Abstract
Receptor-ligand interactions play an important role in many biological processes by triggering specific cellular responses. These interactions are frequently regulated by coreceptors that facilitate, alter, or inhibit signaling. Coreceptors work in parallel with other specific and accessory molecules to coordinate receptor-ligand interactions. Cell surface heparan sulfate proteoglycans (HSPGs) function as unique coreceptors because they can bind to many ligands and receptors through their HS and core protein motifs. Cell surface HSPGs are typically expressed in abundance of the signaling receptors and, thus, are capable of mediating the initial binding of ligands to the cell surface. HSPG coreceptors do not possess kinase domains or intrinsic enzyme activities and, for the most part, binding to cell surface HSPGs does not directly stimulate intracellular signaling. Because of these features, cell surface HSPGs primarily function as coreceptors for many receptor-ligand interactions. Given that cell surface HSPGs are widely conserved, they likely serve fundamental functions to preserve basic physiological processes. Indeed, cell surface HSPGs can support specific cellular interactions with growth factors, morphogens, chemokines, extracellular matrix (ECM) components, and microbial pathogens and their secreted virulence factors. Through these interactions, HSPG coreceptors regulate cell adhesion, proliferation, migration, and differentiation, and impact the onset, progression, and outcome of pathophysiological processes, such as development, tissue repair, inflammation, infection, and tumorigenesis. This review seeks to provide an overview of the various mechanisms of how cell surface HSPGs function as coreceptors.
Keywords: coreceptor, heparan sulfate, proteoglycan, receptor-ligand interaction, signaling
INTRODUCTION
Virtually all cells of eukaryotic, multicellular organisms express one or several types of heparan sulfate proteoglycans (HSPGs) on their cell surface. HSPGs consist of a core protein and one or several covalently attached heparan sulfate (HS) chains. Some HSPGs are hybrid proteoglycans carrying both HS and chondroitin sulfate (CS) chains. HS is a linear polysaccharide comprised of repeating disaccharide units with a signature repeat of (GlcUAβ1-4GlcNAcα1–4)n (1, 2). HS is attached to and polymerized on Ser residues surrounded by a motif containing a hydrophobic amino acid adjacent to the Ser-Gly dipeptide sequence and a cluster of acidic amino acids on the C-terminal side of Ser-Gly sequence in HSPG core proteins (3, 4). The unmodified HS polysaccharides are modified in the Golgi by N-deacetylation, N-sulfation, epimerization, and several O-sulfation reactions that are catalyzed by distinct membrane-bound enzymes (5). The biosynthetic machinery of HS is evolutionarily conserved among vertebrates and invertebrates, suggesting conservation of function for the HS chains. Because HS polymerization and modification reactions do not go to completion, the biosynthetic process generates an exceptionally diverse array of HS structures, both in length and extent of modification. HS can vary in length from 20 to 150 disaccharides with cell type and core protein (6). A mere HS decasaccharide can potentially assume over 106 distinct sequences, which is already in vast excess of the estimated gene products that the whole human genome can generate. This enormous structural diversity largely explains why and how HS can bind to so many proteins.
Cell surface HSPGs include the syndecan (SDC) and glypican (GPC) families with four and six members in mammals, neuropilin-1, CD44, neurexin, and betaglycan (7–11). The latter three are part-time proteoglycans as they may appear in proteoglycan form or as glycoproteins lacking glycosaminoglycans (GAGs). Several HSPGs can harbor HS or CS chains and the type of GAG that they carry can influence their coreceptor function. For example, neuropilin-1 enhances VEGFR2 signaling when it carries HS chains, but inhibits VEGFR2 signaling when it carries CS chains (12). SDCs, neuropilin-1, CD44, neurexin, and betaglycan are type I transmembrane core proteins whereas GPC core proteins are linked to the cell surface by a glycosylphosphatidylinositol (GPI) anchor.
Coreceptors in general are selective for particular receptor-ligand interactions. For example, CD4 is a coreceptor for T cell receptor interactions (13) and CCR5 is a coreceptor for human immuodeficiency virus (HIV) infection (14). HSPG coreceptors are unique in that they can bind to many HS/heparin (HP)-binding proteins through their HS chains and regulate many different types of receptor-ligand interactions. Furthermore, several cell surface HSPGs can interact with ligands independent of their HS chains. For example, betaglycan and neuropilin-1 bind to transforming growth factor (TGF)β family members and vascular endothelial growth factor (VEGF), respectively, through their core proteins. Similarly, the core protein of GPC3 binds with high affinity to sonic Hedgehog (Shh) and Indian Hedgehog (Ihh) (15, 16).
HSPG coreceptors bind soluble and insoluble ligands and screen them, place them at appropriate locations, stabilize them, or distribute them to neighboring cells (Fig. 1). HSPG coreceptors can also increase the avidity of ligands for their signaling receptors by increasing their local concentration, inducing a conformational change, or by serving as a platform for oligomerization (Fig. 1). Similarly, HSPGs can bind to receptors and transport them to appropriate locations, assist them in ligand binding, induce a conformational change, or facilitate their oligomerization (Fig. 2). Furthermore, HSPG coreceptors can regulate signaling components by recruiting them to the receptor complex, altering their stability, and influencing their intracellular trafficking patterns (Fig. 2). In addition, the transmembrane HSPG coreceptors can induce separate signal transduction events that modulate the cellular response. Through these activities, cell surface HSPGs fine tune receptor-ligand interactions and ensuing cellular responses. Insight into functions of cell surface HSPGs as coreceptors was first revealed by studies that discovered the importance of cell surface HS binding to fibroblast growth factor (FGF) 2 for high-affinity interactions with receptor tyrosine kinases (17, 18). These observations served to further galvanize interest in the role of cell surface HSPGs as coreceptors for other growth factors and other HS/HP-binding proteins.
Figure 1.
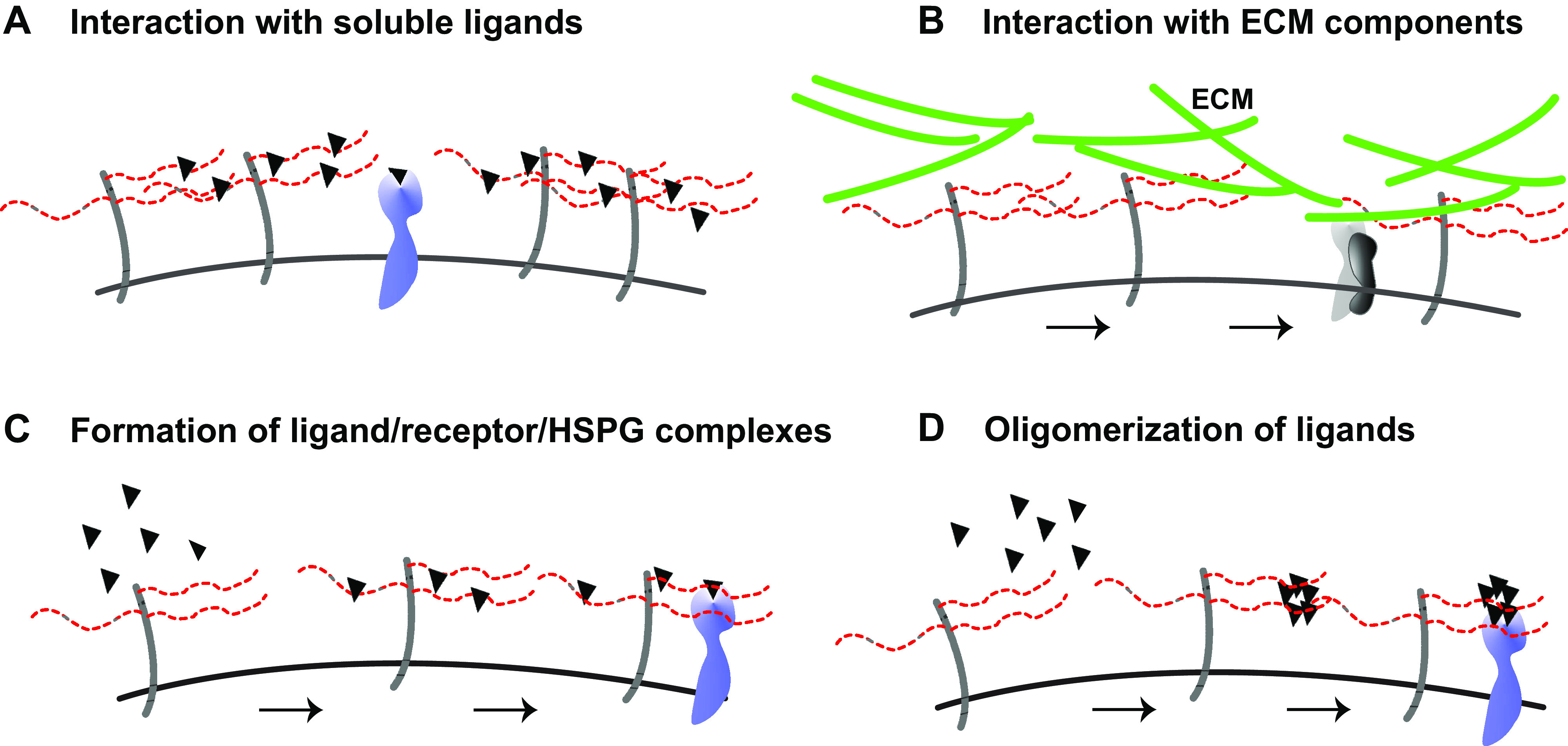
Schematic overview of coreceptor functions of cell surface heparan sulfate proteoglycans (HSPGs) I. A: interaction with soluble ligands: cell surface HSPGs capture soluble ligands, increase their local concentration, and increase their availability for signaling receptors. B: interaction with extracellular matrix (ECM) components: cell surface HSPG binding facilitates binding of ECM components to integrin receptors in a heparan sulfate (HS)-dependent manner. C: formation of ligand/receptor/HSPG complexes: cell surface HSPGs bind to both ligands and signaling receptors to form a tertiary receptor complex. D: oligomerization of ligands: cell surface HSPGs provide a HS platform with multiple binding sites for ligand oligomerization, and ligand oligomers interact more avidly with signaling receptors.
Figure 2.
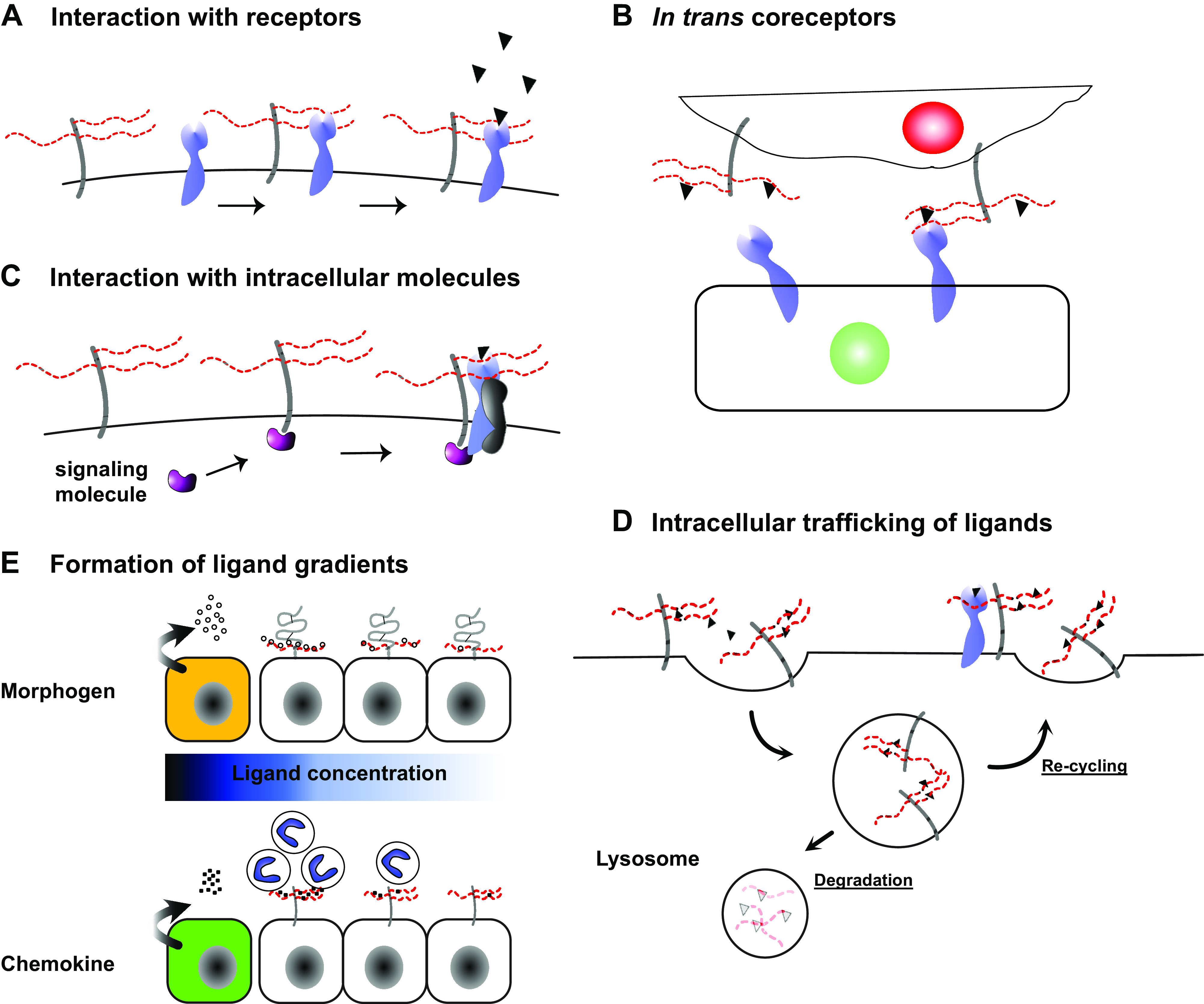
Schematic overview of coreceptor functions of cell surface heparan sulfate proteoglycans (HSPGs) II. A: interaction with receptors: cell surface HSPGs can enhance signaling by directly regulating receptor distribution, stability, oligomerization state, and activity. B: trans coreceptors: cell surface HSPGs present ligands to receptors on neighboring cells thereby functioning as in trans coreceptors. C: interaction with intracellular molecules: cell surface HSPGs with cytoplasmic domains can enhance receptor signaling through recruitment of intracellular signaling molecules to the receptor complex. D: intracellular trafficking of ligands: HSPG coreceptors control ligand availability at the cell surface through endocytosis of ligands and either recycling them back to the cell surface of transporting them to lysosomes for degradation. E: formation of ligand gradients: cell surface HSPGs immobilize morphogens to form morphogen gradients during development and immobilize chemokines to form haptotactic gradients to guide the directional migration of leukocytes.
