Abstract
Hyperglycemic conditions are prodromal to blood-brain barrier (BBB) impairment. The BBB comprises cerebral microvessel endothelial cells (CMECs) that are surrounded by astrocytic foot processes. Astrocytes express high levels of gap junction connexin 43 (Cx43), which play an important role in autocrine and paracrine signaling interactions that mediate gliovascular cross talk through secreted products. One of the key factors of the astrocytic “secretome” is vascular endothelial growth factor (VEGF), a potent angiogenic factor that can disrupt BBB integrity. We hypothesize that high-glucose conditions change the astrocytic expression of Cx43 and increase VEGF secretion leading to impairment of CMEC barrier properties in vitro and in vivo. Using coculture of neonatal rat astrocytes and CMEC, we mimic hyperglycemic conditions using high-glucose (HG) feeding media and show a significant decrease in Cx43 expression and the corresponding increase in secreted VEGF. This result was confirmed by the analyses of Cx43 and VEGF protein levels in the brain cortex samples from the type 2 diabetic rat (T2DN). To further characterize inducible changes in BBB, we measured transendothelial cell electrical resistance (TEER) and tight junction protein levels in cocultured conditioned astrocytes with isolated rat CMEC. The coculture monolayer’s integrity and permeability were significantly compromised by HG media exposure, which was indicated by decreased TEER without a change in tight junction protein levels in CMEC. Our study provides insight into gliovascular adaptations to increased glucose levels resulting in impaired cellular cross talk between astrocytes and CMEC, which could be one explanation for cerebral BBB disruption in diabetic conditions.
Keywords: astrocyte, cerebral microvessel endothelial cell, connexin 43, high-glucose conditions, vascular endothelial growth factor
INTRODUCTION
Prolonged hyperglycemic conditions resulting from diabetes mellitus elicit a progressive impairment of neuronal function in the brain. It is now understood that altered glycemic conditions in patients with diabetes are prodromal to blood-brain barrier (BBB) impairment (1). In the rhesus monkey animal model of type 2 diabetes mellitus, dynamic contrast-enhanced magnetic resonance imaging showed increased BBB permeability in diabetic monkeys compared with controls (2). On a molecular level, in vitro experiments using brain cerebral endothelial cells alone showed barrier dysfunction due to varying glucose concentrations (3). These observations are of paramount relevance for the pathogenesis of brain disorders seen in diabetes mellitus since the BBB acts as a gatekeeper of the brain from potentially harmful substances, regulates the transport of essential molecules, and maintains brain homeostasis. Despite the importance of this problem, the cellular mechanisms of BBB dysfunction that are influenced by hyperglycemia remain poorly understood.
The BBB is supported by the cellular constituents of the neurovascular unit (4). The neurovascular unit is composed of several cells, including the astrocyte. The astrocyte is the most abundant cell in the brain, and until recently, its role in the brain has been assumed to be only supportive (5). Blood vessels in the brain are surrounded primarily by foot processes that emanate from astrocytes. This close approximation of the astrocyte with microvessels allows gliovascular communication via astrocytic-secreted products (6, 7). One of the astrocytic “secretome” critical factors is vascular endothelial growth factor (VEGF), a potent angiogenic factor (8). The role of VEGF in the brain is complex; it directs angiogenic repair to injury by changing the mitogenic activity of endothelial cells. VEGF plays a protective role in neuronal cells by inhibition of caspase 3 activation and activation of the PI3-K signaling pathway (9, 10). At the same time, VEGF can cause occludin and ZO-1 loss from the endothelial cell junctions, disrupting the BBB through mechanisms that alter tight junction protein abundance and function (11, 12). Hyperglycemic conditions have been associated with altered VEGF production in astrocytes in the retina and the brain (13–15). For example, retinal mRNA levels of VEGF were elevated under modeled type 1 diabetic conditions (16). However, the functional changes of the astrocyte in response to high-glucose conditions that regulate VEGF production and secretion remain relatively underinvestigated. It is known that elevated glucose decreases astrocyte viability and promotes apoptosis (17). High-glucose levels observed in diabetes provoke vascular damage while elevated VEGF can be released as an answer to impaired perfusion in microvessels (18).
VEGF expression has been shown to be regulated by connexin proteins (Cx) in cells outside of the central nervous system (CNS) (19–23). Overexpression of Cx43 in retinal pigment epithelial cells resulted in decreased VEGF mRNA levels and reduced VEGF secretion in culture media, whereas Cx43 knockdown resulted in increased VEGF mRNA levels and increased secretion into the culture media (21). Cx are the building blocks to gap junctions and hemichannels for cell-to-cell communication (24). Cx43 is particularly abundant in astrocytes and is located at the gliovascular interface (25). It has been demonstrated that in astrocytes when Cx43 is knocked down, the expression of hypoxia-inducible factor- 1α (HIF-1α) is increased (26). HIF-1α is a master transcription factor for several genes including VEGF (27–30). HIF-1α is highly regulated by hypoxia, but several factors activate it under normoxic conditions. High glucose in the absence of hypoxia has been associated with increased HIF-1α and VEGF levels in retinal epithelial cells (31). These findings taken together suggest that there may be a connection between high-glucose conditions, decreased expression of Cx43, and increased VEGF secretion from astrocytes. Determining if astrocytic Cx43 expression is altered in high-glucose conditions could provide a potential mechanism for VEGF regulation in the astrocyte.
The objective of this study is to investigate the effects of high-glucose conditions on the astrocytic secretome and the resulting changes in barrier formation in cerebral microvessel endothelial cells. We correlate these findings in a novel in vivo rat model of diabetes mellitus. We hypothesize that high-glucose conditions will decrease astrocytic expression of Cx43 protein, which will be associated with an increase in astrocytic VEGF secretion and decreased barrier properties and tight junction protein levels in cocultured cerebral microvessel endothelial cells (CMECs).
MATERIALS AND METHODS
Animal Care
The animal protocols used in this study were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. Brain cortex tissue isolated from age-matched (48 wk old) Wistar (nondiabetic) and T2DN (nonobese type 2 diabetic) male rats were used for experiments. Wistar rats were purchased from Charles River Laboratories and T2DN rats had been inbred for multiple generations at the Medical College of Wisconsin. The type 2 diabetic nephropathy (T2DN) strain was initially developed by Nobrega et al. (32). As we had recently described (33), T2DN strain at the age of 48 wk exhibits severe hyperglycemia, elevated cholesterol, albuminuria, and renal damage. Animals were fed a normal salt diet (No. 5001, LabDiet, Purina) with water and food provided ad libitum. Rats were housed in a temperature (24 ± 2°C), humidity (60 ± 10%), and 12-h light cycle (lights ON: 0600–1800) controlled environment.
Isolation of Cerebral Vessels for Western Blot Analysis
For isolation of cerebral vessels, rats was deeply anesthetized with isoflurane and decapitated. Brain was removed and placed into ice-cold (4°C) oxygenated (95% O2-5% CO2) artificial cerebrospinal fluid (ACSF) of the following composition (mM): 126 NaCl, 3.5 KCl, 2.0 CaCl2, 1.3 MgCl2, 25 NaHCO3, 1.2 NaH2PO4, and 11 glucose (pH 7.4). The cerebral surface vasculature and associated meninges were isolated as previously described (34).
Cell Culture
Astrocytes.
