Abstract
OBJECTIVE
To assess whether risk of severe outcomes among patients with type 1 diabetes mellitus (T1DM) hospitalized for coronavirus disease 2019 (COVID-19) differs from that of patients without diabetes or with type 2 diabetes mellitus (T2DM).
RESEARCH DESIGN AND METHODS
Using the Premier Healthcare Database Special COVID-19 Release records of patients discharged after COVID-19 hospitalization from U.S. hospitals from March to November 2020 (N = 269,674 after exclusion), we estimated risk differences (RD) and risk ratios (RR) of intensive care unit admission or invasive mechanical ventilation (ICU/MV) and of death among patients with T1DM compared with patients without diabetes or with T2DM. Logistic models were adjusted for age, sex, and race or ethnicity. Models adjusted for additional demographic and clinical characteristics were used to examine whether other factors account for the associations between T1DM and severe COVID-19 outcomes.
RESULTS
Compared with patients without diabetes, T1DM was associated with a 21% higher absolute risk of ICU/MV (RD 0.21, 95% CI 0.19–0.24; RR 1.49, 95% CI 1.43–1.56) and a 5% higher absolute risk of mortality (RD 0.05, 95% CI 0.03–0.07; RR 1.40, 95% CI 1.24–1.57), with adjustment for age, sex, and race or ethnicity. Compared with T2DM, T1DM was associated with a 9% higher absolute risk of ICU/MV (RD 0.09, 95% CI 0.07–0.12; RR 1.17, 95% CI 1.12–1.22), but no difference in mortality (RD 0.00, 95% CI −0.02 to 0.02; RR 1.00, 95% CI 0.89–1.13). After adjustment for diabetic ketoacidosis (DKA) occurring before or at COVID-19 diagnosis, patients with T1DM no longer had increased risk of ICU/MV (RD 0.01, 95% CI −0.01 to 0.03) and had lower mortality (RD −0.03, 95% CI −0.05 to −0.01) in comparisons with patients with T2DM.
CONCLUSIONS
Patients with T1DM hospitalized for COVID-19 are at higher risk for severe outcomes than those without diabetes. Higher risk of ICU/MV in patients with T1DM than in patients with T2DM was largely accounted for by the presence of DKA. These findings might further guide recommendations related to diabetes management and the prevention of COVID-19.
Patients with diabetes hospitalized for coronavirus disease 2019 (COVID-19) infection, the majority of whom have type 2 diabetes mellitus (T2DM), have higher risk of intensive care unit (ICU) admission and death than those without diabetes (1–5). Population-based studies in the U.K. have reported increased risk of critical care unit–treated or fatal COVID-19 in patients with either T1DM or T2DM, with greater odds observed among those with T1DM (6,7). However, the difference in risk between patients with T1DM and T2DM was not significant (7).
In the U.S., high rates of diabetic ketoacidosis (DKA) and poor glycemic control have been reported among cohorts of patients with T1DM hospitalized for COVID-19 (8–11). A study of 40 patients with T1DM hospitalized for COVID-19 at Vanderbilt University Medical Center reported higher odds of hospitalization or severe illness as compared with patients without diabetes (12). However, at the time of writing, there is no nationwide study of COVID-19 severity among patients with T1DM compared with those without diabetes in the U.S. Previous studies have been limited by small sample sizes of patients with T1DM and COVID-19, limiting the ability to analyze mortality in patients with T1DM, or by inadequate comparison groups. Given differences in health care systems, payment structures, population demographics, patient profiles, and variance in severe acute respiratory syndrome coronavirus 2 viral subtypes between the U.S. and the U.K., a nationwide analysis of the impact of COVID-19 on patients with T1DM in the U.S. is warranted (13) Furthermore, whether COVID-19 severity is greater in patients with T1DM than in patients with T2DM is unclear, and no studies in the U.S. have directly compared whether COVID-19 severity differs across diabetes subtype.
Using Premier Healthcare electronic medical records, we examined the risk of ICU admission or invasive mechanical ventilation (ICU/MV) and of death among patients with T1DM hospitalized for COVID-19 in the U.S. compared with that among patients without diabetes or among those with T2DM also hospitalized with COVID-19. We also assessed whether demographic, clinical, or hospital characteristics account for differences in COVID-19 severity in patients with T1DM compared with patients without diabetes or with T2DM. We hypothesized that COVID-19 severity may differ by diabetes type.
RESEARCH DESIGN AND METHODS
Patients and Settings
The Premier Healthcare Database Special COVID-19 Release (PHD-CSR) (release date 19 January 2021; Premier, Charlotte, NC) includes discharge records for adult and pediatric patients from >1,000 nongovernmental, teaching, and community hospitals representing ~25% of U.S. hospital admissions (14). Discharge records are for patients who were discharged from the hospital or died. The present analysis included discharge records from 842 hospitals that contributed data for COVID-19 patients discharged during March–November 2020. COVID-19 patients were identified through ICD-10, Clinical Modification (ICD-10-CM) discharge diagnosis codes U07.1 during April–November 2020 and B97.29 during March–April 2020 as either a primary or secondary diagnosis (15). The first hospitalization with a COVID-19 discharge diagnosis was defined as the index hospitalization.
Study Variables
Main Exposure
The main exposures included the diagnosis of T1DM for comparison with groups with the categories no diabetes or T2DM. Diabetes diagnosis codes, medications, and laboratory results from any encounter from January 2019 to the index COVID-19 hospitalization were used to determine diabetes status. Most patients with diabetes had codes present at admission (T1DM, 91.8%; T2DM, 92.9%). Previous visits with diabetes codes were available for 48.0% of patients with T1DM and 42.4% of patients with T2DM. Patients were classified as having T1DM or T2DM with a tiered algorithm approach, as described in Fig. 1. This approach is based on an algorithm developed by Klompas et al. (16) that uses a combination of majority T1DM ICD-10-CM codes of all a patienťs T1DM or T2DM codes (>50% T1DM codes), glucagon prescription, no prescription of a noninsulin antidiabetes drug (excluding metformin), negative C-peptide results, presence of autoantibodies associated with diabetes, and prescription of urine acetone test strips. Schroeder et al. (17) externally validated this algorithm and found the requirement for majority T1DM diagnosis codes alone had a positive predictive value (PPV) of 96.4%, whereas a modified algorithm, excluding urine acetone strips, had PPV of 95.1%. The use of >50% T1DM diagnosis codes alone has previously been shown to have high sensitivity, specificity, and PPV (all >90%) for studies in youth (18–20). As the present data set lacks laboratory results for the majority of patients and medication data mostly come from inpatient settings where treatment is often restricted to insulin, we chose to first apply the simplified criterion of >50% T1DM ICD-10-CM codes in order to increase the sensitivity of T1DM identification. First, we classified individuals as having T1DM if >50% of T1DM (E10.XX) or T2DM (E11.XX) ICD-10-CM codes were T1DM. Second, among patients with overlapping T1DM and T2DM codes not classified as T1DM in the first tier, or those without ICD-10 codes for T1DM or T2DM, the additional criterion with laboratory results was applied (negative C-peptide result or positive autoantibody result). For our purposes, we considered C-peptide results negative if <6 ng/mL, a cutoff associated with diagnosis of T1DM (13,21). Finally, those with any other diabetes, E08.XX (diabetes from underlying condition), E09.XX (drug- or chemical-induced diabetes), E13.XX (other specified diabetes), O24.31X (unspecified preexisting diabetes in pregnancy), O24.32 (unspecified preexisting diabetes in pregnancy), O24.33 (unspecified preexisting diabetes in the puerperium), O24.8XX (other preexisting diabetes in pregnancy, childbirth, or puerperium), and O24.9XX (unspecified diabetes in pregnancy/childbirth/puerperium), were excluded from the no diabetes group. As a sensitivity analysis, the full modified Klompas algorithm (17) was applied to the entire sample, which assigns T1DM on the basis of any of the following three criteria 1) >50% T1DM codes and no dispensing of a noninsulin antidiabetes drug (excluding metformin), 2) >50% T1DM codes and a dispensing for glucagon, and 3) negative C-peptide result or positive diabetes autoantibody result.
