Abstract
Background:
Parasites contribute significantly to the decline of livestock production and productivity and consequently hamper the availability of protein food resources.
Aim:
This study aims to report the prevalence of parasitic diseases in the Eastern Cape Province (ECP), South Africa.
Method:
Retrospective data of animal diseases in the ECP from 2013 to 2018 was obtained from the veterinary unit of the Department of Rural and Agrarian Reform database, decoded analyzed, and interpreted.
Results:
The results reveal a significant association (p < 0.05) between local municipality, seasons, year, and livestock species. Endoparasites (75%) were the highest reported in the year 2015, whereas ectoparasites (38.1%) and fly parasites (30.4%) were mostly reported in the year 2016. The highest prevalence of fly parasites and endoparasites was found in autumn (87%) and spring (75%). The local municipalities with the highest prevalence were Amahlathi (fly parasite, 91.3%), Dr Beyers Naude (ectoparasite, 43.6%), Intsika Yethu (endoparasite, 75%), Makana (protozoa, 45.8%), Mbhashe (hemoparasite, 40%), Raymond Mhlaba municipality (hemoparasite, 12.5%), and Lukhanji (fly parasite, 8.7%). Parasitic diseases diagnosed in the province between 2013 and 2018 were babesiosis (1.7%), anaplasmosis (2.1%), distomatosis (0.1%), goat mange (0.2%), and sheep scab (94%).
Conclusion:
The prevalence of parasitic diseases was found unevenly distributed in the local municipalities of the ECP and mostly diagnosed in autumn and spring compared to summer and winter. This study provides baseline information to guide policy-making on disease preventative actions. The recommended action would include appropriate and timely use of acaricide to mitigate problems associated with parasitic diseases.
Keywords: Sheep scab, endoparasites, ectoparasites, anaplasmosis, Eastern Cape Province
Introduction
Animal parasitosis is a global livestock husbandry problem. Livestock parasites account for 13% of livestock mortality and are generally responsible for reducing animal productivity by reducing the quantity of meat, milk, and other animal products (Grace et al., 2015). Also, parasite infestation leads to poor livestock reproduction and food insecurity (Grace et al., 2015). An estimated $93.1 million is lost to parasite infestation, mainly due to treatment costs and production loss (Zoetis, 2018). A recent South African study reported a loss of 9992.4 USD due to fasciolosis between 2010 to 2012 (Jaja et al., 2017a). Livestock parasites are also associated with human health challenges. Zoonotic parasites like Echinococcus spp, Taenia spp, and Fasciola spp, when present in food, could easily be transmitted to humans, increasing the burden of human infection and mortalities (Torgerson and Macpherson, 2011; Barnes et al., 2017). For instance, a study that investigated the status of neurocysticercosis in Eastern and Southern Africa found that 8.5% were positive for cysticercosis (Mafojane et al., 2003). Previous research established that cysticercosis is associated with epilepsy, and further revealed that 16% of 200 mine workers repatriated because of epilepsy were positive for cysticercosis (Mafojane et al., 2003).
There are 1,000 commercial and 13,000 communal farmers in South Africa, contributing significantly to the gross domestic product. Livestock production generates income through livestock sales, drought power, manure, and other socioeconomic activities (Jaja et al., 2016). Hence, it is regarded as a way out of poverty ( Perry and Grace, 2009; Katiyatiya et al., 2014). Parasites, including sheep scabs, constrain sustainable livestock production (Bath et al., 2016). Such constraints are commonly associated with reduced fertility, skin irritation, anemia, and death (Sanhokwe et al., 2016).
Many factors have been adduced for the high prevalence of parasites and parasitic diseases in many low and medium-income countries. These factors include warm temperatures, lack of understanding of parasite life cycle, limited management and control approaches, as most anti-parasite drugs do not provide permanent solutions due to parasites’ resistance to drugs (Kumar et al., 2013). Also, indiscriminate, prolonged, and unwarranted use of acaricidal products/medicines enhances parasites’ resistance to drugs (Maphosa and Masika, 2010).
Aside from ticks, in South Africa, farmers’ knowledge and ability to manage parasites are very limited. Endoparasites particularly are not perceived as problematic even though animal health authorities responsible for disease management in the region claim the existence of those parasites (Simela, 2012). Ticks are the most reported parasites due to their cosmopolitan nature and ability to cause diverse diseases. Seasonal temperature changes are known to optimize annual tick activity patterns and rapidly influence tick-borne diseases’ dynamics (Hancock et al., 2011).