Sprague-Dawley rat pups 2–3 days of age (male and female) were anesthetized with isoflurane and decapitated. The brain was isolated for the preparation of astrocyte cultures as previously described (35–37). Dissected brain free of meninges was used and isolated hippocampus was cut into small pieces and transferred to a sterile dish containing 20 U/mL papain (Worthington Biochemical Corp., Lakewood, NJ) dissolved in DMEM/F12 media supplemented with 1% penicillin-streptomycin, 0.1% gentamicin, and 0.5% fungizone. The tissue was incubated at 37°C for 40 min with gentle agitation. The tissue was then centrifuged at 400 g for 4 min to remove the papain solution and resuspended in DMEM/F12 media supplemented with 10% FBS, 1% penicillin-streptomycin, and 0.1% gentamicin. The tissues were triturated in the same media with a 1 mL pipette and the resulting cell suspension was filtered using a 40-µm cell strainer. The cell suspension was centrifuged at 400 g for 4 min and resuspended in astrocyte growth media: DMEM normal glucose (NG, 5.5 mM) or high-glucose (HG, 25 mM) media supplemented with 10% FBS and 1% penicillin-streptomycin. Cells were plated at an initial density of ∼2 × 105 cells/cm2. The cells were incubated at 37°C in a 95%-5% mixture of atmospheric air and CO2. The respective astrocyte growth medium (NG vs. HG) was changed after 2 days and subsequently twice weekly. Confluent monolayers of brain astrocytes remained in their respective assigned media for the duration of growth and were studied upon the third passage.
Cerebral microvessel endothelial cells.
Sprague-Dawley male and female rats at (3–4 wk of age) were anesthetized with isoflurane and decapitated. The brain was dissected free of meninges and the cerebral cortex was isolated, removing all large vessels as previously described (8). The tissue was minced and placed into papain solution, underwent trituration, incubation at 37°C, and centrifugation to result in a pellet with two distinct layers. The top layer consists mostly of microvessels and the lower layer contains mostly of nonvascular brain cells. The top layer was carefully removed and resuspended in dissociation media, triturated, and filtered through a 70-μm cell strainer where microvessels remain on the filter and debris is filtered. Microvessels were collected and collagenase (1 mg/mL) was added followed by 1-h incubation at 37°C and then centrifuged at 400 g for 4 min. The resuspended pellet was passed through a 20% sucrose gradient and centrifuged at 600 g for 5 min. The final pellet containing viable microvessel segments was seeded onto dishes coated with attachment factor solution (Cell Applications, San Diego, CA). The cells were incubated at 37°C in a 95%-5% mixture of atmospheric air and CO2. The endothelial cell culture media containing manufacturer-supplied supplementation kit (Cell Biologics, Chicago, IL) was changed 24 h after plating to remove any unattached cell debris. Media was changed twice weekly and confluent monolayer of cerebral microvessel endothelial cells was studied upon third to fifth passage.
Astrocyte and cerebral microvessel endothelial cells coculture.
Cocultures were made according to previously described methods (38) along with modifications for the purposes of our study. Briefly, confluent cerebral microvessel endothelial cells (CMECs) were trypsinized and resuspended in endothelial cell media with supplements (Cell Biologics). Seven days before the coculture was started, CMEC were plated at ∼1 × 104 cells/cm2 on polyester 0.4-μm pore transwell inserts pretreated with attachment factor (Cell Applications). The cells were incubated at 37°C in a 95%-5% mixture of atmospheric air and CO2. The endothelial cell culture media was changed every 3 days over 7 days until confluence was reached. The day before the coculture was started, confluent astrocytes were trypsinized and resuspended in astrocyte growth media containing NG or HG concentrations. Astrocytes were plated at 1.5 × 104 cells/cm2 in a six-well plate. On the day of coculture, confluent CMEC in transwell inserts were washed with phosphate-buffered saline and placed in the wells of a six-well plate in which astrocytes had been cultured the day earlier. Coculture conditions included the following two experimental groups and two control groups: experimental groups: CMEC with HG astrocytes in HG-astrocyte media, CMEC with NG astrocytes in NG-astrocyte media; control groups: CMEC-alone in HG-astrocyte media and CMEC-alone in NG-astrocyte media. CMEC and astrocyte cocultures were incubated at 37°C in a 95%-5% mixture of atmospheric air and CO2 in astrocyte growth media containing NG or HG concentrations as experimental groups. After 72 h of coculture, culture media was analyzed for VEGF secretion, and CMEC was analyzed for barrier formation.
Quantification of Secreted VEGF in Coculture
Cell culture media from four experimental conditions was collected 72 h after commencement of the coculture conditions. Coculture conditions included the following two experimental groups and two control groups: experimental groups: CMEC with HG astrocytes in HG-astrocyte media, CMEC with NG astrocytes in NG-astrocyte media; control groups: CMEC-alone in HG-astrocyte media and CMEC-alone in NG-astrocyte media. Cell-free culture media was collected and concentrated using Amicon Ultra-4 centrifugal filters (Cork Ireland) according to the manufacturer’s instructions. Concentrated cell media was measured in triplicate using a colorimetric solid-phase sandwich ELISA (Rat VEGF Immunoassay, R&D Systems, Minneapolis, MN). The assay was carried out according to the manufacturer’s instructions. Measurements were performed using a microplate reader (FLUOstar Omega, BMG Labtech, Cary, NC).
Transendothelial Electrical Resistance Measurement
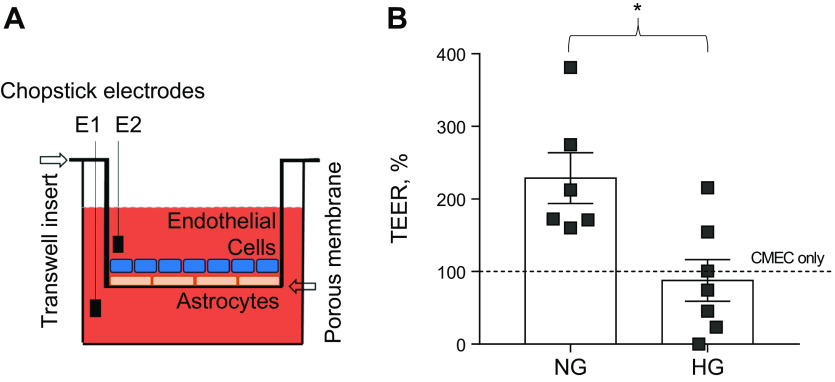
Barrier permeability was assayed by measuring the transendothelial electrical resistance (TEER) across the cell monolayer. TEER is a widely accepted means of measuring barrier properties in vitro with an increase in TEER representing a decrease in permeability (39). In this study, TEER measurement of the CMEC monolayer was obtained before starting coculture conditions with cells in endothelial cells culture media with supplements. Millicell electrode (Millipore Sigma, Burlington, MA) was inserted into each of the three transwell ports for TEER measurements using Millicell-ERS Voltohmmeter (Millipore Sigma, Fig. 3A). The three measurements were averaged to give one overall measurement for the well. Coculture conditions then commenced for the two experimental groups and two control groups: experimental groups: CMEC with HG astrocytes in HG-astrocyte media, CMEC with NG astrocytes in NG-astrocyte media; control groups: CMEC-alone in HG-astrocyte media and CMEC-alone in NG-astrocyte media. Cocultures were incubated for 72 h at which point a plateau phase is reached in TEER measurements (40). After 72 h of coculture conditions, TEER measurements were obtained from each of the three ports in the transwell insert and averaged to give one overall measurement per well. Each well was analyzed using repeated-measures designed within the groups. Generalized linear mixed models with random intercept and unstructured variance/covariance matrix were fitted to the TEER difference.
Figure 3.
