Summary
Background
Non-Hodgkin lymphoma comprises a heterogeneous group of cancers with unresolved aetiology, although risk factors include environmental exposures to toxic chemicals. Although the ubiquitous pollutant benzene is an established leukemogen, its potential to cause non-Hodgkin lymphoma has been widely debated. We aimed to examine the potential link between benzene exposure and risk of non-Hodgkin lymphoma in humans by evaluating a wide array of cohort and case-control studies using electronic systematic review.
Methods
We did a comprehensive systematic review and meta-analysis of all qualified human epidemiological studies that assessed the relationship between benzene exposure and non-Hodgkin lymphoma. We queried the PubMed and Embase databases for relevant articles published before June 5, 2019, and applied the SysRev platform for study selection. All peer-reviewed human cohort and case-control studies that reported non-Hodgkin lymphoma risk estimates specifically for benzene exposure were eligible for inclusion. Studies that calculated relative risks (RRs) for industries or job types without identifying those specifically exposed to benzene, that combined non-Hodgkin lymphoma with other cancer types, or that reported many different solvent exposures together were excluded. From each study, two investigators independently extracted information on the study design, location, years, sample size, participation rates, age, sex, sources of cases and controls, diagnosis, histological verification, exposure assessment, results, adjustment, and statistical analysis, and subsequently assessed study quality. We calculated the meta-analysis relative risk (meta-RR) and CIs using the fixed effect and random effect models, as well as assessing publication bias.
Findings
Our search yielded 2481 articles. After screening and removal of duplicates, 20 case-control studies and eight cohort studies were included in our meta-analysis, which included a total of 9587 patients with non-Hodgkin lymphoma. We reported an increased meta-relative risk (meta-RR) of 33% in highly exposed groups, when data were available (meta-RR 1·33 [95% CI 1·13–1·57], n=28). The meta-RR rose to 1·51 (1·22–1·87, n=18) in the studies that provided results specifically for highly exposed individuals. In particular, we reported a doubling of this risk for diffuse large B-cell lymphoma, a major non-Hodgkin lymphoma subtype (1·67 [1·01–2·77]). We also detected increased risks for follicular lymphoma (1·47 [0·95–2·27]) and hairy cell leukaemia (1·77 [0·99–3·16]), though they were not statistically significant. Funnel plot, Egger’s test (p=0·77) and Begg’s test (p=0·98) did not show evidence of publication bias. We evaluated the major aspects of causal inference and found evidence to support all the Hill considerations for assigning causation.
Interpretation
Our findings suggest a causal link between benzene exposure and non-Hodgkin lymphoma, especially for diffuse large B-cell lymphoma.
Introduction
Non-Hodgkin lymphoma is a diverse and heterogeneous group of blood cancers originating in lymphoid tissue, comprising over 60 cell types, with unresolved disease causes for most. It is a challenging neoplasm to diagnose and classify, and even more difficult to assess epidemiologically. Known risk factors for non-Hodgkin lymphoma include genetics, viral infection (eg, HIV), immunodeficiency disorders, sex, age, and occupational and environmental exposures. Given the diversity of non-Hodgkin lymphoma neoplasms, it is important to evaluate potential agents that could mediate development of non-Hodgkin lymphoma and its respective subtypes. Benzene is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (IARC) because it causes acute myeloid leukaemia and induces a variety of adverse health effects, including possible lymphomagenesis.
Benzene is the backbone of the chemical manufacturing industry. Given benzene’s highly reactive metabolites and simple aromatic structure, it is indispensable in the production of many key chemicals used in the synthesis of plastics, resins, and other fibres.1 Annual production of benzene by the petrochemical industry was estimated at nearly 2 billion tonnes in the USA alone in 2016, with demands expected to increase as dependence upon consumer goods continues to rise.2
Furthermore, the ubiquity of benzene in manufacturing makes exposure widespread, unavoidable, and has been consequently well studied in occupational settings. Environmental exposure in the general population typically occurs through automobile emissions, gasoline, and cigarette smoke; an estimated 50% of household benzene exposure in non-smokers comes from second-hand smoke.3 Given its economic significance, exposure-disease relationships involving benzene are highly controversial.
In its 2018 Monograph, IARC determined that, based on available evidence, there was “limited evidence that benzene causes…non-Hodgkin lymphoma”,4 though the working group did not consider all published epidemiological studies. Further, other studies have reported evidence for benzene-induced lymphoproliferative disorder in experimental animal models, and of a plausible and relevant mechanism of carcinogenicity in humans, as the stem cell known to be at risk for benzene-induced acute myeloid leukaemia is simultaneously responsible for lymphoproliferation.5 Our meta-analysis aimed to resolve the seeming incongruence between epidemiological data and animal and mechanistic data by evaluating a wide array of cohort and case-control studies using the latest methods in systematic review to rigorously examine the potential link between benzene exposure and risk of non-Hodgkin lymphoma.
Methods
Search strategy and selection criteria
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for this study. Details about the search strategy are reported in the appendix (pp 3–4).
Briefly, we queried PubMed and Embase for all epidemiological studies of non-Hodgkin lymphoma and benzene exposure published before June 5, 2019. Our key search terms used included “benzene”, “solvents”, “refinery”, “petroleum industry”, “non-Hodgkin lymphoma”, “lymphoma”, “hematopoietic”, “B-cell lymphoma”, “T-cell lymphoma”, “Mycosis fungoides”, “Sezary syndrome”, and “leukemia”, among others (appendix p 3).
All peer-reviewed human cohort and case-control studies that reported non-Hodgkin lymphoma risk estimates specifically for benzene exposure were eligible for inclusion. Studies that reported relative risks (RRs) by job type or industry without identifying specific exposures to benzene were excluded. Further, studies that reported RR estimates for non-Hodgkin lymphoma combined with other cancer types, or for many different solvent exposures together, were also excluded.
As non-Hodgkin lymphoma is a diverse group of blood cancers with many different subtypes, we also did a cell-type specific analysis of the reported haematological neoplasms from the articles included in the meta-analysis along with the sensitivity analysis.
We describe our a priori selection of the highest exposed category elsewhere.6 Briefly, when multiple RRs or odds ratios (ORs) were presented in the original studies, we selected estimates in the following order: (1) highest average exposure intensity; (2) highest cumulative exposure; (3) longest exposure duration; and (4) ever exposure, defined as individuals with any type of exposure to benzene at any level. We defined high exposure as a group exposed at a level relatively greater than lower exposed counterparts. The effect of our a priori exposure selection criteria was evaluated in the sensitivity analyses.
For studies with overlapping cohorts, the most recent study that reported a high exposure group, as defined by our a priori hypothesis, was prioritised. Only peer-reviewed studies published in scientific journals were eligible for inclusion. From each study, two investigators (IR and SD) independently extracted information on the study design, location, years, sample size, participation rates, age, sex, sources of cases and controls, diagnosis, histological verification, exposure assessment, results (RR or OR), adjustment, and statistical analysis. Any conflicts were resolved by a third reviewer (CS or LZ).
Data analysis
Our electronic systematic review was done and recorded using SysRev, a platform that limits bias by masking reviewers to each other’s responses. Using the Newcastle-Ottawa Scale,7 all studies were reviewed and assessed for methodological quality by two different reviewers (IR and SD) to ensure concordance among score assignments; senior investigators (CS or LZ) were consulted to resolve conflicts. Studies were evaluated on the basis of selection of study population, comparability, outcome (for cohort studies), and exposure (for case-control studies).
We calculated overall meta-analysis relative risk (meta-RR) estimates using both the fixed effect inverse variance method8 and the random effect method.9 If heterogeneity was present (evaluated using the summary variance method),10 the random effect model was used.
The use of the random effect model allowed for the incorporation of between-study variance into the summary variance estimate and CIs, which helped to prevent artificially narrow CIs that can result from using the fixed effect model in the presence of between-study heterogeneity.10 However, a drawback of the random effect model is that greater relative weight is given to smaller studies, which might make the summary results less conservative than the fixed effect model estimates.11 To overcome the limitations presented by the random effect model, we also presented the method first published by Shore and colleagues in which the meta-RR is calculated using the fixed effect model, while between-study heterogeneity is incorporated into the 95% CIs.12 Statistical significance was defined as a p value below 0·05.