INTERACTION WITH SOLUBLE LIGANDS
Cell surface HSPGs bind to soluble ligands and enhance their activity by affecting their concentration, destination, conformation, oligomerization, or stability (7, 10, 19) (Fig. 1A). Two seminal studies published in Science (17) and Cell (18) in 1991 were the first to establish the importance of cell surface HSPG coreceptors in growth factor signaling. Before these studies, it was already known that many growth factors bind to HS/HP (20–24). It was also known that FGF2 binds to HS/HP with low- to mid-nanomolar affinity and that deletion of HS/HP-binding regions in FGF2 dramatically reduces its biological activity (25, 26). The two studies established that cell surface HSPGs function as a coreceptor for FGF2 by showing that 1) mutant CHO cells lacking HS but expressing the high affinity, receptor tyrosine kinase fibroblast growth factor receptor (FGFR)1, and CS are unable to bind FGF2; 2) FGF2 binds to sulfated HS expressed on the surface of fibroblasts and skeletal muscle cells; 3) binding to cell surface HS is required for efficient binding of FGF2 to FGFR1; and 4) cell surface HS is required for FGF2 signaling in fibroblasts and skeletal muscle cells (17, 18). Consistent with these data, FGF2 signaling measured by the activation of ERK MAP kinases is prominent in mouse embryonic fibroblasts (MEFs) expressing SDC1, but markedly reduced in Sdc1−/− MEFs (Fig. 3A). Soluble HP, but not CS, can restore FGF2 binding to cells without sulfated GAGs at concentrations as low as 1 ng/mL (17). These findings suggest that HSPGs not only localize FGF2 to the cell surface, but HSPG binding also induces changes in FGF2 or FGFR1. Indeed, monomeric FGF2 forms oligomers when bound to HS that, in turn, dimerize FGFR1, which is required for signaling (27). Similarly, exogenous HS enhances HB-EGF signaling in a dose-dependent manner in wild-type (Wt) mouse mesenchymal stem cells (MSCs), but CS does not (Fig. 3B). HSPGs also regulate FGF signaling in lower organisms, such as Drosophila (28) and zebrafish (29). Other members of the FGF family are also regulated by cell surface HSPGs, but the structural requirement for HS binding to FGFs is apparently distinct and rather complex. For example, FGF1 and FGF2 require different HS structures for binding (30) and IdoA is required for FGF2 binding but not for FGF10 binding (31).
Figure 3.
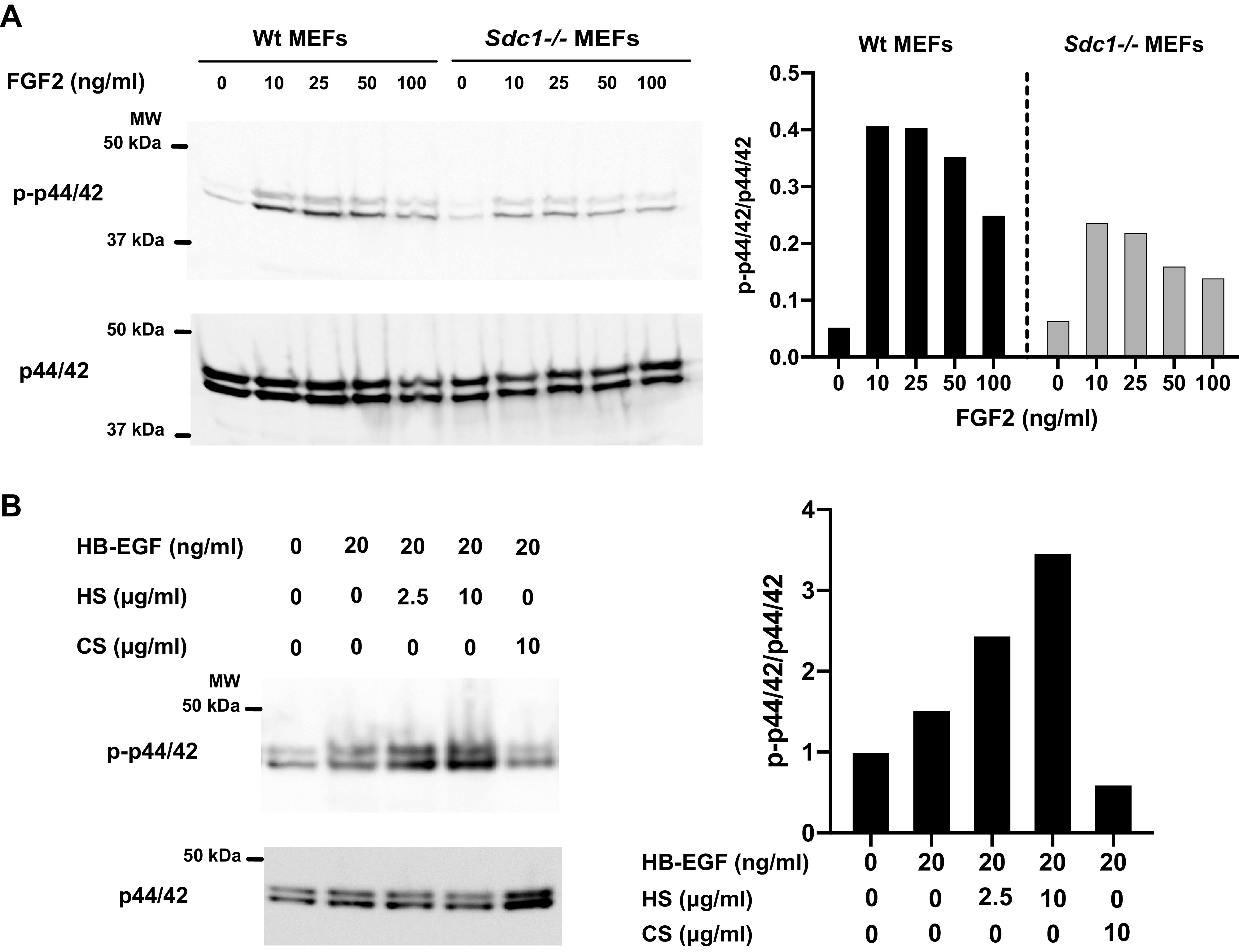
Cell surface syndecan (SDC)1 and exogenous heparan sulfate (HS) enhance growth factor signaling in mouse embryonic fibroblasts (MEFs) and mesenchymal stem cells (MSCs). A: MEFs isolated from Wt or syndecan-1 null (Sdc1−/−) mice on C57BL/6J were stimulated with fibroblast growth factor (FGF)2 at the indicated concentrations for 30 min at 37°C. Whole cell lysates were Western blotted for phospho-p44/42 (Thr202/Tyr204) (p-p44/42) and total p44/42 (Cell Signaling Technology, Danvers, MA). B: MSCs isolated from inguinal fat pads of Wt C57BL/6J mice were incubated with HB-EGF without or with HS or chondroitin sulfate (CS) for 30 min at 37°C. Whole cell lysates were Western blotted for p-p44/42 and total p44/42.
Similar to FGF2, Wingless (Wg), the Drosophila homolog of the proto-oncogene WNT1, binds to HP and Wg bound to cells can be released by exogenous HS (32). Wg signaling is inhibited by treatment of cells with heparinases or chlorate, indicating that sulfated HSPGs bind Wg and enhance Wg activity. Furthermore, exogenous HP can restore Wg activity in chlorate-treated cells. The difference between FGF2 and Wg signaling is that CSPGs can also serve as a coreceptor for Wg (32). However, abrogation of HS synthesis severely impairs Wg, Hedgehog (Hh), and decapentaplegic (Dpp) signaling in Drosophila (33), whereas deletion of the gene for N-deacetylase N-sulfotransferase 1 (Ndst1) impairs Shh and FGF signaling in mice (34), suggesting that proteoglycans carrying HS are the dominant coreceptors. Consistent with these findings, Dally and Dally-like protein (Dlp) are the HSPG coreceptors for Wg in Drosophila (35–37), whereas several GPCs (38–41) and SDCs can serve as coreceptors for WNTs in mammalian cells (42–44). Interestingly, loss of Dlp has a more marked effect on the accumulation of Wg than the loss of Dally (45). Dally binds to Wg via HS, whereas Dlp can bind via both HS and the protein core (46). This biphasic binding is apparently important in shielding the palmitoleate moiety of Wg (45). The human homologs of Dlp, GPC4, and GPC6, also bind to the lipid moiety of WNT, but not GPC3 and GPC5 (45), demonstrating that GPC family members have distinct coreceptor functions.
The HS-modified isoform of CD44, CD44v3, binds to hepatocyte growth factor (HGF) and concentrates HGF at the cell surface, thereby facilitating its interaction with c-Met and activation of signaling in lymphoma cells (47). However, several studies also suggest that CD44 may enhance the activation of Met and other receptor tyrosine kinases in an HS-independent manner as CD44 isoforms lacking HS chains also bind and present HGF and VEGF to their respective signaling receptors (48). Cell surface SDC1 also binds to HGF and enhances HGF signaling through Met in multiple myeloma cells (49). Interestingly, shed SDC1 also binds to HGF and facilitates its interaction with Met in multiple myeloma cells (50), suggesting that SDC1 not only localizes HGF to the cell surface but also boosts HGF activity. In hepatocellular carcinoma (HCC), HCC cell migration and motility do not respond to either canonical or noncanonical Wnt activation but markedly increase with HGF treatment (51). GPC3 binds to HGF in an HS-dependent manner and facilitates HGF-Met signaling in HCC cells, which leads to increased cell migration and motility. Importantly, blocking HS chains of GPC3 inhibits HCC spheroid formation in vitro and liver tumor growth in mice (51). Together, these studies indicate that several HSPG coreceptors positively regulate growth factor interactions to promote cancer cell motility, proliferation, and survival.
Cell surface HSPGs are also used as coreceptors by secreted microbial virulence factors. HIV envelope protein gp120 can be secreted (52) and its interaction with genital epithelial cells triggers innate immune responses that impair mucosal barrier functions (53). Soluble gp120 binds to HS, Toll-like receptor (TLR)2, and TLR4, and stimulates proinflammatory cytokine production via activation of NF-κB in HEK293 cells (53), suggesting that cell surface HSPGs expressed by epithelial cells facilitate gp120 engagement of TLRs. How this is accomplished is not known, but gp120 is activated when it undergoes a conformational change (52) and HS binds and activates TLR4 signaling in dendritic cells (54). These findings suggest that HSPGs may enhance gp120 signaling by inducing a conformational change in gp120, stimulating TLR4, or both.
Other HIV-secreted factors also bind to HSPGs and these interactions are considered to contribute to AIDS pathogenesis. Cell surface HSPGs are coreceptors for p17 matrix protein released by HIV-infected cells (55). Here, HSPG binding via sulfated HS motifs promotes p17 oligomerization and association with its signaling receptors CXCR1 and CXCR2 on leukocytes (56). Colocalization of SDC2 and CXCR2 is seen in hepatic stellate cells when treated with p17 (57), suggesting that SDC2 is one of the HSPG coreceptors for HIV p17.
The HIV transactivating factor Tat is a small, cationic protein released from HIV-infected cells and is found in blood of patients infected with HIV. Cell surface HSPGs bind to Tat, protect Tat from degradation, and induce Tat oligomerization (58–60). Tat-HSPG interaction is important (61), but not essential (62), for clathrin-dependent endocytosis of Tat, indicating involvement of other receptors for internalization (62). Indeed, Tat also binds to αvβ3 integrins and activates focal adhesion kinase (FAK) signaling (63).
Using its HSPG connection, Tat can facilitate the extravasation of lymphoid cells (64). Tat accumulates on the surface of endothelium by binding to HSPGs, and B-lymphoid cells expressing SDC1 or HIV-infected T cells endogenously expressing Tat can attach to the Tat-HSPG complex on the endothelial surface. This heterotypic lymphoid cell-endothelial cell interaction is inhibited by HS inhibition, but not by integrin inhibition, and requires the homodimerization of Tat. Furthermore, Tat-HSPG on endothelial cells promotes the transendothelial migration of PBMCs in vitro and in a zebrafish embryo model of inflammation. Together, these data indicate an interesting mechanism where Tat bound to lymphoid HSPG and Tat bound to endothelial cells dimerize to form a HSPG-Tat-Tat-HSPG quaternary complex (64). This mechanism physically links lymphoid cells to the endothelium and promotes their extravasation.
INTERACTION WITH ECM COMPONENTS
Cell surface HSPGs also function as coreceptors for ECM components (Fig. 1B). Cell surface HSPGs, especially members of the transmembrane SDC family, facilitate formation of a signaling complex that translates information from the extracellular environment to specific cellular responses. Most ECM components contain HS/HP-binding domains and cell surface HSPGs bind to these molecules in an HS-dependent manner. The list is extensive and includes fibrillar collagens (65–67), fibronectin (68–70), thrombospondin (71), laminin (72), vitronectin (73), tropoelastin (74), fibrillin (75), and tenascin (76), among others. Cell surface HSPGs generally facilitate integrin interactions with ECM components. For example, SDC4 coordinates with α5β1 integrins in fibroblasts to attach and spread on fibronectin, and promote focal adhesion formation (69). Both SDC4 and GPC1 can bind to the C-terminal HP-binding HepII domain of fibronectin, but only SDC4 can support focal adhesion formation because the ability of SDC4’s cytoplasmic domain to bind and activate PKCα is important in this process (77). SDC4, but not SDC2, is also associated with focal adhesions formed on laminin, vitronectin, or collagen I in a β1 or β3 integrin-dependent manner (78), suggesting that SDC4 is the primary cell surface HSPG coreceptor that coordinates with integrins in the formation of focal adhesions.
Cell surface HSPGs also participate as coreceptors in cell attachment to other adhesive substrates. For example, cell surface HSPGs mediate mesenchymal cell attachment to immobilized ADAM12 (a disintegrin and metalloproteinase) (79, 80). HS chains of SDC1, SDC2, and SDC4, but not GPC1, bind to the disintegrin domain of ADAM12. After binding via SDCs, cells spread, assemble stress fibers, and form focal adhesions. The postadhesion events are mediated by β1 integrins because blocking or deleting β1 integrins does not inhibit attachment, but inhibits cell spreading. These findings indicate that mesenchymal cells use SDCs for attachment and β1 integrins for signaling when interacting with immobilized ADAM12 (79).
Cell surface HSPGs also positively regulate the assembly of ECM components. Soluble fibronectin is assembled into fibronectin fibrils in a cell-mediated process that involves β1 integrins and cell surface HSPGs (81–83). In plasma, fibronectin exists as a soluble dimer, but in the ECM it is found as an insoluble fibril. Cell surface HSPGs are thought to promote the assembly of fibronectin into detergent-insoluble fibrils by bringing together soluble fibronectin by binding to the C-terminal HepII domain of fibronectin (68), and promoting fibronectin-fibronectin interactions in the N-terminal type I modules (84, 85). Indeed, CHO cells lacking HS do not assemble a fibronectin matrix (85), indicating that HSPGs are important cofactors. Furthermore, SDC2, but not SDC4, promotes fibronectin assembly in cultured mesenchymal cells (86, 87) and in zebrafish (88). SDC1 also interacts with β1 integrins via cytoplasmic domain interactions in epithelial cells (89), and deletion of SDC1 markedly impairs fibronectin assembly in corneal basement membranes (90). Together, these findings indicate that both mesenchymal and epithelial cells use SDCs to facilitate fibronectin assembly in the interstitial ECM and in basement membranes of select tissues.
