Abstract
Background
The neutrophil-to-lymphocyte ratio (NLR) is an index reflecting the overall inflammatory and stress status of patients with major diseases. Many studies associated the NLR with neurological deterioration and a poor prognosis in the spontaneous intracerebral hemorrhage (ICH). However, most previous studies did not further analyze NLR by stratification, and with a relatively small sample size. Besides, the outcome evaluation mostly focused on short-term prognosis or a single timepoint.
Methods
Patients’ basic characteristics and laboratory examination results, including the NLR were taken at baseline, and data from the 1-year follow-up, including the modified Rankin Scale (mRS) and survival status, was obtained for all patients. Patients included in the study were classified into four groups according to NLR quartiles (Q1-Q4). Logistic regression was used to analyze the relationship between different NLR levels and poor outcomes (mRS 3–5 and mRS 3–6).
Results
A total of 594 ICH patients were enrolled. Glasgow Coma Scale (GCS), NIH Stroke Scale (NIHSS) and hematoma volume at first admission were significantly different between different NLR level groups (all P values <0.05). In the multivariate logistic regression model, at the 30-day follow-up, the Q4 (significantly increased NLR) group showed an elevated risk of poor outcomes (OR, 2.37; 95% CI, 1.17–4.83, P=0.02) and functional disability (OR, 2.21; 95% CI, 1.05–4.65, P=0.04). At the 3-month follow-up, the Q4 group still showed an elevated risk of poor outcomes (OR, 2.83; 95% CI, 1.38–5.77, P<0.01) and functional disability (OR, 2.77; 95% CI, 1.28–5.98, P<0.01). At the 1-year follow-up, the Q2 (slightly elevated NLR) group showed significant functional disability (OR, 0.34; 95% CI, 0.16–0.72, P<0.01).
Conclusion
A significantly increased NLR may have an impact on the poor outcomes and functional disability of patients with ICH, while a slightly elevated NLR may play a protective role.
Keywords: neutrophil-to-lymphocyte ratio, functional outcome, spontaneous intracerebral hemorrhage
Introduction
Spontaneous intracerebral hemorrhage (sICH) is a devastating disease that accounts for 10–30% of all strokes and is characterized by high rates of mortality and residual disability among survivors.1 Currently, the application conditions for effective surgical treatments are still relatively limited, so the identification of reliable factors to allow early prognostication and risk stratification of patients still represents a clinical priority. At present, the evaluation of prognosis is mainly based on the primary injury and early mass effect, such as hematoma volume and location, and whether the hematoma extends into the ventricle. Numerous studies have demonstrated that the neuroinflammatory response plays a crucial role in secondary brain injury after ictus of ICH and influences patient prognosis.2 In the early stage of ICH, microglial activation and peripheral leukocyte infiltration play an important role in the secondary brain injury, and with the increase of leukocyte infiltration, it can aggravate secondary brain injury.3
The neutrophil-to-lymphocyte ratio (NLR) is a simple and easily available index to evaluate the overall inflammatory status of patients and has a certain accuracy in predicting the prognosis of many major diseases. Studies have shown that the NLR is associated with neurological deterioration and poor prognosis in ischemic4 and hemorrhagic stroke,5 and when added to the modified ICH score, it can improve the accuracy of prognosis prediction.6 However, most previous studies have been characterized by relatively small sample sizes, and only a few studies have relatively large sample size.7 In addition, these studies on NLR and stroke did not further analyze NLR by stratification and refinement, and the outcome evaluation mostly focused on short-term prognosis or a single timepoint.8 It is worth noting that, survival may not represent the only meaningful endpoint, and residual functionality may have even greater clinical and social relevance.9 Therefore, in this study, we aimed to explore the relationship between NLR and ICH prognosis, including poor outcome, functional disability and death at multiple timepoints in a hierarchical manner.
Method
Patient Selection
The study was a single-center, prospective, observational cohort study conducted in Beijing Tiantan Hospital from January 2014 to September 2016 and was conducted in accordance with the guidelines of the 1975 Declaration of Helsinki World Medical Association and was approved by the Institutional Review Committee (IRB) of Beijing Tiantan Hospital. The ethics committee(s) approved consent in the ethics statement. Written informed consent was obtained from all patients or their relatives.
