Abstract
Pancreatic ductal adenocarcinoma (PDAC) is a highly aggressive cancer with poor prognosis and chemotherapy with gemcitabine has limited effects and is associated with development of drug resistance. Treatment of Panc1 and MiaPaca2 pancreatic cancer cells with gemcitabine induced expression of the orphan nuclear receptor 4A2 (NURR1) and analysis of The Cancer Genome Atlas indicated the NURR1 is overexpressed in pancreatic tumors and is a negative prognostic factor for patient survival. Results of NURR1 knockdown or treatment with the NURR1 antagonist 1,1-bis(3΄-indolyl)-1-(p-chlorophenyl)methane (C-DIM 12) demonstrated that NURR1 was prooncogenic in pancreatic cancer cells and regulated cancer cell and tumor growth and survival. NURR1 is induced by gemcitabine and serves as a key drug resistance factor and is also required for gemcitabine-induced cytoprotective autophagy. NURR1-regulated genes were determined by RNA sequencing of mRNAs expressed in MiaPaCa2 cells expressing NURR1 and in CRISPR/Cas9 gene–edited cells for NURR1 knockdown and Kyoto Encyclopedia of Genes and Genomes enrichment analysis of the differentially expressed genes showed that autophagy was the major pathway regulated by NURR1. Moreover, NURR1 regulated expression of two major autophagic genes, ATG7 and ATG12, which are also overexpressed in pancreatic tumors and like NURR1 are negative prognostic factors for patient survival. Thus, gemcitabine-induced cytoprotective autophagy is due to the NURR1–ATG7/ATG12 axis and this can be targeted and disrupted by NURR1 antagonist C-DIM12 demonstrating the potential clinical applications for combination therapies with gemcitabine and NURR1 antagonists.
Significance:
Gemcitabine induces NURR1-dependent ATG7 and ATG12 cytoprotective autophagy in PDA cells that can be reversed by NURR1 antagonists.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a lethal human malignancy with a five-year survival rate of 9% and it is estimated that by 2030 pancreatic cancer will be the second leading cause of cancer-related deaths in United States (1). Although new therapeutic regimens against PDAC have improved treatment of this disease, the late-stage detection, lack of targeted therapies, and chemoresistance are still some of the major challenges for enhancing patient outcomes. The current treatments include surgical resection, which is followed by adjuvant chemotherapy (2) and palliative chemotherapy [with gemcitabine or FOLFIRINOX (folinic acid, 5-flouorouracil, irinotecan and oxaliplatin)] (3–5). Gemcitabine (Gemzar; 2′,2′-difluorodeoxycytidine), an FDA approved chemotherapeutic drug, is used for treatment of different cancers and overall survival and progression-free survival is enhanced by gemcitabine compared with those receiving non–gemcitabine-based therapy (6). Although, these treatments are initially effective in increasing survival of patients with pancreatic cancer, residual neoplastic cells usually result in relapse and subsequent appearance of more aggressive and lethal tumors (7). The success of cancer treatments for patients with PDA largely depends on diagnosis during initial stages of the disease. Cellular mechanisms such as apoptosis, necroptosis, and autophagy also determine the advancement and response to these cancer treatments (8). Thus, to find a remedy for treating this devastating disease, it is important to understand the mechanism through which pancreatic cancer cells acquire resistance to current therapeutics.
NURR1 (NR4A2) is an orphan nuclear receptor and a transcription factor that activates target genes by binding as monomers or dimers to cognate cis-elements in target gene promoters (9). NURR1 has been characterized as an important regulator in neuronal development and there is also increasing evidence of a prooncogenic role for their receptor in solid tumors (9–11) NURR1 regulates cell growth survival and metabolism (12–14) and studies in this laboratory identified 1,1-bis (3′-indolyl)-1-(p-chlorophenyl) methane (C-DIM12) as prototypical NURR1 ligand (15, 16). A recent study showed that C-DIM12 inhibited glioblastoma cell and tumor growth and also blocked NURR1-dependent prooncogenic pathways in glioblastoma (15). It was previously reported that induction of NURR1 promotes 5-fluorouracil (5-FU) resistance in squamous cell carcinoma (17); however, the mechanism of NURR1-mediated chemoresistance and its role in pancreatic cancer has not been determined.
Autophagy is an evolutionary cellular response to diverse stress and physiologic conditions during cancer progression and has been reported to induce tumor cell survival and drug resistance in cancer cells (18, 19). Autophagy is initiated with the formation of double layer membrane around the cytoplasmic components known as autophagosome which fuses with lysosomes to recycle these components for protein synthesis and energy production. It is closely regulated by a highly conserved set of genes known as autophagy-related genes (ATG; ref. 18). A recent study demonstrates that autophagy regulates the unique properties of cancer stem cells, such as differentiation and self-renewal, which contributes to tumor metastasis, tumor reoccurrence, and chemoresistance (20). A study, combining an autophagy inhibitor with photodynamic therapy, significantly reduced the colorectal tumor size suggesting that autophagy is a cytoprotective process (21), and in another study autophagy inhibition increased the drug sensitivity (22). Thus, understanding the mechanism of autophagy and drug resistance is an unsolved problem and could lead to enhanced therapeutic efficacy.
This study demonstrates that NURR1 is essential for chemotherapeutic agent–induced cytoprotective autophagy through transcriptional regulation of ATGs. We show for first time that ATG7 and ATG12 are NURR1-regulated genes, and high expression of NURR1/ATG7/ATG12 corresponds to the poor survival and prognosis of patients with PDA and the NURR1-ATG7/ATG12 axis can be targeted by NURR1 antagonists.
Materials and Methods
Cell Lines and Transfection with siRNA and Plasmids
MiaPaCa2 and Panc1 pancreatic cancer cell lines were obtained from ATCC and were cultured in in DMEM supplemented with 10% FBS (Gibco/Invitrogen), 1% l-glutamine (Gibco/Invitrogen), and 1% penicillin–streptomycin (Invitrogen) at 37°C in 5% humidified CO2 incubators. CRISPR/Cas9–mediated knockout of NURR1 in MiaPaCa2 cells was accomplished using guide RNAs targeting NURR1, fused with CRISPR/Cas9 and GFP protein (23). CRISPR Universal Negative Control plasmid (CRISPR06–1EA) was purchased from Sigma-Aldrich. Cells were harvested after 48 hours of transfection and GFP-positive cells were single sorted by using FACSCalibur flow cytometer. The guide RNA sequences used were:
NURR1–1(gatcccgggtcgtcccacat),
NURR1–2(gggcttgtagtaaaccgacc),
For all cell culture experiments, Mycoplasma testing (MycoAlert Mycoplasma Detection Kit, Lonza) was performed after each thawing and at least monthly. Cells were also authenticated using short tandem repeat analysis (Biosynthesis)
Cells were plated at 60% confluency in 6-well plates, and transient siRNA transfections (1 μmol/L) were performed using Lipofectamine 3000 (Invitrogen) and Opti-MEM (Invitrogen) according to the manufacturer's protocol; 48 hours after transfection, cells were treated or analyzed, as described previously (24, 25). siRNA oligos were purchased from Life Technologies (siNURR1, 4427038; siATG7, 4392420; siATG12, 4392420; siCTRL, AM4635).
Cell Growth Assays
Cells were plated in 96-well plates at 1 × 103 cells per well. After 5 days of incubation, cell growth was measured using Quant-iT PicoGreen dsDNA Assay Kit (Invitrogen). To estimate cell death, cells were trypsinized and counted after Trypan blue staining (Invitrogen) with a Hausser bright-line hemocytometer (Thermo Fisher Scientific; ref. 26). Annexin V/PI staining was performed using the Dead Cell Apoptosis Kit (Thermo Fisher Scientific, #V13245), according to the manufacturer's instructions. Staining was measured with an Accuri C6 flow cytometer and analyzed with FlowJo Version 10.2 software (26).