Barrier properties of astrocytes and CMEC coculture monolayer conditioned in NG-media vs. HG-media. A: transendothelial electrical resistance (TEER) method schematic to confirm the integrity and permeability of the coculture monolayer. B: X-axis: astrocytes and CMEC coculture monolayer in NG and HG conditions relatively to CMEC monolayer control in NG, Y-axis: TEER difference normalized to control under NG or after 72 h of hyperglycemia conditions (n = 6, P < 0.05, Student’s t test). CMEC, cerebral microvessel endothelial cell; HG, high-glucose; NG, normal glucose.
Protein Isolation and Immunoblot
The procedures used in this section have been used previously by our laboratory and the text is similar to earlier publications with minor modifications (8, 29, 41, 42). The rats were deeply anesthetized by inhalation of isoflurane and decapitated. Brains were quickly removed, and neocortex was isolated, moved to 1 mL tubes, and immediately frozen in liquid nitrogen. Cultured astrocytes or CMEC were lysed in radioimmunoprecipitation assay (RIPA) buffer (No. 20–188, EMD Millipore Corp.) for a 60-min incubation at 4°C and centrifuged for 15 min at 15,000 g. Protein concentration was determined using the Bradford assay (43).
Proteins were separated on 4%–20% polyacrylamide gel (No. 4568094, Mini-PROTEAN TGX Stain-free, Bio-Rad, Hercules, CA). Proteins were transferred to polyvinylidene fluoride membranes with Trans-Blot Turbo Transfer Pack (No. 1704156, Bio-Rad) on Trans-Blot Turbo Transfer System (Bio-Rad). After being blocked with 5% nonfat dried milk-TBST (Tris Buffer Saline-0.1% Tween 20), membranes were incubated overnight at 4°C with primary antibody, goat polyclonal connexin 43 (sc-6561, Santa Cruz Biotechnology, Dallas, TX), mouse monoclonal connexin 43 (No. 610062, BD Biosciences), rabbit polyclonal VEGFA (No. ab46154, Abcam), mouse monoclonal Claudin 5 (35–2500, Invitrogen, Carlsbad, CA), and mouse monoclonal Occludin (33–1500, Invitrogen) with a 1:1,000 dilution in TBST-1% BSA. After 5 min × four rinses with TBST, the membranes were then incubated with donkey anti-goat IgG (H + L) HRP-conjugated secondary antibody (Abcam) or goat anti-mouse IgG (H + L)—HRP-conjugated secondary antibody (No. 170–6516, Bio-Rad), or goat anti-rabbit IgG (H + L) (No. 1706515, Bio-Rad) with 1:5,000 dilution in TBST-1% BSA for 1 h at room temperature, followed by 5 min × four rinses with TBST. The blots were developed in dark with SuperSignal West Pico chemiluminescent Substrate (No. 34080, Thermo Scientific, Waltham, MA) and the signals were captured with ChemiDoc MP Imaging System (Model No: Universal Hood III, Bio-Rad) and were then quantified with Image Lab software (v. 4.0.0, Bio-Rad).
RNA Isolation and Real-Time PCR
The procedures used in this section have been used previously by our laboratory and the text is similar to prior publications with minor modifications (8, 29, 41). Real-time PCR was used to measure the expression of tight junction proteins claudin 5 and occludin mRNA in CMEC cocultured with astrocytes. Analysis of relative gene expression data using Samples’ total RNAs was isolated by RNAeasy Mini Kit (No. 74104, Qiagen Inc., Germantown, MD), and RNA concentrations were determined by Cytation 5 multimode reader (BIO-TEK Instruments, Inc., Winooski, VT). cDNA was synthesized on IQ5 Multicolor Real-Time PCR Detection System (Bio-Rad) using High-Capacity cDNA Reverse Transcription Kit (No. 4368814, Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. PCR was carried out on CFX384 Real-Time System (Model No: CFX384 Optics Module, Bio-Rad). Reaction systems were 3 μL Bulls eye TaqProbe 2X qPCR MasterMix-Low Rox (No. BEQPCR-PL, Midwest Scientific, Valley Park, MO), 2 μL Primer-Probe (diluted from 20X), and 1 μL cDNA (1:20 dilution), loading to MicroAmp Optical 384-well reaction plate (No. 4309849, Applied Biosystems). Applied primers were GAPDH (Ref Seq. No. NM_017008(1), Assay ID: Rn.PT.58.35727291, Integrated DNA Technologies, Inc. Skokie, IL), Occludin (Ref Seq. No. NM_031329(1), Assay ID: Rn.PT.58.11842121, IDT), and Claudin 5 (Ref Seq. No. NM_031701.2, Assay ID: Rn01753146_s1, Thermo Fisher). PCR runs: hot start 10 min at 95°C followed by 40 cycles of 10 sections at 95°C and 60 s at 60°C, terminated by cooling to 4°C. PCR results were obtained by CFX Manager Software (V. 3.1, Bio-Rad) and analyzed with the 2−ΔΔCT method (44).
Statistical Analysis
Data are presented as means ± SD. In the box chart diagram, the ends of the box are presented as ±SE, and the median is marked by a vertical line inside the box. Student’s t test determined statistical significance as demonstrated by a P value of <0.05. All statistical analyses were performed using Statistical Analysis Systems (SAS v.9.4) or OriginPro 9.0 software, under the guidance of the Quantitative Health Sciences section at the Children’s Research Institute.
RESULTS
To determine the ability of the astrocyte to produce Cx43 protein in response to altered glucose concentrations, we measured Cx43 protein levels in astrocyte cell lysate before coculture. Astrocytes cultured in HG media had significantly less Cx43 protein level compared with NG media (Fig. 1A, n = 12/group, P < 0.001). The analyses of brain cortex from control (Wistar, nondiabetic) and diabetic (T2DN) rats confirmed the decline in Cx43 expression during hyperglycemia (Fig. 1B, n = 4 rats/group, P < 0.05), whereas brain vessel analysis did not show any difference in Cx43 expression (Fig. 1C, n = 4).
Figure 1.
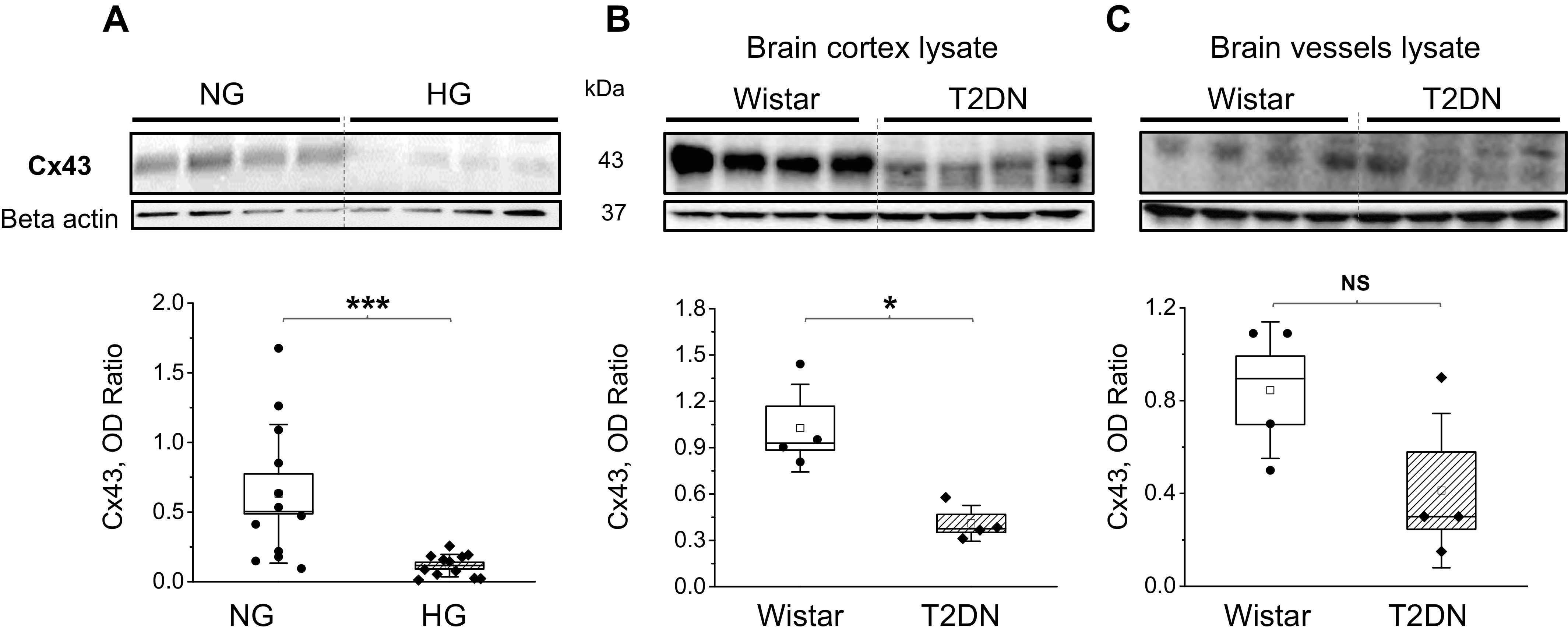
Cx43 protein level expression in chronic high-glucose conditions (HG). A: Western blot analysis of Cx43 expression under normal glucose (NG) or HG. Cx43 OD-optical density relative to β-actin (n = 6 transwell inserts/group, P < 0.001, Student’s t test). Western blot analysis of Cx43 expression in the brain cortex (B) and brain vessels lysate (C) in control Wistar and type 2 diabetes (T2DN) rats (n = 4 rats/group, 48 wk old, Cx43 OD-optical density relative to β-actin; P < 0.05, Student’s t test). Cx43, connexin 43.
