Abstract
Nanoparticles offer great opportunities for precision medicine. However, the use of nanoparticles as smart photosensitizers that target tumor biomarkers and are responsive to the tumor microenvironment has yet to be explored. Herein, prostate cancer (PCa)-selective theranostic gold nanoparticles (AuNPs) for precise cancer imaging and therapy are developed. Silicon phthalocyanine, Pc158, was synthesized and deactivated by conjugating it to AuNPs via a biocleavable linker. In vitro and in vivo, the targeted AuNPs show excellent selectivity for PSMA-positive tumor cells. Triggered release of the therapeutic, Pc158, followed by sequential photodynamic therapy (PDT) results in significant inhibition of tumor growth. Further, we demonstrate that multiple sequential PDT greatly enhances nanoparticle uptake and therapeutic efficacy. PSMA is highly expressed in the neovasculature of most other solid tumors in humans, as well as PCa, making this approach of great practical interest for precision PDT in a wide range of cancers.
Keywords: nanoparticles, photodynamic therapy, PSMA, sequential irradiation, prostate cancer
Graphical Abstract

Photodynamic therapy (PDT), utilizing light to activate photosensitizers for the generation of reactive oxygen species (ROS) and tumor cell killing, is considered a promising therapeutic modality owing to its desirable selectivity, spatiotemporal profile, and its less invasive characteristics.1 For true precision PDT, it is crucial to deliver the photosensitizers accurately to the desired location of action. Any nonspecific accumulation of photosensitizers in healthy tissues causes a decrease in imaging sensitivity/resolution with low signal-to-background ratios and increased PDT side effects.2,3 Phthalocyanines are excellent molecular photosensitizers, which can be directly targeted with ligands,4–6 and clever conjugation with quenchers via tumor environment-responsive linkers, like pH7 or glutathione levels,8,9 has demonstrated that tumor selective switching from a deactivated state (“switched off”) to an activated state (“switched on”) can be achieved.9–13
Nanoparticles (NPs) offer exciting possibilities for the design and delivery of activatable photosensitizers.2,14 By taking advantage of the enhanced permeability and retention (EPR) effects and prolonged blood circulation of NPs, delivering phthalocyanines with NPs can greatly improve delivery efficiency to tumors.15 Gold nanoparticles (AuNPs) have been used for silicon phthalocyanine (Pc4) delivery and cancer therapy16,17 and improved delivery of the payload over 2-fold using targeting peptides specific for tumor biomarkers.18,19 While it is possible to design stimuli-responsive AuNPs for precise tumor imaging,13 exploitation of AuNPs in the design and application of activatable photosensitizers has significant potential benefits. First, the quenching effect by AuNPs protects the photosensitizers from premature activation and the prolonged circulation can maintain a sufficient blood concentration, which may potentially enable secondary or multiple PDT treatments, recruiting more nanoparticles into the tumor by breaking down barriers. This makes the ability to carry out sequential PDT more attractive, especially for larger sized tumors where there may be limited depth penetration of light and/or nanoparticle into the tumor. Second, many tumors have hypoxic regions that will not support PDT (oxygen is required). By utilizing sequential PDT, the time intervals between irradiations could allow sufficient oxygen resupply in hypoxic tumor regions and more nanoparticles to enter the tumor through newly created leaky vasculature or other barrier removal.
Prostate-specific membrane antigen (PSMA), which is overexpressed in most cases of prostate adenocarcinoma and in the neovasculatures of lung, kidney, colon, stomach, breast, and brain carcinomas,20 has been demonstrated to differentiate benign from malignant disease,21 making it an attractive biomarker when designing precisely targeted PDT. Highly upregulated cathepsin activity is strongly associated with tumor invasion and metastasis and has been targeted for cancer theranostics.22 Elevated in many cell types within the tumor microenvironment, cathepsins are highly abundant within intracellular endolysosomal vesicles in cancer cells.23 Therefore, cathepsins would be an excellent tumor associated biomarker to “switch on” photosensitizers for precision PDT. The cathepsin-triggered release of photosensitizers has the advantage of high specificity compared to the other triggered release strategies, due to the high-abundance of cathepsins in prostate tumors, and ease of design and synthesis of the cathepsin-cleavable linker.24
Here, we designed a PSMA-targeted cathepsin activatable AuNP for prostate cancer precision theranostics. Silicon phthalocyanine (Pc158) was synthesized and conjugated to gold nanoparticles (AuNP) via a cathepsin-cleavable linker, GLFGC, taking advantage of AuNPs as both a quencher and delivery vehicle. The constructed AuNP-Pc158 conjugates are completely inactive and nonfluorescent. Upon cathepsin-induced cleavage, Pc158 is released over time, enabling fluorescence and photoswitchable PDT activity. PSMA targeting further enhances the specificity, uptake, and location of release. A sustained release of active Pc158 enables multiple sequential PDT treatments, which also increases further AuNP-Pc158 uptake, compounding the therapeutic efficacy. Here, we demonstrate the striking advantages of protease-activated, phototriggered, targeted nanoparticles and their potential to overcome light penetration and EPR barriers for large-tumor applications.
RESULTS AND DISCUSSION
The design of the theranostic AuNPs, Figure 1, was developed to result in adequate fluorescence and PDT quenching of light sensitizing agents conjugated via cleavable linkers. Silicon phthalocyanine, Pc158, an analogue of the PDT agent Pc4 that has been utilized in clinical trials,25 was synthesized and covalently conjugated to polyethylene glycol (PEG) coated AuNPs using a cathepsin-cleavable linker (Figure 1a).26 Subsequently, the particles were decorated with a ligand (PSMA-1) for selective targeting to PSMA receptors expressed on PCa cells.27 (Details in Figures S1–S6). Tight tethering of Pc158 to the AuNP exploited positional quenching,13 ensuring that Pc158 remains in the quenched (“switched off”) state. Figure 1b summarizes the physical parameters of the conjugates. TEM images reveal a unimodal distribution of nanoparticles without any aggregation and with a PEGylation layer surrounding the particle surface. DLS measurements confirmed a narrow hydrodynamic diameter (HD) distribution (PDI = 0.12) for the AuNPs (Figure S7). AuNPs-Pc158 conjugates were resistant to serum absorption benefiting from the PEG corona at their surface as determined by electrophoresis (Figure S8) and were stable in physiological solutions (10% FBS and PBS) for over 1 month without any aggregations (Figure S9) or undesired Pc158 release (Figure S10).
Figure 1.

Activatable AuNPs-Pc158 conjugates for selective photodynamic therapy. (a) Schematic representation of PSMA-targeted AuNPs-Pc158 conjugates with AuNP core as quencher. Activation occurs with cathepsin, which cleaves the GLFGC linker, releasing Pc158 for PDT. (b) Table shows the size of nanoparticles and Pc158 loading.
By covalently binding Pc158 to AuNPs, the Pc158 molecules were completely quenched and inactivated by efficient energy transfer to the AuNPs17 and could no longer generate reactive oxygen species (ROS) to destroy the singlet oxygen trap, diphenylisobenzofuran (DPBF) upon light irradiation (Figure S11), compared to a nonquenched peptide targeted photosensitizer, PSMA-Pc413 (Figures S11 and S12).28 Further, light irradiation rapidly bleached the free Pc158 and PSMA-Pc413 but had only a marginal effect on AuNPs-Pc158 conjugates (Figure S13). The photochemical reaction during light irradiation is an irreversible process and the Pc158 molecules undergo a structural change during 1O2 generation (Figure S14) depleting fluorescence, which is unrecoverable (Figure S15).