Tick infestations are a growing concern, mainly because of climate change, farming economics, and ecological biodiversity management, which drive changes in their epidemiology and geographical distribution (Taylor, 2012). Hence, communal and resource-poor farmers pay more attention to ticks’ control and underestimate other parasites such as nematodes, trematodes, flies, and protozoa. To the best of our knowledge, there is little research on the epidemiology and prevalence of parasitic diseases in the study area. Hence, this study aimed to determine the prevalence of parasitic diseases in the Eastern Cape Province (ECP), South Africa.
Materials and Methods
Study area
The study was conducted in ECP, South Africa. ECP is one of the nine provinces of South Africa, located in the southern region of the country. The northern part of the province opens to Free State and Lesotho, northeastern part to KwaZulu Natal, south and southeastern part to the Indian Ocean, and western and northern to the Westside. ECP occupies 13.9% of South African land with an estimated population density of 41 persons per square kilometer (Jaja et al., 2016). Eastern Cape's geographic location is 32.2968°S, 26.4194°E and lies 3,019 m above sea level. Major vegetation type in the ECP is Valley thicket and Karoo vegetation, alpine grassland, and subtropical coastal flora (Jaja et al., 2017b). The weather is divided into spring (hot–dry) (August–October), summer (hot–wet) (November–January), winter (cool–dry) (May–July), and autumn (post-rainy) (February–April) seasons (Nantapo and Muchenje, 2013). An average of 1152 mm of annual rainfall is mostly received in the summer season, while the average temperature is 17.8°C.
Description of data
Data of diagnosed parasitic disease cases from 2013 to 2018 was obtained from the Department of Rural and Agrarian Reform (DrDAR), Veterinary Service Unit, in Bhisho in ECP. The data was initially compiled by state veterinary clinics in the province and sent to the provincial veterinary office in Bhisho. All diseases were diagnosed by state veterinarians using regional and national veterinary laboratories. Confirmed cases were transmitted every month to the Veterinary Service Unit in the DrDAR. The captured variables include the type of livestock species (bovine, caprine, and ovine), diseases diagnosed, time of diagnosis, location where diagnoses occurred, and GPS coordinates. The data was then entered into Microsoft Excel and sorted in a variable form such as local municipality, season, year of diagnosis, causative agent, livestock species, and disease diagnosis. Each disease diagnosed was classified as an endoparasite, ectoparasite, fly parasites, hemoparasite, and protozoa to form causative agent variables. The seasons were derived by the month of disease diagnosis. Summer was taken as (November–January), autumn (February–April), winter (May–July), and spring (August–October) (Nantapo and Muchenje, 2013). Areas of diseases diagnosed such as farms, veterinary clinics, and veterinary laboratories were identified and classified according to their respective local municipality. The agroecological zones/natural zones (Table 1) of ECP such as for local municipality, temperature, rainfall, and altitude were generated from Google search engine and modified from the existing literature (Zvinorova et al., 2016).
Table 1. Agroecological zones/natural regions (NR) of the Eastern Cape.
| NR | Local municipality | Rainfall (mmyr1) | Temperature (ºC) | Altitude (m) |
|---|---|---|---|---|
| I | Amahlathi | >700 | 27 | 2,400 |
| II | Buffalo City | >700 | 18 | 850 |
| III | Dr Beyers Naude | 400 | 20 | 1,200 |
| IV | Elundini | >800 | - | 1,276 |
| V | Intsika Yethu | >200 | 24 | 1,020 |
| VI | Inxuba Yethemba | >200 | 20 | 1,000 |
| VII | Lukhanji | >200 | 25 | 1,070 |
| VIII | Makana | 680 | 18 | 2,370 |
| IX | Mbhashe | >1000 | 27 | 2,400 |
| X | Mnquma | >600 | 27 | 1,210 |
| XI | Nelson Mandela Bay | >400 | 17 | 46 |
| XII | Raymond Mhlaba | - | - | 2,400 |
| XIII | Sakhisizwe | >200 | 23 | 2,700 |
Modified from Zvinorova et al. (2016).
Statistical analysis
IBM® Statistical Package for the Social Sciences version 24.0 was used to performed descriptive statistics to determine the prevalence. Chi-square test was used to estimate the association between the local municipality, causative agent, season, and disease livestock species and their interaction with the prevalence of parasite disease. The hypothesis was tested at 95% confidence interval. p ≤ 0.05 was taken as statistically significant.
Ethical approval
The study was conducted under the permit granted by the University of Fort Hare Research and Ethics Committee with ethical clearance certificate number: JAJ011SPHI01.