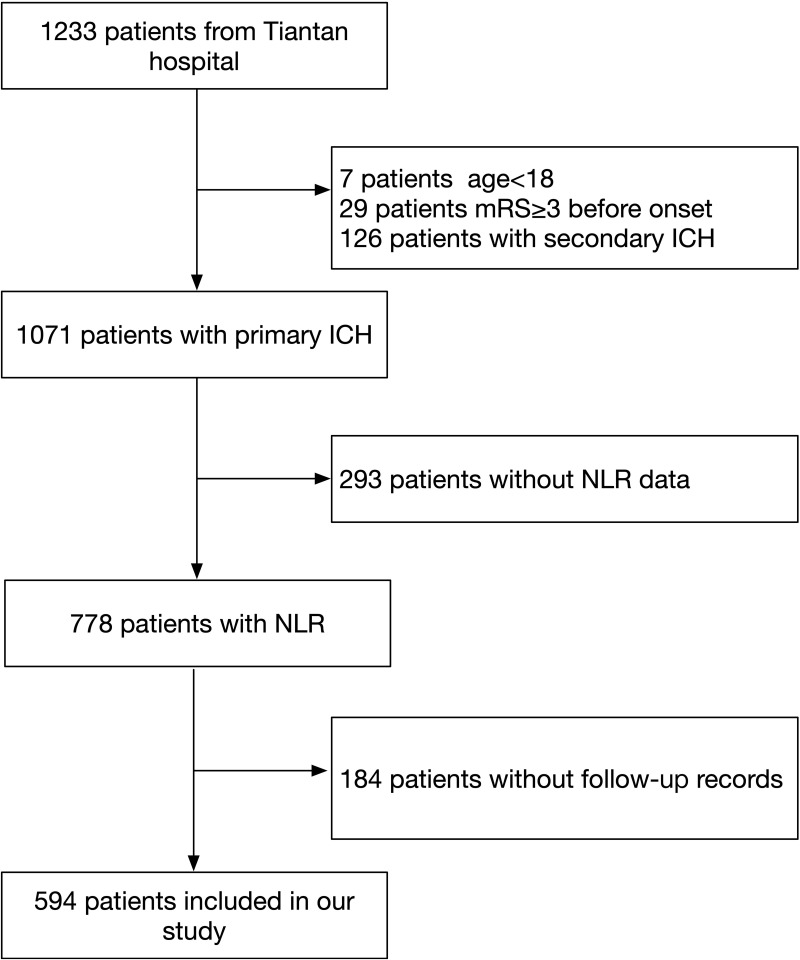
The inclusion criteria were as follows: (1) ICH diagnosed by the WHO standard and confirmed by a computerized tomography (CT) scan; (2) age ≥18 years old; (3) arrival at the hospital within 72 hours after onset; (4) first ever acute-onset ICH; and (5) written informed consent obtained. The exclusion criteria included the following: (1) past history of ICH; (2) congenital or acquired coagulation disorders; (3) secondary ICH resulting from tumor, trauma, aneurysm, arteriovenous malformation, arteriovenous fistula, cavernous hemangioma, venous malformations, telangiectasia, venous sinus thrombosis, moyamoya disease, and coagulation disorders; and (4) disease complicated with major comorbidities or late-stage diseases. A total of 1233 patients were enrolled, 778 of whom had NLR data and were enrolled in the NLR subgroup at baseline. We excluded 184 patients without follow-up records. Finally, 594 patients were enrolled in our study (Figure 1).
Figure 1.
Flow diagram of study patients.
Abbreviations: ICH, intracerebral hemorrhage; mRS, modified Rankin Scale; NLR, neutrophil-to-lymphocyte ratio.
Clinical Variables and Outcome
Baseline clinical characteristics were collected, including demographics (age and sex), medical history (hypertension, diabetes mellitus, stroke due to cerebral ischemia or infarction or aneurysm, smoking and drinking status, and systolic and diastolic blood pressure). Hematologic tests, such as glucose level, hemoglobin, white blood cell (WBC), neutrophil count (NC), monocyte count (MC), lymphocyte count (LC), were also collected. The NLR was measured as the ratio of the NC to the LC on admission. Hypertension was defined as a self-reported history, a systolic blood pressure (SBP) ≥140 mmHg or a diastolic blood pressure (DBP) of ≥90 mmHg at baseline or use of antihypertensive medication. Diabetes mellitus was defined as a self-reported history, use of oral hypoglycemic agents, fasting blood glucose level ≥7.0 mmol/l at baseline, or current treatment with insulin or oral hypoglycemic agents. Dyslipidemia was defined as a self-reported history, total cholesterol ≥6.22 mmol/l or triglyceride ≥2.26 mmol/l, low-density lipoprotein ≥4.14 mmol/l at baseline, or current use of cholesterol-lowering medicine. Smoking was recorded as at least one cigarette per day for over one year. Alcohol consumption was recorded as an intake of at least 80 g of liquor a day for over one year.
