Abstract
Identification of pathogens with pulmonary presentation in patients with hematologic malignancies may be challenging due to diagnostic difficulty related to the underlying malignancy and limitations of conventional microbiologic methods. Herein, we present a case series of three patients with pulmonary consolidations due to Legionella bozemanae necrotizing pneumonia, Pneumocystis jirovecii pneumonia, and disseminated Scedosporium infection, who were diagnosed by microbial cell-free DNA next-generation sequencing. We observed that this new sequencing modality was in agreement with gold-standard diagnostics, posing a potential solution to the problem of limited capability in diagnosing infections in hematological malignancy patients.
Keywords: Karius, cell-free DNA next-generation sequencing, atypical pathogen
INTRODUCTION
Infections are common in patients with hematologic malignancies and recipients of hematopoietic cell transplant (HCT) due to immune defects related to underlying disease or treatments.1 Pulmonary complications occur in up to 70% of patients with hematologic malignancies and HCT recipients and have been associated with inferior survival.2–4
Early and correct identification of causative organisms in infectious diseases is crucial for determining optimal therapy as well as antimicrobial stewardship. A lack of diagnosis among HCT recipients with pulmonary complications is associated with worse survival.5 Invasive procedures for tissue sampling are not often feasible in these patients due to thrombocytopenia or clinical instability. With the recent advent of rapid diagnostics, improvements have been seen in hospital length of stay, mortality, and healthcare costs.6 Rapid diagnostics have been used with particular benefit in patients with sepsis, where culture-independent identification can lead to more expedient tailoring of the antimicrobial regimens.7
