Keywords: brain metabolism and function, cognition, diabetes, magnetic resonance spectroscopy, mood
Abstract
Brain mechanisms underlying the association of diabetes metabolic disorders—hyperglycemia and insulin resistance—with cognitive impairment are unknown. Myoinositol is a brain metabolite involved in cell osmotic balance, membrane phospholipid turnover, and second messenger neurotransmission, which affect brain function. Increased brain myoinositol and altered functional connectivity have been found in diabetes, mild cognitive impairment, and Alzheimer’s disease, but the independent effects of plasma glucose and insulin on brain myoinositol and function are not characterized. We measured myoinositol concentrations in the pregenual anterior cingulate cortex (ACC), a region involved in self-reflective awareness and decision making, using proton magnetic resonance spectroscopy, and whole brain resting-state functional connectivity using fMRI, during acute hyperglycemia (with attendant hyperinsulinemia) and euglycemic-hyperinsulinemia compared with basal fasting-euglycemia (EU) in 11 healthy nondiabetic participants (5 women/6 men, means ± SD, age: 27 ± 7 yr, fasting-glucose: 5.2 ± 0.4 mmol/L, fasting-insulin: 4.9 ± 4.4 μU/mL). Brain MR data were acquired during two separate visits: 1) EU followed by a 60-min hyperglycemic-clamp (glucose: 10.7 ± 0.2 mmol/L, insulin: 33 ± 6 μU/mL); 2) EU followed by a hyperinsulinemic-euglycemic-clamp (glucose: 5.3 ± 0.1 mmol/L, insulin: 27 ± 5 μU/mL) designed to match individual insulin levels achieved during the visit 1 hyperglycemic-clamp. Myoinositol decreased by 14% during the hyperglycemic-clamp (from 7.7 ± 1.5 mmol/kg to 6.6 ± 0.8 mmol/kg, P = 0.031), and by 9% during the hyperinsulinemic-euglycemic-clamp (from 7.1 ± 0.7 mmol/kg to 6.5 ± 0.7 mmol/kg, P = 0.014), with no significant difference between the two clamps. Lower myoinositol was associated with higher functional connectivity of the thalamus and precentral cortex with insula-ACC-related networks, suggesting myoinositol is involved in insulin modulation of cognitive/emotional network function in healthy adults. Regional brain myoinositol levels may be useful biomarkers for monitoring cognitive and mood-enhancing treatment responses.
NEW & NOTEWORTHY Hyperinsulinemia-related decreases of brain anterior cingulate cortex (ACC) myoinositol independent of plasma glucose levels and the association of low ACC myoinositol with increased functional connectivity between sensorimotor regions and ACC/insula-related networks suggest involvement of myoinositol in insulin-modulated brain network function in healthy adults. In diabetes, elevated brain myoinositol may be due to reduced brain insulin levels or action, rather than hyperglycemia, and may be involved in brain network dysfunctions leading to cognitive or mood disorders.
INTRODUCTION
Recent findings of associations between metabolic disorders, depressive symptoms, and cognitive impairment have stimulated increased interest in the effects of pathological variations of plasma glucose and insulin levels on brain metabolism and function (1). Diabetes—with characteristic hyperglycemia due to reduced insulin production in type 1 (T1D) or sensitivity in type 2 (T2D)—is implicated in the pathophysiology of major depressive disorder, cognitive impairment, and dementia. In T1D, elevated glycated hemoglobin (HbA1c) and acute hyperglycemia are associated with altered brain function and metabolism (2–4), and chronic hyperglycemia is implicated in mood and cognitive impairments (5, 6). T2D, prediabetes, and metabolic syndrome are characterized by insulin resistance, chronic hyperglycemia, and hyperinsulinemia, which are associated with altered brain metabolites (7, 8) and function (9–11), and with an elevated risk for mild cognitive impairment and Alzheimer’s disease (12, 13).
It is not known whether brain metabolic and functional alterations are due to the secondary effects of elevated plasma glucose, dysregulation of insulin secretion or action, or to other disease-related causes. Changes in plasma glucose and insulin may affect brain metabolism and function by independent as well as interdependent mechanisms. Therefore, determining the independent brain effects of elevated plasma glucose and insulin should help provide better targets for treatment of the associated mood and cognitive impairments. Plasma glucose and insulin levels are tightly linked by homeostatic metabolic regulation: in healthy individuals, the physiological response to postprandial increases in circulating glucose is a concurrent increase in circulating insulin. Thus, although recent studies using intranasal administration of insulin have helped elucidate the direct effects of insulin on brain function (14–16), the independent effects of hyperglycemia and hyperinsulinemia on the brain are more difficult to study using glucose ingestion or hyperinsulinemic clamps to systematically change circulating insulin and glucose levels (17–20), and the neurochemical mechanisms of insulin or glucose action on brain function are not clearly defined in healthy humans.
Magnetic resonance spectroscopy (MRS) allows the noninvasive measurement of regional brain concentrations of low-molecular-weight metabolites, including myoinositol (MI). We examined brain MI because it is elevated in T2D (21–23), in T1D during hyperglycemia (21, 24, 25), in metabolic syndrome (7), as well as in patients with mild cognitive impairment and Alzheimer’s disease (26). MI is an osmotic regulator maintaining cellular structural integrity, a precursor to cell-membrane phospholipids, and its phosphorylated metabolites play roles as second messengers for signal transduction in the phosphatidyl-inositol-3-kinase (PI3K) system (27). Thus, brain regional variations in MI concentration may constitute direct or indirect responses to diverse metabolic, physiologic, or functional modifications and may also have diverse consequences on brain function.
Functional magnetic resonance imaging (fMRI) noninvasively measures indices of regional brain function (28, 29). We have previously shown altered functional connectivity in the executive control network associated with elevated HbA1c in T1D (3) as well as alterations in the default mode network associated with insulin resistance in T2D (11).
To examine the independent effects of acute hyperglycemia and hyperinsulinemia on regional brain MI and its relation to brain resting-state-network functional connectivity, we measured MI in limbic frontal (pgACC: pregenual anterior cingulate) and visual occipital (OCC) cortex regions, and whole brain resting-state-network functional connectivity during basal fasting euglycemia, a hyperglycemic clamp, and individually matched-insulin level euglycemic clamp conditions in healthy individuals. We also measured total creatine (TCr: creatine + phosphocreatine) to control for potential variation in energetic buffer. We focused on the pgACC because of its implication in cognitive/emotional control. The pgACC is a critical part of the brain’s associative processing network, involved in self-reflective awareness and evaluation of internal and external sensory input for decision making (30). We selected the OCC region to control for global effects. We hypothesized that pgACC MI concentrations would decrease during conditions of increased plasma insulin and would be related to functional connectivity in ACC-related networks.
METHODS
Study Approval
The study was conducted according to the principles of the Declaration of Helsinki. The protocol was approved by the Institutional Review Board of the Beth Israel Deaconess Medical Center (BIDMC), Boston, MA. All participants gave voluntary written informed consent before participation. This study is registered on ClinicalTrials.gov (NCT05355285).
Participants
The study sample consisted of 11 right-handed healthy volunteers (6 men and 5 women), mean (±SD) age 27 ± 7 yr, years of education 17 ± 2, body mass index (BMI) 24.8 ± 3.8 kg/m2, HbA1c 5.5 ± 0.2%, fasting plasma glucose 5.2 ± 0.4 mmol/L, and fasting insulin 4.9 ± 4.4 µU/mL, homeostasis model assessment-insulin resistance (HOMA-IR) 1.16 ± 1.13. The WASI IQ and the grooved pegboard using dominant and nondominant hand assessed cognition and psychomotor speed, respectively. All participants also were assessed for depressive symptoms using the Hamilton (HAM-D) and the SCL-90-R (revised symptom checklist) depression rating scores. Participants were excluded if they had clinically significant cardiovascular, neurologic, or Axis I psychiatric disease, malignancy, drug or alcohol abuse, diabetes, or any contraindications to MRI such as metallic implants, pregnancy, or claustrophobia. All study procedures were performed at the Clinical Research Center and MRI Research Center of the BIDMC. After the screening visit to determine inclusion criteria, all participants came to the BIDMC for two MRI/MRS study visits separated by 2–4 wk.
Experimental Design
The study protocol is shown in Fig. 1 and described in the next paragraph. All studies were performed between 0800 and 1100 after an overnight fast. The clamp technique is described in detail elsewhere (3, 31) and briefly summarized here. An intravenous catheter was inserted into an antecubital vein for the administration of dextrose and/or insulin, and a second catheter was inserted into a distal forearm or hand vein for the withdrawal of blood samples. A heated gel pack was used to warm the hand to arterialize the venous blood. During the entire clamp protocol, glucose levels were measured every 5 min, and insulin was measured every 10 min. Plasma glucose was measured by the glucose oxidase method, and insulin was measured by enzyme-linked immunosorbent assay (ELISA).
