Abstract
Propachlor (2-chloro-N-isopropylacetanilide) is an acetamide herbicide used in preemergence. In this study, we isolated and characterized a soil bacterium, Acinetobacter strain BEM2, that was able to utilize this herbicide as the sole and limiting carbon source. Identification of the intermediates of propachlor degradation by this strain and characterization of new metabolites in the degradation of propachlor by a previously reported strain of Pseudomonas (PEM1) support two different propachlor degradation pathways. Washed-cell suspensions of strain PEM1 with propachlor accumulated N-isopropylacetanilide, acetanilide, acetamide, and catechol. Pseudomonas strain PEM1 grew on propachlor with a generation time of 3.4 h and a Ks of 0.17 ± 0.04 mM. Acinetobacter strain BEM2 grew on propachlor with a generation time of 3.1 h and a Ks of 0.3 ± 0.07 mM. Incubations with strain BEM2 resulted in accumulation of N-isopropylacetanilide, N-isopropylaniline, isopropylamine, and catechol. Both degradative pathways were inducible, and the principal product of the carbon atoms in the propachlor ring was carbon dioxide. These results and biodegradation experiments with the identified metabolites indicate that metabolism of propachlor by Pseudomonas sp. strain PEM1 proceeds through a different pathway from metabolism by Acinetobacter sp. strain BEM2.
Controlled persistence and biodegradation of herbicides in soil and water is highly desirable for reducing contamination and protecting our food and environment (3, 6, 7, 20). Acetamide herbicides are used as preemergence herbicides for selective control of monocotyledon and dicotyledon weeds. These persistent herbicides are necessary for weed control in certain crops, but their phytotoxicity may restrict their use.
Propachlor (2-chloro-N-isopropylacetanilide) is an acylanilide herbicide widely used with corn, onion, cabbage, rose bushes, and ornamental plants. Microbial degradation is the primary mechanism of acylanilide dissipation from soil. Villareal et al. (19) proposed a pathway of propachlor degradation yielding 2-chloro-N-isopropylacetamide as an intermediate. Cometabolism of propachlor, alachlor, and cycloate has been studied by Novick et al. (16), and in this case N-isopropylaniline was identified as an intermediate in propachlor degradation.
We previously reported the isolation of Pseudomonas strain PEM1 (2, 12), which metabolizes the herbicide propachlor in bath suspension and immobilized on ceramic support, as well as in pilot-scale soil experiments. One product of the microbial metabolism was identified as N-isopropylacetanilide. In this paper we report the isolation of Acinetobacter strain BEM2, another soil bacterium which can grow on propachlor as the sole source of carbon and energy. In this study we identified new metabolites in the degradation of propachlor by Pseudomonas strain PEM1, and we suggested two pathways for propachlor metabolism based on the identification of N-isopropylaniline and isopropylamine in Acinetobacter strain BEM2 culture fluids. Our results indicate that initial dehalogenation may occur in both strain PEM1 and strain BEM2 but that the following steps in the degradation pathways are different in the two bacteria. Furthermore, both bacteria liberate the propachlor ring carbon atoms as carbon dioxide.
MATERIALS AND METHODS
Isolation of bacteria.
Ten soil samples were collected from agricultural fields, with a history of propachlor contamination, in central Spain. Minimal medium (PJC) (8) supplemented with 45 mg of propachlor liter−1 was inoculated with 5 g of soil sample and incubated at 28°C without shaking. Aliquots were subcultured every 10 days for 40 days, and the final subculture was plated on PJC agar plates with 1 mM propachlor as the carbon source. A bacterial isolate, designed BEM2, was selected for further analysis of substrate specificity and biochemical reactions (Api2ONE kit; bioMérieux S.A., Marcy l’Etoile, France). The moles percent G+C content was estimated by the spectrometric method of Ulitzur (18) with DNA from Escherichia coli B as a reference standard. DNA was prepared with the Kristal kit (DNA extraction kit; Cambridge Molecular Technologies, Cambridge, United Kingdom). The other propachlor-degrading bacterium used in this work was previously isolated and designed PEM1 and was characterized as a Pseudomonas strain (2, 12).
Media and growth conditions.
