Abstract
Background.
Adult immunocompetent male C57BL/6 mucopolysaccharidosis, type I (MPSI) mice develop aortic insufficiency (AI), dilated ascending aortas and decreased cardiac function, findings not observed in immune incompetent adult male NSG MPSI mice. We sought to determine why.
Methods.
Cardiac ultrasound measurements of ascending aorta and left ventricular dimensions and Doppler interrogation for AI were performed in 6-month-old male B6 MPSI (N=12), WT (N=6), NSG MPSI (N=8), NSG (N=6) mice. Urinary glycosaminoglycans, RNA sequencing with quantitative PCR were performed and aortic pathology assessed by routine and immunohistochemical staining on subsets of murine aortas.
Results.
Ascending aortic diameters were significantly greater, left ventricular function significantly decreased, and AI significantly more frequent in B6 MPSI mice compared to NSG MPSI mice (p <0.0001, p=0.008 and p=0.02, respectively); NSG and B6 WT mice showed no changes. Urinary glycosaminoglycans were significantly greater in B6 and NSG MPSI mice and both were significantly elevated compared to WT controls (p=0.003 and p<0.0001, respectively). By RNA sequencing, all 11 components of the inflammasome pathway were upregulated in B6 MUT, but only Aim2 and Ctsb in NSG MUT mice and none in WT controls. Both B6 and NSG MUT mice demonstrated variably-severe intramural inflammation, vacuolated cells, elastin fragmentation and disarray, and intense glycosami noglycans on histological staining. B6 MPSI mice demonstrated numerous medial MAC2+ macrophages and adventitial CD3+ T-cells while MAC2+ macrophages were sparse and CD3+ T-cells absent in NSG MPSI mice.
Conclusions.
Aortic dilation, AI and decreased cardiac function occur in immunocompetent B6 MPSI male mice but not in immune incompetent NSG MPSI mice, unrelated to GAG excretion, upregulation of Ctsb, or routine histologic appearance. Upregulation of all components of the inflammasome pathway in B6 MUT, but not NSG MUT mice, and abundant medial MAC2 and adventitial CD3 infiltrates in B6, but not NSG, MPSI aortas differentiated the two strains. These results suggest that the innate and adaptive immune systems play a role in these cardiac findings which may be relevant to human MPSI.
Keywords: mucopolysaccharidosis, thoracic aortic aneurysm, inflammasome, cathepsin, innate immunity, adaptive immunity, NSG mouse
1.0. Introduction
Mucopolysaccharidosis, type I (MPS), is an inherited progressive, autosomal recessive lysosomal storage disease affecting somatic and central nervous system tissue, leading to early mortality if left untreated. Hematopoietic cell transplantation (HCT) and enzyme replacement therapy (ERT) have significantly altered the natural history of the disease. Individuals with severe MPS I (Hurler syndrome, MPS IH) who underwent successful HCT more than 35 years ago survive well past their historical life expectancy (1) and the life span of those with attenuated MPS I has been prolonged by ERT (2). Despite these encouraging results, neither of these therapies is curative. Skeletal and cardiac valve abnormalities can persist despite successful HCT or long term ERT and lead to the need for repeated orthopedic intervention and, on occasion, cardiac valve replacement.
In addition to cardiac valve regurgitation and stenosis, aortic root dilation is common in all types of human MPS (3, 4) and there are contradictory results of its ability to be corrected by either hematopoietic cell transplantation or enzyme replacement therapy. As survival of individuals with treated MPS now extends well into young adulthood, the potential for progression of this dilation into an ascending aortic aneurysm is currently unknown although recently the first known adult MPS patient underwent aortic root replacement for respiratory symptoms (5). Ascending aortic aneurysms in non-MPS individuals are both a common and life threatening disease and, in contrast to abdominal aortic aneurysms, are due to genetic mutations of the contractile apparatus of the aorta in upwards of 30% of cases (6) There is currently no medical treatment to reverse ascending aortic aneurysms and the indications for prophylactic aortic root replacement depend upon underlying specific genetic mutations (7). There are no reliable biomarkers to track the progression of ascending aortic aneurysms other than imaging (6). This disease is considered a silent killer as the first symptom is often catastrophic aortic dissection, requiring emergency ascending aortic root replacement performed with a high operative mortality. Murine models of MPS I have the potential to provide insight into this problem since ascending aortic dilation and aortic insufficiency are commonly present in the adult male C57BL/6 mouse constructed with the functional absence of alpha-1-iduronidase (IDUA−/−) genes (8, 9).
The IDUA−/− mouse, first reported by Clarke et al in 1997 (10) and Ohmi et al in 2003 (11), were bred onto the C57BL/6 background strains. All C57BL/6 background strains are thought to be immune competent with excellent wound healing (12) although significant strain-dependent differences in the C57BL/6 phenotype exist (13, 14). Clarke reported that the C57BL/6 IDUA−/− (B6 MUT) mouse had a shortened lifespan, urinary GAG excretion 1.5- to 3-fold greater than controls, GAG accumulation in all tissues and pronounced skeletal and CNS abnormalities (15). We subsequently reported that ascending aortic dilation and aortic insufficiency, increased myocardial GAG content and left ventricular end-diastolic diameter, as well as significantly decreased left ventricular function are universally seen in male B6 MUT mice by 6–8 months of age (8, 9).The invariable presence of a dilated ascending aorta in these male mice makes the model attractive as a biomarker for therapeutic testing strategies that could have potential for human MPS as we and others have noted that the use of angiotensin receptor blockers delays the onset of dilation (16, 17).
In 2015 a long-lived immune-deficient IDUA−/− mouse model was developed by Mendez et al (18) on a NOD/SCID/Il2rγ (NSG) background. This NSG IDUA−/− (NSG MUT) mouse was reported to have absence of IDUA in all tissues examined and the typical phenotypic features of MPS I, including corneal clouding, kyphosis and facial features. The model was envisaged to be suitable for preclinical testing of stem-cell based therapies. The background strain upon which this IDUA−/− model was constructed is extremely immunodeficient, carrying both the severe combined immune deficiency (scid) mutation and a complete null allele of the IL2 receptor common gamma chain (IL2rgnull) (19). These mice lack mature T cells, B cells and NK cells, are long-lived and resist lymphoma development. Additionally, the NOD/LtSz scid/scid mouse, one of the two parent strains used to develop the NSG model, is known to have functionally immature macrophages that show decreased IL-1 secretion in response to lipopolysaccharide stimulation (20).
With plans to use the NSG MUT mouse for ongoing projects, we performed cardiac ultrasounds on adult male NSG MUT mice and were surprised to find the absence of aortic dilation, normal cardiac function and only rare and mild aortic insufficiency. As this was reminiscent of ultrasound findings we had previously reported in short-lived NOD/SCID IDUA−/− mice (21), we sought to discover why the immune-incompetent NSG MUT mouse would be devoid of the cardiac phenotype commonly seen in the immune-competent B6 MUT mouse. Studies of urinary GAG excretion, cardiac ultrasounds, RNA sequencing, Nanostring PCR, routine- and immunohistopathology were performed on adult wild type (WT) and IDUA−/− (MUT) male mice from both C57BL/6 (B6) and NSG strains. These studies, described below, have led us to the conclusion that the inflammasome pathway and the resulting adaptive immune response are involved in the development of aortic dilation in the immune-competent B6 MUT mouse and likely account for its absence in the immune-incompetent NSG MUT mouse. This information may be key to understanding the inflammatory mechanisms underlying ascending aortic dilation in human MPS and possibly in ascending aortic aneurysms in general.
2.0. Materials and Methods
2.1. Mice.
All animal care and handling procedures were approved by and in compliance with the Institutional Animal Care and Use Committee (IACUC) of the University of Minnesota. All study mice were obtained from existing breeding colonies maintained at the University of Minnesota.
The immune-competent MPS I mouse model (IDUA−/−), originally a kind gift from Dr. Elizabeth Neufeld, was constructed on a C57BL/6NHsd (B6N) background strain (Harlan Sprague Dawley, now Envigo, Indianapolis, IN) through disruption of the IDUA gene by insertion of the neomycin resistance gene on a tk (herpes simplex virus thymidine kinase) promoter into the unique BstEII site (blunt-ended in exon 6, in the opposite orientation). Immune-competent B6N IDUA −/− (MUT) and B6N IDUA +/+ (WT) offspring were obtained from ongoing breeding of C57BL/6NHsd IDUA (+/−) heterozygous mice for use in the study.
The immune-incompetent MPS I mouse model (NSG IDUA−/−) was generated from breeding of female NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (strain 005557 (‘NSG’), Jackson Labs, Bar Harbor, ME) mice to heterozygous male NOD.Cg-Prkdcscid Iduatm1Clk/J (strain 004083, Jackson Labs, Bar Harbor, ME) mice as the IL2rg is an X-linked gene. Immune-incompetent NSG IDUA −/− (MUT) and IDUA +/+ (WT) offspring were obtained from ongoing breeding of NSG IDUA (+/−) heterozygous mice for use in the study.