Results
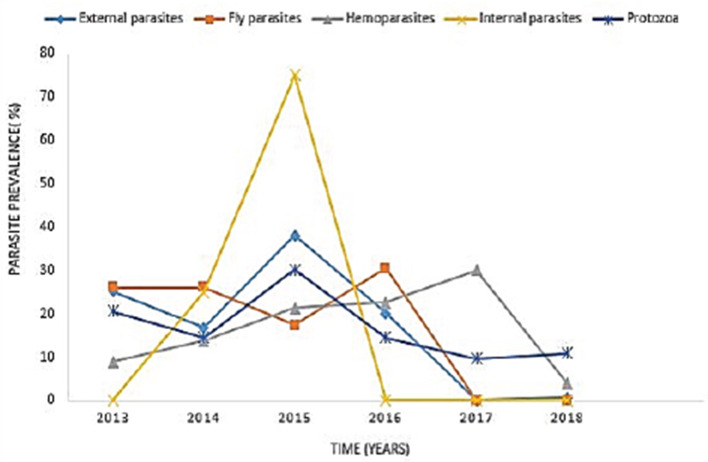
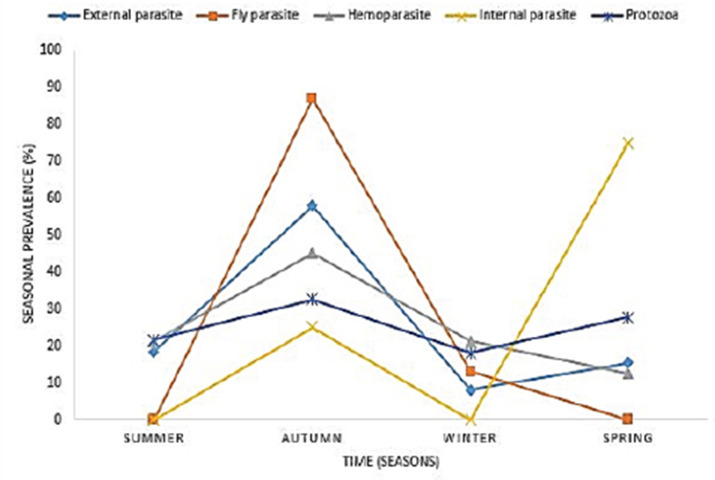
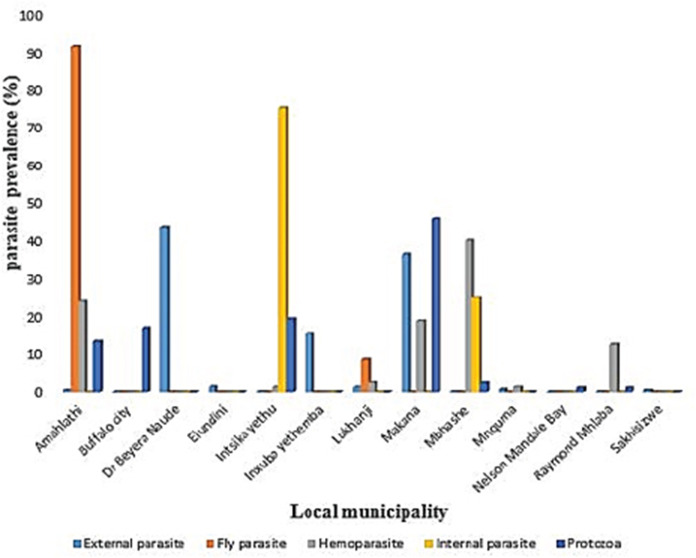
In 2015, the highest reported disease was due to endoparasites (75%), followed by ectoparasites (38.1%) and protozoa (30.1%). In 2016, fly parasite (30.4%) was the highest etiologic agent of disease (Fig. 1). In autumn, fly parasites (87%), ectoparasites (58%), hemoparasites (45%), and protozoa (32.5%) were the most reported parasitic problems. On the other hand, endoparasites (75%) were the most reported in spring (Fig. 2). In Figure 3, the following municipalities had the highest proportion of parasitic disease: Amahlathi (fly parasite, 91.3%), Dr. Beyers Naude (ectoparasite, 43.6%), Intsika Yethu (endoparasite, 75%), Makana (protozoa, 45.8%), Mbhashe (hemoparasite, 40%), Raymond Mhlaba (hemoparasite, 12.5%), and Lukhanji (fly parasite, 8.7%). The results revealed high associations (p < 0.05) between local municipality, season, year of diagnosis, disease diagnosed, and livestock species. Parasites and parasitic diseases mostly diagnosed in ECP from 2013 to 2018 were anaplasmosis (1.7%), babesiosis (2.1%), sheep scab (94%), Goat mange (0.2%), and distomatosis (0.1%) (Tables 2–4).