Immunoblot Analysis
Cells were lysed using 1% Triton in TBS containing protease and phosphatase inhibitors. Tumors were lysed using 1× RIPA buffer containing protease and phosphatase inhibitors. Equal amounts of total protein were separated by electrophoresis on a 4%–12% Bis-Tris gel and transferred to a polyvinylidene difluoride membrane (23). Blots were blocked in 5% BSA, and then probed with antibodies against anti-NURR1 (sc-376984, Santa Cruz Biotechnology), anti-ATG 7 (10088–2AP, ProteinTech), anti-ATG 12(D88H11, Cell Signaling Technology), anti-LC3BI/II (3868, Cell Signaling Technology), anti-cleaved PARP (9532S, Cell Signaling Technology), and anti-α-tubulin (Sigma). Chemiluminescent (32106, Thermo Fisher Scientific) signal was captured using a Syngene G-BOX iChemi XT imager (26).
Clonogenic Assay
1000–2000 cells per well were plated in a 6-well plate. The media were not changed during experiments unless indicated. Upon completion of the experiments, colonies were fixed in reagent containing 80% methanol and stained with 0.5% crystal violet. To determine relative growth, dye was extracted from stained colonies with 10% acetic acid and the associated absorbance measured at 600 nm using a Microplate Reader/Synergy HT BioTek plate reader (23, 27).
Luciferase Reporter Assays
Cells were seeded at 60% confluency in 6-well dishes, then transfected using lipofectamine 3000 (Thermo Fisher Scientific) with 2 μg of the promoter dual reporter plasmid (pCheck2–promoter ATG7 [TSS = 11272324; Upstream = 1265, Downstream = 295; Length = 1561] or ATG12 [TSS = 115841851; Upstream = 1271, Downstream = 228; Length = 1500]), purchased from GeneCopoeia (23). The luciferase activity of the cultured supernatant was measured 48 hours after transfection using Luciferase Assay Reporter Kit (Promega) according to the manufacturer's instructions (23).
Electron Microscopy
Cells were cultured in permanox petri dish and treated with gemcitabine (1 μmol/L) and C-DIM12 (15 μmol/L) for 24 hours. The cells were fixed with 2.5% paraformaldehyde, 2% glutaraldehyde, 0.1 mol/L cacodylate buffer, and embedded using Epon 812. The ultra-thin sections (∼100 nm) were cut using a Leica EM UC6 ultramicrotome and diamond knife. The sections were then placed on copper grids, poststained with saturated Uranyl Acetate and Reynolds Lead Citrate, and imaged using an FEI Morgagni 268 transmission electron microscope equipped with a MegaView III CCD camera.
Quantitative RT-PCR
Total RNA was extracted using RNAeasy Kit (Qiagen). cDNA was synthesized from 1 μg of total RNA using TagMan probes (NURR1, Hs00428691, Hs01117525; ATG7, Hs00893766, Hs04969948; ATG12, Hs00740818, Hs01047860; 18s, 99999901; Actin, Hs00157387, Life Technologies) and MultiScribe Reverse Transcriptase (Life Technologies). qRT-PCR analysis was performed using the Applied Biosystems 7500 Fast Real-Time PCR System (Life Technologies) and TagMan RT-PCR Master Mix (Life Technologies).
RNA Sequencing
RNA quality was assessed via the Agilent 2100 Bioanalyzer (Agilent Technologies). Strand-specific RNA-sequencing (RNA-seq) library was prepared using NEBNext Ultra II Directional RNA Library Prep Kit (NEB) according to the manufacturer's protocols (27). RNA-seq was performed using 150-bp paired-end format on a NovaSeq 6000 (Illumina) sequencer. RNA-seq quality was checked by running FastQC, and TrimGalore was used for adapter and quality trimming (27). Sequence reads were aligned to the hg19 human genome build using the STAR aligning program (28). Quantification of all genes and their isoforms was performed using FPKM normalized values using Cufflinks v2.2.1, DESeq2 analysis with an Padj < 0.05 was used to get a list of differentially expressed genes (29).
Immunofluorescence Assay
Cells were seeded in 24-well plate (50,000 cells/well). After 24 hours, the media were discarded and the cells were then washed with 1× PBS. The cells were then fixed with 10% formaldehyde and permeabilized with 0.1% Triton-X 100. The cells were then incubated with primary antibody, overnight at 4°C and then with secondary antibody (Thermo Fisher Scientific #A11001 and Cell Signaling Technology #4412S)] for 2 hours at room temperature. The images were captured using Zeiss Imager.Z1 AXIO at 40× magnification.
Chromatin Immunoprecipitation Assay
Cells were cross-linked for 10 minutes at room temperature by the addition of one-tenth volume of 11% formaldehyde (11% formaldehyde, 50 mmol/L HEPES pH 7.4, 100 mmol/L NaCl, 1 mmol/L EDTA pH 8.0, 0.5 mmol/L EGTA pH 8.0), followed by 5-minute quenching with 1/20th volume of 2.5 mol/L glycine. Cells were washed twice with PBS, with spins after each rinse, the supernatant was aspirated, and the cell pellet was flash frozen in liquid nitrogen. Frozen crosslinked cells were stored at −80°C (30).
Crosslinked cells were lysed with lysis buffer 1 (50 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, 1 mmol/L EDTA, 10% glycerol, 0.5% NP-40, and 0.25% Triton X-100), pelleted, and resuspended in lysis buffer 2 (10 mmol/L TrisHCl pH 8.0, 200 mmol/L NaCl, 1 mmol/L EDTA, 0.5 mmol/L EGTA), and pelleted again (30). The pellet was resuspended in sonication buffer (50 mmol/L HEPES pH 7.5, 140 mmol/L NaCl, 1 mmol/L EDTA pH 8.0, 1 mmol/L EGTA, 0.1% sodium deoxycholate, 0.1% SDS, and 1% Triton X-100), and sonicated (30) using a Branson Sonifier (power setting 5) for 10 cycles at 30 seconds each on ice (18–21 W) with 60 seconds on ice between cycles.
Dynal magnetic beads (Sigma) (50 μL) were blocked with 0.5% BSA (w/v) in PBS, and then bound with 10 μg of antibody against NURR1, Abcam (AB41917). Sonicated crosslinked lysates were incubated overnight at 4°C with magnetic beads bound with antibody. Beads were pelleted, and then washed several times: two times with sonication buffer, one time with sonication buffer with 500 mmol/L NaCl, one time with LiCl wash buffer (10 mmol/L TrisHCl pH 8.0, 1 mmol/L EDTA, 250 mmol/L LiCl, 0.5% NP-40, 0.5% sodium deoxycholate) and one time with TE (10 mmol/L TrisHCl pH 8.0, 1 mmol/L EDTA). Bound protein and cross-linked DNA were eluted in elution buffer (50 mmol/L TrisHCl pH 8.0, 10 mmol/L EDTA, 1% SDS), and cross-links were reversed by overnight incubation using RNase A (10 mg/mL) and Proteinase K (20 mg/mL) for 1 hour, respectively, and DNA was purified with phenol/chloroform extraction and ethanol precipitation and used for qPCR (primers summarized in Supplementary Table S1).
Mouse Studies
All experiments involving mice were approved by the Texas A&M University's Animal Care and Use Committee. Six-week-old, female, athymic nude mice (Nude-Foxn1nu) were purchased from ENVIGO. MiaPaCa2 (ctrl), CTRL.KO, NURR1.KO cells, were prepared in 100 μL solution comprised of 70% DPBS and 30% Matrigel. Suspensions of 3 × 106 cells were then injected subcutaneously into the left and right flanks of mice. Tumor volumes were measured three times per week using calipers (Volume = Length × Width2/2), along with body weight. Mice with established tumors (after 25 days, mean tumor volume of ∼100 mm3) were randomly divided into four groups, which were then treated with vehicle (20 μL/g of 0.9% NaCl), gemcitabine (5 mg/mL, prepared in vehicle solution) 100 mg/kg intraperitoneally i.p.) twice biweekly, C-DIM12 (30 mg/kg i.p.; Monday/Wednesday/Friday; LC Laboratories #R-5000) or a combination of both. Upon termination of mouse experiments, mice were euthanized using carbon dioxide inhalation followed by cervical dislocation, and tumors were harvested.