All studies were carried out on adult male mice in the following four study groups: immune-competent B6N MUT (N= 12) and WT (N=6) mice; and immune-incompetent NSG MUT(N=8) and IDUA WT (N=6) mice (Figure 1). At the conclusion of cardiac ultrasound, 22 mice were euthanized for RNA sequencing and 10 mice for routine histopathology and immuno-histochemistry. Verification of mutant or wild type status with respect to IDUA and DOCK2 mutations was made through genotype of tail or ear clippings, or myocardium (Transnetyx®, Cordova, TN).
Figure 1.

Anthropometric and urinary GAG data for murine cohort with findings as described in the text.
2.2. Urinary glycosaminoglycans.
Urine was collected from mice at the time of sacrifice and stored at −20°C until analysis. GAG levels were measured using the Blyscan Sulfated Glycosaminoglycan Assay kit (Biocolor Life Science Assays, Accurate Chemical, NY Inc. #CLRB1000)according to the manufacturer’s protocol. GAG level measurements were normalized to urinary creatinine, measured using the Creatinine Assay Kit (Sigma, MAK080) according to the manufacturer’s instructions. Both colorimetric assays were read on a Synergy Mx plate reader (Biotek) by measuring absorbance at 656nm and 570nm respectively (1). Urinary GAGs were reported as GAG ug/mg Creatinine.
2.3. High Resolution Cardiac Ultrasound.
High resolution cardiac ultrasound was performed on all 32 mice, anesthetized with 1–2% inhaled isoflurane, by a single murine sonographer (EB) using a Vevo 2100 (Fujifilm/VisualSonics, Toronto, ON, Canada) with 30–40 MHz transducers and Vevo LAB 3.1.1. analysis software, maintained at the University Imaging Centers. Mouse temperature, heart rate and respiratory rate were constantly monitored during the procedure. At the conclusion of the imaging mice recovered quickly from anesthesia and were returned to their cages.
Ascending aortic (Asc Ao) dimensions were made from a modified high right parasternal view at the level of the right pulmonary artery during peak expansion of the aorta. Pulse-wave and color Doppler interrogation beneath the aortic valve and in the descending thoracic aorta was performed for determination of aortic insufficiency (AI) and descending aortic flow reversal (‘run-off’, RO). Measurement of aortic sinus (Sinus) and sinotubular ridge (STR) were made by two-dimensional imaging performed from a modified apical view during maximal valve opening. Left ventricular chamber dimensions in diastole (LVID) and systole (LVIS) with subsequent calculation of shortening fraction (SF) were obtained in parasternal long- and short-axes by two-dimensional B- and M-mode imaging. Left ventricular dimensions and calculation of ventricular function were made as mice emerged from anesthesia and achieved heart rates of 500 beats/minute (BPM). All linear measurements were made in triplicate and values averaged.
2.4. RNA Extraction.
Within one to two days of cardiac ultrasound, the mice were euthanized. Ascending aortas of 22 mice were quickly removed and placed in RNAlater (Qiagen USA, Germantown, MD) solution for 24 hours at room temperature. The aortas were then dissected free of all perivascular tissue while submerged in RNAlater; the ascending aortic segment from the sinotubular ridge to the innominate artery was isolated and returned to RNAlater solution for subsequent mRNA extraction (Extraction Services; University of Minnesota Genomics Center, Minneapolis, MN) using RNeasy Plus Universal Mini Kit, according to RNEasy® Plus Universal Handbook, December 2014. Tissue samples were homogenized. After addition of gDNA Eliminator Solution and chloroform, the homogenate was separated into aqueous and organic phases by centrifugation. RNA partitioned to the upper, aqueous phase, while DNA partitioned to the interphase and proteins to the lower, organic phase or the interphase. The upper, aqueous phase was collected, mixed with ethanol and RNA was purified using spin columns. Total RNA binds to the spin column membrane, and high-quality RNA was then eluted in RNase-free water and stored at −80°C for further use.
2.5. RNA Sequencing.
Total RNA from murine samples was extracted and quantified using a fluorometric RiboGreen assay. Total RNA integrity (RIN) was assessed using capillary electrophoresis (e.g., Agilent BioAnalyzer 2100), generating an RNA Integrity Number (RIN) with at least 250 pg total RNA and RIN>2–3 recommended for quality control by the library preparation kit manufacturer. Illumina sequencing libraries were created using Takara Bio’s SMARTer Stranded Total RNA-Seq-Pico Mammalian Kit v2 (Cat. # 634414) with final library size distribution being validated by capillary electrophoresis and quantified by fluorimetry (PicoGreen). RNA sequencing was performed using either HiSeq 2500 or Novaseq instrumentation and base call files for run-each sequencing were generated by Illumina Real Time Analysis software. Base call files and run folders were exported to servers at the Minnesota Supercomputing Institute where they were subsequently analyzed (JAL) through the use of Ingenuity Pathway Analysis (IPA) (QIAGEN Inc., https://www.qiagenbioinformatics.com/products/ingenuitypathway-analysis)(22).
2.6. Analysis of RNA sequencing output.
Pathway analysis was done in Ingenuity Pathway Analysis (IPA) using gene symbols, fold change and Bonferroni corrected values. Canonical pathways analysis identified the pathways from the Ingenuity Pathway Analysis library of canonical pathways that were most significant to the data set. Molecules from the data set that met the cutoff of and were associated with a canonical pathway in the Ingenuity Knowledge Base were considered for the analysis. The significance of the association between the data set and the canonical pathway was measured in two ways: 1) A ratio of the number of molecules from the data set that map to the pathway divided by the total number of molecules that map to the canonical pathway is displayed; and 2) A right-tailed Fisher’s Exact Test was used to calculate a p-value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone.
2.7. Nanostring PCR.
Quantitative PCR was carried out at the University Genomics Center using NanoString technology measurement of candidate genes (NanoString Technologies, Seattle, WA) according to the manufacturer’s instructions. Secondary verification of results by Low-input RTPCR was made for all candidate genes.
2.8. Histology.
Within one to 5 days of cardiac ultrasound examination, the mice were euthanized. Heart and great vessels were removed and placed in 10% formalin for 24 hours. On the following day, the ascending aorta was dissected free from the heart and placed separately into 70% ethanol. Ascending aortas were subsequently processed and cut into 4 micron thickness sections for routine histology (hematoxylin-eosin, elastin, trichrome and Alcian blue) and immunohistochemistry. Immunohistochemical staining was performed on a Dako Autostainer (Dako, Carpinteria, CA) with F4/80 and MAC2 (Cedarlane Labs; Burlington, ON, Canada), Iba1 (abcam; Cambridge, MA), B220 (BD; Franklin Lakes, NJ) and CD3 (Dako) antibodies. Slides were reviewed by a two experienced veterinary histopathologists (DS, GOS), one of whom (DS) was blinded to mouse identification.
2.9. Statistical Analysis.
Three different comparisons were made: 1) comparisons by strain (B6 WT versus NSG WT; B6 MUT versus NSG MUT); 2) comparisons of the effect of the IDUA mutation on the specific strain (B6 WT versus B6 MUT; NSG WT versus NSG MUT); and finally, 3) comparisons of the difference between WT and MUT for each strain (Δ B6 [WT-MUT] versus Δ NSG [WT-MUT]) to assess the effect of the IDUA mutation on each strain while minimizing other differences between the B6 and NSG strains. Linear models were fit for each continuous outcome, with terms for strain (B6 or NSG), IDUA mutation (WT or MUT), strain-by-mutation interaction, and (for echocardiogram measures) body weight. From these models, comparisons of strain effects (B6 WT versus NSG WT, B6 MUT versus NSG MUT), mutation effects (B6 WT versus B6 MUT, NSG WT versus NSG MUT), and difference-of-differences (Δ B6 [WT-MUT] versus Δ NSG [WT-MUT]) were obtained. Results are reported using estimated marginal means with 95% confidence intervals. Categorical outcomes were compared by strain and IDUA mutation using Fisher’s exact test, and difference-in-differences for categorical outcomes were obtained from the interaction test from a Firth’s penalized logistic regression model with strain, mutation, and strain-by-mutation interaction terms. Analyses were conducted using R version 3.6.1 (R Foundation for Statistical Computing, Vienna, Austria).
3.0. Results
3.1. B6 MPSI MUT mice have dilated ascending aortas and aortic insufficiency while NSG MPSI MUT mice do not.
Anthropometric, urinary GAG, and cardiac ultrasound data for all 32 study mice, including WT and MUT immunocompetent B6 mice as well as WT and MUT immunodeficient NSG mice are shown in Figures 1–3, and online Supplemental Table 1. Study mice ranged from 166 – 201 (average 191 ± 6.91) days of age at the time of cardiac ultrasound. Although B6 MUT mice were, on average, one week older than the remaining three cohorts (Figure 1a) this difference was considered to be physiologically insignificant and not further investigated as all were within the age range considered to be adult, but not yet middle-aged (23). B6 WT and MUT mice were significantly larger than their respective NSG counterparts (Figure 1b) and within each strain, MUT mice were significantly larger than WT mice.
