Abstract
Background:
Pvs48/45 is a Plasmodium vivax gametocyte surface protein involved in the parasite fertilization process. Previous studies showed that Pvs48/45 proteins expressed in Escherichia coli (E. coli) and Chinese hamster ovary (CHO) cells were highly immunoreactive with sera from malaria-endemic areas and highly immunogenic in animal models. Here the immunogenicity in mice of three different vaccine formulations was compared.
Methods:
Recombinant (r) Pvs48/45 proteins were expressed in E. coli and CHO, purified, formulated in Alhydrogel, GLA-SE and Montanide ISA-51 adjuvants and used to immunize BALB/c mice. Animals were immunized on days 0, 20 and 40, and serum samples were collected for serological analyses of specific antibody responses using ELISA and immunofluorescence (IFAT). Additionally, ex-vivo transmission-reducing activity (TRA) of sera on P. vivax gametocyte-infected human blood fed to Anopheles albimanus in direct membrane feeding assays (DMFA) was evaluated.
Results:
Most immunized animals seroconverted after the first immunization, and some developed antibody peaks of 106 with all adjuvants. However, the three adjuvant formulations induced different antibody responses and TRA efficacy. While GLA-SE formulations of both proteins induced similar antibody profiles, Montanide ISA-51 formulations resulted in higher and longer-lasting antibody titers with CHO-rPvs48/45 than with the E. coli formulation. Although the CHO protein formulated in Alhydrogel generated a high initial antibody peak, antibody responses to both proteins rapidly waned. Likewise, anti-Pvs48/45 antibodies displayed differential recognition of the parasite proteins in IFAT and ex vivo blockade of parasite transmission to mosquitoes. The CHO-rPvs48/45 formulated in Montanide ISA-51 induced the most effective ex vivo parasite blockage.
Conclusions:
Three out of six vaccine formulations elicited antibodies with ex vivo TRA. The CHO-rPvs48/45 Montanide ISA-51 formulation induced the most stable antibody response, recognizing the native protein and the most robust ex vivo TRA. These results encourage further testing of the vaccine potential of this protein.
Keywords: Malaria, Plasmodium vivax, Gametocytes, Immunogenicity, Transmission blocking vaccine, Transmission Reducing Activity, Pvs48/45, Vaccines
Introduction
Malaria is the most important parasitic disease globally, with ~228 million clinical cases and over 405,000 deaths per year [1]. It is caused by Plasmodium parasites that are transmitted to humans by infected Anopheles mosquito vectors. Plasmodium falciparum and P. vivax are the two major species responsible for ~99% of the global malaria cases [1]. Between 2000 and 2015, intense control activities reduced malaria incidence by ~50% worldwide, generating enthusiasm for the possibility of malaria eradication [2]. However, this significant progress was followed by malaria incidence increases in several endemic regions [1], indicating the need to strengthen control activities and develop alternative strategies [3]. Unfortunately, parasite resistance to artemisinin combination therapies (ACT), the current primary antimalarial treatment for P. falciparum, has been identified in several countries of the Greater Mekong subregion [4]. Additionally, reports of emergence and spreading of ACT resistance in Africa and South America are being investigated [3, 5, 6]. Likewise, a reduction of the sensitivity of P. vivax parasites to antimalarials such as chloroquine and primaquine has been observed [7], and mosquitoes have developed resistance to multiple insecticides [8], adding to challenges in malaria elimination.
Among the new malaria control and elimination tools and strategies, vaccines are considered to have great potential due to the proven cost-effectiveness of vaccination against transmissible diseases [9] and the progress achieved with the Pf-RTS,S malaria vaccine candidate [10, 11]. Malaria transmission-blocking (TB) vaccines, designed to interfere with parasite transmission from humans to mosquitoes, have been considered an excellent potential strategy for accelerating malaria elimination [12-14]. Sera of individuals from malaria-endemic areas have the capacity to block parasite development in the mosquito midgut [15, 16]. Additionally, several parasite surface antigens, which are targets of antibodies with TB capacity, have been identified [12, 17, 18]. Some of them are currently considered potential TB vaccine candidates. Pfs25, Pfs230, and Pfs48/45 are well-established P. falciparum vaccine candidates expressed on gametocytes, gametes, zygotes or ookinetes, some of which are essential in parasite fertilization and are being assessed in preclinical and in Phase I clinical trials [19-22]. Likewise, they all have orthologous P. vivax antigens [23].
P48/45 are cysteine-rich conserved proteins expressed on the gametocyte surface found in several Plasmodium species and involved in parasite fertilization [24]. Pvs48/45 protein was initially expressed as a recombinant product in E. coli [25] and more recently in CHO expression systems [15]. Both products displayed significant immunoreactivity with sera of individuals from malaria-endemic regions of Colombia and Guatemala, and some of them demonstrated ex vivo TB capacity [15]. Additionally, pilot immunization studies in BALB/c mice and Aotus monkeys indicated high immunogenicity of the rPvs48/45 E. coli version [25]. Here, we evaluated the immunogenicity in mice and the functional activity of antibodies elicited by different formulations of the CHO and E. coli rPvs48/45 products in three adjuvants acceptable for human use.
Material and Methods
Ethical approval
The Animal Ethical Committee of Universidad del Valle approved the study protocol (Code 031-015). Animals were housed and handled following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. An additional protocol to obtain P. vivax infected blood samples was approved by the Institutional Review Board (IRB) at the Centro Internacional de Vacunas (CECIV, Cali-Colombia) (code CECIV 1506-2017) for IFAT and DMFA assays, and blood donors provided the corresponding consent.
