Abstract
Background:
In addition to its involvement in both the innate and adaptive immune systems, vitamin D has also been found to affect keratinocyte function and proliferation, suggesting a possible role for vitamin D in cutaneous allergic sensitization.
Objective:
To explore the role of circulating vitamin D levels in allergic sensitization.
Methods:
Serum 25-hydroxyvitamin D (25(OH)D) levels were measured in a subset of children (N=323) enrolled in the Mechanisms of Progression from AD to Asthma in Children (MPAACH) cohort, a prospective early-life cohort of children with atopic dermatitis. Allergic sensitization was determined using skin prick testing, and FLG expression in keratinocytes was measured by quantitative PCR. Multiple Poisson regression was used to evaluate interaction effects between serum 25(OH)D levels and FLG expression with sensitization load as the outcome.
Results:
Black participants had significantly lower mean levels of serum 25(OH)D compared with non-Black participants (29.3 vs. 32.9 ng/ml; p < 0.001). FLG expression and sensitization load were negatively correlated in non-Black participants with 25(OH)D levels < 27.2 ng/ml (Rho = −0.45; p=0.026). No association between FLG expression and sensitization load was found in Black participants or participants with 25(OH)D levels ≥ 27.2 ng/ml. Multiple Poisson regression models confirmed that 25(OH)D levels interact with FLG expression to affect sensitization load in non-Black participants.
Conclusion:
Despite lower vitamin D levels in Black participants, sensitization load was associated with non-lesional skin FLG expression in non-Black, but not Black, children with low vitamin D levels. Thus, a complex interplay of factors determines the impact of vitamin D on allergic sensitization.
Keywords: vitamin D, allergic sensitization, children, cohort study, atopic dermatitis, filaggrin expression, racial differences
Introduction
Vitamin D plays key roles in the functioning of keratinocytes, and keratinocytes provide a large share of vitamin D for the body through the non-enzymatic conversion of 7-dehydrocholesterol that is mediated by UVB radiation. In addition, keratinocytes express the vitamin D receptor (VDR) as well as both enzymes involved in the hydroxylation of vitamin D to 1,25-dihydroxyvitamin D (1,25(OH)2D), the biologically active metabolite of vitamin D. In vitro studies have provided evidence that 1,25(OH)2D together with VDR promotes keratinocyte differentiation and inhibits proliferation1–3. VDR has also been shown to increase the production of epidermis-specific glucosylceramides and promote skin barrier formation4.
Consistent with this role of vitamin D in keratinocyte function, epidemiological studies have suggested a link between low circulating vitamin D levels and allergic skin disease, but results have been conflicting5–7. Two recent meta-analyses found significant differences in serum 25-hydroxyvitamin D (25(OH) D) levels, the main circulating form of vitamin D, between pediatric atopic dermatitis (AD) populations and healthy controls, although results varied among included studies8, 9.
In addition to its well-known role in calcium homeostasis, Vitamin D has a wide range of other biological effects. 1,25(OH)2D is involved in both innate and the adaptive immunity10, 11. Further, given the role that the skin barrier plays in allergic sensitization12–14, it may be expected that vitamin D, through its different functions in the skin and in innate immunity, will be associated with cutaneous sensitization in children. Studies have found associations between vitamin D level and sensitization to both food and aeroallergens15, polysensitization to more than one food16, total serum IgE levels17, and elevated IgE levels for specific allergens5. Some studies have found U-shaped or inversely U-shaped associations between serum vitamin D levels and sensitization18, 19, while others have seen associations with sensitization risk mainly at high vitamin D levels20. The causal nature of the observed associations between vitamin D and allergic sensitization is poorly understood, and is likely to involve timing, route of sensitization, epithelial barrier characteristics, exposure dose, and other factors.
Reports have consistently shown that Black Americans have lower circulating vitamin D levels than non-Black Americans21, 22. Black children also have higher rates of most allergic disorders compared with White children23, 24. This study was designed to investigate the relative contributions by, and interactions between, vitamin D and skin barrier-related factors including filaggrin (FLG), trans-epidermal water loss, and SCORAD as risk factors for allergic sensitization in early childhood. We hypothesized that low levels of vitamin D contribute to a compromised skin barrier, which may increase the risk of sensitization through the skin. We used the established Mechanisms of Progression from AD to Asthma in Children (MPAACH) longitudinal cohort of well-phenotyped children aged 0–2 years diagnosed with AD, which includes 67% Black participants. Given the differences in Vitamin D levels and the reports of different allergic phenotypes in Black children, all analyses were stratified by race.
Methods
Study population
The Mechanisms of Progression from AD to Asthma in Children (MPAACH) cohort (N=577), has been described in detail previously25. This study was approved by the Institutional Review Board at Cincinnati Children’s Hospital Medical Center and all subjects signed informed consent/parental permission prior to participation. Briefly, MPAACH is a prospective early-life cohort of children with AD who are being followed with yearly visits. Participants are aged 0 to 2 years at enrollment. The study population in this analysis was a subset of MPAACH participants for whom results from serum 25-hydroxyvitamin D (25(OH)D) measurements and real-time PCR for skin FLG expression were available for the first visit at the time of data collection (N=323). Race was based on parental-report. Given, the low proportion of individuals who did not identify as Black or White, these individuals were classified with Whites into a non-Black group. Presence of AD was evaluated based on a diagnosis of AD (based on the Hanifin and Rajka Criteria for AD26), OR the parent(s)/legal authorized representative indicated a positive response to each of the 3 questions from the Children’s Eczema Questionnaire27. SCORAD (SCORing Atopic Dermatitis) was used to assess AD severity28. Trans-epidermal water loss (TEWL) was measured on non-lesional skin using the DermaLab TEWL probe (Cortex Technolog, Hadsund, Denmark). Total serum 25(OH)D, the main circulating form of vitamin D, was measured using the automated immunoassay LIAISON® 25 OH Vitamin D assay (DiaSorin, Saluggia VC, Italy). Type of insurance (public or private) was obtained from questionnaires and used as a proxy for household income. In this cohort, lower household income was strongly associated with public insurance (p < 0.0001).
Allergic sensitization
Allergic sensitization was assessed at the study visit by skin prick testing (SPT) to 11 aeroallergens and 13 foods25. The aeroallergen panel includes mold mix 1 (Alternaria tenuis, Hormodendrum cladosporioides, Helminthosporium interseminatum, Aspergillus fumigatus, Aspergillus Niger, Penicillium notatum), mold mix 2 (Rhizopus nigricans, Pullularia pullulans, Fusarium vasinfectum, Mucor racemosus), ragweed (Ambrosia trifida, Ambrosia artemisiifolia), grass (Kentucky bluegrass, orchard, redtop, timothy, Sweet Vernon), tree mix 1 (pecan, maple BHR, oak RVW, American sycamore, black willow), tree mix 2 (white ash, birch mix [Red&White], black walnut, common cottonwood, and American elm), weeds (kochia, English plantain, lamb’s quarters, marsh elder/poverty [BPT Mix], common cocklebur, and careless/pigweed [CR Mix]), mite mix (Dermatophagoides pteronyssinus and Dermatophagoides farinae), cockroach (Periplaneta americana and Blattella germanica), and cat and dog (HollisterStier, Spokane, Wash). The food allergen panel includes cow’s milk, egg white, egg yolk, soy, wheat, peanut, cashew, almond, walnut, pistachio, pecan, hazelnut, and brazil nut (Greer, Lenoir, NC). A positive SPT result was defined as a wheal diameter at least 3 mm larger than that of the diluent control. Sensitization load was defined as the number of different allergens a participant was found to be sensitized to at the time of the study visit.