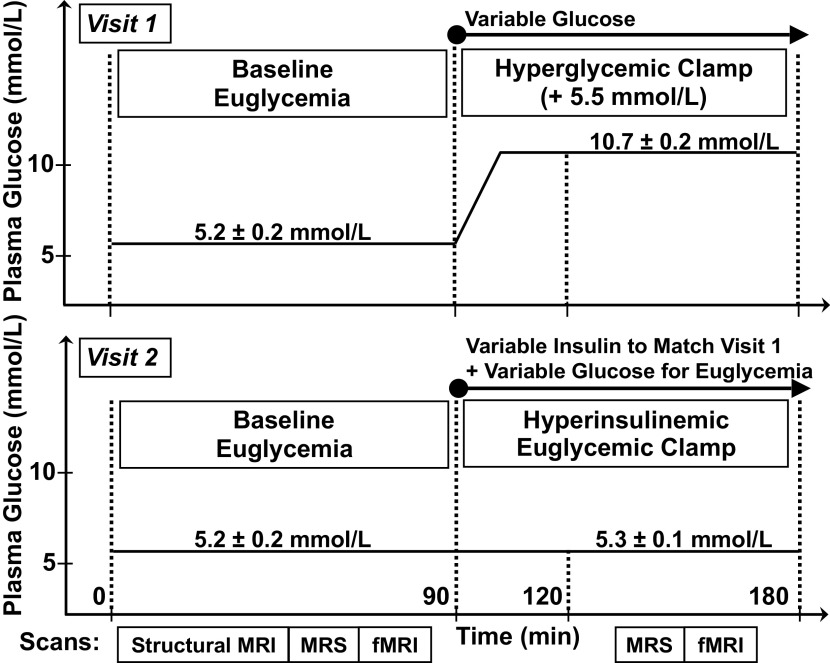
Figure 1.
Experimental protocol—during visit 1 (top), a fasting basal euglycemic period was followed by a hyperglycemic clamp with variable glucose infusion to attain a target of basal +5.5 mmol/L. During visit 2 (bottom), performed at least 15 days after the first visit, a fasting basal euglycemic period was followed by a hyperinsulinemic euglycemic clamp with variable insulin and variable glucose infusion; individual insulin levels were matched to the levels attained during the hyperglycemic clamp of visit 1. Magnetic resonance imaging (MRI)/magnetic resonance spectroscopy (MRS) scans were performed during each of the four plasma glucose/insulin periods, indicated by boxes at the bottom of the figure. The means ± standard error values of plasma glucose are indicated for each visit and condition.
During study visit 1, MRI/MRS was first performed in the fasting euglycemic state; participants then exited the scanner to begin dextrose infusion for the hyperglycemic clamp. After 30 min, when plasma glucose levels were stabilized at the target level of +5.5 mmol/L above the basal euglycemic level, participants were repositioned in the scanner and MRI/MRS was repeated during the hyperglycemic clamp. Study visit 2 was a hyperinsulinemic euglycemic clamp designed to match insulin levels achieved in study visit 1 while maintaining plasma glucose at basal euglycemic levels. During study visit 2, MRI/MRS was again performed in the fasting state; participants exited the scanner to begin a variable insulin infusion ranging from 0.20 to 1.0 mU/kg/min that was administered to reproduce the plasma insulin levels achieved by the participant during study visit 1. Dextrose was infused to maintain the plasma glucose concentration at fasting levels (3, 31). After 30 min, when insulin levels were stabilized at the target level of the participant’s visit 1 clamp level, MRI/MRS was repeated during the euglycemic hyperinsulinemic clamp. The order of the experiments was the same for all participants: the hyperglycemic clamp experiment was performed on the first study visit day followed by the hyperinsulinemic euglycemic clamp experiment on the second study visit day. This allowed measurement of the individual plasma insulin levels attained during hyperglycemia of visit 1, to match these levels during the euglycemic hyperinsulinemic clamp of visit 2 for each individual.
Magnetic Resonance Imaging and Spectroscopy Data Acquisition
MRI and MRS data were acquired using a 3-T Signa HDxt MR scanner (LX 15.0, GE Healthcare, Chicago, IL) with an eight-channel proton head coil. We acquired T1-weighted structural images using a MPRAGE sequence [magnetization prepared rapid gradient echo acquisition with repetition time/echo time (TR/TE) = 7,000/3 ms, two averages, flip angle 8°, in-plane matrix size = 256 × 256, field of view (FOV) = 25.6 cm, slice thickness = 1.6 mm, 124 sagittal slices, fat saturation, inversion recovery inversion time (TI) = 650 ms, and bandwidth = 31.25 kHz]. Structural images were used for accurate MRS voxel positioning and determination of partial volume fractions of gray and white matter and cerebrospinal fluid in the MRS voxel. The partial volume fractions were used for absolute quantitation of metabolites in MRS data analyses. We acquired single-voxel MRS using the point resolved spectroscopy (PRESS) sequence with TR/TE = 2,000/35 ms, 5 kHz spectral width, 4,096 complex points, 128 averages in two volumes of 2 × 2 × 2 cm3 positioned in the pgACC and the OCC centered on the calcarine fissure (Fig. 2). For each water-suppressed metabolite MRS signal acquisition, we acquired the nonsuppressed water signal in the same voxel to be used as a quantitation reference for water scaling. We acquired blood oxygen level-dependent (BOLD) fMRI data using a gradient-echo echo planar imaging (EPI) sequence with TR/TE = 2,000/25 ms, flip angle 90°, in-plane matrix size = 64 × 64, FOV = 24 × 24, axial slice thickness = 4 mm with 1 mm gap; 180 whole head volumes were acquired for a fMRI run duration of 6 min.
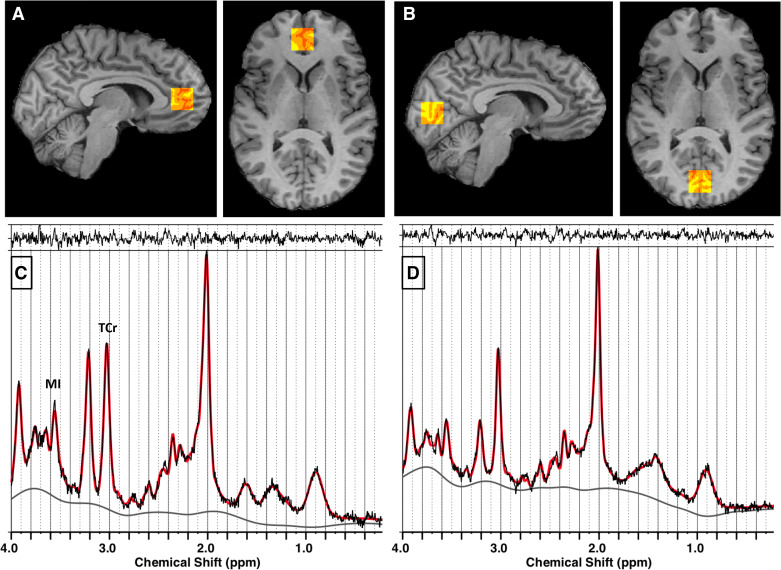
Figure 2.
Localization of voxels and LCModel spectral analyses for magnetic resonance spectroscopy (MRS) in pregenual anterior cingulate cortex (pgACC; A and C) and occipital cortex (OCC; B and D). A and B: red-yellow scale boxes overlaid on a participant’s T1-weighted image in gray scale on sagittal (left) and axial (right) slices show the voxel regions where MRS data were acquired. The colors show the results of the brain tissue segmentation inside the voxel as follows: red, cerebrospinal fluid; orange, gray matter; yellow, white matter. A: pgACC. B: OCC. C and D: MRS analyzed using LCModel—In vivo point resolved spectroscopy (PRESS) spectral data are shown in black, LCModel fit shown in red. The top margin shows the residual to the fit, the bottom gray line is the spectral baseline fit. MRS from pgACC (C) and MRS from OCC (D). MI, myoinositol; TCr, total creatine (creatine + phosphocreatine).
Magnetic Resonance Spectroscopy and Imaging Data Analyses
MRS.