To determine the ability of astrocytes to secrete VEGF in coculture, we measured VEGF protein levels in the media after 72 h of coculture conditions. HG-astrocytes cocultured with CMEC in HG-astrocyte media secreted significantly more VEGF protein compared with NG-astrocytes cocultured with CMEC in NG-astrocyte media (Fig. 2A, n = 6/group, P < 0.001). In the control groups, secreted VEGF protein levels in CMEC alone with either NG- or HG-astrocyte media were nearly undetectable (data not shown). The VEGF expression also was significantly elevated in the brain cortex of type 2 diabetic rats (Fig. 2B, (n = 4 rats/group, P < 0.05) and was not in brain vessels (Fig. 2C, n = 4).
Figure 2.
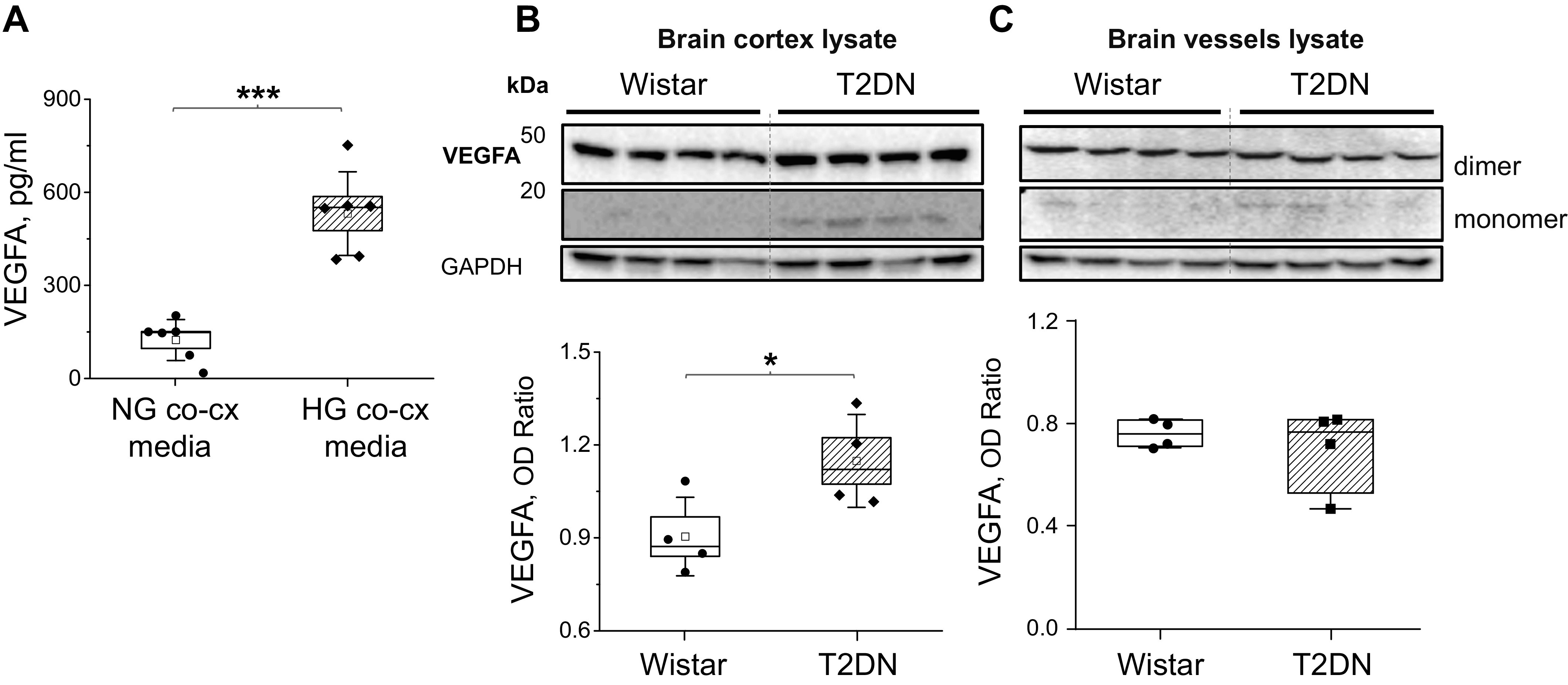
VEGFA protein levels in chronic high-glucose conditions. A: VEGFA protein levels in the media from cocultured astrocytes and CMEC under NG or HG exposure (n = 6 transwell inserts/group, P < 0.001, Student’s t test). Western blot analysis of VEGF expression in the cerebral cortex (B) and brain vessels lysate (C) of control Wistar and type 2 diabetes (T2DN) rats (n = 4 rats/group, 48 wk old, VEGFA OD-optical density relative to GAPDH; P < 0.05, Student’s t test). CMEC, cerebral microvessel endothelial cell; NG, normal glucose; VEGFA, vascular endothelial growth factor A.
To determine the ability of astrocytes to induce barrier properties in CMEC when cocultured in vitro in NG versus HG media, we measured TEER in CMEC monolayers as a surrogate marker of barrier properties. NG-astrocytes cocultured with CMEC in NG astrocyte media had significantly increased TEER compared with HG-astrocytes cocultured with CMEC in HG astrocyte media (Fig. 3B, P < 0.01, 229 ± 35%, n = 6; 88 ± 29%, n = 7). Furthermore, NG-astrocytes cocultured with CMEC in NG astrocyte media also had increased TEER compared with the control of CMEC alone.
