Keywords: intestinal, metaplasia, subsquamous
Abstract
The pathogenesis of subsquamous intestinal metaplasia (SSIM), in which glands of Barrett’s esophagus (BE) are buried under esophageal squamous epithelium, is unknown. In a rat model of reflux esophagitis, we found that columnar-lined esophagus developed via a wound-healing process involving epithelial-mesenchymal plasticity (EMP) that buried glands under ulcerated squamous epithelium. To explore a role for reflux-induced EMP in BE, we established and characterized human Barrett’s organoids and sought evidence of EMP after treatment with acidic bile salts (AB). We optimized media to grow human BE organoids from immortalized human Barrett’s cells and from BE biopsies from seven patients, and we characterized histological, morphological, and molecular features of organoid development. Features and markers of EMP were explored following organoid exposure to AB, with and without a collagen I (COL1) matrix to simulate a wound-healing environment. All media successfully initiated organoid growth, but advanced DMEM/F12 (aDMEM) was best at sustaining organoid viability. Using aDMEM, organoids comprising nongoblet and goblet columnar cells that expressed gastric and intestinal cell markers were generated from BE biopsies of all seven patients. After AB treatment, early-stage Barrett’s organoids exhibited EMP with loss of membranous E-cadherin and increased protrusive cell migration, events significantly enhanced by COL1. Using human BE biopsies, we have established Barrett’s organoids that recapitulate key histological and molecular features of BE to serve as high-fidelity BE models. Our findings suggest that reflux can induce EMP in human BE, potentially enabling Barrett’s cells to migrate under adjacent squamous epithelium to form SSIM.
NEW & NOTEWORTHY Using Barrett’s esophagus (BE) biopsies, we established organoids recapitulating key BE features. During early stages of organoid development, a GERD-like wound environment-induced features of epithelial-mesenchymal plasticity (EMP) in Barrett’s progenitor cells, suggesting that reflux-induced EMP can enable Barrett’s cells to migrate underneath squamous epithelium to form subsquamous intestinal metaplasia, a condition that may underlie Barrett’s cancers that escape detection by endoscopic surveillance, and recurrences of Barrett’s metaplasia following endoscopic eradication therapy.
INTRODUCTION
Barrett’s esophagus (BE) is the condition in which a metaplastic mucosa that has gastric and intestinal features replaces esophageal squamous mucosa damaged by gastroesophageal reflux disease (GERD) (1). BE and GERD, which affects ∼20% of adult Americans, are major risk factors for esophageal adenocarcinoma, a tumor whose frequency has increased more than eightfold over the past four decades in the United States (1, 2). For patients with BE, endoscopic surveillance is recommended with the rationale that it will detect neoplasia in an early, curable form (i.e., dysplasia). There is no proof that this costly practice prevents cancer deaths, however. Furthermore, some high-quality studies have found no such benefit for endoscopic surveillance, citing many examples of patients with BE who developed esophageal adenocarcinomas despite adherence to surveillance protocols (3). It is not clear why such a plausible cancer prevention strategy has performed so poorly.
Radiofrequency ablation (RFA), which inflicts a thermal injury to destroy metaplastic mucosa, is now the endoscopic ablative treatment of choice for dysplasia in BE (4). However, because of a high rate of recurrent metaplasia (5), continued endoscopic surveillance is recommended after RFA (6), adding substantial cost, inconvenience, and risk to patient management. This high recurrence rate also is a major impediment to implementing the potentially cancer preventive practice of RFA for all patients with BE, because RFA for nondysplastic BE cannot be cost-effective unless it eliminates the need for regular endoscopic surveillance (7). The etiology of the intestinal metaplasia that recurs so frequently after endoscopic eradication is not known.
Barrett’s cancers that develop despite endoscopic surveillance and recurrent metaplasia after RFA might be due to a condition called subsquamous intestinal metaplasia (SSIM, also called buried glands or buried Barrett’s) in which metaplastic glands are present in the lamina propria under esophageal squamous epithelium. SSIM initially was assumed to be due to incomplete endoscopic ablation procedures that destroyed a superficial layer of Barrett’s mucosa while leaving a viable, deeper layer of glands to be buried by an overgrowth of new squamous epithelium (8). However, more recent reports suggest that the large majority of treatment-naïve patients with Barrett’s esophagus have SSIM in squamous mucosa near its junction with Barrett’s metaplasia (9, 10). This highly prevalent SSIM might be shielded from destruction by RFA by the overlying squamous epithelium. Thus, SSIM could be the source of tumors missed by endoscopic surveillance, and the nidus for recurrent metaplasia after RFA. As such, SSIM appears to be a frequent and important condition that limits the efficacy of endoscopic surveillance for the millions of patients with BE, and that thwarts attempts to eradicate Barrett’s metaplasia by RFA.
Despite the likely clinical importance of SSIM, very little is known about its pathogenesis. We have proposed that SSIM might be acquired as a result of epithelial mesenchymal transition (EMT), a multifaceted and reversible change in cell phenotype in which epithelial cells lose their typical epithelial features (e.g., cell adhesion), reorganize their cytoskeletons, acquire mesenchymal features, and gain the ability to migrate (11, 12). More recently, the term epithelial-mesenchymal plasticity (EMP) has been proposed to replace EMT due to increased recognition of the plasticity, heterogeneity, and reversibility of this process (11). EMP encompasses hybrid phenotypes whereby cells adopt a mixture of epithelial and mesenchymal features (also referred to as partial EMT) arrayed along a spectrum in which pure epithelial and pure mesenchymal phenotypes mark the extreme endpoints (11).
In earlier studies, we found that rats with reflux esophagitis induced by esophago-jejunostomy developed a columnar-lined esophagus (CLE) via a wound-healing process that involved EMP (12). As part of this wound-healing process, columnar glands were found “buried” in the lamina propria underneath esophageal squamous epithelium close to reflux-induced esophageal ulcerations (13). Using nonneoplastic human Barrett’s epithelial cells grown in organotypic culture, we observed that Barrett’s cells treated with acidic bile salts would migrate deep into the underlying stromal equivalent (i.e., they “buried” themselves) (13). Both the CLE from rats with reflux esophagitis and the acidic bile salt-treated human Barrett’s epithelial cells exhibited both mesenchymal features (i.e., the ability to migrate) and markers of epithelial cells [i.e., E-cadherin (CDH1) expression], suggesting that EMP is the mechanism through which SSIM develops as part of a wound-healing process triggered by reflux-induced inflammation (13, 14). However, animal models and human Barrett’s cell cultures have considerable limitations as models of human BE.
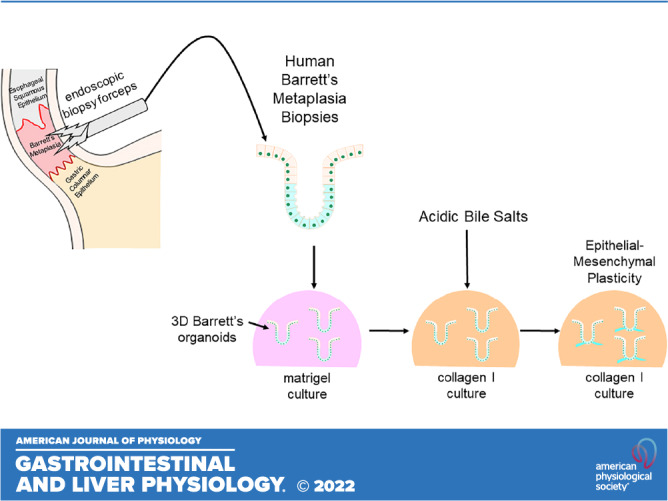
Tissue-relevant organoids are a preferred model for studying human disease processes. The organoid culture system is a three-dimensional (3-D) culture method that relies on putative stem or progenitor cells within a tissue to generate ex vivo cell collections that closely mimic the parent tissue, both structurally and physiologically (15–17). Once established, the organoid culture substrate and medium composition can be altered to simulate a diseased tissue environment, enabling study of the epithelial response to disease (18). Few studies have successfully established human, adult, tissue-derived Barrett's metaplastic organoids and, in those that have, the organoids were not fully characterized (15, 19, 20). Moreover, there is limited understanding of the morphological phases and gene expression patterns that occur during Barrett’s organoid development (19, 20). We have established human Barrett’s organoid cultures from endoscopic biopsies obtained from adults with BE and have characterized their growth and differentiation as well as their histological and molecular phenotype. By modulating culture conditions, we used these human Barrett’s organoids to explore features and markers of EMP developing after treatment with acidic bile salts.
MATERIALS AND METHODS
Patients
These studies were approved by the institutional review boards of Dallas Veterans Affairs Medical Center and Baylor Scott & White Research Institute. Patients scheduled for routine endoscopic surveillance were invited to participate. Endoscopic biopsies of metaplastic Barrett’s mucosa were obtained from seven patients (6 men, 1 woman; median age 49, range 25–67 yr) whose clinical characteristics are shown in Table 1. Six patients had long-segment (≥ 3 cm) Barrett’s esophagus. All patients were on proton pump inhibitors at the time of biopsy. Patients were excluded if they were unwilling or unable to provide informed consent or if they had conditions that precluded safe biopsy of the esophagus.
Table 1.
Patients used to generate Barrett’s primary cell and organoid culture
| Id | Age | Sex | Race | Length of BE | *CM Category | Proton Pump Inhibitor | Dose | Organoid Cultures |
|
|---|---|---|---|---|---|---|---|---|---|
| Primary culture-derived | Biopsy-derived | ||||||||
| BAR-B21 | 67 | M | White | 8 cm | C6M8 | Omeprazole | 20 mg QD | Yes | No |
| BAR-B23 | 66 | M | White | 2 cm | C0M2 | Omeprazole | 20 mg QD | Yes | No |
| BAR-B24 | 47 | M | White | 10 cm | C9M10 | Omeprazole | 40 mg BID | Yes | Yes |
| BAR-B25 | 50 | F | White | 8 cm | C8M8 | Pantoprazole | 40 mg BID | No | Yes |
| BAR-B26 | 25 | M | White | 4 cm | C4M4 | Omeprazole | 20 mg BID | No | Yes |
| BAR-B27 | 38 | M | Hispanic | 4 cm | C4M4 | Omeprazole | 20 mg BID | No | Yes |
| BAR-B28 | 50 | M | White | 8 cm | C8M8 | Omeprazole | 20 mg BID | No | Yes |
*Prague circumferential (C) and maximum (M) extent in centimeters of columnar-appearing mucosa above the gastroesophageal junction. BID, twice per day; QD, once per day.
Barrett’s Metaplastic Cell Lines and Primary Cultures
We used previously characterized, nonneoplastic, telomerase-immortalized human Barrett's epithelial cell lines (BAR-T, BAR-10T) that were developed from endoscopy biopsy specimens of two male patients with nondysplastic Barrett’s metaplasia in our laboratory (21, 22). We also established primary cultures of Barrett’s epithelial cells using techniques previously described by our laboratory (22). Barrett’s primary cultures and cell lines were cocultured with a fibroblast feeder layer and maintained in DMEM/Ham’s F12 (DMEM/F12) medium supplemented with growth factors (Table 2) at 37°C in a 5% CO2 incubator. Primary Barrett’s cultures were also supplemented with 10 µM Y-27632 (Sigma, St. Louis, MO), an inhibitor of Rho-associated, coiled-coil containing protein kinase (ROCK). For individual experiments, primary cultures and cell lines were seeded equally onto collagen IV-coated wells (BD Biosciences, San Jose, CA) in the absence of fibroblast feeder layers and were maintained in growth medium. Short tandem repeat analyses confirmed the identity of cell lines used in this study.
Table 2.