Statistical Analysis
Data were expressed as mean ± SEM of at least three independent experiments. An unpaired, two-tailed Student t test was used to determine the differences between groups (* P < 0.05; ** P < 0.01; *** P < 0.001). ANOVA test was used for the analysis of tumor measurements among treated groups.
Data Availability
RNA-seq files have been deposited in Gene Expression Omnibus (GEO) with accession number GSE159099.
Results
Prognostic Significance of NURR1 in Patients with PDAC
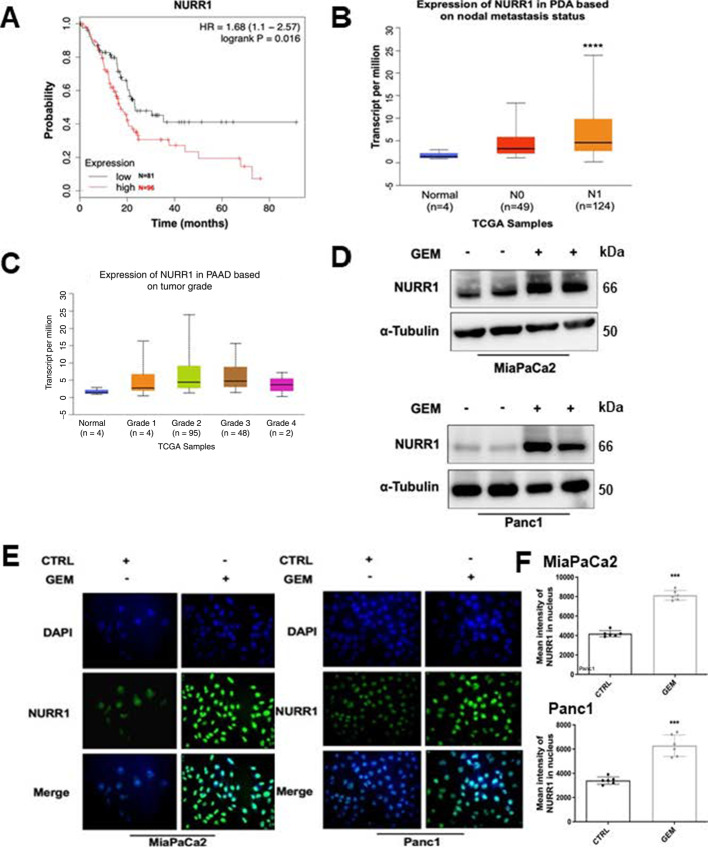
Previous studies showed that NURR1 is highly expressed in glioblastoma (15) and analysis of the published The Cancer Genome Atlas (TCGA) database showed that NURR1 was also more highly expressed in tumor samples from a patient with PDA. Kaplan–Meier analysis of NURR1 expression data showed that NURR1 overexpression of PDA patients’ tumor samples was also significantly associated with their poor survival (Fig. 1A). Lymph node metastasis was studied in 173 patients with PDA and NURR1 expression was significantly higher in patients with N0 and N1 PDA compared with normal individuals (Fig. 1B). NURR1 was also overexpressed in grade 2 tumor samples confirming that high levels of NURR1 are associated with the severity of PDAC (Fig. 1C).
FIGURE 1.
NURR1 confers to PDA chemoresistance. A, TCGA research network for PDA database was used for expression of NURR1 mRNA and correlated with patient's disease-free survival by using Kaplan–Meier survival analysis with log-rank tests (P < 0.016). B,NURR1 mRNA expression levels in patients with PDA based on nodal metastasis status. For each boxplot, median expression value, and 1st and 3rd quartiles are indicated (****, P < 0.00001). C,NURR1 mRNA expression levels in patients with PDA based on tumor grade. D, Immunoblot analysis of NURR1 protein lysates from MiaPaCa2 and Panc1 cells after 48 hours of treatment with gemcitabine (GEM; 1 μmol/L). β-Tubulin serves as a loading control (three independent experiments performed). E, Immunofluorescence staining of MiaPaCa2 and Panc1cells after 24 hours of treatment with gemcitabine (1 μmol/L) NURR1, Alexa Fluor 488 (green); nucleus, DAPI. Merged image, nuclear localization of NURR1 (three independent experiments performed, and at least 10 images per slide were analyzed for each condition). Magnification, 40×. F, Bars represent the quantification of mean intensity of NURR1 in the nucleus. Each data point represents the mean ± SEM of three independent experiments (***, P < 0.001).
PDA is a prime example of a tumor that develops chemoresistance and to investigate the possible relationship between chemotherapeutic drugs and NURR1 expression pancreatic cancer cells were treated with gemcitabine and analysis by immunoblot and immunofluorescence showed that gemcitabine induced expression of NURR1 in both MiaPaCa2 and Panc1 cells (Fig. 1D and E). This demonstrates that NURR1 expression is increased during chemotherapeutic treatment and the mechanisms of gemcitabine induction of NURR1 and GEM – C-DIM12 interactions are unknown and are currently being investigated.
NURR1 has a Cytoprotective Role Against Chemotherapeutic Drug–Induced Cell Death in Pancreatic Cancer Cells
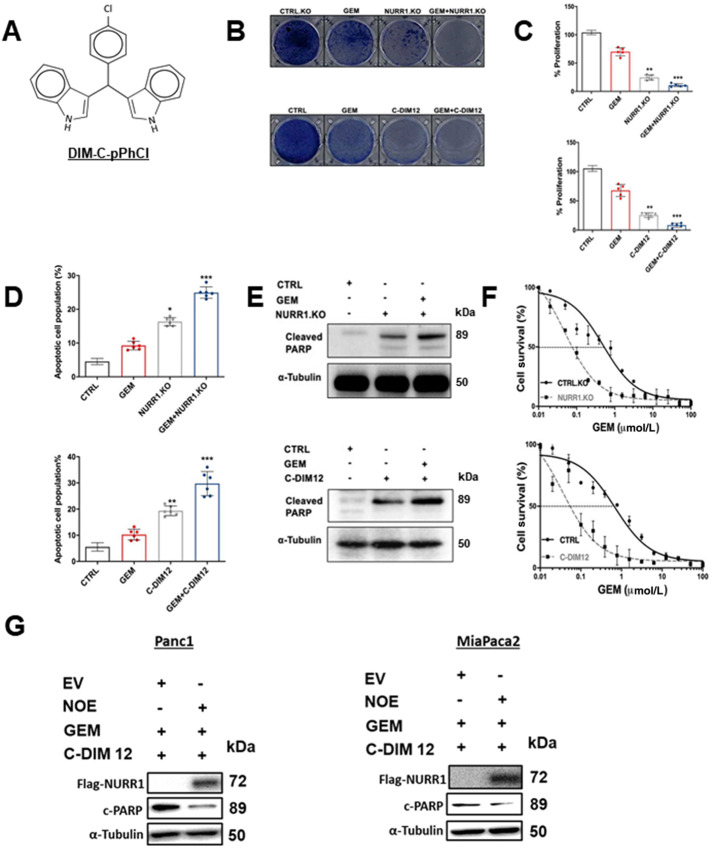
To study the role of NURR1 in drug resistance, we treated the MiaPaCa2 cells with the NURR1 antagonist C-DIM12 (DIM-C-pPhCl; Fig. 2A) and gemcitabine and in parallel studies we also determined the effects of gemcitabine alone and after NURR1 knockout (by RNA interference) in MiaPaCa cells. C-DIM12 has been characterized as an NURR1 antagonist in cancer cells and has been used as a model compound for studying the actions of NURR1 (15, 16). Crystal violet assay, phase contrast microscopy, and Annexin V staining showed that gemcitabine and C-DIM12 alone were effective; however, treatment with gemcitabine in combination with C-DIM12 was a more potent inhibitor of cell proliferation and survival than the individual compounds alone (Fig. 2B–D) and similar results were observed in Panc1 cells (Supplementary Figs. S1A – S1D). Moreover, treating the cells with C-DIM12 in combination with gemcitabine increased apoptosis as determined by cleaved PARP in MiaPaCa2 (Fig. 2E) and Panc1 cells (Supplementary Fig. S2A and S2B). Results summarized in Fig. 2E and 2F also show that cleaved PARP (marker of apoptosis) and inhibition of cell growth by gemcitabine were enhanced by cotreatment with C-DIM12 or by NURR1 knockout using CRISPR/Cas9 gene–edited cells and this response can be attenuated by overexpression of Nurr1 (NOE; Fig. 2G). Electron microscopy images also demonstrate that pancreatic cancer cells were more sensitive to gemcitabine when treated in combination with C-DIM12 and this is evidenced by enhanced cell shrinkage and nuclear condensation in treated compared with control cells. We also observed progressive fragmentation and increase in number of apoptotic bodies, which are indicators of apoptosis in the cells treated with gemcitabine and C-DIM12 (Supplementary Fig. S1C).