Application of next-generation sequencing to detect microbial cell-free DNA (mcfDNA) in plasma has been proposed as an alternative to traditional culture and polymerase-chain reaction (PCR) methods. This strategy enables accurate and timely pathogen identification from plasma samples even in cases of deep or difficult to access foci of infection.8
Herein we describe a series of three cases in which the mcfDNA detection method developed by the Karius test (Karius, Redwood City, CA) was used to provide fast organism detection in difficult-to-establish infectious diseases scenarios.
CASE ONE
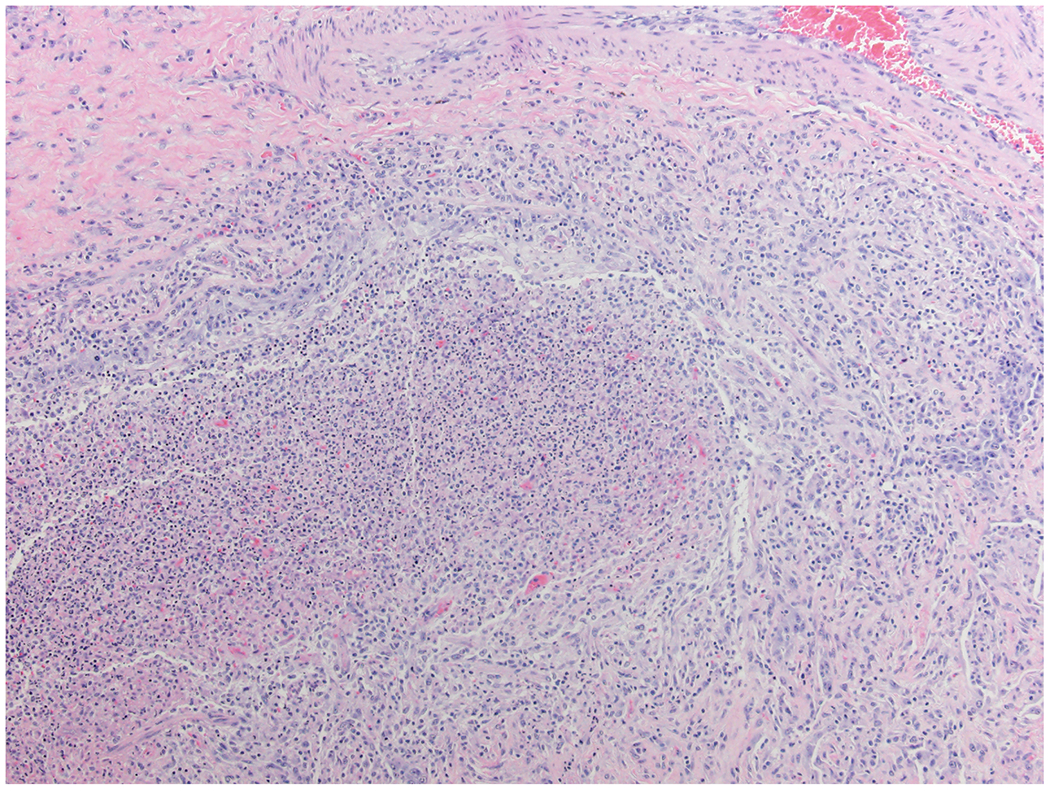
A 31-year-old woman received a T-cell replete nonmyeloablative HCT from a matched unrelated donor for myelodysplastic syndrome associated with GATA2 deficiency. Several months prior to transplant she was admitted to the hospital for hypoxemia and was diagnosed with pulmonary alveolar proteinosis (PAP) via transbronchial lung biopsy. Concurrent bronchioalveolar lavage (BAL) fluid was only positive for Candida albicans. She subsequently had multiple readmissions for low-grade fever, cough, and which improved with whole lung lavage prior to transplant. Her early post-transplant course was complicated by ongoing cough and recurrent hypoxemia with a new right lower lobe consolidation seen on computerized tomography (CT). On day 16 (D+16) post-transplant, she underwent bronchoscopy due to persistent fever while on meropenem and isavuconazole, with BAL fluid subsequently returning negative for fungi or bacteria by stain