Figure 1—
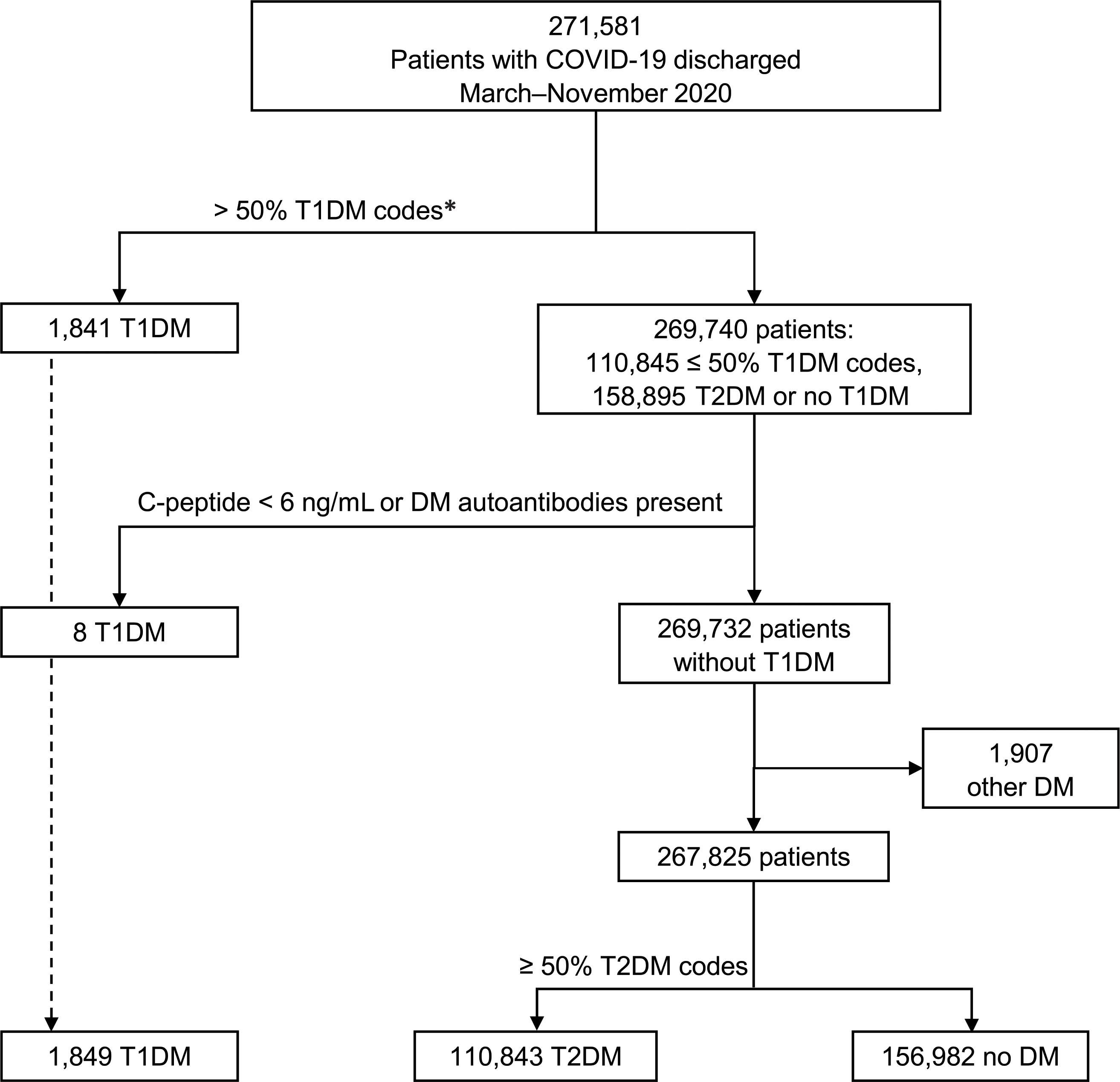
Identification of diabetes diagnosis among patients hospitalized with COVID-19 in the U.S., discharged March–November 2020. DM, diabetes mellitus. *If >50% of the patient's T1DM or T2DM diabetes ICD-10-CM codes were T1DM, the patient was categorized as having T1DM.
Outcomes
A severe COVID-19 outcome was defined as either ICU/MV or death on the basis of hospital records. ICU/MV was coded with use of the PHD-CSR charge master records. Mortality was defined as expired in the hospital or expired in hospice care, with use of PHD-CSR patient discharge records.
Covariates
Information on demographic and clinical characteristics of COVID-19 patients was extracted from PHD-CSR patient discharge records. The presence of selected underlying conditions linked with diabetes or with COVID-19 severity was identified with use of Clinical Classifications Software Refined (CCSR) categories based on all encounters for the cohort from January 2019 through the index hospitalization (22). Categories marked as “nonchronic” were excluded by the Chronic Condition Indicator (23). Underlying medical conditions were defined by aggregation of the chronic ICD-10-CM codes into a smaller number of meaningful categories (i.e., hypertension CIR007, CIR008; disorders of lipid metabolism, END008; coronary atherosclerosis and other heart disease, CIR011; chronic kidney disease [CKD], GEN003; obesity, END009; neoplasms, all CCSR categories starting with “NEO”; chronic obstructive pulmonary disease, RSP008; and DKA, E10.1 or E11.1).