While the AuNP-Pc158 is water-soluble, when liberated, the hydrophobic Pc158 tends toward organic solvent. Using a water–toluene two phase system and activated cathepsin B,29 we were able to demonstrate rapid and significant release of free Pc158 from AuNP-Pc158 (Figure S16). Without active protease there was no release for up to 24 h. The AuNPs-Pc158 conjugates can reduce premature and nonspecific release of free Pc158 and generation of toxic ROS, but Pc158 can be enzymatically released from AuNPs in the presence of cathepsin B, which is desirable for precision photosensitizer delivery and photodynamic therapy.
Confocal fluorescence microscopy demonstrated that PSMA-targeted AuNPs-Pc158 conjugates could be internalized by PSMA-expressing PC3pip cells but not by PSMA-negative PC3flu cells. Pc158 release and ensuing fluorescence was time-dependent (Figure S17). After 1 h incubation there was significant AuNPs internalization by PC3pip cells while absent in PC3flu cells (Figure S18) but intracellular Pc158 fluorescence was still not detectable, Figure 2a.30 By 6 h, the Pc158 fluorescent signal was localized to lysosomes in PC3pip cells, preferentially at the perinuclear space,31 and by 24 h, the signal was distributed around the cell nuclei and overlapping with the mitochondria in PC3pip but not PC3flu cells (Figure 2a and Figure S17). The colocalization of Pc158 with lysosomes increased from 62% (1 h) to 83% (6 h), indicating increased cleavage of Pc158 in lysosomes, and decreased to 41% at 24 h which can be explained by free Pc158 relocalizing to mitochondria. Whereas, the colocalization percentage of Pc158 with mitochondria increased from 4.5% (1 h) to 23.5% (6 h) and to 54.7% after 24 h (Figure S19). The class of silicon phthalocyanine (Pc) localizes to intracellular membranes, especially mitochondria due to its high affinity for phospholipid cardiolipin (CL), which is located almost exclusively in the inner mitochondrial membrane and at the mitochondrial contact sites.32 The binding site of Pc in mitochondria is not dependent on the chemical composition of the carriers.33
Figure 2.
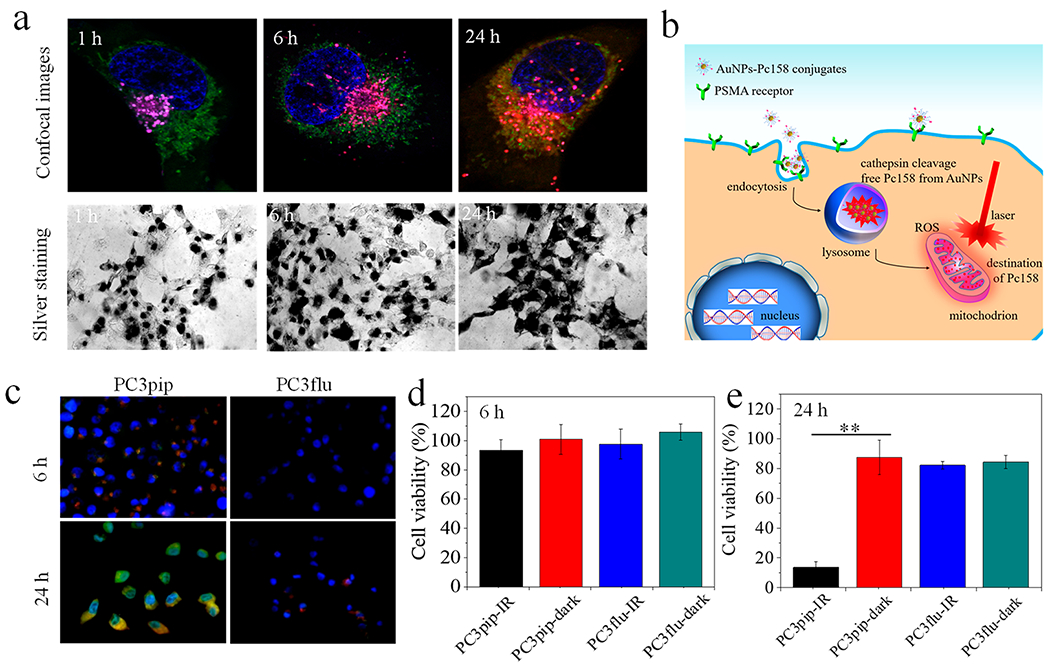
In vitro cell targeting, intracellular Pc158 release and phototoxicity. (a) Selective uptake and intracellular Pc158 release in PC3pip cells after 1, 6, and 24 h incubation times. Lysosomes (magenta), mitochondria (green), and nuclei (blue) were stained with LysoOrange, MitoGreen, and DAPI, respectively, and Pc158 fluorescence (red) was imaged directly. Overlay of Pc158 and lysosomes (pink) at 6 h indicates cleavage of Pc158 into lysosomes and at 24 h, free Pc158 was released from lysosomes to mitochondria (yellow) (for more details see Figure S17). Silver staining assay revealed that there was significant AuNPs-Pc158 uptake by PC3pip cells as early as 1 h. (b) Schematic representation of the uptake sequence of AuNP-Pc158 conjugates and intracellular Pc158 release from lysosomes to mitochondria. (c) Confocal images showing intracellular ROS generation after PDT at 6 and 24 h after incubation with AuNPs-Pc158 conjugates. Intracellular ROS (green) was stained with DCFH-DA (transformed into fluorescent DCF− by ROS) and nuclei were stained with DAPI. (d,e) Phototoxicity shows selective killing of PC3pip cells (PSMA+) over PC3flu cells (PSMA−) at (d) 6 h and (e) 24 h. Data are presented as mean ± SD (n = 5), and differences between groups are compared with two-tailed t-tests, **p ≤ 0.01.
The process of free Pc158 generation could be blocked by adding cathepsin inhibitor E64 to the cells prior to coincubation with AuNPs (Figure S20), similar to non-cleavable covalently bonded AuNP/phthalocyanine conjugates.34 These data suggest that PSMA-targeted AuNPs-Pc158 enters cells via receptor-mediated endocytosis and localizes in lysosomes,35,36 where the GLFGC linker is cleaved, generating free Pc158 that slowly migrates to mitochondria, Figure 2b. This was dramatically different from previous studies using noncovalently adsorbed Pc4 on AuNPs, which released Pc4 into the cytoplasm and transport to mitochondria occurred within 4 h.16,17,19
After 6 h incubation, we irradiated the cells and assessed ROS formation using DCFH-DA. A weak DCF− fluorescence, indicating ROS production, was detectable only in PC3pip cells and colocalized with Pc158 fluorescence in lysosomes, Figure 2c. Viability of the cells remained normal suggesting that this low level of Pc158 activation was not sufficient to kill cells (Figure 2d) and also supported mitochondria localization for efficacy.37 In contrast, after 24 h irradiation resulted in a strong green DCF− fluorescence visible in all PC3pip cells with much less fluorescence in PC3flu cells (Figure 2c). PDT at 24 h resulted in concentration-dependent cell killing, with the maximum dose of PSMA-targeted AuNP-Pc158 killing 86.4% of the PC3pip cells and little to no killing of the PC3flu cells (Figure 2e and Figure S21). Without light excitation Pc158 was nonphototoxic at the same concentration. The highly selective targeting and activation implies that widespread, undesired phototoxicity, and photosensitivity can be avoided.19
To discriminate between PSMA-receptor-mediated targeting and passive tumor accumulation, i.e., EPR,38,39 we subcutaneously implanted both PC3pip and PC3flu tumors in the right and left flanks of nude mice, respectively (Figure 3a). PC3pip tumors had 3× higher Pc158 fluorescence than PC3flu tumors at 48 h (Figure 3a, b). The peak fluorescence in the tumor occurred at 48 h. Importantly, fluorescence imaging of the entire mouse suggested that PSMA-targeted AuNP delivery of Pc158 resulted in little off-target release of Pc158 to other parts of the animal (Figure S22). Tumor-associated proteases were critical to release Pc158. When cathepsin-inhibiting E64 was intratumorally injected before AuNPs-Pc158 administration, no Pc158 fluorescence was measurable in the Pc3pip tumors (Figure S23). To see the distribution of Pc158 in tumor, tumor tissue was sectioned and imaged, showing Pc158 fluorescence around the nuclei (Figure S24), which was very similar to the distribution noted in vitro studies (Figure 2a). This data suggests that the AuNPs-Pc158 penetrated into tumors, was internalized by tumor cells, and released free Pc158 intracellularly.