and culture. On D+34, chest CT was repeated due to ongoing hypoxia and showed increased right middle and lower lobe consolidations along with loculated component of moderate amounts of right pleural effusion. On D+37, she underwent thoracentesis of right-sided pleural effusions with stains and pleural fluid cultures also returning negative. On D+49, she had acute respiratory decompensation with a repeat CT chest revealing necrotizing pneumonia in the right lower lobe. By that point, she had received multiple broad-spectrum antibiotics. She was taken for urgent lobectomy on D+50, and antimicrobial coverage was expanded to include atypical organisms with the addition of azithromycin. Karius testing was requested on D+48, resulting in 6,735 DNA molecules per microliter (MPM) of Fluoribacter bozemanae (Legionella bozemanae) on D+52. Surgical specimens submitted to pathology showed acute and focally necrotizing pneumonia with acute pleuritis and purulent exudates on the pleural surface, but no fungal or bacterial organisms were seen even with special stains for microorganisms including a tissue gram stain and a modified Steiner stain (Figure 1A, Figure 1B). Specimens submitted to microbiology stained positive for 4+ Gram negative rods, however these were not able to be grown in culture. Tissue specimens were sent for broad-range bacterial sequencing (Mayo Clinic Laboratories, Rochester, Minnesota), returning approximately 4 weeks later with a result of Fluoribacter (Legionella) bozemanae. After the Karius test was reported, azithromycin was changed to levofloxacin and she was discharged from the hospital on D+105 post-transplant.
1A –
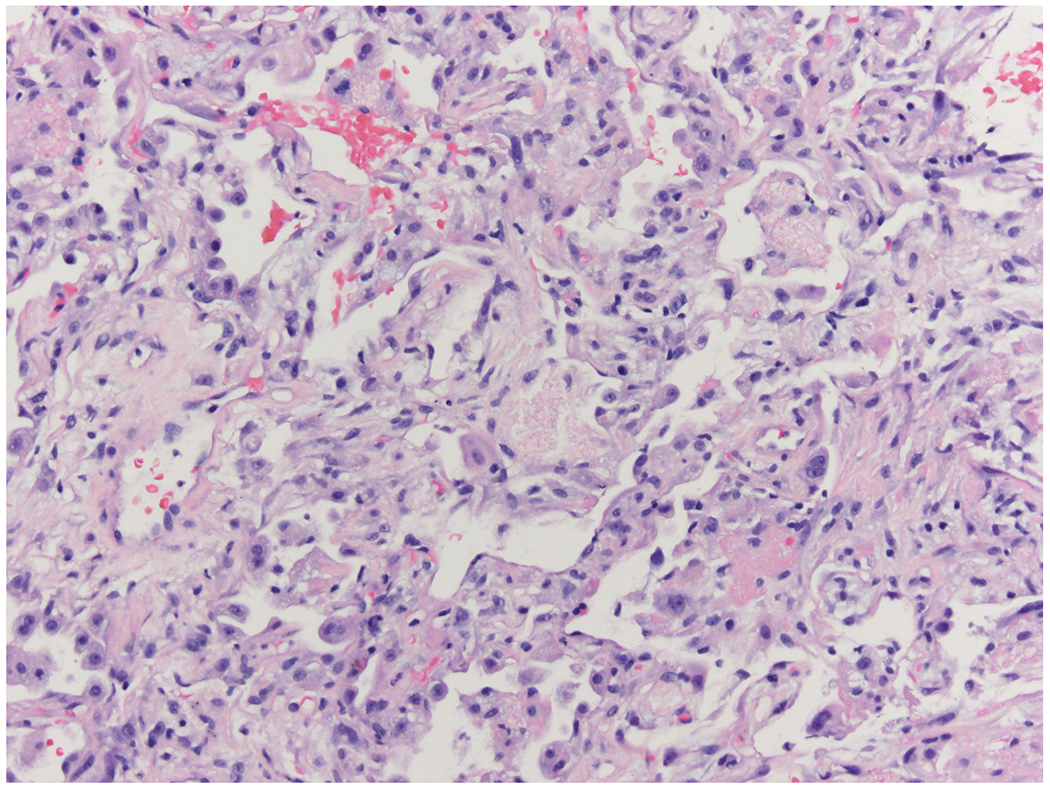