Growth media
| Media | aDMEM/F12 | Mcdb-153 | DMEM/F12 |
|---|---|---|---|
| Base medium | Advanced DMEM/F12 | MCDB-153 | DMEM/F12 |
| Supplements | |||
| Hydrocortisone | – | 0.4 mg/mL | 0.4 mg/mL |
| Insulin/transferrin | – | 5 mg/mL each | 5 mg/mL each |
| Cholera toxin | – | 1.0E-10M | 1.0E-10M |
| Triiodo thyronine | – | 2.0E-11 M | 2.0E-11 M |
| Adenine | – | 20 mg/L | 180 mM |
| FBS | – | 5% | 1% |
| Bovine pituitary extract | – | 140 mg/mL | – |
| l-Glutamine | 1× | 1× | – |
| HEPES | 10 mM | – | – |
| Penicillin-streptomycin | 1× | 1× | 1× |
| Y27632 | 10 mM | – | – |
| N2 supplement | 1× | – | – |
| B27 supplement | 1× | – | – |
| N-acetylcysteine | 1 mM | – | – |
| Noggin/R-Spondin/Wnt3A | 50% (in volume) | – | – |
| (L-WRN-conditioned media) | |||
| A83-01 | 500 nM | 500 nM | – |
| SB202190 | 10 mM | – | – |
| Gastrin | 10 nM | – | – |
| Nicotinamide | 10 mM | – | – |
| EGF | 50 ng/mL | 20 ng/mL | 20 ng/mL |
| FGF | 100 ng/mL | – | – |
Esophageal Squamous Cell Primary Cultures
We established primary cultures of esophageal squamous epithelial cells (NES-D1, NES-D2, NES-D5) obtained from esophagi of heart beating, deceased organ transplant donors with no known history of esophageal disease using techniques as previously described (23, 24). This study was approved by the institutional review board of Baylor Scott & White Research Institute and by Southwest Transplant Alliance. All persons authorizing esophagus organ donation per Revised Uniform Anatomical Gift Act signed the Southwest Transplant Alliance authorization form. Primary esophageal squamous cells were cocultured with a fibroblast feeder layer and maintained in DMEM/F12 medium (Table 2) supplemented with 10 µM Y-27632 at 37°C in a 5% CO2 incubator. Before being seeded into Matrigel, fibroblast feeder layers were removed with EDTA washes (0.02% in 1 time with PBS pH 8.0, Sigma) as previously described (24).
Organoid Cultures
Biopsy tissue specimen-derived organoids were established based on a previously published protocol by Mahe et al (25). Briefly, 2–4 Barrett’s esophageal biopsy tissue specimens were minced into smaller 2- to 3-mm pieces and then immersed in ice-cold PBS containing five times with penicillin-streptomycin. After several rinses, the tissue fragments were digested in a 37°C water bath for 1 h by treating with 1 mL of 2.5 mg/mL collagenase A (Cat. No. 10103578001, Sigma, St. Louis, MO) in Ham’s F12 medium containing 2.5% fetal bovine serum (FBS). The digested fragments were treated with ice-cold dissociation buffer composed of 54.9 mM d-sorbitol (Cat. No. S1876, Sigma) and 43.4 mM sucrose (Cat. No. S7903, Sigma). Finally, the solution containing dissociated cells and tissue debris was filtered through a 70-µM cell strainer, and cell numbers were determined using a Z1 Dual-Threshold Particle Counter (Beckman Coulter, Indianapolis, IN). For organoid cultures, 2,000 cells were mixed in 25-µL Matrigel (Cat. No. 356231, BD Bioscience), seeded into a 48-well plate, and incubated at 37°C for 20 min. After solidification, 250 µL of culture media was added to each well, and then the wells were replenished with fresh culture media every other day by gentle pipetting. Barrett’s epithelial cell lines in 3-D cultures were used to optimize culture media (Table 2) for Barrett’s biopsy-derived tissue specimen and biopsy-derived primary cell culture organoids. Primary esophageal squamous cell organoids were cultured in modified keratinocyte serum-free medium containing 0.6-mM calcium as previously described by Kasagi et al. (26). By optic morphology, spheroids were defined as smoothly shaped, round, hollow structures, and organoids were defined as dense cellular masses with an ellipsoid-shape with or without crypt-like budding, or with a glandular appearance. The proportion of spheroids to organoids was determined by counting the cellular morphologies of 10 randomly selected organoids at 3, 7, and 16 days of growth.
Passaging and Freezing of Barrett’s Organoids
Organoids were passaged between 7 and 10 days of growth. Organoids were recovered from the Matrigel by incubation with the Gentle Cell Dissociation Reagent (Cat. No. 07174, StemCell Technologies, Inc., Vancouver, Canada) for 1–2 min at room temperature followed by dissociation with gentle pipetting. The recovered organoids fragments were collected, washed once with advanced DMEM/Ham’s F12 medium (aDMEM/F12), and then pelleted to the bottom of a conical tube by centrifugation at 1,500 rpm for 5 min. After centrifugation, the organoid fragments were either reseeded at a dilution of ∼1:5 in Matrigel for subsequent passages or resuspended in freezing media (10% DMSO and 90% FBS) for cryopreservation.
Formation Rate, Growth Rate, and Viability of Barrett’s Organoids
Organoid formation rate was calculated as the number of organoids at day 7 expressed as a percentage of the 2,000 seeded cells. The organoid growth rate was determined by measuring the diameter of 10 randomly selected organoids after 3, 5, 8, 10, 13, and 16 days of growth. Organoid viability was assessed by optic morphology using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Cat. No. CT02, EMD Millipore, Billerica, MA), in which tetrazolium salt is converted by viable cells into an insoluble purple formazan and quantitated by spectrophotometry per the manufacturer’s protocol. Cell death in organoids was assessed by live/dead fluorescent staining. Organoids were incubated for 20 min with calcein-acetoxymethyl ester (Calcein-^AM; 1 µg/mL) to detect live cells and propidium iodide (PI; 5 µg/mL) to detect dead cells, followed by fluorescent microscopy and quantitation by manual counting of 10 randomly selected fields.
Organoid Processing, Histological Evaluation, and Immunofluorescent Staining
After dissociation and centrifugation, the organoid pellets were fixed in 4% paraformaldehyde for 1 h at room temperature, followed by overnight incubation in 70% ethanol. The organoids were mounted in Histogel (Cat. No. HG-4000-012, Thermo Fisher Scientific), transferred into tissue cassettes, and embedded in paraffin, and blocks were cut into 5-µm sections. For histological evaluation, sections were stained with hematoxylin and eosin (H&E) or Alcian blue (pH 2.5) counterstained with Nuclear Fast Red. For immunofluorescent staining, the organoid pellets were mounted in 3% agarose, embedded in optimal cutting temperature (OCT) medium, and sectioned at 5 µm. Whole organoids were fixed in 4% paraformaldehyde in PME buffer (50 mM HEPES buffer, 2.5 mM MgCl2, and 5 mM EDTA) for 30 min at 4°C. Next, the fixed organoids were rinsed one time with PBS containing 0.2% Triton X-100 and 0.05% Tween 20 and treated with Permeabilization Solution (1 time with PBS with 0.5% Triton X-100) for 10 min. Immunofluorescent staining of OCT sections and whole organoids was performed using previously established procedures (13). Primary and secondary antibodies are listed in Table 3 along with their Research Resource Identifiers (RRIDs) for validation. The slides were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI) for analysis.
Table 3.
Antibodies and stains used for fluorescent imaging
| Antibody | Source Information (Cat. No., Vendor) | Dilution |
|---|---|---|
| MUC2 | Sc-15334, Santa Cruz RRID:AB_2146667 |
1:50 |
| MUC5AC | No. 61193, Cell Signaling Technology RRID:AB_2799603 |
1:100 |
| CK7 | ab68459, Abcam RRID:AB_1139824 |
1:100 |
| CK8/18 | Sc-52325, Santa Cruz RRID:AB_629848 |
1:100 |
| CK20 | ab76126, Abcam RRID:AB_1310117 |
1:100 |
| CK14 | sc-53253, Santa Cruz RRID:AB_2134820 |
1:100 |
| P63 | sc-8431, Santa Cruz RRID:AB_628091 |
1:500 |
| LEFTY1 | ab22569, Abcam RRID:AB_2135767 |
1:100 |
| OLFM4 | No. 14369, Cell Signaling Technology RRID:AB_2798465 |
1:100 |
| CDH1 | sc-7870, Santa Cruz RRID:AB_2798465 |
1:50 |
| Phalloidin | R415, Thermo Fisher Scientific | 1:400 |
| DAPI | MBD0015, Millipore Sigma | 1:1,000 |
| Alexa Fluor 594 donkey anti-rabbit IgG | A-21207, Thermo Fisher Scientific RRID:AB_141637 |
1:500 |
| Alexa Fluor 594 donkey anti-mouse IgG | A-21203, Thermo Fisher Scientific RRID:AB_141633 |
1:500 |
| Alexa Fluor 488 donkey anti-rabbit IgG | A-21206, Thermo Fisher Scientific RRID:AB_2535792 |
1:500 |
| Alexa Fluor 488 donkey anti-mouse IgG | A-21202, Thermo Fisher Scientific RRID:AB_141607 |
1:500 |
Acidic Bile Salt and Recombinant Human VEGF Treatment
For acidic bile salt treatment, primary Barrett’s cells in monolayer and in organoid culture were exposed to neutral pH growth medium (pH 7.2), or to acidic medium (pH 5.5) containing a mixture of conjugated bile acids [deoxycholic acid, glycocholic acid, glycodeoxycholic acid, taurocholic acid (all from Calbiochem, San Diego, CA), and glycochenodeoxycholic acid (from Colonial Scientific, Richmond, VA) in a 1:1:1:1:1 molar concentration, total concentration 100 µM] as previously described (27, 28). Neutral or acidic bile salt medium was added for 15 min once per day, after which the medium was removed and replaced with neutral pH growth medium for the remainder of the experiment. Recombinant human vascular endothelial growth factor (rhVEGF) (30 ng/mL; R&D systems, Minneapolis, MN) was added to neutral pH growth medium continuously for 24–48 h as a positive control for the wound healing, protrusive migration, and the quantitative reverse transcription polymerase chain reaction assays based on findings from our earlier studies (13).