Publication bias was evaluated using funnel plot, Egger’s test, and Begg’s test.13,14 All statistical analyses were done using Stata (version 15.1)15 and Microsoft Excel 2013.16
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
The screening process and results are shown in figure 1. We queried PubMed and Embase databases and identified 2390 articles for screening by title and abstract, after duplicates were removed. Of these articles, 24 (1%) studies were assigned conflicting labels by two independent reviewers, which were subsequently resolved by one of the principal investigators.
Figure 1: Study selection.
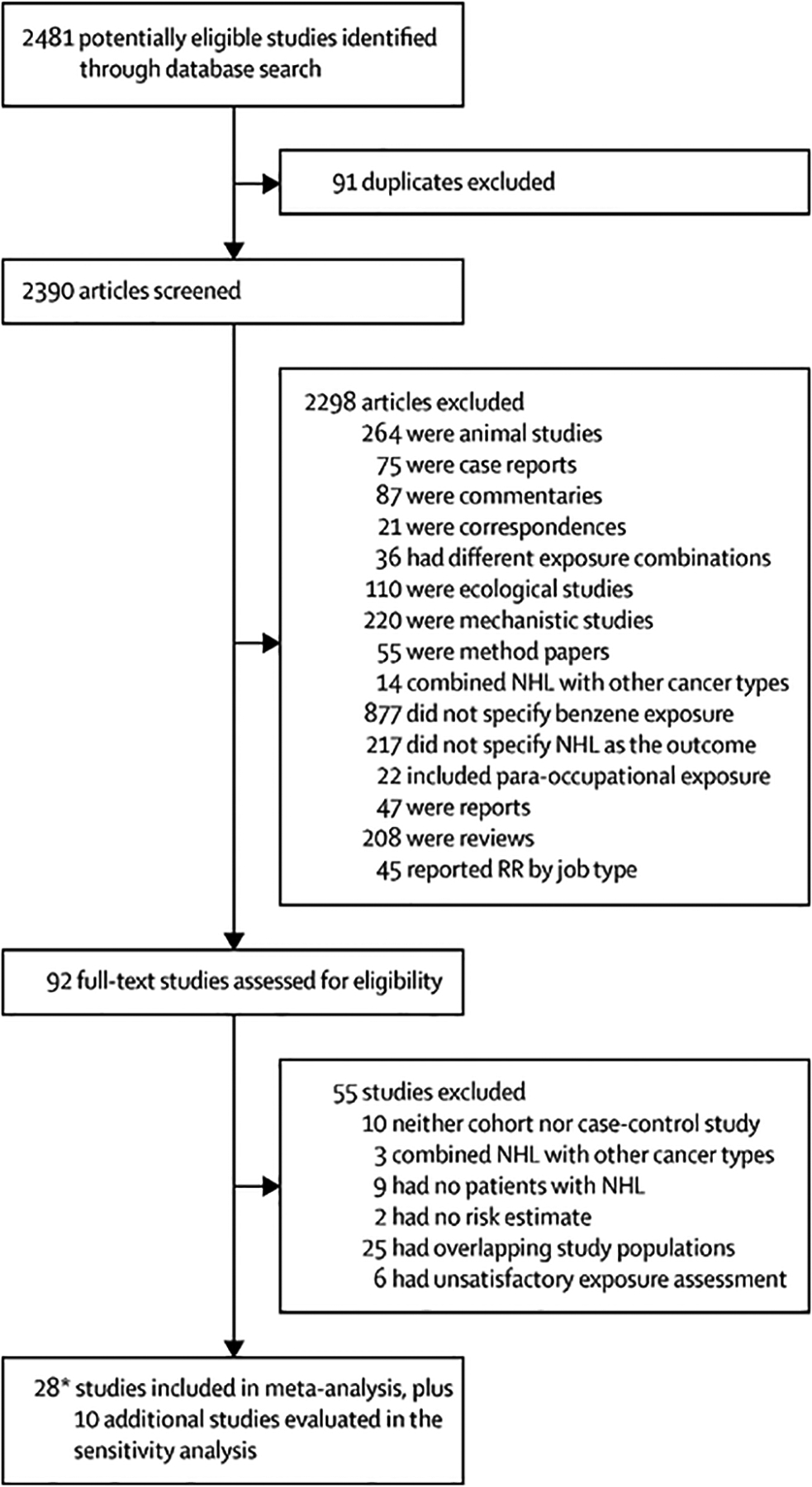
SysRev was used to help identify and screen potential studies for the meta-analysis.
*Two of the case-control studies in our meta-analysis are from the same paper17 because men and women were analysed separately. NHL=non-Hodgkin lymphoma. RR=relative risk.
Following initial screening, 92 papers were reviewed in full and assessed for eligibility. Of these, a total of 28 studies (eight cohort and 20 case-control design, including 9582 patients with non-Hodgkin lymphoma) were eligible for inclusion in the meta-analysis and ten studies were evaluated in the sensitivity analysis. Of note, two of the case-control studies in our meta-analysis are from the same paper17 because men and women were analysed separately. Hence, for clarity, the number of case-control studies has been listed as 20 throughout our manuscript. Three studies were done in China, eight studies were done in the USA, four studies were done in Canada, 11 studies were done in Europe, and two studies were done in Australia.
Table 1 summarises key aspects of the study design, exposure assessment, and results of all the studies evaluated in this meta-analysis. Additional char acteristics of these studies are described in the appendix (pp 5–20). The subsets of data from each study corresponding to each disease analysed including all available types of non-Hodgkin lymphoma, such as diffuse large B-cell lymphoma, follicular lymphoma, chronic lymphocytic leukaemia, and hairy cell leukaemia, as well as other haematopoietic malignancies, such as Hodgkin lymphoma, multiple myeloma, and myeloid leukaemia, are reported in the appendix (p 21).