This bronchiole is massively infiltrated by neutrophils within the lumen and chronic inflammatory cells in the bronchiolar wall. (Hematoxylin and eosin X 10)
1B –
The airway lumen is filled mostly with neutrophils and the bronchiolar wall shows mostly chronic inflammatory cells. (Hematoxylin and eosin X 40)
CASE TWO
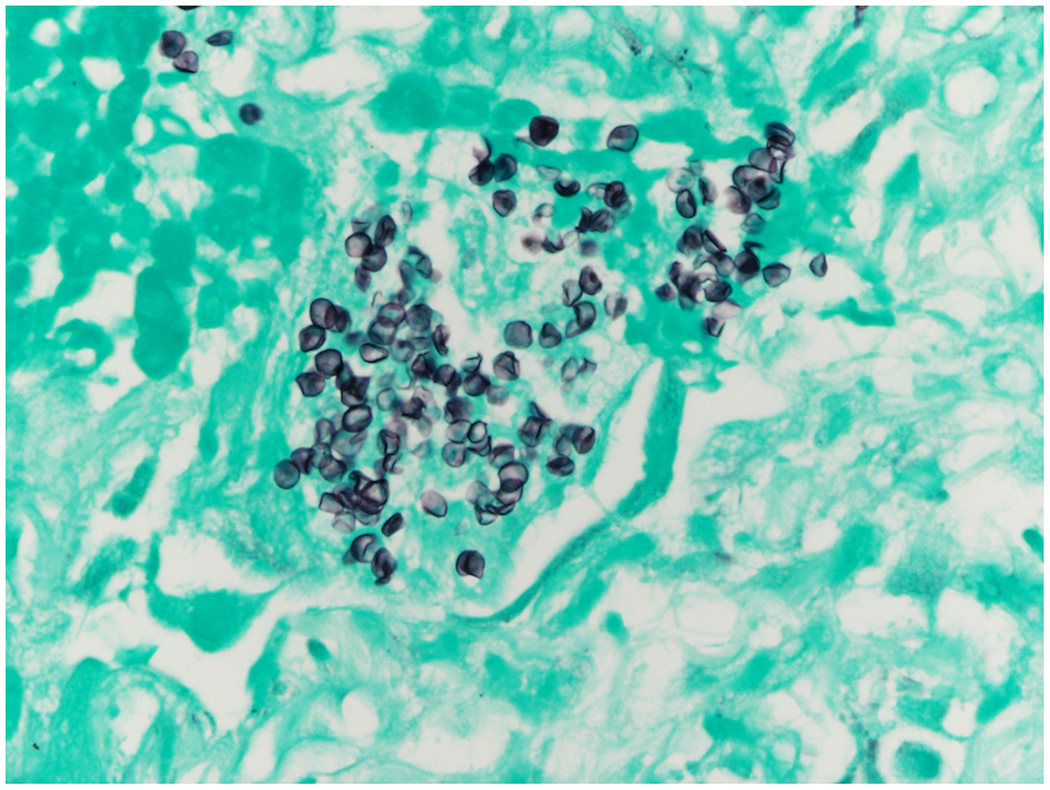
A 60-year-old man received a CD34+ cell selected HCT for B-cell acute lymphoblastic leukemia. His post-transplant course was complicated by grade II acute graft-versus-host disease treated with oral budesonide and tacrolimus as well as adenovirus (ADV) and cytomegalovirus (CMV) viremias. The patient had been experiencing intermittent recurrent febrile episodes requiring hospitalization for up to 6 months post-HCT. Fevers were not felt to be attributable to CMV or ADV and did not respond to multiple antibiotic and antiviral courses. Extensive infectious workups were otherwise negative including positron Emission Tomography-Computed Tomography (PET-CT). Five months post-transplant the patient was admitted to our hospital with recurrent febrile episodes. Approximately 10 days into admission Karius testing was sent due to an ongoing lack of discernible infectious etiologies which subsequently resulted in 1,520 DNA MPM human adenovirus D and 39 DNA MPM of Pneumocystis jirovecii. At the time of the Karius result, P. jirovecii pneumonia (PJP) was considered an unlikely diagnose due to a lack of compatible imaging or clinical syndrome, as well as ongoing prophylaxis with pentamidine. Approximately 2 weeks after the Karius result, the patient was admitted to the hospital with a febrile episode accompanied by new onset dyspnea. CT chest showed new patchy bilateral ground glass opacities. A serum (1→3)-β-D-glucan (BDG) was elevated to 479 pg/mL. P. jirovecii PCR from sputum resulted positive. He underwent bronchoscopy with BAL in which P. jirovecii was also detected by PCR. Cytologic specimens from the BAL were sent with examination of these specimens by silver stain showing fungal forms consistent with P. jirovecii. He was started on PJP treatment with atovaquone and micafungin for possible cystic PJP and defervesced. Two weeks after that admission, he presented again with fevers with CT chest showing increasing bilateral ground glass opacities. A lung biopsy was done showing clusters of P. jirovecii by Gomori methenamine silver stain with acute lung injury (Figure 2A, Figure 2B). He was subsequently switched to clindamycin/primaquine (trimethoprim-sulfamethoxazole was avoided due to concurrent pancytopenia). Because of persistently elevated serum BDG to >500 pg/ml without alternative explanation, he remained on PJP treatment for a prolonged duration, without recurrent febrile episodes.
2A –
The lung shows diffuse thickening of alveolar walls with loose connective tissue and focally alveolar spaces are filled with an eosinophilic exudate. (Hematoxylin and eosin X 20)
2B –
This GMS stain shows numerous round to oval shaped fungal organisms consistent with Pneumocystis jirovecii. These organisms were most conspicuous in alveolar filled with the eosinophilic exudate seen in in Figure 2A. (Gomori methenamine silver X 80)
CASE THREE
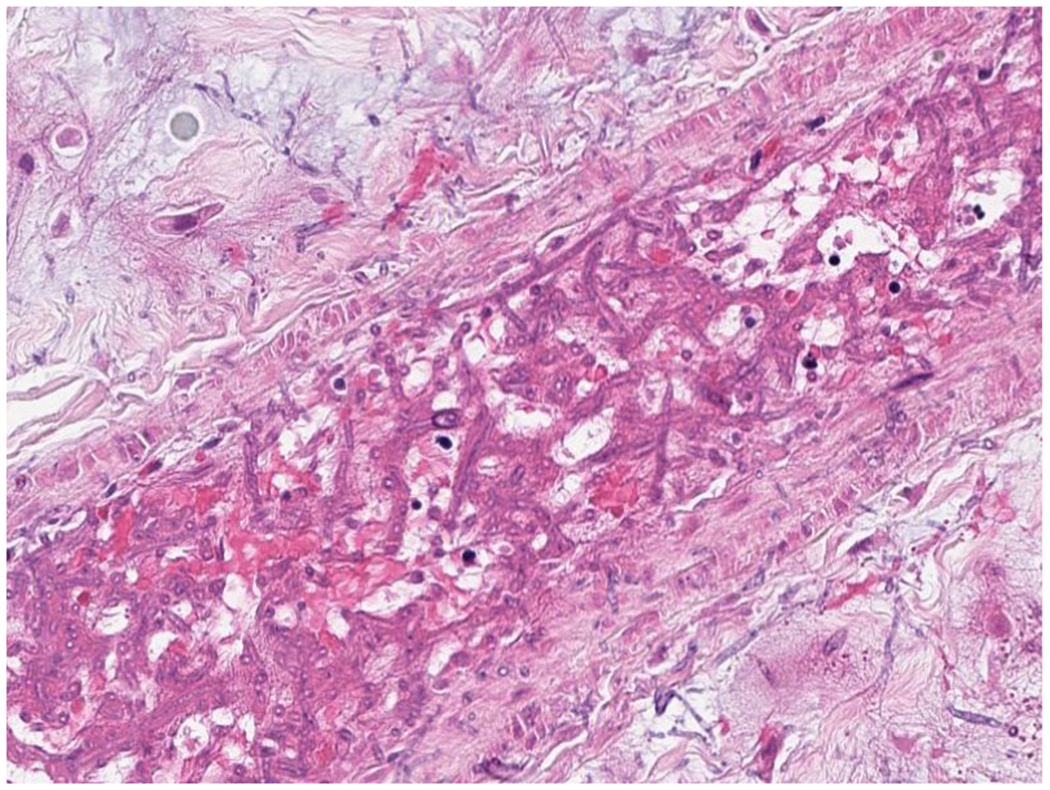
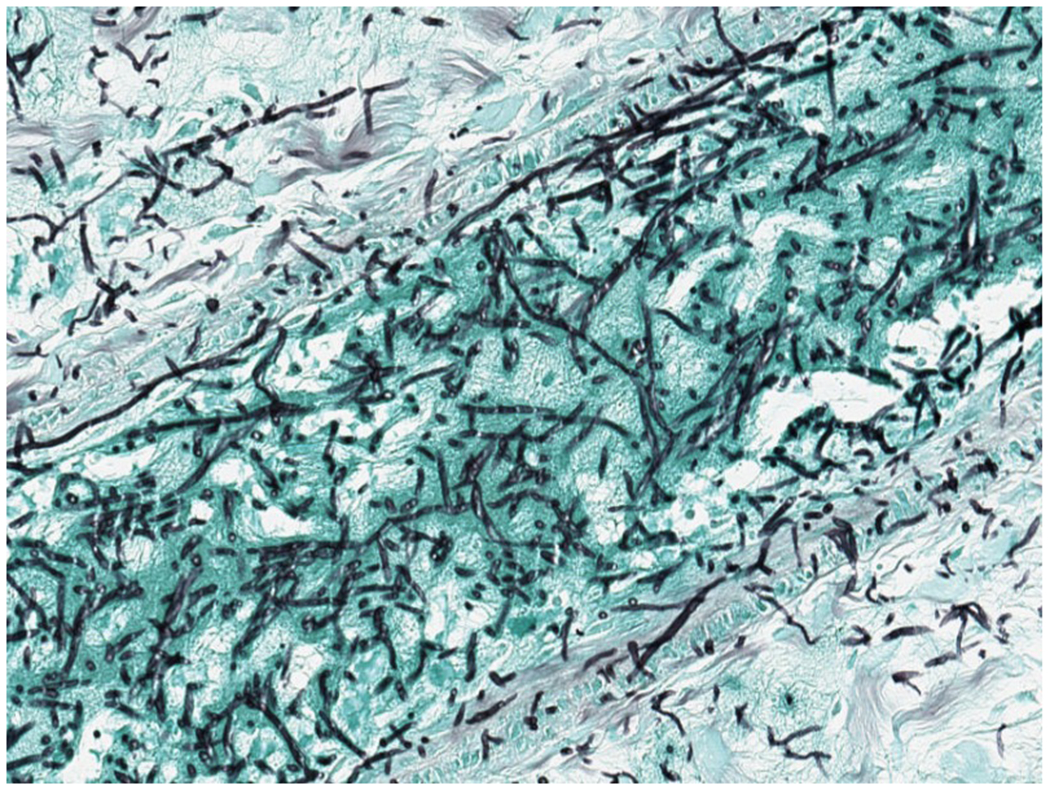
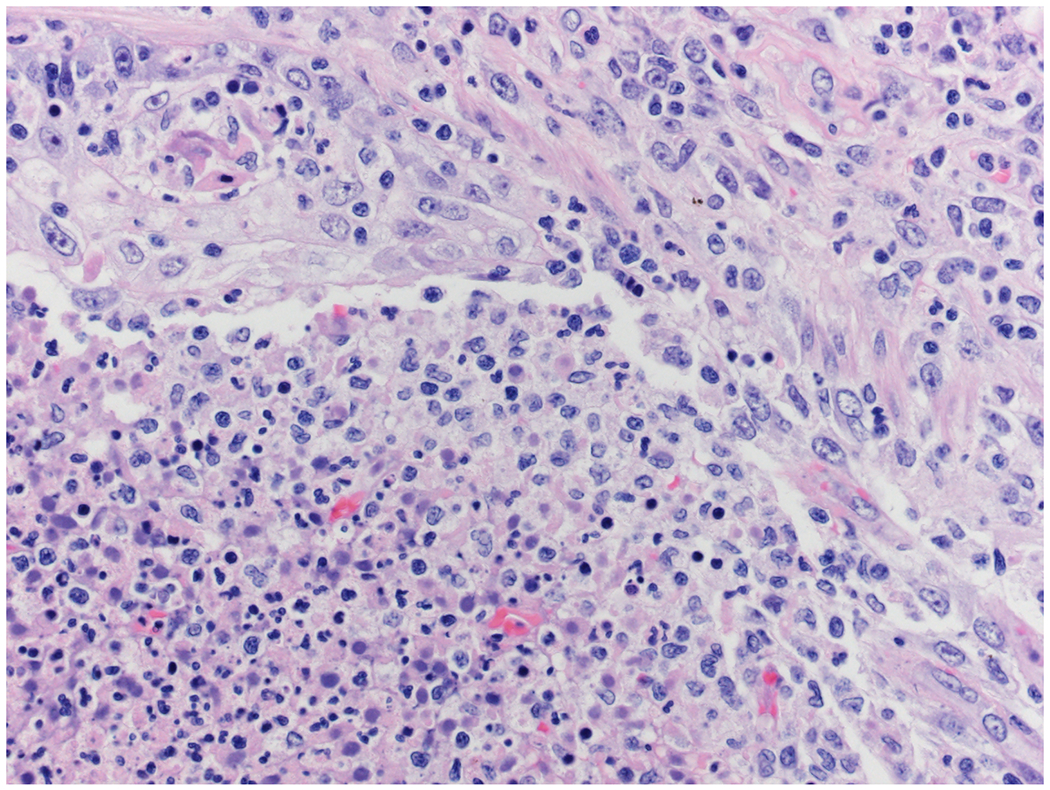