Filaggrin expression
The procedures for sampling keratinocytes from non-lesional skin using SmartSolve water soluble tape (SmartSolve, Bowling Green, OH) and extract RNA for quantitative PCR analysis have been described previously25, 29. Quantitative PCR reactions were performed using the Taqman gene expression assay for FLG: Hs00856927_g1. FLG expression levels were normalized to 18S levels and is a unitless variable.
Statistics
Our goal was to test whether that low levels of vitamin D contribute to a compromised skin barrier, which may increase the risk of sensitization through the skin. Given previous reports showing marked differences between race, especially related to FLG30 and vitamin D21, 22, analyses, unless otherwise specified were stratified by race. The study population was compared with the full MPAACH cohort using Chi-square test for sex, type of insurance, season of study visit, and sensitization. Wilcoxon rank-sum test was used for comparisons of age and SCORAD. The non-Black and Black participants of the study population were compared in the same way. Differences in mean serum 25(OH)D and mean Ln[FLG expression] were analyzed using Student’s t-test and Wilcoxon rank-sum test was used to evaluate differences in median non-lesional TEWL. Black and non-Black participants were further stratified by serum 25(OH)D. The lower tertile for the distribution of serum 25(OH)D values in the study population was 27.2 ng/ml, and this value was used to define low 25(OH)D (< 27.2 ng/ml) and high 25(OH)D (≥ 27.2 ng/ml), respectively. Associations between 25(OH)D levels and clinical and demographic variables were analyzed by comparing participants with high vs. low 25(OH)D levels as described above. Similarly, sensitization to food (FS), aeroallergens (AS), and any allergen (SPT) was analyzed for associations with clinical and demographic variables by comparing sensitized and non-sensitized participants.
To evaluate the relationship between FLG and sensitization and the impact of vitamin D on this relationship, we used a sequential approach. First, we sought to visualize the relationship between FLG and sensitization load. Correlations between Ln[non-lesional FLG expression] and sensitization load were evaluated using Spearman’s rank-order correlation analysis, performing unstratified and stratified analyses by 25(OH)D levels (using the threshold specified above). As sensitization load was also associated with other factors, we then performed multivariate regression to allow us to account for covariates and formally test whether FLG and 25(OH)D exhibited an interactive effect on sensitization load. Given the data distribution a Poisson regression was used. Covariates included sex, age, non-lesional TEWL and SCORAD. Analyses were performed in the whole cohort and stratified by non-Blacks /Blacks. Because of missing non-lesional TEWL data, the sample sizes for the Poisson regression analyses were n=289 for the whole study population, n=121 for non-Black participants, and n=168 for Black participants. To gain additional insight into these relationships we then stratified the Poisson regression models by race and 25(OH)D status adjusting for sex, age, non-lesional TEWL and SCORAD and plotted the theoretical regression lines. In all analyses, a p-value < 0.05 was considered statistically significant.
Results
Study population
At the time of data collection, 577 MPAACH participants had completed year-1 visits. The study population included 323 participants for whom results for 25(OH)D assays and qPCR of FLG were available. As shown in eTable 1, the study population was well representative of the full MPAACH cohort with regard to demographic and clinical parameters. The study population included 126 non-Black and 197 Black participants (Table 1). Among the non-Black participants, 118 were White, two were Asian, two were Pacific Islanders, two were Native American, and two were Other. Median age, percentage of male participants, and median SCORAD did not differ significantly between non-Black and Black children. Non-Black participants were more likely to have private insurance (71.4%), whereas most of the Black participants had public insurance (87.3%). Non-Black children were significantly more likely to have their study visits in the winter, in contrast with Black children, who were more likely to have visits in the summer. (19.0 vs 18.1, p=0.49). Distributions of serum 25(OH)D values in Black and non-Black participant are shown in eFigure 1. Serum 25(OH)D levels were lower in Black than in non-Black children (32.9 vs. 29.3, <0.001), while vitamin D supplementation was more common among non-Blacks than Blacks (23.8% vs. 10.3%, p=0.008). Median non-lesional TEWL was significantly lower in Black compared with non-Black participants (8.7 vs. 10.3, p = 0.003), and the geometric mean of FLG expression was higher (0.0013 vs 8.0x10‒4, p = 0.02). The percentage of participants who were sensitized to any allergen was significantly lower in Black than in non-Black participants (28.4% vs 46.8%, p = 0.03). A non-significant trend towards lower percentage of sensitization in Black participants was found for AS and FS, respectively.
Table 1.
Demographic and clinical characteristics of the study population overall and by race.
| Variable | All (N=323) | Non-Black (N=126) | Black (N=197) | p |
|---|---|---|---|---|
| Median age, years (IQR) | 2.02 (1.45, 2.36) | 2.04 (1.56, 2.43) | 2.02 (1.40, 2.33) | 0.14 |
| Male (%) | 54.5 | 54.8 | 54.3 | 0.96 |
| Insurance | <0.001 | |||
| Private (%) | 32.5 | 71.4 | 7.6 | |
| Public (%) | 63.8 | 27 | 87.3 | |
| Other/unknown (%) | 3.7 | 1.6 | 5.1 | |
| Season of study visit | <0.001 | |||
| Winter (%) | 17.3 | 27.8 | 10.6 | |
| Spring (%) | 28.2 | 31 | 26.4 | |
| Summer (%) | 29.1 | 17.5 | 36.5 | |
| Fall (%) | 25.4 | 23.8 | 26.4 | |
| Vitamin D supplementation (%) | 15.6 | 23.8 | 10.3 | 0.008 |
| Median SCORAD (IQR) | 18.9 (11.1, 28.8) | 19.0 (11.4, 31.2) | 18.1 (11.0, 28.2) | 0.49 |
| Mean serum 25(OH)D (ng/ml) (SD) | 30.7 (9.0) | 32.9 (8.9) | 29.3 (8.8) | <0.001 |
| GM of FLG expression (GCI) | 0.0011(8.8x10−4 …0.0013) | 8.0x10−4 (5.7x10−4 …0.0011) | 0.0013(0.0010…0.0016) | 0.02 |
| Median non-lesional TEWL (IQR) | 9.0 (7.0, 13.2) | 10.3 (7.4, 15.3) | 8.7 (6.6, 11.7) | 0.003 |
| Sensitized to food (%) | 25.4 | 34.1 | 21.3 | 0.07 |
| Sensitized to aeroallergens (%) | 24.4 | 31 | 18.8 | 0.07 |
| Sensitized to any allergen (%) | 35.6 | 46.8 | 28.4 | 0.03 |
GM: geographic mean. GCI: geometric confidence interval.
Given the differences in 25(OH)D levels as well as in other clinical and demographic variables, we stratified our further analyses for Black vs. non-Black participants.
Factors associated with serum 25(OH)D levels in Black vs. non-Black participants
We next characterized associations between low 25(OH)D and demographic and clinical variables, stratified for Black vs. non-Black participants (Table 2). For these analyses, we dichotomized the 25(OH)D variable into low vs. high 25(OH)D levels (below vs. at or above the lower tertile, 27.2 ng/ml). While low 25(OH)D was not associated with age in non-Black participants, Black children with low 25(OH)D were older than children with high 25(OH)D (2.03 yrs vs 1.73 yrs, p < 0.001). Sex, type of insurance, SCORAD, and vitamin D supplementation were not associated with low 25(OH)D in either group. Season of study visit was associated with low 25(OH)D in both non-Black (p = 0.02) and Black (p = 0.01) participants; for both groups, the percentage of participants with low 25(OH)D was higher in the winter and lower in the summer compared with participants with high 25(OH)D. For non-Black, but not Black, participants, low 25(OH)D was associated with higher non-lesional FLG expression (p = 0.01) and lower non-lesional TEWL (p = 0.049).
