Abstract
Mycoplasma ovipneumoniae (M. ovipneumoniae) is a respiratory pathogen associated with mild to moderate respiratory disease in domestic lambs and severe pneumonia outbreaks in wild ruminants such as bighorn sheep. However, whether M. ovipneumoniae by itself causes clinical respiratory disease in domestic sheep in the absence of secondary bacterial pathogens is still unclear. The goal of our study was to better understand the role of M. ovipneumoniae as a respiratory pathogen in domestic sheep and to explore potential antibiotic treatment approaches. Therefore, we inoculated four 4-month-old, specific-pathogen-free lambs with fresh nasal wash fluids from M. ovipneumoniae-infected sheep. The lambs were monitored for M. ovipneumoniae colonization, M. ovipneumoniae-specific antibodies, clinical signs, and cellular and molecular correlates of lung inflammation for eight weeks. All lambs then were treated with gamithromycin and observed for an additional four weeks. M. ovipneumoniae inoculation resulted in stable colonization of the upper respiratory tract in all M. ovipneumoniae-inoculated, but in none of the four mock-infected control lambs. All M. ovipneumoniae-infected lambs developed a robust antibody response to M. ovipneumoniae within 2 weeks. However, we did not observe significant signs of respiratory disease, evidence of lung damage or inflammation in any of the infected lambs. Interestingly, treatment with gamithromycin, which blocked growth of the M. ovipneumoniae in vitro, failed to reduce M. ovipneumoniae colonization. These observations indicate that, in the absence of co-infections, M. ovipneumoniae caused asymptomatic colonization of the upper respiratory tract that was resistant to clearance by the host immune response and by gamithromycin treatment.
Keywords: Sheep, Mycoplasma, respiratory tract, gamithromycin
1. Introduction
Mycoplasma ovipneumoniae (M. ovipneumoniae) is a highly prevalent respiratory pathogen associated with atypical, chronic non-progressive pneumonia in sheep and goats. Colonization of the upper respiratory tract of adult sheep with M. ovipneumoniae is increasingly recognized across the world (Jay et al., 2020). In the United States, active M. ovipneumoniae infection was detected in 88.5% of commercial sheep flocks in a 2011 study (USDA, 2013). While most cases of M. ovipneumoniae infection in adult sheep are thought to be asymptomatic or mild, significant production losses may occur due to reduced weight gains, lower carcass quality and increased mortality in lambs (Besser et al., 2019; Manlove et al., 2019). Moreover, M. ovipneumoniae-infected domestic sheep pose a significant threat to other ruminants such as bighorn sheep (Ovis canadensis) (Besser et al., 2008), Dall’s sheep (Ovis dalli dalli) (Black et al., 1988), , and Norwegian Muskox (Ovibos moschatus) (Handeland et al., 2014), where M. ovipneumoniae infection causes severe pneumonia outbreaks with up to 100% mortality.
However, it is still unclear to what extent M. ovipneumoniae causes clinical respiratory disease in sheep in the absence of other pathogens. Several studies have demonstrated that M. ovipneumoniae greatly increases the susceptibility for infection with other opportunistic pathogens such as Mannheimia haemolytica and Bibersteinia trehalosi, which then leads to interstitial pneumonia with severe clinical symptoms and significant mortality (Besser et al., 2013; Jones et al., 1982a). However, infection studies with M. ovipneumoniae alone have yielded conflicting results. Thus, respiratory symptoms and lung pathology were observed in a subset of sheep following endobronchial application of M. ovipneumoniae in two early studies (Foggie et al., 1976; Jones et al., 1982b), and more recently, Du et al. (2020) described coughing, wheezing and increased body temperatures in Bashbay lambs experimentally inoculated with M. ovipneumoniae . In contrast, Buddle et al. (1984) did not find evidence of pneumonia in colostrum-deprived lambs after experimental M. ovipneumoniae infection. In bighorn sheep, inoculation with cultured strains of M. ovipneumoniae did not result in clinical respiratory disease (Besser et al., 2008), whereas natural transmission of M. ovipneumoniae between animals led to severe bronchopneumonia (Besser et al., 2014). These conflicting findings point to a need for further experimental investigations.
While the contribution of M. ovipneumoniae to sheep respiratory disease is widely acknowledged and elimination of M. ovipneumoniae colonization in domestic sheep could prevent severe spillover-associated disease outbreaks in bighorn sheep and other wild ruminants, no vaccines or treatments to combat the infection are currently approved. While experiments have demonstrated the in vitro susceptibility of most M. ovipneumoniae isolates to a wide range of macrolide, tetracycline and fluoroquinolone antibiotics (Jay et al., 2020; Maksimovic et al., 2020), no studies on antibiotic treatment of M. ovipneumoniae infection in vivo have been published.
To better understand the role of M. ovipneumoniae as a respiratory pathogen in domestic sheep and to explore potential antibiotic treatment approaches, we performed an M. ovipneumoniae infection and antibiotic treatment study in four 4-month-old specific-pathogen-free lambs and four age-matched control lambs. Intranasal inoculation of lambs with M. ovipneumoniae-positive nasal washes collected from lambs with clinical respiratory disease resulted in consistent colonization of the upper respiratory tract and robust M. ovipneumoniae-specific humoral immunity. However, we did not observe clear clinical signs or evidence of lung damage or inflammation in any of the infected lambs. Interestingly, treatment with the macrolide antibiotic gamithromycin, which has been used successfully to treat clinical Mycoplasma-associated pneumonia in cattle and goats (Baggott et al., 2011; Kacar et al., 2018), failed to reduce M. ovipneumoniae colonization in the infected lambs. Overall, these data suggest that, in the absence of other opportunistic pathogens, M. ovipneumoniae behaves like an upper respiratory tract commensal that is not affected by the host antibody response or a macrolide antibiotic gamithromycin, which is commonly used to treat respiratory disease in ruminants.
2. Methods
2.1. Animals and husbandry
All animal experiments in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Montana State University, protocol #2019-95. To generate specific-pathogen-free lambs, 15 mixed-breed ewes (Rambouillet, Suffolk, Targhee, Columbia, and/or Hampshire) 4-5 years of age were purchased several weeks before the projected lambing date from a local sheep farmer. Pregnancy was confirmed using abdominal ultrasound, and M. ovipneumoniae exposure was determined by serology and nasal swab PCR, as described below. Prior to lambing, ewes were fed hay from the Johnson Family Livestock Facility (JFLF) farm, grain, and an appropriate vitamin/mineral supplement.
To derive lambs free from M. ovipneumoniae and other facultative respiratory pathogens, we used supervised lambing and motherless rearing, as previously described(Bimczok et al., 2005). Around the projected lambing date, ewes were monitored around the clock for signs of imminent delivery. Ewes developing signs of labor were separated from the flock and moved into a designated lambing pen, and lambs were manually delivered onto sterile towels placed on top of a clean plastic sheet and then were transferred into a separate, heated nursery area within the JFLF ABSL-2 laboratory. All animal care personnel showered and changed into sterile personal protective equipment prior to entering the nursery, and personnel responsible for the lambs did not have any contact with other non-SPF sheep for the duration of lambing. Lambs were housed in heated animal rooms (15.5-16.8 °C) inside the JFLF in groups of 5-10 animals, with siblings and lambs of similar ages grouped together. During the first 24 h, lambs were bottle-fed a commercial colostrum replacer (Rescue Lamb & Kid Colostrum Replacer, Lifeline Nutrition Solutions/APC, Ankeny, IA), which contains bovine serum as an antibody source. Animals were trained to use bucket feeders and were fed a commercial lamb milk replacer diet (Hubbard Feeds, Mankato, MN) for 5 weeks, with ad libitum access to hay and water. Lambs were gradually weaned onto pelleted lamb food starting at 36-48 days of age days by decreasing the concentration of milk replacer to 50% for two weeks as previously described (Bimczok et al., 2005).