Statistical Analysis
Descriptive analysis of demographic and clinical characteristics is shown by diabetes status. For examination of differences in proportions among diabetes groups, logittransformed CIs were estimated at an a level of 0.05. The percentage of individuals who received ICU/MV treatment or who died was examined by age-group and diabetes diagnosis. For the purpose of estimating the outcome of ICU/MV, we excluded 8,272 patients who died without being in the ICU/MV. Multivariable logistic regression models were used for estimation of absolute risk difference (RD) and risk ratio (RR) of ICU/MV or mortality among patients with T1DM compared with patients without diabetes or patients with T2DM hospitalized for COVID-19. When the T1DM group was compared with no diabetes and T2DM groups, patients with T2DM and those without diabetes were excluded, respectively. Models for ICU/MV had final sample sizes of 154,179 and 109,056 for the no diabetes and T2DM reference groups, respectively. Models for mortality had sample sizes of 158,831 and 112,692 for the no diabetes and T2D reference groups. RD were estimated with Stata’s postestimation command adjrr, which builds on the margins command (24). RD represents the actual RD in outcomes between T1DM and no diabetes or T2DM; RR represents the ratio of risk for ICU/MV or mortality in T1DM and no diabetes or T2DM. RD is estimated as a probability, but we present it as a percentage in the text for clarity (e.g., an RD of 0.25 is equivalent to 25% increased absolute risk). Given the high risk of outcomes based on the selected exposures, we chose to present RR instead of odds ratio (OR) (24). However, for comparison with previous publications, ORs are presented in Supplementary Table 2. Models were clustered on hospital identifier and included covariates for age, sex, and race or ethnicity (model 1). Continuous age was included as linear and quadratic terms to account for nonlinear associations. Additional adjustments controlled for admission month, payer type, hospital census region, and hospital area (urban, rural) (model 2) and selected underlying conditions (model 3). All statistical analyses were conducted by with Stata (version 16.1; StataCorp, College Station, TX). This activity was reviewed by Centers for Disease Control and Prevention (CDC), and its conduct was consistent with applicable federal law and CDC policy (Code of Federal Regulations [C.F.R.] and U.S. code [U.S.C.]): 45 C.F.R. part 46, 21 C.F. R. part 56, 42 U.S.C. section [Sect.] 241 (d), 5 U.S.C. Sect. 552a, and 44 U.S.C. Sect. 3501 et seq.
RESULTS
T1DM Algorithm Results
Among 269,674 patients with COVID-19 in the final cohort, 41.8% had a diagnosis of either T1DM (n = 1,849) or T2DM (n = 110,843) (Fig. 1). Of 271,581 patients discharged during March–November 2020, 1,907 were excluded as having only ICD codes for other diabetes. A limited number of T1DM patients had information on C-peptide (0.04%) or diabetes autoantibodies (0.03%). C-peptide levels <6 ng/mL were reported for 12 patients with T1DM (0.65%), 8 patients with T2DM (0.01%), and 0 patients without diabetes. Autoantibodies associated with diabetes were present for 15 patients with T1DM (0.81%), 7 patients with T2DM (0.01%), and 0 patients without diabetes. Among the eight patients identified as having T1DM through application of the verified Klompas algorithm, three had C-peptide levels <0.6 ng/mL and five had detectable autoantibodies associated with diabetes. One-half of them (n = 4) had overlapping T1DM and T2DM ICD-10 codes (≤50% T1DM codes), and one-half (n = 4) did not have any T1DM or T2DM ICD-10 codes.
The most commonly reported medication was insulin. At the COVID-19 visit, insulin was dispensed to 86.5% of patients with T1DM, 78.4% of patients with T2DM, and 12.9% of patients without diabetes. From January 2019 through the index hospitalization, insulin was dispensed to 35.3%, 22.0%, and 1.3% of patients with T1DM, T2DM, and no diabetes, respectively. Among the noninsulin antidiabetes drugs (16.1% T2DM, 4.3% T1DM, 0.3% no diabetes), the most commonly reported from January 2019 through the index hospitalization were metformin (9.4% T2DM, 2.8% T1DM, 0.2% no diabetes), dipeptidyl peptidase 4 inhibitors (3.7% T2DM, 1.1% T1DM, <0.1% no diabetes), and sulfonylureas (5.7% T2DM, 1.1% T1DM, <0.1% no diabetes). Glucagon was rarely dispensed, as expected in nonendocrinological practices (4.0% T1DM, 1.5% T2DM, 0.5% no diabetes).
Demographic, Clinical, and Hospital Characteristics
Overall, median age was 64 years, 51.6% patients were male, and 47.9% were non-Hispanic White. The highest percentage of patients overall came from the South (45.2%) and from urban hospitals (88.7%). Patients with T1DM were younger than those without diabetes or with T2DM and more likely to have Medicaid (Table 1). Compared with patients without diabetes, those with T1DM more frequently were non-Hispanic Black (24.6% vs. 17.6%) and had higher prevalence of some underlying conditions (i.e., disorders of lipid metabolism, 49.1% vs. 34.1%; CKD, 36.3% vs. 14.9%; heart disease, 24.6% vs. 16.7%). Compared with patients with T2DM, those with T1DM more frequently were non-Hispanic White (49.6% vs. 43.9%), more likely to have DKA (45.7% vs. 4%), and less likely to have additional underlying conditions, except for CKD, of which there was a similar prevalence (36.3% vs. 34.8%). DKA was present on admission in 37.3% of patients with T1DM and 3.2% of patients with T2DM. The percentage of patients with DKA within each age category decreased with age and was higher for patients with T1DM than patients with T2DM within each age category (e.g., 0–17 years, 74.6% DKA in patients with T1DM vs. 24.6% DKA in patients with T2DM, and ≥75 years, 10.3% DKA in patients with T1DM vs. 1.3% DKA in patients with T2DM) (Supplementary Table 1). Among patients with T1DM, 60.2% required ICU/MV treatment, whereas this percentage was lower among those without diabetes (43.6%) or among those with T2DM (54.8%) (Table 1).
