Abstract
Background:
A previous large randomized trial indicated preconception-initiated assignment to low-dose aspirin (LDA) did not positively affect pregnancy outcomes. However, this trial was subject to nonadherence which is not accounted for with the intent-to-treat approach.
Objective:
We estimated per-protocol effects of preconception-initiated LDA on pregnancy loss and livebirth.
Design:
We used the Effects of Aspirin on Gestation and Reproduction Trial to construct a prospective cohort for a post-hoc analysis.
Setting:
Four university medical centers in the U.S.
Participants:
1,227 women between 18-40 years of age with 1-2 prior pregnancy losses attempting pregnancy.
Exposures:
Adherence to low-dose aspirin or placebo, assessed by pill bottle weight measurements at regular intervals over follow-up.
Main Outcomes and Measures:
Primary outcomes were hCG detected pregnancies, pregnancy losses, and live births from pregnancy tests and medical records.
Results:
Relative to placebo, adhering to LDA for 5/7 days per week led to 8 more hCG pregnancies (95% CI 4.64, 10.96), 15 more live births (95% CI 7.65, 21.15), and 6 fewer pregnancy losses (95% CI −12.00, −0.20), for every 100 women in the trial. Additionally, relative to taking placebo, postconception initiation of LDA led to a reduction in the estimated effects. Furthermore, a minimum of 4/7 days per week was needed to obtain effects.
Conclusions and Relevance:
Per protocol results suggest taking LDA prior to conception at least 4 days per week may improve reproductive outcomes for women who have experienced one or two prior pregnancy losses. Increasing adherence to daily LDA appears key to improving effectiveness.
Trial Registration:
INTRODUCTION
Aspirin is a widely available non-steroidal anti-inflammatory drug, with beneficial hemodynamic and immunomodulatory effects (1, 2). Accordingly, low dose aspirin (LDA) is used in perinatal medicine for prevention of preeclampsia in at-risk women (3), and prevention of pregnancy loss among women with antiphospholipid syndrome (4). However prior trials examining pregnancy outcomes initiated aspirin therapy after 12 weeks’ gestation, leaving unstudied the potential effects on the most common reproductive complications of subfertility and early pregnancy loss (5). The Effects of Aspirin in Gestation and Reproduction (EAGeR) trial was designed to fill this gap, examining preconception-initiated low dose aspirin treatment on pregnancy loss and live birth in 1,227 women who were trying to become pregnant after experiencing one or two prior pregnancy losses (6).
EAGeR’s primary intention-to-treat (ITT) results demonstrated a 10% increase in livebirths in the aspirin treatment arm relative to placebo, but without precision warranting changes to clinical recommendations (95% CI: 0.98, 1.22) (7). Furthermore, no impact on pregnancy loss (13% in LDA vs 12% in placebo) was observed (7). Interestingly, secondary analyses indicated aspirin may confer some benefits, including higher incidence of both human chorionic gonadotropin (hCG) and ultrasound confirmed pregnancies (7), a shorter time to pregnancy among women with a single recent pregnancy loss (8), and restoration of decreased pregnancy and live birth rate in women with chronic inflammation (9). However, these prior findings were subject to complications often affecting ITT analyses, most notably, nonadherence with assigned treatment, which may be influenced by common pregnancy symptoms, such as nausea and bleeding, that are also potential side effects of the treatment (10).
Our objective was to estimate the per-protocol effects of LDA on hCG pregnancy, live birth, and pregnancy loss in the EAGeR trial. We also examined the optimal timing of LDA initation (either prior to conception or after pregnancy recognition), and the variation in effects by the average number of days per week LDA was used.
METHODS
Population
We conducted a post-hoc per protocol analysis of data from the EAGeR trial (2007-2011), which was a multicenter, block randomized, double-blind, placebo-controlled trial (Clinical Trials Registration: #NCT00467363) in four University medical centers in the United States (6, 7). Briefly, eligible women were between 18-40 years of age, actively trying to conceive, had a history of one or two documented pregnancy losses, had regular menstrual cycles of 21-42 days in length during the preceding 12 months, and no history of infertility. Women were followed for up to six cycles while attempting pregnancy, and throughout pregnancy if they conceived (the duration of total follow-up ranged from 1 to 60 weeks, median = 37 weeks). Written informed consent was provided by all participants. The data coordinating center and each site obtained Institutional Review Board approvals. The Data Safety and Monitoring Board ensured participant safety.