A 24-year-old woman was transferred from an outside hospital for the treatment of acute myeloid leukemia (AML). Her disease was refractory to multiple induction chemotherapy at an outside hospital and further complicated by an apparent disseminated fungal infection 2 months after AML diagnosis. At outside institution, unidentified hyphal elements were found on a right upper lobe lung fine needle aspirate biopsy with concerns for invasion outwardly into the chest wall (Figure 3A, Figure 3B), and non-biopsied lesions in the liver seen on CT. She was begun on empiric antifungal therapy with liposomal amphotericin B. Broad-range fungal PCR (ARUP Laboratories, Salt Lake City, Utah) was performed on the biopsy specimen and resulted approximately 3 weeks after collection with Scedosporium apiospermum complex. Upon transfer to Memorial Sloan Kettering Cancer Center, CT chest, abdominal pelvis was performed which showed mass like consolidation in the right upper lobe and numerous hepatic and splenic lesions. Spine magnetic resonance imaging (MRI) showed hypertense lesions on T12 and S1 and brain MRI showed a peripherally enhancing lesion in the right frontal lobe. Multiple blood cultures were negative and transthoracic echo was negative for vegetation. Serum BDG was elevated to >500 pg/mL. Her white blood count was <0.1 K/mcL and her platelet was 6000 K/mcL. Due to refractory thrombocytopenia, a biopsy was not feasible. As there was a concern of septic emboli in CT abdomen-pelvis and refractory thrombocytopenia, Karius testing was requested approximately 1 month after the initial biopsy specimen to rule out other etiologies to cause septic emboli. Results showed 548 DNA MPM of Scedosporium boydii. Voriconazole and terbinafine were added for synergy with liposomal amphotericin B.
3A –
Histologic assessment of fungal angioinvasion. (Hematoxylin and eosin X 200).