Table 1:
Description and weight of studies selected for the current meta-analyses of exposures to benzene and risk of non-Hodgkin lymphoma
| Cases of non-Hodgkin lymphoma (exposed/total) | RR or OR (95% Cl) | Location | Self-report | Exposure category | Benzene exposure | Weight* | |
|---|---|---|---|---|---|---|---|
| Cohort (n=8) | |||||||
| Bassig et al (2015)18 | 12/102 | 2·04 (1·08–3·86)† | Shanghai, China | No | High | Cumulative >102·4 mg/m3 per year (10 year lag) | 5·07 |
| Collins et al (2003)19 | 3/25 | 1·80 (0·4–5·1)‡ | Illinois, USA | No | High | >100 ppm per day (>40 days exposed) | 1·54 |
| Collins et al (2015)20 | 3/15 | 0·53 (0·12–1·69)‡ | Michigan, USA | No | High | Cumulative ≥25 ppm per year | 1·43 |
| Hayes et al (1996)21 | 7/19 | 4·7 (1·2–18·1) | China | No | High | Average intensity ≥25 ppm per year | 1·37 |
| Rinsky et al (2002)22 | 5/5 | 0·96 (0·31–2·25)‡ | Ohio, USA | No | All | Ever | 2·43 |
| Sorahan et al (2005)23 | 24/24 | 1·0 (0·64–1·49)§ | UK | Yes | All | Ever | 8·88 |
| Stenehjem et al (2015)24 | 20/85¶ | 1·55 (0·83–2·88)† | Norway | No | High | Average intensity ≥0·013–0·040 ppm | 5·25 |
| Wong (1987)25 | 4/15 | 4·12 (1·11–10·55)|| | USA | No | High | Cumulative ≥720 ppm (adjusted) | 1·93 |
| Case-control (n=20) | |||||||
| Bernard et al (1984)26 | Unknown/158 | 0·49 (0·21–2·00) | Yorkshire, UK | Yes | All | Ever | 1·93 |
| Blair et al (1993)27 | 12/622 | 1·5 (0·7–3·1)** | Iowa, Minnesota, USA | Yes | High | High intensity | 3·96 |
| Cocco et al (2010)28 | 55/1179 | 1·3 (0·98–1·69)**,|| | Czech Republic, France, Germany, Ireland, Italy, and Spain | No | High | High cumulative | 13·55 |
| Dryver et al (2004)29 | 15/859 | 1·95 (0·90–4·21)** | Sweden | Yes | High | High exposure (aromatic hydrocarbons) | 3·73 |
| Fabbro-Peray et al (2001)30 | 8/445 | 5·7 (1·4–23·2)** | France | Yes | High | Cumulative >810 days | 1·28 |
| Franceschi et al (1989)31 | 15/208 | 1·14 (0·57–2·28) | Pordenone, Italy | Yes | All | Benzene and solvents | 4·44 |
| Fritschi et al (2005)32 | 2/694 | 0·31 (0·06–1·50)** | NSW or ACT, Australia | Yes | High | Substantial (>10% TLV >5 days for 5 years) | 0·99 |
| Gerin et al (1998)33 | 9/215 | 0·8 (0·4–1·6)** | Montreal, Canada | Yes | High | Medium to high (probability, frequency, and concentration) | 4·44 |
| Glass et al (2003)34 | 2/31 | 1·48 (0·30–7·16)**,|| | Australia | No | High | Cumulative >16 ppm per year | 1·02 |
| Kato et al (2005)35 | 7/376 | 1·52 (0·41–5·70)** | New York, USA | Yes | All | Ever | 1·45 |
| La Vecchia et al (1989)36 | 4/153 | 0·86|| (0·23–2·2)**,|| | Milan, Italy | Yes | High | Duration >10 years (solvents and benzene) | 1·92 |
| Mao et al (2000)17,†† | 36/764 | 1·2 (0·8–1·9)** | Canada | Yes | All | Ever | 8·63 |
| Mao et al (2000)17,‡‡ | 5/705 | 0·6 (0·2–1·8)** | Canada | Yes | All | Ever | 2·02 |
| Miligi et al (2006)37 | 58/1428 | 1·6 (1·0–2·4)** | Italy | Yes | High | Medium to high intensity | 8·51 |
| Orsi et al (2010)38 | 4/244 | 2·6 (0·6–11·2)** | France | No | High | High average intensity | 1·18 |
| Persson et al (1999)39 | 3/199 | 0·8 (0·1–3·8)** | Sweden | Yes | All | Occupational 1 year, 5–45 years earlier | 0·78 |
| Scherr et al (1992)40 | Unknown/303 | 1·2 (0·5–2·6) | Boston, USA | Yes | All | Ever | 3·34 |
| Schnatter et al (1996)41 | 2/8 | 0·93 (0·08–7·19)** | Canada | No | High | Intensity=0·2–0·49 mean ppm (5 year lag) | 0·52 |
| Wang et al (2009)42 | 30/601 | 1·4 (0·8–2·4)** | Connecticut, USA | No | High | Medium high intensity and probability | 6·31 |
| Xu et al (2003)43 | 27/109 | 2·78 (1·68–14·32)** | Sichuan, China | No | All | Ever | 2·11 |
ACT=Australian Capital Territory. NSW=New South Wales. OR=odds ratio. ppm=parts per million. RR=relative risk. TLV=threshold limit value.
Weight given to each study in the random effect model.
This study reported a hazard ratio.
This study reported a standardised mortality ratio.
This study reported a standardised rate ratio.
Although the study had 85 patients with non-Hodgkin lymphoma overall, only the 81 B-cell non-Hodgkin lymphoma cases were analysed.
Not reported in original study. Value given is from our own calculation.
Study reported odds ratio.
Men only.
Women only.
The results of the meta-analysis are reported in table 2. The meta-RR for all studies combined was 1·33 (95% CI 1·13–1·57, figure 2A). Among all studies, there was no evidence of asymmetry consistent with obvious publication bias (figure 2B). Egger’s (p=0·77) and Begg’s (p=0·98) tests similarly did not show evidence of publication bias.
Table 2:
Major findings from the meta-analysis of benzene exposure and NHL
| N | Fixed effect model meta-RR (95% Cl) | Shore calculated 95% Cl | Random effect model* meta-RR (95% Cl) | Heterogeneity χ2 (p value) | |
|---|---|---|---|---|---|
| Exposure category | |||||
| Main-highest average intensity | 28 | 1·32 (1·16–1·51) | 1·14–1·53 | 1·33 (1·13–1·57) | 33·34 (0·19) |
| Cumulative exposure | 28 | 1·28 (1·13–1·47) | 1·12–1·47 | 1·28 (1·12–1·48) | 28·12 (0·40) |
| Duration | 28 | 1·30 (1·14–1·48) | 1·13–1·50 | 1·31 (1·12–1·52) | 30·24 (0·30) |
| Ever exposure | 28 | 1·16 (1·07–1·26) | 1·04–1·29 | 1·17 (1·04–1·33) | 45·38 (0·01) |
| High exposure only | 18 | 1·46 (1·24–1·72) | 1·21–1·76 | 1·51 (1·22–1·87) | 22·29 (0·17) |
| High exposure without self-report† | 11 | 1·49 (1·22–1·82) | 1·21–1·83 | 1·53 (1·22–1·91) | 10·62 (0·39) |
| Cell-type specific NHL outcomes | |||||
| DLBCL | 6 | 1·62 (1·17–2·26) | 1·01–2·60 | 1·67 (1·01–2·77) | 10·26 (0·07) |
| FL | 6 | 1·47 (0·95–2·27) | ·· | ·· | 1·93 (0·86) |
| HCL | 3 | 1·77 (0·99–3·16) | ·· | ·· | 0·88 (0·65) |
| CLL | 10 | 1·22 (0·93–1·60) | 0·81–1·83 | 1·24 (0·79–1·94) | 20·55 (0·01) |
| Study design | |||||
| Case-control | 20 | 1·29 (1·11–1·51) | 1·11–1·52 | 1·29 (1·09–1·53) | 20·17 (0·38) |
| Cohort | 8 | 1·41 (1·08–1·84) | 0·98–2·02 | 1·55 (1·03–2·33) | 12·88 (0·08) |
| Quality analysis | |||||
| High quality studies | 8 | 1·42 (1·16–1·73) | ·· | ·· | 6·06 (0·53) |
| Low quality studies | 20 | 1·26 (1·05–1·50) | 1·02–1·55 | 1·27 (1·01–1·59) | 26·52 (0·12) |
CLL=chronic lymphocytic leukaemia. DLBCL=diffuse large B-cell lymphoma. FL=follicular lymphoma. HCL=hairy cell leukaemia. Meta-RR=meta-analysis relative risk. NHL=non-Hodgkin lymphoma.
Random effect model was used when χ2 heterogeneity statistic was greater than the degrees of freedom (calculated as the number of studies minus one).
Studies that used self-reported exposure to benzene were excluded.
Figure 2: Forest plot of studies used in meta-analysis of benzene and non-Hodgkin lymphoma using the random effect model (A) and funnel plot of studies (B).