2.2. Health monitoring
Approximately 2 months prior to lambing, ewes were screened for M. ovipneumoniae, Coxiella burnetii and Mycobacterium avium paratuberculosis (MAP) exposure by serum ELISA and for parainfluenza 3 (PI3) exposure by serum-based virus neutralization assay. Nasal swabs from the ewes were also analyzed for M. ovipneumoniae by PCR.
Specific-pathogen-free lambs at the JFLF were screened daily for signs of illness or discomfort. In addition, SPF-status of the lambs was confirmed by testing nasal swab samples for M. ovipneumoniae infection by quantitative PCR at birth, at the age of 1 month, and seven days prior to the start of the experiment. At 3 months of age, nasal swabs from a subset of lambs were analyzed for M. ovipneumoniae by PCR and for the presence of Pasteurellaceae by conventional aerobic culture. All laboratory assays were performed at the Washington Animal Disease Diagnostic Laboratory (WADDL, accredited by the American Association of Veterinary Laboratory Diagnosticians).
Following experimental M. ovipneumoniae infection or mock treatment, lamb health status was assessed twice daily by trained JFLF personnel. The following health parameters were assessed and were used to calculate a clinical disease score: (1) general behavior, (2) appetite, (3) rectal temperature, (4) respiratory symptoms, and (5) any medications administered exclusive of the experimental treatment provided at 8 weeks post infection (p.i.) as detailed in Table 1. Daily scores represent the sum of the two values obtained for each day, and weekly scores represent the sum of all 14 values collected over a 7-day period. In addition, lamb body weights were determined on days 20, 32 and 55 p.i.
Table 1:
Parameters for lamb health scoring during challenge experiment
| Parameter | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Behavior | bright, alert, responsive | small change in attitude/behavior | less active, visibly appears ill | ill, but still responsive | lethargic | unresponsive |
| Appetite | ate well | ate most of the ration | ate 3/4 feed | ate 1/2 feed | ate <1/2 feed | not eating |
| Body temperature | 100°F-102°F | 102.1°F-103°F or below 99.9°F | 103.1°F-104°F | 104.1°F-104.5°F | 104.6°F-105°F | above 105°F |
| Respiratory signs | no nasal or ocular discharge, no cough, normal respirations | Panting/increased respiratory rate, or slight clear nasal/ocular discharge | clear nasal or ocular discharge, or coughed 1-5 times | moderate nasal or ocular discharge or frequent coughing | mucous nasal or ocular discharge, coughing, lamb visibly unwell | discharge, coughing, wheezing, labored breathing |
| Medication | no treatments | electrolytes | NSAID | antibiotic | NSAID and antibiotic | multiple NSAIDS and antibiotics |
2.3. Experimental infection with Mycoplasma ovipneumoniae
Out of thirty live lambs born in our flock, eight SPF lambs aged between 15 and 16 weeks were selected for the experiment and divided into an M. ovipneumoniae treatment group (n=4) and a control group (n=4) based on the following criteria: (1) absence of previous clinical disease or injuries that required treatment; (2) equal distribution of available sibling pairs into the two groups; (3) equal numbers of male and female lambs; and (4) group composition matched for age and sex. Animal details are listed in Table 2. The SPF lambs were infected using pooled nasal wash fluids from lambs previously determined to be infected with M. ovipneumoniae, following a published protocol (Besser et al., 2014). Animals housed at MSU’s Red Bluff Research Ranch, aged 11-13 weeks, were used as donor lambs for nasal washes. We selected eight lambs (4 ewes and 4 wethers) with respiratory disease symptoms including nasal discharge, coughing, labored breathing and low body weights that were confirmed to be infected with M. ovipneumoniae based on PCR analysis of nasal swabs. Nasal wash fluids have been successfully used in previous M. ovipneumoniae infection studies (Besser et al., 2017; Besser et al., 2014), and this approach prevents potential loss of bacterial virulence due to in vitro passaging (Niang et al., 1998). To obtain nasal wash fluids, the M. ovipneumoniae-infected lambs were restrained using halters, with the heads kept in a slightly lowered position. Nasal washes were performed by squirting 2 × 15 mL of sterile PBS into each nostril and collecting the flush fluid into a clean polyethylene bag. Nasal wash fluids were placed on ice and immediately transferred to the laboratory, where they were pooled, diluted 1 : 1 with tris-buffered saline, and then treated with ceftiofur (100 μg/mL, MWI Animal Health, Boise, ID) for 2 h at 37°C to reduce contamination with Pasteurellaceae. Microbiological analysis of the pooled nasal wash fluid confirmed that both Mannheimia haemolytica and Bibersteinia trehalosi were present before ceftiofur treatment, but were undetectable after the treatment. Within 4 h of nasal wash collection, we then inoculated eight of the SPF lambs (Table 2) with 50 mL of the treated nasal wash fluid (M. ovipneumoniae group, n=4) or PBS (control group, n=4) by infusing 15 mL into each nostril, 10 mL into the oral cavity and 5 mL into each conjunctival sac. Following inoculation, the M. ovipneumoniae group and the control group were housed in separate rooms of the JFLF to prevent aerosol transmission of M. ovipneumoniae. Each room had its own set of equipment so that no equipment was shared between the rooms. For the duration of the infection experiment, staff first performed all necessary husbandry procedures on the control animals before entering the room with the infected animals and showered and changed immediately after leaving the room with the infected lambs.
Table 2:
Characteristics of experimental animals
| Animal # | Treatment | Age (days)* | Sex |
|---|---|---|---|
| 12 | M. ovipneumoniae | 109 | F (twin of 15) |
| 19 | M. ovipneumoniae | 108 | F |
| 26 | M. ovipneumoniae | 105 | M# (twin of 25) |
| 30 | M. ovipneumoniae | 103 | M# |
| 15 | PBS | 109 | M# (twin of 12) |
| 17 | PBS | 109 | M# |
| 25 | PBS | 105 | F (twin of 26) |
| 27 | PBS | 104 | F |
age at time of infection;
all males were castrated at 11 weeks.
2.4. Antibiotic treatment
Eight weeks after experimental infection with M. ovipneumoniae or mock infection with PBS, all lambs were treated with 6 mg/kg BW gamithromycin (Zactran®, Boehringer Ingelheim, Ridgefield, CT) by subcutaneous injection, with a second dose given 5 days later.
2.5. M. ovipneumoniae isolation and culture
Isolation media consisting of Mycoplasma Broth Base (Becton Dickinson, Franklin Lakes, NJ) supplemented with dextrose (Thermo Fisher Scientific, Waltham, MA), L-cysteine hydrochloride monohydrate (Thermo Fisher Scientific), beta-NAD (Thermo Fisher Scientific), thallium acetate (Acros Organics, Fair Lawn, NJ), phenol red (Sigma Aldrich, St. Louis, MO), porcine serum (Quad Five, Ryegate, MT), equine serum (Quad Five), and penicillin G potassium salt (Sigma Aldrich) as both broth and agar plates. Mycoplasma plates were inoculated with the nasal wash used in the infection experiment. These plates were then incubated under microaerophilic conditions at 37°C for 10 days. Several of the resulting colonies were then determined to be M. ovipneumoniae through qPCR targeting the p133 gene (Yang et al., 2014) with Ct values <25. These colonies were inoculated into Mycoplasma broth and expanded by incubation under microaerophilic conditions at 37°C until acid production caused a color change in the media with the phenol red.
2.6. Antibiotic broth microdilution assay
The antibiotic broth microdilution assay was performed according to the methods outlined previously by Hannan (2000) for veterinary mycoplasma species. Gamithromycin concentrations from 0.031-128 μg/mL were tested against the nasal wash isolate and the standard type strain Y98 (ATCC 29419™, NCTC 10151). Susceptibility of the mycoplasma to gentamicin (0.031-128 μg/mL, Thermo Fisher Scientific) was analyzed for comparison. 96-well plates containing M. ovipneumoniae cultures initially were incubated for 48 h under microaerophilic conditions at 37°C and were observed every 24 h for 10 – 14 days for a color change in the media that indicated acid production and growth of the organism. The lowest dilution without a color change after two subsequent 24 h readings without change was recorded as the end-point minimum inhibitory concentration (MIC).