FIGURE 2.
NURR1 plays a cytoprotective role in pancreatic cancer. A, Chemical structure of DIM-C-pPhCl (C-DIM12). B, Images of crystal violet stained dishes that were treated with vehicle control, gemcitabine (GEM), C-DIM12, or NURR1 loss for 10 days after plating 1,000 cells per well in a 12-well plate (three independent experiments performed). C, Quantification of crystal violet staining is shown. Error bars indicate SEM of triplicate wells from a representative experiment (three independent experiments performed; **, P < 0.01; ***, P < 0.001). D, Apoptotic cell fraction was determined after treatment control (vehicle or CTRL.KO), gemcitabine, C-DIM12 or NURR1 loss, or a combination of both (gemcitabine + C-DIM12/ gemcitabine +NuRR1.KO for 72 hours. Apoptotic cell death was quantified by Annexin V/propidium iodide (PI) staining and flow cytometry and is shown as the percentage of cells that were PI positive. Each data point represents the mean ± SEM of three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001). E, Immunoblot analysis of cleaved PARP in control, gemcitabine, C-DIM12, or NURR1 loss treated cells with α-tubulin as a loading control in MiaPaCa2. F, Drug sensitivity was measured by PicoGreen DNA quantitation, in MiaPaCa2 cells, under the indicated culture conditions, and with varying doses of gemcitabine. Each data point represents the mean of four independent measurements. G, Panc1 MiaPaca2 cells were treated as indicated in the presence or absence of NOE or expression of empty vector (EV) and whole-cell lysates were analyzed by Western blots as outlined in the Materials and Methods.
ATG7 and ATG12 are the Key Targets of NURR1
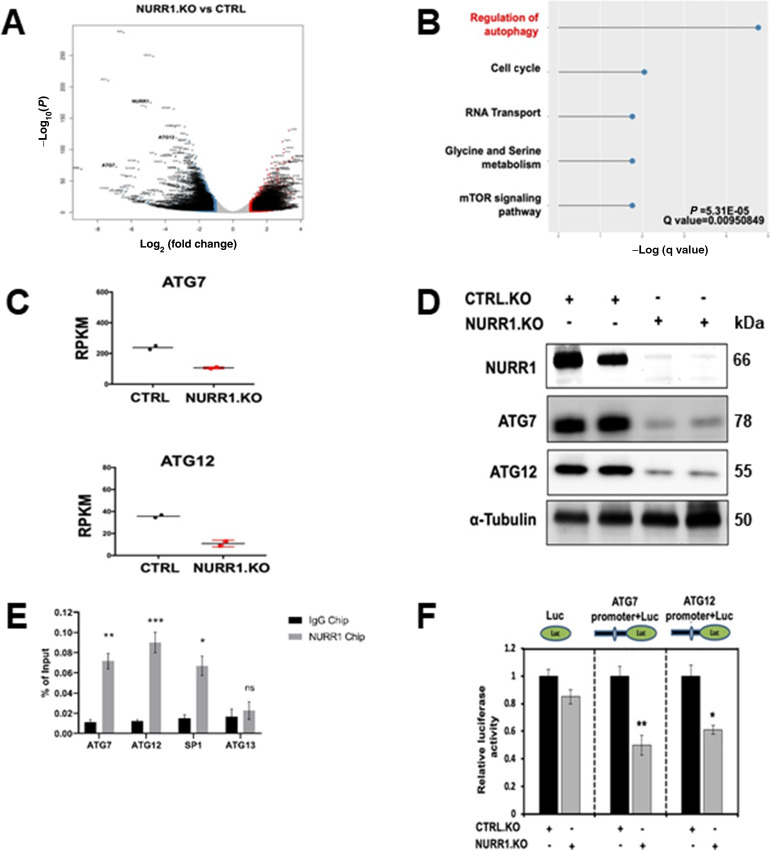
NURR1 regulates specific gene activity and mediates cell survival, migration, invasion, and transformation (9–13, 15, 31). To identify NURR1-regulated genes that may be involved in drug resistance, we performed RNA-seq and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment pathway analysis in MiaPaCa2 cells, modified by CRISPR/Cas9 gene editing for NURR1 expression and in control cells expressing NURR1 (Fig. 3A). To investigate pathways altered by the NURR1 knockout, we performed KEGG enrichment pathway analysis and observed that regulation of genes associated with autophagy was enriched (Fig. 3B; Supplementary Table S2). The expression of the autophagic ATG7 and ATG12 genes (18) was downregulated in NURR1.KO cells when compared with NURR1.CTRL cells (Fig. 3A–C) and therefore the potential roles of ATG7 and ATG12 in NURR1-mediated chemoresistance was further investigated because autophagy plays a key role in cell survival and drug resistance (32). Immunoblot analysis confirmed that NURR1 regulates the expression of ATG7 and ATG12 because knockdown of NURR1 significantly downregulated levels of ATG7 and 12 proteins (Fig. 3D). Further analysis by chromatin immunoprecipitation (ChIP) revealed that NURR1 is associated with the ATG7 and 12 promoters (Fig. 3E). Nurr1 was also associated with the Sp1 but not the ATG13 (negative control) promoter in a ChIP assay (Fig. 3E). We also subcloned the ATG7 and ATG12 promoter sequences into a luciferase reporter plasmid and after transfection into MiaPaCa2 cells the effects of NURR1 knockdown on luciferase activity was determined. Knockdown of NURR1 expression significantly decreased luciferase activity in both AGT7 and AGT12 promoter-luciferase constructs (Fig. 3F) confirming that ATG7 and ATG12 are key autophagic genes regulated by NURR1. Therefore, the role of NURR1 and ATG7/ATG12 in gemcitabine resistance was further investigated.
FIGURE 3.
ATG7 and ATG12 are the key targets of NURR1.A, Volcano plots of log2 fold change versus −log10(P) of RNA-sequencing data for NURR1.KO versus control (CTRL), for MiaPaCa2 cells subject to these manipulations. B, KEGG enrichment pathway analysis of NURR1-regulated pathways by comparing gene expressed wild-type MiaPaca2 cells and NURR1 knockout cells. C, qRT-PCR analysis of ATG7and ATG12 expression in CTRL and NURR1.KO cells. Gene expression is normalized to β-actin. Each data point represents the mean ± SEM of four independent experiments (**, P < 0.01; ***, P < 0.001). D, Immunoblot analysis of NURR1, ATG7, and ATG12 in NURR1.KO cells compared with CTRL MiaPaCa2 cells with α-tubulin as loading control. E, qPCR analysis of a ChIP assay in MiaPaCa2 cells shows a significant increase of NURR1 interactions on the in ATG7 and ATG12 promoters relative to IgG (**, P < 0.01; ***, P < 0.001). Results are expressed as the fold enrichment over input DNA. Error bars represent the mean ± SEM of three independent experiments. F, Dual luciferase assay of MiaPaCa2 cells with empty vector control (EV) and NURR1.KO. Cell lines were transfected with Renilla luciferase reporter constructs fused with ATG7 or ATG12 promoter, as well as a constitutive firefly luciferase expression construct for 24 hours. Renilla luciferase activity was normalized to firefly luciferase activity, and results shown are the average of four experiments ± SEM (*, P < 0.05; **, P < 0.01).