Wound-Healing Assay
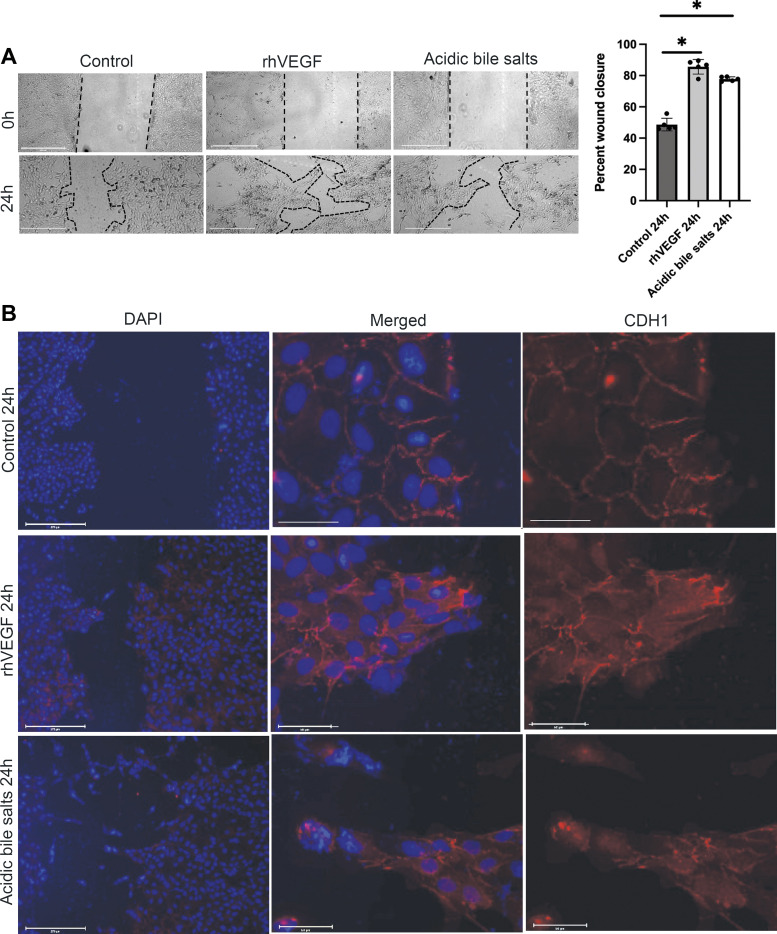
Migration of Barrett’s cells was assessed using a scratch wound assay. Primary cultures of BAR-B24 Barrett’s cells (passage 1 or 2) were grown on chamber slides to confluency and scratched using a 200-µL pipette tip. Monolayers were washed, and the medium was removed and replaced with medium containing rhVEGF or acidic bile salts. Optical images of scratch wound closure were recorded at the time of injury (0 h) and 24 h later. The monolayers were then fixed with 4% paraformaldehyde and subjected to immunofluorescent staining for CDH1.
Protrusive Migration Assay
Protrusive migration was evaluated in Matrigel and in collagen I as a model of the extracellular matrix encountered during tissue regeneration after severe esophagitis (29).Organoids were grown in Matrigel for 3 days and then recovered. The organoids were next mixed with neutralized rat tail collagen I (Cat. No. 08–115, Upstate, Lake Placid, NY) using a previously published protocol by Nguyen-Ngoc et al. (30). A 25-μL suspension of organoids and collagen I was plated in each well of a 48-well plate or on a chamber slide, followed by incubation at 37°C for 45 min to allow for polymerization, after which organoid culture medium was added. After 2 days, organoids were treated with rhVEGF continuously for 2 days or with acidic bile salts given 15 min once a day for 2 days. Organoid protrusions were characterized by sharp-edge protrusive extensions into the collagen I, in contrast to the smooth edges usually observed during organoid growth in Matrigel. Organoids demonstrating protrusions from a total of 4 wells were counted in low-power fields and the percentage of organoids showing protrusive migration was determined after treatment with rhVEGF or acidic bile salts. After fixation, immunofluorescent staining for phalloidin or CDH1 was performed.
Microscopic Imaging Analysis
Microscopic images from control and treatment groups were processed under the same conditions and captured at the same time using the Invitrogen EVOS FL Auto 2.0 Imaging system and its software (Thermo Fisher Scientific). Images of hematoxylin and eosin (H&E) staining and Alcian blue staining were recorded using bright-field microscopy with objectives ×20, ×40, or ×60 at room temperature. For live imaging, organoids were captured with phase-contrast microscopy with objectives ×4, ×10, or ×20 at room temperature. For the wound-healing assay and CDH1 staining, images were captured with phase contrast and fluorescent microscopy techniques with objectives ×10 and ×40 at room temperature. To document differential interference contrast (DIC) and fluorescent staining images, both control and treatment groups were collected at the same time and under the same conditions at room temperature using the Nikon A1R H25 laser scan confocal microscope equipped with Ti2-E inverted microscope and four lasers (405/488/561/640 nm; Nikon Instruments, Inc., Melville, NY). Images were captured using the CFI Plan Fluor ×40 objective (NA 1.3) and standard filter set, i.e., DAPI (405 nm), FITC (488 nm), TRITC (561 nm) in the format of 2,048 × 2,048 pixels. Imaging and analysis used Nikon software. Individual optical section images were presented without further processing. All images were modified in Photoshop (RRID:SCR_014199) to enhance clarity. Any adjustments in contrast, color balance, brightness, or sharpness were applied to the entire image.
Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted using TRIzol (Invitrogen, Waltham, MA) following the manufacturer’s instructions. Reverse transcription was performed using M-MLV reverse transcriptase (Life Technologies, Grand Island, NY) per manufacturer’s instructions. The primer sequences (Table 4) were designed using the Primer Express (Applied BioSystems, Foster City, CA) and manufactured by Sigma (St. Louis, MO). The quantitative real-time polymerase chain reaction (qPCR) reactions were carried out using the QuantStudio 6 Flex Real-Time PCR System and SYBR Green mix (Applied Biosystems, Foster City, CA); cyclophilin was used as the reference gene (13). All qPCR assays were performed in triplicate. The relative quantity of mRNA in the treatment group with respect to the Matrigel control group was calculated using standard equations (Applied BioSystems): Relative mRNA quantity (treatment to control) = 2(−ΔΔCt), where ΔCt = mean threshold cycle (Ct; vimentin, ZEB1, or CDH1) − mean Ct (cyclophilin), and ΔΔ Ct = Δ Ct (treatment) − Δ Ct (Matrigel control).
Table 4.
Oligonucleotide primers for real-time PCR
| Primers for mRNA sequences | ||
|---|---|---|
| Primer | Sense sequence (5′ to 3′) | Antisense sequence (5′ to 3′) |
| SOX2 | CAGTTACGCGCACATGAACG | CCCTGCTGCGAGTAGGACAT |
| p63 | CAGAGTGTGCTGGTACCTTATG | GGTTCATCCCTCCAACACAA |
| CD49f | TTTGAAGATGGGCCTTATGAA | CCCTGAGTCCAAAGAAAAACC |
| TFF2 | CACCATGGAGAACAAGGTGA | CACTGTACACGTCTCTGTCTG |
| MUC5AC | CCATTGCTATTATGCCCTGTGT | TGGTGGACGGACAGTCACT |
| CD44 | CGGACACCATGGACAAGTTT | GAAAGCCTTGCAGAGGTCAG |
| KLF4 | CAGCTTCACCTATCCGATCCG | GACTCCCTGCCATAGAGGAGG |
| OCT4 | CTTGAATCCCGAATGGAAAGGG | GTGTATATCCCAGGGTGATCCTC |
| LGR5 | CACCTCCTACCTAGACCTCAGT | CGCAAGACGTAACTCCTCCAG |
| FOXA2 | AGCGCCCACGTACGACGACATG | AGAGCCCGAGGGCTACTCCT |
| LEFTY1 | CTGCTGATGGACAAATGCTCTG | ACTTTAGCCCAGATCCAGTGAC |
| OLFM4 | TTCTCCTAGCCCTTCTGTTCTTCC | TTCCAAGCGTTCCACTCTGTCC |
| CK7 | CAGGAAGTCATGAGCGTGAA | GAATAAGCCTTCAGGAGCCC |
| TFF3 | CCAAGCAAACAATCCAGAGCA | GCTCAGGACTCGCTTCATGG |
| CDX2 | AGGGGAGAGAGGGACTCAAG | AGTCCAATAACCACCCCCTC |
| MUC2 | AGGATGACACCATCTACCTCAC | CATCGCTCTTCTCAATGAGCA |
| CK8 | GACGGCTCGAAGCAACATGG | GATCTCGTCGGTCAGCCCTT |
| CK18 | CTCCATCTGTAGGGCGTAGC | TCACCACACAGTCTGCTGAGG |
| SOX9 | GACCAGTACCCGCACTT | TTCACCGACTTCCTCCG |
| Villin | AACGAGGAGGAGAAGAAGGC | GTTCACTAGCTGCTCCAGGG |
| AGR2 | TGCTCCTTGTGGCCCTCTCC | AGGGTCTGGGGCAGTTTGGGT |
| CK20 | CAGACACACGGTGAACTATGG | GATCAGCTTCCACTGTTAGACG |
| MUC4 | CGCGGTGGTGGAGGCGTTCTT | GAAGAATCCTGACAGCCTTCA |
| EpCAM | ATAACCTGCTCTGAGCGAGTG | TGCAGTCCGCAAACTTTTACTA |
| CDH1 | CAGCCTATTTTTCCCTCGACAC | GGCCTTTTGACTGTAATCACACC |
| FN1 | TGACCCCTACACAGTTTCCCA | TGATTCAGACATTCGTTCCCAC |
| Vimentin | GGAAGCCGAAAACACCCTG | GAGACGCATTGTCAACATCCT |
| ZEB1 | AGCAGTGAAAGAGAAGGGAATGC | GGTCCTCTTCAGGTGCCTCAG |
| VEGF | ATCTTCAAGCCATCCTGTGTGC | GCTCACCGCCTCGGCTTGT |
| Cyclophilin | CCCACCGTGTTCTTCGACAT | CCAGTGCTCAGAGCACGAAA |
Bioinformatics Analysis
The ΔCt (cycle threshold) method was used to normalize raw Ct values of target gene to reference gene (cyclophilin, Table 4) (31). We first subtracted the ΔCt of each data point from the highest ΔCt of the entire qPCR data set to obtain the log2 relative expression. For each gene, the average of day 3 log2 expression was further subtracted from all three time points to obtain “day-3 mean-subtracted” expression values. The heatmaps were produced using R (32) package pheatmap (33).
Statistical Analyses
All experiments were performed in triplicate and repeated at least three times unless otherwise specified. Quantitative data are expressed as the means ± standard deviation (SD). Statistical analyses were performed using an unpaired Student’s t test with the Instat for Windows statistical software package (GraphPad Software, San Diego, CA; RRID:SCR_000306). For multiple comparisons, an ANOVA and the Student–Newman–Keuls multiple-comparisons test was performed using the Instat for Windows statistical software package (GraphPad). P values ≤ 0.05 were considered significant for all analyses.