The Glasgow Coma Scale (GCS) and the NIH Stroke Scale (NIHSS) were administered at admission. We also documented the hematoma location (lobar, basal ganglia, thalamus, brainstem, cerebellum). The volume of the hematoma was measured by the “ABC/2 method”10 based on the initial CT scan, which was completed on admission.
Follow-Up Information and Outcome Evaluation
Face-to-face interviews were performed when the patient was discharged, and telephone interviews were performed at 30 days, 3 months, and 1 year after ICH onset. The researchers who participated in the follow-up were trained and were blinded to the baseline information and disease characteristics of the patients. During the interview, the functional status of each patient was evaluated, and an mRS score was obtained. For patients who did not answer the phone, we contacted them three times over the next week. If they did not return the call, they were regarded as lost. Poor outcomes, including death and disability, were defined as mRS scores of 3–6. Functional disability was defined as mRS scores of 3–5. A score of 6, which indicates death, was analyzed separately.
Statistical Analysis
SAS software was used for statistical analysis (version 9.4; SAS Institute, Cary, NC, USA). All patients were divided into Q1, Q2, Q3 and Q4 groups according to the quartile and median NLR levels. Continuous variables are expressed as medians (interquartile range, IQR) or means ± standard deviation (SD) and were compared by the Kruskal–Wallis test. The chi-square test was used for the comparison of categorical variables, which are expressed as numbers (proportions). Multivariate logistic regression was used to analyze the association between NLR levels and clinical outcomes. Variables with p values < 0.05 from the comparison of baseline characteristics and selected variables (age, sex, smoking, GCS at first admission, NIHSS at first admission, hematoma volume at first admission, systolic blood pressure) were included in multivariate analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each group with the first quartile (Q1) as a reference for the NLR. The results were considered significant for p values < 0.05 (two sided).
Result
Baseline Characteristics
From January 2014 to September 2016, 594 patients with spontaneous ICH fulfilling the inclusion criteria were enrolled in this study. Among them, the median age was 56 (IQR 49–64) years, and 423 (71.21%) were males. Participants were classified into four groups by NLR quartiles, and the first quartile, median and third quartile of NLR were 3.68, 6.89 and 12.14, respectively. The NLR ranges of the quartile groups were Q1 (NLR<3.68), Q2 (3.68≤NLR<6.89), Q3 (6.89≤NLR<12.14) and Q4 (NLR ≥12.14). The baseline characteristics of the patients are shown in Table 1. The medians and IQRs in these four groups were as follows: GCS scores 14.5 (11–15), 14 (11–15), 13 (8–15), 10.5 (6–15); NIHSS scores, 9 (3–16.5), 10 (3–16), 12 (4–20), 16 (8–27); and hematoma volume, 11.28 (4.70–30.30), 15.80 (7.4–34.60), 23.80 (10.60–48.00), 34.70 (10.85–64.85), respectively; which were all significantly different among the four groups (all P values<0.05). With an increase in the NLR level, the GCS score decreased, and the NIHSS score and hematoma volume increased. In addition, with the increase in the NLR, the leukocyte count increased, and the lymphocyte count decreased gradually. There were also significant differences among the four groups (P<0.05).
Table 1.