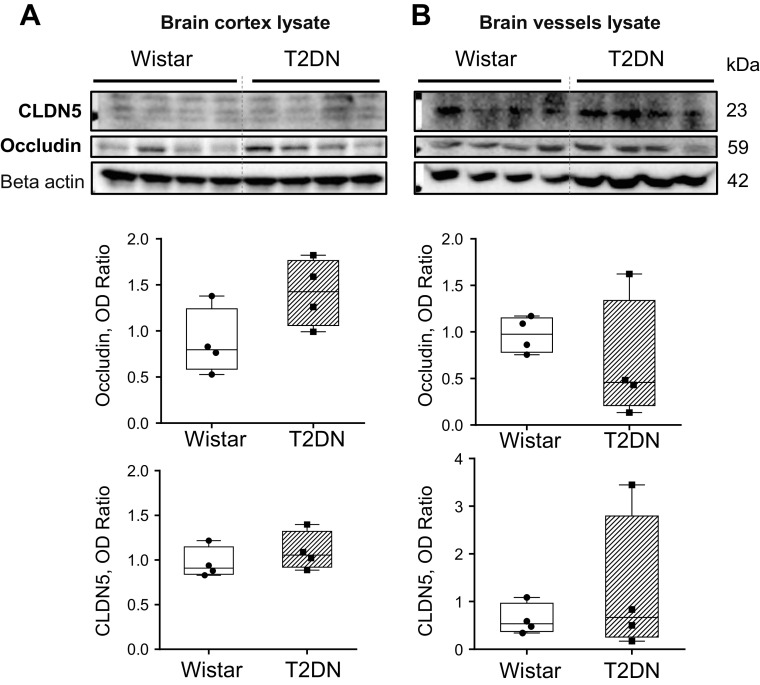
To determine phenotypic changes in high-glucose conditions unique to the BBB, we measured protein levels of tight junction proteins, Occludin and Claudin 5. We used real-time PCR and Western blot analysis of HG and NG coculture (data not shown) and Western blot analysis of brain cortex and vessels lysate in Wistar and T2DN diabetic rats (Fig. 4, A and B). In our experiments, we did not find a difference in Claudin 5 or Occludin expression.
Figure 4.
Claudin (CLDN5) and Occludin expression in the brain of control Wistar and type 2 diabetes (T2DN) rats (48 wk old). Western blot analysis of CLDN5 and Occludin expression in the cerebral cortex (A) and brain vessels lysate (B) of control Wistar and type 2 diabetes (T2DN) rats (n = 4 rats/group, 48 wk old, OD-optical density relative to β-actin; P < 0.05, Student’s t test).
DISCUSSION
The association of diabetes mellitus to cognitive impairment demands careful investigations focusing on the mechanistic effects of high-glucose conditions on the cerebrovascular system. The spatial arrangement of astrocytes to CMEC points to their importance in barrier induction and maintenance. Our laboratory and others have demonstrated that the astrocytic secretome can affect the mitogenic activity of CMEC in vitro (8, 14, 37, 45, 46). Secreted astrocytic products have also been implicated in barrier dysfunction during disease (47, 48). The novel findings in this study are 1) high-glucose conditions change the astrocytic expression of Cx43, a protein known to regulate intracellular VEGF production; 2) high-glucose conditions significantly increase the astrocytic secretion of VEGF in the presence of CMEC; 3) these protein changes were also confirmed using a novel in vivo model of diabetes mellitus; 4) normal glucose conditions allow the astrocyte to induce barrier properties in cocultured CMEC; and 5) these barrier properties are not associated with quantitative changes in tight junction proteins occludin and claudin-5. These results demonstrate that exposure to high-glucose conditions alters critical astrocytic proteins responsible for inducing barrier properties in CMEC (Fig. 5).
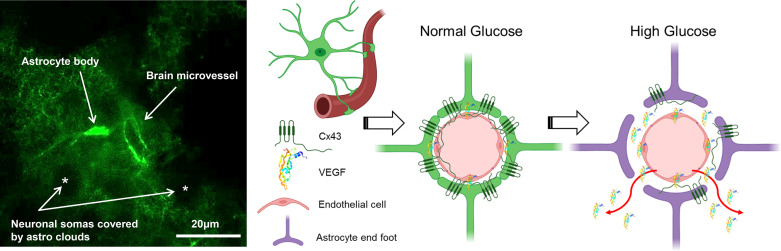
Figure 5.
Pictorial depiction of study hypothesis. Left: astrocyte, expressing an enhanced green fluorescent protein under the control of the human glial fibrillary acidic protein promotor, tightly cover microvessel in the rodent brain cortex (71). Right: astrocyte-endothelial signal transduction exposure to high glucose in diabetic conditions causing overproduction of VEGF and abnormal proliferation of endothelial cells, loss of Cx43 expression, cell membrane gap junctions, and astrocyte function, which correlates with the impairment in blood-brain barrier. Cx43, connexin 43; VEGF, vascular endothelial growth factor.
Hyperglycemia-elicited complications at the cerebral microvascular level include low perfusion rates, thickening of capillary walls, and abnormal proliferation of endothelial cells with increased vascular permeability both in vitro and in vivo (1). The cellular constituents of the BBB regulate vascular permeability in the CNS. At the cellular level, the BBB is constituted by vascular endothelium lining the cerebral microvessels with closely apposed astrocytic end-feet processes (Fig. 5). The astrocytes are highly coupled by gap junctions in areas of the brain with high metabolic rates and are involved in selective syncytial “trafficking” of energy metabolites and cytokines (49). Cx proteins are a family of gap junctional proteins found in most mammalian cells. Astrocytes express high levels of Cx30 and Cx43, and they are suggested to be responsible for determining the autocrine and paracrine signaling interactions that mediate glial communication with its microenvironment. Our findings that high-glucose conditions resulted in significantly decreased Cx43 protein levels in astrocyte cell lysate compared with normal glucose conditions are consistent with previous findings in astrocytes (49) (Fig. 1A). Using a novel in vivo model of nonobese diabetes mellitus, we confirmed this finding in the diabetic cortex (Fig. 1B). In in vitro model mimicking high-glucose conditions during diabetic retinopathy, decreased Cx43 protein levels were restored, which resulted in reduction of apoptotic processes and normalization of mitochondrial morphology confirming that Cx43 is necessary for cell function and survival (50). Several works suggested that this change in Cx43 level was implicated in the impaired gap junctional communication among astrocytes (49, 51). In human umbilical cord endothelial cells, high-glucose conditions diminished the intensity and frequency of Cx43 at cell-cell borders and throughout the cytosol and perinuclear region on confocal microscopy (52). This was not thought to be due to the osmotic effect because the cells in normal glucose conditions reflected a similar expression pattern. In cochlea, inhibition of Cx43 disruption of intercellular tight junctions while using patch-clamp dye loading showed that Cx43 is crucial for cell-cell communication (53). Together with ours, these findings suggest that Cx43 is directly affected by changes in glucose concentration and could have profound effects on intracellular and intercellular communication in the cerebral microenvironment.
Cx43 protein subunits arrange into hemichannels on the astrocyte cell membrane through which metabolic, biochemical, and electrical signals are passed (54). Cx43 form gap junction channels that directly connect the cellular cytoplasm with extracellular environmental triggers that can regulate downstream intracellular signaling pathways (54). Downregulation of Cx43 has been associated with cellular dysfunction including astrocytomas, and restoring Cx43 to tumor cells reduces their rate of proliferation (55). Furthermore, Cx43 knock-down melanoma cells produced increased HIF-1α and VEGF levels suggesting that Cx43 regulates tumor growth by way of angiogenesis (23). HIF-1α is highly regulated by oxygen levels, but it should be noted that several factors activate HIF-1α under normoxic conditions. In the retina, increasing levels of glucose concentration were associated with increasing levels of VEGF mRNA (56). In vascular smooth muscle cells, glucose dose-dependently increases VEGF synthesis and secretion. We found that high glucose conditions resulted in significantly increased VEGF secretion in astrocytes cocultured with CMEC (Fig. 2A) as well as in brain cortex but not vessels in the in vivo model of diabetes mellitus (Fig. 2, B and C). This is opposite to what we measured before in monoculture of astrocytes(8), suggesting that coculture changes the astrocytic secretome. These results taken with the changes seen in Cx43 expression point to the possible role that astrocytes play in sensing the microenvironment with cell membrane gap junctions and responding with changes in intracellular signaling pathways that alter the astrocytic secretome.