RESULTS
Advanced DMEM/F12 Provides Optimal Support for the Generation of Human Barrett’s Cell Line-Derived Organoids
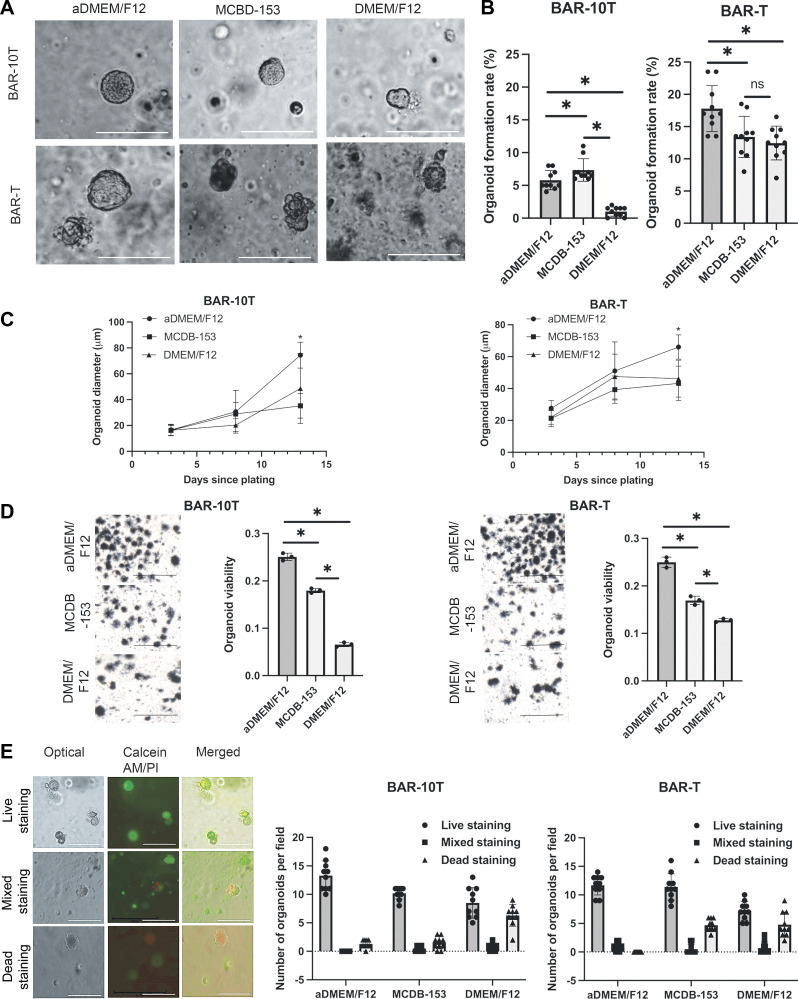
Since the ideal growth medium for Barrett’s organoids was unknown, we used BAR-T and BAR-10T cells to compare organoid growth in three separate culture media: aDMEM/F12 (19), MCDB-153, and DMEM/F12 (Table 2). In all three growth media, Barrett’s cells seeded into Matrigel formed 3-D spheroids (i.e., smooth-shaped, hollow structures) within 3–5 days. By day 7, the spheroids changed their morphology to that of a dense cellular mass with an ellipsoid shape, with some structures showing crypt-like buds (Fig. 1A). At day 7, organoid formation rates for BAR-10T cells were significantly higher in aDMEM/F12 and MCDB-153 compared with DMEM/F12 (Fig. 1B); BAR-T organoid formation rates were significantly higher in aDMEM/F12 than the other two media (Fig. 1B). BAR-10T and BAR-T organoid size significantly increased from days 3 to 13 in all three media (P < 0.01) with formation of significantly larger organoids at day 13 in aDMEM/F12 than in MCDB-153 and DMEM/F12 media (Fig. 1C). Organoid viability can be observed and quantitated using the MTT assay (34). As shown in Fig. 1D, although viable cells were present in organoids grown in all three growth media, organoid viability was significantly better in aDMEM/F12; live/dead staining with calcein-acetoxymethyl ester (Calcein-AM/PI) confirmed that aDMEM/F12 was better at supporting organoid viability than MCDB-153 or DMEM/F12 (Fig. 1E). After reviewing these data, we chose to use aDMEM/F12 medium to establish Barrett’s organoids from endoscopic biopsy specimens of patients with BE.
Figure 1.
Optimization of culture media for human Barrett’s epithelial cell organoids using telomerase-immortalized, nonneoplastic Barrett’s epithelial cell lines (BAR-10T and BAR-T). A: representative phase contrast microscopic images of three-dimensional BAR-10T and BAR-T organoid structures grown in aDMEM/F12, MCDB-153, and DMEM/F12 media after 7 days of culture. Scale bar = 125 µm. B: organoid formation rates in three different media after 7 days of culture. C: organoid growth curves in three different culture media over 13 days. D: representative phase contrast microscopic images and quantification of MTT staining of viable organoids in three different media after 14 days in culture. Scale bars = 275 µm. E: representative dual fluorescence staining for live (Calcein-AM, green), mixed (Calcein-AM and propidium iodide), and dead (propidium iodide, red) organoids from BAR-T or BAR-10T and quantification of live, mixed, and dead staining after 14 days in culture. Scale bars = 275 µm. Bar graphs depict the means ± SD from ≥ 3 technical replicates. *P ≤ 0.05; ns, nonsignificant; one-way ANOVA. All experiments were repeated three times. aDMEM/F12, advanced DMEM/F12.
Establishment and Analyses of Human Barrett’s Organoids Derived from Endoscopic Biopsies
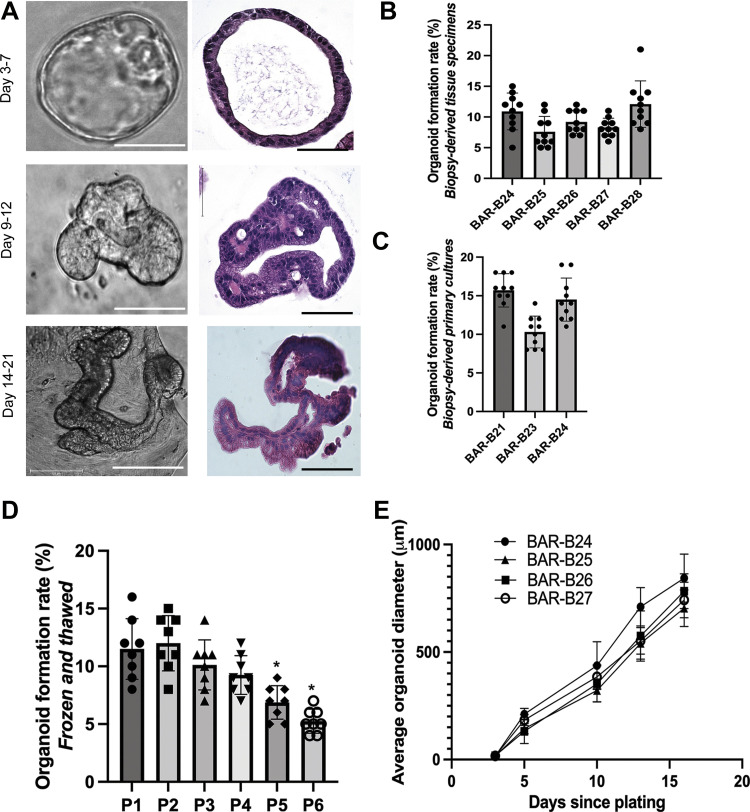
Biopsy-derived primary Barrett’s epithelial cells were established in monolayer culture before being seeded into Matrigel for two patients (BAR-B21 and BAR-B23), four patients had their biopsy tissue specimens directly seeded into Matrigel (BAR-B25, BAR-B26, BAR-B27, and BAR-B28), and one patient had organoids established both from biopsy-derived primary cultures and biopsy-derived tissue specimens (BAR-B24; Table 1). Three-dimensional structures were successfully generated from endoscopic biopsy specimens of all seven patients with BE. Three distinct morphologies were observed during organoid development: 1) spheroid (days 3–7), 2) ellipsoid with crypt-like buds (days 9–12), and 3) glandular-appearing (days 14–21; Fig. 2A). The average organoid formation rate was 9.6% ± 3.1 standard deviation (SD) for biopsy-derived tissue specimens (Fig. 2B) and 13.5% ± 3.3 SD for biopsy-derived primary cultures (Fig. 2C), a difference that achieved statistical significance (P < 0.0001). Organoids from BAR-B24 (derived from biopsy tissue specimens) were passaged for up to six passages and spheroids (between days 5 and 7) were cryopreserved at each passage. Spheroids at all six passages could be cryo-recovered; the organoid formation rate after seven days of culture was maintained at ∼10% for the first four passages, after which the organoid formation rate decreased significantly (Fig. 2D). Organoid size from biopsy-derived tissue specimens increased significantly from days 3 to 16 (P < 0.0001; Fig. 2E).
Figure 2.
Morphology and growth kinetics of human Barrett’s organoids derived from endoscopic biopsy specimens. A: representative phase contrast (scale bar = 125 µm) and H&E-stained microscopic images (scale bar = 75 µm) of BAR-B24 organoids (derived from biopsy tissue specimens) at different time intervals during development. Organoid formation rate at 7 days of culture for biopsy-derived tissue specimens (B) and biopsy-derived primary cultures (C). D: BAR-B24 organoid formation rate at 7 days of culture following cryo-recovery from passages 1–6 (1 biological replicate). E: organoid growth curves for biopsy-derived tissue specimens. Bar graphs depict the means ± SD from 4 BE patients. *P ≤ 0.05; one-way ANOVA. All experiments were repeated three times. H&E, hematoxylin and eosin.
Human Barrett’s Organoids Derived from Endoscopic Biopsies Recapitulate Histological and Molecular Features of In Vivo Barrett’s Metaplasia
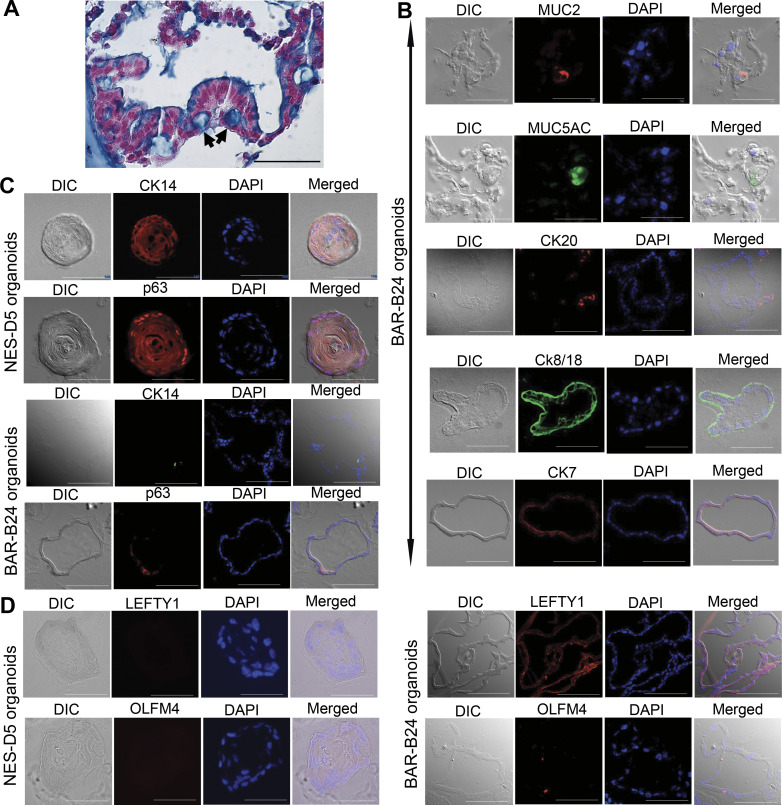
Histological and molecular analyses demonstrated that organoids established from endoscopic biopsies recapitulate features of Barrett’s metaplasia in vivo. As shown in Fig. 3A, at day 14, Barrett’s organoids formed a single layer of columnar epithelial cells with some exhibiting Alcian blue (pH 2.5) staining typical of goblet cells. Immunofluorescent staining demonstrated that Barrett’s organoids express gastric and intestinal columnar cell markers including the goblet cell mucin (MUC) 2, the gastric mucin MUC5AC, and cytokeratins (CK) 20, 8/18, and 7 (Fig. 3B). It has been proposed that progenitor cells native to the esophagus (including basal cells of the squamous epithelium or cells of the esophageal submucosal glands and their ducts) might give rise to Barrett’s metaplasia (35). To explore whether Barrett’s organoids express markers of squamous basal cells, we established esophageal squamous cell organoids derived from the normal esophageal epithelium of organ donors (following protocols established by Kasagi et al.) to serve as positive controls (26). As shown in Fig. 3C, esophageal squamous cell organoids develop as a solid mass with differentiated cells forming the inner layers and basaloid cell layers adjacent to the matrix (26). Immunofluorescent staining of NES-D5 squamous organoids revealed cytoplasmic staining for CK14 and nuclear staining for p63 (markers of squamous progenitor cells) in the basaloid layers of these organoids. In the Barrett’s organoids, we also observed occasional cells that stained for CK14 and p63 (Fig. 3C). Interestingly, the Barrett’s organoids also expressed the putative esophageal submucosal gland markers LEFTY1 and OLFM4 (36); no expression of these esophageal submucosal gland markers was detected in the NES-D5 squamous organoids (Fig. 3D).
Figure 3.