We analyzed MRS data using the LCModel software (v6.3-1L) (32), which performs automatic quantitation of in vivo proton MR spectra as linear combinations of model metabolite spectra. The model basis set used for analysis was composed of 17 metabolite spectra that were simulated in-house using the Vespa (33) software graphical interface to the GAMMA magnetic resonance simulation library (34), as well as macromolecule and lipid spectra that were simulated by LCModel (for details see Ref. 4). To perform absolute quantitation of the selected MI and TCr metabolites in units of mmol/kg wet weight brain tissue, we scaled the metabolite signal to the water signal from the same voxel using LCModel’s water scaling method and estimated the water concentration in each voxel by using the voxel partial volume fractions of gray and white matter and cerebrospinal fluid (CSF) (35, 36) obtained by segmentation of the T1-weighted structural image. T1-weighted images were segmented using the FMRIB software library FSL v5.0.10 (https://fsl.fmrib.ox.ac.uk/fsl) (37, 38) FAST segmentation tool.
The application of spectral quality control exclusion criteria of full width at half maximum (FWHM) > 10 Hz, signal-to-noise ratio (SNR) < 10, and FWHM within-visit changes by condition (visit 1: basal euglycemia to hyperglycemic clamp, or visit 2: basal euglycemia to hyperinsulinemic euglycemic clamp) > 3 Hz, did not result in the exclusion of any spectra (Table 1). The LCModel metabolite Cramér-Rao lower bound (CRLB) relative (%) and absolute (mM) estimates were examined to establish measurement reliability. One spectral data set was excluded from analyses based on excessively high absolute CRLB for MI (>3 SD). During all conditions, the average ± SD (range) relative CRLB was as follows: for MI 6.2 ± 1.2% [4%–11%], for TCr 2.5 ± 0.7% [2%–4%]; the absolute CRLB was as follows: for MI 0.32 ± 0.07 mM [0.22–0.55 mM], for TCr 0.16 ± 0.04 mM [0.13–0.30 mM]. This indicates that MI and TCr concentrations were reliably detected with high precision during all conditions in both regions. Furthermore, there was no significant between-condition difference in LCModel CRLB values in either pgACC or OCC for either MI or TCr, indicating that there was no measurement precision bias in the condition comparison analyses (Table 1).
Table 1.
Reliability of metabolite concentration estimations in pregenual anterior cingulate cortex and occipital lobe cortex
| EU1 | HG | EU2 | HI | P | |
|---|---|---|---|---|---|
| pgACC | |||||
| SNR | 19 ± 5 | 18 ± 3 | 19 ± 4 | 19 ± 4 | 0.944 |
| FWHM, Hz | 7 ± 2 | 6 ± 1 | 6 ± 1 | 6 ± 1 | 0.643 |
| CRLB MI, mM | 0.38 ± 0.09 | 0.38 ± 0.04 | 0.38 ± 0.05 | 0.36 ± 0.07 | 0.909 |
| CRLB TCr, mM | 0.21 ± 0.05 | 0.19 ± 0.03 | 0.19 ± 0.05 | 0.19 ± 0.04 | 0.586 |
| OCC | |||||
| SNR | 31 ± 5 | 29 ± 4 | 30 ± 4 | 30 ± 5 | 0.851 |
| FWHM, Hz | 5 ± 1 | 6 ± 2 | 5 ± 1 | 5 ± 1 | 0.801 |
| CRLB MI, mM | 0.26 ± 0.02 | 0.27 ± 0.02 | 0.28 ± 0.02 | 0.27 ± 0.03 | 0.224 |
| CRLB TCr, mM | 0.14 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.2 | 0.14 ± 0.02 | 0.987 |
Values are means ± SD. CRLB, absolute Cramér-Rao lower bounds from LCModel analysis; EU1, visit 1, basal euglycemia; EU2, visit 2, basal euglycemia; FWHM, full width at half maximum from LCModel analysis; HG, visit 1, hyperglycemic clamp; HI, visit 2, matched hyperinsulinemic euglycemic clamp; MI, myoinositol; OCC, occipital lobe cortex; P, P values from one-way ANOVA across conditions; pgACC, pregenual anterior cingulate cortex; SNR, signal-to-noise ratio from LCModel analysis; TCr, total creatine (sum of creatine and phosphocreatine).
fMRI.
We used the FIX (FMRIB’s ICA-based X-noiseifier) software tool with a training set of 18 hand-classified fMRI time series to de-noise each fMRI data time series (39, 40) before processing and analyses. We used FSL software tools to process and analyze the fMRI time series data according to methods previously described by us (3). Briefly, fMRI data preprocessing included slice time correction, head motion correction, nonbrain removal, spatial smoothing by a Gaussian kernel with full-width at half-height of 8 mm, grand-mean intensity normalization of the entire four-dimensional (4-D) data set by a single multiplicative factor and high-pass temporal filtering by subtraction of a Gaussian-weighted least-squares straight line fitting with sigma = 50 s. All images were registered to MNI-152 standard space with 2 mm × 2 mm × 2 mm resolution. One fMRI data set was excluded from analyses due to excessive head movement (>3 mm translation or >3° rotation).
We used independent component analysis (ICA) with a dual regression approach of fMRI (41) to determine the relationship between pgACC MI concentrations and functional connectivity of resting-state networks. The ICA used the fMRI temporal series of all participants during all conditions to determine temporally correlated independent components (IC) that were common to all participants during all conditions, including functionally relevant resting-state networks as well as artifacts. By setting the number of ICs to 20, we obtained 12 component spatial patterns consistent with the literature for frequently reported resting-state networks (e.g., default mode network, sensorimotor network, etc.) (42), as well as IC patterns that overlapped with the pgACC region used for MRS measures and that have been shown to be relevant to variations in plasma glucose and insulin levels or sensitivity based on our studies (3, 11) and others (43, 44). Five of the component patterns were identified as noise (39, 40, 45). For the pgACC MI concentration by functional-connectivity correlational analysis, we selected a total of four resting-state networks that had spatial overlap with the ACC by visual inspection. In the first stage of the dual regression, each participant’s condition-specific resting-state network was regressed against the full group-condition ICA to obtain the participant-specific time courses corresponding to each IC map. The full set of 20 ICs was used at this stage to help reduce unexplained variance in the multiple regression. The participant-specific time courses from the first stage were then used in a second multiple regression of each participant’s fMRI data against the full set of time courses to obtain each participant’s spatial pattern of the resting-state network identified in the full group-condition ICA during each condition. Estimation and inference of the brain regions with a significant correlation between pgACC MI concentration and resting-state-network functional connectivity was done using a standard group general linear model (GLM) approach with nonparametric permutation testing using FSL Randomise (46, 47) with 5,000 permutations, with the demeaned pgACC MI concentration as the explanatory variable on each of the four selected ACC-related resting-state networks, and with cluster-based thresholding corrected for multiple comparisons by using the null distribution of the maximum cluster size. The cluster-forming threshold was z = 2.3, and a familywise error corrected threshold of P < 0.05 was used. Individual average functional connectivity in regions with significant correlations between functional connectivity and ACC MI was calculated using the FSLSTATS tool.
Statistical Analysis
Statistical analyses were performed using the software package R. Plasma glucose, plasma insulin, and pgACC metabolite concentrations were analyzed using paired t tests between conditions. To assess the relationship between pgACC MI concentrations and average regional functional connectivity, we calculated Pearson correlation coefficients using bivariate linear fitting. Results were considered significant at two-tailed P value <0.05.
RESULTS
Cognition, Mood, and Psychomotor Performance
The participants’ mean (±SD) cognition score on the WASI IQ was 120 ± 8. The depressive symptom rating score on the HAM-D was 0.3 ± 0.4 and on the SCL-90-R depression was 1.1 ± 1.5. The psychomotor speed performance time on the grooved pegboard with dominant hand was 60 ± 8 s, and with nondominant hand was 68 ± 9 s.
