- HRQoLQ
health-related quality of life
- HR
hazard ratio
- IRR
incidence risk ratio
- LSDI
Lumbar Stiffness Disability Index
- mFI
modified Frail Index
- NRS
Numeric Rating Scale
- ODI
Oswestry Disability Index
- OR
Odds Ratio
- PCS
physical component score
- PJK
proximal junctional kyphosis
- PQRS
Post-operative Quality of Recovery Scale
- RBC
red blood cells
- RR
relative risk
- SF-36
Short Form survey
- SRS
Scoliosis Research Society
- SVA
sagittal vertical axis
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Footnotes
Authors’ Note: Carl Laverdière and Miltiadis Georgiopoulos have contributed equally to this manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Miltiadis Georgiopoulos, MD  https://orcid.org/0000-0001-8656-1412
https://orcid.org/0000-0001-8656-1412
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Passias PG, Bortz CA, Lafage V, et al. Durability of satisfactory functional outcomes following surgical adult spinal deformity correction: a 3-year survivorship analysis. Oper Neurosurg (Hagerstown). 2020;18(2):118–125. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy I, O’Brien M, Ames C, et al. Incremental cost-effectiveness of adult spinal deformity surgery: observed quality-adjusted life years with surgery compared with predicted quality-adjusted life years without surgery. Neurosurg Focus. 2014;36(5):E3. [DOI] [PubMed] [Google Scholar]
- 3.Berven SH, Hohenstein NA, Savage JW, Tribus CB. Does the outcome of adult deformity surgery justify the complications in elderly (above 70 y of age) patients? J Spinal Disord Tech. 2015;28(8):271–274. [DOI] [PubMed] [Google Scholar]
- 4.Yagi M, Ames CP, Keefe M, et al. A cost-effectiveness comparisons of adult spinal deformity surgery in the United States and Japan. Eur Spine J. 2018;27(3):678–684. [DOI] [PubMed] [Google Scholar]
- 5.Leven DM, Lee NJ, Kothari P, et al. Frailty index is a significant predictor of complications and mortality after surgery for adult spinal deformity. Spine (Phila Pa 1976). 2016;41(23):E1394–E1401. [DOI] [PubMed] [Google Scholar]
- 6.Miller EK, Neuman BJ, Jain A, et al. An assessment of frailty as a tool for risk stratification in adult spinal deformity surgery. Neurosurg Focus. 2017;43(6):E3. [DOI] [PubMed] [Google Scholar]
- 7.Miller EK, Vila-Casademunt A, Neuman BJ, et al. External validation of the adult spinal deformity (ASD) frailty index (ASD-FI). Eur Spine J. 2018;27(9):2331–2338. [DOI] [PubMed] [Google Scholar]
- 8.Somani S, Capua JD, Kim JS, et al. ASA Classification as a risk stratification tool in adult spinal deformity surgery: a study of 5805 patients. Global Spine J. 2017;7(8):719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joshi RS, Haddad AF, Lau D, Ames CP. Artificial intelligence for adult spinal deformity. Neurospine. 2019;16(4):686–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xue Q-L. The frailty syndrome: definition and natural history. Clin Geriatr Med. 2011;27(1):1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. [DOI] [PubMed] [Google Scholar]
- 12.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung A, Haas B, Ringer TJ, McFarlan A, Wong CL. Canadian Study of Health and Aging Clinical Frailty Scale: does it predict adverse outcomes among geriatric trauma patients? J Am Coll Surg. 2017;225(5):658–665. e653. [DOI] [PubMed] [Google Scholar]
- 14.Yagi M, Fujita N, Okada E, et al. Impact of frailty and comorbidities on surgical outcomes and complications in adult spinal disorders. Spine (Phila Pa 1976). 2018;43(18):1259–1267. [DOI] [PubMed] [Google Scholar]
- 15.Soroceanu A, Burton DC, Oren JH, et al. Medical complications after adult spinal deformity surgery: incidence, risk factors, and clinical impact. Spine (Phila Pa 1976). 2016;41(22):1718–1723. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. [DOI] [PubMed] [Google Scholar]
- 17.Shin JI, Kothari P, Phan K, et al. Frailty index as a predictor of adverse postoperative outcomes in patients undergoing cervical spinal fusion. Spine (Phila Pa 1976). 2017;42(5):304–310. [DOI] [PubMed] [Google Scholar]