Table 2.
Demographic and clinical characteristics in participants with low vs. high serum 25(OH)D levels
| Variable | Non-Black | Black | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Low 25(OH)D (N=24) | High 25(OH)D (N=102) | P | Low 25(OH)D (N=80) | High 25(OH)D (N=117) | p | |
| Median age, years (IQR) | 2.03 | 1.95 | 0.5 | 2.03 | 1.73 | <0.001 |
| Male (%) | 58 | 54 | 0.87 | 50 | 57 | 0.39 |
| Insurance | 0.58 | 0.44 | ||||
| private (%) | 79 | 71 | 11 | 6 | ||
| public (%) | 21 | 29 | 89 | 94 | ||
| Season of study visit | 0.02 | 0.01 | ||||
| winter (%) | 50 | 22.5 | 16.2 | 6.8 | ||
| spring (%) | 33.3 | 30.4 | 33.8 | 21.4 | ||
| summer (%) | 4.2 | 20.6 | 26.2 | 43.6 | ||
| fall (%) | 12.5 | 26.5 | 23.8 | 28.2 | ||
| Vitamin D supplementation (%) | 12 | 26 | 0.24 | 9 | 11 | 0.8 |
| Median SCORAD ((IQR) | 19.4 (12.2, 36.1) | 19.1 (11.1, 28.6) | 0.68 | 18.1 (11.1, 27.8) | 18.1 (11.0, 28.6) | 0.95 |
| GM of FLG expression (GCI) | (9.3x10−4 …0.0028) | (4.5x10−4 …9.8x10−4) | 0.01 | 0.0015 (0.0011 …0.0022) | 0.0011(8.6x10−4 …0.0014) | 0.14 |
| Median non-lesional TEWL ((IQR) | 8.0 (6.9, 12.0) | 11.4 (7.5, 15.5) | 0.049 | 8.2 (6.4, 10.6) | 8.9 (7.0, 12.5) | 0.15 |
GM: geographic mean. GCI: geometric confidence interval
Sensitization to food and aeroallergens in Black vs. non-Black participants
Bivariate analyses were conducted to evaluate potential predictors of sensitization. Outcomes were sensitization (yes/no) to any allergen (SPT), food sensitization (FS), and sensitization to aeroallergens (AS). In non-Black participants, only median SCORAD was consistently associated with all three sensitization types (p = 0.006, < 0.001, and 0.003, respectively), with sensitized participants having significantly higher AD severity defined by SCORAD values (Table 3). Non-Black participants sensitized to aeroallergens were significantly older than non-sensitized participants (median age (yrs) = 2.4 vs. 1.9, p = 0.008), and were significantly less likely to have taken vitamin D supplements (12% vs. 30%, p = 0.046). No differences were found in age or vitamin D supplementation among non-Black participants with SPT or FS vs. non-sensitized participants. As in non-Black participants, Black participants sensitized to any allergen, foods, or aeroallergens had higher SCORAD values compared with non-sensitized participants (p = 0.002, <0.001, and 0.003). Furthermore, they were also more likely to be male (p = <0.001, 0.003, and 0.007, respectively) (Table 4). Black participants with SPT and FS, but not AS, had significantly higher non-lesional TEWL (p = 0.02 and 0.002, respectively).
Table 3.
Factors associated with sensitization in non-Black participants.
| Variable | SPT | FS | AS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No (N=67) | Yes (N=59) | p | No (N=83) | Yes (N=43) | p | No (N=84) | Yes (N=42) | P | |
| Median age, years (IQR) | 1.9 (1.6, 2.3) | 2.1 (1.6, 2.5) | 0.095 | 2.1 (1.6, 2.4) | 2.0 (1.5, 2.5) | 0.79 | 1.9 (1.5, 2.3) | 2.4 (1.8, 2.5) | 0.008 |
| Male (%) | 49 | 61 | 0.25 | 52 | 60 | 0.46 | 51 | 62 | 0.34 |
| Public insurance (%) | 33 | 21 | 0.17 | 31 | 21 | 0.33 | 28 | 27 | 0.91 |
| Season of study visit | 0.45 | 0.99 | 0.24 | ||||||
| Winter (%) | 28 | 19 | 24 | 23 | 27 | 17 | |||
| Spring (%) | 30 | 32 | 31 | 30 | 32 | 29 | |||
| Summer (%) | 13 | 22 | 17 | 19 | 13 | 26 | |||
| Fall (%) | 28 | 27 | 28 | 28 | 27 | 29 | |||
| Vitamin D supplementation (%) | 27 | 20 | 0.52 | 24 | 23 | 0.91 | 30 | 12 | 0.046 |
| Low 25(OH)D (%) | 14.9 | 25.4 | 0.21 | 16.9 | 25.6 | 0.35 | 17.9 | 23.8 | 0.58 |
| Median SCORAD ((IQR) | 18.7 (10.1, 24.8) | 24.9 (13.1, 36.3) | 0.006 | 18.7 (10.1, 25.0) | 26.4 (15.0, 38.6) | <0.001 | 18.6 (11.0, 25.2) | 27.5 (13.6, 39.7) | 0.003 |
| GM of FLG expression (GCI) | 9.3x10−4 (5.7x10−4 …0.0015) | 6.7x10−4 (4.3x10−4 …0.0010) | 0.31 | 9.3x10−4 (6.0x10−4 …0.0014) | 6.0x10−4 (3.6x10−4 …9.8x10−4) | 0.18 | 9.0x10−4 (5.9x10−4 …0.0014−4) | 6.2x10−4 (3.6x10−4 …0.0011) | 0.27 |
| Median non-lesional TEWL ((IQR) | 10.0 (7.3, 13.9) | 10.8 (7.5, 15.5) | 0.76 | 10.2 (7.3, 14.4) | 10.8 (7.5, 15.5) | 0.75 | 10.0 (7.5, 13.8) | 11.1 (7.0, 16.7) | 0.86 |
GM: geographic mean. GCI: geometric confidence interval.
Table 4.
Factors associated with sensitization in Black participants.
| Variable1 | SPT | FS | AS | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| No (N=141) | Yes (N=56) | p | No (N=158) | Yes (N=39) | p | No (N=160) | Yes (N=37) | P | |
| Median age, years (IQR) | 2.0 (1.4, 2.3) | 2.0 (1.5, 2.3) | 0.64 | 2.1 (1.4, 2.3) | 1.8 (1.4, 2.4) | 0.65 | 2.0 (1.4, 2.3) | 2.2 (1.7, 2.3) | 0.17 |
| Male (%) | 46 | 75 | <0.001 | 49 | 77 | 0.003 | 49 | 76 | 0.007 |
| Public insurance (%) | 93 | 91 | 0.77 | 93 | 89 | 0.5 | 92 | 94 | 0.99 |
| Season of study visit | 0.96 | 0.72 | 0.93 | ||||||
| Winter (%) | 26 | 27 | 25 | 33 | 26 | 27 | |||
| Spring (%) | 27 | 25 | 27 | 26 | 27 | 24 | |||
| Summer (%) | 37 | 36 | 38 | 31 | 37 | 35 | |||
| Fall (%) | 10 | 12 | 11 | 10 | 10 | 14 | |||
| Vitamin D supplementation (%) | 12 | 7 | 0.54 | 12 | 5 | 0.4 | 11 | 8 | 0.85 |
| Low 25(OH)D (%) | 40.4 | 44.6 | 0.7 | 43.0 | 35.9 | 0.53 | 38.8 | 54.1 | 0.13 |
| Median SCORAD ((IQR) | 16.9 (10.2, 25.1) | 24.6 (14.6, 35.8) | 0.002 | 16.8 (10.2, 25.1) | 29.5 (16.4, 41.3) | <0.001 | 17.0 (10.4, 26.2) | 25.3 (15.6, 36.2) | 0.003 |
| GM of FLG expression (GCI) | 0.0013 (0.0010…0.0017) | 0.0011 (7.7 x10−4 …0.0016 | 0.39 | 0.0014 (0.0011…0.0018) | 8.7x10−4 (5.5x10−4 …0.0014) | 0.07 | 0.0013 (0.0010…0.0016) | 0.0013 (8.0x10−4 …0.0020) | 0.99 |
| Median non-lesional TEWL ((IQR) | 8.3 (6.6, 10.3) | 9.8 (6.7, 14.1) | 0.02 | 8.3 (6.6, 10.5) | 11.0 (8.0, 14.3) | 0.002 | 8.4 (6.7, 11.0) | 9.3 (6.5, 14.1) | 0.2 |
GM: geographic mean. GCI: geometric confidence interval. SPT: sensitization to any allergen. FS: Food sensitization. AS: sensitization to aeroallergens.