Like fibronectin, laminins are modular proteins with domains that interact with both cells and various ECM components (91, 92). Laminin is a large heterotrimeric protein consisting of an α chain, β chain, and γ chain, and named based on the chain composition of the heterotrimer (e.g., LM-α3β3γ2 = LM332) (92, 93). Laminin is essential for basement assembly because the laminin lattice serves as a scaffold for the assembly and incorporation of other basement membrane components (92–94). Although laminin can self-assemble (95), the cell surface provides a nucleation surface for laminin polymerization (92, 96), and laminin binding to cell surface receptors is critical for laminin assembly (97). Cell surface tethering of laminin is mediated by several receptors, including integrins, α-dystroglycan, sulfated glycolipids, and HSPGs (92, 96). The identity of HSPGs that participate in laminin assembly is still under investigation, but SDC1, SDC2, and SDC4, but not GPC1, bind to laminin β1 chain peptide (98). Similarly, SDC2 and SDC4, but not GPC1, bind to the α3 LG4/5 domain peptide (99), and SDC2 promotes laminin assembly in zebrafish (88). Furthermore, SDC1 binds to the LG4/5 domain of laminin α3 chain (72, 100, 101) and to the N-terminal LE domain of laminin γ2 chain (102). These findings suggest that both epithelial SDC1 and mesenchymal SDC2 and SDC4 might mediate cell attachment to laminin and coordinate with other laminin receptors to assemble laminin networks in the basement membrane.
Elastin is a major fibrillar ECM component with key structural and biological functions in elastic tissues where reversible elasticity is critical, such as major arterial blood vessels, lungs, and skin. Elastin maturation involves the assembly of tropoelastin into a highly cross-linked polymer by cell surface receptors and enzymes. Cell surface HSPGs bind to tropoelastin (74), and SDC4 colocalizes with microfibrillar components of the elastic fiber, suggesting a potential role for cell surface HSPGs in elastin assembly (103). Tropoelastin also contains domains in the N-terminal region where cells attach and spread via αv and α5β1 integrins. Interestingly, cell surface HSPGs bind to a peptide sequence in an adjacent domain that contains Lys residues and induce a conformational ordering of the region (104). These data suggest that cell surface HSPGs might mediate the initial attachment of cells to tropoelastin, which is followed by αv and α5β1 integrin-dependent binding and signaling (104). Whether these HSPG and integrin interactions contribute to elastic fiber assembly is not known.
INTERACTION WITH PATHOGENS
Microbial pathogens interact with cell surface HSPGs in a manner similar to mammalian cells, except here, the objective of the interaction is to promote pathogenesis and survival in the host environment. A large number of viruses, bacteria, parasites, and fungi bind to the HS moiety of HSPGs (7, 105–107). Because there are many HS-binding pathogens, some have questioned the significance and relevance of the microbial interactions and suggested that the ability of pathogens to interact with HSPGs may be a result of cell culture adaptation (106, 108, 109). However, a counterargument is that the ability to exploit a ubiquitous molecule like cell surface HSPGs as attachment and invasion coreceptors is likely to expand cellular targets for infection, strengthen specific interactions of microbes with their primary receptors, and give a significant survival advantage to microbes in the host environment. Furthermore, HSPG-pathogen studies are potentially significant because coreceptor-based therapeutic strategies have the benefit of targeting invariant host determinants, which in principle, should not be vulnerable to the development of resistance from a rapidly mutating pathogen population.
Interaction with cell surface HSPGs is most prominent among intracellular pathogens. For example, several serotypes of adenovirus use cell surface HSPGs as the low affinity, initial binding site to promote its interaction with internalization receptors (110). HIV uses SDC3 as an attachment receptor that cooperates with DC-SIGN and CD4 to facilitate infection of leukocytes that express low levels of CD4 (111). HIV binds to the HS moiety of SDC3 via gp120 and SDC3-bound HIV can infect T cells in trans (111). Ebola virus uses T-cell immunoglobulin and mucin domain 1 (TIM-1) and several other receptors for its internalization (112), but its entry into polarized epithelial cells is inhibited by removal of cell surface HSPGs (113). Interestingly, Ebola virus strictly infects polarized epithelial cells via the basolateral route, suggesting that SDC1, abundantly expressed in these cellular compartments, is the primary coreceptor for Ebola virus.
The coronavirus disease 2019 (COVID-19) pandemic has infected more than 470 million people, killing more than 6.1 million people worldwide as of March 2022. The type I transmembrane angiotensin-converting enzyme 2 (ACE2) is the entry receptor for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the causative agent of COVID-19. Several studies indicate that cell surface HSPGs function as coreceptors for SARS-CoV-2 infection (114, 115). Spike (S) glycoprotein binds to HS and ACE2 and forms a ternary complex. HP binds at long, positively charged patches on the S glycoprotein, thereby masking basic residues of both the receptor-binding domain (RBD) and the multifunctional S1/S2 site (116). HP enhances the open conformation of the RBD in S protein, suggesting that cell surface HSPGs enhance S protein binding to ACE2 (114). Cell surface HSPGs also promote SARS-CoV-2 infection in neighboring cells. Although dendritic cells (DCs) are not infected by SARS-CoV-2, DCs efficiently bind SARS-CoV-2 via HSPGs and distribute the virus to surrounding ACE2-positive cells (115).
Neutralizing antibodies against SARS-CoV-2 isolated from patients with COVID-19 inhibit SARS-CoV-2 binding to HSPGs, suggesting that targeting viral interactions with cell surface HSPGs is a viable therapeutic option. Indeed, unfractionated HP, nonanticoagulant HP, low-molecular-weight HP, 6-O-desulfated HP, and heparinases all block S protein binding and cellular infection by SARS-CoV-2 virus (114, 115, 117). HP not only interferes with SARS-CoV-2 infection by blocking S protein binding but also S protein cleavage by furin (116). Consistent with these data, several randomized clinical trials and retrospective cohort studies have demonstrated beneficial effects of HP and low-molecular-weight HP therapy in COVID-19 (118–121). Although therapeutic doses of HP do not appear to have beneficial effects on survival or cardiovascular or respiratory organ injury in critically ill patients with COVID-19 (122), HP therapy increases the probability of survival to hospital discharge with reduced use of cardiovascular or respiratory organ support in noncritically ill patients (121). These data are consistent with the findings that cell surface HSPGs promote the initial attachment and invasion of SARS-CoV-2 virus, and not the progression of COVID-19, which is primarily mediated by a dysregulated inflammatory response (123, 124).
Bacterial pathogens also exploit cell surface HSPGs as coreceptors for their attachment and invasion. Cell adhesion receptors, such as integrins, cadherins, and immunoglobulin-related cell adhesion molecules are frequently recognized by specific bacterial surface proteins. Binding of bacterial pathogens to these receptors triggers a signaling cascade that leads to bacterial internalization (125). Cell surface HSPGs also frequently interact either directly or indirectly with these cell adhesion receptors (7, 8), suggesting that HSPGs are located at sites where they can function as coreceptors for bacterial pathogens. Indeed, Borrelia burgdorferi, the causative agent of Lyme disease, attaches to cell surface HSPGs and αvβ3 and α5β1 integrins on host cells (126, 127).
Similarly, available data suggest that Listeria monocytogenes, the causative agent of listeriosis, coopts cell surface HSPGs as coreceptors for its attachment and invasion. First, internalin A (InlA) mediates L. monocytogenes entry into intestinal epithelial cells by binding to E-cadherin (128), and E-cadherin expression is associated with that of SDC1 (129). Second, L. monocytogenes internalin B (InlB) also binds to HSPGs (130–132). InlB is a virulence factor that binds to c-Met (133) and C1qR (134). C1qR primarily mediates the uptake of L. monocytogenes in professional phagocytes, whereas Met mediates bacterial internalization in nonphagocytic cells, such as hepatocytes, via clathrin-dependent endocytosis (135). Importantly, InlB binds tightly to HP (132), and HP can potentiate Met activation by InlB by 10-fold (130, 131). Structural studies indicate that the C-terminal region of InlB induces HP-mediated Met clustering, which is required for full Met activation (136). Together, these observations suggest that HSPG coreceptors promote InlA and InlB interactions during L. monocytogenes attachment and internalization.
FORMATION OF LIGAND/RECEPTOR/HSPG COMPLEXES
Cell surface HSPGs can facilitate receptor-ligand interactions by binding both ligand and receptor and forming a ternary ligand/receptor/HSPG complex (Fig. 1C). For example, HSPGs can bind to both FGF ligands and FGF receptors and promote signaling (137). In the Slit-Robo system, GPC1 and SDC bind to both Slit (ligand) and Robo (receptor) via their HS moiety to form a ternary Slit/Robo/HSPG complex and regulate axon guidance (138–140). In B lymphoma cells, SDC1 expression levels are associated with an increased capacity to migrate in response to HIV Tat and to promote lymphoma pathogenesis in AIDS (141). Here, the formation of a receptor complex where SDC1 interacts with Tat via its HS, with αvβ3 integrin via its core protein, and with pp60src via its cytoplasmic domain switches the cellular response from a CXCR4/G-protein/Rac pathway to one that activates FAK and stimulates tumor cell migration.
Several GPCs regulate the Hh signaling pathway by binding to both Hh ligands and their receptors. GPC1 functions as a coreceptor for Shh in commissural neurons where it regulates repulsive guidance cues mediated by Shh signaling (142). GPC5 also enhances Shh signaling in rhabdomyosarcoma cells to promote tumor cell proliferation by binding to both Shh and its signaling receptor Patched (143). GPC5 binds to Shh and Patched in a HS-dependent manner through highly sulfated HS domains containing a critical 2-O-sulfate motif (144). Drosophila Dlp stimulates Hh signaling and interacts with both Hh and Patched during wing development (145). GPC6 also stimulates Hh signaling by binding to both Hh and Patched, and this coreceptor activity is important for the growth of long bones and intestinal elongation during development (39, 146). Furthermore, GPC3 binds to Shh, but in an HS-independent manner (15, 16). The reason why GPC3 binds to Hh via its core protein and not HS is incompletely understood, but the low level of HS sulfation in GPC3 compared with other GPCs is thought to play a role.
Betaglycan (TGFβRIII) is able to bind with high affinity to inhibin, bone morphogenetic proteins (BMPs), and all three isoforms of TGFβ. Betaglycan does not have a kinase domain and its primary function is to facilitate binding of TGFβ ligands to the TGFβRI & II complex and to enhance SMAD2/3 signaling. Betaglycan null mice are embryonic lethal because of severe defects in various organs, including the liver, heart, kidney, thymus, and testis (147–150), underscoring the importance of betaglycan’s coreceptor activities in development. Coreceptor functions of betaglycan are particularly important for TGFβ2 because TGFβ2 alone binds very weakly to TGFβRII compared with TGFβ1 and TGFβ3 (151). Consistent with these observations, TGFβ2 null mice exhibit many of the same phenotypes seen in betaglycan null mice (152).
OLIGOMERIZATION OF LIGANDS
Because mature HS chains contain multiple bindings sites for HS/HP-binding ligands, HSPGs can promote receptor-ligand interactions by serving as a platform for the oligomerization of ligands (Fig. 1D). For example, HSPG binding induces HGF oligomerization into a more stable and active form, which facilitates c-Met receptor dimerization and activation (153). Similarly, HS chains of SDCs and GPCs promote the oligomerization of a proliferation-inducing ligand (APRIL), a TNF superfamily member cytokine. Oligomerization of APRIL is a prerequisite for activating APRIL’s signaling receptors, transmembrane activator and CAML interactor (TACI), and B cell maturation antigen (BCMA), on B and T cells, and to enhance lymphocyte activation, migration, and survival (154). Chemokine oligomerization and binding to HS chains are required for their ability to stimulate cell migration (155). The propensity to bind to cell surface HSPGs and to form higher order oligomers are essential for the in vivo activity of several chemokines (156). Although most chemokines form homodimers on HSPGs, some can form heterodimers with other chemokines (157). Interestingly, viral chemokines, whose function is to misdirect leukocyte recruitment to evade immune responses, also bind and oligomerize on HSPG surfaces (158).
INTERACTION WITH RECEPTORS
Cell surface HSPGs can enhance signaling by interacting directly with receptors and regulating their location, stability, oligomerization state, and activity (Fig. 2A). In Drosophila, Sdc and Dlp have different effects on synapse morphogenesis and function (159). Both Sdc and Dlp bind with high affinity to the protein tyrosine phosphatase leukocyte antigen-related tyrosine phosphatase (LAR), a receptor that controls both neuromuscular junction (NMJ) growth and active zone morphogenesis. In NMJs, the majority of Sdc is found either on the muscle cell surface or in the space between the presynaptic membrane and the subsynaptic reticulum, whereas Dlp is found in a subset of restricted synaptic space. Here they both bind to LAR, but Sdc binding promotes LAR function and NMJ growth, whereas Dlp inhibits LAR function and influence active zone structure and physiology. How this is accomplished is not fully understood, but these findings suggest that cell surface HSPGs can regulate receptor activity by specifying its location.
GPC3 expression is significantly increased in HCC and is considered a biomarker for HCC diagnosis and prognosis (160). GPC3 promotes hepatoma cell proliferation for the progression of HCC, mainly by activating the canonical Wnt pathway (41) and increasing the expression of c-Myc, a classical downstream target of the canonical Wnt pathway (161). GPC3 functions in this manner by interacting directly with Frizzled G-protein coupled receptors (GPCRs) to facilitate the recruitment of Wnt into the signaling complex at the cell membrane and later translocate into the cytoplasm via endocytosis (40). Expression of soluble GPC3 inhibits the canonical Wnt pathway (162), suggesting that shed GPC3 interferes with GPC3-Frizzled interactions at the cell surface. SDC3 also enhances Wnt signaling in osteoblasts by interacting directly with Frizzled 1 (44). This interaction is apparently important in bone formation because Sdc3−/− mice exhibit low bone volume, reduced bone formation, increased bone marrow adipose tissue, increased bone fragility, and a blunted anabolic bone formation response to mechanical loading. These phenotypes were traced to reduced Wnt signaling in osteoblasts, which led to delayed osteoblast maturation, impaired osteoblast function, and increased osteoclast-mediated bone resorption.
Cell surface HSPGS also enhances signaling by facilitating the formation of receptor complexes. In bone morphogenetic protein (BMP) signaling, cell surface HSPGs do not affect BMP binding to type I receptor subunits but instead, enhance the recruitment of type II receptor subunits to BMP-type I receptor complexes to enhance BMP signaling (163). Cell surface HSPGs also directly regulate signaling by receptor for advanced glycation endproducts (RAGE), one of the receptors for HMGB1 that mediates vascular inflammation. Here, HSPGs bind to RAGE and facilitate the oligomerization of RAGE into hexamers, which is believed to play an essential role in signal transduction (164).
HSPGs AS IN TRANS CORECEPTORS
Cell surface HSPGs can also present ligands to receptors on neighboring cells thereby functioning as in trans coreceptors (Fig. 2B). The development of blood capillary structures from ES cells is strictly dependent on signaling through VEGFR-2. Here, VEGF signaling in endothelial cells is stimulated by cell surface HSPGs expressed in trans in adjacent perivascular smooth muscle cells (165), indicating an HSPG-mediated cross talk between adjacent cells. A similar transactivation mechanism is also seen during Xenopus development. In left-right patterning during early gastrulation, ectodermal SDC2 presents Vg 1, an essential TGFβ family member in Xenopus development, to mesodermal cells (166). These mechanisms demonstrate that cell surface HSPGs have a broader coreceptor activity by functioning in trans to modulate receptor-ligand interactions.