Figure 3.

(a-f). Images showing: a) dilated ascending aorta (arrow), b) aortic insufficiency by color and high velocity holodiastolic Doppler signal (arrows), and c) descending thoracic aortic flow reversal (‘run-off’) (arrow) in B6 MUT mice; with d) normal ascending aortic dimension (arrow), e) no aortic insufficiency and f) no runoff in NSG MUT mice.
Urinary GAG excretion did not differ significantly between B6 and NSG WT mice (Figure 1c); urinary GAG excretion was significantly increased in both NSG and B6 MUT mice when compared to their respective WT counterparts but there was no significant difference in the increase of urinary GAG excretion (WT to MUT difference) between strains. There were no significant differences for average heart rate when mice emerged from anesthesia other than NSG MUT mice having significantly slower heart rate than NSG WT (Figure 1d).
Left ventricular dimensions and function.
Left ventricular and aortic root dimensions were weight-adjusted for intrinsic differences in size between the two strains (Figure 2). There were no significant weight-adjusted differences in left ventricular dimensions in diastole or systole (LVID, LVIS) among any of the cohorts (Figures 2a and b).
Figure 2.
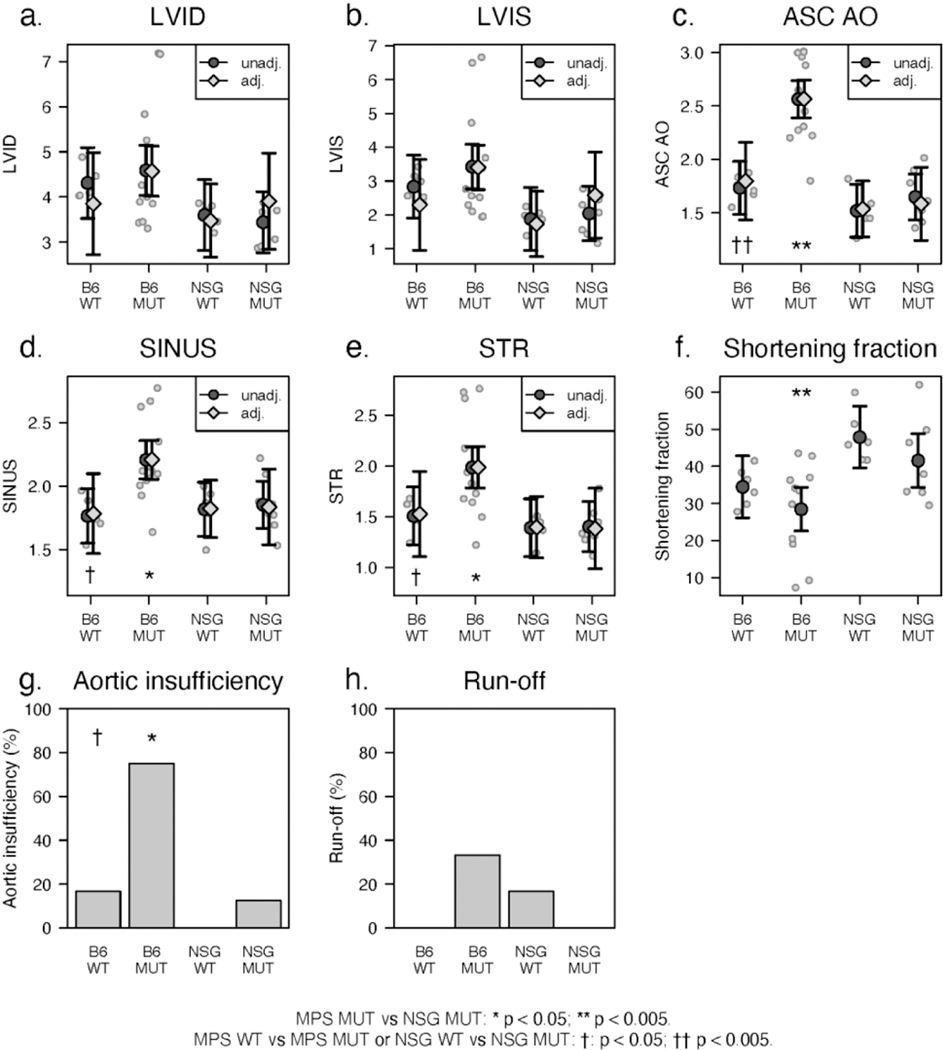
(a-h). Cardiac ultrasound measurements, shortening fractions and Doppler interrogations of aortic valve and descending thoracic aorta with findings as described in the text.
Average shortening fraction, a measure of left ventricular systolic function, (Figure 2f) was within the normal range for B6 WT and MUT mice but was significantly increased for both NSG WT and MUT mice when compared to their respective B6 counterparts and to published normal values (24).
Aortic root and ascending aorta.
Weight-adjusted diameters of the aortic root (SINUS, STR) and ascending aorta (ASC AO) of WT B6 and NSG mice did not differ significantly from each other (Figure 2c-e). Each of these aortic measurements increased significantly in B6 MUT mice when compared to their respective WT counterparts. Aortic dimensions remained within normal limits in NSG MUT mice when compared to their WT controls. Doppler- and color-flow interrogation of the aortic valve for valve insufficiency (AI) and descending thoracic aorta for flow reversal (RUNOFF) showed that AI and RUNOFF were uncommon in WT mice from either strain (Figure 3g,h). The occurrence of aortic insufficiency was significantly increased only in B6 MUT mice (Figure 3h). Aortic RUNOFF remained uncommon in all mice. Representative cardiac ultrasound images from B6 and NSG MPSI are shown in Figure 3.
3.2. Differential expression of mRNA was found in all components of the NLRP3 inflammasome pathway in B6 [WT-MUT], but not the NSG [WT-MUT] murine aortic tissue.
RNA sequencing was performed on the ascending aortas of 22 mice. RNA Integrity Numbers (RINs) were within the manufacturer’s suggested range except for one NSG WT mouse, which nonetheless, produced sequencing data indistinguishable from the other 3 NSG WT mice, and was included in the analysis. A comparison of the RNA transcript differences between B6 WT and MUT aortas and NSG WT and MUT aortas were constructed. There were 1817 transcripts identified with significant p-values <0.05 and false discovery rates of <0.05 in the B6 MUT mice when compared to their WT controls, and 348 mapped genes in the NSG IDUA MUT mice when compared to their respective WT controls (Supplementary Tables 2A and 2B).
A comparison was then made of the differential expression between B6 and NSG mice, i.e., 1817 [B6 WT to MUT] gene differentially expressed transcripts were compared to the 348 [NSG WT to MUT] differentially expressed transcripts. The resulting top five pathways with significant pvalues and Z-scores for both [B6 WT to MUT] and [NSG WT to MUT] mice included: the neuroinflammation signaling pathway, production of nitric oxide and reactive oxygen species in macrophages, role of NFAT in regulation of the immune response, dendritic cell maturation and TREM1 signaling (Table 1).
Table 1.
Top five canonical pathways upregulated in both B6 [WT to MUT] and NSG [WT to MUT] mice
| Pathway | B6 WT to MUT | NSG WT to MUT |
|---|---|---|
| p-value | p-value | |
| B-H value | B-H value | |
| Z-score | Z-score | |
| Neuroinflammation signaling | 4.97E-12 | 2.15E-06 |
| 5.1E-10 | 7.33E-05 | |
| 6.303 | 3.638 | |
|
| ||
| Production of nitric oxide | 3.17E-11 | 3.08E-06 |
| 2.32E-09 | 9.59E-05 | |
| 5.06 | 3.464 | |
|
| ||
| Role of NFAT in regulation of the immune response | 2.26E-08 | 1.21E-05 |
| 4.14E-07 | 2.26E-04 | |
| 5 | 3.464 | |
|
| ||
| Dendritic cell maturation | 4.31E-14 | 1.64E-09 |
| 7.36E-12 | 1.02E-05 | |
| 4.938 | 2.887 | |
|
| ||
| TREM1 signaling | 1.23E-10 | 3.52E-06 |
| 5.73E-09 | 9.59E-05 | |
| 4.707 | 3 | |
Upon further examination of each of these pathways, we noted that, despite upregulation of the total pathway, there were fewer genes upregulated in the NSG pair than the B6 pair. For example, for the neuroinflammation pathway, 63 genes exhibited a change of expression in the [B6 WT to MUT] pair, but only 18 were changed in the [NSG WT to MUT] pair (Supplemental Table 3). Given this finding, we then sought canonical pathways where enrichment had occurred only in the [B6 WT to MUT] dataset with no comparable enrichment within NSG [WT to MUT] dataset (i.e., B-H value was insignificant in both NSG WT and NSG MUT pathway, indicating no significant difference in the pathway between WT and MUT mice).