Figure 3.

In vivo tumor targeting of AuNPs-Pc158 conjugates, intratumoral Pc158 release and PDT of 100 mm3 sized tumors. (a) Black and white image showing mouse with both PC3pip (right) and PC3flu (left) tumors, left; Maestro fluorescence image (at 48 h), middle; and 3D CT image (at 8 h) showing good selectivity of AuNPs-Pc158 conjugates, right. (b) Kinetics of Pc158 fluorescence intensity (top panel) and quantitative CT signals (HU) (bottom panel) of PC3pip and PC3flu tumors shows that Pc158 fluorescence peaked at 48 h and Au accumulation peaked at 8 h (n = 3). (c) Maestro fluorescence images show intratumoral ROS generation. (d) Quantitative Pc158 and ROS fluorescence intensity before and after PDT (n = 3). (e) Representative tumor H&E and immunochemistry images showing the damage by PDT. AuNPs in tumor tissue were stained with silver (red arrows). (f) Photodynamic therapy for small sized tumor (~100 mm3) (n = 5). Inset images show the tumor size before PDT (left) and 30 days after PDT (right). Data are presented as mean ± SD, and tumor growth inhibition are compared with a two-tailed t-test, **p ≤ 0.01.
We used micro CT to monitor AuNP uptake and measured a significantly higher level of AuNP accumulation in PC3pip tumors compared to PC3flu tumors (Figure 3a, right panel; Figure S25, for time course) peaking at 8 h with 315 HU and 201 HU for PC3pip and PC3flu tumors, respectively (Figure 3b). Interestingly, the peak accumulation for AuNPs is significantly earlier than the peak time for Pc158 fluorescence (48 h), which is in line with AuNP-Pc158 uptake preceding enzymatic release and intracellular migration of Pc158. In contrast, previous studies with noncovalently adsorbed Pc4 to AuNPs showed similar accumulation kinetics for both the AuNP and Pc4 fluorescence in tumors, peaking at 4 h.17 The targeted AuNPs distribution in organs was also measured using ICP-MS and showed that there was selective uptake into the tumors that remained constant for at least 7 days (Figure S26). Furthermore, both spleen and liver, organs for NP uptake and excretion, also showed increased levels of the AuNP for 7 days.
To detect the intratumor ROS generation, we intravenously injected ROStar 800CW, an in vivo ROS probe, 30 min before irradiating the tumor with 150 J/cm2. Baseline fluorescence imaging at 24 h postinjection of PSMA-targeted AuNPs-Pc158 demonstrated strong Pc158 fluorescence from the tumor and absence of ROS signals in the tumor (Figure 3c). After light irradiation of the tumor, the Pc158 fluorescence was photobleached, indicating activation of Pc158 to produce ROS,28,40 and, at the same time, the tumor showed significant ROStar 800CW signal, suggesting generation of ROS in the tumor induced by Pc158 and light irradiation (Figure 3c). We quantified the fluorescence signals of Pc158 and ROStar 800CW in the tumor before and after light irradiation, showing a significant decrease of Pc158 signal from 14.0 to 3.8 counts and an increase of ROStar 800CW signal from 0.2 to 17.2 counts (Figure 3d). Since hydrocyanines (ROStar 800CW) are nonfluorescent until being oxidized to fluorescent cyanine dyes by ROS radicals,41 the fluorescence change gives direct evidence of in vivo ROS generation due to light irradiation. The generated ROS induced direct damage to the tumor tissue as severe necrosis with typical nucleus dissociation,42 and large amounts of apoptotic lesions (red fluorescent spots) were identified in the H&E and the immunofluorescence images (Figure 3e). In animals that were administered PSMA-targeted AuNPs-Pc158 but were not irradiated, there was no cell necrosis noticed in the H&E images and very few apoptotic regions, even though a large number of AuNPs clusters were accumulated in the tumor tissue (Figure 3e, red arrows).
We then tested the efficacy of the targeted PDT agent in vivo. Animals bearing PC3pip tumors (100 mm3) were injected with PSMA-targeted AuNPs-Pc158, subjected to PDT, and tumor growth was monitored over 30 days. PC3pip tumor growth was completely inhibited by PDT treatment (Figure 3f). In contrast, control tumors grew rapidly in animals that did not undergo PDT, with the tumor size increasing approximately 13 times the original volume in 30 days. No toxicity to the nude mice was noted after PDT, and their body weight was very well maintained over the 30 day period, while it decreased slightly for the mice from the control group without PDT (Figure S27).
The protease-induced drug release from AuNPs-Pc158 measured at 6 h in vitro in PC3pip cells generated a weak DCF− fluorescence. We therefore surmised that enzymatic release of Pc158 might occur before 24 h when Pc158 fluorescence was visible in cells but remained partially quenched by being encapsulated within the lysosomes. To test this hypothesis, we irradiated the cells at an earlier time point (6 h) in vitro and demonstrated an accelerated escape of liberated Pc158 from lysosomes and rapid accumulation of free Pc158 into mitochondria (Figure 3a and Figure S28). The kinetics of light triggered Pc158 escape from lysosomes was recorded with the same cells over 30 min (Figure S29). Though only a limited PDT effect was observed at 6 h, it could be enhanced to eradicate 62.4% cells by carrying out a second light irradiation 30 min after the first exposure, taking advantage of accelerated diffusion of free Pc158 from lysosomes to mitochondria. When cathepsin-inhibiting E64 was added to block the cleavage, release of free Pc158 and PDT was significantly hindered (Figure 4b).
Figure 4.

Sequential irradiation induces intracellular Pc158 release and enhances efficacy. (a) Light-induced release of protease liberated Pc158 escaping from lysosomes to mitochondria. Confocal image (left) shows mitochondria containing Pc158, yellow, and free Pc158, red. NIR was irradiated (670 nm, 1 J) at 6 h, and lysosome was disrupted showing no staining compared to Figure 2a at 6 h with no PDT. Right image diagrams lysosomal release and accumulation into mitochondria after PDT. (b) Phototoxicity of PC3pip cells incubated with AuNPs-Pc158 showing an enhanced PDT efficacy by carrying out a second irradiation 30 min after the first light exposure. (c) Scheme shows the timeline of the repeated PDT treatments in mice with 500 mm3 tumors. (d) Maestro fluorescence images of mice injected with AuNP-Pc158 and PSMA-Pc413 conjugates before and after each PDT treatment (150 J/cm2). (e) Normalized Pc158 fluorescence intensity for mice injected with AuNPs-Pc158 and PSMA-Pc413 before and after each NIR irradiation. (f) In vivo pharmacokinetics of AuNPs in blood over 7 days. (g) AuNP uptake in tumors before and after each PDT showing a PDT enhanced AuNP accumulation in irradiated tumors compared to tumors without PDT. For all studies, data are presented as mean ± SD (n = 3). Differences of Au content in tumors was compared with a two-tailed t-test, *p ≤ 0.05, **p ≤ 0.01.