Fig. 1. Yearly prevalence rate of parasites from 2013 to 2018.
Fig. 2. Average seasonal prevalence of parasitic diseases from 2013 to 2018.
Fig. 3. Prevalence rate of parasitic diseases in the local municipalities in the Eastern Cape from 2013 to 2018.
Table 2. Seasonal prevalence and distribution of diagnosed parasitic diseases in the Eastern Cape Province, South Africa, from 2013 to 2018.
| Disease classification | Disease | Season % | |||
|---|---|---|---|---|---|
| summer | autumn | winter | Spring | ||
| HP | Anaplasmosis | 17 (21.3) | 36 (45) | 17 (21.3) | 10 (12.5) |
| PP | Babesiosis | 15 (24.2) | 25 (40.3) | 15 (24.2) | 7 (11.3) |
| FP | Crhysomia bezziana | 0 | 18 (85.7) | 3 (14.3) | 0 |
| PP | Coccidiosis | 3 (14.3) | 2 (9.5) | 0 | 16 (76.2) |
| FP | Cochliomia hominivorax | 0 | 2 (100) | 0 | 0 |
| IP | Distomatosis | 0 | 1 (25) | 0 | 3 (75) |
| EP | Goat mange | 3 (33.3) | 0 | 0 | 6 (66.7) |
| EP | Sheep scab | 656 (18.5) | 2067 (58.2) | 258 (8) | 544 (15.3) |
| Total | 694 (18.5) | 2151 (73.3) | 293 (7.8) | 586 (15.6) | |
DO: Disease occurrence; HP: hemoparasite; PP: Protozoa parasite; FP: fly parasite; IP: endoparasite; EP: ectoparasite. Disease variables were compared within seasons. X2 = 147.591, p-value = 0.001.
Table 4. Seasonal distribution of parasitic diseases from 2013 to 2018.
| Season | Livestock species | Causative agents | ||||||
|---|---|---|---|---|---|---|---|---|
| EP% | FP% | HP% | IP% | P% | Total | p-value | ||
| Summer | Bovine | 0 (0) | 0 (0) | 17 (48.6) | 0 (0) | 18 (51.4) | 35 (0.9) | 0.001 |
| Ovine | 656 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 656 (17.5) | 0.001 | |
| Caprine | 3 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (0.1) | 0.001 | |
| Autumn | Bovine | 0 (0) | 18 (22.2) | 36 (44.4) | 0 (0) | 27 (33.3) | 81 (2.2) | 0.001 |
| Ovine | 2,067 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2,067 (55.1) | 0.001 | |
| Caprine | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (0.1) | 0.001 | |
| Winter | Bovine | 0 (0) | 3 (8.6) | 17 (48.6) | 0 (0) | 15 (42.9) | 35 (0.9) | 0.001 |
| Ovine | 285 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 285 (7.6) | 0.001 | |
| Spring | Bovine | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (100) | 7 (0.9) | 0.001 |
| Ovine | 544 (94.9) | 0 (0) | 10 (1.7) | 3 (0.5) | 16 (2.8) | 573 (15.3) | 0.001 | |
| Caprine | 6 (100) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (0.2) | 0.001 | |
HP: hemoparasite; EP: ectoparasite; FP: fly parasite; IP: endoparasite; P: protozoa parasite. p-value >0.05 is significant.
Discussion
Livestock parasites are common worldwide; the disease burden due to parasites is worse in developing countries (Torgerson and Macpherson, 2011). Financial losses due to weight loss, poor feed utilization, general unthriftiness, and loss of reproduction have been reported (Kumar et al., 2013). In this study, endoparasites were the most reported livestock parasites in Intsika Yethu (75%) and Mbhashe (25%) municipalities. The autumn season (87%) also had the highest prevalence of endoparasites in the 2013–2018 period. The current study’s high prevalence of parasites contradicts Colombian research, where 56.5% of endoparasites was reported (Pinilla León et al., 2019). The reason for these differences might be due to ecological discrepancies in the study geographic areas, poor animal husbandry, lack of understanding of parasite life cycle by farmers, and a general weak primary animal health and herd health programs. A further contributing factor could be the acute shortage of veterinarians in ECP, warranting limited access points for veterinary and extension services across the province (Jaja et al., 2017a). Apart from the unavailability of government established veterinary clinics in most communities, most farmers are ignorant of the need for veterinary health management for their livestock (Onuekwusi, 2014).