Morphological and molecular characterization of human Barrett’s organoids derived from endoscopic biopsy specimens. Representative images of BAR-B24 organoids (derived from biopsy tissue specimens) at day 14. A: H&E and Alcian blue staining demonstrating a single layer of columnar cells with some goblet-type cells (arrows). Scale bar = 50 µm; biological replicates = 3. DIC and immunofluorescence images for columnar markers MUC2, MUC5AC, and CKs 20, 8/18, and 7 (B), squamous basal cell markers CK14 (red in NES-D5 organoids, green in BAR-B24 organoids) and p63 (C) and esophageal submucosal gland putative markers LEFTY1 and OLMF4 in BAR-B24 organoids (D); NES-D5 esophageal squamous cell organoids derived from the normal esophageal epithelium of an organ donor served as a control. DAPI was used as a nuclear counterstain. Scale bar = 50 µm. DAPI, 4′,6′-diamidino-2-phenylindole; DIC, differential interference contrast microscopy; H&E, hematoxylin and eosin; MUC2, mucin 2.
Morphologies and Gene Expression Patterns Reflect Step-by-Step Differentiation Programs during Barrett’s Organoid Development
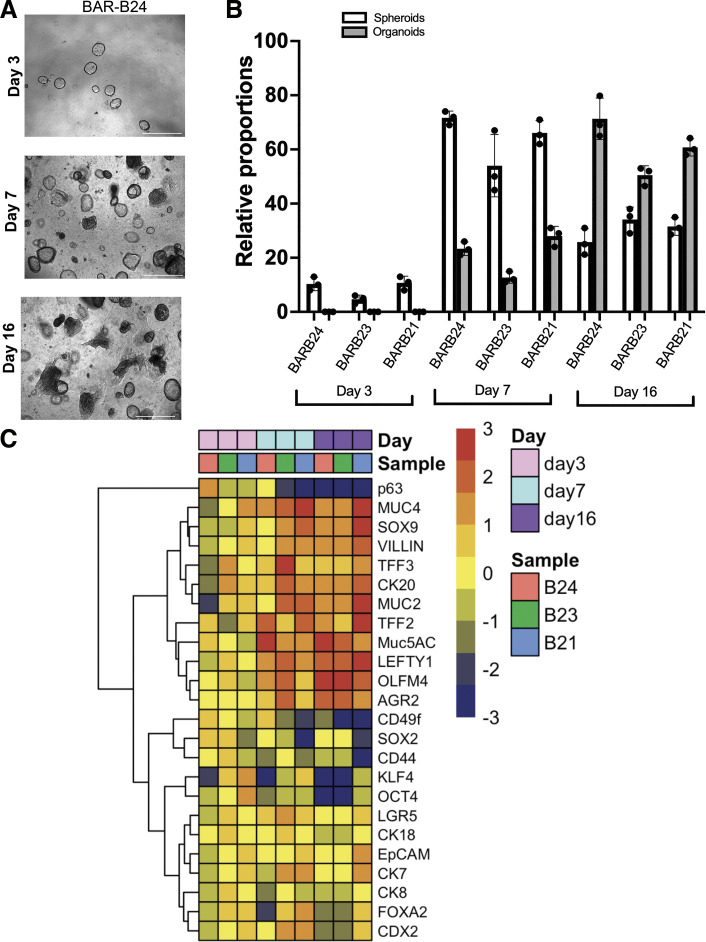
To examine morphology changes and epithelial mRNA gene expression patterns during Barrett’s organoid development, we used BAR-B24, BAR-B23, and BAR-B21 3-D cultures developed from biopsy-derived primary cell cultures. By optic morphology, we observed an increase in spheroid development from days 3 to 7 followed by a decline at day 16, whereas organoid development was first observed at day 7 and increased at day 16 (Fig. 4, A and B). To explore gene expression changes underlying development of our Barrett’s organoids, we evaluated a panel of genes known to be stem cell markers and columnar/intestinal cell differentiation markers by qPCR on 3-D cultures of BAR-B24, BAR-B23, and BAR-B21 at days 3, 7, and 16. Hierarchical clustering revealed gene expression patterns that, during organoid development, demonstrated highest expression of the stem cell markers SOX2, OCT4, KLF4, the gastric progenitor marker CD44, and the squamous esophagus progenitor marker p63 at day 3, all of which declined by day 7. Expression levels of the endodermal marker FOXA2, the hindgut transcription factor CDX2, and the intestinal stem cell marker LGR5 increased at day 7, and then declined at day 16 to levels similar to those at day 3. The transcription factor SOX9, which plays key roles in maintaining stem and progenitor cells and regulating cell fate decisions, was prominently expressed from day 7 onward. By day 16, the columnar/intestinal differentiation markers MUC5AC, TFF2, TFF3, MUC2, and Villin were highly expressed (Fig. 4C). We also found that expression of the stem cell-associated gene OLFM4 and the developmental gene LEFTY1 found in esophageal submucosal glands increased at day 7 and remained elevated at day 16 in our Barrett’s organoids. Thus, during the development of Barrett’s organoids, our 3-D cultures demonstrate patterns of gene expression for stem cell and differentiation markers that resemble those found in Barrett’s metaplastic tissue in vivo (36–39).
Figure 4.
Time course of morphologies and mRNA gene expression patterns during development of Barrett’s organoids derived from endoscopic biopsy specimens. A: representative phase contrast images of BAR-B24 morphology at days 3, 7, and 16 in three-dimensional culture. Scale bar = 650 µm for day 3; scale bar = 275 µm for days 7 and 16. B: the relative proportions of spheroid vs. organoid morphology at days 3, 7, and 16 of culture for BAR-B24, BAR-B23, and BAR-B21. Bar graphs depict the means ± SD from three technical replicates. C: heat-map and hierarchical clustering with dendrogram generated from qPCR gene expression data of three-dimensional cultures of BAR-B24, BAR-B23, and BAR-B21 at days 3, 7, and 16. Red color corresponds to high relative expression and blue color corresponds to low relative expression. qPCR, quantitative real-time polymerase chain reaction.
Primary and Three-Dimensional Cultures of Barrett’s Epithelial Cells Treated with Acidic Bile Salt Medium Maintain Epithelial Features and Acquire Motility via Collective Cell Migration
In earlier studies, we found that acidic bile salts induce EMP in Barrett’s epithelial cell lines, a process that might underlie the pathogenesis of SSIM. In those studies, Barrett’s cell lines treated with acidic bile salt medium exhibited a mixture of epithelial and mesenchymal features as well as expression of mesenchymal markers (i.e., vimentin). To determine whether EMP is induced by acidic bile salts in primary cultures of Barrett’s epithelial cells, we first performed wound-healing (scratch) assays using BAR-B24 to assess cell migration; rhVEGF was used as a positive control (13). Compared with nontreated BAR-B24 primary cells, treatment with acidic bile salts significantly enhanced epithelial wound repair via increases in cell migration (Fig. 5A). Both rhVEGF-treated and acidic bile salt-treated BAR-B24 cells filled in the wound with the migration of cohorts of cells forming finger-like protrusions, a characteristic feature of collective cell migration. We next examined the morphology of the migratory front of BAR-B24 cells and performed immunofluorescence for the adhesion protein CDH1, as its loss is considered a hallmark of the transition from an epithelial to a mesenchymal state. As shown in Fig. 5B, compared with nontreated controls, cells at the leading edge of the finger-like protrusions demonstrated complete or partial loss of membranous CDH1 staining with relocalization of CDH1 staining to the cytoplasm (Fig. 5B). Thus, in agreement with our earlier data in Barrett’s epithelial cell lines, primary cultures of Barrett’s epithelial cells treated with acidic bile salts acquire mesenchymal features (i.e., migration) while maintaining epithelial markers.
Figure 5.
Primary cultures of Barrett’s epithelial cells treated with acidic bile salts increase collective cell migration associated with loss of membranous of CDH1. A: representative phase contrast images of scratch wounds and quantification of wound closure in BAR-B24 cells at time 0 and 24 h after treatment with recombinant human (rh)VEGF or acidic bile salts. Scale bar = 275 µm. Bar graphs depict the means ± SD from four technical replicates. *P ≤ 0.05 compared with nontreated controls; one-way ANOVA. Experiments in BAR-B24 were repeated three times. B: representative fluorescent microscopy images of wound closure 24 h after treatment with rhVEGF or acidic bile salts demonstrating the migration of cohorts of cells forming finger-like protrusions (i.e., collective cell migration). Scale bar = 275 µm. Middle and right panels are magnified images (Scale bar = 50 µm) of cell cohorts at the leading edge of the finger-like protrusions demonstrating complete or partial loss of membranous CDH1 staining following treatment with rhVEGF or acidic bile salts. CDH1, E-cadherin; rhVEGF, recombinant human vascular endothelial growth factor.
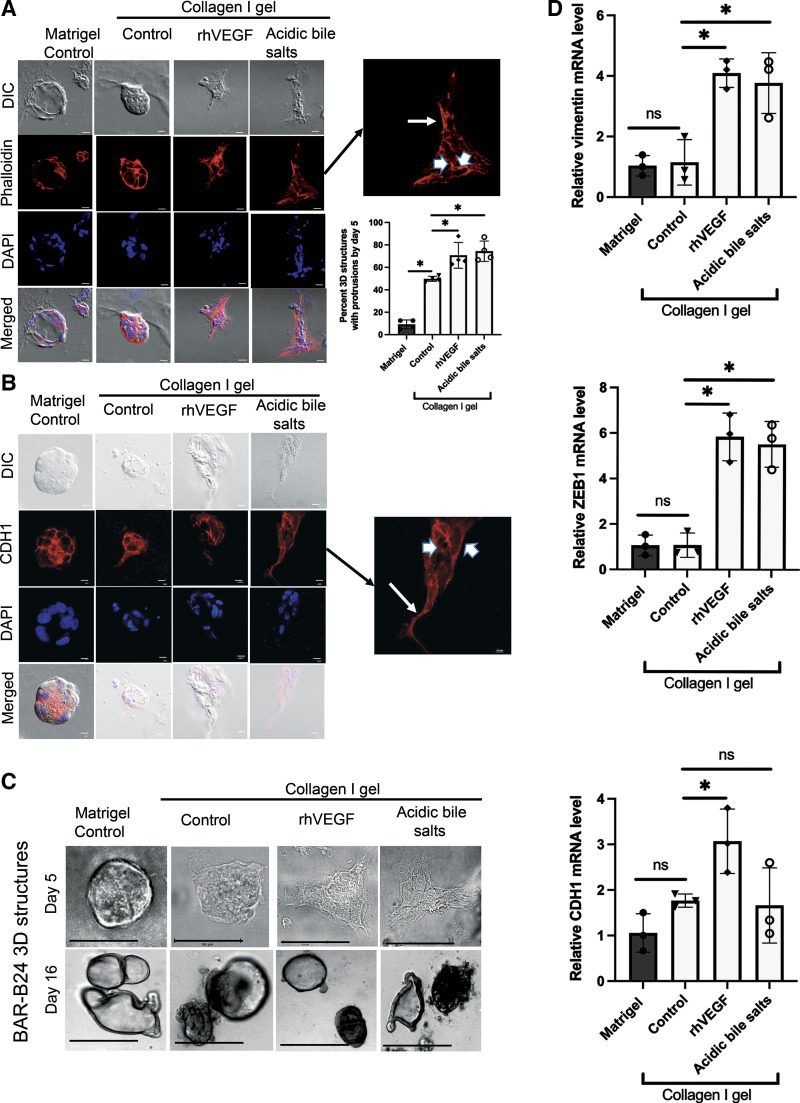
We next sought to determine whether acidic bile salts could induce EMP and migration in Barrett’s 3-D cultures. Primary culture-derived BAR-B24 cells were grown in Matrigel for 3 days, recovered, and then reseeded into Matrigel or collagen I for 2 days (total 5 days; spheroid stage) before a 2-day treatment with acidic bile salts or rhVEGF. Collagen I was selected to model the extracellular matrix encountered during epithelial tissue repair (18, 29). As shown in Fig. 6, A and B, differential interference contrast (DIC) and DAPI images demonstrate that BAR-B24 cells in Matrigel maintain a rounded morphology without evidence of protrusive extensions. In contrast, cells embedded in collagen I demonstrate cell spreading by forming finger-like protrusions into the matrix. Compared with nontreated control cells in collagen I, BAR-B24 cells treated with acidic bile salts or rhVEGF significantly increased the number of spheroids showing cell spreading with protrusions (Fig. 6A). In addition to loss of membranous CDH1, reorganization of the actin cytoskeleton is another central event in cell migration. Therefore, we visualized the filamentous (F) actin network using phalloidin staining along with that for CDH1 in the BAR-B24 spheroids. In untreated, control BAR-B24 spheroids embedded in Matrigel and collagen I, the F actin network was found to encircle the epithelial cell perimeter (Fig. 6A); a similar pattern was observed for CDH1 staining (Fig. 6B). In contrast, BAR-B24 spheroids treated with acidic bile salts or rhVEGF demonstrated cells at the migratory front of the protrusion whose F-actin was redistributed along the long axis of the cell and whose CDH1 was localized primary to the cytoplasm (Fig. 6, A and B, inset arrows); expression of F actin and CDH1 remained encircling the cell perimeter in cells at the nonprotrusive base (Fig. 6, A and B, inset arrowheads). We repeated this experiment using primary culture-derived BAR-B24 cells that were grown in Matrigel for 14 days, recovered, and then reseeded into Matrigel or collagen I for 2 days (total 16 days; organoid stage) before a 2-day treatment with acidic bile salts or rhVEGF. As shown in Fig. 6C, BAR-B24 organoid structures at day 16 do not demonstrate cell protrusions with either acidic bile salt or rhVEGF treatment in contrast to those treated at day 5. These findings demonstrate that acidic bile salts can initiate EMP in the early (spheroid) stage when mRNA expression of putative stem/progenitor cell markers is high (Fig. 4C) but not in late-stage Barrett’s organoids when mRNA expression of columnar/intestinal differentiation marker expression is high and putative stem/progenitor cell marker expression is relatively low (Fig. 4C). In addition to increases in cell protrusions, acidic bile salt and rhVEGF treatments of day 5 BAR-B24 spheroids also increased mRNA expression of the mesenchymal gene vimentin compared with untreated collagen I controls (Fig. 6D).