HIV also exploits HSPGs as trans coreceptors (167). All four SDCs are capable of attaching HIV to the cell surface when expressed in nonpermissive, CD4-negative cells. HIV binds to 6-O-sulfated motifs in SDC HS via gp120 (168). Because Arg298 in gp120 is critical for gp120 binding to both SDCs and the chemokine receptor CCR5, it is thought that 6-O-sulfated motifs in SDC HS mimic sulfated Tyr residues in the N-terminus of CCR5 and that HIV gp120 recognizes these similar structures (168). Importantly, SDC-bound HIV retains its infectivity for 6 days, whereas unbound virus loses its infectivity in less than a day, and HIV bound on SDCs efficiently infects CD4-positive T cells (167). Vascular endothelial cells are thought to present SDC-bound HIV to CD4-positive T cells in vivo (167). Because vascular endothelial cells express SDC2 and SDC4, these SDCs may be the primary HSPGs functioning as in trans coreceptors for HIV transmission. Together, it appears as though HIV has maximized its exploitation of SDC coreceptors. HIV uses SDCs as coreceptors for its virulence factor Tat (58–60), as in cis coreceptors for binding to macrophages and DCs (111), and as in trans coreceptors for infection of T cells (167).
INTERACTION WITH INTRACELLULAR MOLECULES
Cell surface HSPGs with cytoplasmic domains can enhance receptor signaling through regulation of intracellular signaling molecules (Fig. 2C). SDCs, for example, has a short cytoplasmic domain that contains two conserved regions (C1 and C2) separated by a variable (V) region unique to each SDC. The SDC cytoplasmic domain interacts with quite a large number of intracellular mediators, including ERM (ezrin, radixin, moesin) proteins, c-SRC, cortactin, tubulin, PKCα, and several PDZ (PSD95, Dlg, ZO1) binding proteins, among others (7, 8). Apart from GPCs, all transmembrane HSPGs contain a PDZ binding motif in their cytoplasmic domain (8), suggesting that PDZ interactions may mediate common functions of transmembrane HSPG coreceptors.
An elegant study showed that the interaction of SDC1 with the PDZ binding protein calcium/calmodulin-dependent serine kinase (CASK) is critical in assembling cholinergic neuromuscular junctions in C. elegans (169). Here, the anterograde organizer Punctin is secreted by cholinergic motor neurons in the synaptic cleft and activates postsynaptic differentiation. Punctin binds to muscle cell SDC1 and localizes SDC1 at postsynaptic sites. Punctin also concentrates and activates another transmembrane receptor, un-coordinated 40 (UNC-40)/DCC, and SDC1 acts as a coreceptor to strengthen the binding of Punctin to UNC-40 at synaptic sites. The clustering of SDC1 and UNC-40 increases the local concentration of PDZ domain-containing cytoplasmic domains of these transmembrane receptors and stimulates the intracellular recruitment of CASK and FRM-3/FARP by direct interaction with the PDZ domains of SDC1 and UNC-40, respectively. The resulting CASK-FRM-3/FARP complex then triggers synaptic clustering of nicotine-sensitive acetylcholine receptors. SDC-CASK interactions are also seen in neuronal synapses where SDC2-CASK complexes are enriched in postsynaptic density fractions of rat brain (170). These studies suggest the importance of SDC-CASK in organizing postsynaptic structures of neuronal and neuromuscular synapses. Interestingly, neurexins, major synaptic organizing proteins, are transmembrane HSPGs with PDZ binding motifs in their cytoplasmic domains. Presynaptic neurexin binds to neuroligin and LRRTM2 in postsynaptic neurons and presents pleiotrophin to LRRTM2 (11). These HS-mediated neurexin interactions are required for presynaptic differentiation, synapse development, and survival in mice (11). Furthermore, mutations in HS biosynthetic enzymes have been implicated in defects in synaptic functions and autism susceptibility (171, 172). Together, these findings suggest that HSPG coreceptor functions at the cell surface and in intracellular compartments have a strong impact on neuronal and synaptic biology.
Among cell surface HSPGs, SDC4 plays a prominent role in regulating the formation of focal adhesions (70, 173). Cells form focal adhesions when adhering to fibronectin via α5β1 integrins. This process requires support from the SDC4 coreceptor and its ability to recruit PKCα to the signaling complex and to activate this Ser/Thr kinase. Inhibition of PKCα impairs focal adhesion formation and cell migration mediated by α5β1 integrin, demonstrating the importance of SDC4-mediated PKCα activation. Furthermore, the α5β1 integrin and SDC4 complex in focal adhesions coordinate cell migration by providing separate signals that contribute to the suppression of RhoA activity during fibronectin engagement (174). Activation of the guanosine triphosphatase-activating protein p190RhoGAP is critical because it inhibits RhoA in an SRC-dependent manner. Fibronectin engagement stimulates Tyr-phosphorylation of p190RhoGAP, and this process is solely mediated by α5β1 integrin. SDC4 mediates the redistribution of Tyr-phosphorylated pool of p190RhoGAP between membrane and cytosolic fractions through activation of PKCα. By doing so, RhoA is effectively suppressed and focal adhesion formation and cell migration are promoted.
SDC4-mediated PKCα recruitment and activation is also crucial in the formation of focal adhesions when cells attach to immobilized ADAM12 (80). In contrast to cell adhesion on fibronectin, SDC4 binding to ADAM12 triggers β1 integrin-dependent cell spreading, stress fiber assembly, and focal adhesion formation. Pharmacological inhibition of PKCα completely inhibits ADAM12-induced cell spreading and a mutant form of SDC4 deficient in the capacity to activate PKCα is unable to promote cell spreading, indicating the absolute requirement for SDC4-mediated PKCα recruitment and activation in this process. Furthermore, SDC2 is unable to promote cell spreading, suggesting that the capacity to promote β1 integrin-dependent cell spreading and to form focal adhesions is specific to SDC4. However, some integrins can form focal adhesions without coreceptor support from SDC4 and also without PKCα activation (70), suggesting that coreceptor functions of SDC4 might be important in select cell types and in select cellular microenvironments.
INTRACELLULAR TRAFFICKING OF LIGANDS
Cell surface HSPGs can regulate receptor-ligand interactions through endocytosis of ligands thereby controlling the availability of ligands at the cell surface (Fig. 2D). In vascular smooth muscle cells, the presence of cell surface HSPGs extends the half-life of intracellular FGF2 and prolongs the availability of an intracellular pool of bioactive FGF-2 (175). By doing so, HSPGs are thought to function as a cellular switch between immediate and prolonged FGF-2 signaling in vascular smooth muscle cells, which might be important for the regulation of angiogenesis.
Loss of function mutations in GPC3 causes Simpson–Golabi–Behmel overgrowth syndrome (176). Corroborating the clinical features of this syndrome, GPC3 null mice display developmental overgrowth (177). GPC3 null embryos display increased Hh signaling, which positively regulates body size during development. GPC3 inhibits Hh activity in cultured MEFs by inducing Hh endocytosis and degradation, thereby limiting the availability of Hh at the cell surface (15).
Neuropilin-1 promotes α5β1 integrin-dependent endothelial cell adhesion to fibronectin and this interaction is crucial for vascular development (178). This mechanism is dependent on the SEA motif in neuropilin-1’s cytoplasmic domain. Neuropilin-1 interacts with α5β1 integrin at adhesion sites but does not directly mediate cell spreading on fibronectin. Instead, neuropilin-1 stimulates endothelial cell spreading by increasing the Rab5/GIPC1/Myo6-dependent internalization of active α5β1 integrin. The absence of this mechanism is thought to be causal in the angiogenesis defects observed in neuropilin-1 null mice.
FORMATION OF LIGAND GRADIENTS
HSPGs facilitate the formation of morphogen gradients during development (9, 179–182), and chemokine gradients during an inflammatory response to infection or tissue injury (183, 184). Cell surface HSPGs present morphogens and chemokines in trans to recipient cells (Fig. 2E). Morphogen gradients play crucial roles in the patterning and morphogenesis of the early developing embryo. Concentration gradients of morphogens are critical for establishing positional identities of differentiating cells. Several mechanisms of morphogen gradient formation have been proposed, such as restricted diffusion (185), spread of soluble morphogens through a continuous extracellular matrix (186), exosome transport (187), morphogen clustering (188), morphogen shuttling (189), and active transport of morphogens through an actin-based, filopodia-like cellular protrusion called cytoneme (190). Cell surface HSPGs are considered critical mediators in most of these mechanisms. For example, Wg does not move across a strip of cells lacking HS (191). Instead, Wg moves through the restricted diffusion mechanism by binding to cell surface HSPGs via Dally and Dlp (191). Cell surface HSPGs also control FGF gradients by regulating their diffusion in tissues. In midbrain development, binding to HSPGs is essential in the formation of a long range, posterior to anterior FGF8 gradient spanning the midbrain (192). In another study, tracking of FGF2 movement in living cells showed that the spatial organization of HS and the availability of FGF2 binding sites in HS can promote either FGF2 confinement or substantial movement (193). Other members of the FGF family are also bound by cell surface HSPGs. FGF4 and FGF8 are tethered to the cell surface by SDC1 and these interactions are critical for their local retention in the extraembryonic ectoderm (194). The extent of HS sulfation apparently regulates FGF morphogen gradients. For example, regional patterns of HS O-sulfation control FGF10 gradients formed during branching morphogenesis in lung development (195). HSPGs in the lung mesenchyme at sites of prospective budding express low sulfated HS, whereas highly sulfated HS chains are present in basement membranes of branching epithelial tubes (195).
HSPGs, especially those of the GPC family, can also facilitate intercellular transport of morphogens or intercellular communication between germ layers during development in morphogen gradient formation. In Drosophila wing development, Dally and Dlp control the movement of Hh across receiving cells in a functionally redundant manner (196). Interestingly, in regions of active Hh signaling in the Drosophila wing disk and abdominal epidermis, cells generate cytonemes (190). Cytoneme formation associates with Hh gradient establishment in space and time, and conditions that interfere with cytoneme formation reduce Hh gradient length, suggesting that cytonemes are critical for Hh gradient formation in wing and abdominal epithelia. Although the role of cell surface HSPGs in these processes is incompletely understood, cytonemes are thought to transport morphogens in exosomes (197) and SDC1 facilitates exosome biogenesis (198), suggesting that SDC1 might indirectly regulate morphogen gradient formation through its effects on morphogen packaging in cytoneme exosomes. More importantly, GPC4 aids in the formation of cytonemes that transport Wnt from endoderm to mesoderm in zebrafish embryos (199). The exact function of GPC4 in cytoneme formation remains to be defined, but without GPC4, the cytonemes are too short and too few to effectively transport Wnts from cell to cell.
Chemokines are chemotactic cytokines that comprise the most prominent group of migration guidance cues that operate in vertebrates (200, 201). Chemokines are distinguished from other cytokines by their ability to act on GPCRs. Chemokines diffuse from their site of production, and their interactions between their nonreceptor binding domains and cell surface and matrix HSPGs along the way are thought to drive the formation of linear immobilized (haptotactic) gradients that provide directional guidance for leukocyte migration (183, 184). Without this mechanism, chemokines will disperse and result in broad, nonlinear, and soluble gradients, which are less robust and stable, and rather transient in nature compared with immobilized haptotactic gradients. For many chemokines, their HS binding mutants that retain chemotactic activity in vitro have significantly reduced the capacity to induce leukocyte migration in vivo (156, 202), demonstrating that tethering by HSPGs is essential for chemokine activity. However, the chemokine gradient concept is somewhat oversimplified in several aspects. The role of HSPGs in the chemokine gradient concept largely assumes a similar level of expression, similar chemokine binding avidity, and similar effects on chemokine activity by HSPGs expressed in different tissue compartments. These assumptions are likely not true. Furthermore, except for few examples, the identity of cell surface HSPGs that tether chemokines and form chemokine gradients is mostly unknown. Moreover, HSPGs themselves are highly regulated during an inflammatory response, but the impact of these mechanisms on the regulation of chemokine gradients has not yet been rigorously examined.
Concluding Remarks
Studies during the last 30 years have clearly established the role of cell surface HSPGs as important coreceptors that facilitate receptor-ligand interactions and subsequent signaling. We mostly restricted our discussion to stimulatory functions of HSPG coreceptors, but there are many examples where cell surface HSPGs function as inhibitory coreceptors. For example, cell surface HSPGs control the basal activation of macrophages by type I interferons in atherosclerosis and obesity by sequestering IFNβ and inhibiting interferon-α/β receptor (IFNAR) signaling (203). Similarly, GPC3 suppresses Hh signaling by binding Hh and mediating Hh endocytosis and degradation (15, 16). Also, it is important to note that some transmembrane cell surface HSPGs possess the ability to directly signal, and in certain cases it is difficult to distinguish whether the primary function of HSPGs is that of a coreceptor or a signaling receptor. Furthermore, several microbial pathogens and virulence factors have been reported to use cell surface HSPGs as primary receptors (204–207). In addition, while beyond the scope of this review, cell surface HSPGs can regulate receptor-ligand interactions when shed as soluble ectodomains (107). Ectodomain shedding rapidly reduces the amount of cell surface HSPG coreceptors and releases soluble HSPG ectodomains that can regulate ligand binding in an autocrine and paracrine manner (7, 10).
Although mechanisms of cell surface HSPG coreceptors have been extensively studied in vitro and in vivo in lower organisms, comparatively little is known about their contribution in vivo in mammalian organisms. In principle, HSPG coreceptors are likely important in vivo, but not essential, since their primary function is to fine-tune receptor-ligand interactions and cellular responses. In other words, they primarily control the efficiency of an interaction and not the occurrence. Furthermore, even if a particular HSPG coreceptor function is essential, the redundancy of HSPGs may compensate for the loss of a particular HSPG and mask its importance in vivo. Consistent with these ideas, knockout (KO) mice with constitutive deletion of genes for Sdc1, Sdc2, Sdc3, Sdc4, Gpc1, Gpc2, Gpc4, and Cd44 and systemic knockdown of Gpc5 are not lethal in mice (8, 107, 208–212), and the majority of these KO mice show relatively minor phenotypes under unchallenged conditions. On the other hand, deletion of genes for neuropilin-1 (Nrp1), betaglycan (Tgfbr3), neurexin (Nrxn1-3), and Gpc6 are embryonic lethal (147, 213–215) and deletion of Gpc3 is perinatal lethal (177), indicating that these cell surface HSPGs are essential for development. Furthermore, adult Sdc1 and Cd44 KO mice show significant pathological phenotypes when challenged with inflammatory, infectious, and oncogenic agents or conditions (7, 8, 107, 216–218), suggesting that certain postdevelopmental functions of these cell surface HSPGs are specific and essential.
Obviously, cell surface HSPGs have other functions, and it is unclear if these developmental and postdevelopmental phenotypes of HSPG KO mice are solely due to their coreceptor activities. The majority of coreceptor functions of cell surface HSPGs might only be significant and relevant in select cell types and in specific steps of developmental and postdevelopmental processes. Nonetheless, studies so far suggest that coreceptor functions of cell surface HSPGs are critical in providing support to various key steps of a receptor-ligand interaction. Absence of this activity appears to tip the balance toward developmental abnormalities, disease onset and progression, and in the worst case lethality. On the other hand, a large number of pathogens apparently evolved or adapted to exploit coreceptor functions of HSPGs for their pathogenesis. These findings suggest that precise manipulations of HSPG coreceptor functions might have therapeutic value in many hereditary and acquired diseases.