A correlation between skin FLG expression and sensitization load observed in non-Black but not Black children differ by 25(OH)D levels
When treating sensitization as a binary variable, we did not see associations with either skin FLG expression or 25(OH)D levels. However, simply treating children as sensitized or non-sensitized does not capture the impact of sensitization burden (the number of sensitizations a child has). We hypothesized that a gradual increase in barrier dysfunction caused by decreasing FLG expression in non-lesional skin results in a correlation with sensitization load. Indeed, when ignoring 25(OH)D levels, a weak but significant correlation was found between total sensitization load and skin FLG expression in non-Black (Rho = −0.20, p = 0.026) but not Black (Rho = −0.088, p = 0.22) children (Figure 1). Given the interplay between vitamin D and FLG, we next stratified the correlation analyses according to 25(OH)D levels. In non-Black participants with low 25(OH)D, the correlation between total sensitization load and skin FLG expression was still significant (p = 0.026) despite the lower sample size, with an increased effect size (Rho = −0.45) (Figure 2). By contrast, no significant correlation was found in non-Black participants with high 25(OH)D (rho = ‒0.15; p = 0.13). No correlation was found between total sensitization load and skin FLG expression in Black participants regardless of 25(OH)D levels. Similar results were obtained when examining FS load and AS load separately (eFigures 2–3). We next validated the 25(OH)D cutoff at the lower tertile, 27.2 ng/ml by repeating the analysis of the correlation between skin FLG expression and total sensitization load for non-Black participants with low 25(OH)D, and increasing the 25(OH)D cutoff stepwise from 20 to 30 ng/ml. We found indications of a threshold effect close to the lower tertile (eTable 2).
Figure 1.
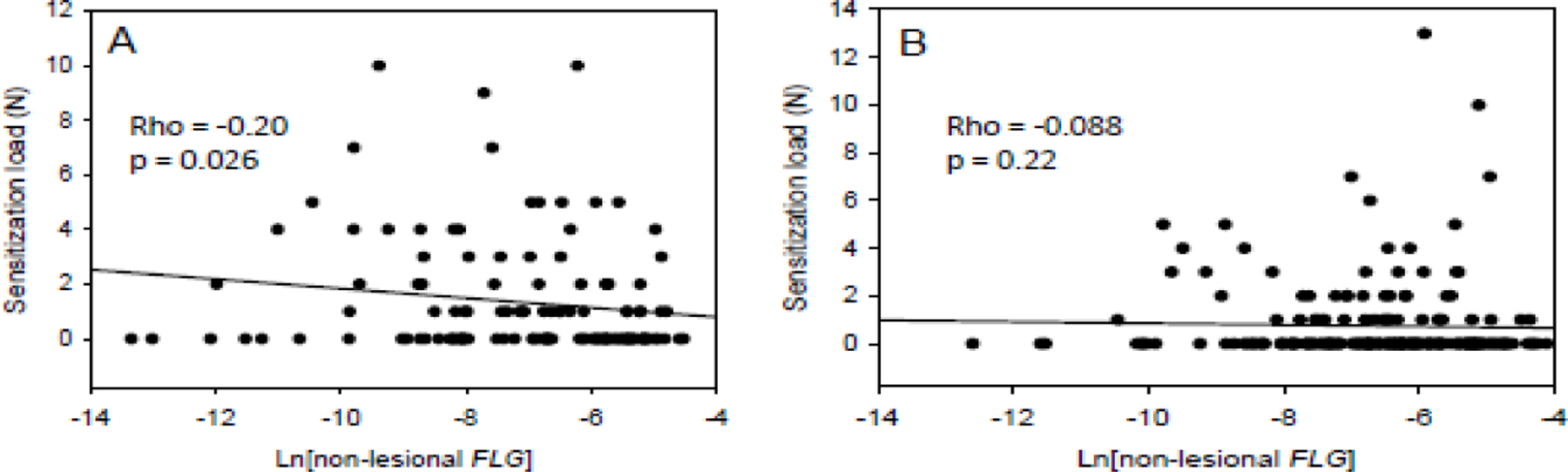
Spearman correlations between FLG expression in non-lesional skin and total sensitization load in (A) non-Black and (B) Black participants.
Figure 2.
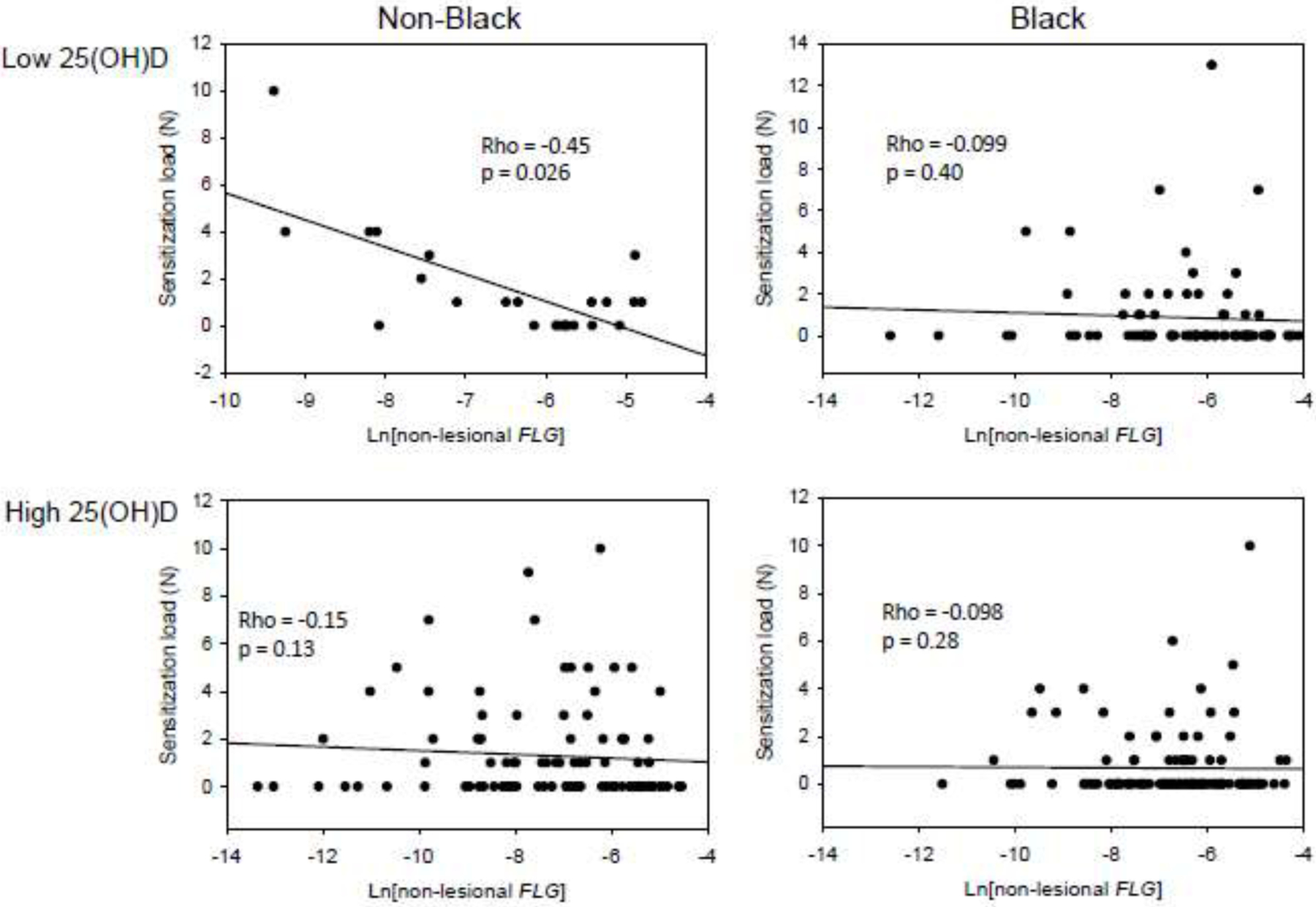
Spearman correlations between FLG expression in non-lesional skin and total sensitization load, stratified for serum 25(OH)D levels.