Cells were grown aerobically at 30°C in PJC minimal medium. The carbon sources were sterilized separately and added to give 0.1 to 1.2 mM propachlor, 1 mM acetanilide, 1 mM N-isopropylaniline, 10 mM acetamide, 10 mM isopropylamine, 1 mM aniline, 1 mM phenol, or 5 mM benzoate.
Analytical methods.
The gas chromatography-mass spectrometry (GC-MS) analyses were performed with a Hewlett-Packard 5890 Series II gas chromatograph equipped with a methyl silicone capillary column (20 m by 0.22 mm [inner diameter]) programmed from 70 to 220°C (4°C/min) and connected to an HP-5971 mass detector.
High-pressure liquid chromatography (HPLC) analysis was performed with a Waters 616PDA996 chromatograph equipped with a data analysis Millennium 20/10. Separation was performed on a Novapack C18 (3.9 by 150 mm) column with a mobile phase consisting of 40% acetonitrile in water at a flow rate of 0.5 ml/min, and the products were monitored at 214 nm. The injection volume was 10 μl.
Mineralization of propachlor.
The kinetic parameters for the mineralization of propachlor by whole cells were determined with glucose- or propachlor-grown cells. Metabolism was determined by measuring 14CO2 released from [ring-U-14C]propachlor. Cells pregrown in PJC medium plus glucose or propachlor were washed and resuspended in 10 ml of phosphate buffer (pH 7.2). To a series of 50-ml flasks was added 5 ml of the used phosphate buffer, containing 1 μCi of [ring-U-14C]propachlor. Different amounts of cold propachlor were added to the flasks to achieve final concentrations ranging from 0.2 to 1.2 mM. To initiate mineralization assays, media were inoculated with 106 cells of early-stationary-phase culture of glucose- or propachlor-grown cells. The flasks were incubated at 30°C for 30 h. 14CO2 formed from the mineralization was trapped in a 1 N NaOH solution located at the top of the bottles. Radioactivity was measured in a 2500 TR Packard scintillation spectrometer. Total initial activity (and concentration) was determined by averaging counts obtained with 1-ml aliquots sampled before, during, and after the incubation. The Ks was calculated from a Hanes plot of the data of nonsaturating propachlor concentrations (1).
Characterization of the propachlor degradation intermediates.
Intermediates were identified by experiments with nongrowing cells. Cultures of glucose- or propachlor-grown cells were centrifuged at 10,000 × g for 10 min at 4°C, and the pellets were washed twice with 10 mM phosphate buffer (pH 7.2) and resuspended in the same buffer. Substrates were added to the cell suspensions, which were then incubated at 30°C. Propachlor and the resulting intermediates in its degradation were analyzed by HPLC and GC-MS. Samples for HPLC were evaporated to dryness under a nitrogen stream and redissolved in ethanol. For GC-MS, samples from the experimental cultures were extracted 1:1 with ethyl acetate (12) and 2-μl aliquots of the ethyl acetate extracts were injected into the column. Metabolites were identified by comparison of their electron impact-MS spectra with those obtained for standards and by coelution in HPLC and GC.
Data analysis.
The shape of the substrate utilization data aimed to fit a Gauss-type curve S(t) = S(0)exp(−t2/2ς2). The rate of substrate utilization, S′(t) = -tS(t)/ς2, reaches its optimum value for t = ς and tends to linearity during the stationary phase. The shape of the growth data caused us to consider the logistic-type curve (Monod). The parameters of the logistic- and Gauss-type curves fitted to the growth data and propachlor degradation were estimated by using NLIN, the nonlinear procedure of the SAS statistical package.
Chemicals.
Propachlor and [14C]propachlor were obtained from Monsanto España S.A. (Madrid, Spain). Acetamide and acetanilide were purchased from Aldrich (Milwaukee, Wis.). N-Isopropylaniline and isopropylamine were from Sigma (St. Louis, Mo.). All the chemicals were of the highest purity commercially available.
RESULTS
Enrichment cultures and strain isolation.