Baseline Characteristics of the Patients Stratified by the NLR
| Total | Q1 (NLR<3.68) | Q2 (3.68≤NLR<6.89) | Q3 (6.89≤NLR<12.14) | Q4 (12.14≤NLR) | P | |
|---|---|---|---|---|---|---|
| N=594 | (n=148) | (n=149) | (n=149) | (n=148) | ||
| Age | 56(49–64) | 56(51–64) | 56(47–65) | 55(49–64) | 56(48–63) | 0.516 |
| Sex (male) | 423(71.21) | 110(74.32) | 109(73.15) | 101(67.79) | 103(69.59) | 0.569 |
| Hypertension, n (%) | 441(74.87) | 111(75.00) | 118(79.73) | 109(74.15) | 103(70.55) | 0.341 |
| Diabetes, n (%) | 81(13.64) | 20(13.51) | 19(12.75) | 25(16.78) | 17(11.49) | 0.587 |
| Stroke history, n (%) | 103(17.34) | 32(21.62) | 22(14.77) | 24(16.11) | 25(16.89) | 0.430 |
| Smoking, n (%) | 322(54.21) | 93(62.84) | 81(54.36) | 73(48.99) | 75(50.68) | 0.078 |
| Drinking, n (%) | 307(52.06) | 66(44.69) | 84(56.38) | 79(53.02) | 78(52.70) | 0.216 |
| SBP (mmHg, IQR) | 168(150–187) | 170(155–189) | 160(144–184) | 163(150–190) | 170(155–184) | 0.058 |
| DBP (mmHg, IQR) | 98(85–110) | 100(87–110) | 92(84–107) | 98(84–110) | 100(88–110) | 0.149 |
| GCS at first admission (IQR) | 14(8–15) | 14.5(11–15) | 14(11–15) | 13(8–15) | 10.5(6–15) | <0.001 |
| NIHSS at first admission (IQR) | 12(4–21) | 9(3–16.5) | 10(3–16) | 12(4–20) | 16(8–27) | <0.001 |
| Hematoma volume (mL IQR) | 18.10(7.60–47.90) | 11.28(4.70–30.30) | 15.80(7.4–34.60) | 23.80(10.60–48.00) | 34.70(10.85–64.85) | <0.001 |
| WBC at first admission (109/L IQR) | 10.41(7.81–13.25) | 7.48(6.06–8.99) | 8.84(7.50–11.13) | 11.38(9.47–14.24) | 13.76(11.44–16.12) | <0.001 |
| Lymphocytes at first admission (109/L IQR) | 1.21(0.84–1.74) | 2.03(1.55–2.68) | 1.37(1.13–1.78) | 1.07(0. 90–1.36) | 0.68(0.55–0.86) | <0.001 |
| Monocytes at first admission (109/L IQR) | 0.39(0.30–0.53) | 0.40(0.33–0.52) | 0.40(0.31–0.53) | 0.39(0.28–0.53) | 0.39(0.26–0.54) | 0.499 |
| Neutrophils at first admission (109/L IQR) | 8.52(5.68–11.65) | 4.53(3.66–5.73) | 6.95(5.86–8.86) | 10.01(8.07–12.22) | 12.74(10.37–14.95) | <0.001 |
Note: Values are median (IQR) or number (%).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; GCS, Glasgow Coma Scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; DBP, diastolic blood pressure; WBC, white blood cell and IQR, interquartile range.
NLR and Outcomes
The Outcome characteristics of the patients are shown in Table 2. During the 30-day follow-up, 387 (65.15%) patients had poor functional outcomes (mRS=3-6), 267 (56.33%) patients had functional disability (mRS=3-5), and 120 (20.20%) patients died. During the 3-month follow-up, 348 (58.59%) patients had poor outcomes, 221 (47.32%) patients had functional disability, and 127 (21.38%) patients died. During the 1-year follow-up, 304 (51.18%) patients had poor outcomes, 161 (35.70%) patients had functional disability, and 143 (24.07%) patients died. There were significant differences in all of the above indexes among the four groups (P<0.05).
Table 2.
Clinical Outcomes of the Patients Stratified by NLR
| Outcome | Total | Q1 (NLR<3.68) | Q2 (3.68≤NLR<6.89) | Q3 (6.89≤NLR<12.14) | Q4 (12.14≤NLR) | P |
|---|---|---|---|---|---|---|
| N=594 | (n=148) | (n=149) | (n=149) | (n=148) | ||
| 30-day follow-up | ||||||
| mRS≥3(3–6) | 387(65.2) | 85(57.4) | 82(55.0) | 99(66.4) | 121(81.8) | <0.001 |
| Disabled (3–5) | 267(56.3) | 59(48.4) | 64(48.9) | 69(58.0) | 75(73.5) | <0.001 |
| Death | 120(20.2) | 26(17.6) | 18(12.1) | 30(20.1) | 46(31.1) | <0.001 |
| 3-month follow-up | ||||||
| mRS≥3(3–6) | 348(58.6) | 73(49.3) | 72(48.3) | 87(58.4) | 116(78.4) | <0.001 |
| Disabled (3–5) | 221(47.3) | 46(38.0) | 53(40.8) | 54(46.6) | 68(68.0) | <0.001 |
| Death | 127(21.4) | 27(18.2) | 19(12.8) | 33(22.2) | 48(32.4) | <0.001 |
| 1-year follow-up | ||||||
| mRS≥3(3–6) | 304(51.2) | 66(44.6) | 55(36.9) | 78(52.4) | 105(71.0) | <0.001 |
| Disabled (3–5) | 161(35.7) | 36(30.5) | 28(23.0) | 43(37.7) | 54(55.7) | <0.001 |
| Death | 143(24.1) | 30(20.3) | 27(18.1) | 35(23.5) | 51(34.5) | 0.005 |
Note: Values are number (%).