Intervention
Women were randomized 1:1 to 81 mg aspirin per day or placebo; all received 400 mcg folic acid. All participants and care providers were blinded to treatment allocation. Women were instructed to take study medication daily throughout six menstrual cycles, and until the 36th week of gestation if they conceived.
Covariate data
Demographic and lifestyle information was assessed via baseline questionnaires. Bleeding and nausea and/or vomiting were assessed prospectively over follow-up, measured via daily diaries for the first two months, and via clinical questionnaires roughly once a month for the remaining follow-up. In any given week of follow-up, bleeding was defined as present if the woman experienced any unusual or excessive bleeding, including vaginal or other bleeding, during that week. Similarly, in any given week nausea and/or vomiting was defined as present if the woman experienced any nausea and/or any vomiting during that week.
Outcome
The primary outcome of interest was live birth. Secondary outcomes included hCG pregnancy and pregnancy losses. hCG pregnancies were identified via (i) a positive result on a “real-time” urine pregnancy test (Quidel Quickvue, Quidel Corporation, San Diego, CA), sensitive to 25 mIU/ml hCG, conducted at home or any study visit during expected menses; and (ii) batched urine hCG testing performed after study completion on stored samples from the last 10 days of each woman’s first and second cycle (n=21 additional pregnancies detected).(11) Pregnancy losses were defined as a 1) positive urine hCG pregnancy test at home or the clinical site followed by absence of signs of clinical pregnancy at the study ultrasound; 2) positive hCG from batched augmented urine testing followed by the absence of a positive pregnancy test at home or in the clinic (11); or 3) loss after ultrasound confirmation. Live birth was defined as a live born infant as indicated on the medical record.
Adherence
Daily adherence with both LDA and placebo was assessed using bottle-weight measurements conducted at regular intervals over follow-up. The median number of days between bottle weight measurements was 27 (IQR: 15, 32). Since less than daily adherence may achieve some biological effect (12), daily adherence measures were averaged over a running 7-day period, yielding the proportion of each week in which either treatment or placebo was taken. A woman was deemed “adherent” if she took the assigned treatment (either LDA or placebo) for ≥ 5/7 days of the running 7 day period.
Statistical Analyses
Descriptive Analyses
Changes in adherence, using the ≥ 5/7 days/week criterion, were evaluated visually using a locally weighted scatterplot smoother. Baseline characteristics were compared between women defined as adherent for ≥70% of their person-time versus <70% of their person-time.
Per Protocol Analysis
We used g computation (also known as the parametric g formula) (13) to quantify the effects of aspirin under ideal conditions in which all women adhere to the protocols we define below. This approach is a generalization of standardization that can adjust for both baseline and time-varying confounders, and is useful when there are time-varying factors (e.g., bleeding, nausea) that may be linked to past and future adherence and be associated with outcomes of interest (14, 15). We specified a causal diagram depicting the assumed causal relations between the outcomes, the time-varying confounders, and adherence to the defined protocol (Supplemental Figure S1). Based on this causal diagram, we modeled each causal relations via GLMs in the sample of 1,227 women with a total of 42,697 person-weeks. A total of eight GLMs were fit to the data, representing models for live birth, pregnancy loss, withdrawal, no pregnancy, hCG pregnancy, adherence, bleeding, and nausea and/or vomiting. All models were stratified by the randomized treatment indicator. Estimates of the per protocol effects were obtained by: 1) obtaining a Monte Carlo resample of M = 10,000 of all baseline data, and time-varying data at the first week on study; 2) using the eight GLMs fit to the original data and the Monte Carlo resample, generate outcomes over all 60 weeks of follow-up that would be observed under the desired protocols; 3) compute the probability of hCG pregnancy, pregnancy loss, and live birth from the follow-up generated from the Monte Carlo resample under the desired protocol. Full details are provided in the supporting documentation.
Using g computation, we estimated the per protocol effects of preconception-initiated LDA taken 5/7 days per week on hCG pregnancy, live birth, and pregnancy loss. Optimal timing of aspirin initiation was explored by estimating the effects of initiating aspirin after the 6th, 8th, 12th or 20th week post-pregnancy recognigtion. We further estimated per protocol effects if adherence was defined as taking the assigned treatment 2/7, 3/7, 4/7, and 6/7 days per week.