GRANTS
This study is supported by the National Institutes of Health (NIH) Grants R01 HL142213, R01 HL132573, and R21 AI156284.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
This article is part of the special collection “Deciphering the Role of Proteoglycans and Glycosaminoglycans in Health and Disease.” Liliana Schaefer, MD, served as Guest Editor of this collection.
AUTHOR CONTRIBUTIONS
K.H. and P.W.P. conceived and designed research; K.H. performed experiments; K.H. and P.W.P. analyzed data; K.H. and P.W.P. interpreted results of experiments; K.H. and P.W.P. prepared figures; K.H., R.S.A., and P.W.P. drafted manuscript; R.S.A. and P.W.P. edited and revised manuscript; K.H., R.S.A., and P.W.P. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank our colleagues in the Park laboratory for helpful discussions. We apologize for not citing studies that may have been relevant but were omitted due to space limitations.
REFERENCES
- 1.Kjellen L, Lindahl U. Specificity of glycosaminoglycan-protein interactions. Curr Opin Struct Biol 50: 101–108, 2018. doi: 10.1016/j.sbi.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, Goddard WA III, Nishi A, Hsieh-Wilson LC. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol 2: 467–473, 2006. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L, David G, Esko JD. Repetitive Ser-Gly sequences enhance heparan sulfate assembly in proteoglycans. J Biol Chem 270: 27127–27135, 1995. doi: 10.1074/jbc.270.45.27127. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Esko JD. Amino acid determinants that drive heparan sulfate assembly in a proteoglycan. J Biol Chem 269: 19295–19299, 1994. doi: 10.1016/S0021-9258(17)32166-X. [DOI] [PubMed] [Google Scholar]
- 5.Esko JD, Selleck SB. Order out of chaos: assembly of ligand binding sites in heparan sulfate. Annu Rev Biochem 71: 435–471, 2002. doi: 10.1146/annurev.biochem.71.110601.135458. [DOI] [PubMed] [Google Scholar]
- 6.Sarrazin S, Lamanna WC, Esko JD. Heparan sulfate proteoglycans. Cold Spring Harb Perspect Biol 3: a004952–a004952, 2011. doi: 10.1101/cshperspect.a004952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, Zako M. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68: 729–777, 1999. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 8.Couchman JR. Transmembrane signaling proteoglycans. Annu Rev Cell Dev Biol 26: 89–114, 2010. doi: 10.1146/annurev-cellbio-100109-104126. [DOI] [PubMed] [Google Scholar]
- 9.Filmus J, Capurro M. The role of glypicans in Hedgehog signaling. Matrix Biol 35: 248–252, 2014. doi: 10.1016/j.matbio.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Park PW, Reizes O, Bernfield M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J Biol Chem 275: 29923–29926, 2000. doi: 10.1074/jbc.R000008200. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Lu H, Peixoto RT, Pines MK, Ge Y, Oku S, Siddiqui TJ, Xie Y, Wu W, Archer-Hartmann S, Yoshida K, Tanaka KF, Aricescu AR, Azadi P, Gordon MD, Sabatini BL, Wong ROL, Craig AM. Heparan sulfate organizes neuronal synapses through neurexin partnerships. Cell 174: 1450–1464.e23, 2018. doi: 10.1016/j.cell.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shintani Y, Takashima S, Asano Y, Kato H, Liao Y, Yamazaki S, Tsukamoto O, Seguchi O, Yamamoto H, Fukushima T, Sugahara K, Kitakaze M, Hori M. Glycosaminoglycan modification of neuropilin-1 modulates VEGFR2 signaling. EMBO J 25: 3045–3055, 2006. doi: 10.1038/sj.emboj.7601188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janeway CA Jr. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol 10: 645–674, 1992. doi: 10.1146/annurev.iy.10.040192.003241. [DOI] [PubMed] [Google Scholar]
- 14.Berger EA, Murphy PM, Farber JM. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol 17: 657–700, 1999. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 15.Capurro MI, Xu P, Shi W, Li F, Jia A, Filmus J. Glypican-3 inhibits Hedgehog signaling during development by competing with patched for Hedgehog binding. Dev Cell 14: 700–711, 2008. doi: 10.1016/j.devcel.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 16.Capurro MI, Li F, Filmus J. Overgrowth of a mouse model of Simpson–Golabi–Behmel syndrome is partly mediated by Indian hedgehog. EMBO Rep 10: 901–907, 2009. doi: 10.1038/embor.2009.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapraeger AC, Krufka A, Olwin BB. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 252: 1705–1708, 1991. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- 18.Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64: 841–848, 1991. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- 19.Couchman JR. Syndecans: proteoglycan regulators of cell-surface microdomains? Nat Rev Mol Cell Biol 4: 926–937, 2003. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 20.Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science 223: 1296–1299, 1984. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 21.Klagsbrun M, Shing Y. Heparin affinity of anionic and cationic capillary endothelial cell growth factors: analysis of hypothalamus-derived growth factors and fibroblast growth factors. Proc Natl Acad Sci USA 82: 805–809, 1985. doi: 10.1073/pnas.82.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sullivan R, Klagsbrun M. Purification of cartilage-derived growth factor by heparin affinity chromatography. J Biol Chem 260: 2399–2403, 1985. [PubMed] [Google Scholar]
- 23.Folkman J, Klagsbrun M, Sasse J, Wadzinski M, Ingber D, Vlodavsky I. A heparin-binding angiogenic protein—basic fibroblast growth factor—is stored within basement membrane. Am J Pathol 130: 393–400, 1988. [PMC free article] [PubMed] [Google Scholar]
- 24.Munaim SI, Klagsbrun M, Toole BP. Developmental changes in fibroblast growth factor in the chicken embryo limb bud. Proc Natl Acad Sci USA 85: 8091–8093, 1988. doi: 10.1073/pnas.85.21.8091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bashkin P, Doctrow S, Klagsbrun M, Svahn CM, Folkman J, Vlodavsky I. Basic fibroblast growth factor binds to subendothelial extracellular matrix and is released by heparitinase and heparin-like molecules. Biochemistry 28: 1737–1743, 1989. doi: 10.1021/bi00430a047. [DOI] [PubMed] [Google Scholar]
- 26.Seno M, Sasada R, Kurokawa T, Igarashi K. Carboxyl-terminal structure of basic fibroblast growth factor significantly contributes to its affinity for heparin. Eur J Biochem 188: 239–245, 1990. doi: 10.1111/j.1432-1033.1990.tb15395.x. [DOI] [PubMed] [Google Scholar]
- 27.Schlessinger J, Lax I, Lemmon M. Regulation of growth factor activation by proteoglycans: what is the role of the low affinity receptors? Cell 83: 357–360, 1995. doi: 10.1016/0092-8674(95)90112-4. [DOI] [PubMed] [Google Scholar]
- 28.Lin X, Buff EM, Perrimon N, Michelson AM. Heparan sulfate proteoglycans are essential for FGF receptor signaling during Drosophila embryonic development. Development 126: 3715–3723, 1999. doi: 10.1242/dev.126.17.3715. [DOI] [PubMed] [Google Scholar]
- 29.Venero Galanternik M, Kramer KL, Piotrowski T. Heparan sulfate proteoglycans regulate Fgf signaling and cell polarity during collective cell migration. Cell Rep 10: 414–428, 2015. doi: 10.1016/j.celrep.2014.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schultz V, Suflita M, Liu X, Zhang X, Yu Y, Li L, Green DE, Xu Y, Zhang F, DeAngelis PL, Liu J, Linhardt RJ. Heparan sulfate domains required for fibroblast growth factor 1 and 2 signaling through fibroblast growth factor receptor 1c. J Biol Chem 292: 2495–2509, 2017. doi: 10.1074/jbc.M116.761585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia J, Maccarana M, Zhang X, Bespalov M, Lindahl U, Li JP. Lack of L-iduronic acid in heparan sulfate affects interaction with growth factors and cell signaling. J Biol Chem 284: 15942–15950, 2009. doi: 10.1074/jbc.M809577200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reichsman F, Smith L, Cumberledge S. Glycosaminoglycans can modulate extracellular localization of the wingless protein and promote signal transduction. J Cell Biol 135: 819–827, 1996. doi: 10.1083/jcb.135.3.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bornemann DJ, Duncan JE, Staatz W, Selleck S, Warrior R. Abrogation of heparan sulfate synthesis in Drosophila disrupts the Wingless, Hedgehog and Decapentaplegic signaling pathways. Development 131: 1927–1938, 2004. doi: 10.1242/dev.01061. [DOI] [PubMed] [Google Scholar]
- 34.Grobe K, Inatani M, Pallerla SR, Castagnola J, Yamaguchi Y, Esko JD. Cerebral hypoplasia and craniofacial defects in mice lacking heparan sulfate Ndst1 gene function. Development 132: 3777–3786, 2005. doi: 10.1242/dev.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin X, Perrimon N. Dally cooperates with Drosophila Frizzled 2 to transduce Wingless signalling. Nature 400: 281–284, 1999. doi: 10.1038/22343. [DOI] [PubMed] [Google Scholar]
- 36.Tsuda M, Kamimura K, Nakato H, Archer M, Staatz W, Fox B, Humphrey M, Olson S, Futch T, Kaluza V, Siegfried E, Stam L, Selleck SB. The cell-surface proteoglycan Dally regulates Wingless signalling in Drosophila. Nature 400: 276–280, 1999. doi: 10.1038/22336. [DOI] [PubMed] [Google Scholar]
- 37.Baeg GH, Lin X, Khare N, Baumgartner S, Perrimon N. Heparan sulfate proteoglycans are critical for the organization of the extracellular distribution of Wingless. Development 128: 87–94, 2001. doi: 10.1242/dev.128.1.87. [DOI] [PubMed] [Google Scholar]
- 38.Pan J, Ho M. Role of glypican-1 in regulating multiple cellular signaling pathways. Am J Physiol Cell Physiol 321: C846–C858, 2021. doi: 10.1152/ajpcell.00290.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi W, Kaneiwa T, Cydzik M, Gariepy J, Filmus J. Glypican-6 stimulates intestinal elongation by simultaneously regulating Hedgehog and non-canonical Wnt signaling. Matrix Biol 88: 19–32, 2020. doi: 10.1016/j.matbio.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Capurro M, Martin T, Shi W, Filmus J. Glypican-3 binds to Frizzled and plays a direct role in the stimulation of canonical Wnt signaling. J Cell Sci 127: 1565–1575, 2014. doi: 10.1242/jcs.140871. [DOI] [PubMed] [Google Scholar]
- 41.Capurro MI, Xiang YY, Lobe C, Filmus J. Glypican-3 promotes the growth of hepatocellular carcinoma by stimulating canonical Wnt signaling. Cancer Res 65: 6245–6254, 2005. doi: 10.1158/0008-5472.CAN-04-4244. [DOI] [PubMed] [Google Scholar]
- 42.Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat Genet 25: 329–332, 2000. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- 43.O’Connell MP, Fiori JL, Kershner EK, Frank BP, Indig FE, Taub DD, Hoek KS, Weeraratna AT. Heparan sulfate proteoglycan modulation of Wnt5A signal transduction in metastatic melanoma cells. J Biol Chem 284: 28704–28712, 2009. doi: 10.1074/jbc.M109.028498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson de Sousa Brito FM, Butcher A, Pisconti A, Poulet B, Prior A, Charlesworth G, Sperinck C, Scotto di Mase M, Liu K, Bou-Gharios G, Jurgen van ’t Hof R, Daroszewska A. Syndecan-3 enhances anabolic bone formation through WNT signaling. FASEB J 35: e21246, 2021. doi: 10.1096/fj.202002024R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McGough IJ, Vecchia L, Bishop B, Malinauskas T, Beckett K, Joshi D, O’Reilly N, Siebold C, Jones EY, Vincent JP. Glypicans shield the Wnt lipid moiety to enable signalling at a distance. Nature 585: 85–90, 2020. doi: 10.1038/s41586-020-2498-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan D, Wu Y, Feng Y, Lin SC, Lin X. The core protein of glypican Dally-like determines its biphasic activity in wingless morphogen signaling. Dev Cell 17: 470–481, 2009. doi: 10.1016/j.devcel.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Voort R, Taher TE, Wielenga VJ, Spaargaren M, Prevo R, Smit L, David G, Hartmann G, Gherardi E, Pals ST. Heparan sulfate-modified CD44 promotes hepatocyte growth factor/scatter factor-induced signal transduction through the receptor tyrosine kinase c-Met. J Biol Chem 274: 6499–6506, 1999. doi: 10.1074/jbc.274.10.6499. [DOI] [PubMed] [Google Scholar]
- 48.Volz Y, Koschut D, Matzke-Ogi A, Dietz MS, Karathanasis C, Richert L, Wagner MG, Mély Y, Heilemann M, Niemann HH, Orian-Rousseau V. Direct binding of hepatocyte growth factor and vascular endothelial growth factor to CD44v6. Biosci Rep 35, 2015. doi: 10.1042/BSR20150093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derksen PW, Keehnen RM, Evers LM, van Oers MH, Spaargaren M, Pals ST. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood 99: 1405–1410, 2002. doi: 10.1182/blood.v99.4.1405. [DOI] [PubMed] [Google Scholar]
- 50.Ramani VC, Yang Y, Ren Y, Nan L, Sanderson RD. Heparanase plays a dual role in driving hepatocyte growth factor (HGF) signaling by enhancing HGF expression and activity. J Biol Chem 286: 6490–6499, 2011. doi: 10.1074/jbc.M110.183277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gao W, Kim H, Ho M. Human monoclonal antibody targeting the heparan sulfate chains of glypican-3 inhibits HGF-mediated migration and motility of hepatocellular carcinoma cells. PLoS One 10: e0137664, 2015. doi: 10.1371/journal.pone.0137664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ugolini S, Mondor I, Sattentau QJ. HIV-1 attachment: another look. Trends Microbiol 7: 144–149, 1999. doi: 10.1016/s0966-842x(99)01474-2. [DOI] [PubMed] [Google Scholar]
- 53.Nazli A, Kafka JK, Ferreira VH, Anipindi V, Mueller K, Osborne BJ, Dizzell S, Chauvin S, Mian MF, Ouellet M, Tremblay MJ, Mossman KL, Ashkar AA, Kovacs C, Bowdish DM, Snider DP, Kaul R, Kaushic C. HIV-1 gp120 induces TLR2- and TLR4-mediated innate immune activation in human female genital epithelium. J Immunol 191: 4246–4258, 2013. doi: 10.4049/jimmunol.1301482. [DOI] [PubMed] [Google Scholar]