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; mRS, modified Rankin Scale.
The risks of adverse clinical outcomes in the NLR quartile groups are shown in Table 3. At the 30-day follow-up, compared with the lowest NLR (Q1) quartile group taken as a reference, the highest quartile (Q4) group showed elevated risks of poor outcomes (OR, 3.32; 95% CI, 1.96–5.64, P<0.01) and functional disability (OR, 2.97; 95% CI, 1.69–5.22, P<0.01). In the multivariate logistic regression model, Q4 remained an indicator of poor outcomes (OR, 2.37; 95% CI, 1.17–4.83, P=0.02) and functional disability (OR, 2.21; 95% CI, 1.05–4.65, P=0.04).
Table 3.
Risks of Adverse Clinical Outcomes Stratified by NLR
| Overall | Q1 (NLR<3.68) | Q2 (3.68≤NLR<6.89) | Q3 (6.89≤NLR<12.14) | Q4 (12.14≤NLR) | P trend | ||||
|---|---|---|---|---|---|---|---|---|---|
| 30-day follow-up | 594 | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | ||
| mRS≥3 (3–6) | 387 (65.15) | ||||||||
| Crude | Ref. | 0.91 (0.57–1.44) | 0.68 | 1.47 (0.92–2.35) | 0.11 | 3.32 (1.96–5.64) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.88 (0.49–1.59) | 0.67 | 1.24 (0.66–2.32) | 0.51 | 2.37 (1.17–4.83) | 0.02 | <0.01 | |
| Disabled (3–5) | 267 (56.33) | ||||||||
| Crude | Ref. | 1.02 (0.62–1.67) | 0.94 | 1.47 (0.89–2.45) | 0.14 | 2.97 (1.69–5.22) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.82 (0.44–1.54) | 0.54 | 1.10 (0.56–2.13) | 0.79 | 2.21 (1.05–4.65) | 0.04 | <0.01 | |
| Death | 120 (20.20) | ||||||||
| Crude | Ref. | 0.65 (0.34–1.24) | 0.19 | 1.18 (0.66–2.12) | 0.57 | 2.12 (1.22–3.66) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.64 (0.30–1.39) | 0.26 | 0.78 (0.38–1.60) | 0.05 | 0.95 (0.47–1.90) | 0.88 | 0.62 | |
| 3-month follow-up | |||||||||
| mRS≥3 (3–6) | 348 (58.59) | ||||||||
| Crude | Ref. | 0.96 (0.61–1.51) | 0.86 | 1.44 (0.91–2.28) | 0.12 | 3.72 (2.24–6.18) | <0.01 | <0.01 | |
| Adjusted | Ref. | 1.00 (0.54–1.84) | 0.99 | 1.15 (0.61–2.18) | 0.67 | 2.83 (1.38–5.77) | <0.01 | <0.01 | |
| Disabled (3–5) | 221 (47.32) | ||||||||
| Crude | Ref. | 1.12 (0.68–1.86) | 0.66 | 1.42 (0.85–2.38) | 0.18 | 3.46 (1.98–6.05) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.92 (0.47–1.80) | 0.81 | 0.99 (0.50–1.99) | 0.99 | 2.77 (1.28–5.98) | <0.01 | <0.01 | |
| Death | 127 (21.38) | ||||||||
| Crude | Ref. | 0.66 (0.35–1.24) | 0.19 | 1.28 (0.72–2.25) | 0.40 | 2.15 (1.25–3.69) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.65 (0.31–1.38) | 0.26 | 0.89 (0.44–1.80) | 0.75 | 0.99 (0.50–1.96) | 0.97 | 0.61 | |
| 1-year follow-up | |||||||||
| mRS≥3 (3–6) | 304 (51.18) | ||||||||
| Crude | Ref. | 0.73 (0.46–1.16) | 0.18 | 1.37 (0.87–2.16) | 0.18 | 3.03 (1.88–4.91) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.59 (0.32–1.08) | 0.09 | 1.01 (0.55–1.86) | 0.96 | 1.75 (0.91–3.34) | 0.09 | 0.01 | |
| Disabled (3–5) | 161 (35.70) | ||||||||
| Crude | Ref. | 0.68 (0.38–1.21) | 0.19 | 1.38 (0.80–2.38) | 0.25 | 2.86 (1.63–5.01) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.34 (0.16–0.72) | <0.01 | 0.83 (0.41–1.65) | 0.59 | 1.61 (0.77–3.37) | 0.21 | 0.02 | |
| Death | 143 (24.07) | ||||||||
| Crude | Ref. | 0.87 (0.49–1.55) | 0.64 | 1.21 (0.70–2.10) | 0.50 | 2.07 (1.22–3.50) | <0.01 | <0.01 | |
| Adjusted | Ref. | 0.96 (0.48–1.90) | 0.90 | 0.88 (0.45–1.74) | 0.72 | 0.99 (0.51–1.92) | 0.98 | 0.66 | |
Note: Adjusted for age, sex, smoking, GCS at first admission, NIHSS at first admission, hematoma volume at first admission and SBP.