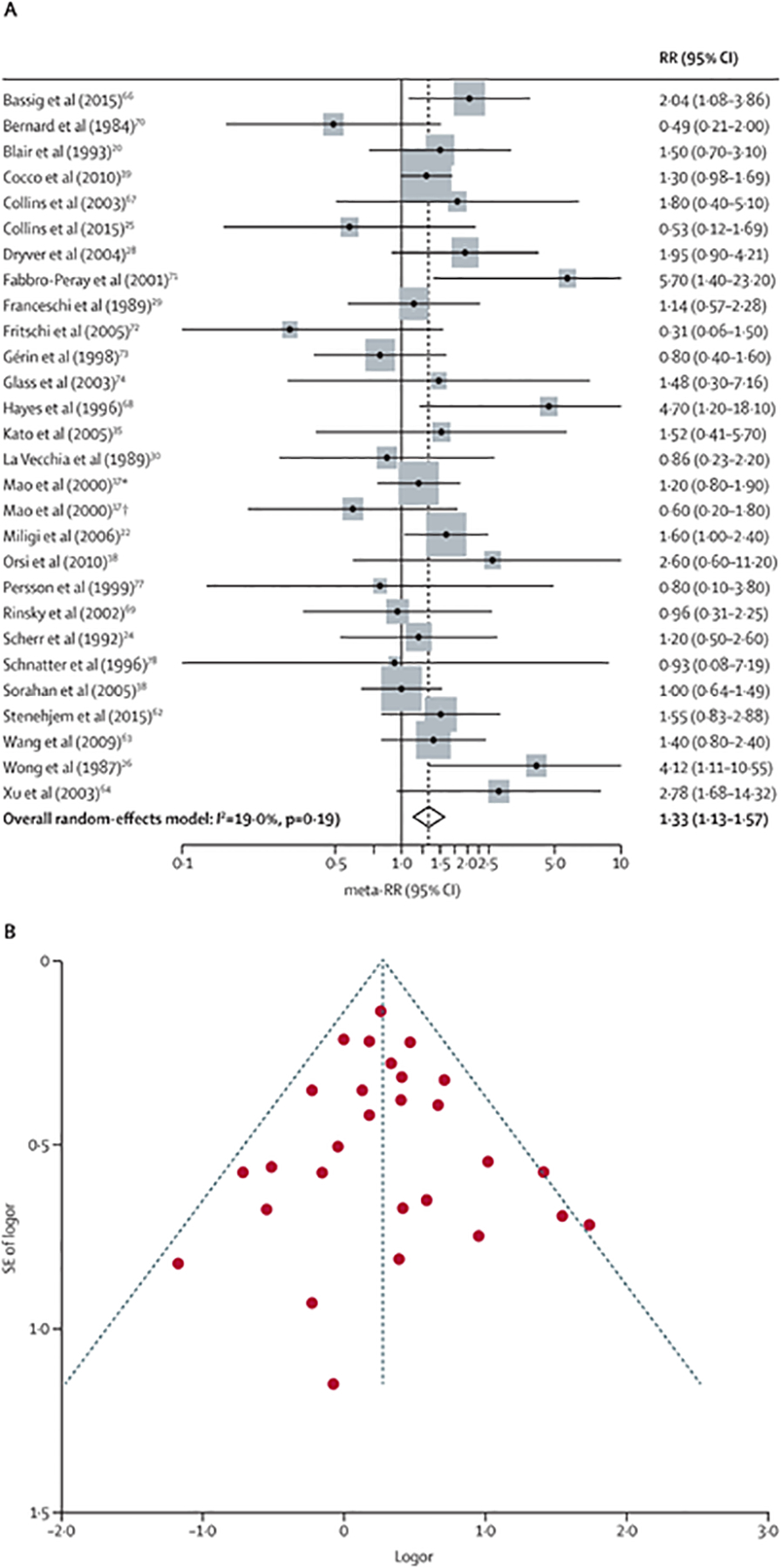
Meta-RR=meta-analysis relative risk. RR=relative risk. *Men only. †Women only.
We evaluated the effect of our a priori selection of highest average intensity of benzene exposure. When highest cumulative exposure was used, the meta-RR nominally decreased to 1·28 (95% CI 1·12–1·48). Similarly, when longest duration was used, the meta-RR remained almost the same at 1·31 (1·12–1·52).
Meta-analysis of ever exposure (any exposure) resulted in a meta-RR of 1·17 (95% CI 1·04–1·33; table 2). Among the 18 studies that provided risk estimates specifically for highly exposed workers beyond ever exposure, the meta-RR was increased to 1·51 (1·22–1·87). 11 of these high exposure studies did not rely on self-reported exposure information for benzene, raising the meta-RR to 1·53 (1·22–1·91). When comparing ever to high exposures, the meta-RR was markedly increased in a dose-dependent manner (figure 3).
Figure 3: Comparison of meta-RR of non-Hodgkin lymphoma when using higher exposures to benzene versus all exposures.
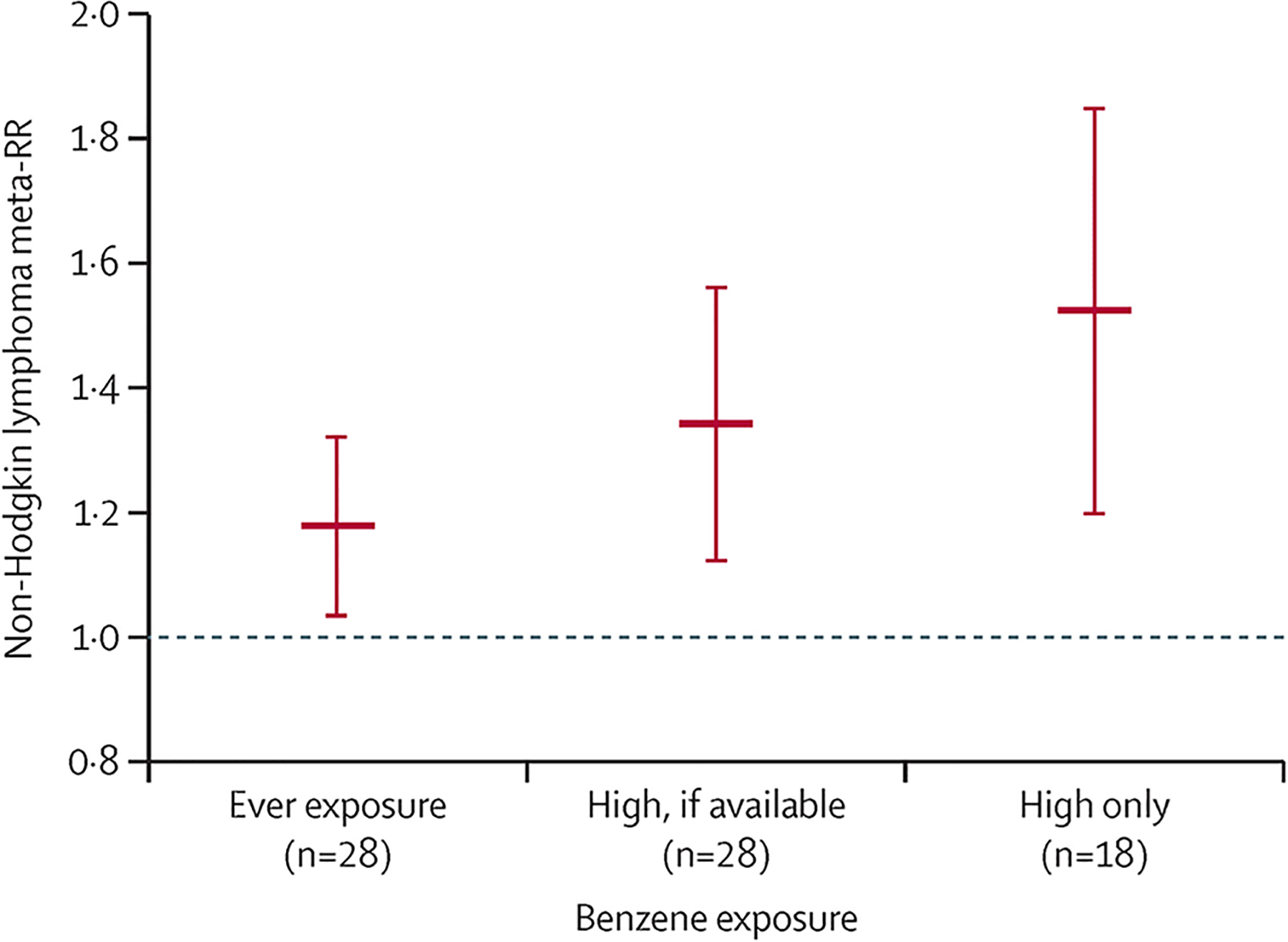
Meta-RR=meta-analysis relative risk.
We did a cell-type specific analysis and found high exposure to benzene was most associated with diffuse large B-cell lymphoma (meta-RR 1·67 [95% CI 1·01–2·77]; table 2). Increased associations were also detected for follicular lymphoma (1·47 [0·95–2·27]) and hairy cell leukaemia (1·77 [0·99–3·16]), which were close to being significant but were not statistically significant.
Consistent with previously established links,44,45 a statistically significant increased meta-RR was detected for myeloid leukaemia (meta-RR 1·59 [95% CI 1·28–1·99]; appendix p 21). There was no association between high exposures to benzene and Hodgkin lymphoma (1·00 [0·77–1·28]). We detected increased meta-RRs for chronic lymphocytic leukaemia (1·24 [0·79–1·94]) and acute lymphoblastic leukaemia (1·53 [0·70–3·32]), though the CIs overlapped the null. We found a non-statistically significant meta-RR for multiple myeloma (1·32 [0·89–1·97]). In our analysis by study design, the meta-RR for case-control studies (1·29 [1·09–1·53, n=20) was lower than that of cohort studies (1·55 [1·03–2·33], n=8; table 2).