- 54.Brennan TV, Lin L, Huang X, Cardona DM, Li Z, Dredge K, Chao NJ, Yang Y. Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD after allogeneic stem cell transplantation. Blood 120: 2899–2908, 2012. doi: 10.1182/blood-2011-07-368720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bugatti A, Giagulli C, Urbinati C, Caccuri F, Chiodelli P, Oreste P, Fiorentini S, Orro A, Milanesi L, D’Ursi P, Caruso A, Rusnati M. Molecular interaction studies of HIV-1 matrix protein p17 and heparin: identification of the heparin-binding motif of p17 as a target for the development of multitarget antagonists. J Biol Chem 288: 1150–1161, 2013. doi: 10.1074/jbc.M112.400077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bugatti A, Paiardi G, Urbinati C, Chiodelli P, Orro A, Uggeri M, Milanesi L, Caruso A, Caccuri F, D’Ursi P, Rusnati M. Heparin and heparan sulfate proteoglycans promote HIV-1 p17 matrix protein oligomerization: computational, biochemical and biological implications. Sci Rep 9: 15768, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Renga B, Francisci D, Schiaroli E, Carino A, Cipriani S, D’Amore C, Sidoni A, Sordo RD, Ferri I, Lucattelli M, Lunghi B, Baldelli F, Fiorucci S. The HIV matrix protein p17 promotes the activation of human hepatic stellate cells through interactions with CXCR2 and Syndecan-2. PLoS One 9: e94798, 2014. doi: 10.1371/journal.pone.0094798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rusnati M, Coltrini D, Oreste P, Zoppetti G, Albini A, Noonan D, d’Adda di Fagagna F, Giacca M, Presta M. Interaction of HIV-1 Tat protein with heparin. Role of the backbone structure, sulfation, and size. J Biol Chem 272: 11313–11320, 1997. doi: 10.1074/jbc.272.17.11313. [DOI] [PubMed] [Google Scholar]
- 59.Rusnati M, Tulipano G, Spillmann D, Tanghetti E, Oreste P, Zoppetti G, Giacca M, Presta M. Multiple interactions of HIV-1 Tat protein with size-defined heparin oligosaccharides. J Biol Chem 274: 28198–28205, 1999. doi: 10.1074/jbc.274.40.28198. [DOI] [PubMed] [Google Scholar]
- 60.Imamura J, Suzuki Y, Gonda K, Roy CN, Gatanaga H, Ohuchi N, Higuchi H. Single particle tracking confirms that multivalent Tat protein transduction domain-induced heparan sulfate proteoglycan cross-linkage activates Rac1 for internalization. J Biol Chem 286: 10581–10592, 2011. doi: 10.1074/jbc.M110.187450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyagi M, Rusnati M, Presta M, Giacca M. Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J Biol Chem 276: 3254–3261, 2001. doi: 10.1074/jbc.M006701200. [DOI] [PubMed] [Google Scholar]
- 62.Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin-dependent endocytosis and heparan sulfate receptors. J Biol Chem 280: 15300–15306, 2005. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- 63.Urbinati C, Bugatti A, Giacca M, Schlaepfer D, Presta M, Rusnati M. α(v)β3-integrin-dependent activation of focal adhesion kinase mediates NF-kappaB activation and motogenic activity by HIV-1 Tat in endothelial cells. J Cell Sci 118: 3949–3958, 2005. doi: 10.1242/jcs.02518. [DOI] [PubMed] [Google Scholar]
- 64.Urbinati C, Nicoli S, Giacca M, David G, Fiorentini S, Caruso A, Alfano M, Cassetta L, Presta M, Rusnati M. HIV-1 Tat and heparan sulfate proteoglycan interaction: a novel mechanism of lymphocyte adhesion and migration across the endothelium. Blood 114: 3335–3342, 2009. doi: 10.1182/blood-2009-01-198945. [DOI] [PubMed] [Google Scholar]
- 65.Koda JE, Rapraeger A, Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells. Cell surface proteoglycan as a receptor for interstitial collagens. J Biol Chem 260: 8157–8162, 1985. [PubMed] [Google Scholar]
- 66.Vuoriluoto K, Jokinen J, Kallio K, Salmivirta M, Heino J, Ivaska J. Syndecan-1 supports integrin α2β1-mediated adhesion to collagen. Exp Cell Res 314: 3369–3381, 2008. doi: 10.1016/j.yexcr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 67.Ishikawa T, Kramer RH. Sdc1 negatively modulates carcinoma cell motility and invasion. Exp Cell Res 316: 951–965, 2010. doi: 10.1016/j.yexcr.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Saunders S, Bernfield M. Cell surface proteoglycan binds mouse mammary epithelial cells to fibronectin and behaves as a receptor for interstitial matrix. J Cell Biol 106: 423–430, 1988. doi: 10.1083/jcb.106.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Woods A, Longley RL, Tumova S, Couchman JR. Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch Biochem Biophys 374: 66–72, 2000. doi: 10.1006/abbi.1999.1607. [DOI] [PubMed] [Google Scholar]
- 70.Mostafavi-Pour Z, Askari JA, Parkinson SJ, Parker PJ, Ng TT, Humphries MJ. Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J Cell Biol 161: 155–167, 2003. doi: 10.1083/jcb.200210176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun X, Mosher DF, Rapraeger A. Heparan sulfate-mediated binding of epithelial cell surface proteoglycan to thrombospondin. J Biol Chem 264: 2885–2889, 1989. [PubMed] [Google Scholar]
- 72.Carulli S, Beck K, Dayan G, Boulesteix S, Lortat-Jacob H, Rousselle P. Cell surface proteoglycans syndecan-1 and -4 bind overlapping but distinct sites in laminin α3 LG45 protein domain. J Biol Chem 287: 12204–12216, 2012. doi: 10.1074/jbc.M111.300061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J Cell Biol 167: 171–181, 2004. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Broekelmann TJ, Kozel BA, Ishibashi H, Werneck CC, Keeley FW, Zhang L, Mecham RP. Tropoelastin interacts with cell-surface glycosaminoglycans via its COOH-terminal domain. J Biol Chem 280: 40939–40947, 2005. doi: 10.1074/jbc.M507309200. [DOI] [PubMed] [Google Scholar]
- 75.Ritty TM, Broekelmann TJ, Werneck CC, Mecham RP. Fibrillin-1 and -2 contain heparin-binding sites important for matrix deposition and that support cell attachment. Biochem J 375: 425–432, 2003. doi: 10.1042/BJ20030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salmivirta M, Elenius K, Vainio S, Hofer U, Chiquet-Ehrismann R, Thesleff I, Jalkanen M. Syndecan from embryonic tooth mesenchyme binds tenascin. J Biol Chem 266: 7733–7739, 1991. [PubMed] [Google Scholar]
- 77.Tumova S, Woods A, Couchman JR. Heparan sulfate chains from glypican and syndecans bind the Hep II domain of fibronectin similarly despite minor structural differences. J Biol Chem 275: 9410–9417, 2000. doi: 10.1074/jbc.275.13.9410. [DOI] [PubMed] [Google Scholar]
- 78.Woods A, Couchman JR. Syndecan 4 heparan sulfate proteoglycan is a selectively enriched and widespread focal adhesion component. Mol Biol Cell 5: 183–192, 1994. doi: 10.1091/mbc.5.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Iba K, Albrechtsen R, Gilpin B, Frohlich C, Loechel F, Zolkiewska A, Ishiguro K, Kojima T, Liu W, Langford JK, Sanderson RD, Brakebusch C, Fassler R, Wewer UM. The cysteine-rich domain of human ADAM 12 supports cell adhesion through syndecans and triggers signaling events that lead to β1 integrin-dependent cell spreading. J Cell Biol 149: 1143–1156, 2000. doi: 10.1083/jcb.149.5.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Thodeti CK, Albrechtsen R, Grauslund M, Asmar M, Larsson C, Takada Y, Mercurio AM, Couchman JR, Wewer UM. ADAM12/syndecan-4 signaling promotes β 1 integrin-dependent cell spreading through protein kinase Cα and RhoA. J Biol Chem 278: 9576–9584, 2003. doi: 10.1074/jbc.M208937200. [DOI] [PubMed] [Google Scholar]
- 81.Singh P, Carraher C, Schwarzbauer JE. Assembly of fibronectin extracellular matrix. Annu Rev Cell Dev Biol 26: 397–419, 2010. doi: 10.1146/annurev-cellbio-100109-104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wolanska KI, Morgan MR. Fibronectin remodelling: cell-mediated regulation of the microenvironment. Biochem Soc Trans 43: 122–128, 2015. doi: 10.1042/BST20140313. [DOI] [PubMed] [Google Scholar]
- 83.McDonald JA. Extracellular matrix assembly. Annu Rev Cell Biol 4: 183–207, 1988. doi: 10.1146/annurev.cb.04.110188.001151. [DOI] [PubMed] [Google Scholar]
- 84.Raitman I, Huang ML, Williams SA, Friedman B, Godula K, Schwarzbauer JE. Heparin-fibronectin interactions in the development of extracellular matrix insolubility. Matrix Biol 67: 107–122, 2018. doi: 10.1016/j.matbio.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chung CY, Erickson HP. Glycosaminoglycans modulate fibronectin matrix assembly and are essential for matrix incorporation of tenascin-C. J Cell Sci 110: 1413–1419, 1997. doi: 10.1242/jcs.110.12.1413. [DOI] [PubMed] [Google Scholar]
- 86.Klass CM, Couchman JR, Woods A. Control of extracellular matrix assembly by syndecan-2 proteoglycan. J Cell Sci 113: 493–506, 2000. doi: 10.1242/jcs.113.3.493. [DOI] [PubMed] [Google Scholar]
- 87.Galante LL, Schwarzbauer JE. Requirements for sulfate transport and the diastrophic dysplasia sulfate transporter in fibronectin matrix assembly. J Cell Biol 179: 999–1009, 2007. doi: 10.1083/jcb.200707150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Arrington CB, Yost HJ. Extra-embryonic syndecan 2 regulates organ primordia migration and fibrillogenesis throughout the zebrafish embryo. Development 136: 3143–3152, 2009. doi: 10.1242/dev.031492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayashida K, Stahl PD, Park PW. Syndecan-1 ectodomain shedding is regulated by the small GTPase Rab5. J Biol Chem 283: 35435–35444, 2008. doi: 10.1074/jbc.M804172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jinno A, Hayashida A, Jenkins HF, Park PW. Syndecan-1 promotes Streptococcus pneumoniae corneal infection by facilitating the assembly of adhesive fibronectin fibrils. mBio 11: e01907–e01920, 2020. doi: 10.1128/mBio.01907-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ekblom P, Timpl R. Cell-to-cell contact and extracellular matrix. A multifaceted approach emerging. Curr Opin Cell Biol 8: 599–601, 1996. doi: 10.1016/s0955-0674(96)80099-8. [DOI] [PubMed] [Google Scholar]
- 92.Yurchenco PD. Integrating activities of laminins that drive basement membrane assembly and function. Curr Top Membr 76: 1–30, 2015. doi: 10.1016/bs.ctm.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Miner JH, Yurchenco PD. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol 20: 255–284, 2004. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 94.Smyth N, Vatansever HS, Murray P, Meyer M, Frie C, Paulsson M, Edgar D. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J Cell Biol 144: 151–160, 1999. doi: 10.1083/jcb.144.1.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cheng YS, Champliaud MF, Burgeson RE, Marinkovich MP, Yurchenco PD. Self-assembly of laminin isoforms. J Biol Chem 272: 31525–31532, 1997. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- 96.Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Curr Pharm Des 15: 1277–1294, 2009. doi: 10.2174/138161209787846766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li S, Edgar D, Fässler R, Wadsworth W, Yurchenco PD. The role of laminin in embryonic cell polarization and tissue organization. Dev Cell 4: 613–624, 2003. doi: 10.1016/s1534-5807(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 98.Katagiri F, Takeyama K, Ohga Y, Hozumi K, Kikkawa Y, Kadoya Y, Nomizu M. Amino acid sequence requirements of laminin β1 chain peptide B133 (DISTKYFQMSLE) for amyloid-like fibril formation, syndecan binding, and neurite outgrowth promotion. Biochemistry 49: 5909–5918, 2010. doi: 10.1021/bi100748s. [DOI] [PubMed] [Google Scholar]
- 99.Utani A, Nomizu M, Matsuura H, Kato K, Kobayashi T, Takeda U, Aota S, Nielsen PK, Shinkai H. A unique sequence of the laminin α 3 G domain binds to heparin and promotes cell adhesion through syndecan-2 and -4. J Biol Chem 276: 28779–28788, 2001. doi: 10.1074/jbc.M101420200. [DOI] [PubMed] [Google Scholar]
- 100.Michopoulou A, Montmasson M, Garnier C, Lambert E, Dayan G, Rousselle P. A novel mechanism in wound healing: laminin 332 drives MMP9/14 activity by recruiting syndecan-1 and CD44. Matrix Biol 94: 1–17, 2020. doi: 10.1016/j.matbio.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 101.Okamoto O, Bachy S, Odenthal U, Bernaud J, Rigal D, Lortat-Jacob H, Smyth N, Rousselle P. Normal human keratinocytes bind to the α3LG4/5 domain of unprocessed laminin-5 through the receptor syndecan-1. J Biol Chem 278: 44168–44177, 2003. doi: 10.1074/jbc.M300726200. [DOI] [PubMed] [Google Scholar]
- 102.Ogawa T, Tsubota Y, Hashimoto J, Kariya Y, Miyazaki K. The short arm of laminin gamma2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin β4 chain. Mol Biol Cell 18: 1621–1633, 2007. doi: 10.1091/mbc.e06-09-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sideek MA, Menz C, Parsi MK, Gibson MA. LTBP-2 competes with tropoelastin for binding to fibulin-5 and heparin, and is a negative modulator of elastinogenesis. Matrix Biol 34: 114–123, 2014. doi: 10.1016/j.matbio.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 104.Bochicchio B, Yeo GC, Lee P, Emul D, Pepe A, Laezza A, Ciarfaglia N, Quaglino D, Weiss AS. Domains 12 to 16 of tropoelastin promote cell attachment and spreading through interactions with glycosaminoglycan and integrins αV and α5β1. FEBS J 288: 4024–4038, 2021. doi: 10.1111/febs.15702. [DOI] [PubMed] [Google Scholar]
- 105.Bomsel M, Alfsen A. Entry of viruses through the epithelial barrier: pathogenic trickery. Nat Rev Mol Cell Biol 4: 57–68, 2003. doi: 10.1038/nrm1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Stroh LJ, Stehle T. Glycan engagement by viruses: receptor switches and specificity. Annu Rev Virol 1: 285–306, 2014. doi: 10.1146/annurev-virology-031413-085417. [DOI] [PubMed] [Google Scholar]