Understanding that sequential PDT could increase the release and efficacy of Pc158, we sought to determine if this approach could be used in the treatment of larger tumors in vivo, which are generally more difficult to achieve complete nanoparticle and light penetration. When the tumors reached approximately 500 mm3, the mice were injected with either PSMA-targeted AuNPs-Pc158 conjugates or PSMA-Pc413, a small molecule PDT agent targeting the PSMA receptor.28,43 Mice were then imaged, the distribution measured, and then PDT was performed for both, Pc158 or Pc-413. This was repeated every 24 h for 3 sequential days (Figure 4c). The choice of the multiple PDT time intervals of 24 h was based on the fluorescence kinetics of Pc158 in vivo, which took up to 24 h to reach the maximum free Pc158 accumulation in tumor. AuNPs-Pc158 conjugates showed good tumor targeting ability and Pc158 was released in the tumor after 24 h. After light irradiation, Pc158 fluorescence in the tumor was bleached. However, it recovered after another 24 h (Figure 4d). When light irradiation was repeated, the Pc158 fluorescence in the tumor was bleached again. At day 3, a similar Pc158 fluorescence recovery was observed and the mice received another PDT treatment. In contrast, PSMA-Pc413 showed good accumulation in the tumor at 24 h and no Pc413 fluorescence recovery was observed after one light irradiation. We quantified the fluorescence intensity after each treatment for both groups and summarized them in Figure 4e. For mice injected with AuNPs-Pc158, repeated PDT resulted in an increased tumor-associated Pc158 fluorescence by day 3. However, for the purely molecular PSMA-Pc413, the Pc413 fluorescence was weakened after each light exposure. Since we and others have already demonstrated an irreversible destruction of phthalocyanine molecules by light irradiation (Figures S14–S16),44 we ascribe the repeated Pc158 fluorescence recovery in tumors to two factors: (1) a sustained Pc158 cleavage/release from AuNPs already within the tumor and (2) PDT enhanced targeted AuNPs-Pc158 accumulation in the tumor.
To verify our first notion, we injected PSMA-targeted AuNPs-Pc158 conjugates intravenously and did the same PDT treatment after 24 h (D1), but immediately after bleaching of the Pc158 fluorescence, we intratumorally injected cathepsin-inhibitor E64. The inhibition of protease activity resulted in fluorescence inhibition in the tumor for the following 48 h (at Day 2 and Day 3, Figure S30). Next, we measured the circulation of AuNPs-Pc158 conjugates in blood and found that there was still a 21.0 ± 4.5% ID AuNPs remaining in blood at 72 h postinjection (Figure 4f). A long circulation time likely allows more targeted AuNPs-Pc158 to accumulate in the tumor via both EPR and active targeting. To demonstrate this possibility, we retrieved the tumors before the first PDT and following each PDT treatment and measured the Au content by ICP-MS (Figure 4g). Au content in tumors increased after each PDT from 0.08 ± 0.03 μg/mg (before PDT) to 0.18 ± 0.04 μg/mg after the third PDT treatment. In contrast, without any PDT, the Au content in tumors was steady and decreased after 3 days. The increased targeted AuNPs-Pc158 accumulation in tumors is likely due to the PDT-induced damage of the tumor vasculature and permeability enhancement,45,46 which is particularly important in larger tumors where deep penetration into the tumor is difficult. To confirm this, we analyzed the blood vessels before and after PDT with CD31 staining, showing significant tumor vasculature damage by PDT (Figure S31). The results above highlight the advantages of targeted AuNPs-Pc158 conjugates over small molecular PDT agents, with an increased and sustained uptake and activatability for precision PDT.
The sequential PDT also improved in vivo efficacy. When PDT was performed after the NP injection, there was a dramatic impact on the tumor growth. For groups that received one or two light irradiations, tumor growth was clearly inhibited in the first 10 days, but then the tumor growth rates of these groups eventually returned to that of the control group. In contrast, mice that were irradiated three consecutive times showed dramatic reductions in tumor size (49.9% of the original volume after 24 days) and maintained almost zero growth rate for the period observed (Figure 5a). Interestingly, mice injected with PSMA-Pc413 that also received 3 PDT treatments did not show a comparable tumor inhibition, likely due to the limited PDT efficiency for the second and third PDT, i.e., PSMA-1-Pc413 was bleached and its short blood half-life did not allow for regeneration of the molecular PDT reagent within the tumor. Repeated PDT alone did not make any difference on tumor growth (Figure S32). Tumor weight after 24 days also evidenced the significant impact of consecutive PDT treatments on tumor eradication (Figure 5b). To evaluate the biosafety of such sequential PDT strategies, H&E staining of heart, lung, spleen, liver, and kidney from the three groups of treatments (blank control, AuNPs-Pc158 + PDT × 3, and PSMA-Pc413 + PDT × 3) was carried out. We did not observe obvious differences among the three treatment groups, suggesting a good safety profile for both the AuNPs-Pc158 and PSMA-Pc413 after sequential PDT (Figure S33).
Figure 5.

Multiple photodynamic therapy enhances the eradication of large tumors. (a) Effects of PDT on growth kinetics of larger tumors (around 500 mm3). (b) Tumor weight for each of the groups at the end of growth monitoring. Data are presented as mean ± SD (n = 5), and tumor growth inhibition is compared with two-tailed t-test, **p ≤ 0.01. (c) Tumor H&E and immunochemistry images showing the damage by PDT. Upper panel shows the H&E staining at low magnification; middle panel shows the silver stained tissue from the highlighted areas (black boxes). The AuNPs were stained black (red arrows); bottom panel shows the immunohistochemistry staining (TUNEL) of tumor tissues taken from the blue box regions demonstrating increase apoptosis with increased iterations of PDT.
To further compare the damage to the tumors induced by PDT, we retrieved the tumors after each treatment and did histological analysis (Figure 5c). These data demonstrate that the targeted AuNP-Pc158 conjugates accumulated in the tumor tissues and increased tissue destruction with increasing number of light irradiations. PDT induced nuclei/cytoplasm dissociation and cytoplasm damage that increased with sequential PDT treatments. As silver staining revealed, each additional PDT treatment resulted in more AuNPs accumulating in the tumors (indicated by red arrows), especially when compared to tumors that did not undergo PDT treatments, in agreement with the ICP measurement results in Figure 4g. Caspase-3 staining (TUNEL assay) also confirmed that sequential PDT treatments induced tumor tissue apoptosis as indicated by the increased red fluorescence after multiple irradiations. For the PSMA-Pc413 treated group, significant tumor tissue damage and apoptosis were also observed (Figure S34).
CONCLUSION
In the work presented here, we combined two biofunctionalities, PSMA targeting and cathepsin-induced drug release for spatiotemporal precise PDT application. In addition, the applied light irradiation further focuses the localization of the PDT drug to the target tumors. Multiple, sequential irradiations led to a gradual increased accumulation of AuNP-Pc158. Altogether a treatment modality emerged that combined the highest selectivity with sustained targeted sensitizer accumulation and a strongly improved treatment efficacy.