Abbreviations: NLR, neutrophil-to-lymphocyte ratio; OR, odds ratio; CI, confidence interval; mRS, modified Rankin Scale.
At the 3-month follow-up, compared with the lowest NLR (Q1) quartile group taken as a reference, the highest quartile (Q4) groups still showed elevated risks of poor outcomes (OR, 3.72; 95% CI, 2.24–6.18, P<0.01) and functional disability (OR, 3.46; 95% CI, 1.98–6.05, P<0.01). These associations were still significant after adjustments (OR, 2.83; 95% CI, 1.38–5.77, P<0.01 and OR, 2.77; 95% CI, 1.28–5.98, P<0.01).
Regarding the outcome at the 1-year follow-up, although the Q2 group did not show significant functional disability (mRS 3–5) in the crude logistic regression analysis (OR, 0.68; 95% CI, 0.38–1.21, P=0.19), functional disability in this group was found to be significant after adjustment (OR, 0.34; 95% CI, 0.16–0.72, P<0.01). We found no significant correlation between the NLR and risk of death at the 30-day (OR, 0.95; 95% CI, 0.47–1.90, P=0.88), 3-month (OR, 0.99; 95% CI, 0.50–1.96, P=0.97), or 1-year (OR, 0.99; 95% CI, 0.51–1.92, P=0.98) follow-ups after adjustments.
Discussion
The current study demonstrated that significantly increased NLR levels were correlated with an increased risk of 30-day and 3-month poor outcomes and functional disability, while slightly elevated NLR levels were correlated with a reduced risk of functional disability. An association between the NLR level and death was not found in this study. Our results suggest that a higher NLR may have an impact on the poor prognosis of patients with ICH, while a slightly elevated NLR may play a protective role.
The NLR is an indicator that reflects the overall inflammatory and stress status of patients.11 Because it is easy to obtain and inexpensive to check, it has attracted extensive attention in a variety of disease fields in recent years. Many studies suggest that the NLR can clearly predict the prognosis of a variety of major diseases, such as bacteremia, acute coronary syndrome, tumors and ischemic stroke.4,9 ICH is an acute major disease, and a series of local and systemic inflammatory reactions will occur on the basis of primary brain injury. Secondary brain injury caused by microglial activation and peripheral leukocyte infiltration in the early stage of ICH, such as the release of cytokines and proinflammatory factors, leads to the destruction of the blood–brain barrier, aggravation of brain edema and an increase in intracranial pressure, which further aggravates the neurological injury of patients and leads to adverse outcomes.3 In clinical work, we often find that patients in the acute stage of ICH (within 24 hours or even earlier) admitted in the emergency department often exhibit an increase in peripheral leukocytes, despite a lack of clear evidence of complications such as infection at that time. At this time, the NLR may reflect the local and systemic inflammatory response and degree in patients with acute ICH.