- 107.Teng YH, Aquino RS, Park PW. Molecular functions of syndecan-1 in disease. Matrix Biol 31: 3–16, 2012. doi: 10.1016/j.matbio.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smit JM, Waarts BL, Kimata K, Klimstra WB, Bittman R, Wilschut J. Adaptation of αviruses to heparan sulfate: interaction of Sindbis and Semliki forest viruses with liposomes containing lipid-conjugated heparin. J Virol 76: 10128–10137, 2002. doi: 10.1128/jvi.76.20.10128-10137.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cagno V, Tseligka ED, Jones ST, Tapparel C. Heparan sulfate proteoglycans and viral attachment: true receptors or adaptation bias? Viruses 11: 596, 2019. doi: 10.3390/v11070596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tuve S, Wang H, Jacobs JD, Yumul RC, Smith DF, Lieber A. Role of cellular heparan sulfate proteoglycans in infection of human adenovirus serotype 3 and 35. PLoS Pathog 4: e1000189, 2008. doi: 10.1371/journal.ppat.1000189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.de Witte L, Bobardt M, Chatterji U, Degeest G, David G, Geijtenbeek TB, Gallay P. Syndecan-3 is a dendritic cell-specific attachment receptor for HIV-1. Proc Natl Acad Sci USA 104: 19464–19469, 2007. doi: 10.1073/pnas.0703747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kondratowicz AS, Lennemann NJ, Sinn PL, Davey RA, Hunt CL, Moller-Tank S, Meyerholz DK, Rennert P, Mullins RF, Brindley M, Sandersfeld LM, Quinn K, Weller M, McCray PB Jr, Chiorini J, Maury W. T-cell immunoglobulin and mucin domain 1 (TIM-1) is a receptor for Zaire Ebolavirus and Lake Victoria Marburgvirus. Proc Natl Acad Sci USA 108: 8426–8431, 2011. doi: 10.1073/pnas.1019030108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tamhankar M, Gerhardt DM, Bennett RS, Murphy N, Jahrling PB, Patterson JL. Heparan sulfate is an important mediator of Ebola virus infection in polarized epithelial cells. Virol J 15: 135, 2018. doi: 10.1186/s12985-018-1045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Clausen TM, Sandoval DR, Spliid CB, Pihl J, Perrett HR, Painter CD, et al. SARS-CoV-2 infection depends on cellular heparan sulfate and ACE2. Cell 183: 1043–1057.e5, 2020. doi: 10.1016/j.cell.2020.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bermejo-Jambrina M, Eder J, Kaptein TM, van Hamme JL, Helgers LC, Vlaming KE, Brouwer PJM, van Nuenen AC, Spaargaren M, de Bree GJ, Nijmeijer BM, Kootstra NA, van Gils MJ, Sanders RW, Geijtenbeek TBH. Infection and transmission of SARS-CoV-2 depend on heparan sulfate proteoglycans. EMBO J 40: e106765, 2021. doi: 10.15252/embj.2020106765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Paiardi G, Richter S, Oreste P, Urbinati C, Rusnati M, Wade RC. The binding of heparin to spike glycoprotein inhibits SARS-CoV-2 infection by three mechanisms. J Biol Chem 298: 101507, 2022. doi: 10.1016/j.jbc.2021.101507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tandon R, Sharp JS, Zhang F, Pomin VH, Ashpole NM, Mitra D, McCandless MG, Jin W, Liu H, Sharma P, Linhardt RJ. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. J Virol 95: e01987-20, 2021. doi: 10.1128/JVI.01987-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sholzberg M, Tang GH, Rahhal H, AlHamzah M, Kreuziger LB, Áinle FN, et al. Effectiveness of therapeutic heparin versus prophylactic heparin on death, mechanical ventilation, or intensive care unit admission in moderately ill patients with covid-19 admitted to hospital: RAPID randomised clinical trial. BMJ 375: n2400, 2021. doi: 10.1136/bmj.n2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Spyropoulos AC, Goldin M, Giannis D, Diab W, Wang J, Khanijo S, Mignatti A, Gianos E, Cohen M, Sharifova G, Lund JM, Tafur A, Lewis PA, Cohoon KP, Rahman H, Sison CP, Lesser ML, Ochani K, Agrawal N, Hsia J, Anderson VE, Bonaca M, Halperin JL, Weitz JI; HEP-COVID Investigators. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med 181: 1612–1620, 2021. doi: 10.1001/jamainternmed.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pereyra D, Heber S, Schrottmaier WC, Santol J, Pirabe A, Schmuckenschlager A, Kammerer K, Ammon D, Sorz T, Fritsch F, Hayden H, Pawelka E, Krüger P, Rumpf B, Traugott MT, Glaser P, Firbas C, Schörgenhofer C, Seitz T, Karolyi M, Pabinger I, Brostjan C, Starlinger P, Weiss G, Bellmann-Weiler R, Salzer HJF, Jilma B, Zoufaly A, Assinger A. Low-molecular-weight heparin use in coronavirus disease 2019 is associated with curtailed viral persistence: a retrospective multicentre observational study. Cardiovasc Res 117: 2807–2820, 2021. doi: 10.1093/cvr/cvab308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, et al., REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med 385: 790–802, 2021. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med 385: 777–789, 2021. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Merad M, Subramanian A, Wang TT. An aberrant inflammatory response in severe COVID-19. Cell host Microbe 29: 1043–1047, 2021. doi: 10.1016/j.chom.2021.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lowery SA, Sariol A, Perlman S. Innate immune and inflammatory responses to SARS-CoV-2: Implications for COVID-19. Cell host Microbe 29: 1052–1062, 2021. doi: 10.1016/j.chom.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hauck CR, Agerer F, Muenzner P, Schmitter T. Cellular adhesion molecules as targets for bacterial infection. Eur J Cell Biol 85: 235–242, 2006. doi: 10.1016/j.ejcb.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 126.Isaacs RD. Borrelia burgdorferi bind to epithelial proteoglycan. J Clin Invest 93: 809–819, 1994. doi: 10.1172/JCI117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coburn J, Magoun L, Bodary SC, Leong JM. Integrins α(v)β3 and α5β1 mediate attachment of lyme disease spirochetes to human cells. Infect Immun 66: 1946–1952, 1998. doi: 10.1128/IAI.66.5.1946-1952.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell 84: 923–932, 1996. doi: 10.1016/S0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 129.Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol Biol Cell 6: 559–576, 1995. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jonquieres R, Pizarro-Cerda J, Cossart P. Synergy between the N- and C-terminal domains of InlB for efficient invasion of non-phagocytic cells by Listeria monocytogenes. Mol Microbiol 42: 955–965, 2001. doi: 10.1046/j.1365-2958.2001.02704.x. [DOI] [PubMed] [Google Scholar]
- 131.Banerjee M, Copp J, Vuga D, Marino M, Chapman T, van der Geer P, Ghosh P. GW domains of the Listeria monocytogenes invasion protein InlB are required for potentiation of Met activation. Mol Microbiol 52: 257–271, 2004. doi: 10.1111/j.1365-2958.2003.03968.x. [DOI] [PubMed] [Google Scholar]
- 132.Hrtska SC, Kemp MM, Munoz EM, Azizad O, Banerjee M, Raposo C, Kumaran J, Ghosh P, Linhardt RJ. Investigation of the mechanism of binding between internalin B and heparin using surface plasmon resonance. Biochemistry 46: 2697–2706, 2007. doi: 10.1021/bi062021x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shen Y, Naujokas M, Park M, Ireton K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103: 501–510, 2000. doi: 10.1016/s0092-8674(00)00141-0. [DOI] [PubMed] [Google Scholar]
- 134.Braun L, Ghebrehiwet B, Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J 19: 1458–1466, 2000. doi: 10.1093/emboj/19.7.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Veiga E, Cossart P. Listeria hijacks the clathrin-dependent endocytic machinery to invade mammalian cells. Nat Cell Biol 7: 894–900, 2005. doi: 10.1038/ncb1292. [DOI] [PubMed] [Google Scholar]
- 136.Niemann HH, Jager V, Butler PJ, van den Heuvel J, Schmidt S, Ferraris D, Gherardi E, Heinz DW. Structure of the human receptor tyrosine kinase met in complex with the Listeria invasion protein InlB. Cell 130: 235–246, 2007. doi: 10.1016/j.cell.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 137.Huhtala MT, Pentikäinen OT, Johnson MS. A dimeric ternary complex of FGFR [correction of FGFR1], heparin and FGF-1 leads to an ‘electrostatic sandwich’ model for heparin binding. Structure 7: 699–709, 1999. doi: 10.1016/S0969-2126(99)80091-4. [DOI] [PubMed] [Google Scholar]
- 138.Fukuhara N, Howitt JA, Hussain SA, Hohenester E. Structural and functional analysis of slit and heparin binding to immunoglobulin-like domains 1 and 2 of Drosophila Robo. J Biol Chem 283: 16226–16234, 2008. doi: 10.1074/jbc.M800688200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ronca F, Andersen JS, Paech V, Margolis RU. Characterization of Slit protein interactions with glypican-1. J Biol Chem 276: 29141–29147, 2001. doi: 10.1074/jbc.M100240200. [DOI] [PubMed] [Google Scholar]
- 140.Johnson KG, Ghose A, Epstein E, Lincecum J, O’Connor MB, Van Vactor D. Axonal heparan sulfate proteoglycans regulate the distribution and efficiency of the repellent slit during midline axon guidance. Curr Biol 14: 499–504, 2004. doi: 10.1016/j.cub.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 141.Urbinati C, Grillo E, Chiodelli P, Tobia C, Caccuri F, Fiorentini S, David G, Rusnati M. Syndecan-1 increases B-lymphoid cell extravasation in response to HIV-1 Tat via α(v)β(3)/pp60src/pp125FAK pathway. Oncogene 36: 2609–2618, 2017. doi: 10.1038/onc.2016.420. [DOI] [PubMed] [Google Scholar]
- 142.Wilson NH, Stoeckli ET. Sonic hedgehog regulates its own receptor on postcrossing commissural axons in a glypican1-dependent manner. Neuron 79: 478–491, 2013. doi: 10.1016/j.neuron.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 143.Li F, Shi W, Capurro M, Filmus J. Glypican-5 stimulates rhabdomyosarcoma cell proliferation by activating Hedgehog signaling. J Cell Biol 192: 691–704, 2011. doi: 10.1083/jcb.201008087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Witt RM, Hecht ML, Pazyra-Murphy MF, Cohen SM, Noti C, van Kuppevelt TH, Fuller M, Chan JA, Hopwood JJ, Seeberger PH, Segal RA. Heparan sulfate proteoglycans containing a glypican 5 core and 2-O-sulfo-iduronic acid function as Sonic Hedgehog co-receptors to promote proliferation. J Biol Chem 288: 26275–26288, 2013. doi: 10.1074/jbc.M112.438937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yan D, Wu Y, Yang Y, Belenkaya TY, Tang X, Lin X. The cell-surface proteins Dally-like and Ihog differentially regulate Hedgehog signaling strength and range during development. Development 137: 2033–2044, 2010. doi: 10.1242/dev.045740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Capurro M, Izumikawa T, Suarez P, Shi W, Cydzik M, Kaneiwa T, Gariepy J, Bonafe L, Filmus J. Glypican-6 promotes the growth of developing long bones by stimulating Hedgehog signaling. J Cell Biol 216: 2911–2926, 2017. doi: 10.1083/jcb.201605119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stenvers KL, Tursky ML, Harder KW, Kountouri N, Amatayakul-Chantler S, Grail D, Small C, Weinberg RA, Sizeland AM, Zhu HJ. Heart and liver defects and reduced transforming growth factor β2 sensitivity in transforming growth factor β type III receptor-deficient embryos. Mol Cell Biol 23: 4371–4385, 2003. doi: 10.1128/MCB.23.12.4371-4385.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarraj MA, Escalona RM, Umbers A, Chua HK, Small C, Griswold M, Loveland K, Findlay JK, Stenvers KL. Fetal testis dysgenesis and compromised Leydig cell function in Tgfbr3 (beta glycan) knockout mice. Biol Reprod 82: 153–162, 2010. doi: 10.1095/biolreprod.109.078766. [DOI] [PubMed] [Google Scholar]
- 149.Walker KA, Sims-Lucas S, Caruana G, Cullen-McEwen L, Li J, Sarraj MA, Bertram JF, Stenvers KL. Betaglycan is required for the establishment of nephron endowment in the mouse. PLoS One 6: e18723, 2011. doi: 10.1371/journal.pone.0018723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Aleman-Muench GR, Mendoza V, Stenvers K, Garcia-Zepeda EA, Lopez-Casillas F, Raman C, Soldevila G. Betaglycan (TβRIII) is expressed in the thymus and regulates T cell development by protecting thymocytes from apoptosis. PLoS One 7: e44217, 2012. doi: 10.1371/journal.pone.0044217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cheifetz S, Hernandez H, Laiho M, ten Dijke P, Iwata KK, Massagué J. Distinct transforming growth factor-β (TGF-β) receptor subsets as determinants of cellular responsiveness to three TGF-β isoforms. J Biol Chem 265: 20533–20538, 1990. [PubMed] [Google Scholar]
- 152.Sanford LP, Ormsby I, Gittenberger-de Groot AC, Sariola H, Friedman R, Boivin GP, Cardell EL, Doetschman T. TGFβ2 knockout mice have multiple developmental defects that are non-overlapping with other TGFβ knockout phenotypes. Development 124: 2659–2670, 1997. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zioncheck TF, Richardson L, Liu J, Chang L, King KL, Bennett GL, Fügedi P, Chamow SM, Schwall RH, Stack RJ. Sulfated oligosaccharides promote hepatocyte growth factor association and govern its mitogenic activity. J Biol Chem 270: 16871–16878, 1995. doi: 10.1074/jbc.270.28.16871. [DOI] [PubMed] [Google Scholar]
- 154.Ingold K, Zumsteg A, Tardivel A, Huard B, Steiner QG, Cachero TG, Qiang F, Gorelik L, Kalled SL, Acha-Orbea H, Rennert PD, Tschopp J, Schneider P. Identification of proteoglycans as the APRIL-specific binding partners. J Exp Med 201: 1375–1383, 2005. doi: 10.1084/jem.20042309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dyer DP, Salanga CL, Volkman BF, Kawamura T, Handel TM. The dependence of chemokine-glycosaminoglycan interactions on chemokine oligomerization. Glycobiology 26: 312–326, 2016. doi: 10.1093/glycob/cwv100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rajasekaran D, Keeler C, Syed MA, Jones MC, Harrison JK, Wu D, Bhandari V, Hodsdon ME, Lolis EJ. A model of GAG/MIP-2/CXCR2 interfaces and its functional effects. Biochemistry 51: 5642–5654, 2012. doi: 10.1021/bi3001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sepuru KM, Rajarathnam K. Structural basis of a chemokine heterodimer binding to glycosaminoglycans. Biochem J 478: 1009–1021, 2021. doi: 10.1042/BCJ20200927. [DOI] [PubMed] [Google Scholar]
- 158.Pontejo SM, Murphy PM. Two glycosaminoglycan-binding domains of the mouse cytomegalovirus-encoded chemokine MCK-2 are critical for oligomerization of the full-length protein. J Biol Chem 292: 9613–9626, 2017. doi: 10.1074/jbc.M117.785121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Johnson KG, Tenney AP, Ghose A, Duckworth AM, Higashi ME, Parfitt K, Marcu O, Heslip TR, Marsh JL, Schwarz TL, Flanagan JG, Van Vactor D. The HSPGs Syndecan and Dallylike bind the receptor phosphatase LAR and exert distinct effects on synaptic development. Neuron 49: 517–531, 2006. doi: 10.1016/j.neuron.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 160.Capurro M, Wanless IR, Sherman M, Deboer G, Shi W, Miyoshi E, Filmus J. Glypican-3: a novel serum and histochemical marker for hepatocellular carcinoma. Gastroenterology 125: 89–97, 2003. doi: 10.1016/s0016-5085(03)00689-9. [DOI] [PubMed] [Google Scholar]
- 161.Li L, Jin R, Zhang X, Lv F, Liu L, Liu D, Liu K, Li N, Chen D. Oncogenic activation of glypican-3 by c-Myc in human hepatocellular carcinoma. Hepatology 56: 1380–1390, 2012. doi: 10.1002/hep.25891. [DOI] [PubMed] [Google Scholar]
- 162.Zittermann SI, Capurro MI, Shi W, Filmus J. Soluble glypican 3 inhibits the growth of hepatocellular carcinoma in vitro and in vivo. Int J Cancer 126: 1291–1301, 2010. doi: 10.1002/ijc.24941. [DOI] [PubMed] [Google Scholar]