3B –
This GMS stain shows abundant fungal hyphae in lung parenchyma and whin bold vessels. (Gomori methenamine silver X 200)
DISCUSSION
Herein, we report three cases in which the Karius test was used as an adjunct to gold-standard diagnostic histopathologic and/or microbiological techniques. In case one, Karius was done first and was concordant with bacterial PCR showing Legionella bozemanae performed on resected lung tissue. In the second case, Karius was also done first and was concordant with later histological evaluation of resected lung tissue showing Pneumocystis jirovecii. In the third case, lung biopsy was performed first showing Scedosporium apiospermum complex by PCR, later confirmed by the Karius test.
The first case highlights the difficulty of diagnosis of atypical pathogen and limitations of conventional microbiologic methods. Cultures from the resected lung failed to grow and organisms were not seen in the lung tissue histologically even with special stains, while bacterial PCR of the lung tissue was positive for legionella; however, the result was not available for weeks as a result of reference laboratory workflow. Atypical and fastidious organisms represent unique diagnostic challenges in infectious disease. Organisms such as Legionella species are exemplary to the extent that they often require invasive means to obtain samples, require special culture media to grow, and do not have timely growth even under optimal conditions.9 Attempts to overcome the challenge of such organisms have included broad-range PCR detection methods; however, while such methods may supplement or occasionally replace culture for diagnosis, they often are even less timely and typically detect bacteria, whereas mcfDNA can detect a broad range of microorganisms including bacteria, DNA viruses, and fungi. Automated PCR based panels have been shown to provide expedient results, with the drawback that such panels have a narrow and limited range of organisms they detect and are also limited by the types of specimens on which they can be run.9 Additionally, extrapulmonary Legionellosis has been documented in the hematologic malignancy population,10 which compounds diagnostic difficulty since the BCYE agar needed to grow Legionella species is not routinely used in extrapulmonary specimens.
The second case demonstrates that mcfDNA test can be used for early identification of organisms in the investigation of fever of unknown origin. Interestingly, P. jirovecii was detected by the Karius in the patient’s blood prior to the development of overt pulmonary consolidations and before the lung biopsy confirmed the diagnosis pathologically. Moreover, mcfDNA also has the potential to guide the preemptive treatment approach. The preemptive approach is widely adapted for cytomegalovirus viremia where a relationship has been established between viral load and mortality.11 There has been demonstration of utilization of Karis for preemptive detection of pathogens causing bloodstream infection in the pediatric oncology patients.12 Further studies are needed for the utility of the mcfDNA sequencing for preemptive therapy.
The third case demonstrates that the Karius test detected Scedosporium even after weeks of prolonged use of antifungal treatment. Invasive fungal infections pose significant morbidities and mortalities in patients with hematologic malignancies. Tissue sampling for deep-seated fungal infections may not be feasible due to clinical conditions and thrombocytopenia. The sensitivities of conventional diagnostic methods decreased after the prolonged use of antifungal agents.13 Noninvasive diagnostic workups such as galactomannan and BDG lack sensitivities and specificities and do not detect all fungal pathogens.14
Our case series suggests that mcfDNA diagnostic techniques such as the Karius test can be very useful in patients with hematologic malignancies who cannot safely undergo invasive diagnostic procedures, or for whom the diagnostic yield of conventional cultures is suboptimal. A clinical trial (NCT04047719) is currently underway to evaluate the added diagnostic utility of the Karius test in immunocompromised patients with pneumonia and will inform the optimal use of the test in this patient population.
Financial support.
This research was funded in part by National Institutes of Health (NIH) award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748.
Disclosures
I.P. has received research funding from Merck and serves as a member on a data and safety monitoring board for ExcellThera. E.M.S. has membership on the board of directors or advisory committee for Agios, Astellas, Celgene, Daiichi Sankyo, Genentech, Novartis, PTC Therapeutics, and Syros, and has acted as consultant for Agios. G.A.P. has served as an investigator for Chimerix, Astellas, Merck, and Shire and has received research support and consulting and other fees from Chimerix, Inc, Astellas, and Merck.
Footnotes
Conflict of Interest
None of the authors of this work have any conflicts of interest.