HIF-1α induction of VEGF gene expression often represents a critical rate-limiting step in angiogenesis as well as BBB dysfunction (29, 57–60). VEGF is one of the most important angiogenic growth factor that binds to receptors on the surface of endothelial cells and activates intracellular tyrosine kinases, triggering multiple downstream signals (28, 30). In vivo, VEGF is an important effector used by reactive astrocytes to mediate disruption of BBB integrity (47). The integrity of the endothelial cell-cell junctions and BBB function are regulated by a series of adhesion molecules that make up tight junctions (61). Interestingly, VEGF can be both protective and dangerous and its changes can be related to protective and pathological processes (62). VEGF is known to increase BBB permeability by downregulation of tight junction proteins occludin and claudin-5, the major components of the functional barrier in the brain (63). It is well known that BBB permeability and tight junction proteins’ integrity may be compromised by different factors, whereas VEGF could alter expression and arrangement of tight junction proteins (64, 65). VEGF is elevated in high-glucose conditions in culture and in diabetic models. However, there are interesting data that suggest that VEGF levels and effects can be different in type 1 and 2 diabetes (62). Insulin itself also can affect VEGF expression (66). In our study, CMEC cocultured in high-glucose conditioned astrocytes resulted in significantly less barrier properties compared with CMEC cocultured in normal glucose conditioned astrocytes (Fig. 3B). These findings suggest that the presence of cellular cross talk between astrocytes and CMEC under normal glucose conditions is needed for barrier formation. This speculation is supported by other studies that investigated the effects of the astrocytic secretome on endothelial cell monolayers in vitro (67, 68). Interestingly, high-glucose conditions in a model of type 2 diabetes were not associated with any significant change in occludin or claudin-5 protein levels (Fig. 4). Our findings are similar to those of other studies that have investigated the effects of glucose on tight junction protein expression in the eye and brain (3, 69); however, occludin and other junction proteins expression were shown to increase in cell culture under high-glucose treatment (3). This suggests that any changes in barrier function may be due to qualitative rather than quantitative changes such as posttranslational modifications including phosphorylation of tight junction proteins (70). Investigating these changes was outside the scope of this study but may lead to future research directions.
Perspectives and Significance
High glucose promotes a decrease in the expression of Cx43 protein in astrocytes and is associated with elevated secretion of VEGF both in coculture and animal models of nonobese diabetes mellitus. Furthermore, these results are associated with a failure to induce barrier properties in the CMEC monolayer compared with normal glucose conditions. This inability to induce barrier properties (i.e., increased TEER) was surprisingly unrelated to a quantitative difference in key tight junction proteins, both in culture and animal models. Additional studies are needed to evaluate if unique and nuanced phenotypic changes in tight junctional proteins explain this process in diabetes. Our study further proves that normal glucose conditions are necessary for appropriate astrocyte-CMEC cross talk and barrier formation.
GRANTS
This work was supported by the National Institutes of Health Grants R01 HL033833-32 (to D. Harder) and DK126720 (to O. Palygin), endowed funds from the SC SmartState Centers of Excellence (to O. Palygin), Medical College of Wisconsin Neuroscience Research Center Imagine More Award (to S. Cohen), and Children’s Hospital of Wisconsin Research Institute Pilot Award funding (to S. Cohen).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.G., K.R., and S.C. conceived and designed research; J.G., M.S., Q.L., E.I., V.L., and O.P. performed experiments; J.G., M.S., Q.L., K.R., E.I., V.L., A.S., O.P., D.H., and S.C. analyzed data; J.G., M.S., Q.L., K.R., E.I., V.L., A.S., O.P., D.H., and S.C. interpreted results of experiments; J.G., M.S., K.R., E.I., A.S., O.P., and S.C. prepared figures; J.G. and S.C. drafted manuscript; J.G., M.S., Q.L., K.R., O.P., D.H., and S.C. edited and revised manuscript; J.G., M.S., K.R., V.L., O.P., and S.C. approved final version of manuscript.
ACKNOWLEDGMENTS
Statistical assistance was provided by Quantitative Health Sciences at the Medical College of Wisconsin (MCW).
REFERENCES
- 1.Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes mellitus and blood-brain barrier dysfunction: an overview. J Pharmacovigil 2: 125, 2014. doi: 10.4172/2329-6887.1000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu Z, Zeng W, Sun J, Chen W, Zhang R, Yang Z, Yao Z, Wang L, Song L, Chen Y, Zhang Y, Wang C, Gong L, Wu B, Wang T, Zheng J, Gao F. The quantification of blood-brain barrier disruption using dynamic contrast-enhanced magnetic resonance imaging in aging rhesus monkeys with spontaneous type 2 diabetes mellitus. Neuroimage 158: 480–487, 2017. doi: 10.1016/j.neuroimage.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 3.Yan J, Zhang Z, Shi H. HIF-1 is involved in high glucose-induced paracellular permeability of brain endothelial cells. Cell Mol Life Sci 69: 115–128, 2012. doi: 10.1007/s00018-011-0731-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173–185, 2005. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 5.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol 119: 7–35, 2010. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature 325: 253–257, 1987. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 7.Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26: 523–530, 2003. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Cohen S, Liu Q, Wright M, Garvin J, Rarick K, Harder D. High glucose conditioned neonatal astrocytes results in impaired mitogenic activity in cerebral microvessel endothelial cells in co-culture. Heliyon 5: e01795, 2019. doi: 10.1016/j.heliyon.2019.e01795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jin K, Mao XO, Batteur SP, McEachron E, Leahy A, Greenberg DA. Caspase-3 and the regulation of hypoxic neuronal death by vascular endothelial growth factor. Neuroscience 108: 351–358, 2001. doi: 10.1016/S0306-4522(01)00154-3. [DOI] [PubMed] [Google Scholar]
- 10.Wu KW, Yang P, Li SS, Liu CW, Sun FY. VEGF attenuated increase of outward delayed-rectifier potassium currents in hippocampal neurons induced by focal ischemia via PI3-K pathway. Neuroscience 298: 94–101, 2015. doi: 10.1016/j.neuroscience.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 11.Alvarez JI, Katayama T, Prat A. Glial influence on the blood brain barrier. Glia 61: 1939–1958, 2013. doi: 10.1002/glia.22575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang W, Dentler WL, Borchardt RT. VEGF increases BMEC monolayer permeability by affecting occludin expression and tight junction assembly. Am J Physiol Heart Circ Physiol 280: H434–H440, 2001. doi: 10.1152/ajpheart.2001.280.1.H434. [DOI] [PubMed] [Google Scholar]
- 13.Allen CL, Bayraktutan U. Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood–brain barrier hyperpermeability. Diabetes Obes Metab 11: 480–490, 2009. doi: 10.1111/j.1463-1326.2008.00987.x. [DOI] [PubMed] [Google Scholar]