Low 25(OH)D levels interact with decreased skin FLG expression to increase allergic sensitization load
While the stratified analyses show different effect sizes for the correlation between FLG and sensitization load depending on 25(OH)D, these analyses do not directly test whether the effect sizes between the groups differ. To address whether effect sizes for the relationship between FLG and sensitization load differed in those with low versus higher 25(OH)D, we performed Poisson regression analysis with an interaction term Ln[non-lesional FLG]*Low 25(OH)D as well as the main effects of FLG expression and low 25(OH)D were included, accounting for other factors (sex, age, non-lesional TEWL and SCORAD) as covariates. In both non-Black and Black participants, the main effect of FLG is not significant. In contrast in non-Black participants, low 25(OH)D and the interaction effect between 25(OH)D are significant (p = 0.0015 and 0.00042, respectively), but not in Black participants (Table 5). In non-Blacks, the lack of an association between FLG and sensitization load but the presence of a significant interaction suggests that FLG is only associated when 25(OH)D is low. Similar results were obtained when the regression analysis was restricted to FS (eTable 3) or AS (eTable 4). The interaction term between serum 25(OH)D levels and skin FLG expression was significant among participants with FS and AS in the non-Black but not the Black group (p = 0.025 and 0.0083 for FS and AS, respectively, among non-Black participants). It should be noted that since a large portion of the sensitized participants were sensitized to both foods and aeroallergens, we were not able to determine if AS or FS was driving the associations. Therefore, it cannot be ruled out that one type of sensitization is confounded by the other type.
Table 5.
Multiple Poisson regression analysis of total sensitization load in non-Black vs. Black participants.
| Variable | All participants (N=289) | Non-Black (N=121) | Black (N=168) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| Estimate | p value | Estimate | p value | Estimate | p value | |
| Intercept | −1.5 | 0.00049 | −1.3 | 0.016 | −1.9 | 0.019 |
| Ln[non-lesional FLG] | −0.017 | 0.70 | −0.034 | 0.48 | 0.087 | 0.32 |
| Low 25(OH)D | −0.74 | 0.16 | −3.3 | 0.0015 | 0.12 | 0.88 |
| Male sex | 0.23 | 0.067 | −0.11 | 0.51 | 1.1 | 1.1E-06 |
| Age (yrs) | 0.25 | 0.047 | 0.35 | 0.035 | 0.31 | 0.14 |
| Non-lesional TEWL | −0.0084 | 0.17 | −0.012 | 0.28 | −0.0037 | 0.63 |
| SCORAD | 0.032 | 9.1E-21 | 0.03 | 8E-10 | 0.032 | 1.2E-09 |
| Ln[non-lesional FLG]*Low 25(OH)D | −0.099 | 0.17 | −0.47 | 0.00042 | 0.014 | 0.91 |
Since effect estimates in interaction models can be conditional on interaction terms, Poisson regression models of sensitization load were further performed in the stratified data (High 25(OH)D and Low 25(OH)D) for non-Black and Black participants separately, adjusting for sex, age, non-lesional TEWL and SCORAD. The expected number of sensitization loads were plotted against FLG expression levels for all four groups (Figure 3). The expected total number of sensitizations increased sharply with decreasing FLG expression in non-Black participants with low 25(OH)D levels, but not in non-Black participants with high 25(OH)D levels or in Black participants at either 25(OH)D category where the lines are nearly horizontal. These results in combination with the interaction analyses above suggest that low skin FLG expression is associated with higher sensitization load, but only in non-Black children with low 25(OH)D.
Figure 3.
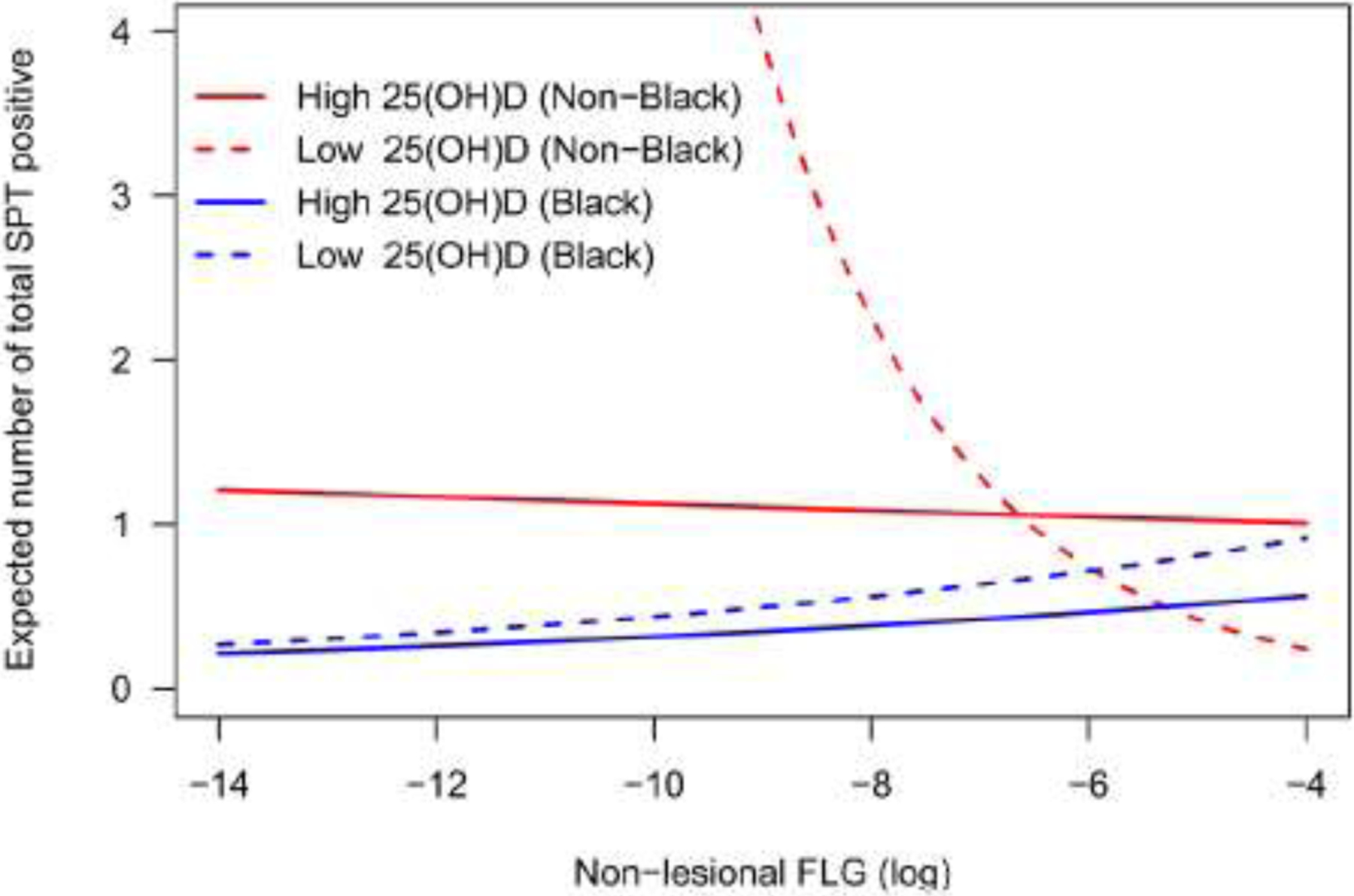
Stratified adjusted regression plots showing the dependence of expected total sensitization load on FLG expression and 25(OH)D levels. Expected total sensitization load was adjusted for sex, age, non-lesional TEWL, and SCORAD, and calculated by holding these covariates at their overall mean.