The top 6 canonical pathways (z-score >3 and p-value E-02) where RNA transcripts were enriched in the B6 pair but not the NSG pair included: Type I Diabetes Mellitus signaling pathway, the inflammasome pathway, acute phase response pathway, IL-6 pathway, iNOS pathway and the interferon signaling pathway (Table 2). Due to its known involvement in thoracic aortic aneurysm development (25), the inflammasome pathway was further evaluated (Table 3). Inflammasomes are ancient cytosolic defense pathways found in plants and mammals, that are central to an organism’s early response to pathogen or stress signals and are key components of innate immunity (26).
Table 2.
Top 6 canonical pathways significantly upregulated in B6 [WT to MUT] pair, but not NSG [WT to MUT] pair.
| Pathway | B6 WT to MUT | NSG WT to MUT |
|---|---|---|
| p-value | p-value | |
| B-H value | B-H value | |
| Z-score | Z-score | |
| Type I Diabetes Mellitus Signaling | 1.12E-06 | 1.08E-01 |
| 1.19E-05 | 2.33E-01 | |
| 3.873 | N/A | |
|
| ||
| IL-6 Signaling | 1.55E-03 | 3.69E-01 |
| 5.39E-03 | 4.88E-01 | |
| 3.71 | N/A | |
|
| ||
| Inflammasome Pathway | 1.476E-07 | 4.41E-02 |
| 2.05E-06 | 1.43E-01 | |
| 3.319 | N/A | |
|
| ||
| iNOS Signaling | 1.66E-03 | iNOS signaling is |
| 5.71E-03 | not involved in | |
| 3.162 | NSG 354 data set | |
|
| ||
| Acute Phase Response Signaling | 5.82E-04 | 3.45E-01 |
| 2.53E-03 | 4.69E-01 | |
| 3.13 | N/A | |
|
| ||
| Interferon Signaling | 1.43E-03 | 4.27E-01 |
| 5.08E-03 | 4.69E-01 | |
| 3 | N/A | |
Table 3.
Gene expression in the inflammasome pathway for B6 [WT to MUT] and NSG [WT to MUT] datasets.
| Gene Symbol | B6 [WT-MUT] dataset | NSG [WT-MUT] dataset |
|---|---|---|
| Expression fold change (Z-score) | Expression fold change (Z-score) | |
| Expression false discovery rate | Expression false discovery rate | |
| Aim2 | 13.882 | 28.069 |
| 5.81E-16 | 0.000733 | |
|
| ||
| Casp1 | 8.809 | |
| 2.13E-09 | ||
|
| ||
| Casp8 | 2.358 | |
| 0.000000141 | ||
|
| ||
| Ctsb | 3.032 | 4.8 |
| 1.56E-18 | 0.00125 | |
|
| ||
| Naip2 | 5.15 | |
| 4.93E-08 | ||
|
| ||
| Nfkb2 | 2.006 | |
| 0.00247 | ||
|
| ||
| Nlrc4 | 4.629 | |
| 0.00816 | ||
|
| ||
| Nlrp1b | 12.317 | |
| 1.65E-18 | ||
|
| ||
| Nlrp3 | 4.745 | |
| 0.000654 | ||
|
| ||
| P2rx7 | 3.397 | |
| 3.02E-20 | ||
|
| ||
| Pycard | 5.142 | |
| 3.06E-10 | ||
Absence of values for NSG [WT -MUT] pair indicates that there was no significant different by Bonferroni between WT and MUT values, i.e – gene expressions were not significantly different.
Of the 11 genes within the pathway, two (cathepsin B (Ctsb) and Absent in Melanoma 2 (Aim2)) were significantly enriched in both B6 [WT to MUT) and NSG [WT to MUT] datasets while the remaining nine genes were significantly upregulated in only the B6 [WT to MUT] dataset (Table 3). The 11 genes enriched in the B6 [WT to MUT] pair include: components of four inflammasomes (Nlrp3, Aim2, Naip/Nlrc4, Nlrp1, Pycard, Casp1), imputed activators of the inflammasome (P2×7, Ctsb), and a nuclear (Nf-κb) and a cytoplasmic (Casp8) regulator of inflammasomes (Figure 4).
Figure 4.

Upregulation of inflammasome pathway in B6 [WT-MUT] and NSG [WT-MUT] murine aortas (22).
Despite the difference in upregulation of inflammasome components and in the Il6 pathway (online Supplemental Table 4), no significant differences were found in isolated Il1b, Il18 and Il6 mRNA expression (data not shown). However, in conjunction with upregulation of Il1 and Il18 receptors, the Il1, and Il6 clusters were upregulated in the IL6 pathway only for B6 [WT-MUT] cohort (online Supplemental Figure 1A and B).
3.3. Cathepsin B mRNA was increased in both B6 and NSG [WT-MUT] aortic tissue by PCR
Quantitative PCR nCounter gene expression of the inflammasome targets and three housekeeping genes are shown in Figure 5A and 5B. When normalized absolute values of mRNA of each group for each gene are seen, (Figure 5A), the ~4-fold greater quantity of Ctsb mRNA in both B6 and NSG strains in comparison to the remaining inflammasome targets is apparent. By fold-change analysis (Figure 5B, Table 4) the inflammasome components Naip and Nlrc4 were significantly upregulated in the B6 [WT to MUT] versus the NSG [WT-MUT] pair (p=.0159 and 0.0483, respectively). There was a trend for preferential upregulation of the B6 [WT – MUT] pair compared to the NSG pair for inflammasome components Casp1 (p=0.1238), NfKb2 (p=0.1364), Nlrp1 (p=0.0983), Nlrp3 (p=0.0583), and P2rx7 (p=0.0750). (see Table 4). There was no significant difference in upregulation of Aim2 (p=0.8752), Ctsb (p=0.5808), Pycard (0.7832), or Gapdh (p=0.8720). As noted for RNA sequencing, there was no upregulation noted for Il1, IL18 or IL6 by N Counter PCR. Two highly variable housekeeping genes (Hprt and Pgk1) were downregulated in the B6 versus the NSG pair (p=0.0585, p=0.0365, respectively) but are included for completeness.
Figure 5A.

NCounter PCR normalized absolute RNA values for B6 WT (light peach), B6 MUT (dark peach), NSG WT (light blue) and NSG MUT (darker blue) aortic tissue RNA. Note the 3–4-fold difference in Ctsb values from other inflammasome components.
Figure 5B. Fold changes for inflammasome and housekeeping genes for B6[WT-MUT] (peach) and NSG[WT-MUT] (blue) pairs. NAIP, NLRC4 are significantly upregulated for B6 versus the NSG pair. Casp 1, Nfkb2, Nlrp1, Nlrp3 and P2rx7 trend toward significance for upregulation of the B6 pair over the NSG pair. There is no significant upregulation by the B6 pair in Aim2, Ctsb, Pycard, Casp 8, Il1, Il18 or Il6 targets (see Table 4 below).
Table 4.
P-values for fold-changes in nCounter PCR for difference between B6[WT-MUT] AND NSG[WT-MUT] groups. Concordance of results to RNA sequencing values exists for all gene except Casp8 and Pycard, the former showing equal upregulation of both groups, the latter showing no change in expression between the two groups. No significant difference was noted between any of Il18, Il1 or Il6 values.
| Gene target | p-value B6[WT-MUT] versus NSG[WT-MUT] |
|---|---|
| Aim2 | 0.8752 |
| Casp1 | 0.1238 |
| Casp8 | 0.9022 |
| Ctsb | 0.5808 |
| Naip | 0.0159 |
| Nfkb2 | 0.1364 |
| Nlrc4 | 0.0483 |
| Nlrp1 | 0.0983 |
| Nlrp3 | 0.0583 |
| P2rx7 | 0.0750 |
| Pycard | 0.7832 |
| Il18 | 0.1558 |
| Il1b | 0.2096 |
| Il6 | 0.7497 |
| Gapdh | 0.8720 |
| Hprt | 0.0585 |
| Pgk1 | 0.0365 |
3.4. B6 and NSG MUT aortic tissue appeared similar by routine histologic staining.
Both the B6 and NSG MUT mice demonstrate a similar spectrum of histologic pathology across the H&E, ELVG, and Alcian Blue stains Figure 6 (A-F). The wall thicknesses of the ascending aortas differed by strain and by measured aortic diameter (Supplemental Table 5). Aortic wall thickness of B6 WT mouse (average, 116.2 microns) increased in thickness in the B6 MUT cohort to an average of 197.6 microns as aortic diameters increased from an average of 1.8 mm to 2.57 mm, respectively. By comparison, there was no apparent difference in either the average wall thickness or the ascending aortic diameter when comparing the NSG WT mice (136.1 microns, 1.54 mm) to the NSG MUT cohort (148.4 microns, 1.58 mm). On the H&E-stained sections, both strains demonstrate the presence of vacuolated smooth muscle cells (“Hurler” cells) throughout the tunica media and adventitia (Figure 6 A, D). With ELVG staining, elastin fragmentation, elastin loss and tissue disarray are present in both B6 and NSG MUT murine aortas (Figure 6 B, E). ). The severity of the histologic changes for B6 and NSG MUT mice appeared to form a continuum with lesser elastin fiber disarray and laminar collapse generally occurring in the NSG MUT mice with more severe changes in the B6 MUT mice.