A variety of soil samples (5 g suspended in 50 ml of minimal medium) taken from El Encin (Madrid) were incubated at 28°C, and propachlor was added to each tube. Samples were plated on Luria-Bertani medium (13), and the resulting isolates were tested for their capability to grow on propachlor as the sole carbon source. By using this technique, a pure culture designated strain BEM2, which resulted in complete utilization of propachlor, was isolated. The organism was a bacterium on the basis of its morphological and biochemical properties. Strain BEM2 exhibited characteristics of the genus Acinetobacter: it was nonmotile, oxidase negative, obligately aerobic, and 0.9 to 1.4 μm in diameter and 1.6 to 2.4 μm long. Electron microscopy of the sections of cells showed a cell wall ultrastructure that is typical of gram-negative bacteria. The colonies became spherical in the stationary phase. The G+C content of the DNA was 45.2% ± 1.6%. The organism was not able to reduce nitrate to nitrite and did not hydrolyze gelatin. Growth of the isolate did not require the addition of vitamins to the growth medium.
Propachlor metabolism by Acinetobacter strain BEM2.
Bacterial growth was studied kinetically (Table 1); in batch cultures, strain BEM2 showed a growth yield of 2.1 g (dry weight)/mol and a mean generation time during growth on 0.6 mM propachlor at 30°C of 3.1 h during the early exponential phase.
TABLE 1.
Growth yields and kinetic parametersa when different carbon sources are used
| C source | Strain | Ks (mM) | Km (cell/mol) | μ (h−1)b | Y (g [dry wt]/mol)b |
|---|---|---|---|---|---|
| Propachlor | PEM1 | 0.17 | 0.40 | 0.16 (0.01) | 2.6 (0.1) |
| Acetanilide | PEM1 | 0.20 (0.02) | 1.2 (0.1) | ||
| Propachlor | BEM2 | 0.3 | 0.32 | 0.18 (0.01) | 2.1 (0.1) |
| N-Isopropylaniline | BEM2 | 0.17 (0.02) | 1.2 (0.1) |
μ, maximum specific growth rate, [dX/dt]/X; Ks, substrate utilization-associated half-velocity constant; Km, growth rate at saturating concentration; Y, yield.
Numbers in parentheses are standard deviations.
The parameter ς of the curve fitted to propachlor degradation data was estimated to be 13.13. The rate of propachlor utilization at 25 h of incubation was estimated to be 4.9 μmol · h−1.
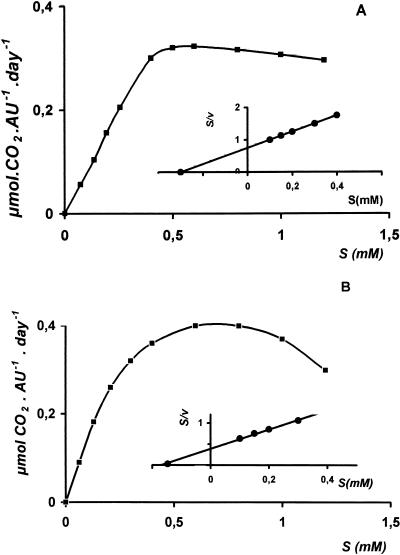
Mineralization of propachlor by strain BEM2 was monitored as described above, by using cells pregrown in glucose or propachlor (Fig. 1A). [ring-U-14C]propachlor was added to the flasks, and different amounts of propachlor were used to obtain final concentrations (0.1 to 1.2 mM). Glucose-grown cells did not metabolize propachlor, suggesting that propachlor metabolism was inducible. Propachlor-grown whole cells produced 14CO2 from [ring-U-14C]propachlor, with a Ks of 0.3 ± 0.07 mM (Table 1). The principal product from the carbon atoms in the propachlor ring was CO2; no significant 14CO2 was released in control experiments without cells, and no counts were measured in controls without radioactivity. Thus, propachlor could be completely degraded by Acinetobacter strain BEM2.
FIG. 1.
Kinetics of propachlor metabolism by propachlor-grown cells. (A) Acinetobacter sp. strain BEM2. (B) Pseudomonas sp. strain PEM1. Linear regression analysis of the data in a Hanes plot (insert) revealed the Ks values shown in Table 1.