There is a close relationship between the immune system and the pathophysiology of major diseases. Usually, after the occurrence of such diseases, a rapid increase in neutrophils and decrease in lymphocytes lead to an increase in the NLR in the early stage of the disease. Accordingly, an early increase in NLR values can result in the reliable prediction of the growth of perihemorrhagic edema,12 the risk of developing infections,13 and the occurrence of early neurological deterioration.14 Studies have shown that an increase in peripheral neutrophils can increase central neutrophil infiltration and aggravate the inflammatory response.15 Moreover, the decrease in lymphocyte levels is caused by an increase in catecholamine and steroid levels.11,16 The overactivation of the sympathetic nervous system and hypothalamic pituitary adrenal axis in patients with acute intracerebral hemorrhage leads to apoptosis and functional deactivation of peripheral lymphocytes; thus, there is a certain degree of immunosuppression and an impaired antibacterial immune response after stroke.17 Activated neutrophils induce the production of various molecules related to tissue injury and participate in oxidative stress, and high-level oxidative stress exposure may be related to prognosis. This also explains why the NLR can reasonably reflect the possibility of secondary brain injury after intracerebral hemorrhage and the susceptibility to poststroke complications. The results of animal experiments confirmed that if peripheral neutrophils were selectively consumed before intracerebral hemorrhage, the activation of microglia and the infiltration of neutrophils and monocytes from peripheral blood after intracerebral hemorrhage could be reduced, and the inflammatory reaction and destruction of the blood–brain barrier could be alleviated.18
In addition, it is well known that the inflammatory response is an important mechanism of bodily self-protection and damage repair. The earliest stage after intracerebral hemorrhage is the activation of microglia and infiltration of peripheral blood neutrophils. Neutrophils are often viewed as a proinflammatory cell population but are known to play a vital role in wound healing through their involvement in phagocytosis, metalloproteinase release, and growth factor production. After tissue injury, neutrophils can help prepare the damaged environment for repair.19 Actually, the contribution of neutrophils to a central nervous system (CNS) lesion may depend on their state of activation, and overactivation of neutrophils and the inflammatory response cascade are important factors in tissue damage caused by the inflammatory response. Our study found that when the NLR is at a high level, it increases the risk of poor prognosis and functional disability, which is consistent with the common results of recent research; that is, most studies report that when the NLR is greater than 5–7, its increase is related to poor prognosis.20–22 Some studies have shown that the normal NLR in healthy adults is between 0.78 and 3.53,23 which is basically consistent with the Q1 quartile in our study population. When the NLR is higher than Q1 and less than 5–7, it is close to the Q2 quartile in our study, so we call it a slight increase. In our study, we found that a slight increase in the NLR (Q2) may have a protective effect on the functional prognosis of ICH patients for 1 year, which may be related to the inherent promotion of neuroprotection and phagocytic repair of neutrophils, and the relatively stable number of lymphocytes also provides a certain preventive effect on the occurrence of infectious complications. In addition, compared with the populations with higher NLR levels, these patients do not have the material basis for strong inflammatory reactions, so they experience a certain protective effect, which may also indicate that the immune system of this population is relatively stable under the attack of major and serious diseases.
At the early stage of follow-up, there was no prognostic difference in patients with a slightly elevated NLR. It may be that there are many factors affecting the functional prognosis in the early stage of the disease, while the impact of the NLR is relatively weak. In addition, the recovery of neural function is a long process, during which sound and stable immune function may be an important factor. It has been reported in the literature that 30-day mortality is related to the NLR level,22 but our study has not confirmed this. An increased cohort size and a large-scale study are needed to confirm this hypothesis. In addition, our results do not include more detailed data on infratentorial hemorrhage and ventricular hemorrhage, so more detailed data may be needed to support the conclusion in the future.
Conclusion
A significant increase in the NLR in patients with spontaneous intracerebral hemorrhage in the acute stage may be related to poor prognosis and functional disability at 30 days and 3 months, while a slightly increased NLR may be a protective factor against functional disability at 1 year.
Acknowledgments
We thank the study quality coordinators’ meticulous work for data quality control. Thank all the participants and investigators who took part in our study.
Funding Statement
This study was supported by the National Key Research and Development Program of China (2018YFC1312200/2018YFC1312204), the National Science and Technology Major Project (2017ZX09304018), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2019-I2M-5-029), Beijing Natural Science Foundation (Z200016), and Beijing Municipal Committee of Science and Technology (Z201100005620010).