Discussion
In this study, we have demonstrated a protective effect of sufficient vitamin D levels on allergic sensitization in non-Black children when FLG expression levels in the skin were taken into account. We found that there was a complex relationship between sensitization load and non-lesional skin FLG expression, which is conditional on race and 25(OH)D levels. Specifically, while Black children had lower 25(OH)D levels, sensitization load was negatively associated with non-lesional skin FLG expression in non-Black, but not Black, children with low 25(OH)D levels. Using multiple Poisson regression analysis, we showed that there was a significant interaction between FLG expression and 25(OH)D levels in non-Black children for the outcome total sensitization load, as well as for FS and AS load. This is to our knowledge the first study demonstrating interactions between circulating vitamin D levels and a skin barrier-related marker, and this interaction was race-specific. While Vitamin D deficiency is more prevalent in Black children, its negative impact on sensitization is evident only the non-Black children, who were primarily White. Given the small sample sizes in some of these groups, future studies will need to replicate these findings and evaluate additional racial groups.
We found that Black children were more likely to have low 25(OH)D levels than non-Black children. Importantly, the lower levels of 25(OH)D in Black compared with non-Black participants were independent of season of visit, as Black participants were more likely than non-Black participants to have their study visits in the summer when vitamin D levels are the highest. The racial differences in 25(OH)D are in agreement with a previous study that showed an association between low circulating 25(OH)D levels and the degree of skin pigmentation as well as with African ancestry in an inner-city population of 6-mo- to 3-y-old children22. The lower levels of circulating vitamin D in individuals of African ancestry are believed to be the result of higher melanin content in the skin21. Melanin absorbs ultraviolet B radiation, leading to decreased photo-mediated vitamin D synthesis.
We found that Black participants in the MPAACH cohort had lower rates of allergic sensitization at the first study visit compared with non-Black participants, and appeared to be protected from the increase in sensitization despite low 25(OH)D levels. This is an intriguing finding as it is well established that Black Americans suffer from higher rates of most allergic disorders including asthma and AD23, 24. We speculate that the reason for the reduced sensitization in Blacks may have to do with factors related to the skin barrier other than FLG. Although low doses of UV radiation have been found to have beneficial effects on AD31, other studies have also found that UV radiation produces changes in skin associated with decreased barrier function, including changes in mechanical strength, tight junctions and ceramide composition32–35. It is possible that the protection from UVB radiation by melanin may protect the structure of the skin barrier in individuals with dark skin tone. Similar to a previous report36, Black participants in this study had lower TEWL than non-Black participants, which suggests overall better barrier function in Black participants. Our results are supported by a previous study showing that FLG was down-regulated in European Americans, but not African Americans, with AD37. Although AD and skin barrier dysfunction are risk factors for asthma, previous studies from our lab indicated that Black children are more likely than non-Black children to develop asthma without preceding AD38 and that Black and non-Black children with AD have different allergic trajectories30. One reason for this could be that Black children are more likely to get sensitized through the airways than through the skin. Moreover, although FLG has been found to be a major contributor to skin barrier functioning, other factors are also involved. It is worth noting that we found an association between TEWL and sensitization in Black children even though the interaction between FLG expression and 25(OH)D levels were not associated with sensitization load. This suggests that in Black children other factors not yet understood are important to the functioning of the skin barrier. Our results should not be interpreted to suggest that low vitamin D levels in Black Americans are not a matter of concern, and much remains to be learned about the health effects of low vitamin D in individuals of African ancestry39. For instance, a number of studies have shown that Black Americans have higher bone density compared with White Americans40–42 despite Blacks having lower vitamin D levels. Lower urinary calcium excretion and differences in vitamin D metabolism in individuals of African ancestry have been suggested as explanations for this paradox43.
Our finding that SCORAD in children with AD is associated allergic sensitization has been observed previously44, 45. In our study, SCORAD was a strong independent risk factor for sensitization, and it is worth noting that the effect sizes for the association between SCORAD and sensitization load in the multiple regression analyses were very similar for Black and non-Black participants.
Beyond skin biology, vitamin D is involved in both innate and adaptive immune responses, and timing of exposure may also be an important factor. Results from The Vitamin D Antenatal Asthma Reduction Trial indicated that maternal supplementation of vitamin D during pregnancy reduces asthma and recurrent wheeze in the offspring, and the authors did not find differences in this effect between Black and non-Black mothers46. Given the many diverse functions of vitamin D, it is likely that the role of race and vitamin D differs for different health outcomes and may be confounded by factors related to socioeconomic status and environment as well. Clearly additional studies are warranted.
Although associations between low vitamin D levels and allergic sensitization have been reported previously5, 15–17, in this study the relationship was only observed in non-Black participants with low levels of FLG expression in the skin. It is worth noting that although allergic sensitization as a binary variable was not associated with FLG expression, we found negative correlations between FLG expression and sensitization load as a quantitative variable, indicating that the risk of sensitization to additional allergens increases with gradually decreasing amounts of FLG in the skin in non-Black children. We also found that this correlation was conditional on serum 25(OH)D levels. Our finding that the total number of sensitizing allergens was associated with the quantitative level of FLG expression supports the importance of the skin barrier in allergic sensitization.
In this study we measured FLG expression directly from skin keratinocyte samples. Intriguingly, some studies have found associations between serum vitamin D levels and the genotype of FLG, a critical component of the skin barrier47, 48. This could be due to the UV-protective properties of urocanic acid, a filaggrin degradation product49, such that mutations that impact filaggrin, and thus urocanic acid, levels in the skin may also lead to greater UV-mediated synthesis of vitamin D. FLG expression may, however, be affected by factors other than genotype50, 51. Therefore, one major advantage of our study is that we measured FLG expression directly in the skin and thus were able to investigate the relationship between FLG levels in the skin and low 25(OH)D levels. We found that low 25(OH)D was associated with higher non-lesional FLG expression and lower non-lesional TEWL in non-Black children, which is in agreement with the earlier studies mentioned above. It is unclear how these associations are related to the protective effect of vitamin D against low FLG levels, but the relationship between FLG and vitamin D is likely complex and may involve several different pathways and mechanisms.