NURR1 Induces Autophagy, ATG7, and ATG12 in Pancreatic Cancer Cells
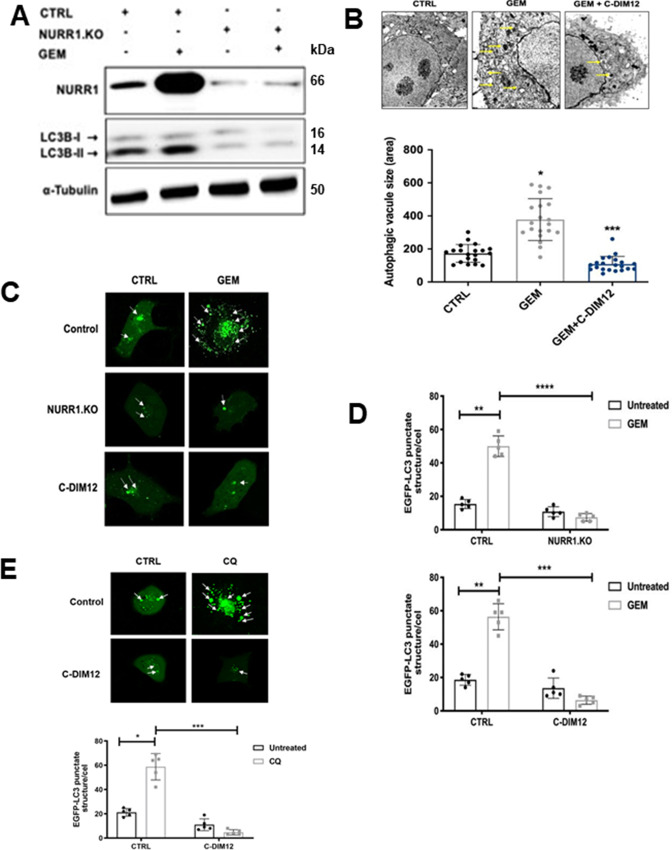
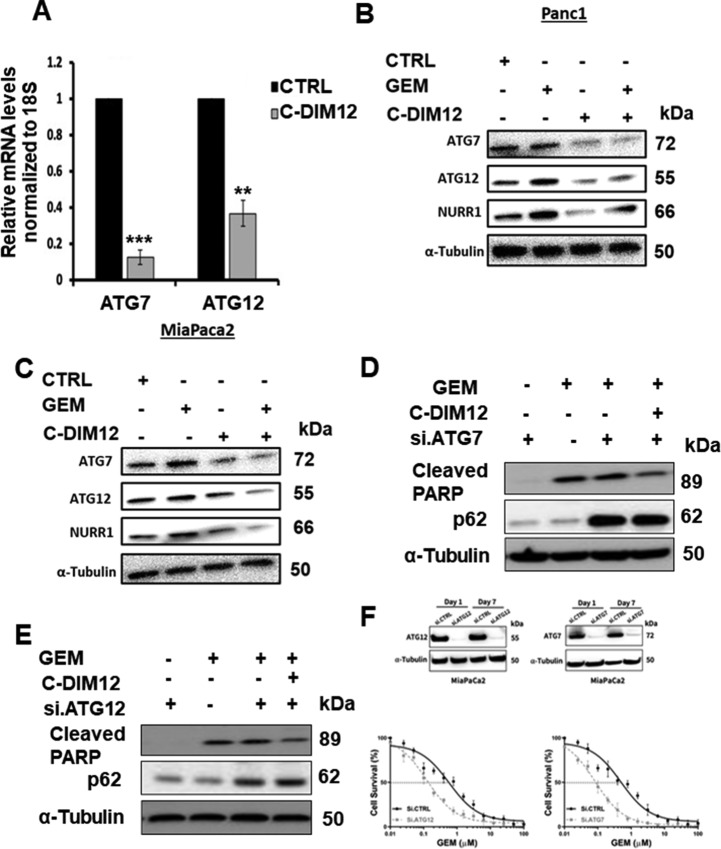
Autophagy is a cellular self-degradation process that reduces cellular damage in response to stressful conditions and the link between NURR1 expression and autophagy was further investigated in MiaPaCa2 cells treated with gemcitabine. Figure 1D and E show that gemcitabine induced NURR1 expression in Panc1 and MiaPaCa2 cells and this is also observed in Fig. 4A where gemcitabine-induced NURR1 expression is accompanied by an increase in the autophagic marker LC3B-II, whereas in NURR-KO cells ± gemcitabine, low levels of LC3B-II were expressed (Fig. 4A). Next, electron microscopy supports the immunoblot data showing that gemcitabine induced autophagy, which is characterized by autophagosomes and autophagic compartments; however, treatment with gemcitabine in combination with the NURR1 antagonist C-DIM12 reduced evidence for autophagy (Fig. 4B). Confocal microscopy also demonstrated that gemcitabine induced autophagy and that knockdown of NURR1 or treating the cells with C-DIM12 significantly reduced autophagy marked by decreased punctate staining of LC3-II (Fig. 4C). The bar graph representation revealed that LC3-II punctate staining was decreased 2–3-fold when expression of NURR1 was inhibited (Fig. 4D) and as a control we show that chloroquine increased LC3-II punctate staining (Fig. 4E). These data suggest that NURR1 is essential for induction of autophagy in pancreatic cancer cells. We next investigated the effects of the NURR1 antagonist C-DIM12 on expression of ATG7 and ATG12 in MiaPaCa2 cells and showed that C-DIM12 decreased expression of ATG7 and ATG12 mRNA levels (Fig. 5A). Treatment with C-DIM12 in combination with gemcitabine significantly downregulated expression of ATG7 and ATG12 in immunoblot analysis in MiaPaCa2 and Panc1 cells (Fig. 5B and C) and in the latter cell line, C-DIM12 decreased Nurr1 expression. Gemcitabine alone induced PARP cleavage in MiaPac2 cells (Fig. 5D and E), whereas knockdown of ATG7 or ATG12 did not induce this response. In contrast, gemcitabine alone and gemcitabine plus knockdown of ATG7 (Fig. 5D) or ATG12 (Fig. 5E) induced PARP cleavage and p62, and these responses were not enhanced by C-DIM12. The unexpected synergistic interactions of gemcitabine plus knockdown of ATG7 and ATG12 on induction of p62 are being further investigated. We also observed that silencing of ATG7 or ATG12 enhanced the cytotoxicity of gemcitabine (Fig. 5F) and these results are consistent with enhanced gemcitabine cytotoxicity after treatment with C-DIM12 or knockdown of Nurr1 (Fig. 2F).
FIGURE 4.
NURR1 induces autophagy in pancreatic cancer. A, Immunoblot analysis of NURR1 and LC3-I and LC3-II in NURR1.KO cells compared with control (empty vector; EV) MiaPaCa2 cells with α-tubulin as loading control. B, MiaPaCa2 cells were treated with gemcitabine (GEM) or C-DIM12 and autophagosomes were observed using transmission electron microscopy. The yellow arrow head indicates the autophagosomes and autolysosomes. The size of autophagosome was analyzed by measuring the area of autophagic vacuoles. C, Confocal microscopy images of MiaPaCa2 cells for EGFP-LC3 punctate staining in control (EV) and NURR1.KO cells treated with gemcitabine and C-DIM12 for 24 hours; white arrows indicate punctate EGFP-LC3 structures. D, As a control experiment, we show that chloroquine induces punctate screening. E, Bar graphs representation of the average EGFP-LC3 punctate in the cells (*, P < 0.05; **, P < 0.01; ****, P < 0.0001; three independent experiments performed, and at least 10 images per slide were analyzed for each condition). Scale bar, 10 μm. Magnification, 40×.
FIGURE 5.
NURR1 regulates the expression of ATG7 and ATG12.A, qPCR analysis of ATG7 and ATG12 expression after treatment with C-DIM12 (**, P < 0.01; ***, P < 0.001). B, Immunoblot analysis of ATG7 and ATG12 and cleaved PARP in gemcitabine (GEM) and C-DIM12–treated MiaPaCa2 cells. C, Immunoblot analysis of ATG7 and ATG12 in gemcitabine and C-DIM12–treated Panc1 cells. D and E, Immunoblot analysis of cleaved PARP and p62 in the presence of si.ATG7 or si.ATG12 MiapaCa2 cells in the presence or absence of treatment with gemcitabine, C-DIM12, and combination of both. α-Tubulin serves as a loading control. F, Effects of ATG7 and ATG12 knockdown on gemcitabine-induced survival of MiaPaca2 cells was determined as outlined in Fig. 2F. Each data point represents the mean of four independent measurements.