REFERENCES
- 1.Safdar A, Armstrong D. Infections in patients with hematologic neoplasms and hematopoietic stem cell transplantation: neutropenia, humoral, and splenic defects. Clin Infect Dis. 2011;53(8):798–806. [DOI] [PubMed] [Google Scholar]
- 2.Diab KJ, Yu Z, Wood KL, Shmalo JA, Sheski FD, Farber MO, Wilkes DS, Nelson RP Jr. Comparison of pulmonary complications after nonmyeloablative and conventional allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 2012;18(12):1827–34. [DOI] [PubMed] [Google Scholar]
- 3.Kotloff RM, Ahya VN, Crawford SW. Pulmonary complications of solid organ and hematopoietic stem cell transplantation. Am J Respir Crit Care Med. 2004;170(1):22–48. [DOI] [PubMed] [Google Scholar]
- 4.Harris B, Morjaria SM, Littmann ER, Geyer AI, Stover DE, Barker JN, Giralt SA, Taur Y, Pamer EG. Gut Microbiota Predict Pulmonary Infiltrates after Allogeneic Hematopoietic Cell Transplantation. Am J Respir Crit Care Med. 2016;194(4):450–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruson D, Hilbert G, Valentino R, Vargas F, Chene G, Bebear C, Allery A, Pigneux A, Gbikpi-Benissan G, Cardinaud JP. Utility of fiberoptic bronchoscopy in neutropenic patients admitted to the intensive care unit with pulmonary infiltrates. Crit Care Med. 2000;28(7):2224–30. [DOI] [PubMed] [Google Scholar]
- 6.Bauer Karri A., Perez Katherine K., Forrest Graeme N., Goff Debra A.. Review of Rapid Diagnostic Tests Used by Antimicrobial Stewardship Programs, Clin Infect Dis. 2014;59(Suppl 3):S134–S145. [DOI] [PubMed] [Google Scholar]
- 7.Eubank Taryn A, Long SW, Perez Katherine K,. Role of Rapid Diagnostics in Diagnosis and Management of Patients With Sepsis, J Infect Dis. 2020;222(Suppl 2): S103–S109. [DOI] [PubMed] [Google Scholar]
- 8.Huang TD, Melnik E, Bogaerts P, Evrard S, Glupczynski Y. Evaluation of the ePlex Blood Culture Identification Panels for Detection of Pathogens in Bloodstream Infections. J Clin Microbiol. 2019;57(2):e01597–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doern Gary V., Detection of Selected Fastidious Bacteria, Clin Infect Dis. 2000;30(1):166–173. [DOI] [PubMed] [Google Scholar]
- 10.Franco-Garcia A, Varughese TA, Lee YJ, Papanicolaou G, Rosenblum MK, Hollmann TJ, Koehne G, Boulad F, Babady NE, Tang YW, Seo SK. Diagnosis of Extrapulmonary Legionellosis in Allogeneic Hematopoietic Cell Transplant Recipients by Direct 16S Ribosomal Ribonucleic Acid Sequencing and Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry. Open Forum Infect Dis. 2017;4(3):ofx140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duke ER, Williamson BD, Borate B, Golob JL, Wychera C, Stevens-Ayers T, Huang ML, Cossrow N, Wan H, Mast TC, Marks MA, Flowers ME, Jerome KR, Corey L, Gilbert PB, Schiffer JT, Boeckh M. CMV viral load kinetics as surrogate endpoints after allogeneic transplantation. J Clin Invest. 2021;131(1):e133960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goggin KP, Gonzalez-Pena V, Inaba Y, Allison KJ, Hong DK, Ahmed AA, Hollemon D, Natarajan S, Mahmud O, Kuenzinger W, Youssef S, Brenner A, Maron G, Choi J, Rubnitz JE, Sun Y, Tang L, Wolf J, Gawad C. Evaluation of Plasma Microbial Cell-Free DNA Sequencing to Predict Bloodstream Infection in Pediatric Patients With Relapsed or Refractory Cancer. JAMA Oncol. 2020;6(4):552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dadwal SS, Kontoyiannis DP. Recent advances in the molecular diagnosis of mucormycosis. Expert Rev Mol Diagn. 2018;18(10):845–854. [DOI] [PubMed] [Google Scholar]
- 14.White PL, Barnes RA, Springer J, Klingspor L, Cuenca-Estrella M, Morton CO, Lagrou K, Bretagne S, Melchers WJ, Mengoli C, Donnelly JP, Heinz WJ, Loeffler J; EAPCRI. Clinical Performance of Aspergillus PCR for Testing Serum and Plasma: a Study by the European Aspergillus PCR Initiative. J Clin Microbiol. 2015;53(9):2832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