Plasma Glucose and Insulin
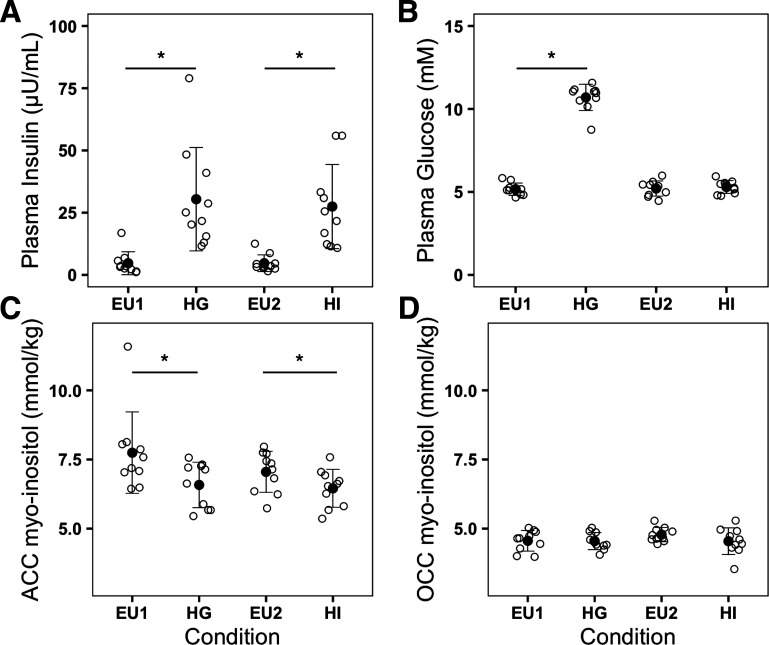
During visit 1, the mean (±SE) plasma glucose concentration during basal euglycemia was 5.2 ± 0.1 mmol/L and increased to 10.7 ± 0.2 mmol/L during the +5.5 mmol/L hyperglycemic clamp. Visit 1 mean (±SE) plasma insulin during basal euglycemia was 4.9 ± 1.3 µU/mL and increased to 32.5 ± 6.3 µU/mL during the hyperglycemic clamp. During visit 2, plasma glucose was 5.2 ± 0.1 mmol/L during basal euglycemia and 5.3 ± 0.1 mmol/L during the hyperinsulinemic euglycemic clamp. Visit 2 plasma insulin concentration was 4.7 ± 1.1 µU/mL during basal euglycemia and 27.5 ± 5.3 µU/mL during the hyperinsulinemic euglycemic clamp (Fig. 3). There was no significant difference between plasma glucose concentrations during both visits’ basal euglycemic periods (visit 1 vs. visit 2, P = 0.786). Plasma insulin concentrations were significantly increased during both visits’ clamp periods compared with their respective baseline concentrations (visit 1 basal euglycemia vs. hyperglycemic clamp: P = 0.0009; visit 2 basal euglycemia vs. hyperinsulinemic euglycemic clamp: P = 0.0007); there was no significant difference of plasma insulin during the hyperglycemic clamp compared with the hyperinsulinemic euglycemic clamp (P = 0.329) (Fig. 3). Thus, as per experimental design, high plasma insulin levels were matched between the hyperglycemic and hyperinsulinemic euglycemic clamps, whereas plasma glucose levels were significantly different.
Figure 3.
Plasma insulin, plasma glucose, and brain myoinositol concentrations—black empty circles represent the individual plasma insulin concentrations (µU/mL) (A); plasma glucose concentrations (mM) (B); pregenual anterior cingulate cortex (pgACC) myoinositol concentrations (mmol/kg brain tissue) (C); occipital cortex (OCC) myoinositol concentrations (mmol/kg brain tissue) (D) during each condition. Black filled circles and error bars represent the means ± SD of variables during each condition. Conditions: EU1 and EU2, basal euglycemia during visits 1 and 2, respectively; HG, hyperglycemic clamp during visit 1; HI, hyperinsulinemic euglycemic clamp during visit 2 with individual insulin levels matched to those of visit 1. *P < 0.05 two-sided paired t test.
Brain Metabolites by MRS
Brain metabolite concentrations were comparable with values found in the literature for healthy individuals using similar 3 T MRS acquisition and analysis methods, as well as those found at higher field strengths (48, 49). Basal euglycemic concentrations of MI and TCr in the pgACC were significantly higher than those in the OCC (Table 2). Instrumental factors, such as radiofrequency field inhomogeneity or spatial variation in coil sensitivity, as well as biological factors, such as brain tissue metabolite and water relaxation times, the difference in ratios of gray to white matter between the pgACC and the OCC voxels in this study, and the reported tendency of higher concentrations of MI in gray than in white matter (50), could have potentially contributed to the observed metabolite concentration differences between frontal and occipital lobe voxels.
Table 2.
Concentrations of metabolites in mmol/kg of wet-weight brain tissue in pregenual anterior cingulate cortex and occipital lobe cortex
| EU1 | HG | HG—EU1 | P | EU2 | HI | HI—EU2 | P | HG-HI | P | |
|---|---|---|---|---|---|---|---|---|---|---|
| Metabolite | pgACC | |||||||||
| MI | 7.7 ± 1.5 | 6.6 ± 0.8 | −1.1 ± 1.4 | 0.031 | 7.1 ± 0.7 | 6.5 ± 0.7 | −0.6 ± 0.6 | 0.014 | 0.1 ± 0.9 | 0.663 |
| TCr | 8.3 ± 0.5 | 8.2 ± 0.7 | −0.1 ± 0.6 | 0.452 | 8.6 ± 0.7 | 8.2 ± 0.5 | −0.4 ± 0.7 | 0.133 | 0.0 ± 0.7 | 0.934 |
| Metabolite | OCC | |||||||||
| MI | 4.6 ± 0.4 | 4.6 ± 0.3 | 0.0 ± 0.4 | 0.944 | 4.8 ± 0.3 | 4.5 ± 0.5 | −0.3 ± 0.4 | 0.062 | 0.0 ± 0.3 | 0.953 |
| TCr | 6.4 ± 0.3 | 6.5 ± 0.2 | 0.1 ± 0.3 | 0.368 | 6.4 ± 0.2 | 6.5 ± 0.2 | 0.1 ± 0.2 | 0.511 | 0.1 ± 0.2 | 0.560 |
Values are means ± SD. EU1, visit 1, basal euglycemia; EU2, visit 2, basal euglycemia; HG, visit 1, hyperglycemic clamp; HG—EU1, change from EU1 to HG; HG-HI, difference between HI and HG; HI, visit 2, matched hyperinsulinemic euglycemic clamp; HI—EU2, change from EU2 to HI; MI, myoinositol; P, P values from paired t tests between conditions, P values in bold < 0.05; TCr, total creatine (sum of creatine and phosphocreatine).
Myoinositol and total creatine concentrations.
In the pgACC, mean (±SD) MI decreased significantly during the hyperglycemic clamp (6.6 ± 0.8 mmol/kg brain tissue) compared with the visit 1 basal euglycemic period (7.7 ± 1.5 mmol/L brain tissue, P = 0.031), and also decreased significantly during the hyperinsulinemic euglycemic clamp (6.5 ± 0.7 mmol/kg brain tissue) compared with the visit 2 basal euglycemic period (7.1 ± 0.7 mmol/kg brain tissue, P = 0.014). No significant MI difference was found between the hyperglycemic and hyperinsulinemic euglycemic clamps (P = 0.663) or between both visits’ basal euglycemic periods (P = 0.293) (Table 2 and Fig. 3). There were no significant between-condition MI differences in the OCC. Thus, in the pgACC, MI was equally decreased during hyperglycemic and hyperinsulinemic euglycemic clamps, during which plasma insulin levels were equivalently high. There was no significant between-condition TCr difference found in either pgACC or OCC (Table 2).
Brain Resting-State-Network Functional Connectivity by fMRI
We examined the relationship of pgACC MI concentrations to the functional connectivity of four resting-state networks of interest that contained an ACC node: 1) RSN A, comprised of subgenual ACC, insula, and lingual gyrus (salience network); 2) RSN B, comprised of dorsal ACC, thalamus, striatum (putamen, pallidum), insula, and temporal lobe (ACC-insula network); 3) RSN C, comprised of pregenual and subgenual ACC, frontal cortex, and caudate nucleus (ACC-caudate network); 4) RSN D, comprised of dorsal ACC, superior frontal gyrus, and thalamus (executive control network).
Relationship between MRS measures of pgACC MI concentrations and functional connectivity.
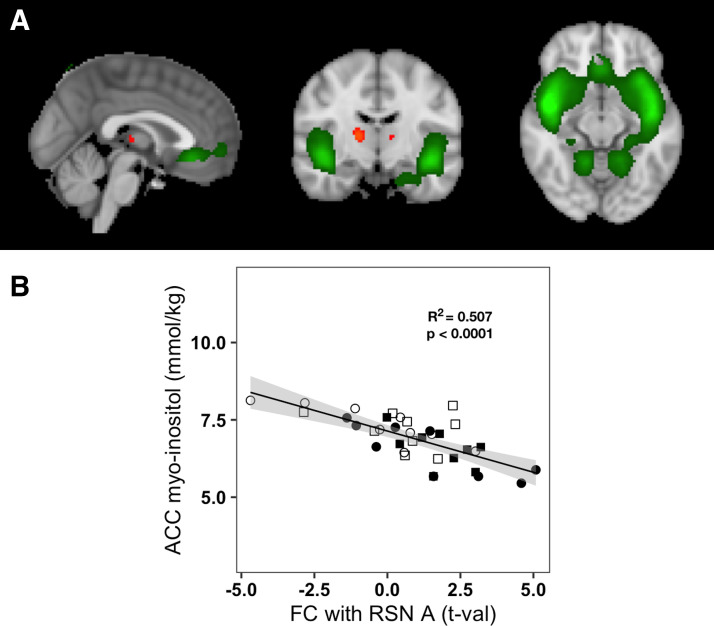
Pregenual ACC MI concentrations were correlated with functional connectivity between the salience network (RSN A) and bilateral regions in the thalamus (Fig. 4A) across all participants and conditions. Lower pgACC MI was associated with increased functional connectivity (Pearson R = −0.712, P = 6.85 × 10−7) (Fig. 4B). Pregenual ACC MI concentrations were also correlated with functional connectivity between the ACC-insula network (RSN B) and a region in the left precentral cortex across all participants and conditions (Fig. 5A). Lower ACC MI was associated with increased functional connectivity (Pearson R = −0.666, P = 5.65 × 10−6) (Fig. 5B). No other resting-state network examined showed any significant correlation between pgACC MI concentrations and its regional FC.