In Vivo Confirmation of NURR1/ATG7/ATG12 Axis
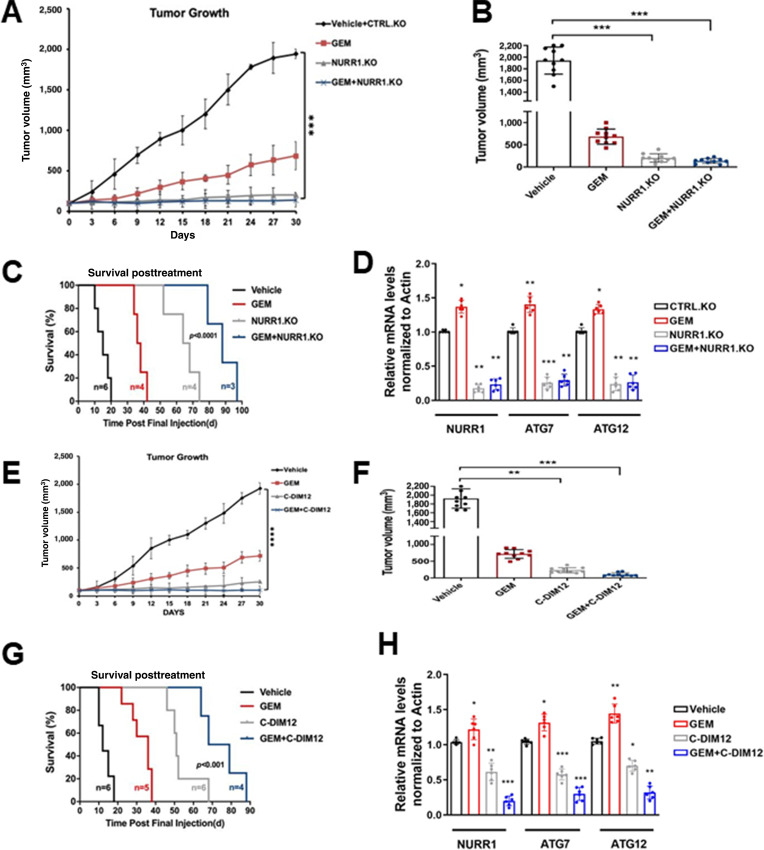
In athymic nude mice bearing MiaPaCa2 cells (Ctrl and NURR1-KO), gemcitabine alone decreased tumor volume (Fig. 6A and B), and both tumor volumes and weights were decreased in mice bearing NURR1-KO cells alone and after treatment with gemcitabine. Compared with the vehicle-treated group, the combination of gemcitabine, and NURR1 knockdown, also extended the median survival of the mice from 20 to 97 days (Fig. 6C). Both ATG7 and ATG12 mRNA levels were lower in tumors derived from mice bearing NURR1-KO cells and treated with gemcitabine compared with control cells (Fig. 6D). A comparable set of data were obtained in tumors from mice bearing wild-type MiaPaCa2 cells and treated with vehicle, gemcitabine, C-DIM12 and their combination. Both gemcitabine and C-DIM12 and their combination inhibited tumor growth (Fig. 6E and F) to a similar extent; however, posttreatment survival (Fig. 6G) shows that gemcitabine plus C-DIM12 was more effective than either compound alone. Gemcitabine plus C-DIM12 also inhibited ATG7 and ATG12 mRNA levels (Fig. 6H) and this corresponds to results observed in the in vitro studies (Fig. 5).
FIGURE 6.
Gemcitabine (GEM) in combination with C-DIM12 inhibits tumor growth in vivo. A, Tumor growth curves of MiaPca2 CTRL and NURR1.KO xenografts in nude mice treated with vehicle, gemcitabine, or combined NURR1.KO and gemcitabine for 30 days, when control tumors reached 100 mm3 in size. Tumor size was assessed every 3 days using digital calipers. (n = 5 per group, two tumors per mouse; ***, P < 0.001). Mean and SD are shown. B, Average tumor volume of MiaPaca2 (vehicle, gemcitabine, NURR1.KO, and combination of both) xenografts at the end of the experiment (day 30; n = 10 tumors per group; ***, P < 0.001). C, Survival was assessed after gemcitabine cessation. Mice were removed from the group when tumors achieved a volume of 1,500 mm3. Statistical significance was determined by log-rank test. D, Tumor RNA was extracted from vehicle or combination of gemcitabine and NURR1.KO xenografts and analyzed for mRNA expression of NURR1, ATG7, or ATG12. Normalized values are shown as mean ± SEM (n = 6 tumors per group; **, P < 0.01). E, Tumor growth curves of MiaPca2 xenografts in nude mice treated with vehicle, gemcitabine, C-DIM, or combined for 30 days (n = 5 per group, two tumors per mouse; ****, P < 0.0001). F, Average tumor volume of MiaPacA2 (vehicle, gemcitabine, C-DIM, and combination of both) xenografts at the end of the experiment (day 30; n = 10 tumors per group; **, P < 0.01; ***, P < 0.001). G, Survival was assessed after treatment cessation. Mice were removed from the group when tumors achieved a volume of 1,500 mm3. Statistical significance was determined by log-rank test. H, Tumor RNA was extracted from vehicle or combination of gemcitabine and C-DIM xenografts and analyzed for mRNA expression of NURR1, ATG7, or ATG12. β-Actin was used as normalization control. Normalized values are shown as mean ± SEM (n = 6 tumors per group; *, P < 0.05; **, P < 0.01).
Prognostic Significance of ATG7 and ATG12 and Association of NURR1 and ATG7 and ATG12 Expressions in Clinical Specimens
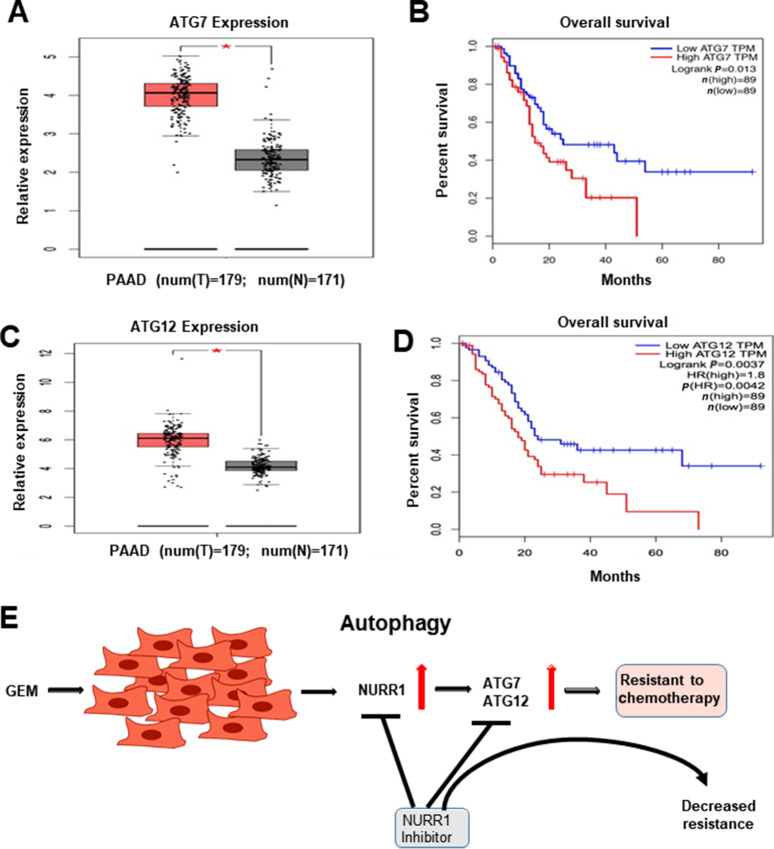
To study the prognostic significance of ATG7 and ATG12 in PDA, we examined TCGA, which showed a significant increase in expression of ATG7 in pancreatic tumors (T = 179) compared with normal tissue samples (n = 171; Fig. 7A). Kaplan–Meier analysis survival curve showed that high expression of ATG7 correlated with poor survival rate of patients with PDA (Fig. 7B). This analyses also demonstrated a significant increase in the expression level of ATG12 in pancreatic tumors, (T = 179) compared with normal tissue samples (N = 171; Fig. 7C) and Kaplan–Meier analysis shows that high expression of ATG12 corresponds to poor patient survival rate (Fig. 7D). We also investigated the correlation between the expression of NURR1 and ATGs (ATG7 and ATG12) in pancreatic tumor samples by performing correlation analysis. NURR1 expression was strongly correlated with ATG7 expression (R = 0.2, P = 6.2e-05; Supplementary Fig. S2C) and ATG12 expression (R = 0.31, P = 2e-05; Supplementary Fig. S2D) in PDA patient samples. Together, these data indicate prognostic significance of ATG7 and ATG12 in PDA and their correlation with NURR1 expression. These data coupled with mechanistic studies suggest a role for NURR1 in gemcitabine-induced resistance, which is linked to enhanced autophagy and the potential clinical applications of combination therapies using gemcitabine and NURR1 antagonists (Fig. 7E). The linkage between NURR1 and ATG7/ATG12 has been clearly demonstrated; however, the possible interactions with other important autophagic factors (e.g., TFEB) has not been determined and is currently been investigated.