Table 1—
Demographic and clinical characteristics of patients hospitalized with COVID-19, by diabetes diagnosis—PHD-CSR, U.S., discharged March–November 2020
| Total (N = 269,674) |
T1DM (N = 1,849) |
T2DM (N = 110,843) |
No diabetes (N = 156,982) |
|||||
|---|---|---|---|---|---|---|---|---|
| n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | n | % (95% CI) | |
|
| ||||||||
| Sex | ||||||||
| Male | 139,266 | 51.6 (51.5–51.8) | 940 | 50.8 (48.6–53.1) | 59,149 | 53.4 (53.1–53.7) | 79,177 | 50.4 (50.2–50.7) |
| Female | 130,223 | 48.3 (48.1–48.5) | 909 | 49.2 (46.9–51.4) | 51,630 | 46.6 (46.3–46.9) | 77,684 | 49.5 (49.2–49.7) |
| Unknown | 185 | 0.1 (0.1–0.1) | 0 | 0.0 (0.0–0.0) | 64 | 0.1 (0.0–0.1) | 121 | 0.1 (0.1–0.1) |
|
| ||||||||
| Age, years§ | ||||||||
| 0–17 | 2,570 | 1.0 (0.9–1.0) | 71 | 3.8 (3.1–4.8) | 57 | 0.1 (0.0–0.1) | 2,442 | 1.6 (1.5–1.6) |
| 18–39 | 32,638 | 12.1 (12.0–12.2) | 697 | 37.7 (35.5–39.9) | 4,994 | 4.5 (4.4–4.6) | 26,947 | 17.2 (17.0–17.4) |
| 40–49 | 28,352 | 10.5 (10.4–10.6) | 290 | 15.7 (14.1–17.4) | 9,832 | 8.9 (8.7–9.0) | 18,230 | 11.6 (11.5–11.8) |
| 50–64 | 73,573 | 27.3 (27.1–27.5) | 435 | 23.5 (21.6–25.5) | 33,918 | 30.6 (30.3–30.9) | 39,220 | 25.0 (24.8–25.2) |
| 65–74 | 57,514 | 21.3 (21.2–21.5) | 200 | 10.8 (9.5–12.3) | 29,973 | 27.0 (26.8–27.3) | 27,341 | 17.4 (17.2–17.6) |
| ≥75 | 75,027 | 27.8 (27.7–28.0) | 156 | 8.4 (7.3–9.8) | 32,069 | 28.9 (28.7–29.2) | 42,802 | 27.3 (27.0–27.5) |
|
| ||||||||
| Race/ethnicity | ||||||||
| White, non-Hispanic | 129,086 | 47.9 (47.7–48.1) | 917 | 49.6 (47.3–51.9) | 48,706 | 43.9 (43.6–44.2) | 79,463 | 50.6 (50.4–50.9) |
| Black, non-Hispanic | 54,180 | 20.1 (19.9–20.2) | 455 | 24.6 (22.7–26.6) | 26,085 | 23.5 (23.3–23.8) | 27,640 | 17.6 (17.4–17.8) |
| Hispanic | 52,184 | 19.4 (19.2–19.5) | 297 | 16.1 (14.5–17.8) | 21,596 | 19.5 (19.3–19.7) | 30,291 | 19.3 (19.1–19.5) |
| Other, non-Hispanic | 26,995 | 10.0 (9.9–10.1) | 152 | 8.2 (7.1–9.6) | 11,769 | 10.6 (10.4–10.8) | 15,074 | 9.6 (9.5–9.7) |
| Unknown | 7,229 | 2.7 (2.6–2.7) | 28 | 1.5 (1.0–2.2) | 2,687 | 2.4 (2.3–2.5) | 4,514 | 2.9 (2.8–3.0) |
|
| ||||||||
| Underlying conditions | ||||||||
| Essential or secondary hypertension | 136,577 | 50.7 (50.5–50.8) | 812 | 43.9(41.7–46.2) | 67,233 | 60.7(60.4–60.9) | 68,532 | 43.7(43.4–43.9) |
| Disorders of lipid metabolism | 125,105 | 46.4 (46.2–46.6) | 908 | 49.1 (46.8–51.4) | 70,628 | 63.7 (63.4–64.0) | 53,569 | 34.1 (33.9–34.4) |
| Obesity | 85,335 | 31.6 (31.5–31.8) | 467 | 25.3 (23.3–27.3) | 45,461 | 41.0 (40.7–41.3) | 39,407 | 25.1 (24.9–25.3) |
| CKD | 62,577 | 23.2 (23.0–23.4) | 671 | 36.3 (34.1–38.5) | 38,598 | 34.8 (34.5–35.1) | 23,308 | 14.9 (14.7–15.0) |
| Coronary atherosclerosis and other heart disease | 62,000 | 23.0 (22.8–23.1) | 455 | 24.6 (22.7–26.6) | 35,335 | 31.9 (31.6–32.2) | 26,210 | 16.7 (16.5–16.9) |
| COPD and bronchitis | 27,997 | 10.4 (10.3–10.5) | 132 | 7.1 (6.1–8.4) | 13,809 | 12.5 (12.3–12.7) | 14,056 | 9.0 (8.8–9.1) |
| Neoplasms | 18,875 | 7.0 (6.9–7.1) | 105 | 5.7 (4.7–6.8) | 8,061 | 7.3 (7.1–7.4) | 10,709 | 6.8 (6.7–6.9) |
| DKA | 5,279 | 2.0 (1.9–2.0) | 845 | 45.7 (43.4–48.0) | 4,434 | 4.0 (3.9–4.1) | 0 | 0.0 (0.0–0.0) |
| Present at admission | 4,195 | 1.6 (1.5–1.6) | 690 | 37.3 (35.1–39.5) | 3,505 | 3.2 (3.1–3.3) | 0 | 0.0 (0.0–0.0) |