Insects such as horn flies, face flies, horseflies, cattle grubs, lice, ticks, and mites are the major ectoparasites that cause obvious discomfort to livestock (Kaufman, 2018). Such discomfort often leads to weight loss, production losses, due to irritation (tick worries) that keep infested animals off feed, immune suppression, disease, and mortality. The present study found the prevalence of ectoparasites scattered and distributed in various parts of the province. The municipalities with the highest prevalence were Dr Beyers Naude (43.6%), Inxuba Yethemba (15.3%), and Makana (36.5%). The results in the current study were higher than a study investigating the prevalence of ectoparasites infestation in Bench Maji zone, southwest Ethiopia. The Ethiopian study reported an overall prevalence of 27.3% (Shiferaw and Onu, 2013). The high infestation rates might be attributed to favorable climatic conditions in some parts of the province. Also, malnutrition occasioned by the ongoing drought in the Province is a contributory factor. Farmer's lack of knowledge about the effects of ectoparasites and the inadequate animal health services such as dipping and head health programs are other factors promoting the high levels of parasitism seen in the study area.
Table 3. Percentage distribution of parasitic diseases in various local municipalities in the Eastern Cape from 2013 to 2018.
| Local Municipality | Livestock species | Causative agents | ||||||
|---|---|---|---|---|---|---|---|---|
| EP% | FP% | HP% | IP% | P% | Total count | P-value | ||
| Amahlathi | Bovine | - | 21(41.2) | 19(37.3) | - | 11(21.6) | 51(1.4) | 0.001 |
| Ovine | 8(100) | - | - | - | - | 9(100) | 0.001 | |
| Caprine | 9(100) | - | - | - | - | 9(0.2) | 0.001 | |
| Buffalo city | Ovine | - | - | - | - | 14(100) | 14(0.4) | - |
| Dr Beyers Naude | Ovine | 1554(100) | - | - | - | - | 1554(41.5) | - |
| Elundini | Ovine | 49(100) | - | - | - | - | 49(1.3) | - |
| Intsika yethu | Bovine | - | - | 1(100) | - | - | 1(.0.02) | - |
| Ovine | 3(13.6) | - | - | 3(13.6) | 16(72.7) | 22(0.6) | - | |
| Inxuba yethemba | Ovine | 544(100) | - | - | - | - | 544(14.5) | 0.001 |
| Lukhanji | Bovine | - | - | 2(100) | - | - | 2(0.1) | 0.001 |
| Ovine | 45(100) | - | - | - | - | 45(1.2) | 0.001 | |
| Makana | Bovine | - | - | 15(28.3) | - | 38(71.7) | 53(1.4) | 0.001 |
| Ovine | 1300(100) | - | - | - | - | 1300(34.7) | 0.001 | |
| Mbhashe | Bovine | - | - | 32(94.1) | - | 2(5.9) | 34(0.9) | 0.001 |
| Ovine | 2(66.7) | - | - | 1(33.3) | - | 3(0.1) | 0.001 | |
| Mnquma | Bovine | - | - | - | - | 1(100) | 1(0.02) | 0.001 |
| Ovine | 26(100) | - | - | - | - | 26(0.7) | 0.001 | |
| Nelson Mandela Bay | Bovine | - | - | - | - | 1(100) | 1(0.02) | 0.157 0.157 |
| Ovine | 1(100) | - | - | - | - | 1(0.02) | ||
| Raymond Mhlaba | Bovine | - | - | - | - | 1(100) | 1(0.02) | 0.001 |
| Ovine | 2(16.7) | - | 10(83.3) | - | - | 12(0.3) | 0.001 | |
| Sakhisizwe | Ovine | 18(100) | - | - | - | - | 18(0.5) | 0.001 |
HP: hemoparasite; EP: ectoparasite; FP: fly parasite; IP: endoparasite; P: protozoa parasite. p-value >0.05 is significant.
Fly parasites were reported high in the autumn season in Amahlathi and Lukhanji local municipalities. These findings concurred with the report in the study investigating prevalence and distribution of tsetse fly in Nigeria. These studies revealed that fly parasites are more prevalent in areas where the same fly parasites had earlier been eradicated (Oluwafemi, 2012). They show monthly changes in abundance throughout the year and peak in July, one season before the onset of the autumn season (Oluwafemi, 2012). The high prevalence in these regions in ECP could be due to seasonal factors such as altitude and temperature. Also, changing weather patterns, climate variability, and animal movements could shift some parasites and diseases into higher elevations (Scasta, 2015).