2.7. Collection and analysis of bronchoalveolar lavage fluids
To collect bronchoalveolar lavage fluid (BAL), a flexible fiber-optic endoscope measuring 6.6 mm x 100 cm (VFS-2B VetVu, Swiss Precision Products, Inc, Oxford, MA) was introduced into the trachea under local anesthesia with lidocaine. Lavage was performed by instilling 60 mL of sterile saline into the lungs and then aspirating the liquid again; approximately 20 mL of fluid were routinely recovered from the sheep. Bronchoalveolar lavage fluid was stored on ice until transfer to the laboratory, where cells were harvested by centrifugation at 400 g for 10 min. Supernatants were then analyzed for evidence of lung damage using the Lactate Dehydrogenase Assay Kit (Abcam, Cambridge, UK). Cell pellets were processed for absolute cell counts using a hemocytometer and for differential cell counts using cytospin preparations stained with a DippKwik stain (ThermoFisher, Waltham, MA). At least 300 cells from each sample were classified by a scientist blinded to the treatment of the animals.
2.8. Detection of M. ovipneumoniae infection
Detection of M. ovipneumoniae infection by PCR was performed at the Washington Animal Disease Diagnostic Laboratory (WADDL, Pullman, WA) using nasal swab samples and standard protocols. Briefly, probe based quantitative PCRs were performed with the following primers and probe: forward: 59-GGG GTG CGC AAC ATT AGT TA-39; reverse: 59-CTT ACT GCT GCC TCC CGT AG-39; and probe: 59-6-FAM-TTA GCG GGG CCA AGA GGC TGT A-BHQ-1-39 derived from GenBank sequences EU290066 and NR_025989 of M. ovipneumoniae. Samples with Ct values <40 were considered positive. Data are shown as 40 minus the measured Ct value to enable semi-quantitative comparison between samples.
2.9. M. ovipneumoniae serology
Analysis of serum samples for the presence of M. ovipneumoniae-reactive antibodies was performed at WADDL using the laboratory’s monoclonal antibody-based competitive enzyme-linked immunosorbent assay (cELISA) test, which has a diagnostic sensitivity of 88% and a diagnostic specificity of 99.3%. Validation data for the assays may be obtained directly from the laboratory at http://www.vetmed.wsu.edu/depts_waddl/). Data are shown as % inhibition and represent the reduction in binding of the labelled monoclonal antibody to the M. ovipneumoniae test antigen caused by competitive binding of serum antibodies from the diagnostic samples. Inhibition of >50% was considered a positive result, and inhibition between 40% and 50% was considered indeterminate.
2.10. Statistical analyses
Data were analyzed by GraphPad Prism, version 9.0 (San Diego, CA) and are shown as mean ± standard deviation (SD). Differences between groups were analyzed by 2-way ANOVA with Sidak’s, Tukey’s or Dunnett’s multiple comparisons test or by Student’s t test and were considered significant at P≤0.05.
3. Results
3.1. Supervised lambing and motherless rearing successfully prevented colonization of domestic lambs with M. ovipneumoniae and Pasteurellaceae.
Specific-pathogen-free lambs were derived by supervised lambing and artificial rearing from a domestic sheep flock with a history of M. ovipneumoniae infection. Pathogen exposure of the ewes was determined by serological analysis prior to lambing. The ewes were free from C. burnetii and M. avium ssp. paratuberculosis, but had variable serological responses to M. ovipneumoniae and parainfluenza virus (PI-3), with 7/15 ewes serologically positive for M. ovipneumoniae and serologically positive for PI-3. Notably, all nasal swabs collected at the same time as the serum samples tested negative for M. ovipneumoniae by PCR, indicating previous exposure of the sheep, but no active pathogen shedding.
All thirty lambs born from our ewe flock including the eight experimental lambs selected for our study were free from M. ovipneumoniae on days 0 and 30 after birth and one week prior to the experimental inoculation. We also confirmed the absence of upper respiratory tract colonization by Pasteurellaceae, which include the facultative respiratory pathogens Mannheimia haemolytica and Bibersteinia trehalosi, in all eight experimental lambs at 3 months of age.
3.2. Application of nasal wash fluids from M. ovipneumoniae-infected lambs led to successful colonization of specific-pathogen free lambs and induces M. ovipneumoniae-specific serum antibodies.
To analyze the effects of experimental M. ovipneumoniae infection in specific pathogen-free sheep, four lambs each were inoculated with M. ovipneumoniae-positive nasal wash fluids or with PBS. Following inoculation, lambs were monitored for clinical signs for twelve weeks, and nasal swabs, serum and BALs for laboratory analyses were collected as shown in Figure 1A. All lambs in the M. ovipneumoniae group, but none of the lambs in the control group, showed positive PCR results for M. ovipneumoniae at two weeks p.i. (Fig. 1B). M. ovipneumoniae levels peaked at 2–4 weeks, declined at 6 weeks and then plateaued. Colonization with M. ovipneumoniae was confirmed by successful culture of viable mycoplasma in nasal swab samples from two of the experimental lambs at 4 weeks p.i. We did not detect Pasteurellaceae in any nasal swab samples collected on days 0, 56, and 84 of the infection experiment using standard microbiology techniques.
Figure 1: Mycoplasma ovipneumoniae colonization and antibody responses in experimentally infected lambs.
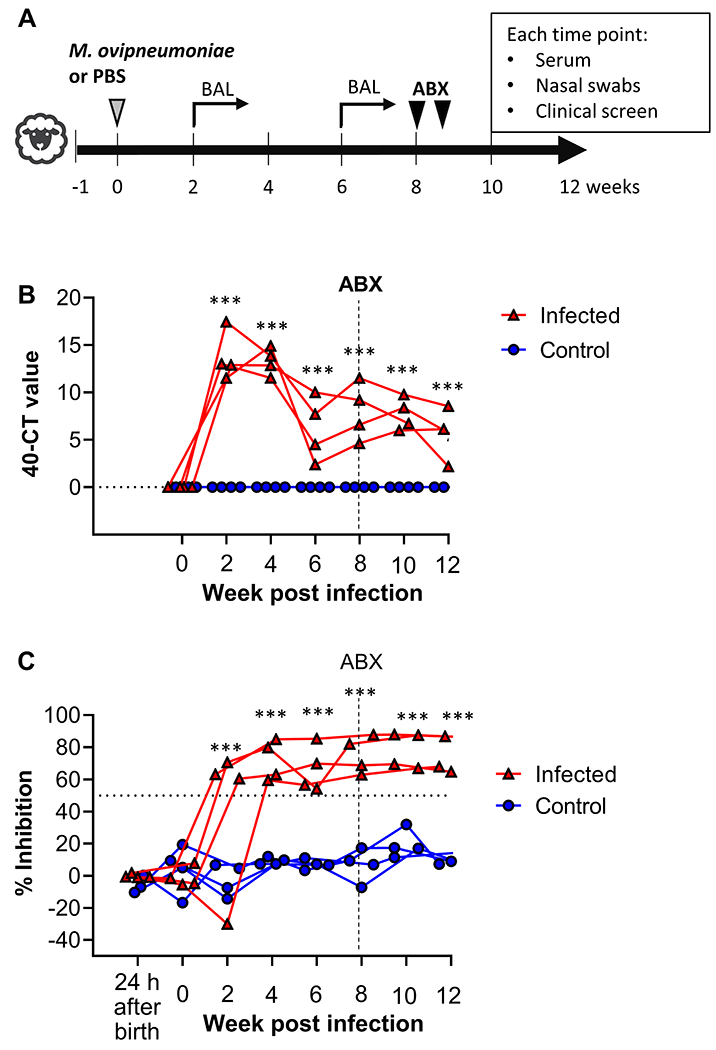
(A) Experimental schedule. Four SPF lambs aged 3 – 4 months were inoculated with M. ovipneumoniae-positive nasal washes, and four control lambs were mock-infected with PBS. All lambs received an antibiotic treatment (gamithromycin) after 8 weeks and were monitored for a total of 12 weeks. (B) M. ovipneumoniae infection levels in nasal swab samples were determined by qPCR. Data are shown as 40 minus Ct value. Triangles represent individual lambs from M. ovipneumoniae infection group, circles represent lambs from control group. Data were analyzed by 2-way ANOVA with Dunnett’s multiple comparisons test for differences between week 0 and other time points. ***P≤0.001 for the M. ovipneumoniae-infected lambs. (C) Mycoplasma ovipneumoniae-specific antibody levels were determined by competitive ELISA analysis of serum samples. Red triangles represent individual lambs from M. ovipneumoniae infection group, blue circles represent lambs from control group. Data were analyzed by 2-way ANOVA with Dunnett’s multiple comparisons test for differences between week 0 and other time points. ***P≤0.001 for the M. ovipneumoniae-infected lambs.