|
| ||||||||
| Payer type | ||||||||
| Medicare | 132,683 | 49.2 (49.0–49.4) | 608 | 32.9 (30.8–35.1) | 63,032 | 56.9 (56.6–57.2) | 69,043 | 44.0 (43.7–44.2) |
| Commercial | 68,764 | 25.5 (25.3–25.7) | 530 | 28.7 (26.6–30.8) | 23,612 | 21.3 (21.1–21.5) | 44,622 | 28.4 (28.2–28.6) |
| Medicaid | 40,048 | 14.9 (14.7–15.0) | 491 | 26.6 (24.6–28.6) | 14,226 | 12.8 (12.6–13.0) | 25,331 | 16.1 (16.0–16.3) |
| Charity/indigent/self-pay | 14,619 | 5.4 (5.3–5.5) | 143 | 7.7 (6.6–9.0) | 4,929 | 4.5 (4.3–4.6) | 9,547 | 6.1 (6.0–6.2) |
| Other | 13,560 | 5.0 (4.9–5.1) | 77 | 4.2 (3.3–5.2) | 5,044 | 4.6 (4.4–4.7) | 8,439 | 5.4 (5.3–5.5) |
|
| ||||||||
| Admission month | ||||||||
| 2019–February | 267 | 0.1 (0.1–0.1) | 0 | 0.0 (0.0–0.0) | 112 | 0.1 (0.1–0.1) | 155 | 0.1 (0.1–0.1) |
| March–April | 65,803 | 24.4 (24.2–24.6) | 378 | 20.4 (18.7–22.3) | 27,002 | 24.4 (24.1–24.6) | 38,423 | 24.5 (24.3–24.7) |
| May–June | 45,571 | 16.9 (16.8–17.0) | 326 | 17.6 (16.0–19.4) | 18,802 | 17.0 (16.7–17.2) | 26,443 | 16.8 (16.7–17.0) |
| July–August | 70,662 | 26.2 (26.0–26.4) | 478 | 25.9 (23.9–27.9) | 29,805 | 26.9 (26.6–27.2) | 40,379 | 25.7 (25.5–25.9) |
| September–November | 87,371 | 32.4 (32.2–32.6) | 667 | 36.1 (33.9–38.3) | 35,122 | 31.7 (31.4–32.0) | 51,582 | 32.9 (32.6–33.1) |
|
| ||||||||
| Hospital area | ||||||||
| Urban | 239,205 | 88.7 (88.6–88.8) | 1,629 | 88.1 (86.5–89.5) | 97,783 | 88.2 (88.0–88.4) | 139,793 | 89.1 (88.9–89.2) |
| Rural | 30,469 | 11.3 (11.2–11.4) | 220 | 11.9 (10.5–13.5) | 13,060 | 11.8 (11.6–12.0) | 17,189 | 11.0 (10.8–11.1) |
|
| ||||||||
| Hospital census region | ||||||||
| South | 121,947 | 45.2 (45.0–45.4) | 800 | 43.3 (41.0–45.5) | 51,344 | 46.3 (46.0–46.6) | 69,803 | 44.5 (44.2–44.7) |
| Northeast | 54,964 | 20.4 (20.2–20.5) | 355 | 19.2 (17.5–21.1) | 21,710 | 19.6 (19.4–19.8) | 32,899 | 21.0 (20.8–21.2) |
| Midwest | 60,478 | 22.4 (22.3–22.6) | 471 | 25.5 (23.5–27.5) | 23,965 | 21.6 (21.4–21.9) | 36,042 | 23.0 (22.8–23.2) |
| West | 32,285 | 12.0 (11.8–12.1) | 223 | 12.1 (10.7–13.6) | 13,824 | 12.5 (12.3–12.7) | 18,238 | 11.6 (11.5–11.8) |
|
| ||||||||
| Severity markers | ||||||||
| ICU/MV | 126,271 | 48.3 (48.1–48.5) | 1,095 | 60.2 (58.0–62.5) | 58,736 | 54.8 (54.5–55.1) | 66,440 | 43.6 (43.4–43.9) |
| Mortality | 39,843 | 14.8 (14.6–14.9) | 177 | 9.6 (8.3–11.0) | 20,540 | 18.5 (18.3–18.8) | 19,126 | 12.2 (12.0–12.3) |
Data are percentages of column totals and 95% CI unless otherwise specified. Patients admitted to the hospital prior to 2019 are included in the 2019–February category. COPD, chronic obstructive pulmonary disease.
Median age by patient category: all, 64 years (interquartile range 50–76); T1DM, 45 years (30–61); T2DM, 67 years (56–76); and no diabetes, 67 years (56–76).
Age Distribution of COVID-19 Outcomes
Age distribution of outcomes by diabetes status is shown in Fig. 2. Among patients with T1DM, the largest proportion of outcomes occurred among persons aged <50 years, whereas the opposite was observed among patients with T2DM or among those without diabetes.
Figure 2—
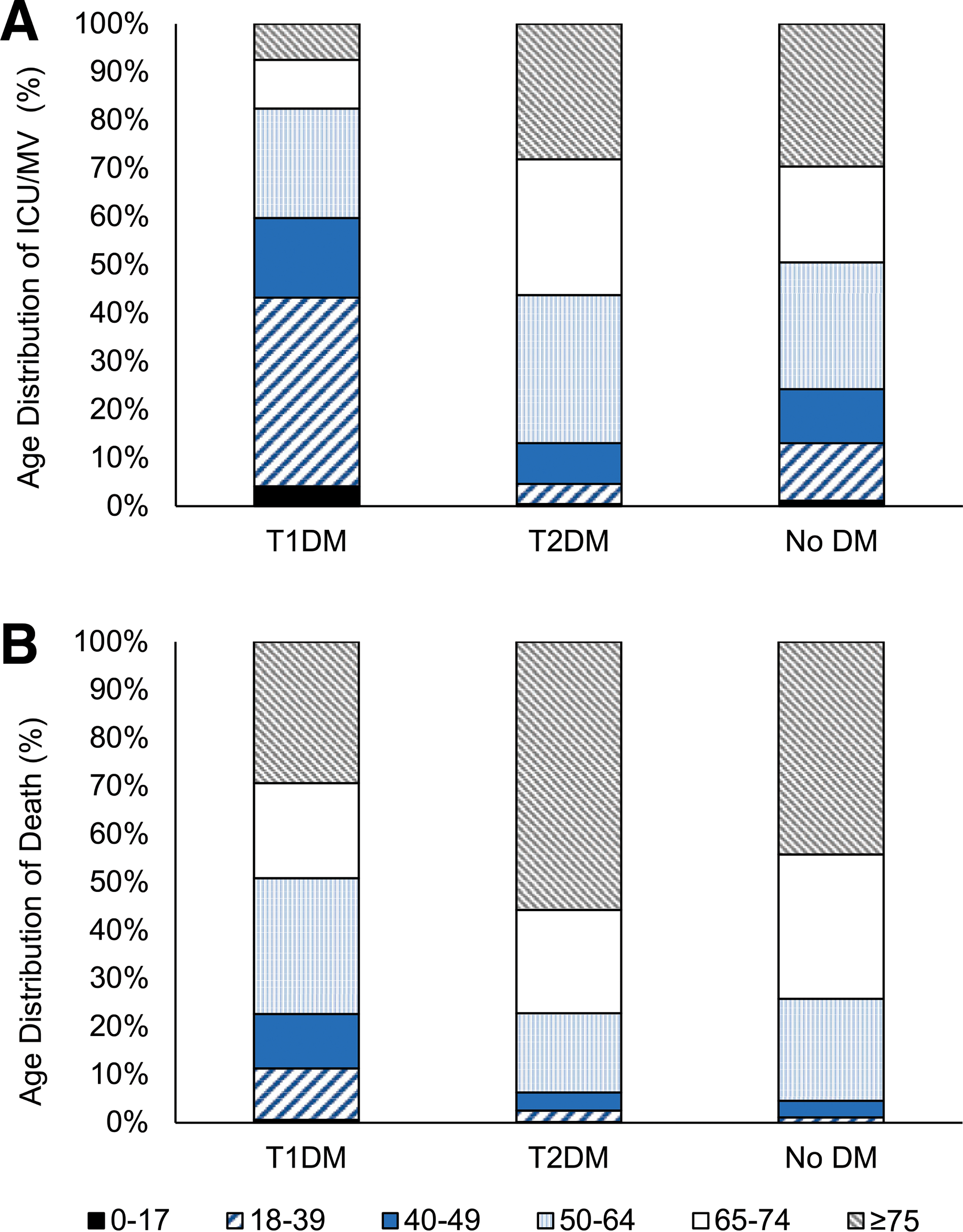
Age distribution (in years) by diabetes status of patients with COVID-19 who received ICU/MV treatment (A)ordied(B). DM, diabetes mellitus.