Figure 4.
Relation of pregenual anterior cingulate cortex (pgACC) myoinositol to functional connectivity of the salience network (RSN A) with thalamus. A: RSN A, common to all participants and conditions, is overlaid in green on the standard MNI-152 brain; regions overlaid in red-yellow scale show the thalamic region where increased functional connectivity with RSN A was associated with lower pgACC myoinositol (MI) concentrations. Images are shown using the radiological convention: left—sagittal (x = 0 cm); middle—coronal (y = −11.6 cm); right—axial (z = −11.4 cm). B: plot of pgACC MI concentrations vs. functional connectivity of RSN A with thalamus for all participants and conditions. Empty shapes indicate the basal euglycemia conditions of visits 1 and 2: empty circle = visit 1, empty square = visit 2; filled shapes indicate the clamps; filled circles indicate the hyperglycemic clamp; filled squares indicate the euglycemic hyperinsulinemic clamp. The solid black line represents the linear fit of pgACC MI concentrations to functional connectivity with RSN A (Pearson R2 = 0.507, P < 0.0001) and the gray area represents the 95% confidence interval.
Figure 5.
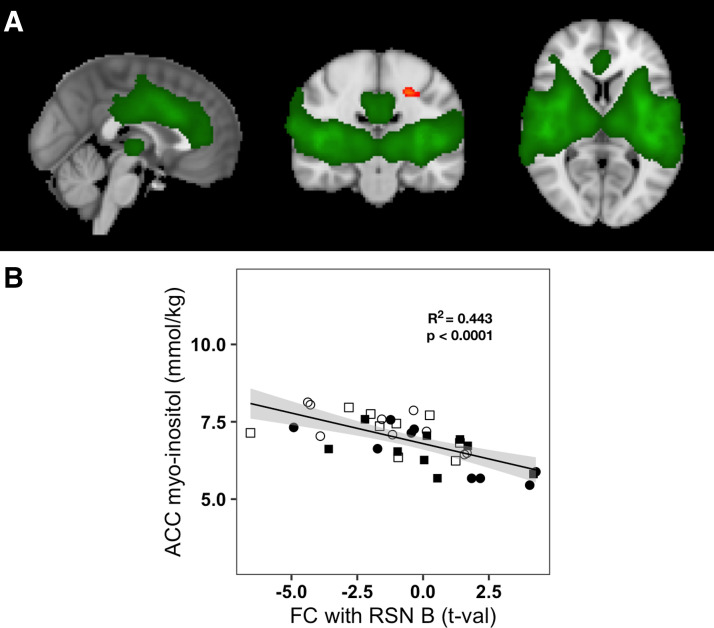
Relation of anterior cingulate cortex (ACC) myoinositol to functional connectivity of ACC-insula network (RSN B) with precentral cortex. A: RSN B, common to all participants and conditions, overlaid in green on the standard MNI-152 brain; regions in red-yellow scale show the precentral gyrus region where increased functional connectivity with RSN B was associated with lower pregenual anterior cingulate cortex (pgACC) myoinositol (MI) concentrations (P < 0.05). Images are shown using the radiological convention: left—sagittal (x = 0 cm); middle—coronal (y = −20 cm); right—axial (z = 6 cm). B: plot of pgACC MI concentrations vs. functional connectivity of RSN B with the precentral gyrus region for all participants and conditions. Empty shapes indicate the basal euglycemia conditions of visits 1 and 2: empty circle = visit 1, empty square = visit 2; filled shapes indicate the clamps; filled circles indicate the hyperglycemic clamp; filled squares indicate the euglycemic hyperinsulinemic clamp. The solid black line represents the linear fit of pgACC MI concentrations to functional connectivity with RSN B (Pearson R2 = 0.433, P < 0.0001) and the gray area represents the 95% confidence interval.
DISCUSSION
Our study shows that elevated plasma insulin decreases pregenual ACC MI levels, whereas elevated plasma glucose has no significant independent effect on MI in this region in healthy individuals. In addition, lower pregenual ACC MI is associated with higher functional connectivity of thalamic and primary motor regions with large-scale resting-state networks involved in internal and external sensory processing, voluntary motor control, salience, and cognition.
These findings may have particular relevance to many recent studies demonstrating the effects of exogenously administered insulin on affective and cognitive function in humans. Hyperinsulinemia induced while maintaining plasma glucose at fasting baseline levels has been shown to improve memory in patients with Alzheimer’s disease (51, 52). More recently, intranasal administration of insulin, which provides a more direct pathway to the brain bypassing the blood-brain barrier (53), has been shown to enhance cognition, memory, and mood in healthy individuals, T2D, mild cognitive impairment, and Alzheimer’s disease (54–56), and is currently being proposed as a potential cognitive enhancing treatment (57–60). The results of our study may provide insight on the insulin-mediated biochemical mechanisms underlying improvement of clinical cognitive and mood symptoms with insulin-based treatments.
The density of insulin receptors in the brain is predominant in the limbic and hippocampal regions (61, 62). Consistent with the heterogeneous distribution of insulin receptors, no effect on MI of either elevated plasma insulin or glucose was found in the OCC, suggesting that insulin-dependent MI reductions are region-specific and may be related to a higher density of insulin receptors in the pgACC. This lack of an insulin effect on metabolites in the primary visual OCC area with a concurrent effect in ACC is consistent with a similar lack of insulin effect on visual evoked potentials with a concurrent effect on cognitive task-related potentials observed by EEG, suggesting region- and task-specific effects of insulin on brain function (63). Notably, only the two networks that contained the insula showed a correlation between functional connectivity and pgACC MI concentrations, whereas the two networks that overlapped with ACC but not insula showed no correlations. The insula is another brain region with a particularly high insulin receptor density relative to other regions. This supports the notion that the relationships between pgACC MI concentration and resting-state-network functional connectivity observed here could be mediated by insulin.
MI serves multiple functions in the brain. MI is one of the main osmotically active particles inside the cell, and changes in MI levels contribute to the regulation of brain cell volume under chronic pathological conditions of tonicity such as extended hypo- or hypernatremia (27). It is also a precursor to the cytosolic second messenger inositol phosphates, to cell membrane phosphoinositide phospholipids, which mediate important functions in signal transduction, and to highly phosphorylated forms of inositol implicated in the regulation of nuclear function and endo/exocytosis (27). The few studies that have applied MRS to study brain metabolism in metabolic disorders show consistent alterations of MI. Notably, brain MI was shown to be elevated in patients with T1D during hyperglycemia (21, 24, 25), in patients with T2D (21–23), in metabolic syndrome (7), and also in patients with mild cognitive impairment and Alzheimer’s disease (26). MI was often considered to be a glial marker because increases in brain MI have been associated with gliosis and inflammatory states (64). The PI3K pathway is one of the three major nodes of the insulin receptor-mediated signaling pathway in the brain, which targets multiple downstream pathways involved in neuronal survival, modulation of memory formation processes, synaptic plasticity, and regulation of autophagy or neurodegenerative processes (65, 66). Myoinositol 1,4,5-triphosphate plays a crucial role in the mobilization of intracellular calcium (27). Our findings of decreased brain MI associated with increased plasma insulin levels in healthy young adults suggest that the elevated brain MI levels that are found in patients with diabetes or metabolic syndrome with associated cognitive or mood impairment may be related to reduced insulin levels or insulin action in the brain.
In our study, elevated plasma insulin decreased pregenual ACC MI independently of plasma glucose, suggesting that MI changes are due to the insulin effect rather than an osmotic effect secondary to increased osmotic pressure from hyperglycemia. Insulin receptor stimulation could increase metabolism of cytosolic MI, possibly by increasing inositol-phospholipid synthesis or increased phosphorylation via the phosphoinositol second messenger metabolic cascade. Our results could be explained by the incorporation of MI into phospholipids or phosphorylation of MI to phosphoinositol, which would reduce the MI MRS detectable signal through chemical shift effects and reduced molecular rotational mobility. Thus, it is possible that the insulin-dependent MI reductions that we observe reflect an increase in insulin receptor signaling via the PI3K second messenger pathway. Insulin-dependent MI changes related to PI3K signaling independent of hyperglycemia are consistent with the early experiments showing memory enhancement during elevated plasma insulin without hyperglycemia in Alzheimer’s disease (52).