Although the proportion of protozoa parasites in this study was low, protozoan parasites are a significant cause of abortion and infertility in domestic ruminants resulting in considerable economic losses (Kaltungo and Musa, 2013). Hastutiek et al. (2019) studied protozoa parasites prevalence in low and upland regions and found the highest prevalence (88.23%) in the higher regions. The relatively very low prevalence of protozoa parasites observed in this study could probably be because animals that recovered from some protozoa parasitic diseases might become immune to reinfection (Kaltungo and Musa, 2013).
In the present study, the leading hemoparasitic infections were anaplasmosis and babesiosis. Cattle and goats are the most susceptible to hemoparasites, and these parasites cause severe clinical diseases and production losses (Weny et al., 2017). Hemoparasites in the present study were low and widely distributed in many local municipalities and were highest in the autumn season. The low prevalence of hemoparasites in this study is similar to the study conducted in Nigeria investigating the prevalence of hemoparasites in livestock. The Nigerian study recorded a regional prevalence of 38% and a summer prevalence of 64% (Onuekwusi, 2014). The relatively low prevalence of hemoparasitic diseases might be due to underreporting. Also, goats in the study area browse invasive plant species such as Acacia karoo. These plants have been proven as potent anthelminthic agents (Sanhokwe et al., 2016). Communal farmers lack financial resources to cover the veterinary cost (Jaja et al., 2017a). Hence, they make use of ethnoveterinary sources for the management of livestock parasites.
In the current study, the prevalence of goat mange was 0.2%. This is in contrast with the findings recorded in the East Harerghe zone (3.4%), Eastern Ethiopia. Mange is a zoonotic parasitic disease caused by mites, which are obligate parasites that affect all warm-blooded animals and man (Anyogu, 2020). It is transmitted through contact with affected animals and or contaminated materials (Zoetis, 2018). Three genera that affect small ruminants include Sarcoptes, Psoroptes, and Demodex. Parasite activities in the host’s skin elicit intense pruritus, which causes a wide range of complications such as mechanical injury from scratching, irritation, dermatitis, and abscess formation due to secondary bacterial infection, weight loss, lameness, anemia, and death in severe cases (Nyangiwe and Horak, 2007). The mange disease is generally seen in animals that are in poor conditions in the pre-summer and summers season (Mana, 2018).
Sheep scab is one of the oldest known skin diseases of sheep, and it is caused by either of the mange mites Psoroptes ovis or Sarcoptes scabiei. It is a serious animal welfare condition as parasite activities lead to allergic dermatitis through fecal deposition on the animal’s skin (Dunn et al., 2016). In the present study, sheep scab prevalence rate was 94.5%. This result tallies with a Brazilian study where 66.6%–100% mean intensity of Psoroptes ovis infestation in goats was reported (Amorim et al., 2015). On the contrary, a survey of ectoparasites of goats in the UK only found seven mite and lice infections (Cornall and Wall, 2015). Sheep scab is a controlled disease in terms of the South African Animal Diseases Act of 1984. However, sheep-keeping farmers in the ECP do not fully comply with government regulation on early disease reporting. Sheep scab can only be effectively controlled if all farmers make an effort to obey the law and report incidences timeously.
Conclusion
This study found the prevalence of parasitic diseases significantly high in autumn and spring than in summer and winter. Amahlathi, Dr. Beyers Naude, Intsika Yethu, Makana, Mbhashe, and Mhlaba municipalities recorded the highest prevalence of fly parasite, ectoparasite, endoparasite, protozoa disease, and hemoparasitic disease, respectively. On the other hand, Raymond Mhlaba, Lukhanji, Ulundi, Mnquma, Nelson Mandela Bay, and Sakhisizwe municipalities recorded a very low prevalence of parasitic disease. The highest prevalence was reported in 2015 for endoparasites but lowest in 2013, 2016, 2017, and 2018. Parasites are a significant cause of disease and livestock production loss, frequently causing consequential economic loss and affecting animal welfare. Regular and proper use of insecticides, dewormers, and acaricides to control the parasites in ECP is highly recommended an effective preventive or control measures to tame the tides of high parasitic disease prevalence during season active activity. Farmers should be adequately trained on the effective management of animal parasites, acaricides, and veterinary medicines for chemoprophylaxis. Furthermore, strict implementation/enforcement of the South African Animal Disease Act or provision of incentives to farmers who report sheep scabies help to improve the disease reporting system in the study area.
Conflict of interest
The authors declare that there is no conflict of interest.