We evaluated the methodological quality of each study using the Newcastle-Ottawa scale to determine the effect of including both high-quality and low-quality studies in our meta-analysis. According to our quality assessment (appendix pp 22–23), the highest quality studies in either design category were those done by Blair and colleagues,27 Kato and colleagues,35 Miligi and colleagues,37 Orsi and colleagues,38 and Scherr and colleagues,40 which were all case-control studies. The two lowest quality studies were of cohort design.20,25 Our analysis of high-quality studies produced a meta-RR of 1·42 (95% CI 1·16–1·73; table 2), whereas the meta-RR of low-quality studies was 1·27 (1·01–1·59). The high-quality studies showed an additional 15% (0·15) increased meta-RR compared with the low-quality studies, indicating that the true risk of developing non-Hodgkin lymphoma after exposure to benzene is possibly higher than our calculated meta-RR. Higher quality studies tended to control for other factors and were more likely to have adequate case definition. Factors that contributed to low-quality studies included shorter follow-up time, poor exposure assessment, and absence of histological verification, among other metrics that would be expected to attenuate the meta-RR, should a true association exist.
Other sensitivity analyses are reported in the appendix (p 24). When we analysed men only, we found the meta-RR remained the same (1·32 [95% CI 1·02–1·71]), whereas the meta-RR for women was increased, though not significantly (1·43 [0·93–2·19]).
On average, studies done in North America (meta-RR 1·21 [95% CI 0·96–1·53]) and Europe and Australia (1·29 [1·03–1·62]) had lower meta-RRs than those done in China (2·46 [1·48–4·08]). This difference might be attributable to more stringent occupational exposure limits of benzene in high-income countries (1 part per million [ppm] averaged over 8 h in the USA) versus China’s extremely high limits of 12 ppm (1979–2002) and 2 ppm (2002–present).46 The widened 95% CIs for the studies done in China were possibly the result of a smaller number of studies (n=3).
Three studies included benzene in a mixture with other solvents, such as toluene and xylene.29,31,36 We determined the effect of confounding from these co-exposures would be minimal, given neither toluene nor xylene are reported to be associated with non-Hodgkin lymphoma. We evaluated the effect of this decision by excluding all three studies and found almost no change in the meta-RR (1·34 [95% CI 1·12–1·60]; appendix p 24).
We excluded Vlaanderen and colleagues’ study47 because exposures were calculated using census information. When this study was included, the meta-RR decreased slightly to 1·28 (95% CI 1·09–1·52; appendix p 24). Further, we excluded Tranah and colleagues’ study48 because the study included a population with a high risk of AIDS.49 When this study was included, the meta-RR decreased slightly to 1·28 (1·09–1·51). When both Vlaanderen and colleagues’ study47 and Tranah and colleagues’ study48 were included, the meta-RR decreased to 1·24 (1·06–1·43).
Although Linet and colleagues’ study50 done in 2015 reported the most updated follow-up of a large Chinese cohort, we used relative risks reported in Hayes and colleagues’ study51 because they stratified by high exposure. When Linet and colleagues’ 2015 study50 was used instead, the meta-RR increased nominally to 1·34 (95% CI 1·14–1·58). During the review of our paper, the Chinese cohort was updated once more with stratification by high exposure in Linet and colleagues’ study done in 2020;52 when this study was used, the results of our main meta-analysis remained almost unchanged (appendix p 25).
To assess the relative influence of each study on the meta-analysis, we removed each study one at a time (appendix p 26). Results remained almost unchanged after removal of each study, with the lowest meta-RR being 1·30 following removal of Bassig and colleagues’ study53 (95% CI 1·10–1·54) and Wong’s study25 (1·12–1·52). The meta-RR raised to 1·37 (1·16–1·63) following removal of Sorahan and colleagues’ study.23 Removing Cocco and colleagues’ study,28 the most highly weighted study, had almost no effect on our results (1·34 [1·11–1·61]). Overall, the robust sensitivity analyses indicate that our main findings are sound and rigorous.
Discussion
The results of our meta-analysis provide new collective evidence to suggest that exposures to benzene can induce non-Hodgkin lymphoma in humans at high levels, as defined in our a priori hypothesis. The increases in meta-RRs were both statistically significant and consistent across numerous robust sensitivity analyses, suggesting epidemiological evidence of a true causal relationship.
Although a single study cannot establish causation, to our knowledge, this meta-analysis is the first to provide new data that collectively met the Hill considerations54 to establish a causal relationship between benzene exposure and non-Hodgkin lymphoma. First, our meta-analysis of 257 173 participants and 9587 cases indicated a strong, statistically significant, positive association between high exposures to benzene and non-Hodgkin lymphoma (meta-RR 1·33 [95% CI 1·13–1·57]). Second, this association was consistent across cohort and case-control study designs, differential exposure metrics (table 2), sex, and geographical location (appendix p 24). Third, we qualitatively showed evidence of a dose-response relationship between benzene exposure and non-Hodgkin lymphoma. In our sensitivity analysis, we detected a 34% increased meta-RR of non-Hodgkin lymphoma when comparing ever exposure to studies that only reported high exposure groups (meta-RR increased from 1·17 to 1·51; figure 3) Fourth, these studies are well powered and sufficiently establish an appropriate temporal relationship between benzene exposure and onset of non-Hodgkin lymphoma. Fifth, the association detected was specific to non-Hodgkin lymphoma, and not to other haematological malignancies, such as Hodgkin lymphoma (appendix p 21). Sixth, experimental studies in rodent models have detected lymphomas in rats55,56 and mice57–59 following benzene exposure in a dose-dependent manner.
The final criterion of biological plausibility is satisfied given current knowledge regarding the causes and mechanisms of non-Hodgkin lymphoma. Key risk factors for non-Hodgkin lymphoma include immunosuppression and pre-existing autoimmune disease.60 There are several epidemiological and experimental studies that indicate that chronic benzene exposure through inhalation or oral consumption targets the immune system,61 by decreasing number of circulating B-lymphocytes,62 decreasing immunoglobulin levels,63,64 decreasing T-cells,63–66 and decreasing IL-2 production.67 The suppression of crucial immunologic cells could contribute to the susceptibility of invading pathogens, such as Helicobacter pylori.68 Indeed, the findings of our meta-analysis, coupled with the evidence from the literature, compellingly met Hill’s considerations.54
To the best of our knowledge, our meta-analysis is the most comprehensive and updated analysis done to date. Compared with previously published meta-analyses6,69–71 (table 3), our findings show a higher meta-RR that is statistically significant. This difference could be attributable to the addition of several new studies38,36,53,28,24,42 and extended follow-up of a cohort study,20 allowing malignancies with longer latency periods to be reported.
Table 3:
Comparison of findings between current and previous meta-analyses
| Number of studies* | Study years† | Exposure category‡ | Meta-RR (95% Cl) | |
|---|---|---|---|---|
| Rana et al (2021) | 28 | 1984–2015 | A priori | 1·33 (1·13–1·57) |
| Vlaanderen et al (2011)69 | 33 | 1983–2008 | Ever | 1·32 (0·97–1·80) |
| Kane and Newton (2010)70 | 26 | 1979–2008 | Ever | 1·11 (0·94–1·30) |
| Alexander and Wagner (2010)71 | 26 | 1984–2009 | A priori | 1·08 (0·93–1·24) |
| Steinmaus et al (2008)6 | 22 | 1984–2007 | A priori | 1·22 (1·02–1·47) |
Meta-RR=meta relative-risk.
Number of studies was counted as number of separately reported cohorts in each meta-analysis forest plot, or number of studies reported, if no forest plot was presented.
Range of publication years of studies included in meta-analysis.
Benzene exposure category evaluated in determining meta-RR.