To characterize the metabolites formed during propachlor degradation by strain BEM2, samples of the culture liquid were taken periodically. HPLC analyses of organic extracts from cultures revealed a number of products (Table 2), and the most significant were identified by GC-MS. Metabolite I had M at m/z = 177 and a M+ peak at 178. The ion peak at m/z = 162 represented the fragmentation of the molecular ion by loss of a methyl radical, and the ion at m/z 120 was the most characteristic and corresponded to M+ − CO(CH3)2. The MS spectrum of metabolite IIa exhibited a molecular ion at m/z = 136 (M+) and a fragmentation pattern consistent with the loss of methyl (M+ −15; m/z = 120), isopropyl (M+ −43; m/z = 93), and isopropylamino (M+ −59; m/z = 77) groups. The significant ion at m/z = 120 was also found in the mass spectra of authentic N-isopropylaniline.
TABLE 2.
Range of growth substrates tested and product formation by Pseudomonas strain PEM1 and Acinetobacter strain BEM2
| Substrate | Strain | Growth | Product(s)a |
|---|---|---|---|
| Propachlor | PEM1 | + | I, IIa, IIIa, IV, CO2 + H2O |
| BEM2 | + | I, IIb, IIIb, IV, CO2 + H2O | |
| Acetanilide | PEM1 | + | IIIa, IV |
| BEM2 | − | ||
| N-Isopropylaniline | PEM1 | − | |
| BEM2 | + | IIIb, IV | |
| Aniline | PEM1 | − | |
| BEM2 | − | ||
| Phenol | PEM1 | − | |
| BEM2 | − | ||
| Benzoate | PEM1 | + | IV |
| BEM2 | + | IV | |
| Acetamide | PEM1 | + | nd |
| BEM2 | + | nd | |
| Isopropylamine | PEM1 | − | |
| BEM2 | + | nd |
I, N-isopropylacetanilide; IIa, N-isopropylaniline; IIIa, isopropylamine; IIb, acetanilide; IIIb, acetamide; IV, catechol; nd, not detected.
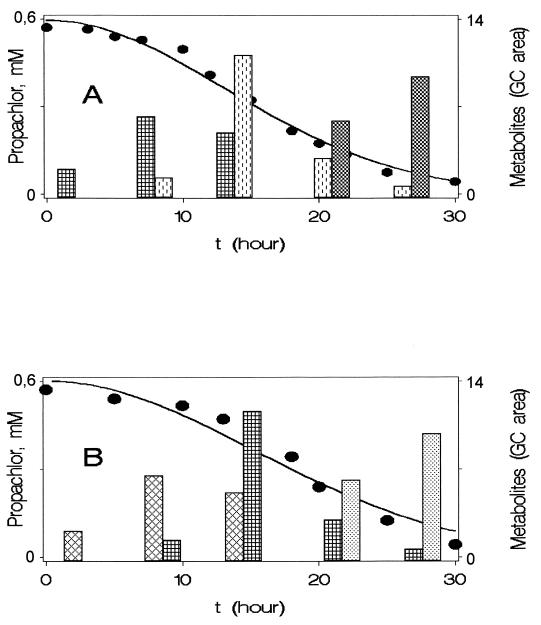
Figure 2A shows the appearance and disappearance of the metabolic intermediates during growth of BEM2 cells on propachlor. After 3 h of incubation, N-isopropylacetanilide is the metabolite accumulated from BEM2 metabolism of propachlor. When the culture had reached exponential phase, this intermediate reached its highest concentration in the medium; the concentration then decreased, and at the same time N-isopropylaniline could be detected. After the culture had reached stationary phase, isopropylamine was formed from the cleavage at the bond between the C atom of the aromatic ring and the N atom.
FIG. 2.
Utilization of propachlor by Acinetobacter sp. strain BEM2 (A) and by Pseudomonas sp. strain PEM1 (B) and appearance of the metabolic intermediates. Metabolites were measured by GC analysis as described in Materials and Methods. Propachlor (•), N-isopropylacetanilide (▩), N-isopropylaniline ( ), isopropylamine ( ), acetanilide ( ), and acetamide (░⃞) concentrations are shown.