All infected lambs developed a strong M. ovipneumoniae-specific antibody response that peaked at 4 weeks p.i. and remained high throughout the experimental period (Fig. 1C), whereas no significant M. ovipneumoniae-reactive antibodies were detected in the control group. Likewise, no M. ovipneumoniae-reactive antibodies were present in any of the animals one day after birth, demonstrating that the colostrum replacer used did not contain any cross-reactive antibodies that might have contributed to M. ovipneumoniae-resistance through passive antibody transfer.
To assess whether pathogen loads were similar in experimentally and in naturally infected lambs, we compared M. ovipneumoniae colonization levels detected at different time points throughout the experiment in the experimentally infected lambs to those seen in the nasal wash donor lambs, which were sampled at one time point. No significant difference in M. ovipneumoniae colonization was detected between the two groups (data not shown). Likewise, antibody levels detected by ELISA in the experimentally infected lambs at multiple time points did not differ significantly from antibody levels detected in the ewes. These observations suggest that our experimental M. ovipneumoniae infection closely replicated natural infection with regards to pathogen load and immune response.
3.3. M. ovipneumoniae infection in domestic SPF lambs did not cause overt respiratory disease.
Interestingly, careful monitoring of M. ovipneumoniae-inoculated and control lambs for signs of illness including loss of appetite, altered behavior, and respiratory changes did not reveal consistent signs of respiratory disease in response to M. ovipneumoniae infection (Fig. 2A–F). Our comprehensive and objective scoring system (Table 1) showed increased respiratory signs in the M. ovipneumoniae-infected lambs during weeks 1-3 p.i. and a similar increase in the control group during weeks 6-8 p.i. A detailed review of our animal records showed that one of the four lambs in the infected group displayed nasal mucus accumulation in week 1 after inoculation, and coughing was observed on two separate days during week 3, possibly representing a mild, transient respiratory illness in this animal (Fig. 2E). However, coughing was also observed in two control lambs on various days during the experiment. Other respiratory scores were assigned for panting respirations, which again were observed in both the M. ovipneumoniae-infected and the control group. Appetite and body temperatures also varied widely in both groups, and decreased appetite was seen in the control group at several time points (Fig. 2C,D). As described in more detail below (Fig. 6), lambs in both groups had increased clinical scores and received anti-inflammatory treatment following administration of the antibiotic gamithromycin (Fig. 2A,F). Lamb body weights and daily gains measured between days 20 and 55 of the experiment, prior to administration of antibiotics, also did not differ significantly between M. ovipneumoniae-infected and control lambs (data not shown). Overall, clinical signs and scores in the M. ovipneumoniae-infected lambs did not differ markedly from the scores in the non-infected control lambs, indicating that M. ovipneumoniae alone did not consistently cause clinical disease in domestic SPF-lambs under controlled laboratory conditions.
Figure 2: Clinical scoring of lambs following experimental inoculation with M. ovipneumoniae.
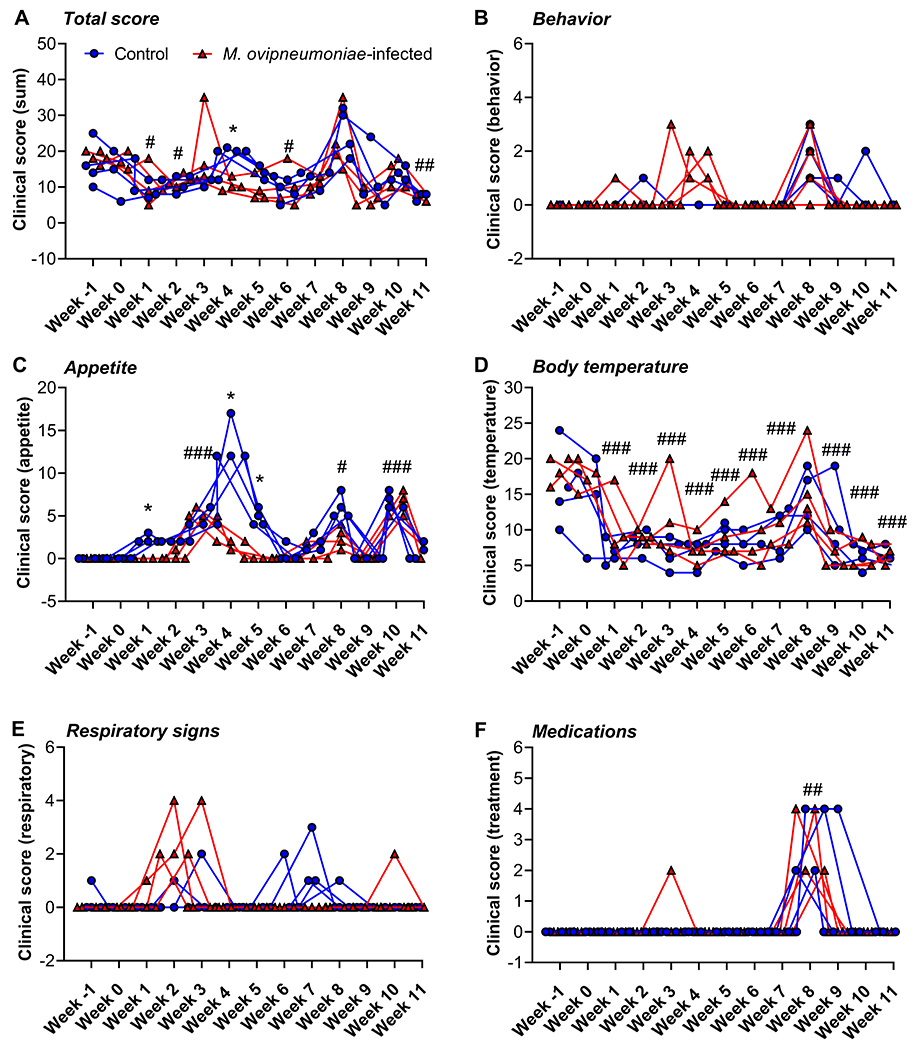
All lambs were screened twice daily for changes in behavior and appetite and for signs of respiratory disease; body temperatures and administered medications were also recorded. Scores were determined using the criteria listed in Table 1. Weekly scores are sums of all 14 scores obtained for each lamb per week and per category. Red triangles represent individual lambs from M. ovipneumoniae infection group, blue circles represent lambs from control group. (A) Total clinical scores, sum of B-F; (B) Behavior scores, (C) Appetite scores, (D) Body temperature scores; (E) Respiratory score; (F) Medication score. Data were analyzed by 2-way ANOVA with Sidak’s multiple comparisons test for differences between the M. ovipneumoniae-infected and control groups. *P≤0.05; **P≤0.01; ***P≤0.001. Differences between individual time points and baseline values (week −1 p.i.) for all lambs are indicated by #P≤0.05; ##P≤0.01; ###P≤0.001.