We used ICA as opposed to seed-based correlational analysis (SBCA) because it has advantages in methodology and power of interpretation. In the SBCA, intersubject variations in anatomy could lead to seed selection bias (67) even if care were taken to place MRS voxels in the same regions for all subjects according to anatomical landmarks, whereas ICA avoids this potential bias. Also, network amplitude effects are not accurately reflected by the SBCA correlation coefficients obtained, as opposed to ICA (29). Furthermore, ICA provides a more global view of the brain’s functional-connectivity changes in response to the insulinemic/glycemic conditions. As opposed to SBCA, ICA allows analyzing and identifying whole brain networks that are well known, consistent, and reproducible in humans, and functionally relevant (68). ICA thus allows interpretation of effects in context of known networks relatable to sensorimotor, cognitive, and emotional function.
Decreased pgACC MI was associated with increased functional connectivity of the salience network (RSN A) with the thalamus, and of the ACC-insula network (RSN B) with the precentral gyrus. The thalamus is the sensory relay and the precentral gyrus is the primary motor region of the brain involved in voluntary control of movement and cognition. Resting-state networks A and B, which contain, respectively, the ventral-frontal and dorsal-posterior insula, are likely involved in integrating external and internal stimuli and emotional valuation. The insula is thought to play a role in integration across multiple functional systems, including social-emotional, sensorimotor, olfacto-gustatory, and cognitive functions (69). Our results suggest that increased activation of brain insulin receptors increases functional connectivity of networks that include insula and ACC and may increase between-network functional connectivity as well. Increased ACC/insula network functional connectivity with the thalamic and precentral cortex regions could reflect increased internal/external sensory, cognitive or emotional processing, or increased integration between these functional systems.
Our results are consistent with studies (70–72) showing effects of intranasal insulin on functional activity in ACC, putamen, orbitofrontal cortex, and insular cortex—all brain regions included in the resting-state networks affected in our study—suggesting that insulin may affect behavior regulating food intake by action on functional activity in these regions. However, another study (17) found that a hyperinsulinemic-euglycemic clamp did not affect either the insula/striatal/reward-circuit functional activity response to food images or food-craving scores and suggested that a modern food cue saturated environment may overpower homeostatic hormonal signals. Further studies are needed to determine the behavioral significance of the brain functional changes induced by insulin.
Our findings suggest that in diabetes, dysfunctional phosphoinositide metabolism (decreased MI phosphorylation or increased inositol-phosphate dephosphorylation) at the membrane-cytosol interface could mediate dysfunctional downstream-related signaling. The resulting disruption of neuronal cell regulation signaling in key nodes of resting-state networks, such as the pregenual ACC, known to be implicated in mood regulation and depression, could underlie cognitive and mood dysfunction. A similar MRS study by Karczewska-Kupczewska et al. (73) comparing metabolite levels during fasting baseline to those after a 240-min euglycemic hyperinsulinemic clamp showed decreased MI in the left frontal and temporal lobes in healthy participants with high-insulin sensitivity but not in participants with low-insulin sensitivity (73). Insulin sensitivity was inversely correlated with temporal MI assessed during the clamp. Their findings are consistent with ours and support the suggestion that the MI changes are due to insulin receptor signaling. The longer duration of the hyperinsulinemic clamp before MRS in their study (∼240 min vs. ∼40 min in our study) suggest it is possible that our study only shows early changes related to insulin signaling, which may persist after longer exposure to insulin.
The small sample size, young age, and good physical health of the participants limited our study in several ways. The small number of participants did not allow sufficient power to observe significant correlations of interest of MI with resting-state-network functional connectivity during each condition separately. This correlation attained significance when we combined results from baseline and clamp conditions. We did not observe any significant effect of the clamps on functional connectivity using paired t tests comparing conditions for each visit separately. Our observation of associations of MI with functional connectivity was limited to the pregenual ACC region where MRS measures were performed, thus, correlations were only examined in resting-state networks that include ACC. Also, we did not control for menstrual cycle in female participants, thus we cannot exclude that hormonal variation in female participants may have impacted the results. Given the young age and good physical health of participants, our findings may not apply in older individuals with compromised metabolism or cognition. Future studies including older individuals and longitudinal assessments of these brain and metabolic variables may further inform us on the mechanisms underlying cognitive decline with age.
Recent reviews suggest that MRS may be used as a tool for monitoring disease progression or treatment response for mild cognitive impairment and Alzheimer’s disease, and that MRS MI measures may constitute potential biomarkers of brain dysfunction and may contribute to diagnosis of Alzheimer’s disease and other dementias (74). In a longitudinal study of cognitively normal older adults, the N-acetyl-aspartate/MI (NAA/MI) ratio was a significant predictor of mild cognitive impairment and was associated with cognitive decline (75). In mild cognitive impairment, the MI/TCr ratio predicted the conversion to Alzheimer’s disease with 70% sensitivity and 85% specificity (76). In T2D with depression, elevations in prefrontal white matter MI correlated with greater impairment of visual-spatial cognitive function (22). MRS measures of MI may also be of particular interest for following the brain effects of intranasal insulin, which is proposed as a cognitive enhancer (77). Our results of insulin-dependent MI changes and the association of MI to resting-state network functional connectivity give further support to the usefulness of MRS measures of regional brain MI as biomarkers for improvement of diagnoses, for monitoring of treatment response and for development of novel treatments and medications targeting cognitive and mood disorders associated with diabetes and related metabolic disorders.
GRANTS
This study was supported by National Institutes of Health (NIH) Grant R01-DK084202 (to N. R. Bolo), the Harvard Catalyst and Harvard Clinical and Translational Science Center (National Center for Advancing Translational Sciences, National Institutes of Health Award UL1-TR001102 and National Center for Research Resources, National Institutes of Health Award UL1-RR025758), and financial contributions from Harvard University and its affiliated academic healthcare centers.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
N.R.B., A.M.J., G.M., and D.C.S. conceived and designed research; N.R.B. and D.C.S. performed experiments; N.R.B. and D.C.S. analyzed data; N.R.B. and D.C.S. interpreted results of experiments; N.R.B. prepared figures; N.R.B., A.M.J., and D.C.S. drafted manuscript; G.M. edited and revised manuscript; N.R.B., A.M.J., G.M., and D.C.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the study participants, the BIDMC CRC staff for their nursing assistance during the clamp studies, Fotini Papadopoulou for technological assistance during the MRI/MRS scans, and Brandon Hager for assistance with recruiting participants, cognitive and mood assessments, and coordination of the study protocol.