Ethical Approval
This study was conducted in accordance with the guidelines from the Helsinki Declaration and was approved by the Ethics Committees of Beijing Tiantan Hospital.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Feigin VL, Lawes CMM, Bennett DA, Anderson CS. Stroke epidemiology: a review of population-based studies of incidence, prevalence, and case-fatality in the late 20th century. Lancet Neurol. 2003;2(1):43–53. doi: 10.1016/S1474-4422(03)00266-7 [DOI] [PubMed] [Google Scholar]
- 2.Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang QW. Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol. 2014;115:25–44. doi: 10.1016/j.pneurobio.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 3.Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012;11(8):720–731. doi: 10.1016/S1474-4422(12)70104-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quan K, Wang A, Zhang X, et al. Neutrophil to lymphocyte ratio and adverse clinical outcomes in patients with ischemic stroke. Ann Transl Med. 2021;9(13):1047. doi: 10.21037/atm-21-710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang F, Ren Y, Fu W, et al. Association between neutrophil to lymphocyte ratio and blood glucose level at admission in patients with spontaneous intracerebral hemorrhage. Sci Rep. 2019;9(1):15623. doi: 10.1038/s41598-019-52214-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lattanzi S, Cagnetti C, Rinaldi C, Angelocola S, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci. 2018;387:98–102. doi: 10.1016/j.jns.2018.01.038 [DOI] [PubMed] [Google Scholar]
- 7.Menon G, Johnson SE, Hegde A, Rathod S, Nayak R, Nair R. Neutrophil to lymphocyte ratio - A novel prognostic marker following spontaneous intracerebral haemorrhage. Clin Neurol Neurosurg. 2021;200:106339. doi: 10.1016/j.clineuro.2020.106339 [DOI] [PubMed] [Google Scholar]
- 8.Zhang F, Ren Y, Fu W, et al. Predictive accuracy of neutrophil-to-lymphocyte ratio on long-term outcome in patients with spontaneous intracerebral hemorrhage. World Neurosurg. 2019;125:e651–e657. doi: 10.1016/j.wneu.2019.01.143 [DOI] [PubMed] [Google Scholar]
- 9.Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. 2019;10(2):137–145. doi: 10.1007/s12975-018-0649-4 [DOI] [PubMed] [Google Scholar]
- 10.Kothari RU, Brott T, Broderick JP, et al. The ABCs of measuring intracerebral hemorrhage volumes. Stroke. 1996;27(8):1304–1305. doi: 10.1161/01.STR.27.8.1304 [DOI] [PubMed] [Google Scholar]
- 11.Arbel Y, Shacham Y, Ziv-Baran T, et al. Higher neutrophil/lymphocyte ratio is related to lower ejection fraction and higher long-term all-cause mortality in ST-elevation myocardial infarction patients. Can J Cardiol. 2014;30(10):1177–1182. doi: 10.1016/j.cjca.2014.05.010 [DOI] [PubMed] [Google Scholar]
- 12.Volbers B, Giede-Jeppe A, Gerner ST, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. 2018;90(12):e1005–e1012. doi: 10.1212/WNL.0000000000005167 [DOI] [PubMed] [Google Scholar]
- 13.Giede-Jeppe A, Bobinger T, Gerner ST, et al. Neutrophil-to-lymphocyte ratio is an independent predictor for in-hospital mortality in spontaneous intracerebral hemorrhage. Cerebrovasc Dis. 2017;44(1–2):26–34. doi: 10.1159/000468996 [DOI] [PubMed] [Google Scholar]
- 14.Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. 2017;8(34):57489–57494. doi: 10.18632/oncotarget.15423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zeng Hanhai QB, Shengjun Z, Jingbo L, et al. Neutrophil extracellular traps, released from neutrophil, promote microglia inflammation and contribute to poor outcome in subarachnoid hemorrhage. Aging. 2021;13(9):13108–13123. doi: 10.18632/aging.202993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6(10):775–786. doi: 10.1038/nrn1765 [DOI] [PubMed] [Google Scholar]
- 17.Dirnagl U, Klehmet J, Braun JS, et al. Stroke-induced immunodepression: experimental evidence and clinical relevance. Stroke. 2007;38(2 Suppl):770–773. doi: 10.1161/01.STR.0000251441.89665.bc [DOI] [PubMed] [Google Scholar]
- 18.Moxon-Emre I, Schlichter LC. Neutrophil depletion reduces blood-brain barrier breakdown, axon injury, and inflammation after intracerebral hemorrhage. J Neuropathol Exp Neurol. 2011;70(3):218–235. doi: 10.1097/NEN.0b013e31820d94a5 [DOI] [PubMed] [Google Scholar]
- 19.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 20.Kotani K. Neutrophil/lymphocyte ratio and the oxidative stress burden. Can J Cardiol. 2015;31(3):365 e369. doi: 10.1016/j.cjca.2014.11.026 [DOI] [PubMed] [Google Scholar]
- 21.Capone M, Giannarelli D, Mallardo D, et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi: 10.1186/s40425-018-0383-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang F, Hu S, Ding Y, et al. Neutrophil-to-lymphocyte ratio and 30-day mortality in patients with acute intracerebral hemorrhage. J Stroke Cerebrovasc Dis. 2016;25(1):182–187. doi: 10.1016/j.jstrokecerebrovasdis.2015.09.013 [DOI] [PubMed] [Google Scholar]
- 23.Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes. 2017;10(1):12. doi: 10.1186/s13104-016-2335-5 [DOI] [PMC free article] [PubMed] [Google Scholar]