- 18.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 2014; Updated February 11, 2021. Accessed December 10, 2020. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 19.Yagi M, Michikawa T, Hosogane N, et al. Treatment for frailty does not improve complication rates in corrective surgery for adult spinal deformity. Spine (Phila Pa 1976). 2019;44(10):723–731. [DOI] [PubMed] [Google Scholar]
- 20.Yagi M, Hosogane N, Fujita N, et al. Surgical risk stratification based on preoperative risk factors in adult spinal deformity. Spine J. 2019;19(5):816–826. [DOI] [PubMed] [Google Scholar]
- 21.Yagi M, Michikawa T, Hosogane N, et al. The 5-item modified frailty index is predictive of severe adverse events in patients undergoing surgery for adult spinal deformity. Spine (Phila Pa 1976). 2019;44(18):E1083–E1091. [DOI] [PubMed] [Google Scholar]
- 22.Passias PG, Bortz CA, Pierce KE, et al. Decreased rates of 30-day perioperative complications following ASD-corrective surgery: a modified Clavien analysis of 3300 patients from 2010 to 2014. J Clin Neurosci. 2019;61:147–152. [DOI] [PubMed] [Google Scholar]
- 23.Miller EK, Lenke LG, Neuman BJ, et al. External validation of the adult spinal deformity (ASD) frailty index (ASD-FI) in the Scoli-RISK-1 patient database. Spine (Phila Pa 1976). 2018;43(20):1426–1431. [DOI] [PubMed] [Google Scholar]
- 24.Rothrock RJ, Steinberger JM, Badgery H, et al. Frailty status as a predictor of 3-month cognitive and functional recovery following spinal surgery: a prospective pilot study. Spine J. 2019;19(1):104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reid DBC, Daniels AH, Ailon T, et al. Frailty and health-related quality of life improvement following adult spinal deformity surgery. World Neurosurg. 2018;112:e548–e554. [DOI] [PubMed] [Google Scholar]
- 26.Scheer JK, Passias PG, Sorocean AM, et al. Association between preoperative cervical sagittal deformity and inferior outcomes at 2-year follow-up in patients with adult thoracolumbar deformity: analysis of 182 patients. J Neurosurg Spine. 2016;24(1):108–115. [DOI] [PubMed] [Google Scholar]
- 27.Scheer JK, Smith JS, Schwab F, et al. Development of a preoperative predictive model for major complications following adult spinal deformity surgery. J Neurosurg Spine. 2017;26(6):736–743. [DOI] [PubMed] [Google Scholar]
- 28.Sciubba DM, Scheer JK, Smith JS, et al. Which daily functions are most affected by stiffness following total lumbar fusion: comparison of upper thoracic and thoracolumbar proximal endpoints. Spine (Phila Pa 1976). 2015;40(17):1338–1344. [DOI] [PubMed] [Google Scholar]
- 29.Theologis AA, Ailon T, Scheer JK, et al. Impact of preoperative depression on 2-year clinical outcomes following adult spinal deformity surgery: the importance of risk stratification based on type of psychological distress. J Neurosurg Spine. 2016;25(4):477–485. [DOI] [PubMed] [Google Scholar]
- 30.Whitmore RG, Stephen JH, Vernick C, et al. ASA grade and Charlson comorbidity index of spinal surgery patients: correlation with complications and societal costs. Spine J. 2014;14(1):31–38. [DOI] [PubMed] [Google Scholar]
- 31.Soroceanu A, Diebo BG, Burton D, et al. Radiographical and implant-related complications in adult spinal deformity surgery: incidence, patient risk factors, and impact on health-related quality of life. Spine (Phila Pa 1976). 2015;40(18):1414–1421. [DOI] [PubMed] [Google Scholar]
- 32.Zou HB, Wu CH, Mehbod AA, Lick C, Transfeldt EE. Prediction of health status based on postoperative radiographic variables in adult scoliosis. Orthop Surg. 2014;6(3):196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bess S, Line B, Fu K-M, et al. The health impact of symptomatic adult spinal deformity: comparison of deformity types to United States population norms and chronic diseases. Spine (Phila Pa 1976). 2016;41(3):224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith JS, Shaffrey CI, Berven S, et al. Operative versus nonoperative treatment of leg pain in adults with scoliosis: a retrospective review of a prospective multicenter database with two-year follow-up. Spine (Phila Pa 1976). 2009;34(16):1693–1698. [DOI] [PubMed] [Google Scholar]