References
- Amorim M.G.R, de, Azevedo S.S, Riet-Correa F. Seasonal prevalence and mean intensity of Psoroptes ovis infestation in goats in the Brazilian semiarid region. Rev. Bras. Parasitol. Veterinária. 2015;24:59–65. doi: 10.1590/S1984-29612014094. [DOI] [PubMed] [Google Scholar]
- Anyogu D.C. Diagnosis of mange in West African Dwarf ( WAD ) and Red Sokoto (RS) goats. Int. J. Infect. Dis. 2018;73S:314. doi: 10.1016/j.ijid.2018.04.4128. [DOI] [Google Scholar]
- Barnes A.N, Davaasuren A, Baasandagva U, Gray G.C. A systematic review of zoonotic enteric parasitic diseases among nomadic and pastoral people. PLoS One. 2017;12:1–22. doi: 10.1371/journal.pone.0188809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bath G.F, Penrith, M.-L. and Leask R. A questionnaire survey on diseases and problems affecting sheep and goats in communal farming regions of the Eastern Cape province, South Africa. J. S. Afr. Vet. Assoc. 2016;87:10. doi: 10.4102/jsava.v87i1.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornall K, Wall R. externas of goats in the UK. Vet. Parasitol. 2015;207:176–179. doi: 10.1016/J.VETPAR.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Dunn J.A, Prickett J.C, Collins D.A, Weaver R.J. Primary screen for potential sheep scab control agents. Vet. Parasitol. 2016;224:68–76. doi: 10.1016/j.vetpar.2016.05.019. [DOI] [PubMed] [Google Scholar]
- Grace D, Songe M, Knight-jones T. Nairobi: 2015. [05 June 2020]. Impact of Neglected Diseases on Animal Productivity and Public Health in Africa, Africa-OIE Regional Commission. Available via https://cgspace.cgiar.org/handle/10568/56836. [Google Scholar]
- Hancock P.A, Brackley R, Palmer S.C.F. Modelling the effect of temperature variation on the seasonal dynamics of Ixodes ricinus tick populations. Int. J. Parasitol. 2011;41:513–522. doi: 10.1016/j.ijpara.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Hastutiek P, Yuniarti W.M, Djaeri M, Lastuti N.D.R, Suprihati E, Suwanti L.T. Prevalence and diversity of gastrointestinal protozoa in Madura cattle at Bangkalan Regency, East Java, Indonesia. Vet. World. 2019;12:198–204. doi: 10.14202/vetworld.2019.198-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja I.F, Mushonga B, Green E, Muchenje V. Financial loss estimation of bovine fasciolosis in slaughtered cattle in South Africa. Parasite Epidemiol. Control. 2017a;2:27–34. doi: 10.1016/j.parepi.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja I.F, Mushonga B, Green E, Muchenje V. Seasonal prevalence, body condition score and risk factors of bovine fasciolosis in South Africa. Vet. Anim. Sci. 2017b;4:1–7. doi: 10.1016/j.vas.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja I.F, Mushonga B, Green E, Muchenje V. Prevalence of lung lesions in slaughtered cattle in the Eastern Cape Province, South Africa. J. S. Afr. Vet. Assoc. 2016;87:1–9. doi: 10.4102/jsava.v87i1.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltungo B.Y, Musa I.W. A review of some protozoan parasites causing infertility in farm animals. ISRN Trop. Med. 2013:1–6. doi: 10.1155/2013/782609. [DOI] [Google Scholar]
- Katiyatiya C.L.F, Muchenje V, Mushunje A. Farmers’ perceptions and knowledge of cattle adaptation to heat stress and tick resistance in the eastern cape, South Africa. Asian-Australasian J. Anim. Sci. 2014;27:1663–1670. doi: 10.5713/ajas.2014.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman P, Koehler P, Butler J. External parasites in beef cattle. 2018. [06/04/2020]. pp. 1–10. Availble via https://edis.ifas.ufl.edu/pdf/IG/IG13000.pdf.