Our results complement several lines of evidence reported in the IARC Monograph.4 IARC’s analysis regarding non-Hodgkin lymphoma was very general, summarising the findings from several (but not all) epidemiological studies. Using our thorough search and more transparent screening methods via SysRev, we were able to identify additional studies for evaluation that were not included in the IARC analysis. Given individual epidemiological studies often vary and can appear to conflict, IARC noted the benefits of meta-analysis in consolidating results from multiple epidemiological studies so that an overall conclusion about the effects of the exposure can be drawn. However, IARC’s meta-analysis analysed only chronic lymphocytic leukaemia and not non-Hodgkin lymphoma. By contrast, our extensive meta-analysis of all non-Hodgkin lymphoma outcomes with robust sensitivity analyses documented a strong statistically significant association that was punctuated by a dose-response relationship (figure 3). Further, our cell-type specific investigation was the first to uncover associations for the diffuse large B-cell lymphoma, follicular lymphoma, and hairy cell leukaemia subtypes, while also corroborating IARC’s findings for chronic lymphocytic leukaemia. When evaluated against the Hill considerations for a causal relationship, these new levels of our analysis were found to satisfy each requirement.
Our novel approach used to do this meta-analysis contributed to its core strengths. First, our meta-analysis was the first to follow PRISMA guidelines, which allowed us to identify several new studies36,53,28,43 that had not been evaluated in previous meta-analyses of benzene and non-Hodgkin lymphoma. Second, the use of a blinded and securely recorded review system (SysRev) helped minimise any selection bias by independent reviewers in screening studies for inclusion. Third, application of our novel a priori hypothesis using the highest exposure groups maximised our ability to detect exposure-response relationships. Lastly, our strategy to review cell-type specific associations allowed us to detect whether exposure to benzene affects a particular subtype more strongly than others.
All meta-analyses inherently have limitations, given that they demand expertise in both clinical and statistical realms.72 Limitations of our meta-analysis lie mainly within the individual studies that were included. Given the evolving pathophysiology of non-Hodgkin lymphoma and the wide spectrum of lymphomas that are included in its definition, a basic analysis of the findings of our meta-analysis might suggest that not all subtypes of non-Hodgkin lymphoma are statistically associated with benzene exposure. However, bias might have masked the strength of the associations detected. The studies included in our meta-analysis were published between 1984 and 2015. The WHO classification of lymphoid neoplasms changed during this time period in 2008 and, more recently, in 2016 to incorporate clinical findings, molecular genetics, and morphology to further elaborate on what constitutes a B-cell or T-cell neoplasm. These diagnostic changes of non-Hodgkin lymphoma possibly contributed to non-differential outcome misclassifications, which would usually bias the meta-RR towards the null.
More specifically, we identified a large statistically significant association between benzene and diffuse large B-cell lymphoma, increased associations between follicular lymphoma and hairy cell leukaemia, and an increased meta-RR for chronic lymphocytic leukaemia that was not statistically significant. Of note, the results for specific non-Hodgkin lymphoma subtypes were likely to be more uncertain both within and among studies due to sample size and potential differential classification across studies; hence, the significance levels were likely to decrease. Our finding of a high risk for diffuse large B-cell lymphoma is thus a stronger conclusion as it is imbued with more intrinsic uncertainty.
Further, given that clinical outcomes of chronic lymphocytic leukaemia are variable and that small lymphocytic leukaemia is a diagnostically equivalent neoplasm,73 it is possible that studies underreported the total number of patients in this group, and hence underestimated the true risk. Other potential considerations could be that chronic lymphocytic leukaemia is considered to be more related to genetics than environmental exposures74 as it is a very rare disease in Asian populations.75,76 Among our studies, only one examined chronic lymphocytic leukaemia in benzene exposed Chinese individuals and reported too few patients (n=2) to compute a relative risk.74
Lastly, all case-control studies can be affected by recall bias, in which cases more vividly remember exposures than controls. The resulting differential exposure misclassification can bias the risk estimate towards or away from null. However, when analysed separately, the meta-RRs of case-control studies versus cohort studies were similar, indicating that study design was not a significant source of bias.
An important next step in comprehending how benzene can modulate non-Hodgkin lymphoma disease progression is to provide mechanistic context. There is strong evidence that benzene exhibits at least five of the ten key characteristics of carcinogens. Further inquiry into the mechanisms of benzene-related metabolic activation and electrophilicity, genotoxicity, altered DNA repair and genomic instability, immunosuppression, and modulation of receptor-mediated effects could strengthen evidence of biological plausibility and better elucidate the process of chemically initiated lymphomagenesis.
To conclude, our study examined whether benzene exposure is linked to increased non-Hodgkin lymphoma risk through a comprehensive systematic review and meta-analysis. Using our a priori hypothesis, we reported that exposure to benzene increased non-Hodgkin lymphoma risk in a dose-dependent manner, a finding that is both statistically robust and biologically plausible. Moreover, we reported a doubling of this risk for the diffuse large B-cell lymphoma subtype, and increased risks for follicular lymphoma and hairy cell leukaemia. Overall, the findings of our new meta-analysis combined with the totality of evidence in the literature satisfied the Hill considerations for causation, strongly indicating that benzene exposure not only causes myeloid leukaemia, but also non-Hodgkin lymphoma.
Supplementary Material
Research in context.
Evidence before this study
The International Agency for Research on Cancer (IARC) classified benzene as a human carcinogen that causes leukaemia in 1979. In 2018, the agency determined there was “limited evidence that benzene causes… non-Hodgkin lymphoma”. Their evaluation of mechanistic and experimental animal studies provided evidence for benzene-induced lymphoma, though epidemiological studies and previous meta-analyses of these studies examining the potential association between exposure to benzene and non-Hodgkin lymphoma have reported incongruent findings.
To resolve this ambiguity, we searched PubMed and Embase to do a blinded systematic review in which we identified and analysed all retrieved human epidemiological studies of benzene and non-Hodgkin lymphoma using meta-analysis. Numerous sensitivity and quality analyses were used to assess the validity of our findings.
Added value of this study
This study updates our understanding of the association between benzene exposure and non-Hodgkin lymphoma risk through use of meta-analysis. Our study complements IARC’s more general analysis regarding non-Hodgkin lymphoma, which was limited to summarising the findings of epidemiological studies. Using our thorough search and more transparent screening methods via the next-generation online review platform SysRev, we identified additional studies for evaluation that were neither included in IARC qualitative analysis, nor their singular meta-analysis of chronic lymphocytic leukaemia. Our extensive meta-analysis of all non-Hodgkin lymphoma outcomes with robust sensitivity analyses documented a strong significant association that was further punctuated by a dose-response relationship. Moreover, our investigation of cell-type specific outcomes was, to our knowledge, the first to uncover associations for the diffuse large B-cell lymphoma subtype, follicular lymphoma, and hairy cell leukaemia. Our new analysis of human studies, coupled with the totality of scientific and observed evidence in the literature, was found to satisfy each of Bradford Hill considerations for a causal relationship. Overall, our findings provide new evidence to support our understanding of benzene as a carcinogen.
Implications of all the available evidence
Our study provides evidence that benzene is not only a human leukemogen but is also linked to non-Hodgkin lymphoma. An important next step in comprehending the exposure-disease relationship is to investigate how benzene can modulate non-Hodgkin lymphoma disease progression using lymphoma-specific hallmarks, risk factors, and biomarkers, as well as key characteristics of carcinogens (ie, immunosuppression and chronic inflammation).
Acknowledgments
This project originated from a University of California Berkeley Practical Toxicology course project (PH270C); we thank all other students who were involved, including Allen Louie and Grace Sheeran. We would also like to thank Dr Yun Zhao for procurement and careful translation of Chinese studies. The two principal investigators (LZ and CS) were partially supported by the UC Berkeley Superfund Research Program (P42ES004705), which had no role in data collection, analysis, interpretation, writing of the manuscript, and the decision to submit.
Funding
National Institute of Environmental Health Sciences.
Footnotes
See Online for appendix
For more on SysRev see https://sysrev.com/
Declaration of interests
We declare no competing interests.
Data sharing
This manuscript makes use of publicly available data from published studies; therefore, no original data are available for sharing.
References
- 1.National Library of Medicine. Benzene. 2020. https://pubchem.ncbi.nlm.nih.gov/compound/Benzene (accessed Feb 20, 2020).