Differential cell counts performed on BAL samples in order to determine a potential influx inflammatory cells into the lungs showed that >90% of cells present in BAL from both M. ovipneumoniae-infected and uninfected lambs had a typical alveolar macrophage morphology, with oval or round nuclei and cytoplasmic vacuoles (Fig. 3A–C). A small number of lymphocytes, neutrophils and eosinophils were also detected (Fig. 3C). Neither cell composition nor total cell counts differed significantly between the M. ovipneumoniae-infected and non-infected lambs (Fig. 3C, D). We also analyzed the cell-free supernatants for the presence of lactate dehydrogenase (LDH) as a marker of lung injury (Drent et al., 1996). Although there was a trend for increased LDH release upon M. ovipneumoniae infection 14 days after experimental inoculation, no statistically significant differences between the two groups were found at either of the time points analyzed (Fig. 3E), although this may have been due to the small group size in our study. These data suggest that the experimental infection did not cause overt lung inflammation in the SPF lambs, consistent with the clinical findings presented above.
Figure 3: Bronchoalveolar lavage fluid from SPF-lambs experimentally infected with M. ovipneumoniae showed no evidence of lung inflammation.
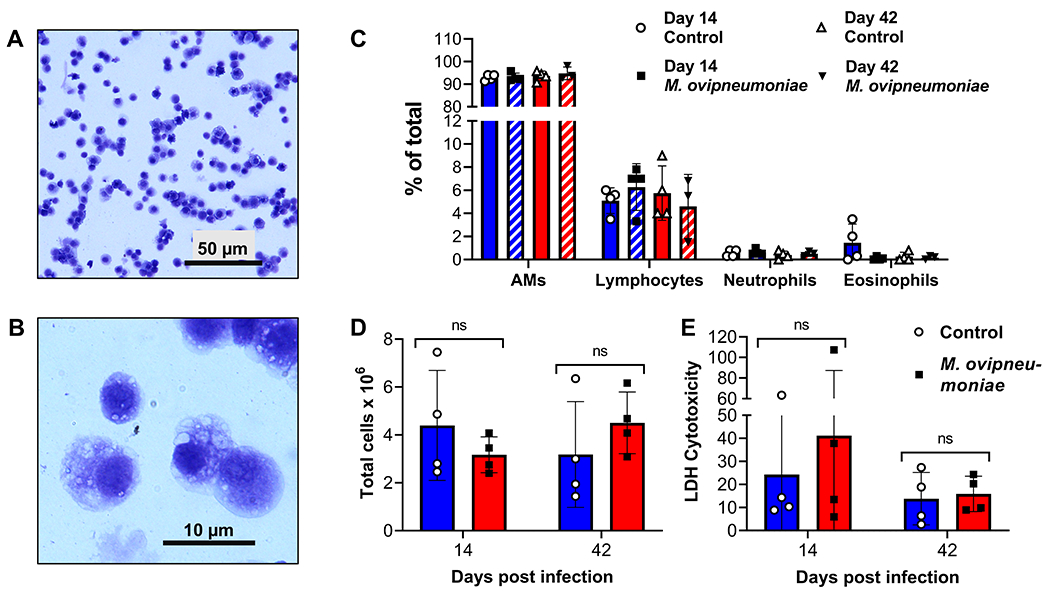
(A, B) Cellular composition of BAL from an M. ovipneumoniae-infected lamb 14 days after experimental inoculation. Representative image from one of four infected sheep shows typical macrophage morphology of the majority of the cells at (A) low and (B) high magnification. (C) Differential cell counts of BAL collected on days 14 and 42 after experimental inoculation from M. ovipneumoniae-infected and non-infected lambs (n=4). (D) Total cell counts of BAL collected on days 14 and 42 after experimental inoculation from M. ovipneumoniae-infected and mock-infected lambs (n=4). (E) Analysis of lactate dehydrogenase in cell-free supernatants of BAL collected on days 14 and 42 p.i.. Individual data points and mean ± SD are shown. Differences between groups were analyzed by Student’s t test.
3.4. High dose treatment with the macrolide antibiotic gamithromycin failed to eliminate M. ovipneumoniae colonization.
We next sought to determine whether M. ovipneumoniae infection could be eliminated by antibiotic treatment with gamithromycin, which is currently used therapeutically to treat M. bovis infection in cattle (Baggott et al., 2011). M. ovipneumoniae that we cultured from the pooled nasal wash fluids used to inoculate the lambs were analyzed for susceptibility to antibiotics using a standard broth microdilution assay (Hannan, 2000). As shown in Fig. 4A,B, both gentamicin and gamithromycin inhibited growth of the nasal wash isolate of M. ovipneumoniae with MICs of 8 μg/mL and 16 μL, respectively. Similarly, minimum inhibitory concentrations of 4 μg/mL (gentamicin) and 32 μg/mL (gamithromycin) were effective against the M. ovipneumoniae type strain Y98.
Figure 4: Antibiotic treatment with gamithromycin did not eliminate asymptomatic M. ovipneumoniae infection.
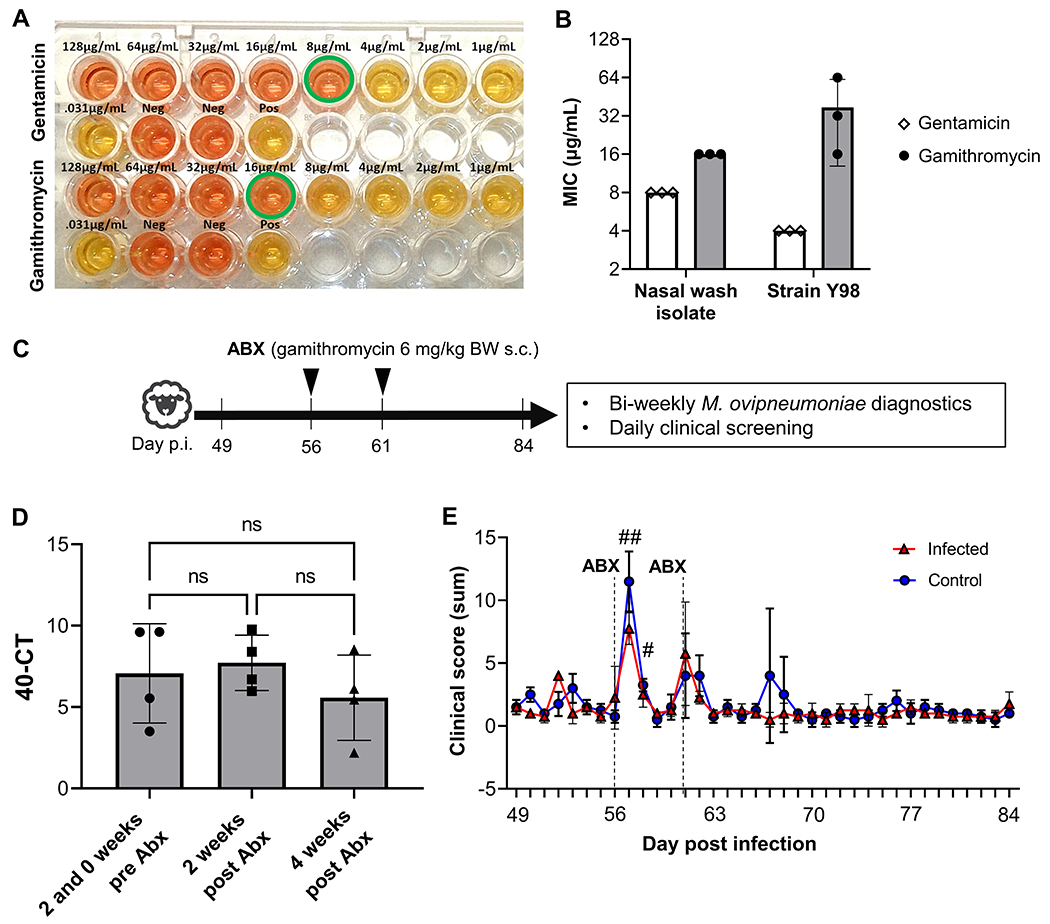
(A) Gamithromycin and gentamicin inhibit growth of M. ovipneumoniae in vitro in a broth microdilution assay. Representative image of a culture plate showing color change from red to yellow reflecting growth of M. ovipneumoniae (nasal wash isolate). Wells with minimum inhibitory concentrations are highlighted by a green circle. (B) Minimum inhibitory concentration (MIC) for gentamicin and gamithromycin detected in three independent broth microdilution experiments performed with the nasal wash isolate and strain Y98. Individual values, mean ± SD are shown. (C) Detailed timeline for antibiotic administration. (D) M. ovipneumoniae infection levels in nasal swab samples of lambs inoculated with M. ovipneumoniae-positive nasal washes were determined by qPCR at 14 and 0 days before administration of gamithromycin (average of two time points) and at 14 and 28 days post administration of gamithromycin. Data are shown as 40 minus Ct value. Differences between time points were analyzed by 1-way ANOVA with, Tukey’s multiple comparisons test; ns: not significant. (E) Clinical scores of M. ovipneumoniae-positive and negative lambs after gamithromycin treatment show side effects of antibiotic administration. All lambs were screened twice daily for changes in behavior and appetite and for signs of respiratory disease; body temperatures and administered medications were also recorded. Scores were determined using the criteria listed in Table 1. Daily scores reflect the sum of two scores per day for all five criteria. Red triangles represent individual lambs from M. ovipneumoniae infection group, blue circles represent lambs from control group. Significant differences (P≤0.05) between individual time points and baseline values (day 49 p.i.) for all lambs are indicated by #.