- 14.Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One 5: e11863, 2010. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Li G, Wang Z, Zhang X, Yao L, Wang F, Liu S, Yin J, Ling EA, Wang L, Hao A. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience 202: 58–68, 2012. doi: 10.1016/j.neuroscience.2011.11.062. [DOI] [PubMed] [Google Scholar]
- 16.Shehata AS, Mohamed DA, Hagras SM, El-Beah SM, Elnegris HM. The role of hesperidin in ameliorating retinal changes in rats with experimentally induced type 1 diabetes mellitus and the active role of vascular endothelial growth factor and glial fibrillary acidic protein. Anat Cell Biol 54: 465–478, 2021. doi: 10.5115/acb.21.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang L, Dong DL, Gao JH, Wang AK, Shao YP. β-HB inhibits the apoptosis of high glucose-treated astrocytes via activation of CREB/BDNF axis. Brain Inj 35: 1201–1209, 2021. doi: 10.1080/02699052.2021.1959061. [DOI] [PubMed] [Google Scholar]
- 18.Rask-Madsen C, King GL. Vascular complications of diabetes: mechanisms of injury and protective factors. Cell Metab 17: 20–33, 2013. doi: 10.1016/j.cmet.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laws MJ, Taylor RN, Sidell N, DeMayo FJ, Lydon JP, Gutstein DE, Bagchi MK, Bagchi IC. Gap junction communication between uterine stromal cells plays a critical role in pregnancy-associated neovascularization and embryo survival. Development 135: 2659–2668, 2008. doi: 10.1242/dev.019810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLachlan E, Shao Q, Wang HL, Langlois S, Laird DW. Connexins act as tumor suppressors in three-dimensional mammary cell organoids by regulating differentiation and angiogenesis. Cancer Res 66: 9886–9894, 2006. doi: 10.1158/0008-5472.CAN-05-4302. [DOI] [PubMed] [Google Scholar]
- 21.Pocrnich CE, Shao Q, Liu H, Feng MM, Harasym S, Savage M, Khimdas S, Laird DW, Hutnik CM. The effect of connexin43 on the level of vascular endothelial growth factor in human retinal pigment epithelial cells. Graefes Arch Clin Exp Ophthalmol 250: 515–522, 2012. doi: 10.1007/s00417-011-1871-x. [DOI] [PubMed] [Google Scholar]
- 22.Shao Q, Wang H, McLachlan E, Veitch GI, Laird DW. Down-regulation of Cx43 by retroviral delivery of small interfering RNA promotes an aggressive breast cancer cell phenotype. Cancer Res 65: 2705–2711, 2005. doi: 10.1158/0008-5472.CAN-04-2367. [DOI] [PubMed] [Google Scholar]
- 23.Wang W-K, Chen M-C, Leong H-F, Kuo Y-L, Kuo C-Y, Lee C-H. Connexin 43 suppresses tumor angiogenesis by down-regulation of vascular endothelial growth factor via hypoxic-induced factor-1α. Int J Mol Sci 16: 439–451, 2014. doi: 10.3390/ijms16010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giaume C, Leybaert L, Naus CC, Sáez JC. Connexin and pannexin hemichannels in brain glial cells: properties, pharmacology, and roles. Front Pharmacol 4: 88, 2013. doi: 10.3389/fphar.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Decrock E, De Bock M, Wang N, Bultynck G, Giaume C, Naus CC, Green CR, Leybaert L. Connexin and pannexin signaling pathways, an architectural blueprint for CNS physiology and pathology? Cell Mol Life Sci 72: 2823–2851, 2015. doi: 10.1007/s00018-015-1962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valle-Casuso JC, González-Sánchez A, Medina JM, Tabernero A. HIF-1 and c-Src mediate increased glucose uptake induced by endothelin-1 and connexin43 in astrocytes. PLoS One 7: e32448, 2012. doi: 10.1371/journal.pone.0032448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benderro GF, Lamanna JC. Hypoxia-induced angiogenesis is delayed in aging mouse brain. Brain Res 1389: 50–60, 2011. doi: 10.1016/j.brainres.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benderro GF, Sun X, Kuang Y, Lamanna JC. Decreased VEGF expression and microvascular density, but increased HIF-1 and 2α accumulation and EPO expression in chronic moderate hyperoxia in the mouse brain. Brain Res 1471: 46–55, 2012. doi: 10.1016/j.brainres.2012.06.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cohen SS, Powers BR, Lerch-Gaggl A, Teng RJ, Konduri GG. Impaired cerebral angiogenesis in the fetal lamb model of persistent pulmonary hypertension. Int J Dev Neurosci 38: 113–118, 2014. doi: 10.1016/j.ijdevneu.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dore-Duffy P, LaManna JC. Physiologic angiodynamics in the brain. Antioxid Redox Signal 9: 1363–1371, 2007. doi: 10.1089/ars.2007.1713. [DOI] [PubMed] [Google Scholar]
- 31.Chang M-L, Chiu C-J, Shang F, Taylor A. High glucose activates ChREBP-mediated HIF-1α and VEGF expression in human RPE cells under normoxia. Adv Exp Med Biol 801: 609–621, 2014. doi: 10.1007/978-1-4614-3209-8_77. [DOI] [PubMed] [Google Scholar]
- 32.Nobrega MA, Fleming S, Roman RJ, Shiozawa M, Schlick N, Lazar J, Jacob HJ. Initial characterization of a rat model of diabetic nephropathy. Diabetes 53: 735–742, 2004. doi: 10.2337/diabetes.53.3.735. [DOI] [PubMed] [Google Scholar]
- 33.Palygin O, Spires D, Levchenko V, Bohovyk R, Fedoriuk M, Klemens CA, Sykes O, Bukowy JD, Cowley AW Jr, Lazar J, Ilatovskaya DV, Staruschenko A. Progression of diabetic kidney disease in T2DN rats. Am J Physiol Renal Physiol 317: F1450–F1461, 2019. doi: 10.1152/ajprenal.00246.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bowyer JF, Thomas M, Patterson TA, George NI, Runnells JA, Levi MS. A visual description of the dissection of the cerebral surface vasculature and associated meninges and the choroid plexus from rat brain. J Vis Exp 14: e4285, 2012. doi: 10.3791/4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebremedhin D, Zhang DX, Carver KA, Rau N, Rarick KR, Roman RJ, Harder DR. Expression of CYP 4A ω-hydroxylase and formation of 20-hydroxyeicosatetreanoic acid (20-HETE) in cultured rat brain astrocytes. Prostaglandins Other Lipid Mediat 124: 16–26, 2016. doi: 10.1016/j.prostaglandins.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Cossette SM, Rarick KR, Gershan J, Dwinell MB, Harder DR, Ramchandran R. Astrocytes directly influence tumor cell invasion and metastasis in vivo. PLoS One 8: e80933, 2013. [Erratum in PLoS One 10: e0137369, 2015]. doi: 10.1371/journal.pone.0080933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Hong G, Lee KS, Hammock BD, Gebremedhin D, Harder DR, Koehler RC, Sapirstein A. Inhibition of soluble epoxide hydrolase augments astrocyte release of vascular endothelial growth factor and neuronal recovery after oxygen-glucose deprivation. J Neurochem 140: 814–825, 2017. doi: 10.1111/jnc.13933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Helms HC, Abbott NJ, Burek M, Cecchelli R, Couraud P-O, Deli MA, Förster C, Galla HJ, Romero IA, Shusta EV, Stebbins MJ, Vandenhaute E, Weksler B, Brodin B. In vitro models of the blood-brain barrier: an overview of commonly used brain endothelial cell culture models and guidelines for their use. J Cereb Blood Flow Metab 36: 862–890, 2016. doi: 10.1177/0271678X16630991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Wang N, Cai B, Wang G-Y, Li J, Piao X-X. In vitro model of the blood-brain barrier established by co-culture of primary cerebral microvascular endothelial and astrocyte cells. Neural Regen Res 10: 2011–2017, 2015. doi: 10.4103/1673-5374.172320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czupalla CJ, Liebner S, Devraj K. In vitro models of the blood–brain barrier. In: Cerebral Angiogenesis: Methods and Protocols, edited by Milner R. New York, NY: Humana Press, 2014, p. 415–437. doi: 10.1007/978-1-4939-0320-7_34. [DOI] [PubMed] [Google Scholar]