Risk of ICU/MV
Age-, sex-, and race- or ethnicity-adjusted absolute risk of ICU/MV among patients with T1DM was 21% (95% CI 0.19–0.24; absolute risk 65% vs. 44%) higher than among those without diabetes (Fig. 2 and Supplementary Table 2) and did not change with further adjustments for demographic and clinical characteristics (Supplementary Fig. 1). Absolute risk of ICU/MV was 9% (95% CI 0.07–0.12) higher than among T2DM patients; however, additional adjustment for DKA reduced this difference to 1% (95% CI −0.01 to 0.03; absolute risk 56% vs. 55%) (Fig. 3 and Supplementary Table 2). The age-, sex-, and race- or ethnicity-adjusted RR for ICU/MV among patients with T1DM was 1.49 (95% CI 1.43–1.56) in comparison with patients without diabetes and 1.17 (95% CI 1.12–1.22) in comparison with patients with T2DM (OR 2.48, 95% CI 2.23–2.76, and 1.47, 95% CI 1.32–1.64, respectively (Fig. 3 and Supplementary Table 2). Additional adjustments marginally reduced the point estimates relative to no diabetes; however, DKA fully accounted for the higher risk in comparisons with T2DM (RR 1.02, 95% CI 0.98–1.06).
Figure 3—
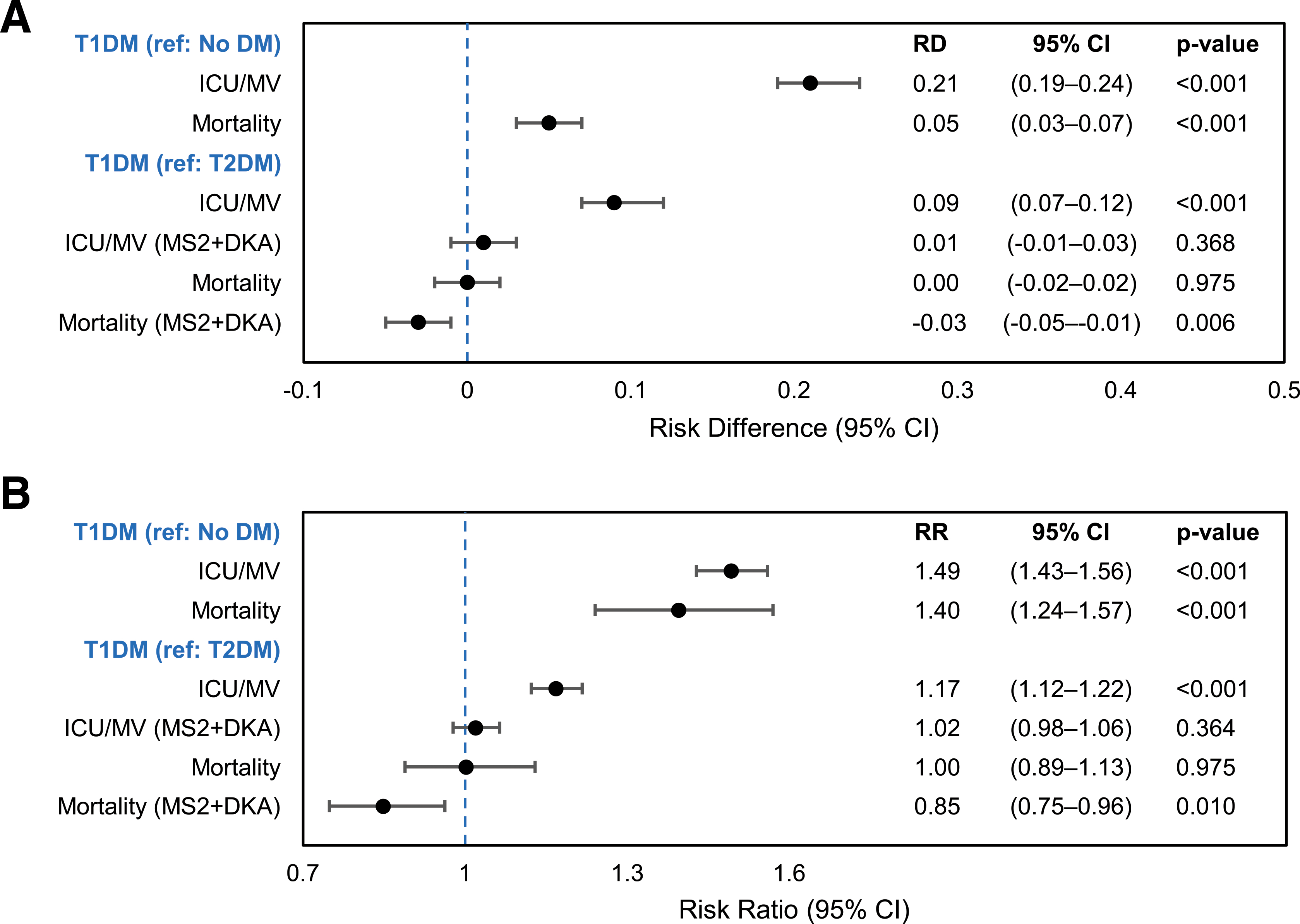
Adjusted absolute RDs (A) and risk ratios (RR) B) for( ICU/MV and mortality among 269,674 patients hospitalized with COVID-19 in the U.S., discharged March–November 2020. Each estimate represents results from a separate model, clustered on hospital identifier and controlling for age, sex, and race/ethnicity (model set 1); model set 2 additionally controls for payer type, census region, hospital area (urban, rural), admission month, and DKA. DM, diabetes mellitus; MS2, model set 2; ref, referent.
Risk of Mortality
The age-, sex-, and race- or ethnicity-adjusted absolute risk of death among patients with T1DM was 5% higher (95% CI 0.03–0.07; absolute risk 17% vs. 12%) than among those without diabetes (Fig. 3 and Supplementary Table 2) but not significantly different from that among patients with T2DM (Fig. 3). In fact, after the presence of DKA was accounted for among patients with diabetes, absolute mortality was 3% lower (95% CI −0.05 to −0.01; absolute risk 16% vs. 18%) among those with T1DM than among those with T2DM (Fig. 3 and Supplementary Table 2). The age-, sex-, and race- or ethnicity-adjusted RR for mortality was 1.40 (95% CI 1.24–1.57) in comparison with no diabetes (OR 1.54, 95% CI 1.31–1.81) (Fig. 3 and Supplementary Table 2) and slightly but not significantly lower with additional adjustments (Supplementary Fig. 2). Relative to mortality among patients with T2DM, DKA adjustment reduced mortality in patients with T1DM by 15% (RR 0.85, 95% CI 0.75–0.96) (Fig. 3).
Overall, however, patients with T1DM represented <1% of hospitalizations and those with T2DM represented 41%; most patients did not have diabetes. The absolute percentage of deaths was lowest among patients with T1DM (9.6%) and highest among those with T2DM (18.5%).
Sensitivity Analysis
With application of the full modified Klompas algorithm (17) we identified 180 fewer patients with T1DM (n = 1,669 T1DM; n = 111,189 T2DM). Age-, sex-, and race- or ethnicity-adjusted estimated risks of ICU/MV or death in patients with T1DM compared with patients without diabetes or with T2DM were similar, with no difference in significance (Supplementary Table 3).