Figure 6.
Acidic bile salt treatment of early stage, three-dimensional structures of Barrett’s epithelial cells increase cell spreading via formation of finger-like protrusions associated with reorganization of the actin cytoskeleton, cytoplasmic relocalization of CDH1, and increased expression of mesenchymal markers. Representative DIC and fluorescence microscopy images of phalloidin (A) or CDH1 staining (B) in day 5. BAR-B24 3-D structures embedded in Matrigel and collagen I. Note that acidic bile salt treatment significantly increased the percentage of 3-D structures with protrusions compared with collagen I control cells. Scale bar = 275 µm. Magnified insets demonstrate cytoplasmic staining for phalloidin and CDH1 in cells at the leading edge of the protrusions (arrows), and membranous staining for these proteins that encircle the cell perimeter in cells at the nonprotrusive base (arrowheads). DAPI was used as a nuclear counterstain. C: representative phase contrast morphological images in day 5 and day 16. BAR-B24 3-D structures embedded in Matrigel and collagen I. Note that treatment with acidic bile salts caused protrusions in the day 5 structures but not in the day 16 structures. Scale bar = 50 µm for day 5; Scale bar = 275 µm for day 16. D: representative qPCRs for vimentin, ZEB1, and CDH1 mRNA levels in day 5 BAR-B24. 3-D structures embedded in Matrigel or collagen I with or without acidic bile salts. In all panels, rhVEGF served as a positive control. Bar graphs depict the means±SD from ≥ 3 technical replicates. *P≤ 0.05; ns, nonsignificant; one-way ANOVA. All experiments in BAR-B24 were repeated three times. CDH1, E-cadherin; DAPI, 4′,6′-diamidino-2-phenylindole; DIC, differential interference contrast; qPCR, quantitative real-time polymerase chain reaction; rhVEGF, recombinant human vascular endothelial growth factor; ZEB, zinc finger transcription factor.
EMP involves some degree of transcriptional regulation by a group of transcription factors known as EMT activators including members of the zinc finger family of transcription factors (ZEBs). In earlier studies, we found that acidic bile salts induce transcriptional upregulation of ZEB1 in Barrett’s epithelial cell lines (13). In agreement with those earlier studies, acidic bile salts increased mRNA expression of ZEB1 in the day 5 BAR-B24 spheroids compared with untreated collagen I controls (Fig. 6D). Although ZEBs are known to be transcriptional repressors of epithelial genes including CDH1, our day 5 BAR-B24 spheroids treated with acidic bile salts or rhVEGF did not demonstrate significant reductions in CDH1 mRNA expression despite an increase in ZEB1 mRNA expression compared with untreated, collagen I-embedded controls (Fig. 6D). These finding suggest that cytoplasmic relocalization of CDH1, rather than transcriptional repression, is an early feature of acidic bile salt-induced initiation of EMP in Barrett’s 3-D cultures.
DISCUSSION
We have demonstrated that organoids that closely recapitulate key histologic and molecular features of Barrett’s epithelium can be established either directly from BE biopsies or from primary Barrett’s epithelial cell cultures. These organoids, composed of both nongoblet and goblet columnar epithelial cells, express gastric and intestinal molecular markers typical of Barrett’s metaplasia. During organoid development, progression from the spheroid to organoid stage is associated with downregulation of transcription factors involved in self-renewal of undifferentiated stem cells (e.g., SOX2, OCT4) and upregulation of genes encoding more tissue-specific transcription factors and differentiation markers (e.g., CDX2, OLFM4, MUC2). We have found that Barrett’s organoids and primary Barrett’s cells treated with acidic bile salts exhibit EMP characterized by loss of membranous CDH1 and onset of protrusive (i.e., collective) cell migration. Moreover, compared with Barrett’s organoids embedded in Matigel, those embedded in type I collagen (modeling the extracellular matrix of wound healing) exhibit enhanced EMP features and markers. Thus, our Barrett’s organoids appear to be high-fidelity models for studying the biology of human Barrett’s metaplasia, and the pathophysiology of the EMP that might underlie the development of SSIM.
An organoid comprises a three-dimensional collection of organ-specific cell types that form in vitro from stem cells, and that recapitulate the parent organ’s histological structure and function. Using insights acquired from studies on organoid culture of mouse intestinal epithelium, in 2011 Sato et al. demonstrated that they could create human intestinal, colonic, and Barrett’s organoids, but the Barrett’s organoids were not characterized extensively (15). Since then, techniques of organoid culture have evolved considerably. Schlaermann et al. (40) found that spheroid formation efficiency of human gastric organoids could be increased significantly by adding Y-27632 [a Rho-associated protein kinase (ROCK) inhibitor that prevents anoikis] to the culture medium. Murine esophageal squamous organoids could be established using the medium originally described by Sato et al., but this medium failed to support growth of human esophageal squamous organoids (15, 26, 41). However, cultures of human esophageal squamous cells grown in keratinocyte serum-free medium (a medium used for monolayer cultures) yielded robust generation of 3-D structures (26). Thus, successful formation of organoids is highly dependent on the culture medium chosen.
To determine the optimal culture medium for human Barrett’s organoids, we grew two human, telomerase-immortalized Barrett’s cell lines in Matrigel using aDMEM/F12 medium (supplemented with Wnt3a, R-spondin, Noggin, and growth factors), (20) and using two media conventionally used for monolayer cultures of human telomerase-immortalized Barrett’s cell lines (MCDB-153 and DMEM-F12) (42). All three media performed well in initiating organoid formation from Barrett’s cell lines, but aDEM/F12 was the best for sustaining organoid growth and viability after 1 wk in culture (a time when crypt buds appear and Wnt signaling becomes essential for crypt proliferation) (15). When we used BE biopsies grown in aDEM/F12, we observed three distinct morphologies during organoid development (spheroid days 3–7, ellipsoid with crypt-like buds days 9–12, and glandular-appearing days 14–21). Since Barrett’s epithelium is an intestinal-type metaplasia, it is not surprising that the crypt bud formation observed after week 1 is Wnt dependent, as it is in the intestine and previously described by Sato et al. (15). Using aDEM/F12, we were able to successfully establish and propagate organoids from BE biopsies in all seven patients with BE, irrespective of whether the organoids were derived directly from biopsy tissue specimens or indirectly from monolayer cultures of primary Barrett’s epithelial cells.
As in the crypts of Barrett’s epithelium (43), our biopsy-derived Barrett’s organoids comprised a single layer of nongoblet and goblet columnar epithelial cells surrounding a lumen, with immunofluorescence staining positive for the goblet cell mucin MUC2 and the gastric mucin MUC5AC. Immunofluorescence staining also revealed rare cells that expressed markers of squamous basal cells (CK14 and p63) and putative markers of esophageal submucosal glands (LEFTY1 and OLFM4) (36). At day 3 of organoid development, there was relatively high expression of genes for pluripotent stem cell markers (KLF4 and OCT4), squamous esophageal progenitor markers (SOX2, p63, and CD49f), and a gastric progenitor marker (CD44), whereas at day 16 there was higher expression of gastric/intestinal differentiation markers (MUC5AC, TFF2, TFF3, MUC2, and Villin), which are well described in human Barrett’s tissue (37, 38, 44, 45). At day 7, when crypt buds appear, we found higher levels of putative stem/progenitor cell markers of adult stomach or intestine (SOX9, LGR5, FOXA2, CDX2) than at day 3 or day 16 (46–48). We also noted relatively high levels of LEFTY1 and OLFM4 mRNA from day 7 onward, and rare cells showing immunofluorescence staining for LEFTY1 and OLFM4 protein. OLFM4 has been shown to associate with LGR5 and is a putative marker of BE progenitor cells (15, 38, 44, 45). Moreover, the LEFTY1-enriched populations of human Barrett’s esophageal cells exhibit considerable transcriptional profile overlap with esophageal submucosal gland cells (36). Thus, our Barrett’s organoids contain the diverse cell types and molecular markers found in human BE biopsy specimens, supporting their utility as tissue surrogate models.
Barrett’s organoids provide a unique model for the study of epithelial responses to pathological environments, such as those that might contribute to the development of SSIM. Although tissue-relevant organoids are a preferred model for studying human disease processes, there are some limitations. For example, organoids comprising epithelial cells alone do not allow for interrogation of the interplay between epithelial cells and certain factors that can modulate disease presentations such as the local microbiome and nonepithelial cells (e.g., fibroblasts, immune cells, nerves) (18, 49). In earlier studies, we found that organotypic cultures of human Barrett’s cell lines treated with acidic bile salts, and the columnar-lined esophagus of rats with reflux esophagitis induced by esophago-jejunostomy both exhibited individual columnar cells “buried” deep in the underlying stroma. These buried cells showed features of mesenchymal cells (e.g., migratory ability) as well as markers of epithelial cells (e.g., CDH1), consistent with development via EMP (13). In the rat model, furthermore, we found that the columnar-lined esophagus developed via a wound-healing process in which cells at the esophago-jejunostomy, in deep portions of the jejunal crypts that express Msi-1 and Dcamkl1 (putative gastrointestinal stem cell markers), appeared to migrate up the esophagus and under ulcerated squamous epithelium (12, 50). These observations in cell line and animal models suggested that EMP is a plausible mechanism for SSIM development in patients with BE. In the present studies, we used our Barrett’s organoids to explore the influence of environmental and extracellular matrix features on EMP.