FIGURE 7.
Prognostic significance of ATG7 and ATG12 in PDA. A,ATG7 expression in PDA patient tumor samples as compared with normal pancreas tissue using TCGA database (*, P <0.05). B, Kaplan–Meier analysis to demonstrate the prognostic significance of ATG7 in PDA patient (P = 0.013). C,ATG12 expression in PDA patient tumor samples as compared with normal pancreas tissue using TCGA database (*, P <0.05). D, Kaplan–Meier analysis to demonstrate the prognostic significance of ATG12 in PDA patient (P = 0.0042). E, Model for the role of NURR1 in gemcitabine (GEM) drug resistance in PDAC and effects of NURR1 antagonists to decrease drug resistance.
Discussion
PDA is usually detected in later stages and patients with PDA have low survival rates and current treatment options are limited in their effectiveness. Improvements in PDA patient survival will require development of validated biomarkers that appear early in the formation of these tumors and also new mechanism-based drugs and drug combinations that increase efficacy and decrease drug resistance. We initially examined the TCGA database and observed that expression of the orphan nuclear receptor NURR1 was more highly expressed in pancreatic tumors compared with the normal pancreas and patients with pancreatic cancer expressing high levels of NURR1 exhibited decreased survival (Fig. 1) and this was similar to our recent studies on the expression and prognostic value of NURR1 in glioblastoma (15). The functions of NURR1 have been investigated in multiple tumors (9, 33–38) and results of knockdown or overexpression studies have characterized this receptor as a prooncogenic factor that regulates cancer cell proliferation, survival, migration, and invasion. Although an endogenous ligand for NURR1 has not been identified, a recent study shows that the bis-indole–derived C-DIM12, a known NURR1 ligand, acted as a receptor antagonist in glioblastoma cells and inhibited cell growth and invasion and increased apoptosis (15). Another report showed that overexpression of NURR1 in squamous cell carcinoma cells increased resistance to 5-FU treatment (17) suggesting a possible role for NURR1 in drug resistance. Gemcitabine has replaced 5-FU for treatment of pancreatic cancer and based on the prognostic and functional characteristics of NURR1 in cancer cells this study focused on determining the functions of NURR1 in pancreatic cancer and its role in gemcitabine resistance.
Knockout of NURR1 in pancreatic cancer cells or treatment with C-DIM12–induced apoptosis and inhibited proliferation (Fig. 2). Moreover, in athymic nude mice bearing MiaPaCa2 (NURR1+/+) or NURR1-KO cells, it was clear that loss of NURR1 or treatment with C-DIM12 inhibited tumor growth and enhanced survival (Fig. 6). These results confirmed the prooncogenic activity of NURR1 in pancreatic cancer cells as previously observed in other cancer cell lines (15) and demonstrated that the NURR1 antagonist C-DIM12 was an effective anticancer agent that blocked NURR1-mediated responses.
We also investigated the role of NURR1 in drug resistance and observed that treatment of pancreatic cancer cells with gemcitabine resulted in induction of NURR1 (Fig. 1). Thus, although gemcitabine alone induced apoptosis and inhibited growth of pancreatic cancer cells and tumors (Figs. 1 and 6) this was accompanied by induction of NURR1, which exhibits tumor promoter–like activity. This counterintuitive effect of gemcitabine could be a component of drug resistance and this was confirmed in combination treatments of cells and mice with gemcitabine alone and in combination with NURR1 knockdown or receptor antagonist (C-DIM12; Figs. 1 and 6). Results of the in vitro and in vivo effects of these combination therapies are complementary and demonstrate the NURR1 inactivation or treatment with C-DIM12 enhanced the effectiveness of gemcitabine thus demonstrating that induction of NURR1 by gemcitabine is associated with drug resistance.
The mechanism of NURR1 as a drug-resistant factor was further investigated by RNA-seq in wild-type and NURR1-KO cells and this resulted in identification of several genes/pathways regulated by NURR1; however, the major pathway was associated with changes in expression of genes involved in autophagy (Fig. 3; refs. 39–43). Because autophagy has previously been linked to cytoprotection in colon cancer cells (21), we examined this gene set and identified two genes, namely ATG7 and ATG12, that were not only regulated by NURR1 (Fig. 5A and B) but also exhibit clinical characteristics similar to that observed for NURR1 in patients with pancreatic cancer (Figs. 1 and 7). These results confirm that NURR1 exhibits prooncogenic-like activity in pancreatic cancer cells and this receptor also plays a role in gemcitabine-induced drug resistance (Fig. 7E). These observations coupled with the anticarcinogenic effects of C-DIM12 suggest that the effectiveness of therapeutic regimens including gemcitabine for treatment of PDAC can be significantly enhanced using combination therapies that include a NURR1 antagonist such as C-DIM12.
Supplementary Material
Supplementary text Figs. 1 and 2 Supplementary text Table S1 and S2.
Acknowledgments
This work was supported by the Department of Defense (DOD-W81XWH-18–0592, to M. Zarei) and the NIH P30-ES029067 (to S. Safe).
Footnotes
Note: Supplementary data for this article are available at Cancer Research Communications Online (https://aacrjournals.org/cancerrescommun/).
Authors' Disclosures
No disclosures were reported by the authors.
Authors' Contributions
Mehrdad Zarei: Investigation, methodology. R. Shrestha: Investigation, methodology. S. Johnson: Investigation, methodology, writing-original draft. Z. Yu: Investigation, methodology. K. Karki: Investigation, methodology. A. Vasiri-Gohar: Investigation, methodology. J. Epps: Investigation, methodology. H. Du: Investigation, methodology. L. Suva: Funding acquisition, project administration, supervision. Masha Zarei: Conceptualization, funding acquisition, supervision, project administration, writing - review and editing. S. Safe: Funding acquisition, supervision, project administration, writing - review and editing.