CONCLUSIONS
Patients with T1DM hospitalized for COVID-19 and discharged during March–November 2020 were at significantly higher adjusted risk for ICU/MV treatment and experienced significantly higher mortality than patients without diabetes hospitalized for COVID-19. After adjustment for age, sex, and race or ethnicity, patients with T1DM had 65% absolute risk of ICU/MV and 17% absolute risk of death, which were 21% and 5% higher, respectively, than the absolute risk in patients without diabetes. Patients with T1DM had 9% higher absolute risk of ICU/MV, but no difference in mortality, compared with patients with T2DM.
After history of DKA was accounted for, patients with T1DM had similar risk of ICU/MV and lower risk of mortality in comparison with patients with T2DM. DKA among T1DM patients hospitalized for COVID-19 accounted for 89% of the absolute RD (from 9 to 1%) of ICU/MV in comparison with patients with T2DM, regardless of demographic and other comorbid conditions. Adjustment for DKA reduced the RD for mortality in patients with T1DM compared with T2DM (from 0% to −3%). Indeed, DKA was the single most powerful predictor of the RD in outcomes between those with T1DM and those with T2DM. Nearly one-half of patients with T1DM had a history of DKA–most being present at admission for COVID-19 hospitalization (82% of DKA codes). It is possible that the threshold for hospital admission among the population with T1DM, who are younger, was lower than for the older populations of patients without diabetes or with T2DM, which is supported by the high rates of DKA at admission. A bidirectional relationship between COVID-19 infection and diabetes (25), delayed access to medical care during the lockdown, or both, may exacerbate the risk and severity of DKA among patients with known (8,9,11) or new-onset T1DM (8). Increased rates of DKA have been reported among patients with newly diagnosed T1DM during the pandemic in both patients with and patients without COVID-19, suggesting that delays in seeking care have exacerbated problems related to diabetes (8).
Published data from the U.S. on COVID-19 severity and mortality among patients with T1DM are limited and inconsistent because of the small number of hospitalizations and the lack of consistent data on nonhospitalized COVID-19 patients (6,7,12,26–31). A small prospective U.S. study of 40 COVID-19–positive patients with T1DM reported a nearly fourfold higher odds of hospitalization and a threefold higher odds of severe illness or death in comparison with patients without diabetes, after adjustment for demographic and clinical differences (12). The same study indicated a similar adjusted risk of these outcomes among COVID-19–positive patients with T2DM compared with those with no diabetes but provided no direct comparisons with T1DM (12). Another U.S. study of pediatric ICU patients across 48 states reported increased duration of high-flow nasal cannula and intubation among patients with T1DM and COVID-19 but provided no sample size for T1DM and no comparison group (29).
The largest studies outside the U.S. are from the U.K., which similarly reported greater risk of COVID-19 severity in patients with T1DM than in those without diabetes (6,7). In-hospital mortality in England was 3.5-fold higher among patients with T1DM compared with those with no diabetes, after adjustments for age, sex, social deprivation, ethnicity, and geographic region (6). After further adjustment for previous hospital admissions for cardiovascular disease, the OR was reduced to 2.9 but still significant. Higher risk of death was reported among Black and Asian patients than among White patients with T1DM (6,32). A similar study of hospital discharge and mortality registration data in Scotland reported 2.4-fold higher age- and sex-adjusted odds of composite fatal or critical care unit–treated COVID-19 among patients with T1DM compared with those without diabetes (7). After an additional adjustment for diabetes duration, T1DM was not associated with higher risk of COVID-19 severity in comparison with T2DM. Previous hospitalizations for DKA were associated with threefold increased odds of severe outcomes among patients with diabetes, although models were not adjusted for other previous comorbidities, as in the current study. The measures of association in the studies from the U.K. (OR 2.4–3.5) are greater than the 1.5-fold increased risk reported here for ICU/MV or 1.4-fold increased risk for death for patients with T1DM compared with patients without diabetes. The present estimated ORs were greater than risk ratios but also still lower than in U.K. studies. However, this comparatively lower risk is expected, as the denominator in the current study is people hospitalized for COVID-19, whereas the studies from the U.K. used population-based denominators. The U.S. does not have a nationwide surveillance system similar to that in the U.K.; thus, we are unable to assess risk of COVID-19 severity among all people with diabetes. However, the current study 1) demonstrates that for those hospitalized, having T1DM confers an additional risk and 2) provides useful information for health care providers in hospital settings.
By contrast, a Belgian study found no data indicating increased COVID-19 hospitalization or mortality among 2,336 patients with T1DM compared with the general population, with only 5 patients with T1DM having COVID-19 (27). Similarly, a nationwide, multicenter, observational study in France found a comparable prevalence of the composite outcome of MV or death by day 7 among 56 patients with T1DM or 2,373 patients with T2DM hospitalized for COVID-19 (31). However, these studies are limited by small sample sizes of patients with T1DM and COVID-19, thus precluding detection of associations with severe outcomes.
The findings of this report are subject to several limitations. First, COVID-19 cases were identified by ICD-10-CM diagnostic codes alone, which may misclassify cases. However, COVID-19 coding in the PHD-CSR shows high sensitivity and specificity with molecular testing (33). Second, ICD-10-CM diagnostic codes may not accurately capture diabetes diagnosis or type and may vary by hospital system. The absence of laboratory data also limits accurate identification of DKA and inclusion of covariates (e.g., HbA1c, blood glucose) that may affect COVID-19 outcomes. Although use of the ratio of T1DM to all T1DM and T2DM ICD-10-CM codes has been shown to have high accuracy in multiple studies (17–20), it is an imperfect measure and may have poor performance (34). Furthermore, patients with undiagnosed diabetes with uncontrolled blood glucose may be at increased risk of COVID-19 severity but were included in the no diabetes group, which may bias toward the null. Third, due to a lack of laboratory data, we are unable to further assess severity of COVID-19 at admission or adjust for biochemical indicators of COVID-19 severity at hospital admission. It is possible that the threshold for admission in patients with T1DM is higher and that this sample represents more severe cases of COVID-19 in comparison with those of patients without diabetes or with T2DM. Fourth, longer diabetes duration may account for higher ICU/MV among those with T1DM compared with those with T2DM (7); however, the present data lack information on diabetes duration. Finally, this study is based on observational data and cannot determine causality.
The current findings suggest that patients with T1DM hospitalized for COVID-19 are at higher risk of ICU/MV and mortality compared with patients without diabetes. We found that 46% of patients with T1DM had a history of DKA, mostly related to the COVID-19 admission. After we accounted for history of DKA, patients with T1DM had similar risk of ICU/MV and significantly lower mortality rate than patients with T2DM. Knowledge of risk among patients with T1DM and associated demographic factors can guide clinical care and resource allocation in a hospital setting, as well as diabetes management and COVID-19 prevention measures. Public health messaging could emphasize risk of COVID-19 severity among people with T1DM.