Our findings suggest that Black children are less sensitive to low levels of vitamin D. One potential explanation for this is that the clinically relevant cutoffs for vitamin D may be different in Black children. We found that associations with 25(OH)D levels in the bivariate analyses and the multivariate regression analyses were stronger when 25(OH)D levels were dichotomized at the lower tertile rather than treated as a continuous variable (data not shown). This finding supports the concept of a vitamin D threshold effect and has been observed before15. Additionally, a post-hoc analysis of the 25(OH)D cutoff for the stratified Spearman correlations between Ln[non-lesional FLG expression] and total sensitization load for non-Black participants, showed a sharp drop-off of significance between 28 and 29 ng/ml (eTable 2). Contrary to some previous studies, we did not detect an association between SCORAD and serum 25(OH)D levels. This could be due to the small number of participants in the lower range of serum 25(OH)D < 20 ng/ml discussed above, which is why we used a mathematical cutoff of 27.2 ng/ml at the lower tertile. We were therefore not able to explore the effect of more severe vitamin D deficiency, and our results may not be applicable to very low 25(OH)D levels. As stated, it is possible that the 25(OH)D threshold is lower in Black children compared with non-Black children. Therefore, we repeated the stratified analysis of correlation between FLG expression and sensitization load in Black participants using the conventional 25(OH)D cutoff for vitamin D deficiency, 20 ng/ml. The results were not significant but reach a statistical trend (p=0.057). 25(OH)D cutoff values for vitamin D deficiency have been developed using populations of mainly European ancestry, which may lead to effects at very low levels of vitamin D in individuals of African ancestry remaining undetected. Therefore, existing definitions of vitamin D may not be appropriate for individuals with darker skin. Larger studies are needed to explore this important question and to validate appropriate 25(OH)D cutoffs for darker-skinned populations. For this study, we utilized a cohort of children diagnosed with AD. The higher risk of sensitization among this group of children increased the power to detect associations in the analyses, but the results may not be generalizable to the general population.
This study provides compelling evidence that 25(OH)D and FLG expression interact with each other with respect to risk of allergic sensitization among non-Black participants. The group of non-Black children was primarily made up of children of European ancestry, and the results cannot be extrapolated to other non-Black racial and ethnic groups. Although the mechanism behind the interaction is not fully clear, it is possible that non-Black children with impaired skin barrier function due to low FLG expression could be protected against allergic sensitization by ensuring adequate levels of circulating vitamin D using supplements when needed.
In conclusion, we found that skin FLG expression is negatively associated with sensitization load in children with low 25(OH)D levels. This association was only seen in non-Black participants, despite significantly lower levels of 25(OH)D in Black, compared with non-Black, participants. One potential explanation is that the clinically relevant thresholds for vitamin D deficiency in Black individuals may differ from non-Black individuals and may vary depending on the clinical outcome. Our results may help explain the often conflicting findings from earlier studies of the role of vitamin D in allergic disease. Identifying specific risk groups could lead to more individualized interventions in the future.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health U19AI070235 (J.M.B., L.J.M., and G.K.K.H.) and R01AI127392–04 (J.M.B., L.J.M., and G.K.K.H.).
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- AD
atopic dermatitis
- AS
aeroallergen sensitization
- FLG
filaggrin
- FS
food sensitization
- IgE
Immunoglobulin E
- PCR
polymerase chain reaction
- SCORAD
SCORing Atopic Dermatitis
- TEWL
trans-epidermal water loss
- UV
ultraviolet
- VDR
vitamin D receptor
Footnotes
None of the authors have any conflicting financial interests to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hu L, Bikle DD, Oda Y. Reciprocal role of vitamin D receptor on beta-catenin regulated keratinocyte proliferation and differentiation. J Steroid Biochem Mol Biol 2014;144 Pt A:237–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gurlek A, Pittelkow MR, Kumar R. Modulation of growth factor/cytokine synthesis and signaling by 1alpha, 25-dihydroxyvitamin D(3): implications in cell growth and differentiation. Endocr Rev 2002;23:763–786. [DOI] [PubMed] [Google Scholar]
- 3.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol 2011;347:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol 2009;129:1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollams EM, Teo SM, Kusel M, et al. Vitamin D over the first decade and susceptibility to childhood allergy and asthma. J Allergy Clin Immunol 2017;139:472–481 e479. [DOI] [PubMed] [Google Scholar]
- 6.Jones AP, Palmer D, Zhang G, Prescott SL. Cord blood 25-hydroxyvitamin D3 and allergic disease during infancy. Pediatrics 2012;130:e1128–1135. [DOI] [PubMed] [Google Scholar]
- 7.Chawes BL, Bonnelykke K, Jensen PF, Schoos AM, Heickendorff L, Bisgaard H. Cord blood 25(OH)-vitamin D deficiency and childhood asthma, allergy and eczema: the COPSAC2000 birth cohort study. PLoS One 2014;9:e99856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim MJ, Kim SN, Lee YW, Choe YB, Ahn KJ. Vitamin D Status and Efficacy of Vitamin D Supplementation in Atopic Dermatitis: A Systematic Review and Meta-Analysis. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hattangdi-Haridas SR, Lanham-New SA, Wong WHS, Ho MHK, Darling AL. Vitamin D Deficiency and Effects of Vitamin D Supplementation on Disease Severity in Patients with Atopic Dermatitis: A Systematic Review and Meta-Analysis in Adults and Children. Nutrients 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D’s Effect on Immune Function. Nutrients 2020;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun RF, Liu PT, Modlin RL, Adams JS, Hewison M. Impact of vitamin D on immune function: lessons learned from genome-wide analysis. Front Physiol 2014;5:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkataraman D, Soto-Ramirez N, Kurukulaaratchy RJ, et al. Filaggrin loss-of-function mutations are associated with food allergy in childhood and adolescence. J Allergy Clin Immunol 2014;134:876–882 e874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sherenian MG, Kothari A, Biagini JM, et al. Sensitization to peanut, egg or pets is associated with skin barrier dysfunction in children with atopic dermatitis. Clin Exp Allergy 2021;51:666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brough HA, Nadeau KC, Sindher SB, et al. Epicutaneous sensitization in the development of food allergy: What is the evidence and how can this be prevented? Allergy 2020;75:2185–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharief S, Jariwala S, Kumar J, Muntner P, Melamed ML. Vitamin D levels and food and environmental allergies in the United States: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol 2011;127:1195–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baek JH, Shin YH, Chung IH, et al. The link between serum vitamin D level, sensitization to food allergens, and the severity of atopic dermatitis in infancy. J Pediatr 2014;165:849–854 e841. [DOI] [PubMed] [Google Scholar]