There are potential issues that can restrict the success of nanoparticle delivery of photosensitizers. First, nanoparticles often are unable to fully penetrate tumors to deliver sensitizers deeper into the tumor. Second, NIR light has a limited penetration depth. Third, when tumors reach a larger size, lack of light penetration prevents complete eradication of the tumor cells. Our delivery approach of PDT using photostable AuNPs and sequential irradiation targeted to the tumors allows for single dose drug administration and cumulative destruction of the tumor, potentially solving the aforementioned drug delivery and therapy problems. Histological results verified the increased PDT efficacy for prostate cancer and that AuNPs-Pc158 enabled multiple irradiation of tumors. This approach offers the possibility to completely and permanently eradicate cancer and potentially could be applied to other tumors such as lung, kidney, colon, stomach, breast, and brain cancers, due to the high expression of PSMA in their neovasculature.
Finally, additionally tethering other drugs with cleavable linkers to the PSMA-targeted AuNP-Pc158 might allow exploitation of increased nanoparticle uptake and drug release induced by sequential light irradiation to more efficiently deliver the traditional chemotherapy drugs.
METHODS
Materials.
All the chemicals were purchased from Sigma-Aldrich unless otherwise stated. Milli-Q water (18.2 MΩ cm) was used in all the experiments.
Synthesis of Silicon Phthalocyanine, Pc158, and GFLGC-Pc158 Conjugations.
Pc158 was synthesized using a reported method with slight modification.47 GFLGC-Fmoc (Gly-Phe-Leu-Gly-Cys) was synthesized using standard Fmoc chemistry, and the Cys was protected with pyridinethiol (PDS). Next, GFLGC(PDS)-Fmoc linker was reacted with Pc158 via a typical NH2 and COOH reaction. After reaction, the Fmoc was removed with 20% piperidine DMF solution, and PDS was removed by tris(2-carboxyethyl)phosphine (TCEP). The final product was purified by HPLC and characterized with ESI-MS. Details of the synthesis and reaction schemes are in the Supporting Information.
Synthesis of PSMA-Targeted AuNPs-Pc158 Conjugates.
Gold nanoparticles (AuNPs) with a core diameter of 5 nm were synthesized according to a previous reported method30 and then PEGylated with PEG5k and PEG5k-PSMA-1 at a ratio of 4:1. PSMA-1 ligands were synthesized as previously described,27 and conjugated to OPSS-PEG5k-NHS, deprotected and purified, yielding PEG5k-PSMA-1 (Supporting Information, Scheme 3). The AuNPs were purified by centrifuging with 30 kDa cutoff Vivaspin tubes (GE Healthcare). Next, the GFLGC-Pc158 was added to AuNPs at a 40:1 ratio and stirred for 2 days. After 2 days, the NPs were purified again by centrifugation with the same procedure. AuNPs concentration was measured by UV–vis spectroscopy (TECAN, infinite M200), and the Pc158 loading rate was calculated according to the absorbance peak of AuNPs and Pc158. The hydrodynamic size was determined by a dynamic light scattering system (DynaPro Nanostar). The absolute size and polymer shell was visualized by transmission electron microscopy (FEI Tecnai F300 kV) with 2% phosphotungstic acid staining. The stability of AuNPs in serum was examined via gel electrophoresis with AuNPs preincubated with 10% FBS at 37 °C for 30 min. Long-term stability in different mediums was monitored by UV–vis spectroscopy. The ability of AuNPs-Pc158 conjugates to generate ROS upon NIR light illumination was tested by the DPBF assay.19
Synthesis of PSMA-Pc413.
Silicon phthalocyanine, Pc413, was synthesized as previously reported and conjugated to PMSA-1 ligands via sulfosuccinimidyl-4-(N-maleimidomethyl)cyclohexiane-1-carbocylate (sulfo-SMCC) linker.28 The synthesis is shown in Supporting Information, Scheme 4. Absorbance of PSMA-Pc413 conjugation, free Pc158 and AuNPs-Pc158 before and after light irradiation was measured by UV–vis.
Cathepsin Cleavage and Release of Pc158.
The release of Pc158 upon cleavage of the stimuli responsive linker was tested with a toluene-aqueous biphase system,48 AuNPs-Pc158 conjugates were present in the aqueous phase. An equal volume of toluene was subsequently added in the cuvette, and the organic phase was monitored by UV–vis spectroscopy. Aqueous solutions were prepared by mixing AuNPs-Pc158 conjugates with 1 mL of activated cathepsin B buffer. Cathepsin B (human liver, Athens Research & Technology) was activated by adding 3 μL of enzyme to 6 μL of activation buffer (50 mM sodium acetate, 20 mM DTT, and 1 mM EDTA) and incubated at 37 °C for 15 min and then diluted to 1 mL with diluting buffer (50 mM sodium acetate and 1 mM EDTA). The mixture was stirred gently at 37 °C, and the absorbance of the organic phase was measured at 1, 2, 6, 18, and 24 h. AuNPs-Pc158 in the same buffer but without the addition of cathepsin B was used as the control and measured at the same time points.
In Vitro Targeting and Intracellular Pc158 Release.
Retrovirally transformed PSMA-positive PC3pip cells and transfection control PSMA-negative PC3flu cells27 were incubated for specific uptake and intracellular release studies. Both PC3pip and PC3flu cells were cultured in RPMI1640 medium (Invitrogen Life Technology) with 2 mmol/L L-glutamine, 10% FBS at 37 °C, and 5% CO2. PC3pip and PC3flu cells were seeded in μ-Slide 8-Well Chamber Slide (ibidi) at 2000 cells/well. When the cells grew to 70% confluence, AuNPs-Pc158 conjugations were added at a Pc158 concentration of 1 μmol/L and coincubated for 1 h, 6 h, and 24 h. Then the cells were washed with PBS and stained with DAPI, LysoOrange, and MitoGreen (all from abcam) for 30 min at 37 °C and 5% CO2, after which they were washed again with PBS and replete medium added. The release of Pc158 and localization of nuclei, lysosomes, and mitochondria was observed under a Leica HyVolution SP8 confocal microscope (Leica Microsystem Inc.).
In Vitro PDT and ROS Generation in Cells.
In vitro PDT was evaluated by CCK8 assay. PC3pip and PC3flu cells were seeded in 96-well plates at 1 × 104 cells/well. After 1 day of incubation, AuNPs-Pc158 conjugates were added at Pc158 concentrations of 0.0625, 0.125, 0.25, 0.5, and 1 μmol/L. After coincubation for 6 or 24 h, the medium was removed and cells were washed with PBS, then another 200 μL of medium was added to each well prior to light irradiation. The 96-well plate was irradiated (Appolo Horizon projector, Acco Brands) with radiant exposure at 1 J/cm2, after which the cells were incubated overnight. After incubation, CCK8 agent (DojinDo Laboratories) was added to each well (10 μL/well) and incubated for 3 h at 37 °C, and absorbance at 450 nm was measured for each well.
The intracellular ROS generation after light irradiation was evaluated with a DCFH-DA assay. PC3pip and PC3flu cells were cultured in μ-Slide 8-Well Chamber Slide (ibidi) and incubated with AuNPs-Pc158 conjugates for 6 and 24 h at a Pc158 dose of 1 μmol/L. Culture medium was removed, and cells were washed with PBS and incubated with 20 μM DCFH-DA HEPES buffer for 30 min. After incubation, the cells were washed again with PBS and irradiated with light at 1 J/cm2. The cells were counterstained with DAPI and fixed for fluorescence imaging.