We computed the probability of hCG pregnancy, pregnancy loss, and live birth under several protocols: (i) all women assigned and adhered to placebo from the preconception period through to 36 weeks gestation (the referent protocol); (ii) all women assigned and adhered to aspirin from the preconception period through to 36 weeks gestation; and (protocols iii-vi) all women were assigned and adhered to placebo prior to pregnancy detection, then switched and adhered to aspirin beginning at 6, 8, 12, or 20 weeks post pregnancy detection and continued through to 36 weeks gestation.
Comparing the outcomes from scenarios i (adherence to placebo) and ii (adherence to aspirin) yields an estimate of the per protocol effect of aspirin. Comparing the outcomes from scenarios ii and iii-vi yields an estimate of the effect of aspirin when therapy was initiated only after the 6th, 8th, 12th, or 20th week post-conception. All 1,227 women were used to compute these effects. We quantified all effects on the risk difference and risk ratio scales. The normal-interval bootstrap was used to obtain 95% confidence intervals (16).
Baseline confounders consisted of age at study entry (years), income (≥$40,000 versus <$40,000), race (white versus other), education (≥ high-school versus other), marital status (married versus other), employment status (employed versus other), body mass index (kg/m2), exercise history (low, moderate, high), tobacco consumption history (ever versus never), and alcohol consumption history (ever versus never).
Time-varying confounders included bleeding (any versus none in a given week), gastrointestinal symptoms (any versus none in a given week). Additionally, hCG-pregnancy was also considered a time-varying confounder of the relation between adherence and live birth and pregnancy loss (14, 15). All continuous and categorical variables were coded using natural cubic splines and dummy variable coding, respectively. Bleeding, nausea, and adherence were also lagged by one week to account for potential feedback relationships (17).
Full details on the g computation procedure, including all models fit, the Monte Carlo resampling procedure, as well as the data structure used for the analysis, are provided in the supporting documentation. Information on model validation strategies and the handling and impact of missing data is also provided in the supporting documentation. All analyses were done in R version 4.0.2 (R Foundation, Vienna, Austria). Code to reproduce all results is available on Github (https://github.com/ainaimi/EAGeR-PerProtocol).
The funding sources played no role in the design, conduct, and analysis of this study, nor in the decision to submit the manuscript for publication.
RESULTS
Overall, 1,228 women were recruited into the trial (615 aspirin, 613 placebo). One woman was excluded from the analysis due to missing follow-up data (615 aspirin, 612 placebo). In the initial week of follow up, 96% of women were adherent with study protocol. No summary difference was observed in adherence over all follow up between women assigned to aspirin or placebo (68% versus 66%, p for difference = 0.130). However, among those who conceived, adherence dropped from an average of 74% prior to conception, to 64% following conception (p<0.001, Figure 1). Overall, 54% (N = 664) of the 1,227 women in the trial adhered with the protocol at ≥70% of their person-time on study. These women were more likely to be married, white, had higher income, and were less likely to smoke or withdraw from the study (Table 1).
Figure 1.
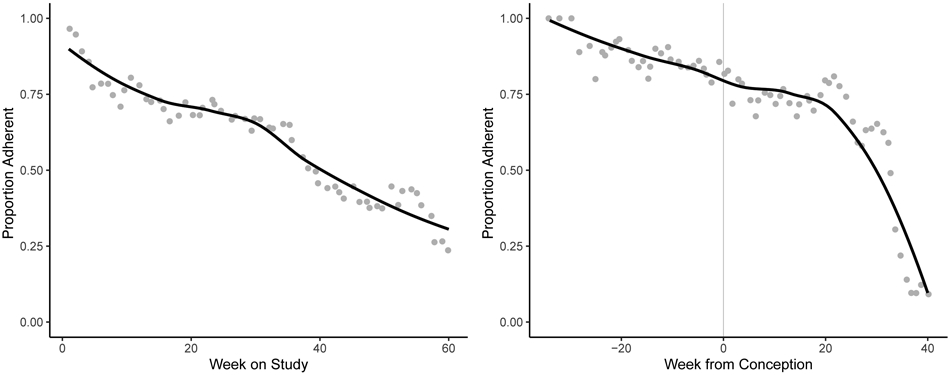
Proportion of all 1,227 women who adhered with the randomized treatment assignment over all person-weeks of the study ranging from the first week to the 60th week post randomization (left panel), and the person-weeks prior to and proceeding hCG pregnancy (right panel).