In the early, spheroid stage of organoid development, our Barrett’s 3-D structures embedded in collagen I (simulating the extracellular matrix of reflux esophagitis) demonstrated spreading into the open matrix via the formation of finger-like protrusions. These protrusions, which were augmented by acidic bile salt exposure, comprised clusters of epithelial cells forming a leading migratory front and a nonmigratory base. Cells within the migratory front exhibited redistribution of F-actin along the long axis and relocalization of CDH1 from the membrane to the cytoplasm, whereas both F-actin and CDH1 were located along the perimeter of cells in the nonmigratory base. Such cytoskeletal and cell-cell adhesion remodeling of cells into “leaders and followers” is characteristic of collective cell migration, a form of cell movement prevalent during embryogenesis and wound healing (51). In addition to this migratory/nonmigratory polarization, acidic bile salt-treated Barrett’s spheroids showed increased mRNA levels of the EMT transcription factor ZEB1 and the mesenchymal gene vimentin. These acidic bile salt-induced finger-like protrusions were observed during the early, Wnt-dependent stage of Barrett’s organoid development associated with the formation of crypt buds, a time at which we observed relatively high mRNA expression of the putative stem/progenitor cell marker LGR5. In addition to regulating the intestinal stem cell niche, Wnt signaling is known to drive EMP (52, 53). Thus, it is not surprising that acidic bile salt treatment of late-stage Barrett’s organoids (in which columnar/intestinal differentiation marker expression is relatively high and Wnt signaling is less critical) did not induce development of finger-like protrusions. These findings suggest that the propensity to undergo EMP is a feature of immature, less differentiated progenitor cells located at the base of Barrett’s crypts rather than in the differentiated cells residing at the luminal surface.
In conclusion, using endoscopic biopsy specimens of human BE, we have successfully established organoids that recapitulate the histological and molecular features of Barrett’s metaplasia. Progression from the spheroid to organoid stage of development was associated with upregulation of tissue-resident stem cell and differentiation markers of gastric-type, intestinal-type, and esophageal submucosal gland-type cells. Modeling of a wound-healing stromal environment during the early, spheroid stage of organoid development induced features of EMP including loss of membranous CDH1 and reorganization of the actin cytoskeleton increased mRNA expression of the mesenchymal gene vimentin and of the EMT transcriptional repressor ZEB1, and collective cell migration. These events that were profoundly increased by treatment with acidic bile salts during the early stages of organoid development, but not in late stage, differentiated Barrett’s organoids. For patients with BE, our findings suggest that the gastroesophageal reflux of acid and bile salts can induce plasticity in Barrett’s progenitor cells that enable them to migrate under adjacent squamous epithelium, resulting in SSIM, a condition that may underlie Barrett’s cancers that escape detection by endoscopic surveillance and recurrences of Barrett’s metaplasia following endoscopic eradication therapy.
GRANTS
This work was supported by the National Institutes of Health (R01-DK124185 to R. F. Souza and S. J. Spechler; P30-CA168524 to A. Bansal), Baylor Scott and White Research Institute, the Merit Review Award CX001668 from the US Department of Veterans Affairs (VA) Clinical Science Research Program (to K. B. Dunbar) and resources at the Dallas VA Medical Center.
DISCLAIMERS
The contents do not represent the views of the US Department of Veterans Affairs or the US Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Q.Z., S.J.S., and R.F.S. conceived and designed research; Q.Z., K.B.D., Y.C., J.Z., X.Z., E.P., and Z.P. performed experiments; Q.Z., A.B., Y.C., U.B., J.G., Z.P., and R.F.S. analyzed data; Q.Z., A.B., Y.C., J.Z., U.B., J.G., Z.P., and R.F.S. interpreted results of experiments; Q.Z., A.B., Y.C., U.B., J.G., Z.P., and R.F.S. prepared figures; Q.Z., A.B., and R.F.S. drafted manuscript; Q.Z., A.B., K.B.D., Y.C., J.Z., U.B., J.G., X.Z., E.P., Z.P., S.J.S., and R.F.S. edited and revised manuscript; Q.Z., A.B., K.B.D., Y.C., J.Z., U.B., J.G., X.Z., E.P., Z.P., S.J.S., and R.F.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Elizabeth Cook, H.T. (ASCP) and Sharon K. Sims, H.T. (ASCP), QIHC, histopathology core technicians at the Center for Esophageal Research, Baylor University Medical Center and Baylor Scott & White Research Institute for assistance in preparation of organoid samples. We also thank Daniel Kim for assistance in patient enrollment and clinical data collection. This work used Nikon A1R H25 confocal microscope that was purchased with funding from a National Institutes of Health Shared Instrumentation Grant (S10 OD025230).
REFERENCES
- 1.Spechler SJ, Souza RF. Barrett's esophagus. N Engl J Med 371: 836–845, 2014. doi: 10.1056/NEJMra1314704. [DOI] [PubMed] [Google Scholar]
- 2.Thrift AP, Pandeya N, Whiteman DC. Current status and future perspectives on the etiology of esophageal adenocarcinoma. Front Oncol 2: 11, 2012. doi: 10.3389/fonc.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corley DA, Mehtani K, Quesenberry C, Zhao W, de Boer J, Weiss NS. Impact of endoscopic surveillance on mortality from Barrett's esophagus-associated esophageal adenocarcinomas. Gastroenterology 145: 312–319, 2013. doi: 10.1053/j.gastro.2013.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaheen NJ, Sharma P, Overholt BF, Wolfsen HC, Sampliner RE, Wang KK, Galanko JA, Bronner MP, Goldblum JR, Bennett AE, Jobe BA, Eisen GM, Fennerty MB, Hunter JG, Fleischer DE, Sharma VK, Hawes RH, Hoffman BJ, Rothstein RI, Gordon SR, Mashimo H, Chang KJ, Muthusamy VR, Edmundowicz SA, Spechler SJ, Siddiqui AA, Souza RF, Infantolino A, Falk GW, Kimmey MB, Madanick RD, Chak A, Lightdale CJ. Radiofrequency ablation in Barrett's esophagus with dysplasia. N Engl J Med 360: 2277–2288, 2009. doi: 10.1056/NEJMoa0808145. [DOI] [PubMed] [Google Scholar]
- 5.Sami SS, Ravindran A, Kahn A, Snyder D, Santiago J, Ortiz-Fernandez-Sordo J, Tan WK, Dierkhising RA, Crook JE, Heckman MG, Johnson ML, Lansing R, Ragunath K, di Pietro M, Wolfsen H, Ramirez F, Fleischer D, Wang KK, Leggett CL, Katzka DA, Iyer PG. Timeline and location of recurrence following successful ablation in Barrett's oesophagus: an international multicentre study. Gut 68: 1379–1385, 2019. doi: 10.1136/gutjnl-2018-317513. [DOI] [PubMed] [Google Scholar]
- 6.Shaheen NJ, Falk GW, Iyer PG, Gerson LB. ; American College of Gastroenterology. ACG clinical guideline: diagnosis and management of Barrett's esophagus. Am J Gastroenterol 111: 30–50, 2016. doi: 10.1038/ajg.2015.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inadomi JM, Saxena N. Screening and surveillance for Barrett's esophagus: is it cost-effective?. Dig Dis Sci 63: 2094–2104, 2018. doi: 10.1007/s10620-018-5148-7. [DOI] [PubMed] [Google Scholar]
- 8.Gray NA, Odze RD, Spechler SJ. Buried metaplasia after endoscopic ablation of Barrett's esophagus: a systematic review. Am J Gastroenterol 106: 1899–1908, 2011. doi: 10.1038/ajg.2011.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou C, Tsai TH, Lee HC, Kirtane T, Figueiredo M, Tao YK, Ahsen OO, Adler DC, Schmitt JM, Huang Q, Fujimoto JG, Mashimo H. Characterization of buried glands before and after radiofrequency ablation by using 3-dimensional optical coherence tomography (with videos). Gastrointest Endosc 76: 32–40, 2012. doi: 10.1016/j.gie.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anders M, Lucks Y, El-Masry MA, Quaas A, Rosch T, Schachschal G, Bahr C, Gauger U, Sauter G, Izbicki JR, Marx AH. Subsquamous extension of intestinal metaplasia is detected in 98% of cases of neoplastic Barrett's esophagus. Clin Gastroenterol Hepatol 12: 405–410, 2014. doi: 10.1016/j.cgh.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 11.Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 21: 341–352, 2020. [Erratum in Nat Rev Mol Cell Biol 22: 834, 2021]. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agoston AT, Pham TH, Odze RD, Wang DH, Das KM, Spechler SJ, Souza RF. Columnar-lined esophagus develops via wound repair in a surgical model of reflux esophagitis. Cell Mol Gastroenterol Hepatol 6: 389–404, 2018. doi: 10.1016/j.jcmgh.2018.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Q, Agoston AT, Pham TH, Zhang W, Zhang X, Huo X, Peng S, Bajpai M, Das K, Odze RD, Spechler SJ, Souza RF. Acidic bile salts induce epithelial to mesenchymal transition via VEGF signaling in non-neoplastic Barrett's cells. Gastroenterology 156: 130–144.e10, 2019. doi: 10.1053/j.gastro.2018.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest 119: 1420–1428, 2009. [Erratum in J Clin Invest 120: 1786, 2010]. doi: 10.1172/JCI39104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology 141: 1762–1772, 2011. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 16.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med 17: 313–319, 2011. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 17.Kopper O, de Witte CJ, Lõhmussaar K, Valle-Inclan JE, Hami N, Kester L, Balgobind AV, Korving J, Proost N, Begthel H, van Wijk LM, Revilla SA, Theeuwsen R, van de Ven M, van Roosmalen MJ, Ponsioen B, Ho VWH, Neel BG, Bosse T, Gaarenstroom KN, Vrieling H, Vreeswijk MPG, van Diest PJ, Witteveen PO, Jonges T, Bos JL, van Oudenaarden A, Zweemer RP, Snippert HJG, Kloosterman WP, Clevers H. An organoid platform for ovarian cancer captures intra- and interpatient heterogeneity. Nat Med 25: 838–849, 2019. doi: 10.1038/s41591-019-0422-6. [DOI] [PubMed] [Google Scholar]
- 18.Fujii M, Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater 20: 156–169, 2021. doi: 10.1038/s41563-020-0754-0. [DOI] [PubMed] [Google Scholar]