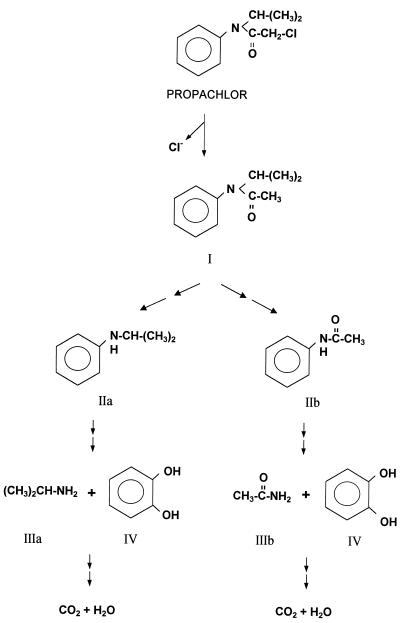
Some intermediates of propachlor degradation by strain BEM2 were tested as growth substrates (Table 2). N-Isopropylaniline (metabolite IIa) could be used as the sole carbon source (Table 1), and isopropylamine (IIIa) and catechol (IV) were identified by HPLC as products of the catabolism. Acetamide (IIIb), isopropylamine (IIIa), benzoate, and catechol were also substrates for strain BEM2, but acetanilide (IIb), aniline, and phenol were not degraded (Table 2). These results support the propachlor degradative pathway for strain BEM2 (Fig. 3).
FIG. 3.
Schematic pathways proposed for the degradation of propachlor. Metabolites IIa and IIIa were specifically produced by Acinetobacter sp. strain BEM2, and metabolites IIb and IIIb were produced by Pseudomonas sp. strain PEM1. Metabolites I and IV were produced by both strains during propachlor degradation. Metabolite I (N-isopropylacetanilide) was identified in a previous publication (12). Chemical designations: IIa, N-isopropylaniline; IIIa, isopropylamine; IIb, acetanilide; IIIb, acetamide; IV, catechol.
Propachlor metabolism by Pseudomonas strain PEM1.
We previously reported the isolation of Pseudomonas strain PEM1, which metabolizes propachlor (2, 12). When PEM1 strain grew on 0.6 mM propachlor as the carbon source, the generation time was 3.4 h (Table 1) and the growth yield obtained was slightly higher than that obtained by strain BEM2. The parameter ς was estimated to be 15.26, and the rate of propachlor utilization was 5.5 μmol · h−1 at 25 h of incubation.
The kinetics of propachlor metabolism by PEM1 cells grown under carbon limitation was studied. Glucose- or propachlor-grown cells were incubated at different propachlor concentrations in the presence of [ring-U-14C]propachlor. Glucose-grown cells did not metabolize propachlor, but propachlor-grown whole cells produced 14CO2 from [ring-U-14C]propachlor (Fig. 1B). The yield of 14CO2 in strain PEM1 cells was similar to the yield of 14CO2 in strain BEM2 cells grown on propachlor.
GC analyses of the spent supernatants of propachlor-grown cultures indicated that propachlor disappeared during cell incubation and that simultaneously other organic compounds appeared in the media (Fig. 2B). N-Isopropylacetanilide (metabolite I), the dehalogenated metabolite of propachlor, was characterized in our laboratory (12) as the first intermediate in the degradative pathway by strain PEM1. During the early exponential phase, this compound was transformed into acetanilide (IIb). The resulting mass spectrum of this intermediate was consistent with M at m/z = 135 and M+ at m/z = 136. The major fragment at m/z = 93 was due to fragmentation of the molecular ion by loss of the H3CC+0 radical, which gives an ion peak at m/z = 43. The significant ion at m/z = 93 was also found in the mass spectra of authentic acetaniline.
Acetanilide could be detected in the liquid medium during the exponential phase (10 to 20 h of incubation) and then was transformed into acetamide (metabolite IIIb) and catechol (IV), which were identified by HPLC analysis.
The ability of Pseudomonas strain PEM1 to degrade a variety of compounds was examined (Table 2). Acetanilide (IIb), acetamide (IIIb), and catechol were products of the metabolism of propachlor and are also growth substrates for this strain. Some of the products formed when strain PEM1 metabolizes these substrates have been analyzed by HPLC (Table 2). N-Isopropylaniline (IIa), isopropylamine (IIIa), aniline, and phenol were not degraded. Thus, propachlor appears to induce its own catabolism when strain PEM1 uses this compound as the sole carbon source, presumably through the pathway shown in Fig. 3.