References
- 1. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol 2018;24:4846–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Orth M, Metzger P, Gerum S, Mayerle J, Schneider G, Belka C, et al. Pancreatic ductal adenocarcinoma: biological hallmarks, current status, and future perspectives of combined modality treatment approaches. Radiat Oncol 2019;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817–25. [DOI] [PubMed] [Google Scholar]
- 4. Cunningham D, Chau I, Stocken DD, Valle JW, Smith D, Steward W, et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J Clin Oncol 2009;27:5513–8. [DOI] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xie Z, Zhang Y, Jin C, Fu D. Gemcitabine-based chemotherapy as a viable option for treatment of advanced breast cancer patients: A meta-analysis and literature review. Oncotarget 2018;9:7148–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Capurso G, Sette C. Drug resistance in pancreatic cancer: New player caught in act. EBioMedicine 2019;40:39–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xie L, Xia L, Klaiber U, Sachsenmaier M, Hinz U, Bergmann F, et al. Effects of neoadjuvant FOLFIRINOX and gemcitabine-based chemotherapy on cancer cell survival and death in patients with pancreatic ductal adenocarcinoma. Oncotarget 2019;10:7276–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ke N, Claassen G, Yu DH, Albers A, Fan W, Tan P, et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res 2004;64:8208–12. [DOI] [PubMed] [Google Scholar]
- 10. Komiya T, Yamamoto S, Roy A, McDonald P, Perez RP. Drug screening to target nuclear orphan receptor NR4A2 for cancer therapeutics. Transl Lung Cancer Res 2017;6:600–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohan HM, Aherne CM, Rogers AC, Baird AW, Winter DC, Murphy EP. Molecular pathways: the role of NR4A orphan nuclear receptors in cancer. Clin Cancer Res 2012;18:3223–8. [DOI] [PubMed] [Google Scholar]
- 12. Riggins RB, Mazzotta MM, Maniya OZ, Clarke R. Orphan nuclear receptors in breast cancer pathogenesis and therapeutic response. Endocr Relat Cancer 2010;17:R213–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitagawa H, Ray WJ, Glantschnig H, Nantermet PV, Yu Y, Leu CT, et al. A regulatory circuit mediating convergence between Nurr1 transcriptional regulation and Wnt signaling. Mol Cell Biol 2007;27:7486–96. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14. Nordzell M, Aarnisalo P, Benoit G, Castro DS, Perlmann T. Defining an N-terminal activation domain of the orphan nuclear receptor Nurr1. Biochem Biophys Res Commun 2004;313:205–11. [DOI] [PubMed] [Google Scholar]
- 15. Karki K, Li X, Jin UH, Mohankumar K, Zarei M, Michelhaugh SK, et al. Nuclear receptor 4A2 (NR4A2) is a druggable target for glioblastomas. J Neurooncol 2020;146:25–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Li X, Tjalkens RB, Shrestha R, Safe S. Structure-dependent activation of gene expression by bis-indole and quinoline-derived activators of nuclear receptor 4A2. Chem Biol Drug Des 2019;94:1711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shigeishi H, Higashikawa K, Hatano H, Okui G, Tanaka F, Tran TT, et al. PGE2 targets squamous cell carcinoma cell with the activated epidermal growth factor receptor family for survival against 5-fluorouracil through NR4A2 induction. Cancer Lett 2011;307:227–36. [DOI] [PubMed] [Google Scholar]
- 18. Chen N, Debnath J. Autophagy and tumorigenesis. FEBS Lett 2010;584:1427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lorente J, Velandia C, Leal JA, Garcia-Mayea Y, Lyakhovich A, Kondoh H, et al. The interplay between autophagy and tumorigenesis: exploiting autophagy as a means of anticancer therapy. Biol Rev Camb Philos Soc 2018;93:152–65. [DOI] [PubMed] [Google Scholar]
- 20. Yun CW, Lee SH. The roles of autophagy in cancer. Int J Mol Sci 2018;19:3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiong L, Liu Z, Ouyang G, Lin L, Huang H, Kang H, et al. Autophagy inhibition enhances photocytotoxicity of photosan-II in human colorectal cancer cells. Oncotarget 2017;8:6419–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cheng CY, Liu JC, Wang JJ, Li YH, Pan J, Zhang YR. Autophagy inhibition increased the anti-tumor effect of cisplatin on drug-resistant esophageal cancer cells. J Biol Regul Homeost Agents 2017;31:645–52. [PubMed] [Google Scholar]
- 23. Zarei M, Giannikou K, Du H, Liu HJ, Duarte M, Johnson S, et al. MITF is a driver oncogene and potential therapeutic target in kidney angiomyolipoma tumors through transcriptional regulation of CYR61. Oncogene 2021;40:112–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Lee SO, Safe S. Structure-dependent activation of NR4A2 (Nurr1) by 1,1-bis(3'-indolyl)-1-(aromatic)methane analogs in pancreatic cancer cells. Biochem Pharmacol 2012;83:1445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Karki K, Harishchandra S, Safe S. Bortezomib targets Sp transcription factors in cancer cells. Mol Pharmacol 2018;94:1187–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lal S, Burkhart RA, Beeharry N, Bhattacharjee V, Londin ER, Cozzitorto JA, et al. HuR posttranscriptionally regulates WEE1: implications for the DNA damage response in pancreatic cancer cells. Cancer Res 2014;74:1128–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zarei M, Lal S, Parker SJ, Nevler A, Vaziri-Gohar A, Dukleska K, et al. Posttranscriptional upregulation of IDH1 by HuR establishes a powerful survival phenotype in pancreatic cancer cells. Cancer Res 2017;77:4460–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 2014;15:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zarei M, Lal S, Vaziri-Gohar A, O'Hayer K, Gunda V, Singh PK, et al. RNA-Binding protein HuR regulates both mutant and wild-type IDH1 in IDH1-mutated cancer. Mol Cancer Res 2019;17:508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Beard JA, Tenga A, Hills J, Hoyer JD, Cherian MT, Wang YD, et al. The orphan nuclear receptor NR4A2 is part of a p53-microRNA-34 network. Sci Rep 2016;6:25108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Degenhardt K, Mathew R, Beaudoin B, Bray K, Anderson D, Chen G, et al. Autophagy promotes tumor cell survival and restricts necrosis, inflammation, and tumorigenesis. Cancer Cell 2006;10:51–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Han YF, Cao GW. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol 2012;18:6865–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ji L, Gong C, Ge L, Song L, Chen F, Jin C, et al. Orphan nuclear receptor Nurr1 as a potential novel marker for progression in human pancreatic ductal adenocarcinoma. Exp Ther Med 2017;13:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Komiya T, Coxon A, Park Y, Chen WD, Zajac-Kaye M, Meltzer P, et al. Enhanced activity of the CREB co-activator Crtc1 in LKB1 null lung cancer. Oncogene 2010;29:1672–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holla VR, Mann JR, Shi Q, DuBois RN. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem 2006;281:2676–82. [DOI] [PubMed] [Google Scholar]
- 37. Han Y, Cai H, Ma L, Ding Y, Tan X, Chang W, et al. Expression of orphan nuclear receptor NR4A2 in gastric cancer cells confers chemoresistance and predicts an unfavorable postoperative survival of gastric cancer patients with chemotherapy. Cancer 2013;119:3436–45. [DOI] [PubMed] [Google Scholar]
- 38. Han Y, Cai H, Ma L, Ding Y, Tan X, Liu Y, et al. Nuclear orphan receptor NR4A2 confers chemoresistance and predicts unfavorable prognosis of colorectal carcinoma patients who received postoperative chemotherapy. Eur J Cancer 2013;49:3420–30. [DOI] [PubMed] [Google Scholar]
- 39. Sun K, Deng W, Zhang S, Cai N, Jiao S, Song J, et al. Paradoxical roles of autophagy in different stages of tumorigenesis: protector for normal or cancer cells. Cell Biosci 2013;3:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu J, Li Y, Tian Z, Hua X, Gu J, Li J, et al. ATG7 overexpression is crucial for tumorigenic growth of bladder cancer in vitro and in vivo by targeting the ETS2/miRNA196b/FOXO1/p27 axis. Mol Ther Nucleic Acids 2017;7:299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Desai S, Liu Z, Yao J, Patel N, Chen J, Wu Y, et al. Heat shock factor 1 (HSF1) controls chemoresistance and autophagy through transcriptional regulation of autophagy-related protein 7 (ATG7). J Biol Chem 2013;288:9165–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun S, Wang Z, Tang F, Hu P, Yang Z, Xue C, et al. ATG7 promotes the tumorigenesis of lung cancer but might be dispensable for prognosis predication: a clinicopathologic study. Onco Targets Ther 2016;9:4975–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhu J, Tian Z, Li Y, Hua X, Zhang D, Li J, et al. ATG7 promotes bladder cancer invasion via autophagy-mediated increased ARHGDIB mRNA stability. Adv Sci 2019;6:1801927. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary text Figs. 1 and 2 Supplementary text Table S1 and S2.
Data Availability Statement
RNA-seq files have been deposited in Gene Expression Omnibus (GEO) with accession number GSE159099.