- 163.Kuo WJ, Digman MA, Lander AD. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell 21: 4028–4041, 2010. doi: 10.1091/mbc.E10-04-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu D, Young JH, Krahn JM, Song D, Corbett KD, Chazin WJ, Pedersen LC, Esko JD. Stable RAGE-heparan sulfate complexes are essential for signal transduction. ACS Chem Biol 8: 1611–1620, 2013. doi: 10.1021/cb4001553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jakobsson L, Kreuger J, Holmborn K, Lundin L, Eriksson I, Kjellén L, Claesson-Welsh L. Heparan sulfate in trans potentiates VEGFR-mediated angiogenesis. Dev Cell 10: 625–634, 2006. doi: 10.1016/j.devcel.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 166.Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell 2: 115–124, 2002. doi: 10.1016/s1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 167.Bobardt MD, Saphire AC, Hung HC, Yu X, Van der Schueren B, Zhang Z, David G, Gallay PA. Syndecan captures, protects, and transmits HIV to T lymphocytes. Immunity 18: 27–39, 2003. doi: 10.1016/S1074-7613(02)00504-6. [DOI] [PubMed] [Google Scholar]
- 168.de Parseval A, Bobardt MD, Chatterji A, Chatterji U, Elder JH, David G, Zolla-Pazner S, Farzan M, Lee TH, Gallay PA. A highly conserved arginine in gp120 governs HIV-1 binding to both syndecans and CCR5 via sulfated motifs. J Biol Chem 280: 39493–39504, 2005. doi: 10.1074/jbc.M504233200. [DOI] [PubMed] [Google Scholar]
- 169.Zhou X, Vachon C, Cizeron M, Romatif O, Bülow HE, Jospin M, Bessereau JL. The HSPG syndecan is a core organizer of cholinergic synapses. J Cell Biol 220: e202011144, 2021. doi: 10.1083/jcb.202011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses. J Cell Biol 142: 139–151, 1998. doi: 10.1083/jcb.142.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang K, Zhang H, Ma D, Bucan M, Glessner JT, Abrahams BS, et al. Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459: 528–533, 2009. doi: 10.1038/nature07999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Irie F, Badie-Mahdavi H, Yamaguchi Y. Autism-like socio-communicative deficits and stereotypies in mice lacking heparan sulfate. Proc Natl Acad Sci USA 109: 5052–5056, 2012. doi: 10.1073/pnas.1117881109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lim ST, Longley RL, Couchman JR, Woods A. Direct binding of syndecan-4 cytoplasmic domain to the catalytic domain of protein kinase C α (PKC α) increases focal adhesion localization of PKC α. J Biol Chem 278: 13795–13802, 2003. doi: 10.1074/jbc.M208300200. [DOI] [PubMed] [Google Scholar]
- 174.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from α5β1 integrin and syndecan-4. J Cell Biol 181: 1013–1026, 2008. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sperinde GV, Nugent MA. Mechanisms of fibroblast growth factor 2 intracellular processing: a kinetic analysis of the role of heparan sulfate proteoglycans. Biochemistry 39: 3788–3796, 2000. doi: 10.1021/bi992243d. [DOI] [PubMed] [Google Scholar]
- 176.Pilia G, Hughes-Benzie RM, MacKenzie A, Baybayan P, Chen EY, Huber R, Neri G, Cao A, Forabosco A, Schlessinger D. Mutations in GPC3, a glypican gene, cause the Simpson-Golabi-Behmel overgrowth syndrome. Nat Genet 12: 241–247, 1996. doi: 10.1038/ng0396-241. [DOI] [PubMed] [Google Scholar]
- 177.Cano-Gauci DF, Song HH, Yang H, McKerlie C, Choo B, Shi W, Pullano R, Piscione TD, Grisaru S, Soon S, Sedlackova L, Tanswell AK, Mak TW, Yeger H, Lockwood GA, Rosenblum ND, Filmus J. Glypican-3-deficient mice exhibit developmental overgrowth and some of the abnormalities typical of Simpson–Golabi–Behmel syndrome. J Cell Biol 146: 255–264, 1999. doi: 10.1083/jcb.146.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Valdembri D, Caswell PT, Anderson KI, Schwarz JP, König I, Astanina E, Caccavari F, Norman JC, Humphries MJ, Bussolino F, Serini G. Neuropilin-1/GIPC1 signaling regulates α5β1 integrin traffic and function in endothelial cells. PLoS Biol 7: e25, 2009. doi: 10.1371/journal.pbio.1000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yan D, Lin X. Shaping morphogen gradients by proteoglycans. Cold Spring Harb Perspect Biol 1: a002493, 2009. doi: 10.1101/cshperspect.a002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fuerer C, Habib SJ, Nusse R. A study on the interactions between heparan sulfate proteoglycans and Wnt proteins. Dev Dyn 239: 184–190, 2010. doi: 10.1002/dvdy.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Makarenkova HP, Hoffman MP, Beenken A, Eliseenkova AV, Meech R, Tsau C, Patel VN, Lang RA, Mohammadi M. Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci Signal 2: ra55, 2009. doi: 10.1126/scisignal.2000304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kornberg TB, Roy S. Cytonemes as specialized signaling filopodia. Development 141: 729–736, 2014. doi: 10.1242/dev.086223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Middleton J, Neil S, Wintle J, Clark-Lewis I, Moore H, Lam C, Auer M, Hub E, Rot A. Transcytosis and surface presentation of IL-8 by venular endothelial cells. Cell 91: 385–395, 1997. doi: 10.1016/s0092-8674(00)80422-5. [DOI] [PubMed] [Google Scholar]
- 184.Handel TM, Johnson Z, Crown SE, Lau EK, Proudfoot AE. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu Rev Biochem 74: 385–410, 2005. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- 185.Crick F. Diffusion in embryogenesis. Nature 225: 420–422, 1970. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- 186.Li P, Markson JS, Wang S, Chen S, Vachharajani V, Elowitz MB. Morphogen gradient reconstitution reveals Hedgehog pathway design principles. Science 360: 543–548, 2018. doi: 10.1126/science.aao0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Gross JC, Chaudhary V, Bartscherer K, Boutros M. Active Wnt proteins are secreted on exosomes. Nat Cell Biol 14: 1036–1045, 2012. doi: 10.1038/ncb2574. [DOI] [PubMed] [Google Scholar]
- 188.Mii Y, Takada S. Heparan sulfate proteoglycan clustering in Wnt signaling and dispersal. Front Cell Dev Biol 8: 631, 2020. doi: 10.3389/fcell.2020.00631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Shilo BZ, Haskel-Ittah M, Ben-Zvi D, Schejter ED, Barkai N. Creating gradients by morphogen shuttling. Trends Genet 29: 339–347, 2013. doi: 10.1016/j.tig.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 190.Bischoff M, Gradilla AC, Seijo I, Andrés G, Rodríguez-Navas C, González-Méndez L, Guerrero I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol 15: 1269–1281, 2013. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Han C, Yan D, Belenkaya TY, Lin X. Drosophila glypicans Dally and Dally-like shape the extracellular Wingless morphogen gradient in the wing disc. Development 132: 667–679, 2005. doi: 10.1242/dev.01636. [DOI] [PubMed] [Google Scholar]
- 192.Chen Y, Mohammadi M, Flanagan JG. Graded levels of FGF protein span the midbrain and can instruct graded induction and repression of neural mapping labels. Neuron 62: 773–780, 2009. doi: 10.1016/j.neuron.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Duchesne L, Octeau V, Bearon RN, Beckett A, Prior IA, Lounis B, Fernig DG. Transport of fibroblast growth factor 2 in the pericellular matrix is controlled by the spatial distribution of its binding sites in heparan sulfate. PLoS Biol 10: e1001361, 2012. doi: 10.1371/journal.pbio.1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Shimokawa K, Kimura-Yoshida C, Nagai N, Mukai K, Matsubara K, Watanabe H, Matsuda Y, Mochida K, Matsuo I. Cell surface heparan sulfate chains regulate local reception of FGF signaling in the mouse embryo. Dev Cell 21: 257–272, 2011. doi: 10.1016/j.devcel.2011.06.027. [DOI] [PubMed] [Google Scholar]
- 195.Izvolsky KI, Shoykhet D, Yang Y, Yu Q, Nugent MA, Cardoso WV. Heparan sulfate-FGF10 interactions during lung morphogenesis. Dev Biol 258: 185–200, 2003. doi: 10.1016/s0012-1606(03)00114-3. [DOI] [PubMed] [Google Scholar]
- 196.Han C, Belenkaya TY, Wang B, Lin X. Drosophila glypicans control the cell-to-cell movement of Hedgehog by a dynamin-independent process. Development 131: 601–611, 2004. doi: 10.1242/dev.00958. [DOI] [PubMed] [Google Scholar]
- 197.Gradilla AC, González E, Seijo I, Andrés G, Bischoff M, González-Mendez L, Sánchez V, Callejo A, Ibáñez C, Guerra M, Ortigão-Farias JR, Sutherland JD, González M, Barrio R, Falcón-Pérez JM, Guerrero I. Exosomes as Hedgehog carriers in cytoneme-mediated transport and secretion. Nat Commun 5: 5649, 2014. doi: 10.1038/ncomms6649. [DOI] [PubMed] [Google Scholar]
- 198.Baietti MF, Zhang Z, Mortier E, Melchior A, Degeest G, Geeraerts A, Ivarsson Y, Depoortere F, Coomans C, Vermeiren E, Zimmermann P, David G. Syndecan-syntenin-ALIX regulates the biogenesis of exosomes. Nat Cell Biol 14: 677–685, 2012. doi: 10.1038/ncb2502. [DOI] [PubMed] [Google Scholar]
- 199.Hu B, Rodriguez JJ, Kakkerla Balaraju A, Gao Y, Nguyen NT, Steen H, Suhaib S, Chen S, Lin F. Glypican 4 mediates Wnt transport between germ layers via signaling filopodia. J Cell Biol 220: e202009082, 2021. doi: 10.1083/jcb.202009082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gerard C, Rollins BJ. Chemokines and disease. Nat Immunol 2: 108–115, 2001. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 201.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 32: 659–702, 2014. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 202.Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci USA 100: 1885–1890, 2003. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Gordts P, Foley EM, Lawrence R, Sinha R, Lameda-Diaz C, Deng L, Nock R, Glass CK, Erbilgin A, Lusis AJ, Witztum JL, Esko JD. Reducing macrophage proteoglycan sulfation increases atherosclerosis and obesity through enhanced type I interferon signaling. Cell Metab 20: 813–826, 2014. doi: 10.1016/j.cmet.2014.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rajan A, Robertson MJ, Carter HE, Poole NM, Clark JR, Green SI, Criss ZK, Zhao B, Karandikar U, Xing Y, Margalef-Català M, Jain N, Wilson RL, Bai F, Hyser JM, Petrosino J, Shroyer NF, Blutt SE, Coarfa C, Song X, Prasad BV, Amieva MR, Grande-Allen J, Estes MK, Okhuysen PC, Maresso AW. Enteroaggregative E. coli adherence to human heparan sulfate proteoglycans drives segment and host specific responses to infection. PLoS Pathog 16: e1008851, 2020. doi: 10.1371/journal.ppat.1008851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.van Putten JP, Paul SM. Binding of syndecan-like cell surface proteoglycan receptors is required for Neisseria gonorrheae entry into human mucosal cells. EMBO J 14: 2144–2154, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Zimmermann N, Saiga H, Houthuys E, Moura-Alves P, Koehler A, Bandermann S, Dorhoi A, Kaufmann SH. Syndecans promote mycobacterial internalization by lung epithelial cells. Cell Microbiol 18: 1846–1856, 2016. doi: 10.1111/cmi.12627. [DOI] [PubMed] [Google Scholar]
- 207.Beaussart A, Brandhorst T, Dufrêne YF, Klein BS. Blastomyces virulence adhesin-1 protein binding to glycosaminoglycans is enhanced by protein disulfide isomerase. mBio 6: e01403–e01415, 2015. doi: 10.1128/mBio.01403-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Aikawa T, Whipple CA, Lopez ME, Gunn J, Young A, Lander AD, Korc M. Glypican-1 modulates the angiogenic and metastatic potential of human and mouse cancer cells. J Clin Invest 118: 89–99, 2008. doi: 10.1172/JCI32412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Jen YH, Musacchio M, Lander AD. Glypican-1 controls brain size through regulation of fibroblast growth factor signaling in early neurogenesis. Neural Dev 4: 33, 2009. doi: 10.1186/1749-8104-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Amor DJ, Stephenson SEM, Mustapha M, Mensah MA, Ockeloen CW, Lee WS, Tankard RM, Phelan DG, Shinawi M, de Brouwer APM, Pfundt R, Dowling C, Toler TL, Sutton VR, Agolini E, Rinelli M, Capolino R, Martinelli D, Zampino G, Dumic M, Reardon W, Shaw-Smith C, Leventer RJ, Delatycki MB, Kleefstra T, Mundlos S, Mortier G, Bahlo M, Allen NJ, Lockhart PJ. Pathogenic variants in GPC4 cause Keipert syndrome. Am J Hum Genet 104: 914–924, 2019. doi: 10.1016/j.ajhg.2019.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Schmits R, Filmus J, Gerwin N, Senaldi G, Kiefer F, Kundig T, Wakeham A, Shahinian A, Catzavelos C, Rak J, Furlonger C, Zakarian A, Simard JJ, Ohashi PS, Paige CJ, Gutierrez-Ramos JC, Mak TW. CD44 regulates hematopoietic progenitor distribution, granuloma formation, and tumorigenicity. Blood 90: 2217–2233, 1997. [PubMed] [Google Scholar]
- 212.Okamoto K, Tokunaga K, Doi K, Fujita T, Suzuki H, Katoh T, Watanabe T, Nishida N, Mabuchi A, Takahashi A, Kubo M, Maeda S, Nakamura Y, Noiri E. Common variation in GPC5 is associated with acquired nephrotic syndrome. Nat Genet 43: 459–463, 2011. doi: 10.1038/ng.792. [DOI] [PubMed] [Google Scholar]
- 213.Jones EA, Yuan L, Breant C, Watts RJ, Eichmann A. Separating genetic and hemodynamic defects in neuropilin 1 knockout embryos. Development 135: 2479–2488, 2008. doi: 10.1242/dev.014902. [DOI] [PubMed] [Google Scholar]
- 214.Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Südhof TC. α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423: 939–948, 2003. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- 215.Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, Grant D, Solloway M, Parker L, Ye W, Forrest W, Ghilardi N, Oravecz T, Platt KA, Rice DS, Hansen GM, Abuin A, Eberhart DE, Godowski P, Holt KH, Peterson A, Zambrowicz BP, de Sauvage FJ. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol 28: 749–755, 2010. doi: 10.1038/nbt.1644. [DOI] [PubMed] [Google Scholar]
- 216.van der Windt GJ, Florquin S, de Vos AF, van’t Veer C, Queiroz KC, Liang J, Jiang D, Noble PW, van der Poll T. CD44 deficiency is associated with increased bacterial clearance but enhanced lung inflammation during Gram-negative pneumonia. Am J Pathol 177: 2483–2494, 2010. doi: 10.2353/ajpath.2010.100562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Púre E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science 296: 155–158, 2002. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 218.Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol 161: 2219–2228, 2002. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