- 2.Argus Media Group. Argus benzene annual 2017. London, UK, 2017. https://www.argusmedia.com/-/media/Files/sample-reports/argus-benzene-annual-2017-brochure-full-toc.ashx?la=pt&hash=DB24CCE59DC101302A210EA721933758EFC57165 (accessed Aug 22, 2019). [Google Scholar]
- 3.Edwards RD, Jantunen M. Benzene exposure in Helsinki, Finland. Atmos Environ 2001; 35: 1411–20. [Google Scholar]
- 4.International Agency for Research on Cancer. Monograph 120. Lyon, France, 2018. https://www.iarc.who.int/news-events/iarc-monographs-volume-120-benzene/ (accessed Aug 23, 2019). [Google Scholar]
- 5.Goldstein B, Smith M. Benzene and haematological cancers. In: Baan R, Stewart B, Straif K, eds. Tumour site concordance and mechanisms of carcinogenesis. Lyon, France: International Agency for Research on Cancer, 2019: 65–69. [PubMed] [Google Scholar]
- 6.Steinmaus C, Smith AH, Jones RM, Smith MT. Meta-analysis of benzene exposure and non-Hodgkin lymphoma: biases could mask an important association. Occup Environ Med 2008; 65: 371–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells GSB, O’connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hospital Research Institute. 2009. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed Aug 14, 2019). [Google Scholar]
- 8.Greenland S Meta-analysis. In: Rothman K, Greenland S, eds. Modern epidemiology, 2nd edn. Philadelphia: Lippincott Raven, 1998: 643–73. [Google Scholar]
- 9.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–88. [DOI] [PubMed] [Google Scholar]
- 10.Petitti D Statistical methods in meta-analysis. Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. New York, NY: Oxford University Press, 1994: 94–118. [Google Scholar]
- 11.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol 1999; 150: 469–75. [DOI] [PubMed] [Google Scholar]
- 12.Shore RE, Gardner MJ, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br J Ind Med 1993; 50: 971–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–101. [PubMed] [Google Scholar]
- 14.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.StataCorp L. Stata Statistical Software. Release 15. College Station, TX: StataCorp, 2017. [Google Scholar]
- 16.Microsoft Corporation. Microsoft Excel Version 3. Redmond, Washington, 2013. [Google Scholar]
- 17.Mao Y, Hu J, Ugnat AM, White K. Non-Hodgkin’s lymphoma and occupational exposure to chemicals in Canada. Ann Oncol 2000; 11 (suppl 1): 69–73. [PubMed] [Google Scholar]
- 18.Bassig B, Friesen M, Vermeulen R, et al. Occupational exposure to benzene and non-Hodgkin lymphoma in a population-based cohort: The Shanghai Women’s Health Study. Environ Health Perspect 2015; 23: 971–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collins JJ, Ireland B, Buckley CF, Shepperly D. Lymphohaematopoeitic cancer mortality among workers with benzene exposure. Occup Environ Med 2003; 60: 676–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins JJ, Anteau SE, Swaen GM, Bodner KM, Bodnar CM. Lymphatic and hematopoietic cancers among benzene-exposed workers. J Occup Environ Med 2015; 57: 159–63. [DOI] [PubMed] [Google Scholar]
- 21.Hayes R, Yin S, Dosemeci M, et al. Mortality among benzene-exposed workers in China. Environ Health Perspect 1996; 104: 1349–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rinsky R, Hornung R, Silver S, Tseng C. Benzene exposure and hematopoietic mortality: a long-term epidemiologic risk assessment. Am J Ind Med 2002; 42: 474–80. [DOI] [PubMed] [Google Scholar]
- 23.Sorahan T, Kinlen LJ, Doll R. Cancer risks in a historical UK cohort of benzene exposed workers. Occup Environ Med 2005; 62: 231–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stenehjem JS, Kjærheim K, Bråtveit M, et al. Benzene exposure and risk of lymphohaematopoietic cancers in 25 000 offshore oil industry workers. Br J Cancer 2015; 112: 1603–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wong O An industry wide mortality study of chemical workers occupationally exposed to benzene. II. Dose response analyses. Br J Ind Med 1987; 44: 382–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernard SM, Cartwright RA, Bird CC, Richards ID, Lauder I, Roberts BE. Aetiologic factors in lymphoid malignancies: a case-control epidemiological study. Leuk Res 1984; 8: 681–89. [DOI] [PubMed] [Google Scholar]
- 27.Blair A, Linos A, Stewart PA, et al. Evaluation of risks for non-Hodgkin’s lymphoma by occupation and industry exposures from a case-control study. Am J Ind Med 1993; 23: 301–12. [DOI] [PubMed] [Google Scholar]
- 28.Cocco P, t’Mannetje A, Fadda D, et al. Occupational exposure to solvents and risk of lymphoma subtypes: results from the Epilymph case-control study. Occup Environ Med 2010; 67: 341–47. [DOI] [PubMed] [Google Scholar]
- 29.Dryver E, Brandt L, Kauppinen T, Olsson H. Occupational exposures and non-Hodgkin’s lymphoma in Southern Sweden. Int J Occup Environ Health 2004; 10: 13–21. [DOI] [PubMed] [Google Scholar]
- 30.Fabbro-Peray P, Daures JP, Rossi JF. Environmental risk factors for non-Hodgkin’s lymphoma: a population-based case-control study in Languedoc-Roussillon, France. Cancer Causes Control 2001; 12: 201–12. [DOI] [PubMed] [Google Scholar]
- 31.Franceschi S, Serraino D, Bidoli E, et al. The epidemiology of non-Hodgkin’s lymphoma in the north-east of Italy: a hospital-based case-control study. Leuk Res 1989; 13: 465–72. [DOI] [PubMed] [Google Scholar]
- 32.Fritschi L, Benke G, Hughes AM, et al. Risk of non-Hodgkin lymphoma associated with occupational exposure to solvents, metals, organic dusts and PCBs (Australia). Cancer Causes Control 2005; 16: 599–607. [DOI] [PubMed] [Google Scholar]
- 33.Gérin M, Siemiatycki J, Désy M, Krewski D. Associations between several sites of cancer and occupational exposure to benzene, toluene, xylene, and styrene: results of a case-control study in Montreal. Am J Ind Med 1998; 34: 144–56. [DOI] [PubMed] [Google Scholar]
- 34.Glass D, Gray C, Jolley D, et al. Leukemia risk associated with low-level benzene exposure. Epidemiology 2003; 14: 569–77. [DOI] [PubMed] [Google Scholar]
- 35.Kato I, Koenig KL, Watanabe-Meserve H, et al. Personal and occupational exposure to organic solvents and risk of non-Hodgkin’s lymphoma (NHL) in women (United States). Cancer Causes Control 2005; 16: 1215–24. [DOI] [PubMed] [Google Scholar]
- 36.La Vecchia C, Negri E, D’Avanzo B, Franceschi S. Occupation and lymphoid neoplasms. Br J Cancer 1989; 60: 385–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miligi L, Costantini AS, Benvenuti A, et al. Occupational exposure to solvents and the risk of lymphomas. Epidemiology 2006; 17: 552–61. [DOI] [PubMed] [Google Scholar]
- 38.Orsi L, Monnereau A, Dananche B, et al. Occupational exposure to organic solvents and lymphoid neoplasms in men: results of a French case-control study. Occup Environ Med 2010; 67: 664–72. [DOI] [PubMed] [Google Scholar]
- 39.Persson B, Fredrikson M. Some risk factors for non-Hodgkin’s lymphoma. Int J Occup Med Environ Health 1999; 12: 135–42. [PubMed] [Google Scholar]
- 40.Scherr PA, Hutchison GB, Neiman RS. Non-Hodgkin’s lymphoma and occupational exposure. Cancer Res 1992; 52 (suppl): 5503s–09. [PubMed] [Google Scholar]
- 41.Schnatter A, Armstrong T, Nicolich M, et al. Lymphohaematopoietic malignancies and quantitative estimates of exposure to benzene in Canadian petroleum distribution workers. Occup Environ Med 1996; 53: 773–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang R, Zhang Y, Lan Q, et al. Occupational exposure to solvents and risk of non-Hodgkin lymphoma in Connecticut women. Am J Epidemiol 2009; 169: 176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu C, Zheng S, Huang J, Wu J. [A case-control study for assessing the relation between the incidence of malignant lymphomas and environmental factors in Sichuan province]. Zhonghua Liu Xing Bing Xue Za Zhi 2003; 24: 875–78. [PubMed] [Google Scholar]