Table 1:
Baseline characteristics and event status by proportion of compliant person-time among 1,227 women in the EAGeR Trial, 2006-2012.a
| Adherent Person-Time | |||
|---|---|---|---|
| <70 % (N = 563) | ≥70 % (N = 664) | p Valueb | |
| Mean (SD) | Mean (SD) | ||
| Age, years | 28.4 (4.5) | 29.2 (4.7) | 0.002 |
| BMI, kg/m2 | 26.3 (6.4) | 26.3 (6.7) | 0.85 |
| N (%) | N (%) | ||
| Assigned to LDA Treatment | 302 (52) | 313 (48) | 0.20 |
| Original Eligibility Stratum | 228 (43) | 320 (47) | 0.171 |
| At Least High School Education | 473 (84) | 584 (89) | 0.030 |
| Married | 493 (89) | 584 (96) | <0.001 |
| Employed | 419 (75) | 500 (75) | 0.89 |
| Non Hispanic White | 525 (93) | 636 (97) | 0.011 |
| Exercise (moderate or vigorous) | 426 (73) | 479 (75) | 0.46 |
| Income (≥ $40,000) | 348 (63) | 473 (72) | 0.002 |
| Alcohol (ever consumed) | 197 (35) | 211 (31) | 0.21 |
| Smoke (ever smoked) | 99 (16) | 53 (7) | <0.001 |
| Outcome on trial | |||
| Live Birth | 252 (48) | 343 (55) | 0.013 |
| Pregnancy Loss | 100 (18) | 89 (13) | 0.033 |
| No Pregnancy | 84 (15) | 223 (34) | <0.001 |
| Withdrawal | 127 (22) | 9 (1) | <0.001 |
Abbreviations: BMI, body mass index.
Dichotomous variables compared via proportions. Continuous variables (age, BMI) compared via mean and standard deviation.
p values obtained from two-sided tests. Proportion comparisons conducted via binomial tests. Wilcox rank sum tests performed to compare distributions of age, BMI.
We also observed that adherence with either placebo or LDA was associated with an increased odds of hCG detected pregnancy (odds ratio = 5.70, 95% CI: 4.34, 7.52) and live birth (odds ratio = 1.33, 95% CI: 1.07, 1.67), and a decreased odds of pregnancy loss (odds ratio = 0.70, 95% CI: 0.51, 0.95). In line with the original ITT results,(7) adherence was not associated with side effects including bleeding (Risk Ratio: 1.10, 95% CI: 0.92, 1.40) and nausea and/or vomiting (Risk Ratio: 0.99, 95% CI 0.80, 1.21). However, bleeding, nausea and/or vomiting, and hCG detected pregnancy were strongly associated with reduced subsequent adherence (bleeding in the previous week 0.80, 95% CI: 0.76, 0.83; nausea and/or vomiting 0.84, 95% CI: 0.80, 0.88; and hCG pregnancy 0.60, 95% CI: 0.57, 0.62).
Per protocol effect estimates suggested that taking aspirin over the entire course of follow-up resulted in more hCG pregnancies (Risk Ratio: 1.12; 95% CI: 1.02, 1.23), fewer pregnancy losses (Risk Ratio: 0.69; 95% CI: 0.50, 0.95), and more live births (Risk Ratio: 1.33; 95% CI: 1.08, 1.64) compared to taking placebo throughout. In additive terms, these amounted to eight more hCG pregnancies (95% CI: 4.64, 10.96), a reduction of six pregnancy lossess (95% CI: −12.00, −0.20), and 15 more live births (95% CI: 7.65, 21.15), for every 100 women in the study (Table 2). In contrast, ITT effects suggested that there would be four more hCG detected pregnancies (95% CI: −1.18, 9.56), one more pregnancy loss (95% CI: −3.09, 4.68), and four more live births (95% CI: −2.13, 9.21) for every 100 women assigned to treatment relative to placebo.
Table 2.