- 41.Cohen SS, Min M, Cummings EE, Chen X, Sadowska GB, Sharma S, Stonestreet BS. Effects of interleukin-6 on the expression of tight junction proteins in isolated cerebral microvessels from yearling and adult sheep. Neuroimmunomodulation 20: 264–273, 2013. doi: 10.1159/000350470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isaeva E, Isaev D, Savrasova A, Khazipov R, Holmes GL. Recurrent neonatal seizures result in long-term increases in neuronal network excitability in the rat neocortex. Eur J Neurosci 31: 1446–1455, 2010. doi: 10.1111/j.1460-9568.2010.07179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 44.Cohen S, Ke X, Liu Q, Fu Q, Majnik A, Lane R. Adverse early life environment increases hippocampal microglia abundance in conjunction with decreased neural stem cells in juvenile mice. Int J Dev Neurosci 55: 56–65, 2016. doi: 10.1016/j.ijdevneu.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Munzenmaier DH, Harder DR. Cerebral microvascular endothelial cell tube formation: role of astrocytic epoxyeicosatrienoic acid release. Am J Physiol Heart Circ Physiol 278: H1163–H1167, 2000. doi: 10.1152/ajpheart.2000.278.4.H1163. [DOI] [PubMed] [Google Scholar]
- 46.Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS. Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 58: 1177–1185, 2010. doi: 10.1002/glia.20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG, Ferrara N, Sofroniew MV, John GR. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122: 2454–2468, 2012. doi: 10.1172/JCI60842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Argaw AT, Gurfein BT, Zhang Y, Zameer A, John GR. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc Natl Acad Sci USA 106: 1977–1982, 2009. doi: 10.1073/pnas.0808698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gandhi GK, Ball KK, Cruz NF, Dienel GA. Hyperglycaemia and diabetes impair gap junctional communication among astrocytes. ASN Neuro 2: e00030, 2010. doi: 10.1042/AN20090048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sankaramoorthy A, Roy S. High glucose-induced apoptosis is linked to mitochondrial connexin 43 Level in RRECs: implications for diabetic retinopathy. Cells 10: 3102, 2021. doi: 10.3390/cells10113102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ball KK, Harik L, Gandhi GK, Cruz NF, Dienel GA. Reduced gap junctional communication among astrocytes in experimental diabetes: contributions of altered connexin protein levels and oxidative-nitrosative modifications. J Neurosci Res 89: 2052–2067, 2011. doi: 10.1002/jnr.22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Willmott T, Leach L. The effects of high glucose on connexin 43 in human endothelial cells. J Anat 200: 532–532, 2002. doi: 10.1046/j.1469-7580.2002.00047_25.x. [DOI] [Google Scholar]
- 53.Zhang J, Wang X, Hou Z, Neng L, Cai J, Zhang Y, Shi X. Suppression of connexin 43 leads to strial vascular hyper-permeability, decrease in endocochlear potential, and mild hearing loss. Front Physiol 11: 974, 2020. doi: 10.3389/fphys.2020.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Bock M, Leybaert L, Giaume C. Connexin channels at the glio-vascular interface: gatekeepers of the brain. Neurochem Res 42: 2519–2536, 2017. doi: 10.1007/s11064-017-2313-x. [DOI] [PubMed] [Google Scholar]
- 55.Tabernero A, Gangoso E, Jaraíz-Rodríguez M, Medina JM. The role of connexin43–Src interaction in astrocytomas: a molecular puzzle. Neuroscience 323: 183–194, 2016. doi: 10.1016/j.neuroscience.2015.02.029. [DOI] [PubMed] [Google Scholar]
- 56.Mu H, Zhang X-M, Liu J-J, Dong L, Feng Z-L. Effect of high glucose concentration on VEGF and PEDF expression in cultured retinal Müller cells. Mol Biol Rep 36: 2147–2151, 2009. doi: 10.1007/s11033-008-9428-8. [DOI] [PubMed] [Google Scholar]
- 57.Ferrara N. Vascular endothelial growth factor: basic science and clinical progress. Endocr Rev 25: 581–611, 2004. doi: 10.1210/er.2003-0027. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 59.Yeh W-L, Lu D-Y, Lin C-J, Liou H-C, Fu W-M. Inhibition of hypoxia-induced increase of blood-brain barrier permeability by YC-1 through the antagonism of HIF-1alpha accumulation and VEGF expression. Mol Pharmacol 72: 440–449, 2007. doi: 10.1124/mol.107.036418. [DOI] [PubMed] [Google Scholar]
- 60.Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen Nv, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest 106: 829–838, 2000. doi: 10.1172/JCI9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen SS, Malaeb SN, Virgintino D, Stonestreet BS. Development of the blood-brain barrier. In: Fetal and Neonatal Physiology, 2011, pp. 1763–1774. [Google Scholar]
- 62.Doronzo G, Viretto M, Russo I, Mattiello L, Anfossi G, Trovati M. Effects of high glucose on vascular endothelial growth factor synthesis and secretion in aortic vascular smooth muscle cells from obese and lean Zucker rats. Int J Mol Sci 13: 9478–9488, 2012. doi: 10.3390/ijms13089478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Almutairi MMA, Gong C, Xu YG, Chang Y, Shi H. Factors controlling permeability of the blood–brain barrier. Cell Mol Life Sci 73: 57–77, 2016. doi: 10.1007/s00018-015-2050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bauer AT, Bürgers HF, Rabie T, Marti HH. Matrix metalloproteinase-9 mediates hypoxia-induced vascular leakage in the brain via tight junction rearrangement. J Cereb Blood Flow Metab 30: 837–848, 2010. doi: 10.1038/jcbfm.2009.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res 63: 70–80, 2002. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- 66.Chou E, Suzuma I, Way KJ, Opland D, Clermont AC, Naruse K, Suzuma K, Bowling NL, Vlahos CJ, Aiello LP, King GL. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic states: a possible explanation for impaired collateral formation in cardiac tissue. Circulation 105: 373–379, 2002. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 67.Hurwitz AA, Berman JW, Rashbaum WK, Lyman WD. Human fetal astrocytes induce the expression of blood-brain barrier specific proteins by autologous endothelial cells. Brain Res 625: 238–243, 1993. doi: 10.1016/0006-8993(93)91064-y. [DOI] [PubMed] [Google Scholar]
- 68.Siddharthan V, Kim YV, Liu S, Kim KS. Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res 1147: 39–50, 2007. doi: 10.1016/j.brainres.2007.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Villarroel M, García-Ramírez M, Corraliza L, Hernández C, Simó R. Effects of high glucose concentration on the barrier function and the expression of tight junction proteins in human retinal pigment epithelial cells. Exp Eye Res 89: 913–920, 2009. doi: 10.1016/j.exer.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 70.Huber JD, Egleton RD, Davis TP. Molecular physiology and pathophysiology of tight junctions in the blood–brain barrier. Trends Neurosci 24: 719–725, 2001. doi: 10.1016/s0166-2236(00)02004-x. [DOI] [PubMed] [Google Scholar]
- 71.Palygin O, Lalo U, Pankratov Y. Distinct pharmacological and functional properties of NMDA receptors in mouse cortical astrocytes. Br J Pharmacol 163: 1755–1766, 2011. doi: 10.1111/j.1476-5381.2011.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]