Figure 6.

(A-F). Histologic findings in B6 MUT (A, B, C) and NSG MUT (D, E, F) ascending aortas. Note the presence of vacuolated cells by H&E staining (panels A, D: black arrows), varying degrees of elastin fiber fragmentation, loss and disarray (panels B, E: white arrows) and intense staining for glycosaminoglycan (C, F) in both B6 and NSG MUT mice. 20x.
With Alcian blue staining, the vacuolated cells seen in both B6 MUT and NSG MUT mice (Figure 6 (C, F) appear enrobed within the stain. Neither B6 nor NSG WT mice had similar findings with any of these three stains, and Alcian blue staining appeared much less intense although tissue GAG was not quantitated in either WT or MUT mice (see online supplemental Figure S2).
3.5. B6 MPSI MUT mice had marked macrophage and T-cell infiltration of aortic tissue while NSG MPSI MUT mice did not.
B6 MUT murine aortas could be differentiated from NSG MUT aortas by immunohistochemical staining for macrophages, T- and B-cells. Staining of aortic tissue for macrophages (Figure 7 A-D) showed the MAC2 signal to be abundantly present throughout the media of B6 MUT mice (Figure 7 A, C) but sparse in NSG MUT mice (Figure 7 B, D). MAC2 positive cells were adjacent to an adventitial mononuclear cell infiltration (Figure 7C, white arrow). No macrophage infiltration was apparent in either B6 or NSG WT mice (see online supplemental Figure S3). CD3 staining for lymphocytes (Figure 8 A-D) demonstrated an adventitial infiltrate of CD3 lymphocytes in B6 MUT mice (panel 8A, C, arrows) that was not seen in NSG MUT mice (panel 8 B, D). No CD3 staining was identified in B6 or NSG WT mice (see online supplemental Figure S4). Very rare B cells were identified in the adventitia of B6 MUT mice while no B cells were found in NSG MUT mice (Figure 9 A-D). WT mice from both strains showed no significant reactivity for B220 or CD3 (see online supplemental Figure S5).
Figure 7.

(A-D). Macrophage (MAC2) staining in B6 MUT (A, C) and NSG MUT (B, D) mice at 10x (A, C) and 20x (B, D) power, respectively. Note diffuse abundant (B6 MUT (A,B), black arrows) and sparse (NSG MUT (C,D), black arrow) MAC2 positive cells that are geographically adjacent to underlying adventitial monocytic infiltrate (white arrow) in B6 MUT. Non-specific adventitial staining is present in NSG MUT aorta.
Figure 8.

(A-D). Lymphocyte (CD3) staining in B6 MUT (A, C) and NSG MUT (B, D) mice at 10x (A, B) and 20x (C, D) power, respectively. Note the adventitial CD3 staining for lymphocytes in the B6 MUT murine aorta (panel 8 A and C, arrows) that is not present in NSG MUT aorta (panel 8 B and D).
Figure 9.

(A-D). B-cell (B220) staining in B6 MUT (A, B) and NSG MUT (C, D) mice at 10x (A, C) and 20x (B, D) power, respectively. Note the single adventitial B cell in the B6 MUT mice (panel 5B, arrow) while none are present in NSG MUT mice (panel 5D).
4.0. Discussion.
We have found that the typical cardiac phenotype of aortic dilation and aortic insufficiency is present in the immunocompetent B6 MPS adult male mouse but absent from the corresponding immune incompetent NSG MPS mouse. Absence of the phenotype in the NSG MUT mouse is not due to differences in urinary GAGs, or the upregulation of CTSB within the aorta, nor is it due to elastin fiber fragmentation or the presence of vacuolated smooth muscle cells in the aortic media. The presence of aortic dilation in the aortas of immunocompetent B6 MUT mice is associated with upregulation of multiple components of the inflammasome pathways that are not equally upregulated in the NSG MUT mice. Ascending aortic dilation is also associated with a robust inflammatory infiltrate composed of macrophages and T-cells throughout the B6 MPS aorta which is not present in the NSG MPS aorta. Taken together, this suggests that aortic dilation in the B6 MPS mouse is the result of an inflammatory process initiated by activation of the inflammasome, a central component of the innate immune system, with resultant response from a fully functional adaptive immune system. Inflammasome activation has been hypothesized to be part of the inflammatory response seen in MPS (27) and has been reported to be present in brain tissue of MPS IIIB mice. Our work strengthens this hypothesis by confirming the presence of inflammasome activation in the cardiovascular system of the immune competent MPS I mouse and thus may offer new avenues for therapeutic intervention as current therapies, ERT, HCT and experimental HSC gene therapy have yet to show total cure of MPS.
4.1. Inflammasomes are the first line of defense of the innate immune system
Inflammasomes are cytosolic multiprotein platforms that, upon sensing intracellular pathogen- or danger-associated molecular patterns (PAMPs, DAMPs), trigger the maturation and release of cytokines IL-1B and Il-18 leading to inflammation and/or cell death by pyroptosis (28, 29). Inflammasomes are highly conserved throughout nature, occurring even in plants which, lacking adaptive immunity, must rely on them entirely for defense (26). Inflammasomes occur in both human disease and in murine experimental models of disease in cells most likely to encounter foreign invaders: macrophages, monocytes, dendritic cells, neutrophils, epithelial cells and cells of the adaptive immune system (28). More recently, and not unexpectedly, inflammasomes have also been identified in many other cells such as brain microglia (30), renal podocytes (31), osteoclasts (32) and vascular endothelial and smooth muscle cells (33). Inflammasomes have been implicated in a wide-ranging assortment of diseases including atherosclerosis (34), cancer (35), Alzheimer’s disease (36), abdominal and thoracic aortic aneurysms (25, 37), and more recently, murine models of mucopolysaccharidosis (27, 38, 39).
Inflammasomes following a canonical pathway are composed of a sensor protein (pattern recognition receptor, PRR), the adaptor protein (ASC), and the effector (inflammatory protease, caspase-1) that, upon activation, trigger the cleavage of pro-IL-1b and pro-IL18 into their active forms (40, 41). Caspase-1 associated inflammasomes have (25) the ability to additionally activate Gasdermin D, a pore forming protein that can cause cell swelling and release of cell contents resulting in recruitment of inflammatory cells such as phagocytes to further drive the inflammatory response (29, 40). Several classes of PRRs are known: the nucleotide-binding and leucine-rich repeat receptors (NLRs: NLRP1, NLRP3), the absent in melanoma 2-like receptor (AIM2), NOD-like receptor family apoptosis inhibitory protein (NAIP)-NLRC4, and pyrin, with each PRR responding to one or more specific pathogen- or danger-associated molecular patterns (40, 42–45). In the present study, upregulation of Nlrp1, Nlrp3, Naip-Nlrc4 and Aim2 inflammasome genes was found by RNA sequencing and was concordant by PCR in immunocompetent B6 MUT mice but only Aim2 was upregulated in both the B6 MPS MUT and the immune incompetent NSG MUT aortas (Figure 2, Table 4). Upregulation of the NLRP3 inflammasome is notable not only because it contains a heparan-sulfated binding motif (46) and has been shown to be upregulated in MPSIIIA murine brain tissue (38) but also because it has also been shown to be upregulated in ascending aortic aneurysms in both humans and mice (25). In sporadically occurring human ascending aortic aneurysm tissue, particularly macrophages and SM22-α+ smooth muscle cells, contractile protein degradation was associated with upregulation of the NLRP3 inflammasome cascade (25). Angiotensin II (AngII) infusion in C57BL/6 mice fed a high fat diet, developed ascending aortic aneurysms that were associated with activation of the NLRP3 inflammasome complex while Nlrp3−/− or caspase-1 deficiency in mice reduced ascending aortic aneurysm formation with AngII challenge (25). Blockade of the NLRP-3-caspase-1 inflammasome pathway by glyburide, a specific pharmacologic inhibitor of this pathway, attenuated ascending aortic formation in mice (25). The AIM2 inflammasome, activated by double-stranded DNA and necrotic cell debris, has been found in abdominal aortic aneurysms in both humans and mice (43, 47), but, to date, has not been reported in either MPS or ascending aortic aneurysms. Although the source of upregulation in both B6 and NSG mice may be due to inflammatory cells within aortic tissue (see below), activation of the inflammasomes within smooth muscle cells cannot be ruled out. The NLRP1 and NAIP-NLRC4 inflammasomes, first reported to respond to signaling from intracellular bacterial components (such as bacillus anthracis lethal toxin (NLRP1) or flagellin from salmonella (NAIP-NLRC4)) have more recently been found to be associated with auto-immune human diseases involving dyskeratosis and arthritis (48) and Macrophage Activation Syndrome (45). To the best of our knowledge, neither the NLRP1 nor NAIP-NLRC4 inflammasomes have been associated with MPS or ascending aortic aneurysms to date.