- 17.Kannan S, Perzanowski MS, Ganguri HB, et al. Complex relationships between vitamin D and allergic sensitization among Puerto Rican 2-year-old children. Ann Allergy Asthma Immunol 2018;120:84–89. [DOI] [PubMed] [Google Scholar]
- 18.Rosendahl J, Pelkonen AS, Helve O, et al. High-Dose Vitamin D Supplementation Does Not Prevent Allergic Sensitization of Infants. J Pediatr 2019;209:139–145 e131. [DOI] [PubMed] [Google Scholar]
- 19.Savilahti EM, Makitie O, Kukkonen AK, et al. Serum 25-Hydroxyvitamin D in Early Childhood Is Nonlinearly Associated with Allergy. Int Arch Allergy Immunol 2016;170:141–148. [DOI] [PubMed] [Google Scholar]
- 20.Wawro N, Heinrich J, Thiering E, et al. Serum 25(OH)D concentrations and atopic diseases at age 10: results from the GINIplus and LISAplus birth cohort studies. BMC Pediatr 2014;14:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor MY, Thoreson CK, Ramsey NL, Ricks M, Sumner AE. The uncertain significance of low vitamin D levels in African descent populations: a review of the bone and cardiometabolic literature. Prog Cardiovasc Dis 2013;56:261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carpenter TO, Herreros F, Zhang JH, et al. Demographic, dietary, and biochemical determinants of vitamin D status in inner-city children. Am J Clin Nutr 2012;95:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brunner PM, Guttman-Yassky E. Racial differences in atopic dermatitis. Ann Allergy Asthma Immunol 2019;122:449–455. [DOI] [PubMed] [Google Scholar]
- 24.Akinbami LJ, Simon AE, Rossen LM. Changing Trends in Asthma Prevalence Among Children. Pediatrics 2016;137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biagini Myers JM, Sherenian MG, Baatyrbek Kyzy A, et al. Events in Normal Skin Promote Early-Life Atopic Dermatitis-The MPAACH Cohort. J Allergy Clin Immunol Pract 2020;8:2285–2293 e2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol Suppl (Stockh) 1980;92:44–47. [Google Scholar]
- 27.von Kobyletzki LB, Berner A, Carlstedt F, Hasselgren M, Bornehag CG, Svensson A. Validation of a parental questionnaire to identify atopic dermatitis in a population-based sample of children up to 2 years of age. Dermatology 2013;226:222–226. [DOI] [PubMed] [Google Scholar]
- 28.Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993;186:23–31. [DOI] [PubMed] [Google Scholar]
- 29.Stevens ML, Gonzalez T, Schauberger E, et al. Simultaneous skin biome and keratinocyte genomic capture reveals microbiome differences by depth of sampling. J Allergy Clin Immunol 2020;146:1442–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Biagini JM, Kroner JW, Baatyrbek Kyzy A, et al. Longitudinal atopic dermatitis endotypes: An atopic march paradigm that includes Black children. J Allergy Clin Immunol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thyssen JP, Zirwas MJ, Elias PM. Potential role of reduced environmental UV exposure as a driver of the current epidemic of atopic dermatitis. J Allergy Clin Immunol 2015;136:1163–1169. [DOI] [PubMed] [Google Scholar]
- 32.Alhasaniah A, Sherratt MJ, O’Neill CA. The Impact of Ultraviolet Radiation on Barrier Function in Human Skin: Molecular Mechanisms and Topical Therapeutics. Curr Med Chem 2018;25:5503–5511. [DOI] [PubMed] [Google Scholar]
- 33.Biniek K, Levi K, Dauskardt RH. Solar UV radiation reduces the barrier function of human skin. Proc Natl Acad Sci U S A 2012;109:17111–17116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuki T, Hachiya A, Kusaka A, et al. Characterization of tight junctions and their disruption by UVB in human epidermis and cultured keratinocytes. J Invest Dermatol 2011;131:744–752. [DOI] [PubMed] [Google Scholar]
- 35.Meguro S, Arai Y, Masukawa Y, Uie K, Tokimitsu I. Relationship between covalently bound ceramides and transepidermal water loss (TEWL). Arch Dermatol Res 2000;292:463–468. [DOI] [PubMed] [Google Scholar]
- 36.Gupta J, Grube E, Ericksen MB, et al. Intrinsically defective skin barrier function in children with atopic dermatitis correlates with disease severity. J Allergy Clin Immunol 2008;121:725–730 e722. [DOI] [PubMed] [Google Scholar]
- 37.Sanyal RD, Pavel AB, Glickman J, et al. Atopic dermatitis in African American patients is TH2/TH22-skewed with TH1/TH17 attenuation. Ann Allergy Asthma Immunol 2019;122:99–110 e116. [DOI] [PubMed] [Google Scholar]
- 38.Johansson E, Biagini Myers JM, Martin LJ, et al. Identification of two early life eczema and non-eczema phenotypes with high risk for asthma development. Clin Exp Allergy 2019;49:829–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ames BN, Grant WB, Willett WC. Does the High Prevalence of Vitamin D Deficiency in African Americans Contribute to Health Disparities? Nutrients 2021;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Looker AC, Melton LJ, 3rd, Borrud LG, Shepherd JA. Lumbar spine bone mineral density in US adults: demographic patterns and relationship with femur neck skeletal status. Osteoporos Int 2012;23:1351–1360. [DOI] [PubMed] [Google Scholar]
- 41.Gutierrez OM, Farwell WR, Kermah D, Taylor EN. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 2011;22:1745–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Ballegooijen AJ, Robinson-Cohen C, Katz R, et al. Vitamin D metabolites and bone mineral density: The multi-ethnic study of atherosclerosis. Bone 2015;78:186–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsu S, Hoofnagle AN, Gupta DK, et al. Race, Ancestry, and Vitamin D Metabolism: The Multi-Ethnic Study of Atherosclerosis. J Clin Endocrinol Metab 2020;105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoos AM, Chawes BL, Bonnelykke K, Stokholm J, Rasmussen MA, Bisgaard H. Increasing severity of early-onset atopic dermatitis, but not late-onset, associates with development of aeroallergen sensitization and allergic rhinitis in childhood. Allergy 2021. [DOI] [PubMed] [Google Scholar]
- 45.Ha EK, Kim JH, Lee SW, et al. Atopic dermatitis: Correlation of severity with allergic sensitization and eosinophilia. Allergy Asthma Proc 2020;41:428–435. [DOI] [PubMed] [Google Scholar]
- 46.Wolsk HM, Harshfield BJ, Laranjo N, et al. Vitamin D supplementation in pregnancy, prenatal 25(OH)D levels, race, and subsequent asthma or recurrent wheeze in offspring: Secondary analyses from the Vitamin D Antenatal Asthma Reduction Trial. J Allergy Clin Immunol 2017;140:1423–1429 e1425. [DOI] [PubMed] [Google Scholar]
- 47.Thyssen JP, Thuesen B, Huth C, et al. Skin barrier abnormality caused by filaggrin (FLG) mutations is associated with increased serum 25-hydroxyvitamin D concentrations. J Allergy Clin Immunol 2012;130:1204–1207 e1202. [DOI] [PubMed] [Google Scholar]
- 48.Skaaby T, Husemoen LL, Martinussen T, et al. Vitamin D status, filaggrin genotype, and cardiovascular risk factors: a Mendelian randomization approach. PLoS One 2013;8:e57647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mildner M, Jin J, Eckhart L, et al. Knockdown of filaggrin impairs diffusion barrier function and increases UV sensitivity in a human skin model. J Invest Dermatol 2010;130:2286–2294. [DOI] [PubMed] [Google Scholar]
- 50.Thyssen JP, Kezic S. Causes of epidermal filaggrin reduction and their role in the pathogenesis of atopic dermatitis. J Allergy Clin Immunol 2014;134:792–799. [DOI] [PubMed] [Google Scholar]
- 51.Drislane C, Irvine AD. The role of filaggrin in atopic dermatitis and allergic disease. Ann Allergy Asthma Immunol 2020;124:36–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