REFERENCES
- 1.Stoeckel LE, Arvanitakis Z, Gandy S, Small D, Kahn CR, Pascual-Leone A, Pawlyk A, Sherwin R, Smith P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Res 5: 353, 2016. doi: 10.12688/f1000research.8300.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolo NR, Musen G, Jacobson AM, Weinger K, McCartney RL, Flores V, Renshaw PF, Simonson DC. Brain activation during working memory is altered in patients with type 1 diabetes during hypoglycemia. Diabetes 60: 3256–3264, 2011. doi: 10.2337/db11-0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolo NR, Musen G, Simonson DC, Nickerson LD, Flores VL, Siracusa T, Hager B, Lyoo IK, Renshaw PF, Jacobson AM. Functional connectivity of insula, basal ganglia, and prefrontal executive control networks during hypoglycemia in type 1 diabetes. J Neurosci 35: 11012–11023, 2015. doi: 10.1523/JNEUROSCI.0319-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolo NR, Jacobson AM, Musen G, Keshavan MS, Simonson DC. Acute hyperglycemia increases brain pregenual anterior cingulate cortex glutamate concentrations in type 1 diabetes. Diabetes 69: 1528–1539, 2020. doi: 10.2337/db19-0936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson AM, Ryan CM, Braffett BH, Gubitosi-Klug RA, Lorenzi GM, Luchsinger JA, Trapani VR, Bebu I, Chaytor N, Hitt SM, Farrell K, Lachin JM; DCCT/EDIC Research Group. Cognitive performance declines in older adults with type 1 diabetes: results from 32 years of follow-up in the DCCT and EDIC Study. Lancet Diabetes Endocrinol 9: 436–445, 2021. doi: 10.1016/S2213-8587(21)00086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lyoo IK, Yoon SJ, Musen G, Simonson DC, Weinger K, Bolo N, Ryan CM, Kim JE, Renshaw PF, Jacobson AM. Altered prefrontal glutamate-glutamine-γ-aminobutyric acid levels and relation to low cognitive performance and depressive symptoms in type 1 diabetes mellitus. Arch Gen Psychiatry 66: 878–887, 2009. doi: 10.1001/archgenpsychiatry.2009.86. [DOI] [PubMed] [Google Scholar]
- 7.Haley AP, Gonzales MM, Tarumi T, Miles SC, Goudarzi K, Tanaka H. Elevated cerebral glutamate and myo-inositol levels in cognitively normal middle-aged adults with metabolic syndrome. Metab Brain Dis 25: 397–405, 2010. doi: 10.1007/s11011-010-9221-y. [DOI] [PubMed] [Google Scholar]
- 8.Sahin I, Alkan A, Keskin L, Cikim A, Karakas HM, Firat AK, Sigirci A. Evaluation of in vivo cerebral metabolism on proton magnetic resonance spectroscopy in patients with impaired glucose tolerance and type 2 diabetes mellitus. J Diabetes Complications 22: 254–260, 2008. doi: 10.1016/j.jdiacomp.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Belfort-DeAguiar R, Constable RT, Sherwin RS. Functional MRI signal fluctuations: a preclinical biomarker for cognitive impairment in type 2 diabetes? Diabetes 63: 396–398, 2014. doi: 10.2337/db13-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marder TJ, Flores VL, Bolo NR, Hoogenboom WS, Simonson DC, Jacobson AM, Foote SE, Shenton ME, Sperling RA, Musen G. Task-induced brain activity patterns in type 2 diabetes: a potential biomarker for cognitive decline. Diabetes 63: 3112–3119, 2014. doi: 10.2337/db13-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musen G, Jacobson AM, Bolo NR, Simonson DC, Shenton ME, McCartney RL, Flores VL, Hoogenboom WS. Resting-state brain functional connectivity is altered in type 2 diabetes. Diabetes 61: 2375–2379, 2012. doi: 10.2337/db11-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 3: 169–178, 2004. doi: 10.1016/S1474-4422(04)00681-7. [DOI] [PubMed] [Google Scholar]
- 13.Luchsinger JA, Tang M-X, Shea S, Mayeux R. Hyperinsulinemia and risk of Alzheimer disease. Neurology 63: 1187–1192, 2004. doi: 10.1212/01.wnl.0000140292.04932.87. [DOI] [PubMed] [Google Scholar]
- 14.Kullmann S, Heni M, Fritsche A, Preissl H. Insulin action in the human brain: evidence from neuroimaging studies. J Neuroendocrinol 27: 419–423, 2015. doi: 10.1111/jne.12254. [DOI] [PubMed] [Google Scholar]
- 15.Kullmann S, Veit R, Peter A, Pohmann R, Scheffler K, Häring H-U, Fritsche A, Preissl H, Heni M. Dose-dependent effects of intranasal insulin on resting-state brain activity. J Clin Endocrinol Metab 103: 253–262, 2018. doi: 10.1210/jc.2017-01976. [DOI] [PubMed] [Google Scholar]
- 16.Hallschmid M. Intranasal insulin. J Neuroendocrinol 33: e12934, 2021. doi: 10.1111/jne.12934. [DOI] [PubMed] [Google Scholar]
- 17.Belfort-DeAguiar R, Seo D, Naik S, Hwang J, Lacadie C, Schmidt C, Constable RT, Sinha R, Sherwin R. Food image-induced brain activation is not diminished by insulin infusion. Int J Obes 40: 1679–1686, 2016. doi: 10.1038/ijo.2016.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heni M, Kullmann S, Ketterer C, Guthoff M, Bayer M, Staiger H, Machicao F, Häring H-U, Preissl H, Veit R, Fritsche A. Differential effect of glucose ingestion on the neural processing of food stimuli in lean and overweight adults. Hum Brain Mapp 35: 918–928, 2014. doi: 10.1002/hbm.22223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heni M, Wagner R, Kullmann S, Gancheva S, Roden M, Peter A, Stefan N, Preissl H, Häring HU, Fritsche A. Hypothalamic and striatal insulin action suppresses endogenous glucose production and may stimulate glucose uptake during hyperinsulinemia in lean but not in overweight men. Diabetes 66: 1797–1806, 2017. doi: 10.2337/db16-1380. [DOI] [PubMed] [Google Scholar]
- 20.Tschritter O, Preissl H, Hennige AM, Stumvoll M, Porubska K, Frost R, Marx H, Klösel B, Lutzenberger W, Birbaumer N, Häring HU, Fritsche A. The cerebrocortical response to hyperinsulinemia is reduced in overweight humans: a magnetoencephalographic study. Proc Natl Acad Sci USA 103: 12103–12108, 2006. doi: 10.1073/pnas.0604404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geissler A, Fründ R, Schölmerich J, Feuerbach S, Zietz B. Alterations of cerebral metabolism in patients with diabetes mellitus studied by proton magnetic resonance spectroscopy. Exp Clin Endocrinol Diabetes 111: 421–427, 2003. doi: 10.1055/s-2003-44289. [DOI] [PubMed] [Google Scholar]
- 22.Haroon E, Watari K, Thomas A, Ajilore O, Mintz J, Elderkin-Thompson V, Darwin C, Kumaran S, Kumar A. Prefrontal myo-inositol concentration and visuospatial functioning among diabetic depressed patients. Psychiatry Res 171: 10–19, 2009. doi: 10.1016/j.pscychresns.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Santhakumari R, Reddy IY, Archana R. Effect of type 2 diabetes mellitus on brain metabolites by using proton magnetic resonance spectroscopy—a systematic review. Int J Pharma Bio Sci 5: 1118–1123, 2014. [PMC free article] [PubMed] [Google Scholar]
- 24.Heikkilä O, Lundbom N, Timonen M, Groop P-H, Heikkinen S, Mäkimattila S. Hyperglycaemia is associated with changes in the regional concentrations of glucose and myo-inositol within the brain. Diabetologia 52: 534–540, 2009. doi: 10.1007/s00125-008-1242-2. [DOI] [PubMed] [Google Scholar]
- 25.Mäkimattila S, Malmberg-Cèder K, Häkkinen A-M, Vuori K, Salonen O, Summanen P, Yki-Järvinen H, Kaste M, Heikkinen S, Lundbom N, Roine RO. Brain metabolic alterations in patients with type 1 diabetes-hyperglycemia-induced injury. J Cereb Blood Flow Metab 24: 1393–1399, 2004. doi: 10.1097/01.WCB.0000143700.15489.B2. [DOI] [PubMed] [Google Scholar]
- 26.Griffith HR, Okonkwo OC, den Hollander JA, Belue K, Copeland J, Harrell LE, Brockington JC, Clark DG, Marson DC. Brain metabolic correlates of decision making in amnestic mild cognitive impairment. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 17: 492–504, 2010. doi: 10.1080/13825581003646135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 82: 736–754, 2002. doi: 10.1046/j.1471-4159.2002.01041.x. [DOI] [PubMed] [Google Scholar]
- 28.Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA 107: 4734–4739, 2010. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nickerson LD, Smith SM, Ongür D, Beckmann CF. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front Neurosci 11: 115, 2017. doi: 10.3389/fnins.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vogt BA. Submodalities of emotion in the context of cingulate subregions. Cortex 59: 197–202, 2014. doi: 10.1016/j.cortex.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 31.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E214–E223, 1979. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 32.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30: 672–679, 1993. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- 33.Soher BJ, Semanchuck P, Young K, Todd D. Vespa—Simulation User Manual and Reference. https://vespa-mrs.github.io/vespa.io/user_manuals/simulation_user_manual.html [2022 Apr 29].