To evaluate the efficacy of gamithromycin in vivo, all lambs were treated with two doses of gamithromycin on days 56 and 61 after inoculation with M. ovipneumoniae or mock-treatment. (Fig. 4C). However, the antibiotic treatment neither eliminated nor significantly decreased the level of M. ovipneumoniae infection as determined by qPCR in any of the lambs either at 2 or at 4 weeks after the treatment (Fig. 4D). Notably, statistically significant side effects of the gamithromycin treatment including changes in behavior, decreased appetite, and increased body temperatures, were observed after treatment in both the M. ovipneumoniae-infected lambs and the control group (Fig. 4E). To counteract these side effects, anti-inflammatory treatment was administered to some of the animals (Fig. 2F). These data strongly indicate that gamithromycin is unsuitable for treatment of M. ovipneumoniae infection in domestic lambs, since it does not eliminate infection, but has considerable adverse effects.
4. Discussion
Mycoplasma ovipneumoniae is a key co-factor in the pathogenesis of chronic, atypical pneumonia in sheep, which has been associated with production losses in domestic lambs and significant deaths in wild sheep populations. In our study, we sought to define whether M. ovipneumoniae infection causes clinical respiratory disease in immunologically naïve, SPF domestic lambs. We also tested whether established M. ovipneumoniae infection could be eliminated by antibiotic treatment. We found that M. ovipneumoniae mostly caused stable, asymptomatic colonization of the upper respiratory tract that induced a significant antibody response, but that could not be cleared by gamithromycin application.
Our SPF protocol resulted in lambs that were initially free from M. ovipneumoniae-infection and M. ovipneumoniae-specific antibodies, with no indication of transplacental transfer of M. ovipneumoniae or antibodies to the lambs. In humans, vertical transmission of Mycoplasmas that colonize the genitourinal tract across the placenta has been described (Chu et al., 2020), and in cattle, blood-borne Mycoplasma species such as Candidatus M. haemobos and M. wenyonii can be transmitted in utero (Hornok et al., 2011), in spite of the multi-layered synepitheliochorial placenta found in ruminants. Since M. ovipneumoniae is a respiratory tract bacterium that is not known to spready systemically in sheep, vertical transmission is unlikely, but cannot be excluded. However, we did not find any evidence that such transmission occurred.
Whether M. ovipneumoniae causes respiratory disease in healthy domestic lambs has been a matter of debate (Du et al., 2020; Foggie et al., 1976; Jones et al., 1982b). Our experimental conditions that included supervised lambing followed by artificial rearing inside an ABSL-2 facility were specifically designed to prevent exposure of the experimental lambs to respiratory pathogens other than the M. ovipneumoniae used in the infections. Repeated testing confirmed that Pasteurellaceae, which are major causative agents of pneumonia in lambs, were not present in any of the lambs before or at any time during the experimental period. While it was not possible to screen for all relevant respiratory pathogens, our protocol likely eliminated additional viruses, parasites and bacteria that may have been present in the ewes, strongly suggesting that M. ovipneumoniae was the only relevant respiratory pathogen present in our experiment. The twice-daily clinical screens and cellular and molecular analyses of the bronchoalveolar lavage fluid demonstrated that active M. ovipneumoniae colonization of the upper airways did not consistently lead to overt clinical disease or lung pathology in our model. Although we detected mild respiratory signs, i.e. very occasional coughing, in one out of four M. ovipneumoniae-infected lambs, identical signs were also observed in the uninfected control lambs at a similar frequency, albeit at different times. Therefore, it is unclear whether these mild signs were associated with the ongoing M. ovipneumoniae infection. In contrast to recent studies (Besser et al., 2019; Manlove et al., 2019), we also did not observe any differences in lamb body weights or daily gains associated with M. ovipneumoniae infection .
Multiple different hypotheses have been proposed to explain why M. ovipneumoniae causes asymptomatic airway colonization in some instances, but severe pneumonia in other cases: (1) differences in M. ovipneumoniae strain virulence; (2) age-related differences in disease susceptibility; (3) differences in the immune response to M. ovipneumoniae; and (4) presence of additional facultative pathogens such as Mannheimia haemolytica. Asymptomatic or mild M. ovipneumoniae infections as seen in our study may be caused by experimental inoculation with non-pathogenic M. ovipneumoniae variants, since M. ovipneumoniae strains are highly diverse and can differ in their virulence (Niang et al., 1998). Moreover, Mycoplasma may lose their virulence upon prolonged culture (Niang et al., 1998). Such unintended, culture-dependent attenuation of M. ovipneumoniae isolates has been discussed as the cause for asymptomatic M. ovipneumoniae infection in several previous studies (Besser et al., 2014; Buddle et al., 1984). Therefore, we used fresh nasal washes obtained from M. ovipneumoniae-positive lambs that showed signs of respiratory disease at the time of nasal wash collection as the infectious inoculum in our study. Because of this approach, that the lack of consistent clinical signs upon experimental M. ovipneumoniae infection likely was not due to the use of an avirulent or attenuated M. ovipneumoniae strain. A limitation of using nasal washes as a source of M. ovipneumoniae is that the exact bacterial strain and its characteristics remain unknown, and infections with multiple strains of M. ovipneumoniae or other pathogenic contaminants cannot be excluded.
With regard to the hypothesis that age-related differences are crucial, it is generally accepted that clinical infections with M. ovipneumoniae most commonly occur in lambs under one year of age, while adult sheep serve as asymptomatic M. ovipneumoniae carriers (Martin, 1996; USDA, 2013). Plowright et al. (2017). showed that, in bighorn sheep, prevalence of M. ovipneumoniae was highest in lambs and aged animals, but low in adults, pointing to age-dependent infection dynamics. However, it is unclear whether the increased susceptibility of lambs was due to the lack of established adaptive immune responses to M. ovipneumoniae or other age-related differences in respiratory anatomy, physiology, or immunity. Interestingly, Gilmour et al. (1979) found increased lung pathology in seven-month-old lambs compared to five-week-old lambs upon experimental M. ovipneumoniae infection. In our study, M. ovipneumoniae infection in four-month old lambs led to colonization in the absence of overt respiratory disease. In bighorn sheep, yearlings and older adults with no previous M. ovipneumoniae infection were equally susceptible to M. ovipneumoniae-induced pneumonia(Besser et al., 2014). Thus, age-dependent mechanisms alone do not explain the variable susceptibility of sheep to M. ovipneumoniae disease.