4.2. Inflammasome activation and adaptive immunity
The path from inflammasome activation to T- and B-cell response is complex, likely disease specific, and a focus of current aneurysm research (49). In models of atherosclerosis, for example, the first response involves an inflammatory infiltrate from macrophages responding to signals from the innate immune system (50). Thereafter tissue dendritic cells trigger detection and processing of damaged extra- or intracellular proteins for recognition and loading onto major histocompatibility receptors for presentation to antigen specific T cells. Subsequently co-regulatory receptors on the T cells fine tune the T cell response and ultimately, T cell differentiation is determined by dendritic cell cytokine production (51). Finally, as a result of the continued presence of activated macrophages and dendritic cells from ongoing insults, an adaptive immune response from T- and B-cells may occur (50).
4.3. Inflammatory cells occur in both abdominal and ascending aortic aneurysms.
Thoracic and abdominal aortic aneurysms are defined histologically by the presence of fragmentation of elastic fibers, loss of smooth muscle cells, pooling of glycosaminoglycans and remodeling of fibrillar collagen (52); glycosaminoglycan ‘lakes’ within the media appear to be found only in thoracic, but not abdominal or cranial, aneurysms and may lead to wall weakness (52). Whether these histologic findings represent inciting events for, or the end result of, inflammation are subjects of active investigation (53). However, more than 20 years ago the presence of excess dermatan sulfate in cultured skin fibroblasts from children with Hurler syndrome was shown to impair assembly of elastin from tropo-elastin subunits (54) suggesting that, at least in MPS, this underlying metabolic derangement may drive subsequent inflammation. Both the B6 MUT and NSG MUT aortas reported herein have the classic histologic findings of aortic aneurysms on H&E, ELVG, and Alcian blue staining and, given the overlap in histologic pathology, it is difficult to distinguish B6 MUT mice from NSG MUT mice based upon routine histology alone. On Alcian blue stain, we could find no GAG “lakes” in either B6 or NSG MUT mice in accordance with the classic definition of ascending aortic aneurysms (52).
In our study, immunohistochemical staining significantly advanced our ability to differentiate immunocompetent B6 MUT mice from immune incompetent NSG MUT mice. B6 MUT mice have a robust medial macrophage infiltrate in proximity to an underlying adventitial lymphocytic infiltrate while macrophages in NSG MUT mice are much less common and lymphocytes are absent, as is expected with the NSG strain of mice (19). Inflammatory infiltrates have long been considered central to the development of abdominal aortic aneurysms (37). Upregulated NLRP3 inflammasome markers have been found within adventitial macrophages from both human and murine abdominal aortic aneurysms (55) while components of the AIM2 inflammasome were found within macrophages, T cells, B cells, activated B cells and lymphatic endothelial cells of human abdominal aortic aneurysms (56). Inflammatory infiltrates had been thought to play a minimal role in ascending aortic aneurysms (57) but this view has been convincingly refuted by ample evidence of macrophage and T-cell infiltration in ascending aortic aneurysm tissue from both syndromic (Marfan) and non-syndromic (familial) types of ascending aortic aneurysms (53, 58). Based upon current thinking, ascending aortic aneurysm development leads to increased inflammatory infiltrates mediated in part by increased chemotactic signaling from connective tissue fragmentation and the expression of chemotactic cytokines (58). It is tempting, but certainly far from proven, to attribute the initiation of this process in the B6 MPSI mice as a response to the long-ago reported elastin fragmentation caused by excess dermatan sulfate in MPS smooth muscle cells (54).
4.4. Urinary GAGs and Cathepsin B upregulation are not associated with ascending aortic dilation.
In our studies, urinary GAGs are increased equally in B6 and NSG MUT mice. Previous studies have confirmed that urinary GAGs are significantly increased in both immunocompetent B6 MPS mice and immune incompetent NSG MPS mice (18, 59). Our study is the first to directly compare the increase in urinary GAG between B6 and NSG MPS mice and shows that there is no difference in the change of the amount excreted between WT and MPS. Additionally, Ctsb mRNA is significantly upregulated in both B6 and NSG MPS mice. This finding is notable as increased Cathepsin B has previously been implicated in the development of aortic dilation in MPS mice (60, 61). The presence of upregulation of Ctsb in the absence of aortic dilation, as seen in the NSG MPS mouse, would suggest that increased cathepsin alone is not sufficient to produce aortic dilation but that cathepsin signaling likely requires an inflammatory response mediated downstream by inflammasomes and the adaptive immune system to produce the cardiac phenotype.
4.5. Cardiac findings are more common in adult male, rather than in adult female, B6 MPSI mice
In two previous studies we have shown that aortic insufficiency is much more common in male MPSI mice than females. In the first study (9), 20/26 males had AI while only 11/26 females did. In subsets of mice with AI, a dilated aorta was, however, equally present in both males and females. It is not currently known if a dilated aorta precedes the development of AI. We found MMP12 increased in the males but not the females in that study confirming the difference between males and females by a biochemical method in addition to the echo findings. In the second study (16), we found that perturbation of the renin-angiotensin system was greater in male MPSI mice than in the females, as a partial explanation for gender dimorphism seen in the cardiac phenotype. Going forward, it will be important to include both male and female mice in studies because there may be other unknown protective factors in female mice that prevent full expression of the cardiac phenotype.
4.6. Murine MPSI and human MPSI cardiac features are not identical.
Human and murine cardiac features are not identical in MPSI with respect to coronary artery involvement or most commonly affected valve but ascending aortic dilation appears to occur commonly in humans, mice, and dogs with MPSI. Ascending aortic dilation was not appreciated in human MPSI (8) until recently (3) but is present in both murine and human MPSI (3, 60) with similar histopathological features consisting of elastin fragmentation, glycosaminoglycan deposition and smooth muscle cell vacularization (8, 60, 62). Myo-intimal proliferation within epicardial coronary arteries leading to diffuse coronary stenosis is a common finding in human MPSI (62) but is not present in MPSI mice (8), where murine coronary disease, in general, is uncommon. Aortic valve regurgitation is common in the murine model of MPSI (8); an earlier report of mitral regurgitation in MPSI mice (63) may be more consistent with a mitral inflow signal due to its low velocity, whereas a high velocity signal would be expected for aortic regurgitation (see Figure 3, this paper). By contrast mitral regurgitation is the most common valve finding in human MPSI, followed by aortic regurgitation (64).
4.7. Study limitations.
This study’s limitations are primarily due to the downstream effects of small sample size (65) – especially for the NSG WT and MUT cohorts as well as small size of the murine aortas, variable RNA integrity numbers and repeated freeze-thaw cycles for determination of QT PCR, that was performed well after the RNA sequencing. All may adversely affect the consistency of the RNA sequencing and QT PCR data and be responsible for differences between RNA sequencing and PCR data. The necessity for performing both quantitative PCR and RNA sequencing has been recently questioned as 15–20% of genes may be non-concordant between the two techniques (66). Lack of confirmation of plasma or tissue protein levels of Il18, Il1 and Il6 are a further limitation of this preliminary descriptive study as is further characterization of the types of macrophages and T-cells within the aortic infiltrates. Although we noted differences in wall thickness among the cohorts, we were unable to provide any biomechanical phenotype, such as wall stress, for these mice since measurement of blood pressure was not part of this protocol (67). Prevention of aneurysm formation by inflammasome blocking drugs represents another avenue of study not yet undertaken. In addition to technical limitations, there are other inflammatory pathways seen by RNA sequencing that have not been evaluated herein and likely participate in development of the ‘cardiac phenotype’ seen in the B6 MPSI mouse. The work reported herein should be considered as a preliminary foray into a novel area of interest for MPSI.