- 44.Schnatter AR, Glass DC, Tang G, Irons RD, Rushton L. Myelodysplastic syndrome and benzene exposure among petroleum workers: an international pooled analysis. J Natl Cancer Inst 2012; 104: 1724–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vlaanderen J, Lan Q, Kromhout H, Rothman N, Vermeulen R. Occupational benzene exposure and the risk of chronic myeloid leukemia: a meta-analysis of cohort studies incorporating study quality dimensions. Am J Ind Med 2012; 55: 779–85. [DOI] [PubMed] [Google Scholar]
- 46.Xing C, Marchetti F, Li G, et al. Benzene exposure near the U.S. permissible limit is associated with sperm aneuploidy. Environ Health Perspect 2010; 118: 833–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vlaanderen J, Straif K, Pukkala E, et al. Occupational exposure to trichloroethylene and perchloroethylene and the risk of lymphoma, liver, and kidney cancer in four Nordic countries. Occup Environ Med 2013; 70: 393–401. [DOI] [PubMed] [Google Scholar]
- 48.Tranah GJ, Holly EA, Bracci PM. Solvent exposure and non-Hodgkin lymphoma: no risk in a population-based study in the San Francisco Bay Area. Cancer Epidemiol Biomarkers Prev 2009; 18: 3130–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moss AR, Osmond D, Bacchetti P, Chermann JC, Barre-Sinoussi F, Carlson J. Risk factors for AIDS and HIV seropositivity in homosexual men. Am J Epidemiol 1987; 125: 1035–47. [DOI] [PubMed] [Google Scholar]
- 50.Linet MS, Yin SN, Gilbert ES, et al. A retrospective cohort study of cause-specific mortality and incidence of hematopoietic malignancies in Chinese benzene-exposed workers. Int J Cancer 2015; 137: 2184–97. [DOI] [PubMed] [Google Scholar]
- 51.Hayes RB, Yin SN, Dosemeci M, et al. Benzene and the dose-related incidence of hematologic neoplasms in China. Chinese Academy of Preventive Medicine—National Cancer Institute Benzene Study Group. J Natl Cancer Inst 1997; 89: 1065–71. [DOI] [PubMed] [Google Scholar]
- 52.Linet MS, Gilbert ES, Vermeulen R, et al. Benzene exposure-response and risk of lymphoid neoplasms in Chinese workers: a multicenter case-cohort study. Am J Ind Med 2020; 63: 741–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bassig BA, Friesen MC, Vermeulen R, et al. Occupational exposure to benzene and non-Hodgkin lymphoma in a population-based cohort: The Shanghai Women’s Health Study. Environ Health Perspect 2015; 123: 971–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill AB. The environment and disease: association or causation? 1965. J R Soc Med 2015; 108: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Maltoni C, Ciliberti A, Cotti G, Conti B, Belpoggi F. Benzene, an experimental multipotential carcinogen: results of the long-term bioassays performed at the Bologna Institute of Oncology. Environ Health Perspect 1989; 82: 109–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maltoni C, Conti B, Cotti G. Benzene: a multipotential carcinogen. Results of long-term bioassays performed at the Bologna Institute of Oncology. Am J Ind Med 1983; 4: 589–630. [DOI] [PubMed] [Google Scholar]
- 57.Farris GM, Everitt JI, Irons RD, Popp JA. Carcinogenicity of inhaled benzene in CBA mice. Fundam Appl Toxicol 1993; 20: 503–07. [DOI] [PubMed] [Google Scholar]
- 58.Kawasaki Y, Hirabayashi Y, Kaneko T, et al. Benzene-induced hematopoietic neoplasms including myeloid leukemia in Trp53-deficient C57BL/6 and C3H/He mice. Toxicol Sci 2009; 110: 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Snyder CA, Sellakumar AR, James DJ, Albert RE. The carcinogenicity of discontinuous inhaled benzene exposures in CD-1 and C57Bl/6 mice. Arch Toxicol 1988; 62: 331–35. [DOI] [PubMed] [Google Scholar]
- 60.Shiels MS, Engels EA, Linet MS, et al. The epidemic of non-Hodgkin lymphoma in the United States: disentangling the effect of HIV, 1992–2009. Cancer Epidemiol Biomarkers Prev 2013; 22: 1069–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Guo H, Ahn S, Zhang L. Benzene-associated immunosuppression and chronic inflammation in humans: a systematic review. Occup Environ Med 2021; 78: 377–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bogadi-Sare A, Zavalic M, Trosić I, Turk R, Kontosić I, Jelcić I. Study of some immunological parameters in workers occupationally exposed to benzene. Int Arch Occup Environ Health 2000; 73: 397–400. [DOI] [PubMed] [Google Scholar]
- 63.Uzma N, Kumar BS, Hazari MA. Exposure to benzene induces oxidative stress, alters the immune response and expression of p53 in gasoline filling workers. Am J Ind Med 2010; 53: 1264–70. [DOI] [PubMed] [Google Scholar]
- 64.Kirkeleit J, Ulvestad E, Riise T, Bråtveit M, Moen BE. Acute suppression of serum IgM and IgA in tank workers exposed to benzene. Scand J Immunol 2006; 64: 690–98. [DOI] [PubMed] [Google Scholar]
- 65.Farris GM, Robinson SN, Wong BA, Wong VA, Hahn WP, Shah R. Effects of benzene on splenic, thymic, and femoral lymphocytes in mice. Toxicology 1997; 118: 137–48. [DOI] [PubMed] [Google Scholar]
- 66.Hsieh GC, Sharma RP, Parker RD. Subclinical effects of groundwater contaminants. IV. Effects of repeated oral exposure to combinations of benzene and toluene on regional brain monoamine metabolism in mice. Arch Toxicol 1990; 64: 669–76. [DOI] [PubMed] [Google Scholar]
- 67.Fan XH. Effect of exposure to benzene on natural killer (NK) cell activity and interleukin-2 (IL-2) production of C57BL/6 mice. Nippon Ika Daigaku Zasshi 1992; 59: 393–99. [DOI] [PubMed] [Google Scholar]
- 68.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013; 13: 800–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vlaanderen J, Lan Q, Kromhout H, Rothman N, Vermeulen R. Occupational benzene exposure and the risk of lymphoma subtypes: a meta-analysis of cohort studies incorporating three study quality dimensions. Environ Health Perspect 2011; 119: 159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kane EV, Newton R. Benzene and the risk of non-Hodgkin lymphoma: a review and meta-analysis of the literature. Cancer Epidemiol 2010; 34: 7–12. [DOI] [PubMed] [Google Scholar]
- 71.Alexander DD, Wagner ME. Benzene exposure and non-Hodgkin lymphoma: a meta-analysis of epidemiologic studies. J Occup Environ Med 2010; 52: 169–89. [DOI] [PubMed] [Google Scholar]
- 72.Bailar JC 3rd. The promise and problems of meta-analysis. N Engl J Med 1997; 337: 559–61. [DOI] [PubMed] [Google Scholar]
- 73.Fabbri G, Dalla-Favera R. The molecular pathogenesis of chronic lymphocytic leukaemia. Nat Rev Cancer 2016; 16: 145–62. [DOI] [PubMed] [Google Scholar]
- 74.Yang SM, Li JY, Gale RP, Huang XJ. The mystery of chronic lymphocytic leukemia (CLL): why is it absent in Asians and what does this tell us about etiology, pathogenesis and biology? Blood Rev 2015; 29: 205–13. [DOI] [PubMed] [Google Scholar]
- 75.Gale RP, Cozen W, Goodman MT, Wang FF, Bernstein L. Decreased chronic lymphocytic leukemia incidence in Asians in Los Angeles County. Leuk Res 2000; 24: 665–69. [DOI] [PubMed] [Google Scholar]
- 76.Redaelli A, Laskin BL, Stephens JM, Botteman MF, Pashos CL. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004; 13: 279–87. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This manuscript makes use of publicly available data from published studies; therefore, no original data are available for sharing.