In bighorn sheep, introduction of M. ovipneumoniae into herds with no prior exposure to the pathogen leads to particularly devastating disease outbreaks, suggesting that immunologically naïve populations are especially prone to symptomatic infections. In vitro studies have shown that M. ovipneumoniae specific antibodies can have protective functions by mediating opsonization and phagocytosis of the Mycoplasma (Al-Kaissi and Alley, 1983). Likewise, Niang et al. (1999) found increased levels of M. ovipneumoniae-reactive antibodies in lambs that had recovered from the clinical M. ovipneumoniae infection. In our study, lambs had no detectable M. ovipneumoniae specific antibodies at any point before experimental inoculation, consistent with the absence of M. ovipneumoniae-specific antibodies in the colostrum replacer and the lack of placental antibody transfer in sheep. However, all inoculated lambs developed a significant, stable antibody response within two weeks after infection, confirming previous studies that have tracked humoral responses to M. ovipneumoniae(Thirkell et al., 1990). These observations indicate that lack of existing immunity does not lead to more severe disease in M. ovipneumoniae infected sheep. Interestingly, the development of an antibody response was not associated with decreased M. ovipneumoniae colonization in our study. A possible explanation for this phenomenon is that antibody levels measured in serum consist mainly of IgG, which may have protective functions within the lungs, whereas a strong mucosal IgA response may be necessary to eliminate asymptomatic M. ovipneumoniae colonization from the upper respiratory tract. Antibody isotypes and mucosal antibodies were not analyzed in this study but may be a subject of future investigations.
Overall, our findings that M. ovipneumoniae application in domestic lambs did not cause significant respiratory disease were in line with earlier studies that showed that pneumonia develops as a result of M. ovipneumoniae co-infections with other facultative bacterial pathogens (Besser et al., 2013; Buddle et al., 1984; Jones et al., 1982a). In bighorn sheep, where a large number of studies have been performed, M. ovipneumoniae is likely essential for causing severe respiratory disease (Besser et al., 2012), but consistent clinical disease and lung pathology only developed when additional agents such as Mannheimia haemolytica, Bibersteinia trehalosi or Pasteurella multocida (Besser et al., 2013; Dassanayake et al., 2010) also were present. In further support of this hypothesis, the lambs that served as donors for the nasal wash fluids that we used to inoculate the experimental animals in our study showed clinical signs of respiratory disease and tested positive for Mannheimia haemolytica and Bibersteinia trehalosi in addition to the M. ovipneumoniae.
Even if M. ovipneumoniae alone does not cause clinical respiratory disease in domestic sheep, an effective treatment strategy that eliminates M. ovipneumoniae infection from domestic sheep flocks could reduce production losses due to complex respiratory disease and protect bighorn sheep from devastating pneumonia epizootics. To address this issue, we analyzed whether antibiotic therapy could eliminate M. ovipneumoniae colonization in our asymptomatically infected lambs. We chose the macrolide antibiotic gamithromycin, because gamithromycin application in goats infected with Mycoplasma and Mannheimia was successful in treatment of clinical pneumonia (Kacar et al., 2018), and preventive treatment with gamithromycin in cattle was successful for reducing bovine respiratory disease, which commonly involves Mycoplasma spp. (Baggott et al., 2011). A recent study by Jay et al. (2020) on the antibiotic susceptibility of M. ovipneumoniae showed homogenously low minimum inhibitory concentrations for a wide range of antimicrobials including macrolides, with no indication of significant antimicrobial resistance. While the minimum inhibitory concentrations identified in our assay may not be directly comparable to the data in Jay’s paper, since a different experimental protocol was used and our antimicrobial susceptibility test was not fully validated, our analysis also demonstrated that the challenge strain used here was susceptible to gamithromycin in vitro. Since gamithromycin is currently not approved for use in sheep in the US, the presence of resistant M. ovipneumoniae strains in the infected donor sheep was unlikely. However, our in vivo data showed no significant reduction in M. ovipneumoniae colonization levels in our experimental lambs following two injections of the recommended gamithromycin dose. It remains to be investigated whether failure of the antibiotic treatment was due to bacterial biofilm formation (McAuliffe et al., 2006), intracellular location of the mycoplasma (Einarsdottir et al., 2018) or an alternative antimicrobial resistance mechanism. Notably, all lambs developed significant side effects following gamithromycin administration, including increased body temperatures, decreased appetites and altered behavior as well as local inflammation at the injection sites that warranted the application of analgesics. These side effects were greatly more severe than the transient mild to moderate swellings at the site of injection described for this drug by the European Medicines Agency (EMEA).
Why domestic lambs tolerate M. ovipneumoniae infection, while bighorn lambs suffer severe clinical disease may thus be due to host genetics and will require further investigations. The inoculation approach that involved nasal, oral and ocular application of four-month-old domestic SPF lambs with ceftiofur-treated nasal washes from naturally infected, symptomatic lambs led to 100% successful upper airway colonization with M. ovipneumoniae, but not with Pasteurellaceae, confirming earlier studies (Besser et al., 2017; Besser et al., 2014). Colonization levels as determined based on the Ct values in the qPCR reactions was similar in experimentally and naturally infected lambs, and antibody levels measured by M. ovipneumoniae-specific ELISA also closely replicated antibody levels seen upon natural infection.
5. Conclusion
In conclusion, our infection model represents a valid tool to investigate the role of M. ovipneumoniae in sheep respiratory disease. In future studies, we plan to utilize this M. ovipneumoniae infection model to explore novel preventative or treatment approaches and to define how interactions between M. ovipneumoniae and other facultative respiratory pathogens lead to clinical respiratory disease and pulmonary pathology in domestic sheep.
Acknowledgements
We gratefully acknowledge support by NIFA AFRI Animal Health & Disease Program grant no. # 2018-06895 from the USDA National Institute of Food and Agriculture for this work. This study was also supported by the Montana Agricultural Experiment Station, USDA/NIFA Animal Health funds and the Johnson Family Foundation. Our sincerest thanks go to the sheep team at the Johnson Family Livestock Facility and to Dr. Bruce Sorensen for providing excellent care of our animals. We also would like to thank Andy Sebrell, Marziah Hashimi and Farimah Moghimpour for assisting with sample processing throughout our study. We also thank Dr. Patrick Hatfield, MSU Department of Animal and Range Sciences, for providing access to lambs naturally infected with M. ovipneumoniae. Many thanks go to Dr. Martin Ganter, Veterinary University of Hannover, Germany, for helpful discussions during the planning phase of this study. Finally, we would like to thank Daniel Bradway and all the other staff from the Washington Animal Disease Diagnostic Laboratory for sample analysis.