Intent to treat and adherence adjusted effects of low-dose aspirin on hCG detected pregnancy, live birth, and pregnancy loss among 1,227 women in the EAGeR Trial, 2006-2012. Adherence was defined as following protocol (take low dose aspirin or take placebo) 5/7 days per week.
| Risk | RD* (95% CI) | RR (95% CI) | |
|---|---|---|---|
| Intent to Treat | |||
| Assigned to Placebo | |||
| Live Birth (ref) | 0.48 | - | - |
| Pregnancy Loss (ref) | 0.15 | - | - |
| hCG-Pregnancy (ref) | 0.62 | - | - |
| Assigned to Aspirin | |||
| Live Birth | 0.50 | 3.51 (−2.13, 9.21) | 1.08 (0.94, 1.19) |
| Pregnancy Loss | 0.16 | 0.74 (−3.09, 4.68) | 1.05 (0.74, 1.29) |
| hCG-Pregnancy | 0.66 | 4.25 (−1.18, 9.56) | 1.07 (0.97, 1.15) |
| Per Protocol | |||
| Placebo Throughout | |||
| Live Birth (ref) | 0.43 | - | - |
| Pregnancy Loss (ref) | 0.20 | - | - |
| hCG-Pregnancy (ref) | 0.65 | - | - |
| Aspirin Throughout | |||
| Live Birth | 0.58 | 14.50 (7.65, 21.15) | 1.33 (1.08, 1.64) |
| Pregnancy Loss | 0.14 | −6.10 (−12.00, −0.20) | 0.69 (0.50, 0.95) |
| hCG-Pregnancy | 0.73 | 7.80 (4.64, 10.96) | 1.12 (1.02, 1.23) |
| Start at 6 Weeks | |||
| Live Birth | 0.49 | 5.90 (0.23, 11.75)) | 1.14 (1.03, 1.26) |
| Pregnancy Loss | 0.14 | −5.95 (−12.93, 1.03) | 0.70 (0.47, 1.04) |
| hCG-Pregnancy | 0.63 | −1.40 (−5.34, 2.54) | 0.98 (0.92, 1.04) |
| Start at 8 Weeks | |||
| Live Birth | 0.50 | 6.45 (1.34, 11.56) | 1.15 (0.83, 1.59) |
| Pregnancy Loss | 0.14 | −5.35 (−15.70, 5.00) | 0.73 (0.52, 1.03) |
| hCG-Pregnancy | 0.65 | 0.10 (−4.69, 4.89) | 1.00 (0.92, 1.08) |
| Start at 12 Weeks | |||
| Live Birth | 0.49 | 5.95 (−0.72, 12.62) | 1.14 (0.83, 1.57) |
| Pregnancy Loss | 0.17 | −3.10 (−13.96, 7.76) | 0.84 (0.59, 1.21) |
| hCG-Pregnancy | 0.66 | 1.60 (−3.12, 6.32) | 1.02 (0.92, 1.13) |
| Start at 20 Weeks | |||
| Live Birth | 0.47 | 3.85 (−1.92, 9.62) | 1.09 (0.90, 1.32) |
| Pregnancy Loss | 0.18 | −1.35 (−11.83, 9.13) | 0.93 (0.65, 1.33) |
| hCG-Pregnancy | 0.66 | 1.30 (−4.32, 6.92) | 1.02 (0.86, 1.21) |
Abbreviations: RD, risk difference; RR, risk ratio; hCG, human chorionic gonadotropin.
The effects of aspirin on live birth and pregnancy loss were observed when aspirin was taken sufficiently early after conception (Table 2). This trend was particularly notable for pregnancy loss. Taking aspirin after the 6th, 8th, 12th, or 20th week post conception led to attentuating risk ratios for pregnancy loss that ranged from between 0.70 (95% CI: 0.47, 1.04) if initiated 6 weeks post conception, to 0.93 (95% CI: 0.65, 1.33) if initiated 20 weeks post conception. The effect of aspirin on live birth was even more impactful when used throughout (beginning pre-pregnancy) and notably lower when aspirin initiation was delayed to 6 or more weeks’ gestation.
Over all of follow-up, the percentage of women deemed adherent ranged from 74% for 2/7 days to 62% for 6/7 days. Relative to taking aspirin 5/7 days per week, no meaningful difference in the magnitude of the effect was observed if the adherence threshold was reduced to 4/7 days per week, or increased to 6/7 days per week (Table 3). However, the estimated effect of aspirin on live birth, pregnancy loss, and hCG pregnancy was attenuated if adherence was less than 4/7 days per week.