- 35.Youssef J, Orndorff D, Patty C, et al. Current status of adult spinal deformity. Global Spine J. 2013;3(1):51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of operative and nonoperative treatment for adult spinal deformity: a prospective, multicenter, propensity-matched cohort assessment with minimum 2-year follow-up. Neurosurgery. 2016;78(6):851–861. [DOI] [PubMed] [Google Scholar]
- 37.Bridwell KH, Glassman S, Horton W, et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa 1976). 2009;34(20):2171–2178. [DOI] [PubMed] [Google Scholar]
- 38.Smith JS, Kasliwal MK, Crawford A, Shaffrey CI. Outcomes, expectations, and complications overview for the surgical treatment of adult and pediatric spinal deformity. Spine Deformity. 2012;1(1):4–14. [Google Scholar]
- 39.Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158(3):280–287. [DOI] [PubMed] [Google Scholar]
- 40.Pellisé F, Vila-Casademunt A, Núñez-Pereira S, et al. The adult deformity surgery complexity index (ADSCI): a valid tool to quantify the complexity of posterior adult spinal deformity surgery and predict postoperative complications. Spine J. 2018;18(2):216–225. [DOI] [PubMed] [Google Scholar]
- 41.Buchlak QD, Yanamadala V, Leveque J-C, Edwards A, Nold K, Sethi R. The Seattle spine score: predicting 30-day complication risk in adult spinal deformity surgery. J Clin Neurosci. 2017;43:247–255. [DOI] [PubMed] [Google Scholar]
- 42.Yagi M, Hosogane N, Fujita N, et al. Predictive model for major complications 2 years after corrective spine surgery for adult spinal deformity. Eur Spine J. 2019;28(1):180–187. [DOI] [PubMed] [Google Scholar]
- 43.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210(6):901–908. [DOI] [PubMed] [Google Scholar]
- 44.Galbusera F, Casaroli G, Bassani T. Artificial intelligence and machine learning in spine research. JOR Spine. 2019;2(1):e1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Suzuki S, Yamashita T, Sakama T, et al. Comparison of risk models for mortality and cardiovascular events between machine learning and conventional logistic regression analysis. PLoS One. 2019;14(9):e0221911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ames CP, Smith JS, Gum JL, et al. Utilization of predictive modeling to determine episode of care costs and to accurately identify catastrophic cost nonwarranty outlier patients in adult spinal deformity surgery: a step toward bundled payments and risk sharing. Spine (Phila Pa 1976). 2020;45(5):E252–E265. [DOI] [PubMed] [Google Scholar]
- 47.Ames CP, Smith JS, Pellisé F, et al. Artificial intelligence based hierarchical clustering of patient types and intervention categories in adult spinal deformity surgery: towards a new classification scheme that predicts quality and value. Spine (Phila Pa 1976). 2019;44(13):915–926. [DOI] [PubMed] [Google Scholar]
- 48.Ames CP, Smith JS, Pellisé F, et al. Development of predictive models for all individual questions of SRS-22 R after adult spinal deformity surgery: a step toward individualized medicine. Eur Spine J. 2019;28(9):1998–2011. [DOI] [PubMed] [Google Scholar]
- 49.Durand WM, DePasse JM, Daniels AH. Predictive modeling for blood transfusion after adult spinal deformity surgery: a tree-based machine learning approach. Spine (Phila Pa 1976). 2018;43(15):1058–1066. [DOI] [PubMed] [Google Scholar]
- 50.Ames CP, Smith JS, Pellisé F, et al. Development of deployable predictive models for minimal clinically important difference achievement across the commonly used health-related quality of life instruments in adult spinal deformity surgery. Spine (Phila Pa 1976). 2019;44(16):1144–1153. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, sj-docx-1-gsj-10.1177_21925682211004250 for Adult Spinal Deformity Surgery and Frailty: A Systematic Review by Carl Laverdière, Miltiadis Georgiopoulos, Christopher P. Ames, Jason Corban, Pouyan Ahangar, Khaled Awadhi and Michael H. Weber in Global Spine Journal