4.8. Summary.
In summary, we have shown that the cardiac phenotype, consisting of ascending aortic dilation and aortic valve insufficiency, occurs in the immune competent B6 adult male MUT mouse but is absent in the NSG counterpart. Both strains of mice have increased excretion of urinary GAGs and classic features of elastin fiber fragmentation, extracellular matrix disarray, the presence of vacuolated cells within the media and increased Alcian blue staining within aortic tissue and are indistinguishable on routine histology. By RNA sequencing, all components of the inflammasome pathway are upregulated in the B6 MUT mouse while only a single inflammasome (Aim2) is upregulated in the NSG mouse. Importantly, Ctsb mRNA is upregulated in both B6 and NSG mice suggesting that events further downstream, rather than Cathepsin B, may be responsible for aneurysm development. Clarification would require measurement of Cathepsin B in NSG MUT murine aortic tissue. The presence of a robust macrophage infiltrate within the aortic media, subjacent to an adventitial lymphocytic infiltrate, differentiates the B6 MUT from the NSG MUT where T-cells are absent and macrophages are sparse. These findings lead to the conclusion that the innate and adaptive immune systems play an important role in the development of the typical cardiac phenotype seen in murine models of MPS I. It is possible that these findings are consistent with chronic sterile inflammation, a finding that may also be important in humans and partially explain why current therapies have not resulted in a complete cure in this, as well as other, lysosomal storage diseases. Additional investigations of the inflammatory pathways in these mouse models may lead to further insights and to novel treatments for both MPS and for ascending aortic aneurysms in general.
Supplementary Material
Acknowledgments
Ultrasound imaging and analysis was performed at the University Imaging Centers, University of Minnesota
Guillermo Marques, PhD
Informatics support came from the Minnesota Supercomputing Institute (MSI)
RNA extraction and sequencing and PCR were carried out by the University of Minnesota Genomics Center
Darrell Johnson, MS
Evan Forsberg, MS
Paige Marsolek, BS
Elyse Froehling, MS
Comparative Pathology Shared Resource
Paula Overn, HT, QIHC (ASCP)
Tim Carlson, DVM
McIvor Lab
Andrea Karlen, BS
Kelli Podertz-Pedersen, MN, RN, PHN
Whitley Lab
Brenda Koniar, AAS, CVT, LAT
This research was supported by a generous grant from the David I. Sit Foundation and by the National Institutes of Health’s National Center for Advancing Translational Sciences, grant UL1TR002494. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences
Footnotes
Supplementary data
Supplemenatry material
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodgers NJ, Kaizer AM, Miller WP, Rudser KD, Orchard PJ, Braunlin EA. Mortality after hematopoietic stem cell transplantation for severe mucopolysaccharidosis type I: the 30-year University of Minnesota experience. J Inherit Metab Dis. 2017;40(2):271–80. [DOI] [PubMed] [Google Scholar]
- 2.Eisengart JB, Rudser KD, Xue Y, Orchard P, Miller W, Lund T, et al. Long-term outcomes of systemic therapies for Hurler syndrome: an international multicenter comparison. Genet Med. 2018. [DOI] [PMC free article] [PubMed]
- 3.Bolourchi M, Renella P, Wang RY. Aortic Root Dilatation in Mucopolysaccharidosis I-VII. Int J Mol Sci. 2016;17(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Poswar FO, de Souza CFM, Giugliani R, Baldo G. Aortic root dilatation in patients with mucopolysaccharidoses and the impact of enzyme replacement therapy. Heart Vessels. 2019;34(2):290–5. [DOI] [PubMed] [Google Scholar]
- 5.Poitier B, Amrane M, Bruneval P, Achouh P. Surgical management of an aortic root dilatation in a patient suffering from Hunter syndrome. Interact Cardiovasc Thorac Surg. 2021;33(5):819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeyeldin AA, Velasquez CA, Mahmood SUB, Brownstein AJ, Zafar MA, Ziganshin BA, et al. Thoracic aortic aneurysm: unlocking the “silent killer” secrets. Gen Thorac Cardiovasc Surg. 2019;67(1):1–11. [DOI] [PubMed] [Google Scholar]
- 7.Ziganshin BA, Zafar MA, Elefteriades JA. Descending threshold for ascending aortic aneurysmectomy: Is it time for a “left-shift” in guidelines? J Thorac Cardiovasc Surg. 2019;157(1):37–42. [DOI] [PubMed] [Google Scholar]
- 8.Braunlin E, Mackey-Bojack S, Panoskaltsis-Mortari A, Berry JM, McElmurry RT, Riddle M, et al. Cardiac functional and histopathologic findings in humans and mice with mucopolysaccharidosis type I: implications for assessment of therapeutic interventions in hurler syndrome. Pediatr Res. 2006;59(1):27–32. [DOI] [PubMed] [Google Scholar]
- 9.Tolar J, Braunlin E, Riddle M, Peacock B, McElmurry RT, Orchard PJ, et al. Gender-related dimorphism in aortic insufficiency in murine mucopolysaccharidosis type I. J Heart Valve Dis. 2009;18(5):524–9. [PMC free article] [PubMed] [Google Scholar]
- 10.Clarke LA, Russell CS, Pownall S, Warrington CL, Borowski A, Dimmick JE, et al. Murine mucopolysaccharidosis type I: targeted disruption of the murine alpha-L-iduronidase gene. Hum Mol Genet. 1997;6(4):503–11. [DOI] [PubMed] [Google Scholar]
- 11.Ohmi K, Greenberg DS, Rajavel KS, Ryazantsev S, Li HH, Neufeld EF. Activated microglia in cortex of mouse models of mucopolysaccharidoses I and IIIB. Proc Natl Acad Sci U S A. 2003;100(4):1902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glowacki J, Mizuno S, Kung J, Goff J, Epperly M, Dixon T, et al. Effects of mouse genotype on bone wound healing and irradiation-induced delay of healing. In Vivo. 2014;28(2):189–96. [PMC free article] [PubMed] [Google Scholar]
- 13.Sellers RS. Translating Mouse Models. Toxicol Pathol. 2017;45(1):134–45. [DOI] [PubMed] [Google Scholar]
- 14.Mahajan A, Rodan AR, Le TH, Gaulton KJ, Haessler J, Stilp AM, et al. Trans-ethnic Fine Mapping Highlights Kidney-Function Genes Linked to Salt Sensitivity. Am J Hum Genet. 2016;99(3):636–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Russell C, Hendson G, Jevon G, Matlock T, Yu J, Aklujkar M, et al. Murine MPS I: insights into the pathogenesis of Hurler syndrome. Clin Genet. 1998;53(5):349–61. [DOI] [PubMed] [Google Scholar]
- 16.Osborn MJ, Webber BR, McElmurry RT, Rudser KD, DeFeo AP, Muradian M, et al. Angiotensin receptor blockade mediated amelioration of mucopolysaccharidosis type I cardiac and craniofacial pathology. J Inherit Metab Dis. 2017;40(2):281–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez EA, Tavares AM, Poletto E, Giugliani R, Matte U, Baldo G. Losartan improves aortic dilatation and cardiovascular disease in mucopolysaccharidosis I. J Inherit Metab Dis. 2017;40(3):311–2. [DOI] [PubMed] [Google Scholar]
- 18.Mendez DC, Stover AE, Rangel AD, Brick DJ, Nethercott HE, Torres MA, et al. A novel, long-lived, and highly engraftable immunodeficient mouse model of mucopolysaccharidosis type I. Mol Ther Methods Clin Dev. 2015;2:14068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174(10):6477–89. [DOI] [PubMed] [Google Scholar]
- 20.Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, et al. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–91. [PubMed] [Google Scholar]
- 21.Sandberg S, Gunther R, Cooksley R, Koniar B, Whitley C, Braunlin E. Comparison of cardiac pathology in three strains of murine mucopolysaccharidosis type I: Considering the role of auto-immune pathogenesis in Hurler syndrome.: Mol Gen Metab; 2009. p. S38.
- 22.Krämer A, Green J, Pollard J, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics. 2014;30(4):523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagan C JAX Blog: When are mice considered old? [Internet]. Bar Harbor, Maine: The Jackson Laboratory. Nov. 6, 2017. [cited 2021].