Table 3:
Impact of the selected threshold for defining whether a woman complied with assigned protocol in a given week on the overall adherence adjusted effect estimates among 1,227 women in the EAGeR Trial, 2006-2012.
| Risk Ratio (95% CI) | ||||
|---|---|---|---|---|
| Compliant Threshold (compliant days per week) |
Proportion Compliant Observed |
Live Birth | Pregnancy Loss | hCG Pregnancy |
| 2 | 0.74 | 1.12 (0.91, 1.39) | 0.89 (0.64, 1.24) | 1.06 (0.97, 1.16) |
| 3 | 0.72 | 1.10 (0.89, 1.36) | 0.84 (0.62, 1.14) | 1.04 (0.93, 1.17) |
| 4 | 0.70 | 1.28 (1.06, 1.55) | 0.71 (0.52, 0.97) | 1.09 (1.00, 1.19) |
| 5 | 0.67 | 1.33 (1.08, 1.64) | 0.69 (0.50, 0.95) | 1.12 (1.02, 1.23) |
| 6 | 0.63 | 1.30 (1.09, 1.55) | 0.71 (0.52, 0.97) | 1.10 (1.00, 1.20) |
Abbreviations: hCG, human chorionic gonadotropin.
Sensitivity analyses suggested that missing adherence, bleeding, and nausea data were unlikely to explain the differences observed between the per protocol and intent to treat effects (Supplemental Table 1). Indeed, the majority of scenarios supported the conclusions implied by the per protocol effects in Table 2. Importantly, the scenarios that did not represented extremes corresponding to unrealistic data scenarios (e.g., 100% of missing adherence data set to “non-adherent”, see Supplemental Table 1).
DISCUSSION
We found that taking aspirin at least four times per week during the preconception period and through 36 weeks’ gestation was associated with increased hCG-detected pregnancies and reduced pregnancy losses, thereby increasing live births by over 30%. These per protocol findings supplement the previous ITT results of the EAGeR trial by adjusting for nonadherence with the study protocol while accounting for time-varying, postrandomization confounding and suggest a preventative effect of LDA on pregnancy loss. This evidence supports the need to focus on improving adherence to daily LDA to maximize the efficacy on pregnancy outcomes.
The per-protocol and prior ITT effects estimated using EAGeR data answer different questions about the impact of aspirin on pregnancy outcomes. Per protocol analyses quantify effects under ideal conditions in which all women adhere with the defined protocol. As such, they provide information on the potential impact of aspirin for individual women were they to fully adhere to clinical recommendations. The validity of such analyses rests on the usual (unverifiable) assumptions required for observational studies (e.g., no confounding bias). In contrast, the ITT results quantify the effect of assigning aspirin. They provide information on on the potential impact in practice of recommending aspirin if adherence patterns were similar to those in the trial. The validity of this analysis depends on the (verifiable) assumptions required for randomized trials (e.g., blinding).
In EAGeR, the differences observed between per protocol and ITT results can be attributed to many factors. First, the overall nonadherence rate of 25%, changed over time on study and particularly between pre- and post-pregnancy recognition time-periods. Indeed, 85% of women met the adherent definition of 5 out of 7 days during the preconception phase of the trial, which dropped to 67% after pregnancy detection. This differentially observed adherence helps clarify previous findings in this trial. Specifically, some secondary findings using the ITT approach revealed that preconception-initaited LDA increased pregnancy rate and shortened time to pregnancy in certain subgroups (8, 9), which may reflect the relatively high compliance while trying to become pregnant.
In contrast, no analyses of the EAGeR trial using the ITT approach illustrated impacts on pregnancy loss (11), which may reflect the declining compliance once women became pregnant or began experiencing pregnancy symptoms (e.g., nausea). Second, nonadherence at any given time was associated with variables that were highly predictive of both subsequent adherence, as well as the outcomes studied (18). Indeed, such post-randomization confounding represents an important challenge in quantifying per-protocol effects in randomized trials, accounted for with g computation (10). Lastly, adherence was also strongly predictive of pregnancy outcomes and withdrawal. Indeed, women with better adherence were more likely to conceive and have a live birth. Furthermore, bleeding, nausea and/or vomiting, and hCG detection of pregnancy were all strongly predictive of whether a woman would adhere with her assigned treatment in the subsequent week. Taken together, these features may also explain why similar findings were not observed with previous ITT analyses (19).