In Vivo Tumor Targeting and Intratumor Pc158 Release.
All animal procedures were performed according to Institutional Animal Care and Use Committee (IACUA)-approved protocols (2015-0033). Four- to five-week-old male athymic nude mice were subcutaneously implanted with PC3pip or PC3flu cells (100 μL, cells suspended in PBS/matrigel at 1 × 107 cells/mL) on the right and left flanks, respectively. When the tumors grew to a sufficient size, AuNPs-Pc158 conjugations were intravenously injected via the tail vein with a Pc158 dose of 0.1 mg/kg. The mice were preimaged before, and at 0.5 h, 1 h, 4 h, 8 h, 24 h, 48 h, 72 h, 96 h, and 120 h after NPs injection using the Maestro In Vivo Imaging System (PerkinElmer) to monitor the delivery and release of Pc158. Multispectral images were unmixed into their component spectra (Pc158 autofluorescence and background). Component images were used to quantitatively determine the average Pc158 fluorescence intensity of the PC3pip and PC3flu tumors. Tumor tissues at 24 h postinjection were also collected, cut, and imaged to see the Pc158 distribution (details in the histology analysis section).
CT imaging was also performed to monitor the accumulation of AuNPs in tumors. PC3pip and PC3flu tumor bearing mice were anesthetized under isoflurane and scanned at the same time points by a preclinical Siemens Inveon Positron Emission Tomography-Computed Tomography system (Siemens). CT scanning was carried out at a tube voltage of 70 kV, current of 300 μA, and gantry rotation time of 140 ms. Hounsfield units (HU) were quantified at the tumor areas, and 3D images were reconstructed. The Au contents in tumors, blood, and organs were also measured by ICP-MS as described previously.31
In Vivo ROS Detection and PDT.
The intratumor ROS generation upon light irradiation after AuNPs-Pc158 injection was detected by a ROStar CW800 probe. PC3pip tumor bearing athymic nude mice were injected with AuNPs-Pc158 conjugates at 0.1 mg/kg Pc158 dose and 24 h later, the mice were intravenously injected with ROStar CW800 (100 nmol, LI-COR) via the tail vein. After 30 min, the tumors were subjected to NIR light irradiation at 150 J/cm2 (model 525 Laser Diode Driver, 1–5 mW/cm2 of 672 nm light from a diode laser (Applied Optronics Corp.) equipped with a GRIN-lens-terminated multimode fiber (OZ Optics)). Mice were imaged by Maestro before and after light irradiation. Multispectral images were unmixed into their component spectra (Pc158 autofluorescence and ROStar CW800), and the component images were used to quantitatively determine the average Pc158 and ROStar CW800 fluorescence before and after irradiation of the PC3pip tumor areas.
For PDT, PC3pip tumor-bearing mice were injected with AuNPs-Pc158 conjugations at 0.1 mg/kg Pc158 dose. For mice with small tumors (around 100 mm3), PDT was given only once at 24 h post injection, the mice were randomly divided into two groups, one group underwent PDT and the other served as a no-treatment control. Tumor size and mouse body weight was monitored every other day for a 30 day period. For multiple PDT treatments, mice with tumor size around 500 mm3 were divided into five groups, receiving A) PBS and irradiated three times with light, i.e., PDT; PSMA-targeted AuNPs-Pc158 conjugates and irradiated (B) one time, (C) two times, or (D) three times with light irradiation and (E) PSMA-Pc413 irradiated three times with light. PDT was performed at 24 h, 2 days, and 3 days postinjection. Fluorescence before and after each PDT was monitored with Maestro, and intensity was quantified. One group of mice that received AuNPs-Pc158 were intratumorally injected with cathepsin inhibitor E64 after the first PDT exposure. After PDT, tumor size and mice body weight were monitored over 24 days.
Histology, Detection of AuNPs, and Immunofluorescent Analysis.
Following imaging, samples were snap-frozen in optimum cutting temperature compound for cryosectioning (Leica CM3050S). Sections, 10-μm thick, were serially collected directly onto slides and stored at −80 °C for processing. For immune-histochemical analysis, the slides were warmed to room temperature (RT) for 10 min, fixed with 10% buffered formalin, and blocked in blocking buffer (5% normal goat serum/0.3% Triton X-100 in 1 × PBS) for 1-h at RT and incubated in primary antibody overnight at +4 °C followed by three 5 min washes in 1 × PBS. The presence of apoptosis in the tumor was evaluated by rabbit antihuman of Cleaved Caspase-3 antibody (Cell Signal Tech) at a 1:400 dilution. After washing, the slides were treated with the secondary ready-to-use antibody (goat antirabbit polyclonal antibody labeled by Alexa Fluor-594 (Invitrogen, Inc.) for 20 min at RT followed by triple washing with 1 × PBS for 5 min. Tissue nuclei were contrasted with Fluoro-Gel-II with DAPI (Electron Microscopy Sciences, Hatfield, PA). Additionally, all adjacent slides were stained for presence of AuNPs (Sigma Silver Enhancer Kit) followed by H&E standard procedures. Fluorescent images were viewed with a Leica-DM4000B microscope (bandpass = 560/645, anti-Cleaved Caspase-3) and analyzed with QCapturePro-7 software. An Olympus-VS120/S5 versatile microscope-based scanner was used to generate histological images larger than a single field of view.
Statistical Analysis.
All the in vitro experiments and in vivo biodistribution studies were performed in triplicate unless stated otherwise, and for PDT treatment each group had 5 mice. All numerical results are expressed as mean ± SD. Descriptive statistics and significant differences between groups were analyzed using twotailed Student’s t tests, and the difference was considered significant if *p < 0.05 and **p < 0.01.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge the use of the core facility provided by Imaging Research Core at Case Western Reserve University and Dr. Meade of Northwestern University for consultation on precursor purification strategies. This research was supported by the National Institute of Health Grant, RO1 EB020353-03. J.P.B. is a fellow of the National Foundation for Cancer Research.
Footnotes
The authors declare no competing financial interest.
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.0c05425.
Additional experimental data including detailed synthesis of Pc158, GFLGC-Pc158, PSMA-1, PEG5K-PSMA-1, and PSMA-Pc413 and their characterization (Figures S1–S6, S12); AuNPs-Pc158 synthesis and characterization (Figure S7); AuNPs-Pc158 stability measurement and PDT activity (Figures S8–S11) and their reaction to NIR light compared to free Pc158 and PSMA-Pc413 (Figure S13–S15); Pc158 release (Figure S16); cell targeting and intracellular Pc158 release (Figures S17–S20); in vitro PDT activity (Figure S21); in vivo tumor targeting and Pc158 release (Figures S22–S25) and biodistribution (Figure S26); mice body weight change after PDT (Figure S27); NIR light induced Pc158 release (Figures S28 and S29); inhibited Pc158 release in tumors by E64 (Figure S30); PDT damage to blood vessels (Figure S31); tumor growth for blank control groups with different PDT exposure times (Figure S32); biosafety evaluation for AuNPs-Pc158 and PSMA-Pc413 (Figure S33); and tumor H&E and immunochemistry images of PSMA-Pc413 treated group after PDT (Figure S34) (PDF)
Complete contact information is available at: https://pubs.acs.org/10.1021/acsnano.0c05425
Contributor Information
Dong Luo, Department of Radiology, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Xinning Wang, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Ethan Walker, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Jing Wang, Department of Radiology, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Sarah Springer, Department of Chemistry, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Jason Lou, Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Gopalakrishnan Ramamurthy, Department of Radiology, Case Western Reserve University, Cleveland, Ohio 44106, United States.