- Kumar N, Rao T.K.S, Varghese A, Rathor V.S. Internal parasites management in grazing livestock. J. Parasit. Dis. 2013;37:151–157. doi: 10.1007/s12639-012-0215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafojane N.A, Appleton C.C, Krecek R.C, Michael L.M, Willingham A.L. The current status of neurocysticercosis in Eastern and Southern Africa. Acta Trop. 2003;87:25–33. doi: 10.1016/S0001-706X(03)00052-4. [DOI] [PubMed] [Google Scholar]
- Mana Y. Study on Prevalence of mange mites and associated risk factors on small ruminants in Kindo Koysha District of Wolaita Zone. Int. J. Res. Stud. Biosci. 2018;6:31–37. [Google Scholar]
- Maphosa V, Masika P.J. Ethnoveterinary uses of medicinal plants: A survey of plants used in the ethnoveterinary control of gastro-intestinal parasites of goats in the Eastern Cape Province, South Africa. Pharm. Biol. 2010;48:697–702. doi: 10.3109/13880200903260879. [DOI] [PubMed] [Google Scholar]
- Nantapo C.T.W, Muchenje V. Winter and spring variation in daily milk yield and mineral composition of Jersey, Friesian cows and their crosses under a pasture-based dairy system. South African J. Anim. Sci. 2013;1:43. doi: 10.4314/sajas.v43i5.3. [DOI] [Google Scholar]
- Nyangiwe N, Horak I.G. Goats as alternative hosts of cattle ticks. Onderstepoort J. Vet. Res. 2007;74:1–7. doi: 10.4102/ojvr.v74i1.133. [DOI] [PubMed] [Google Scholar]
- Oluwafemi R. Prevalence and distribution of Tsetse fly (mainly Glossina palpalis palpalis and Glossina tachinoides) in BICOT Project area in Lafia Local Government of Nassarawa State, Nigeria – Implication for sustainable Agricultural development. Internet J. Parasit. Dis. 2012;4:1–4. doi: 10.5580/e5c. [DOI] [Google Scholar]
- Onuekwusi G. Prevalence of Haemoparasites in Livestock in Ikwuano Local Government Area of Abia State. J. Fish. Livest. Prod. 2014;2:2–4. doi: 10.4172/2332-2608.1000109. [DOI] [Google Scholar]
- Perry B, Grace D. The impacts of livestock diseases and their control on growth and development processes that are pro-poor. Philos. Trans. R. Soc. B Biol. Sci. 2009;364:2643–2655. doi: 10.1098/rstb.2009.0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla León J.C, Delgado N.U, Florez A.A. Prevalence of gastrointestinal parasites in cattle and sheep in three municipalities in the Colombian Northeastern Mountain. Vet. World. 2019;12:48–54. doi: 10.14202/vetworld.2019.48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanhokwe M, Mupangwa J, Masika P.J, Maphosa V, Muchenje V. Medicinal plants used to control internal and ectoparasites in goats. Onderstepoort J. Vet. Res. 2016;83:7. doi: 10.4102/ojvr.v83i1.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scasta J.D. Livestock parasite management on high-elevation rangelands: Ecological interactions of climate, habitat, and wildlife. J. Integr. Pest Manag. 2015;6:1–12. doi: 10.1093/jipm/pmv008. [DOI] [Google Scholar]
- Shiferaw T.Z, Onu S.H. Prevalence of ectoparasite infestations of cattle in Bench Maji Zone, southwest Ethiopia. Vet. World. 2013;6:291–294. doi: 10.5455/vetworld2013.291-294. [DOI] [Google Scholar]
- Simela L. university of pretoria; 2012. [09 March 2020]. Options for the delivery of primary animal health care for livestock farmers on communal land in South Africa: Mnisi community case study By A dissertation submitted in partial fulfilment of the requirements for the degree MSc (Veterinary Tropical Disea. Available via https://repository.up.ac.za/handle/2263/27647. [Google Scholar]
- Taylor M.A. Emerging parasitic diseases of sheep. Vet. Parasitol. 2012;189:2–7. doi: 10.1016/j.vetpar2012.03.027. [DOI] [PubMed] [Google Scholar]
- Torgerson P.R, Macpherson C.N.L. The socioeconomic burden of parasitic zoonoses: global trends. Vet. Parasitol. 2011;182:79–95. doi: 10.1016/j.vetpar2011.07.017. [DOI] [PubMed] [Google Scholar]
- Weny G, Okwee-Acai J, Okech S.G, Tumwine G, Ndyanabo S, Abigaba S, Goldberg T.L. Prevalence and Risk Factors Associated with Hemoparasites in Cattle and Goats at the Edge of Kibale National Park, Western Uganda. J. Parasitol. 2017;103:69–74. doi: 10.1645/16-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoetis. Cost of parasites. [02 March 2020];2018 Available via https://www.zoetis.com.au/livestock-solutions/northern-beef/effective-parasite-management/cost-of-parasites.aspx. [Google Scholar]
- Zvinorova P.I, Halimani T.E, Muchadeyi F.C, Matika O, Riggio V, Dzama K. Prevalence and risk factors of gastrointestinal parasitic infections in goats in low-input low-output farming systems in Zimbabwe. Small Rumin. Res. 2016;143:75–83. doi: 10.1016/j.smallrumres2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]