In light of the changing adherence patterns prior to and following pregnancy observed in our study, researchers and clinicians should be aware of such changes in adherence behaviors to LDA. Because of these adherence patterns, universal recommendations for assigning women to LDA may not lead to meaningful changes in pregnancy outcomes. Research on improving adherence through, for example, alternative routes of LDA delivery, or provider and patient education on the need to maximize adherence, could be of use to increasing the potentially beneficial effects of LDA on pregnancy outcomes.
Aspirin is well-established as a safe and efficacious therapy during pregnancy to reduce preeclampsia and recurrent pregnancy loss attributed to anti-phospholipid syndrome (4). Moreover, safety findings within the EAGeR trial indicated LDA initiated prior to conception and continued through pregnancy was well tolerated (20). Furthermore, a recent randomized trial of roughly 12,000 women from six countries found lower rates of preterm birth, perinatal mortality, fetal loss, as well as fewer hypertensive disorders of pregnancy among women assigned to daily LDA relative to placebo (21). In light of this recent trial, and the present study, there is growing evidence of the positive effects of daily LDA on pregnancy outcomes.
Our study is the first to shed light on the optimal timing of LDA therapy, suggesting maximum benefits by initiating therapy prior to and early in pregnancy. Indeed, the very early stages of pregnancy, prior to a women’s usual first encounter with prenatal care, may be an early window for preventing pregnancy loss and improving the chances of live birth. Current guidelines recommending the initiation of aspirin between 12 and 28 weeks for preeclampsia prevention may be suboptimal for the prevention of pregnancy loss (3). Thus, preconception initiation may be the best course of action to optimize public health impact.
The observation of a plateau of aspirin’s effect at using four or more days per week is consistent with prior research investigating a variety of aspirin dosing regimens on cardiovascular protection. Indeed, LDA (ranging from 40 to 100 mg per day) used every third, every other, and every day produced similar cardiovascular outcomes (12, 22). It may be that approximately every other day dosing of 81mg is sufficient to improve pregnancy rates and reduce pregnancy loss, which may be desirable if fewer doses per week also reduces side effects such as nausea and bleeding. However, because we defined compliance on a weekly basis, we were not able to quantify the impact of variation in daily patterns (i.e., taking aspirin every day versus every other day in the week). Trials specifically investigating differing dose regimens will be required to confirm efficacy of alternate aspirin dose regimens.
Our findings should be considered in light of important limitations. First, the EAGeR trial consisted of women who were mostly well educated and living in households with a high median income, limiting generalizability. Second, there were too few cases of rare but adverse events to be able to evaluate the per protocol effect of aspirin on these outcomes, including preterm birth and preeclampsia. Finally, though our analyses were subject to a large degree of missing weekly data, our sensitivity analyses showed that only extreme scenarios of the underlying missing data (i.e., all missing data take on a single value) led to estimates that would substantively change the interpretation of our findings.
In summary, following the recent call for per protocol analyses of randomized trials (10), our results suggest preconception LDA use in women with prior pregnancy loss may improve pregnancy outcomes. Specifically, early adoption of LDA with maximal daily adherence promoted pregnancy and live birth, and was protective against pregnancy loss in women trying to become pregnant after a history of pregnancy loss. Efforts geared towards improving daily adherence to LDA may yield improvements in the effectiveness of aspirin on reproductive outcomes for women trying to conceive.
Supplementary Material
Acknowledgments
Funded by the NIH R01 HD093602, and by the Intramural Research Program of the Eunice Kennedy Shriver National Institutes of Child Health and Human Development, National Institutes of Health, Bethesda, Maryland, contract numbers HHSN267200603423, HHSN267200603424, and HHSN267200603426.