Clemens Burda, Department of Chemistry, Case Western Reserve University, Cleveland, Ohio 44106, United States.
James P. Basilion, Department of Radiology and Department of Biomedical Engineering, Case Western Reserve University, Cleveland, Ohio 44106, United States
REFERENCES
- (1).Van Straten D; Mashayekhi V; de Bruijn HS; Oliveira S; Robinson DJ Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Li X; Yu S; Lee D; Kim G; Lee B; Cho Y; Zheng BY; Ke MR; Huang JD; Nam KT; Chen X; Yoon J Facile Supramolecular Approach to Nucleic-Acid-Driven Activatable Nanotheranostics That Overcome Drawbacks of Photodynamic Therapy. ACS Nano 2018, 12, 681–688. [DOI] [PubMed] [Google Scholar]
- (3).Sharman WM; Allen CM; van Lier JE Role of Activated Oxygen Species in Photodynamic Therapy. Methods Enzymol. 2000, 319, 376–400. [DOI] [PubMed] [Google Scholar]
- (4).Wang BS; Wang J; Chen J-Y Conjugates of Folic Acids with Zinc Aminophthalocyanine for Cancer Cell Targeting and Photodynamic Therapy by One-Photon and Two-Photon Excitations. J. Mater. Chem. B 2014, 2, 1594–1602. [DOI] [PubMed] [Google Scholar]
- (5).Ranyuk E; Cauchon N; Klarskov K; Guerin B; van Lier JE Phthalocyanine-Peptide Conjugates: Receptor-Targeting Bifunctional Agents for Imaging and Photodynamic Therapy. J. Med. Chem 2013, 56, 1520–1534. [DOI] [PubMed] [Google Scholar]
- (6).Luan L; Fang W; Liu W; Tian M; Ni Y; Chen X; Yu X Phthalocyanine-cRGD Conjugate: Synthesis, Photophysical Properties and in Vitro Biological Activity for Targeting Photodynamic Therapy. Org. Biomol. Chem 2016, 14, 2985–2592. [DOI] [PubMed] [Google Scholar]
- (7).Wang Y; Zhou K; Huang G; Hensley C; Huang X; Ma C; Zhao T; Sumer BD; DeBerardinis RJ; Gao J A Nanoparticle-Nased Strategy for the Imaging of a Broad Range of Tumours by Nonlinear Amplification of Microenvironment Signals. Nat. Mater 2014, 13, 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Gamcsik MP; Kasibhatla MS; Teeter SD; Colvin OM Glutathione Levels in Human Tumors. Biomarkers 2012, 17, 671–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Li X; Kolemen S; Yoon J; Akkaya EU Activatable Photosensitizers: Agents for Selective Photodynamic Therapy. Adv. Funct. Mater 2017, 27, 1604053. [Google Scholar]
- (10).Tian J; Zhou J; Shen Z; Ding L; Yu JS; Ju H A pH-Activatable and Aniline-Substituted Photosensitizer for Near-Infrared Cancer Theranostics. Chem. Sci 2015, 6, 5969–5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Chen J; Stefflova K; Niedre MJ; Wilson BC; Chance B; Glickson JD; Zheng G Protease-Triggered Photosensitizing Beacon Based on Singlet Oxygen Quenching and Activation. J. Am. Chem. Soc 2004, 126, 11450–11451. [DOI] [PubMed] [Google Scholar]
- (12).Zhao J; Huang L; Cui X; Li S; Wu H Maximizing the Thiol-Activated Photodynamic and Fluorescence Imaging Functionalities of Theranostic Reagents by Modularization of Bodipy-Based Dyad Triplet Photosensitizers. J. Mater. Chem. B 2015, 3, 9194–9211. [DOI] [PubMed] [Google Scholar]
- (13).Zhao X; Yang CX; Chen LG; Yan XP Dual-Stimuli Responsive and Reversibly Activatable Theranostic Nanoprobe for Precision Tumor-Targeting and Fluorescence-Guided Photothermal Therapy. Nat. Commun 2017, 8, 14998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Li X; Kim CY; Lee S; Lee D; Chung HM; Kim G; Heo SH; Kim C; Hong KS; Yoon J Nanostructured Phthalocyanine Assemblies with Protein-Driven Switchable Photoactivities for Biophotonic Imaging and Therapy. J. Am. Chem. Soc 2017, 139, 10880–10886. [DOI] [PubMed] [Google Scholar]
- (15).Vankayala R; Hwang KC Near-Infrared-Light-Activatable Nanomaterial-Mediated Phototheranostic Nanomedicines: An Emerging Paradigm for Cancer Treatment. Adv. Mater 2018, 30, No. 1706320. [DOI] [PubMed] [Google Scholar]
- (16).Cheng Y; Meyers JD; Agnes RS; Doane TL; Kenney ME; Broome AM; Burda C; Basilion JP Addressing Brain Tumors with Targeted Gold Nanoparticles: A New Gold Standard for Hydrophobic Drug Delivery? Small 2011, 7, 2301–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Cheng Y; Meyers JD; Broome AM; Kenney ME; Basilion JP; Burda C Deep Penetration of a PDT Drug into Tumors by Noncovalent Drug-Gold Nanoparticle Conjugates. J. Am. Chem. Soc 2011, 133, 2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Meyers JD; Cheng Y; Broome AM; Agnes RS; Schluchter MD; Margevicius S; Wang X; Kenney ME; Burda C; Basilion JP Peptide-Targeted Gold Nanoparticles for Photodynamic Therapy of Brain Cancer. Part. Part. Syst. Charact 2015, 32, 448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Mangadlao JD; Wang X; McCleese C; Escamilla M; Ramamurthy G; Wang Z; Govande M; Basilion JP; Burda C Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Evans JC; Malhotra M; Cryan JF; O’Driscoll CM The Therapeutic and Diagnostic Potential of the Prostate Specific Membrane Antigen/Glutamate Carboxypeptidase II (PSMA/GCPII) in Cancer and Neurological Disease. Br. J. Pharmacol 2016, 173, 3041–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Mhawech-Fauceglia P; Zhang S; Terracciano L; Sauter G; Chadhuri A; Herrmann FR; Penetrante R Prostate-Specific Membrane Antigen (PSMA) Protein Expression in Normal and Neoplastic Tissues and Its Sensitivity and Specificity in Prostate Adenocarcinoma: An Immunohistochemical Study Using Mutiple Tumour Tissue Microarray Technique. Histopathology 2007, 50, 472–483. [DOI] [PubMed] [Google Scholar]
- (22).Weiss-Sadan T; Ben-Nun Y; Maimoun D; Merquiol E; Abd-Elrahman I; Gotsman I; Blum G A Theranostic Cathepsin Activity-Based Probe for Noninvasive Intervention in Cardiovascular Diseases. Theranostics 2019, 9, 5731–5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Tsvirkun D; Ben-Nun Y; Merquiol E; Zlotver I; Meir K; Weiss-Sadan T; Matok I; Popovtzer R; Blum G CT Imaging of Enzymatic Activity in Cancer Using Covalent Probes Reveal a Size-Dependent Pattern. J. Am. Chem. Soc 2018, 140, 12010–12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Gong F; Yang N; Wang X; Zhao Q; Chen Q; Liu Z; Cheng L Tumor Microenvironment-Responsive Intelligent Nano-platforms for Cancer Theranostics. Nano Today 2020, 32, 100851. [Google Scholar]