Supplementary Material
Acknowledgments.
The authors thank Tegan K. Boehmer, Adi Gundlapalli, Hussain Yusuf, Sebastian Romano, Alyson Goodman, and Sachin Agnihotri from the COVID-19 Response Team, CDC.
Funding.
This work was supported by CDC contract 75D-301-20-R-68109.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Footnotes
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
References
- 1.Bode B, Garrett V, Messler J, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol 2020;14:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Killerby ME, Link-Gelles R, Haight SC, et al. CDC COVID-19 Response Clinical Team. Characteristics associated with hospitalization among patients with COVID-19 - metropolitan Atlanta, Georgia, March–April 2020. MMWR Morb Mortal Wkly Rep 2020;69:790–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Yang D, Cheng B, et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care 2020;43:1399–1407 [DOI] [PubMed] [Google Scholar]
- 4.Zhu L, She Z-G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020; 31:1068–1077.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim L, Garg S, O’Halloran A, et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus Disease 2019 (COVID-19)-Associated Hospitalization Surveillance Network (COVID-NET). Clin Infect Dis 2021;72:e206–e214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol 2020;8:813–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGurnaghan SJ, Weir A, Bishop J, et al. ; Public Health Scotland COVID-19 Health Protection Study Group; Scottish Diabetes Research Network Epidemiology Group. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol 2021;9:82–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beliard K, Ebekozien O, Demeterco-Berggren C, et al. Increased DKA at presentation among newly diagnosed type 1 diabetes patients with or without COVID-19: Data from a multi-site surveillance registry. J Diabetes 2021;13:270–272 [DOI] [PubMed] [Google Scholar]
- 9.Ebekozien O, Agarwal S, Noor N, et al. Inequities in diabetic ketoacidosis among patients with type 1 diabetes and COVID-19: data from 52 US clinical centers. J Clin Endocrinol Metab 2021;106:e1755–e1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ebekozien OA, Noor N, Gallagher MP, Alonso GT. Type 1 diabetes and COVID-19: preliminary findings from a multicenter surveillance study in the U.S. Diabetes Care 2020;43:e83–e85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Malley G, Ebekozien O, Desimone M, et al. COVID-19 hospitalization in adults with type 1 diabetes: results from the T1D Exchange multicenter surveillance study. J Clin Endocrinol Metab 2021; 106:e936–e942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory JM, Slaughter JC, Duffus SH, et al. COVID-19 severity is tripled in the diabetes community: a prospective analysis of the pandemic’s impact in type 1 and type 2 diabetes. Diabetes Care 2021;44:526–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okui T, Nojiri C, Kimura S, et al. Performance evaluation of case definitions of type 1 diabetes for health insurance claims data in Japan. BMC Med Inform Decis Mak 2021;21:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Premier Applied Sciences. Premier Healthcare Database: data that informs and performs, 2020. Accessed 17 March 2021. Available from https://products.premierinc.com/downloads/PremierHealthcareDatabaseWhitepaper.pdf [Google Scholar]
- 15.Centers for Disease Control and Prevention. International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM, 2021. Accessed 10 January 2021. Available from https://www.cdc.gov/nchs/icd/icd10cm.htm
- 16.Klompas M, Eggleston E, McVetta J, Lazarus R, Li L, Platt R. Automated detection and classification of type 1 versus type 2 diabetes using electronic health record data. Diabetes Care 2013;36:914–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder EB, Donahoo WT, Goodrich GK, Raebel MA. Validation of an algorithm for identifying type 1 diabetes in adults based on electronic health record data. Pharmacoepidemiol Drug Saf 2018;27:1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong VW, Obeid JS, Craig JB, et al. An efficient approach for surveillance of childhood diabetes by type derived from electronic health record data: the SEARCH for Diabetes in Youth Study. J Am Med Inform Assoc 2016;23:1060–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhong VW, Pfaff ER, Beavers DP, et al. Search for Diabetes in Youth Study Group. Use of administrative and electronic health record data for development of automated algorithms for childhood diabetes case ascertainment and type classification: the SEARCH for Diabetes in Youth Study. Pediatr Diabetes 2014;15:573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chi GC, Li X,Tartof SY, Slezak JM, Koebnick C, Lawrence JM. Validity of ICD-10-CM codes for determination of diabetes type for persons with youth-onset type 1 and type 2 diabetes. BMJ Open Diabetes Res Care 2019;7:e000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leighton E, Sainsbury CA, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther 2017;8:475–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical Classifications Software Refined (CCSR). Healthcare Cost and Utilization Project (HCUP). March 2021. Agency for Healthcare Research and Quality, Rockville, MD. Available from https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp [Google Scholar]
- 23.Healthcare Cost and Utilization Project (HCUP). Chronic Condition Indicators for ICD-10-CM (beta version), 2020. Accessed 10 December 2020. Available from https://www.hcup-us.ahrq.gov/toolssoftware/chronic_icd10/chronic_icd10.jsp
- 24.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. Stata J 2013;13:492–509 [Google Scholar]
- 25.Singh AK, Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res Clin Pract 2020;167:108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duarte-Salles T, Vizcaya D, Pistillo A, et al. Baseline characteristics, management, and outcomes of 55,270 children and adolescents diagnosed with COVID-19 and 1,952,693 with influenza in France, Germany, Spain, South Korea and the United States: an international network cohort study. 30 October 2020. [preprint]. medRxiv:2020.10.29.20222083
- 27.Vangoitsenhoven R, Martens P-J, van Nes F, et al. No evidence of increased hospitalization rate for COVID-19 in community-dwelling patients with type 1 diabetes. Diabetes Care 2020;43:e118–e119 [DOI] [PubMed] [Google Scholar]
- 28.Cardona-Hernandez R, Cherubini V, Iafusco D, Schiaffini R, Luo X, Maahs DM. Children and youth with diabetes are not at increased risk for hospitalization due to COVID-19. Pediatr Diabetes 2021;22:202–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loomba RS, Villarreal EG, Farias JS, Bronicki RA, Flores S. Pediatric intensive care unit admissions for COVID-19: insights using state-level data. Int J Pediatr 2020;2020:9680905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cariou B, Hadjadj S, Wargny M, et al. ; CORONADO Investigators. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia 2020;63:1500–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wargny M, Gourdy P, Ludwig L, et al. ; CORONADO Investigators. Type 1 diabetes in people hospitalized for COVID-19: new insights from the CORONADO study. Diabetes Care 2020;43:e174–e177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol 2020;8:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kadri SS, Gundrum J, Warner S, et al. Uptake and accuracy of the diagnosis code for COVID-19 among US hospitalizations. JAMA 2020;324:2553–2554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ke C, Stukel TA, Luk A, et al. Development and validation of algorithms to classify type 1 and 2 diabetes according to age at diagnosis using electronic health records. BMC Med Res Methodol 2020;20:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