DISCUSSION
The results presented here support and extend the metabolic pathway previously proposed for propachlor degradation by Pseudomonas strain PEM1 (12). We also reported in this study the isolation from soil of an Acinetobacter strain, called BEM2, with the ability to degrade propachlor. Our data show that both Pseudomonas strain PEM1 and Acinetobacter strain BEM2 initially attacked propachlor on the acetamide group with a chlorine as substituent (at C-2) to yield N-isopropylacetanilide (Fig. 3). Thus, the sequence of reactions in both degradative pathways involves dehalogenation as a first step. Villarreal et al. (19) reported the isolation of two microbial species which degrade propachlor, forming 2-chloro-N-isopropylacetamide as a metabolite; in this case, dehalogenation was a subsequent step in the catabolic pathway and the aromatic portion (catechol) of the molecule was degraded by a second isolate.
Novel reactions in the N-isopropylacetanilide degradation for strain PEM1 are the subsequent cleavage at the bond between the N atom and the C atom of the aromatic ring, the clearing off of the isopropyl side chain, and the accumulation in the medium of acetanilide and, later, acetamide. Acetanilide and acetamide were growth substrates for strain PEM1; moreover, when acetanilide was used as the carbon source, acetamide was formed as the product. The formation of acetamide and catechol as intermediates in propachlor degradation by strain PEM1 and the release of 14CO2 from the propachlor ring suggest that the aromatic ring could be metabolized by some of the pathways involved in the aromatic-compound degradation and described for Pseudomonas strains (5, 17). Catechols have been described as products of propachlor degradation in studies with soil bacteria (19).
Strain BEM2 metabolizes the first dehalogenated metabolite by cleavage at the bond between the N atom and the C atom of the acetyl group, yielding N-isopropylaniline (Fig. 3). This intermediate is the growth substrate for strain BEM2, and catechol and isopropylamine were formed as products of the degradation. N-Isopropylaniline has been described by Novick et al. (16) as a dehalogenated intermediate in propachlor catabolism; these authors described a microbial consortium that metabolized propachlor, and they identified N-isopropylaniline as an intermediate after initial cleavage at the amide bond, but no other intermediates were identified and the specific roles of each strain were not elucidated. The release of 14CO2 from the propachlor ring confirms the mineralization of the compound.
An examination of the substrate range metabolized indicates that both strains have the ability to degrade chemicals containing an aniline linked to a carbonyl group via an amide bond. Some aromatic compounds are also growth substrates, but phenol and aniline could not be metabolized. The inability of both bacteria to metabolize these compounds could have been due to different factors such as transport or enzyme specificity required to initiate an attack. The Ks values (Table 1) found for the metabolism of propachlor by whole cells of both strains are quite similar, showing a similar affinity of these bacteria for propachlor. We have observed differences in the yield of cells grown on acetanilide or N-isopropylaniline compared with those grown on propachlor.
The release of organic chemicals into water and soil can have dire consequences for wildlife, ecosystem integrity, and water quality. As a result, there is an increasing interest in the exploitation of microorganisms for the cleanup of soils and sediments in situ (2, 3, 7, 11, 12, 14). In general, it is reasonable to expect that conditions conducive to the growth of inoculate strains in aqueous culture will also be conducive to growth in soil. Field tests showed that Pseudomonas strain PEM1 degraded 50 nmol of propachlor per g per day; therefore, these organisms could be used in bioremediation technology to degrade contaminants of soils and aquifers in situ. Immobilization of strain PEM1 by adsorption onto a ceramic support (2) resulted in a higher tolerance to propachlor and alachlor and provided more stability to the cells, keeping them viable for longer. These results also confirm studies on immobilized cells, which are distinguished from cells growing in suspension by their higher tolerance for different phenol derivatives (10).
The potential of elevating the tolerance of Pseudomonas strain PEM1 and Acinetobacter strain BEM2 against the toxic pollutants and the range of substrates utilized by both strains suggests that these bacteria could be used in the biotreatment of soils and waters contaminated with aromatic compounds, acetamides, and other related compounds.
ACKNOWLEDGMENTS
This work was supported by grant AMB98-0501 from CYCIT (Comisión Interministerial de Ciencia y Tecnología) and by grant CAM07M/0620/1997 from Comunidad Autónoma de Madrid.
We thank Jesús Sanz for the GC-MS analysis. We also thank Jaime Costa (Monsanto España S.A.) for providing propachlor and [14C]propachlor.