References:
- 1.Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110(5-6):255–8. [DOI] [PubMed] [Google Scholar]
- 2.James AH, Brancazio LR, Price T. Aspirin and reproductive outcomes. Obstet Gynecol Surv. 2008;63(1):49–57. [DOI] [PubMed] [Google Scholar]
- 3.Low-dose aspirin use during pregnancy. ACOG Committee Opinion No. 743. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2018;132:e44–52. [DOI] [PubMed] [Google Scholar]
- 4.de Jong P, Kaandorp S, Di Nisio M, Goddijn M, Middeldorp S. Aspirin and/or heparin for women with unexplained recurrent miscarriage with or without inherited thrombophilia. Cochrane Database Syst Rev. 2014;7(CD004734). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. [DOI] [PubMed] [Google Scholar]
- 6.Schisterman EF, Silver RM, Perkins NJ, Mumford SL, Whitcomb BW, Stanford JB, et al. A randomised trial to evaluate the effects of low-dose aspirin in gestation and reproduction: design and baseline characteristics. Paediatr Perinat Epidemiol. 2013;27(6):598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schisterman EF, Silver RM, Lesher LL, Faraggi D, Wactawski-Wende J, Townsend JM, et al. Preconception low-dose aspirin and pregnancy outcomes: results from the EAGeR randomised trial. Lancet. 2014;384(9937):29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schisterman EF, Mumford SL, Schliep KC, Sjaarda LA, Stanford JB, Lesher LL, et al. Preconception low dose aspirin and time to pregnancy: findings from the effects of aspirin in gestation and reproduction randomized trial. J Clin Endocrinol Metab. 2015;100(5):1785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sjaarda LA, Radin RG, Silver RM, Mitchell E, Mumford SL, Wilcox B, et al. Preconception Low-Dose Aspirin Restores Diminished Pregnancy and Live Birth Rates in Women With Low-Grade Inflammation: A Secondary Analysis of a Randomized Trial. J Clin Endocrinol Metab. 2017;102(5):1495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernan MA, Robins JM. Per-Protocol Analyses of Pragmatic Trials. N Engl J Med. 2017;377(14):1391–8. [DOI] [PubMed] [Google Scholar]
- 11.Mumford SL, Silver RM, Sjaarda LA, Wactawski-Wende J, Townsend JM, Lynch AM, et al. Expanded findings from a randomized controlled trial of preconception low-dose aspirin and pregnancy loss. Hum Reprod. 2016;31(3):657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman M, Cryer B, Rushin K, Betancourt J. A comparison of every-third-day versus daily low-dose aspirin therapy on serum thromboxane concentrations in healthy men and women. Clin Appl Thromb Hemost. 2001;7(1):53–7. [DOI] [PubMed] [Google Scholar]
- 13.Hernan MA, Hernandez-Diaz S, Robins JM. Randomized trials analyzed as observational studies. Ann Intern Med. 2013;159(8):560–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naimi AI, Cole SR, Kennedy EH. An introduction to g methods. Int J Epidemiol. 2017;46(2):756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robins J, Hernan MA. Estimation of the causal effects of time-varying exposures. In: Fitzmaurice G DM, Verbeke G, Molenberghs G, ed. Longitudinal Data Analysis. 1st Edition ed. New York: Chapman & Hall/CRC; 2009:553–99. [Google Scholar]
- 16.Efron B TR. An Introduction to the Bootstrap 1st Edition ed. Boca Raton FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 17.Mansournia MA, Naimi AI, Greenland S. The implications of using lagged and baseline exposure terms in the longitudinal-causal and regression models. Am J Epidemiol. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hinkle SN, Mumford SL, Grantz KL, Silver RM, Mitchell EM, Sjaarda LA, et al. Association of Nausea and Vomiting During Pregnancy With Pregnancy Loss: A Secondary Analysis of a Randomized Clinical Trial. JAMA Intern Med. 2016;176(11):1621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahrens KA, Silver RM, Mumford SL, Sjaarda LA, Perkins NJ, Wactawski-Wende J, et al. Complications and Safety of Preconception Low-Dose Aspirin Among Women With Prior Pregnancy Losses. Obstet Gynecol. 2016;127(4):689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoffman MK, Goudar SS, Kodkany BS, Metgud M, Somannavar M, Okitawutshu J, et al. Low-dose aspirin for the prevention of preterm delivery in nulliparous women with a singleton pregnancy (ASPIRIN): a randomised, double-blind, placebo-controlled trial. Lancet. 2020;395(10220):285–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bem D, Lordkipanidze M, Hodgkinson J, Stevens S, Bayliss S, Moore D, et al. The Effects of Different Aspirin Dosing Frequencies and the Timing of Aspirin Intake in Primary and Secondary Prevention of Cardiovascular Disease: A Systematic Review. Clin Pharmacol Ther. 2016;100(5):500–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