- (25).Kinsella TJ; Baron ED; Colussi VC; Cooper KD; Hoppel CL; Ingalls ST; Kenney ME; Li X; Oleinick NL; Stevens SR; Remick SC Preliminary Clinical and Pharmacologic Investigation of Photodynamic Therapy with the Silicon Phthalocyanine Photosensitizer Pc 4 for Primary or Metastatic Cutaneous Cancers. Front. Oncol 2011, 1, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Li J Silicon Phthalocyanines for Photodynamic Therapy Studies. Ph.D. Thesis Case Western Reserve University, Cleveland, OH, 2008. [Google Scholar]
- (27).Wang X; Huang SS; Heston WD; Guo H; Wang BC; Basilion JP Development of Targeted Near-Infrared Imaging Agents for Prostate Cancer. Mol. Cancer Ther 2014, 13, 2595–2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Wang X; Tsui B; Ramamurthy G; Zhang P; Meyers J; Kenney ME; Kiechle J; Ponsky L; Basilion JP Theranostic Agents for Photodynamic Therapy of Prostate Cancer by Targeting Prostate-Specific Membrane Antigen. Mol. Cancer Ther 2016, 15, 1834–1844. [DOI] [PubMed] [Google Scholar]
- (29).Hong R; Han G; Fernádez JM; Kim B; Forbes NS; Rotello VM Glutathione-Mediated Delivery and Release Using Monolayer Protected Nanoparticle Carriers. J. Am. Chem. Soc 2006, 128, 1078–1079. [DOI] [PubMed] [Google Scholar]
- (30).Luo D; Wang X; Zeng S; Ramamurthy G; Burda C; Basilion JP Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Prostate Cancer Radiotherapy: Does Size Matter for Targeted Particles? Chem. Sci 2019, 10, 8119–8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Luo D; Wang X; Zeng S; Ramamurthy G; Burda C; Basilion JP Targeted Gold Nanocluster-Enhanced Radiotherapy of Prostate Cancer. Small 2019, 15, 1900968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Morris RL; Azizuddin K; Lam M; Berlin J; Nieminen A; Kenney ME; Samia ACS; Burda C; Oleinick NL Fluorescence Resonance Energy Transfer Reveals a Binding Site of a Photosensitizer for Photodynamic Therapy. Cancer Res. 2003, 63, 5194–5197. [PubMed] [Google Scholar]
- (33).Ricchelli F; Nikolov P; Gobbo S; Jori G; Moreno G; Salet C Interaction of Phthalocyanines with Lipid Membranes: A Spectroscopic and Functional Study on Isolated Rat Liver Mitochondria. Biochim. Biophys. Acta, Biomembr 1994, 1196, 165–171. [DOI] [PubMed] [Google Scholar]
- (34).Cheng Y; Samia AC; Li J; Kenney ME; Resnick A; Burda C Delivery and Efficacy of a Cancer Drug as a Function of the Bond to the Gold Nanoparticle Surface. Langmuir 2010, 26, 2248–2255. [DOI] [PubMed] [Google Scholar]
- (35).Chithrani BD; Chan WCW Elucidating the Mechanism of Cellular Uptake and Removal of Protein-Coated Gold Nanoparticles of Different Sizes and Shapes. Nano Lett. 2007, 7, 1542–1550. [DOI] [PubMed] [Google Scholar]
- (36).Mosquera J; Garcia I; Liz-Marzan LM Cellular Uptake of Nanoparticles versus Small Molecules: A Matter of Size. Acc. Chem. Res 2018, 51, 2305–2313. [DOI] [PubMed] [Google Scholar]
- (37).Master AM; Rodriguez ME; Kenney ME; Oleinick NL; Gupta AS Delivery of the Photosensitizer Pc 4 in PEG-PCL Micelles for in Vitro PDT Studies. J. Pharm. Sci 2010, 99, 2386–2398. [DOI] [PubMed] [Google Scholar]
- (38).Sykes EA; Chen J; Zheng G; Chan WCW. Investigating the Impact of Nanoparticle Size on Active and Passive Tumor Targeting Efficiency. ACS Nano 2014, 8, 5696–5706. [DOI] [PubMed] [Google Scholar]
- (39).Donahue ND; Acar H; Wilhelm S Concepts of Nanoparticle Cellular Uptake, Intracellular Trafficking, and Kinetics in Nanomedicine. Adv. Drug Delivery Rev 2019, 143, 68–96. [DOI] [PubMed] [Google Scholar]
- (40).Maruoka Y; Nagaya T; Sato K; Ogata F; Okuyama S; Choyke PL; Kobayashi H Near Infrared Photoimmunotherapy with Combined Exposure of External and Interstitial Light Sources. Mol. Pharmaceutics 2018, 15, 3634–3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Kundu K; Padhye N; Volcheck W Application of Reduced Dyes in Imaging. U.S. Patent 9,470,691, 2016.
- (42).Zhu H; Li J; Qi X; Chen P; Pu K Oxygenic Hybrid Semiconducting Nanoparticles for Enhanced Photodynamic Therapy. Nano Lett. 2018, 18, 586–594. [DOI] [PubMed] [Google Scholar]
- (43).Wang X; Ramamurthy G; Shirke AA; Walker E; Mangadlao J; Wang Z; Wang Y; Shan L; Schluchter MD; Dong Z; Brady-Kalnay SM; Walker NK; Gargesha M; MacLennan G; Luo D; Sun R; Scott B; Roy D; Li J; Basilion JP Photodynamic Therapy Is an Effective Adjuvant Therapy for Image-Guided Surgery in Prostate Cancer. Cancer Res. 2020, 80, 156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Sato K; Ando K; Okuyama S; Moriguchi S; Ogura T; Totoki S; Hanaoka H; Nagaya T; Kokawa R; Takakura H; Nishimura M; Hasegawa Y; Choyke PL; Ogawa M; Kobayashi H Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci 2018, 4, 1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Gao W; Wang Z; Lv L; Yin D; Chen D; Han Z; Ma Y; Zhang M; Yang M; Gu Y Photodynamic Therapy Induced Enhancement of Tumor Vasculature Permeability Using an Upconversion Nanoconstruct for Improved Intratumoral Nanoparticle Delivery in Deep Tissues. Theranostics 2016, 6, 1131–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Zhen Z; Tang W; Chuang YJ; Todd T; Zhang W; Lin X; Niu G; Liu G; Wang L; Pan Z; Chen X; Xie J Tumor Vasculature Targeted Photodynamic Therapy for Enhanced Delivery of Nanoparticles. ACS Nano 2014, 8, 6004–6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Li J; Yang Y; Zhang P; Sounik JR; Kenney ME Synthesis, Properties and Drug Potential of the Photosensitive Alkyl- and Alkylsiloxy-Ligated Silicon Phthalocyanine Pc 227. Photochem. Photobiol Sci 2014, 13, 1690–1698. [DOI] [PubMed] [Google Scholar]
- (48).Cheng Y; Samia AC; Meyers JD; Panagopoulos I; Fei B; Burda C Highly Efficient Drug Delivery with Gold Nanoparticle Vectors for in Vivo Photodynamic Therapy of Cancer. J. Am. Chem. Soc 2008, 130, 10643–10647. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