- 19.Liu X, Cheng Y, Abraham JM, Wang Z, Wang Z, Ke X, Yan R, Shin EJ, Ngamruengphong S, Khashab MA, Zhang G, McNamara G, Ewald AJ, Lin D, Liu Z, Meltzer SJ. Modeling Wnt signaling by CRISPR-Cas9 genome editing recapitulates neoplasia in human Barrett epithelial organoids. Cancer Lett 436: 109–118, 2018. doi: 10.1016/j.canlet.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jin RU, Brown JW, Li QK, Bayguinov PO, Wang JS, Mills JC. Tropism of severe acute respiratory syndrome coronavirus 2 for Barrett's esophagus may increase susceptibility to developing coronavirus disease 2019. Gastroenterology 160: 2165–2168.e4, 2021. doi: 10.1053/j.gastro.2021.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X, Yu C, Wilson K, Zhang HY, Melton SD, Huo X, Wang DH, Genta RM, Spechler SJ, Souza RF. Malignant transformation of non-neoplastic Barrett's epithelial cells through well-defined genetic manipulations. PLoS one 5: e13093, 2010. doi: 10.1371/journal.pone.0013093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaiswal KR, Morales CP, Feagins LA, Gandia KG, Zhang X, Zhang HY, Hormi-Carver K, Shen Y, Elder F, Ramirez RD, Sarosi GA Jr, Spechler SJ, Souza RF. Characterization of telomerase-immortalized, non-neoplastic, human Barrett's cell line (BAR-T). Dis Esophagus 20: 256–264, 2007. doi: 10.1111/j.1442-2050.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- 23.Nelson M, Zhang X, Genta RM, Turner K, Podgaetz E, Paris S, Cardenas J, Gu J, Leeds S, Ward M, Nguyen A, Konda V, Furuta GT, Pan Z, Souza RF, Spechler SJ. Lower esophageal sphincter muscle of patients with achalasia exhibits profound mast cell degranulation. Neurogastroenterol Motil 33: e14055, 2021. doi: 10.1111/nmo.14055. [DOI] [PubMed] [Google Scholar]
- 24.Zhang HY, Zhang X, Chen X, Thomas D, Hormi-Carver K, Elder F, Spechler SJ, Souza RF. Differences in activity and phosphorylation of MAPK enzymes in esophageal squamous cells of GERD patients with and without Barrett's esophagus. Am J Physiol Gastrointest Liver Physiol 295: G470–G478, 2008. doi: 10.1152/ajpgi.90262.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahe MM, Aihara E, Schumacher MA, Zavros Y, Montrose MH, Helmrath MA, Sato T, Shroyer NF. Establishment of gastrointestinal epithelial organoids. Curr Protoc Mouse Biol 3: 217–240, 2013. doi: 10.1002/9780470942390.mo130179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasagi Y, Chandramouleeswaran PM, Whelan KA, Tanaka K, Giroux V, Sharma M, Wang J, Benitez AJ, DeMarshall M, Tobias JW, Hamilton KE, Falk GW, Spergel JM, Klein-Szanto AJ, Rustgi AK, Muir AB, Nakagawa H. The esophageal organoid system reveals functional interplay between notch and cytokines in reactive epithelial changes. Cell Mol Gastroenterol Hepatol 5: 333–352, 2018. doi: 10.1016/j.jcmgh.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhat AA, Lu H, Soutto M, Capobianco A, Rai P, Zaika A, El-Rifai W. Exposure of Barrett's and esophageal adenocarcinoma cells to bile acids activates EGFR-STAT3 signaling axis via induction of APE1. Oncogene 37: 6011–6024, 2018. doi: 10.1038/s41388-018-0388-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kauer WK, Peters JH, DeMeester TR, Feussner H, Ireland AP, Stein HJ, Siewert RJ. Composition and concentration of bile acid reflux into the esophagus of patients with gastroesophageal reflux disease. Surgery 122: 874–881, 1997.doi: 10.1016/S0039-6060(97)90327-5. [DOI] [PubMed] [Google Scholar]
- 29.Ozçelik MF, Pekmezci S, Saribeyoğlu K, Unal E, Gümüştaş K, Doğusoy G. The effect of halofuginone, a specific inhibitor of collagen type 1 synthesis, in the prevention of esophageal strictures related to caustic injury. Am J Surg 187: 257–260, 2004. doi: 10.1016/j.amjsurg.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen-Ngoc KV, Cheung KJ, Brenot A, Shamir ER, Gray RS, Hines WC, Yaswen P, Werb Z, Ewald AJ. ECM microenvironment regulates collective migration and local dissemination in normal and malignant mammary epithelium. Proc Natl Acad Sci USA 109: E2595–2604, 2012. doi: 10.1073/pnas.1212834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schefe JH, Lehmann KE, Buschmann IR, Unger T, Funke-Kaiser H. Quantitative real-time RT-PCR data analysis: current concepts and the novel “gene expression's CT difference” formula. J Mol Med 84: 901–910, 2006. doi: 10.1007/s00109-006-0097-6. [DOI] [PubMed] [Google Scholar]
- 32.Team RC. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2020. [Google Scholar]
- 33.Kolde R. pheatmap: Pretty Heatmaps. R package version 1.0.12. 2019. https://cran.r-project.org/package=pheatmap.
- 34.Grabinger T, Luks L, Kostadinova F, Zimberlin C, Medema JP, Leist M, Brunner T. Ex vivo culture of intestinal crypt organoids as a model system for assessing cell death induction in intestinal epithelial cells and enteropathy. Cell death Dis 5: e1228, 2014. doi: 10.1038/cddis.2014.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Que J, Garman KS, Souza RF, Spechler SJ. Pathogenesis and cells of origin of Barrett's esophagus. Gastroenterology 157: 349–364.e1, 2019. doi: 10.1053/j.gastro.2019.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Owen RP, White MJ, Severson DT, Braden B, Bailey A, Goldin R, Wang LM, Ruiz-Puig C, Maynard ND, Green A, Piazza P, Buck D, Middleton MR, Ponting CP, Schuster-Böckler B, Lu X. Single cell RNA-seq reveals profound transcriptional similarity between Barrett's oesophagus and oesophageal submucosal glands. Nat Commun 9: 4261, 2018. doi: 10.1038/s41467-018-06796-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lavery DL, Nicholson AM, Poulsom R, Jeffery R, Hussain A, Gay LJ, Jankowski JA, Zeki SS, Barr H, Harrison R, Going J, Kadirkamanathan S, Davis P, Underwood T, Novelli MR, Rodriguez-Justo M, Shepherd N, Jansen M, Wright NA, McDonald SA. The stem cell organisation, and the proliferative and gene expression profile of Barrett's epithelium, replicates pyloric-type gastric glands. Gut 63: 1854–1863, 2014. doi: 10.1136/gutjnl-2013-306508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowicki-Osuch K, Zhuang L, Jammula S, Bleaney CW, Mahbubani KT, Devonshire G, Katz-Summercorn A, Eling N, Wilbrey-Clark A, Madissoon E, Gamble J, Di Pietro M, O'Donovan M, Meyer KB, Saeb-Parsy K, Sharrocks AD, Teichmann SA, Marioni JC, Fitzgerald RC. Molecular phenotyping reveals the identity of Barrett's esophagus and its malignant transition. Science 373: 760–767, 2021. doi: 10.1126/science.abd1449. [DOI] [PubMed] [Google Scholar]
- 39.Singh H, Ha K, Hornick JL, Madha S, Cejas P, Jajoo K, Singh P, Polak P, Lee H, Shivdasani RA. Hybrid stomach-intestinal chromatin states underlie human Barrett's metaplasia. Gastroenterology 161: 924–939.e11, 2021. doi: 10.1053/j.gastro.2021.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schlaermann P, Toelle B, Berger H, Schmidt SC, Glanemann M, Ordemann J, Bartfeld S, Mollenkopf HJ, Meyer TF. A novel human gastric primary cell culture system for modelling Helicobacter pylori infection in vitro. Gut 65: 202–213, 2016. doi: 10.1136/gutjnl-2014-307949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DeWard AD, Cramer J, Lagasse E. Cellular heterogeneity in the mouse esophagus implicates the presence of a nonquiescent epithelial stem cell population. Cell Rep 9: 701–711, 2014. doi: 10.1016/j.celrep.2014.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palanca-Wessels MCA, Klingelhutz A, Reid BJ, Norwood TH, Opheim KE, Paulson TG, Feng Z, Rabinovitch PS. Extended lifespan of Barrett's esophagus epithelium transduced with the human telomerase catalytic subunit: a useful in vitro model. Carcinogenesis 24: 1183–1190, 2003. doi: 10.1093/carcin/bgg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett's esophagus. N Engl J Med 295: 476–480, 1976. doi: 10.1056/NEJM197608262950904. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto Y, Wang X, Bertrand D, Kern F, Zhang T, Duleba M, Srivastava S, Khor CC, Hu Y, Wilson LH, Blaszyk H, Rolshud D, Teh M, Liu J, Howitt BE, Vincent M, Crum CP, Nagarajan N, Ho KY, McKeon F, Xian W. Mutational spectrum of Barrett's stem cells suggests paths to initiation of a precancerous lesion. Nat Commun 7: 10380, 2016. doi: 10.1038/ncomms10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X, Yamamoto Y, Wilson LH, Zhang T, Howitt BE, Farrow MA, Kern F, Ning G, Hong Y, Khor CC, Chevalier B, Bertrand D, Wu L, Nagarajan N, Sylvester FA, Hyams JS, Devers T, Bronson R, Lacy DB, Ho KY, Crum CP, McKeon F, Xian W. Cloning and variation of ground state intestinal stem cells. Nature 522: 173–178, 2015. doi: 10.1038/nature14484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang BG, Lee BL, Kim WH. Intestinal stem cell markers in the intestinal metaplasia of stomach and Barrett's esophagus. PLoS One 10: e0127300, 2015. doi: 10.1371/journal.pone.0127300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang DH, Clemons NJ, Miyashita T, Dupuy AJ, Zhang W, Szczepny A, Corcoran-Schwartz IM, Wilburn DL, Montgomery EA, Wang JS, Jenkins NA, Copeland NA, Harmon JW, Phillips WA, Watkins DN. Aberrant epithelial-mesenchymal Hedgehog signaling characterizes Barrett's metaplasia. Gastroenterology 138: 1810–1822, 2010. doi: 10.1053/j.gastro.2010.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang DH, Tiwari A, Kim ME, Clemons NJ, Regmi NL, Hodges WA, Berman DM, Montgomery EA, Watkins DN, Zhang X, Zhang Q, Jie C, Spechler SJ, Souza RF. Hedgehog signaling regulates FOXA2 in esophageal embryogenesis and Barrett's metaplasia. J Clin Invest 124: 3767–3780, 2014. doi: 10.1172/JCI66603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pastuła A, Middelhoff M, Brandtner A, Tobiasch M, Höhl B, Nuber AH, Demir IE, Neupert S, Kollmann P, Mazzuoli-Weber G, Quante M. Three-dimensional gastrointestinal organoid culture in combination with nerves or fibroblasts: a method to characterize the gastrointestinal stem cell niche. Stem cells Int 2016: 3710836, 2016. doi: 10.1155/2016/3710836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vega KJ, May R, Sureban SM, Lightfoot SA, Qu D, Reed A, Weygant N, Ramanujam R, Souza R, Madhoun M, Whorton J, Anant S, Meltzer SJ, Houchen CW. Identification of the putative intestinal stem cell marker doublecortin and CaM kinase-like-1 in Barrett's esophagus and esophageal adenocarcinoma. J Gastroenterol Hepatol 27: 773–780, 2012. doi: 10.1111/j.1440-1746.2011.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10: 445–457, 2009. doi: 10.1038/nrm2720. [DOI] [PubMed] [Google Scholar]
- 52.Dunbar K, Valanciute A, Lima ACS, Vinuela PF, Jamieson T, Rajasekaran V, Blackmur J, Ochocka-Fox AM, Guazzelli A, Cammareri P, Arends MJ, Sansom OJ, Myant KB, Farrington SM, Dunlop MG, Din FVN. Aspirin rescues Wnt-driven stem-like phenotype in human intestinal organoids and increases the Wnt antagonist Dickkopf-1. Cell Mol Gastroenterol Hepatol 11: 465–489, 2021. doi: 10.1016/j.jcmgh.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, Bell G, Guo W, Rubin J, Richardson AL, Weinberg RA. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell states in the breast. Cell 145: 926–940, 2011. doi: 10.1016/j.cell.2011.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]