References
- Al-Kaissi A, Alley MR, 1983. Electron microscopic studies of the interaction between ovine alveolar macrophages and Mycoplasma ovipneumoniae in vitro. Vet Microbiol 8, 571–584. [DOI] [PubMed] [Google Scholar]
- Baggott D, Casartelli A, Fraisse F, Manavella C, Marteau R, Rehbein S, Wiedemann M, Yoon S, 2011. Demonstration of the metaphylactic use of gamithromycin against bacterial pathogens associated with bovine respiratory disease in a multicentre farm trial. Vet Rec 168, 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser TE, Cassirer EF, Potter KA, Foreyt WJ, 2017. Exposure of bighorn sheep to domestic goats colonized with Mycoplasma ovipneumoniae induces sub-lethal pneumonia. PLoS One 12, e0178707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser TE, Cassirer EF, Potter KA, Lahmers K, Oaks JL, Shanthalingam S, Srikumaran S, Foreyt WJ, 2014. Epizootic pneumonia of bighorn sheep following experimental exposure to Mycoplasma ovipneumoniae. PLoS One 9, e110039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser TE, Cassirer EF, Potter KA, VanderSchalie J, Fischer A, Knowles DP, Herndon DR, Rurangirwa FR, Weiser GC, Srikumaran S, 2008. Association of Mycoplasma ovipneumoniae infection with population-limiting respiratory disease in free-ranging Rocky Mountain bighorn sheep (Ovis canadensis canadensis). J Clin Microbiol 46, 423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besser TE, Cassirer EF, Yamada C, Potter KA, Herndon C, Foreyt WJ, Knowles DP, Srikumaran S, 2012. Survival of bighorn sheep (Ovis canadensis) commingled with domestic sheep (Ovis aries) in the absence of Mycoplasma ovipneumoniae. J Wildl Dis 48, 168–172. [DOI] [PubMed] [Google Scholar]
- Besser TE, Frances Cassirer E, Highland MA, Wolff P, Justice-Allen A, Mansfield K, Davis MA, Foreyt W, 2013. Bighorn sheep pneumonia: sorting out the cause of a polymicrobial disease. Prev Vet Med 108, 85–93. [DOI] [PubMed] [Google Scholar]
- Besser TE, Levy J, Ackerman M, Nelson D, Manlove K, Potter KA, Busboom J, Benson M, 2019. A pilot study of the effects of Mycoplasma ovipneumoniae exposure on domestic lamb growth and performance. PLoS One 14, e0207420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bimczok D, Roehl FW, Ganter M, 2005. Evaluation of lamb performance and costs in motherless rearing of German Grey Heath sheep under field conditions using automatic feeding systems. Small Rum. Res 60 255–265. [Google Scholar]
- Black SR, Barker IK, Mehren KG, Crawshaw GJ, Rosendal S, Ruhnke L, Thorsen J, Carman PS, 1988. An epizootic of Mycoplasma ovipneumoniae infection in captive Dall’s sheep (Ovis dalli dalli). J Wildl Dis 24, 627–635. [DOI] [PubMed] [Google Scholar]
- Buddle BM, Herceg M, Davies DH, 1984. Experimental infection of sheep with Mycoplasma ovipneumoniae and Pasteurella haemolytica. Vet Microbiol 9, 543–548. [DOI] [PubMed] [Google Scholar]
- Chu A, de St Maurice A, Sim MS, Kallapur SG, 2020. Neonatal Mycoplasma and Ureaplasma Infections. Pediatr Ann 49, e305–e312. [DOI] [PubMed] [Google Scholar]
- Dassanayake RP, Shanthalingam S, Herndon CN, Subramaniam R, Lawrence PK, Bavananthasivam J, Cassirer EF, Haldorson GJ, Foreyt WJ, Rurangirwa FR, Knowles DP, Besser TE, Srikumaran S, 2010. Mycoplasma ovipneumoniae can predispose bighorn sheep to fatal Mannheimia haemolytica pneumonia. Vet Microbiol 145, 354–359. [DOI] [PubMed] [Google Scholar]
- Drent M, Cobben NA, Henderson RF, Wouters EF, van Dieijen-Visser M, 1996. Usefulness of lactate dehydrogenase and its isoenzymes as indicators of lung damage or inflammation. Eur Respir J 9, 1736–1742. [DOI] [PubMed] [Google Scholar]
- Du Z, Sun Y, Wang J, Liu H, Yang Y, Zhao N, 2020. Comprehensive RNA-Seq profiling of the lung transcriptome of Bashbay sheep in response to experimental Mycoplasma ovipneumoniae infection. PLoS One 15, e0214497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einarsdottir T, Gunnarsson E, Hjartardottir S, 2018. Icelandic ovine Mycoplasma ovipneumoniae are variable bacteria that induce limited immune responses in vitro and in vivo. J Med Microbiol 67, 1480–1490. [DOI] [PubMed] [Google Scholar]
- Foggie A, Jones GE, Buxton D, 1976. The experimental infection of specific pathogen free lambs with Mycoplasma ovipneumoniae. Res Vet Sci 21, 28–35. [PubMed] [Google Scholar]
- Gilmour JS, Jones GE, Rae AG, 1979. Experimental studies of chronic pneumonia of sheep. Comp Immunol Microbiol Infect Dis 1, 285–293. [DOI] [PubMed] [Google Scholar]
- Handeland K, Tengs T, Kokotovic B, Vikoren T, Ayling RD, Bergsjo B, Sigurethardottir OG, Bretten T, 2014. Mycoplasma ovipneumoniae--a primary cause of severe pneumonia epizootics in the Norwegian Muskox (Ovibos moschatus) population. PLoS One 9, e106116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan PC, 2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet Res 31, 373–395. [DOI] [PubMed] [Google Scholar]
- Hornok S, Micsutka A, Meli ML, Lutz H, Hofmann-Lehmann R, 2011. Molecular investigation of transplacental and vector-borne transmission of bovine haemoplasmas. Vet Microbiol 152, 411–414. [DOI] [PubMed] [Google Scholar]
- Jay M, Ambroset C, Tricot A, Colin A, Tardy F, 2020. Population structure and antimicrobial susceptibility of Mycoplasma ovipneumoniae isolates in France. Vet Microbiol 248, 108828. [DOI] [PubMed] [Google Scholar]
- Jones GE, Gilmour JS, Rae AG, 1982a. The effect of Mycoplasma ovipneumoniae and Pasteurella haemolytica on specific pathogen-free lambs. J Comp Pathol 92, 261–266. [DOI] [PubMed] [Google Scholar]
- Jones GE, Gilmour JS, Rae AG, 1982b. The effects of different strains of Mycoplasma ovipneumoniae on specific pathogen-free and conventionally-reared lambs. J Comp Pathol 92, 267–272. [DOI] [PubMed] [Google Scholar]
- Kacar Y, Batmaz H, Yilmaz OE, Mecitoglu Z, 2018. Comparing clinical effects of marbofloxacin and gamithromycin in goat kids with pneumonia. J S Afr Vet Assoc 89, e1–e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovic Z, Bacic A, Rifatbegovic M, 2020. Antimicrobial Susceptibility of Caprine and Ovine Mycoplasma ovipneumoniae Isolates. Microb Drug Resist 26, 1271–1274. [DOI] [PubMed] [Google Scholar]
- Manlove K, Branan M, Baker K, Bradway D, Cassirer EF, Marshall KL, Miller RS, Sweeney S, Cross PC, Besser TE, 2019. Risk factors and productivity losses associated with Mycoplasma ovipneumoniae infection in United States domestic sheep operations. Prev Vet Med 168, 30–38. [DOI] [PubMed] [Google Scholar]
- Martin WB, 1996. Respiratory infections of sheep. Comp Immunol Microbiol Infect Dis 19, 171–179. [DOI] [PubMed] [Google Scholar]
- McAuliffe L, Ellis RJ, Miles K, Ayling RD, Nicholas RAJ, 2006. Biofilm formation by mycoplasma species and its role in environmental persistence and survival. Microbiology (Reading) 152, 913–922. [DOI] [PubMed] [Google Scholar]
- Niang M, Rosenbusch RF, DeBey MC, Niyo Y, Andrews JJ, Kaeberle ML, 1998. Field isolates of Mycoplasma ovipneumoniae exhibit distinct cytopathic effects in ovine tracheal organ cultures. Zentralbl Veterinarmed A 45, 29–40. [DOI] [PubMed] [Google Scholar]
- Niang M, Rosenbusch RF, Lopez-Virella J, Kaeberle ML, 1999. Differential serologic response to Mycoplasma ovipneumoniae and Mycoplasma arginini in lambs affected with chronic respiratory disease. J Vet Diagn Invest 11, 34–40. [DOI] [PubMed] [Google Scholar]
- Plowright RK, Manlove KR, Besser TE, Paez DJ, Andrews KR, Matthews PE, Waits LP, Hudson PJ, Cassirer EF, 2017. Age-specific infectious period shapes dynamics of pneumonia in bighorn sheep. Ecol Lett 20, 1325–1336. [DOI] [PubMed] [Google Scholar]
- Thirkell D, Spooner RK, Jones GE, Russell WC, 1990. The humoral immune response of lambs experimentally infected with Mycoplasma ovipneumoniae. Vet Microbiol 24, 143–153. [DOI] [PubMed] [Google Scholar]
- USDA 2013. Sheep 2011, “Part III: Health and Management Practices on U.S. Sheep Operations, 2011” (USDA–APHIS–VS–CEAH–NAHMS. Fort Collins, CO: ). [Google Scholar]
- Yang F, Dao X, Rodriguez-Palacios A, Feng X, Tang C, Yang X, Yue H, 2014. A real-time PCR for detection and quantification of Mycoplasma ovipneumoniae. J Vet Med Sci 76, 1631–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]