- 24.Hinton RB, Yutzey KE. Heart valve structure and function in development and disease. Annu Rev Physiol. 2011;73:29–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D, Ren P, Zheng Y, Zhang L, Xu G, Xie W, et al. NLRP3 (Nucleotide Oligomerization Domain-Like Receptor Family, Pyrin Domain Containing 3)-Caspase1 Inflammasome Degrades Contractile Proteins: Implications for Aortic Biomechanical Dysfunction and Aneurysm and Dissection Formation. Arterioscler Thromb Vasc Biol. 2017;37(4):694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bayless AM, Nishimura MT. Reinventing the wheel with a synthetic plant inflammasome. Proc Natl Acad Sci U S A. 2020;117(34):20357–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parker H, Bigger BW. The role of innate immunity in mucopolysaccharide diseases. J Neurochem. 2019;148(5):639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–32. [DOI] [PubMed] [Google Scholar]
- 29.de Vasconcelos NM, Lamkanfi M. Recent Insights on Inflammasomes, Gasdermin Pores, and Pyroptosis. Cold Spring Harb Perspect Biol. 2020;12(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milner MT, Maddugoda M, Götz J, Burgener SS, Schroder K. The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer’s disease. Curr Opin Immunol. 2021;68:116–24. [DOI] [PubMed] [Google Scholar]
- 31.Conley SM, Abais JM, Boini KM, Li PL. Inflammasome Activation in Chronic Glomerular Diseases. Curr Drug Targets. 2017;18(9):1019–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Detzen L, Cheat B, Besbes A, Hassan B, Marchi V, Baroukh B, et al. NLRP3 is involved in long bone edification and the maturation of osteogenic cells. J Cell Physiol. 2021;236(6):4455–69. [DOI] [PubMed] [Google Scholar]
- 33.Hakimi M, Peters A, Becker A, Böckler D, Dihlmann S. Inflammation-related induction of absent in melanoma 2 (AIM2) in vascular cells and atherosclerotic lesions suggests a role in vascular pathogenesis. J Vasc Surg. 2014;59(3):794–803. [DOI] [PubMed] [Google Scholar]
- 34.Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ Res. 2020;126(9):1260–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fang Y, Tian S, Pan Y, Li W, Wang Q, Tang Y, et al. Pyroptosis: A new frontier in cancer. Biomed Pharmacother. 2020;121:109595. [DOI] [PubMed] [Google Scholar]
- 36.Venegas C, Heneka MT. Inflammasome-mediated innate immunity in Alzheimer’s disease. FASEB J. 2019;33(12):13075–84. [DOI] [PubMed] [Google Scholar]
- 37.Yuan Z, Lu Y, Wei J, Wu J, Yang J, Cai Z. Abdominal Aortic Aneurysm: Roles of Inflammatory Cells. Front Immunol. 2020;11:609161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker H, Ellison SM, Holley RJ, O’Leary C, Liao A, Asadi J, et al. Haematopoietic stem cell gene therapy with IL-1Ra rescues cognitive loss in mucopolysaccharidosis IIIA. EMBO Mol Med. 2020;12(3):e11185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azambuja AS, Pimentel-Vera LN, Gonzalez EA, Poletto E, Pinheiro CV, Matte U, et al. Evidence for inflammasome activation in the brain of mucopolysaccharidosis type II mice. Metab Brain Dis. 2020;35(7):1231–6. [DOI] [PubMed] [Google Scholar]
- 40.Xue Y, Enosi Tuipulotu D, Tan WH, Kay C, Man SM. Emerging Activators and Regulators of Inflammasomes and Pyroptosis. Trends Immunol. 2019;40(11):1035–52. [DOI] [PubMed] [Google Scholar]
- 41.Schroder K What is an inflammasome? Institute for Molecular Bioscience | https://imb.uq.edu.au/ The University of Queensland; March 3, 2020. [Available from: https://www.youtube.com/watch?v=2812ion3aUk. [Google Scholar]
- 42.Takahashi M NLRP3 Inflammasome as a Common Denominator of Atherosclerosis and Abdominal Aortic Aneurysm. Circ J. 2021. [DOI] [PubMed]
- 43.Wortmann M, Xiao X, Wabnitz G, Samstag Y, Hakimi M, Böckler D, et al. AIM2 levels and DNA-triggered inflammasome response are increased in peripheral leukocytes of patients with abdominal aortic aneurysm. Inflamm Res. 2019;68(4):337–45. [DOI] [PubMed] [Google Scholar]
- 44.Zhao ZZ, Zheng XL, Jiang ZS. Emerging roles of absent in melanoma 2 in cardiovascular diseases. Clin Chim Acta. 2020;511:14–23. [DOI] [PubMed] [Google Scholar]
- 45.Duncan JA, Canna SW. The NLRC4 Inflammasome. Immunol Rev. 2018;281(1):115–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simon Davis DA, Parish CR. Heparan sulfate: a ubiquitous glycosaminoglycan with multiple roles in immunity. Front Immunol. 2013;4:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wortmann M, Arshad M, Hakimi M, Böckler D, Dihlmann S. Deficiency in Aim2 affects viability and calcification of vascular smooth muscle cells from murine aortas and angiotensin-II induced aortic aneurysms. Mol Med. 2020;26(1):87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grandemange S, Sanchez E, Louis-Plence P, Tran Mau-Them F, Bessis D, Coubes C, et al. A new autoinflammatory and autoimmune syndrome associated with NLRP1 mutations: NAIAD (Ann Rheum Dis. 2017;76(7):1191–8. [DOI] [PubMed] [Google Scholar]
- 49.Hachim MY, Khalil BA, Elemam NM, Maghazachi AA. Pyroptosis: The missing puzzle among innate and adaptive immunity crosstalk. J Leukoc Biol. 2020;108(1):323–38. [DOI] [PubMed] [Google Scholar]
- 50.Tabas I, Lichtman AH. Monocyte-Macrophages and T Cells in Atherosclerosis. Immunity. 2017;47(4):621–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatscher L, Amon L, Heger L, Dudziak D. Inflammasomes in dendritic cells: Friend or foe? Immunol Lett. 2021;234:16–32. [DOI] [PubMed] [Google Scholar]
- 52.Humphrey JD. Possible mechanical roles of glycosaminoglycans in thoracic aortic dissection and associations with dysregulated transforming growth factor-beta. J Vasc Res. 2013;50(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinesh NEH, Reinhardt DP. Inflammation in thoracic aortic aneurysms. Herz. 2019;44(2):138–46. [DOI] [PubMed] [Google Scholar]
- 54.Hinek A, Wilson SE. Impaired elastogenesis in Hurler disease: dermatan sulfate accumulation linked to deficiency in elastin-binding protein and elastic fiber assembly. Am J Pathol. 2000;156(3):925–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Usui F, Shirasuna K, Kimura H, Tatsumi K, Kawashima A, Karasawa T, et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35(1):127–36. [DOI] [PubMed] [Google Scholar]
- 56.Dihlmann S, Erhart P, Mehrabi A, Nickkholgh A, Lasitschka F, Böckler D, et al. Increased expression and activation of absent in melanoma 2 inflammasome components in lymphocytic infiltrates of abdominal aortic aneurysms. Mol Med. 2014;20:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Collins MJ, Dev V, Strauss BH, Fedak PW, Butany J. Variation in the histopathological features of patients with ascending aortic aneurysms: a study of 111 surgically excised cases. J Clin Pathol. 2008;61(4):519–23. [DOI] [PubMed] [Google Scholar]
- 58.Malecki C, Hambly BD, Jeremy RW, Robertson EN. The Role of Inflammation and Myeloperoxidase-Related Oxidative Stress in the Pathogenesis of Genetically Triggered Thoracic Aortic Aneurysms. Int J Mol Sci. 2020;21(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Garcia-Rivera MF, Colvin-Wanshura LE, Nelson MS, Nan Z, Khan SA, Rogers TB, et al. Characterization of an immunodeficient mouse model of mucopolysaccharidosis type I suitable for preclinical testing of human stem cell and gene therapy. Brain Res Bull. 2007;74(6):429–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma X, Tittiger M, Knutsen RH, Kovacs A, Schaller L, Mecham RP, et al. Upregulation of elastase proteins results in aortic dilatation in mucopolysaccharidosis I mice. Mol Genet Metab. 2008;94(3):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baldo G, Tavares AM, Gonzalez E, Poletto E, Mayer FQ, Matte UD, et al. Progressive heart disease in mucopolysaccharidosis type I mice may be mediated by increased cathepsin B activity. Cardiovasc Pathol. 2017;27:45–50. [DOI] [PubMed] [Google Scholar]
- 62.Renteria VG, Ferrans VJ, Roberts WC. The heart in the Hurler syndrome: gross, histologic and ultrastructural observations in five necropsy cases. Am J Cardiol. 1976;38(4):487–501. [DOI] [PubMed] [Google Scholar]
- 63.Jordan MC, Zheng Y, Ryazantsev S, Rozengurt N, Roos KP, Neufeld EF. Cardiac manifestations in the mouse model of mucopolysaccharidosis I. Mol Genet Metab. 2005;86(1–2):233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wippermann CF, Beck M, Schranz D, Huth R, Michel-Behnke I, Jungst BK. Mitral and aortic regurgitation in 84 patients with mucopolysaccharidoses. Eur J Pediatr. 1995;154(2):98–101. [DOI] [PubMed] [Google Scholar]
- 65.Li CI, Samuels DC, Zhao YY, Shyr Y, Guo Y. Power and sample size calculations for high-throughput sequencing-based experiments. Brief Bioinform. 2018;19(6):1247–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Coenye T Do results obtained with RNA-sequencing require independent verification? Biofilm. 2021;3:100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Humphrey JD, Tellides G. Central artery stiffness and thoracic aortopathy. Am J Physiol Heart Circ Physiol. 2019;316(1):H169-H82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