REFERENCES
- 1.Cornish-Bowden A. Fundamentals of enzyme kinetics. London, United Kingdom: Portland Press Ltd.; 1995. [Google Scholar]
- 2.Ferrer E, Blanco J, Alonso R, Martín M. Propachlor and alachlor degradation by immobilized and suspended Pseudomonas cells. In: Wijffels R M, et al., editors. Immobilized cells: basics and applications. Oxford, United Kingdom: Elsevier Science Publishing; 1996. pp. 762–769. [Google Scholar]
- 3.Fetzner S, Lingens F. Bacterial dehalogenase: biochemistry, genetics and biotechnological applications. Microbiol Rev. 1994;58:641–685. doi: 10.1128/mr.58.4.641-685.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerhardt P, Murray R G, Costilow R N, Nester E W, Wood W A, Krieg N R, Phillips G B, editors. Manual of methods for general and molecular bacteriology. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 5.Gottschalk G. Bacterial metabolism. Berlin, Germany: Springer-Verlag KG; 1979. Catabolic activities of aerobic heterotrophs; pp. 126–130. [Google Scholar]
- 6.Greer L E, Robinson J A, Shelton D R. Kinetic comparison of seven strains of 2,4-dichlorophenoxyacetic acid-degrading bacteria. Appl Environ Microbiol. 1992;58:1027–1030. doi: 10.1128/aem.58.3.1027-1030.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greer L E, Shelton D R. Effect of inoculant strain and organic matter content on kinetics of 2,4-dichlorophenoxyacetic acid degradation in soil. Appl Environ Microbiol. 1992;58:1459–1465. doi: 10.1128/aem.58.5.1459-1465.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hareland W A, Crawford R L, Chapman P J, Dagley S. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975;121:272–285. doi: 10.1128/jb.121.1.272-285.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayaishi O, Katagiri M, Rothger S. Studies on oxygenases. J Biol Chem. 1957;229:905–920. [PubMed] [Google Scholar]
- 10.Heipieper H J, Keweloh H, Rehm H J. Influence of phenols on growth and membrane permeability of free and immobilized Escherichia coli. Appl Environ Microbiol. 1991;57:1213–1217. doi: 10.1128/aem.57.4.1213-1217.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J K, Minard R D, Bollag J M. Microbial metabolism of propachlor (2-chloro-N-isopropylacetanilide) in soil suspension. Han’guk Nonghwa Hakhoechi. 1982;25:44–50. [Google Scholar]
- 12.Martin M, Fernández J, Ferrer E, Alonso R. Bioremediation of soil contaminated by Propachlor using native bacteria. Int Biorem Biodeterior. 1995;35:213–225. [Google Scholar]
- 13.Miller J M. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 14.Morgan P, Watkinson R. Microbiological methods for the clean-up of soils and ground water contaminated with halogenated organic compounds. FEMS Microbiol Res. 1989;63:277–300. doi: 10.1111/j.1574-6968.1989.tb03401.x. [DOI] [PubMed] [Google Scholar]
- 15.Novick N J, Alexander M. Cometabolism of low concentrations of propachlor, alachlor, and cycloate in sewage and lake water. Appl Environ Microbiol. 1985;49:737–743. doi: 10.1128/aem.49.4.737-743.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Novick N J, Mukherjee R, Alexander M. Metabolism of alachlor and propachlor in suspensions of pretreated soils and in samples from ground water aquifers. J Agric Food Chem. 1986;34:721–725. [Google Scholar]
- 17.Stanier R Y, Ornston L N. The β-ketoadipate pathway. Adv Microb Physiol. 1973;9:89–151. [PubMed] [Google Scholar]
- 18.Ulitzur S. Rapid determination of DNA base composition by ultraviolet spectroscopy. Biochim Biophys Acta. 1972;272:1–11. doi: 10.1016/0005-2787(72)90025-1. [DOI] [PubMed] [Google Scholar]
- 19.Villareal D T, Turco R F, Konopka A. Propachlor degradation by a soil bacterial community. Appl Environ Microbiol. 1991;57:2135–2140. doi: 10.1128/aem.57.8.2135-2140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilber G G, Wang G. Biotransformation of herbicides in the presence of various electron acceptors. J Air Waste Manag Assoc. 1997;47:690–696. [Google Scholar]