- 34.Smith SA, Levante TO, Meier BH, Ernst RR. Computer simulations in magnetic resonance. An object-oriented programming approach. J Magn Reson Ser A 106: 75–105, 1994. doi: 10.1006/jmra.1994.1008. [DOI] [Google Scholar]
- 35.Ernst T, Kreis R, Ross BD. Absolute quantitation of water and metabolites in the human brain. I. Compartments and water. J Magn Reson B 102: 1–8, 1993. doi: 10.1006/jmrb.1993.1055. [DOI] [Google Scholar]
- 36.Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med 55: 1219–1226, 2006. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- 37.Beckmann CF, DeLuca M, Devlin JT, Smith SM. Investigations into resting-state connectivity using independent component analysis. Philos Trans R Soc Lond B Biol Sci 360: 1001–1013, 2005. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, Suppl 1: S208–S219, 2004. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 39.Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CE, Moeller S, Xu J, Yacoub E, Baselli G, Ugurbil K, Miller KL, Smith SM. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. NeuroImage 95: 232–247, 2014. doi: 10.1016/j.neuroimage.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. NeuroImage 90: 449–468, 2014. doi: 10.1016/j.neuroimage.2013.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc Natl Acad Sci USA 106: 7209–7214, 2009. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc Natl Acad Sci USA 106: 13040–13045, 2009. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kullmann S, Giel KE, Teufel M, Thiel A, Zipfel S, Preissl H. Aberrant network integrity of the inferior frontal cortex in women with anorexia nervosa. Neuroimage Clin 4: 615–622, 2014. doi: 10.1016/j.nicl.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kullmann S, Heni M, Hallschmid M, Fritsche A, Preissl H, Häring H-U. Brain insulin resistance at the crossroads of metabolic and cognitive disorders in humans. Physiol Rev 96: 1169–1209, 2016. doi: 10.1152/physrev.00032.2015. [DOI] [PubMed] [Google Scholar]
- 45.Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J Neurosci Methods 189: 233–245, 2010. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15: 1–25, 2002. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SM, Beckmann CF, Ramnani N, Woolrich MW, Bannister PR, Jenkinson M, Matthews PM, McGonigle DJ. Variability in fMRI: a re-examination of inter-session differences. Hum Brain Mapp 24: 248–257, 2005. doi: 10.1002/hbm.20080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terpstra M, Cheong I, Lyu T, Deelchand DK, Emir UE, Bednařík P, Eberly LE, Öz G. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med 76: 1083–1091, 2016. doi: 10.1002/mrm.26022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Bogaard SJA, Dumas EM, Teeuwisse WM, Kan HE, Webb A, Roos RAC, van der Grond J. Exploratory 7-Tesla magnetic resonance spectroscopy in Huntington’s disease provides in vivo evidence for impaired energy metabolism. J Neurol 258: 2230–2239, 2011. doi: 10.1007/s00415-011-6099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baker EH, Basso G, Barker PB, Smith MA, Bonekamp D, Horská A. Regional apparent metabolite concentrations in young adult brain measured by (1)H MR spectroscopy at 3 Tesla. J Magn Reson Imaging 27: 489–499, 2008. doi: 10.1002/jmri.21285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Craft S, Newcomer J, Kanne S, Dagogo-Jack S, Cryer P, Sheline Y, Luby J, Dagogo-Jack A, Alderson A. Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 17: 123–130, 1996. doi: 10.1016/0197-4580(95)02002-0. [DOI] [PubMed] [Google Scholar]
- 52.Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, Cherrier M, Lofgreen C, Latendresse S, Petrova A, Plymate S, Raskind M, Grimwood K, Veith RC. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry 56: 1135–1140, 1999. doi: 10.1001/archpsyc.56.12.1135. [DOI] [PubMed] [Google Scholar]
- 53.Hanson LR, Frey WH. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci 9, Suppl 3: S5, 2008. doi: 10.1186/1471-2202-9-S3-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Benedict C, Hallschmid M, Hatke A, Schultes B, Fehm HL, Born J, Kern W. Intranasal insulin improves memory in humans. Psychoneuroendocrinology 29: 1326–1334, 2004. doi: 10.1016/j.psyneuen.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, Manor B, Roberson P, Craft S, Abduljalil A. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care 37: 751–759, 2014. doi: 10.2337/dc13-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shemesh E, Rudich A, Harman-Boehm I, Cukierman-Yaffe T. Effect of intranasal insulin on cognitive function: a systematic review. J Clin Endocrinol Metab 97: 366–376, 2012. doi: 10.1210/jc.2011-1802. [DOI] [PubMed] [Google Scholar]
- 57.Claxton A, Baker LD, Hanson A, Trittschuh EH, Cholerton B, Morgan A, Callaghan M, Arbuckle M, Behl C, Craft S. Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer’s disease dementia. J Alzheimers Dis 44: 897–906, 2015. doi: 10.3233/JAD-141791. [DOI] [PubMed] [Google Scholar]
- 58.Craft S, Claxton A, Baker LD, Hanson AJ, Cholerton B, Trittschuh EH, Dahl D, Caulder E, Neth B, Montine TJ, Jung Y, Maldjian J, Whitlow C, Friedman S. Effects of regular and long-acting insulin on cognition and Alzheimer’s disease biomarkers: a pilot clinical trial. J Alzheimers Dis 57: 1325–1334, 2017. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Craft S. Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment. Arch Neurol 69: 29, 2012. doi: 10.1001/archneurol.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Freiherr J, Hallschmid M, Frey WH, Brünner YF, Chapman CD, Hölscher C, Craft S, De Felice FG, Benedict C. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS Drugs 27: 505–514, 2013. doi: 10.1007/s40263-013-0076-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Derakhshan F, Toth C. Insulin and the brain. Curr Diabetes Rev 9: 102–116, 2013. [PubMed] [Google Scholar]
- 62.Unger JW, Livingston JN, Moss AM. Insulin receptors in the central nervous system: localization, signalling mechanisms and functional aspects. Prog Neurobiol 36: 343–362, 1991. doi: 10.1016/0301-0082(91)90015-s. [DOI] [PubMed] [Google Scholar]
- 63.Benedict L, Nelson CA, Schunk E, Sullwold K, Seaquist ER. Effect of insulin on the brain activity obtained during visual and memory tasks in healthy human subjects. Neuroendocrinology 83: 20–26, 2006. doi: 10.1159/000093338. [DOI] [PubMed] [Google Scholar]
- 64.Mader I, Rauer S, Gall P, Klose U. (1)H MR spectroscopy of inflammation, infection and ischemia of the brain. Eur J Radiol 67: 250–257, 2008. doi: 10.1016/j.ejrad.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 65.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes 63: 2232–2243, 2014. doi: 10.2337/db14-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao WQ, Alkon DL. Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 177: 125–134, 2001. doi: 10.1016/s0303-7207(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 67.Cole DM, Smith SM, Beckmann CF. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front Syst Neurosci 4: 8, 2010. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Damoiseaux JS, Rombouts SARB, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci USA 103: 13848–13853, 2006. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214: 519–534, 2010. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heni M, Kullmann S, Ketterer C, Guthoff M, Linder K, Wagner R, Stingl KT, Veit R, Staiger H, Häring HU, Preissl H, Fritsche A. Nasal insulin changes peripheral insulin sensitivity simultaneously with altered activity in homeostatic and reward-related human brain regions. Diabetologia 55: 1773–1782, 2012. doi: 10.1007/s00125-012-2528-y. [DOI] [PubMed] [Google Scholar]
- 71.Kroemer NB, Krebs L, Kobiella A, Grimm O, Vollstädt-Klein S, Wolfensteller U, Kling R, Bidlingmaier M, Zimmermann US, Smolka MN. (Still) longing for food: insulin reactivity modulates response to food pictures. Hum Brain Mapp 34: 2367–2380, 2013. doi: 10.1002/hbm.22071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kullmann S, Frank S, Heni M, Ketterer C, Veit R, Häring H-U, Fritsche A, Preissl H. Intranasal insulin modulates intrinsic reward and prefrontal circuitry of the human brain in lean women. Neuroendocrinology 97: 176–182, 2013. doi: 10.1159/000341406. [DOI] [PubMed] [Google Scholar]
- 73.Karczewska-Kupczewska M, Tarasów E, Nikołajuk A, Stefanowicz M, Matulewicz N, Otziomek E, Górska M, Strączkowski M, Kowalska I. The effect of insulin infusion on the metabolites in cerebral tissues assessed with proton magnetic resonance spectroscopy in young healthy subjects with high and low insulin sensitivity. Diabetes Care 36: 2787–2793, 2013. doi: 10.2337/dc12-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao F, Barker PB. Various MRS application tools for Alzheimer disease and mild cognitive impairment. AJNR Am J Neuroradiol 35: S4–11, 2014. doi: 10.3174/ajnr.A3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kantarci K. Proton MRS in mild cognitive impairment. J Magn Reson Imaging 37: 770–777, 2013. doi: 10.1002/jmri.23800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Targosz-Gajniak MG, Siuda JS, Wicher MM, Banasik TJ, Bujak MA, Augusciak-Duma AM, Opala G. Magnetic resonance spectroscopy as a predictor of conversion of mild cognitive impairment to dementia. J Neurol Sci 335: 58–63, 2013. doi: 10.1016/j.jns.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 77.Lioutas V-A, Alfaro-Martinez F, Bedoya F, Chung C-C, Pimentel DA, Novak V. Intranasal insulin and insulin-like growth factor 1 as neuroprotectants in acute ischemic stroke. Transl Stroke Res 6: 264–275, 2015. doi: 10.1007/s12975-015-0409-7. [DOI] [PMC free article] [PubMed] [Google Scholar]