Production of recombinant E. coli and CHO Pvs48/45 proteins
The full-length rPvs48/45 gene was expressed in E. coli [25] and in CHO [15]. Briefly, rPvs48/45 expressed in E. coli was produced by Integrated DNA Technologies (Skokie, IL) in the IDT Blue vector by cloning the P. vivax Sal I pvs48/45 gene into the pET32a vector for protein expression in the Origami 2 E. coli using a heat shock method as described [25]. The protein was purified by affinity chromatography, using immobilized metal ion affinity chromatography with a histidine select cobalt resin (Pierce Inc., USA), and endotoxins were removed using High-Capacity Endotoxin Removal Resin (Pierce, USA). The full-length CHO Pvs48/45 protein was produced by Transient Gene Expression (Excellgene SA, Lausanne, Switzerland) in CHO-Express™ cells [26]. The CHO product has a signal peptide at its N-terminus, allowing secretion and a 6-His tag at its C-terminus to allow IMAC purification (or Western detection with an anti-6-His antibody). All production cultures (post-transfection) were performed in serum-free, animal protein-free medium (low protein content), and the protein was purified using IMAC-FPLC.
Vaccine formulation and mice immunization
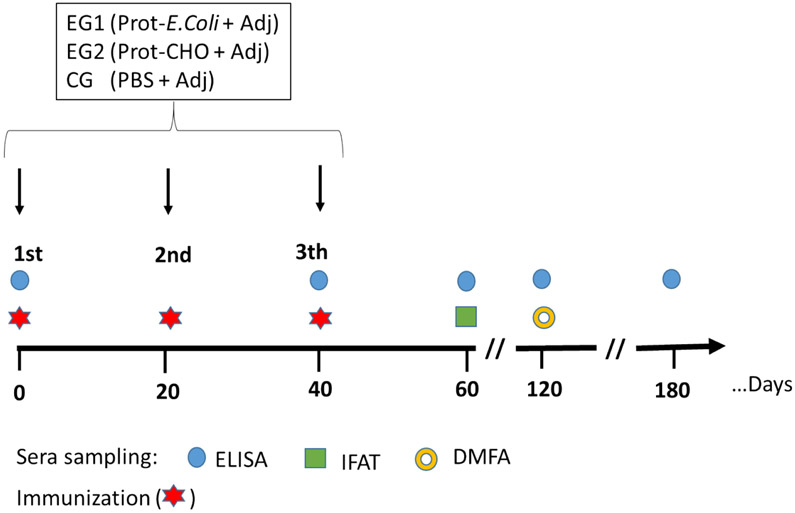
A total of 60 male and female, 6-8 weeks old BALB/c mice of 20 ± 5 g of body weight were randomly selected and distributed in three adjuvant groups (A, B and C) for each of the two proteins, 10 animals per each group. Each group was further divided into test (t) and control (c) sub-groups of five mice each (See flowchart in supplement material). Animals were subcutaneously immunized at the base of the tail on days 0, 20 and 40, using 20 μg/dose with either E. coli or CHO rPvs48/45 products formulated in Alhydrogel for Group A (subgroup A-test) or Montanide ISA-51, emulsified at 50% (vol/vol) for Group B (subgroup B-test). Both vaccines were injected with a final volume of 100 μL/dose. For Group C (subgroup C-test), immunogens were emulsified in 5 μg of GLA-SE with a final volume of 50 μL/dose. Control groups (subgroups A-, B- and C-control) were immunized with a saline solution formulated in the corresponding adjuvant (Figure 1).
Figure 1. Immunization and bleeding schedule.
Mice were immunized three times at 20 days intervals with 20 μg of rPvs48/45 formulated separately in Alhydrogel, GLA-SE or Montanide ISA-51 adjuvants. Blood samples were obtained at the indicated time points until day 180 for serology and functional assays. For days 0 and 40, the blood samples were collected before immunizations. EG: Experimental group; CG: Control group; Prot: recombinant protein
Mice bleeding
All the mice were bled from submandibular veins on days 1-2 before the first and third immunization and then 20 days after the third dose and every 60 days until day 180. No blood was taken before the second immunization (Figure 1). Whole blood (~100 μL) was collected, and sera separated by centrifugation and stored frozen at −20°C until serological analyses and evaluation of the ex vivo TB activity were performed
Serological analysis
Enzyme-Linked ImmunoSorbent Assay (ELISA) was performed using both E. coli and CHO rPvs48/45 proteins, as described before [27]. Briefly, 96-well plates (Nunc-Immuno Plate, Maxisorp, Roskilde, Denmark) were coated with one μg/mL of rPvs48/45 produced in CHO or E. coli in PBS, pH 7.4 at 4°C, overnight. After plates were blocked with 5% skim milk solution [PBS 1X, 0.05% Tween 20, (PBS-T)], sera samples were added at three-fold serial dilutions starting at 1:300 in 2.5% skim milk in PBS-T and were incubated for 1 hour. All sera were pre-adsorbed in E. coli powder to remove any anti-E. coli antibodies potentially present. After the reaction, plates were washed and incubated with alkaline phosphatase-conjugated anti-mouse IgG antibody (Sigma Chemical Co., St Louis, MO) at a 1:1000 dilution for 1 hour. Reactions were revealed with para-nitrophenyl phosphate substrate (p-NPP) (Sigma Aldrich) and read at 405 nm wavelength (Dynex Technologies, Inc., MRX Chantilly, VA). Cut-off points for ELISA were calculated as three SD above the mean absorbance value at 405 nm of sera from naive mice.
Avidity test
A standard ELISA method was used to measure the avidity of specific antibodies elicited by both recombinant proteins, as described before [28]. In brief, duplicate wells of polystyrene microtiter plates were coated with 1 μg/mL of either CHO rPvs48/45 or E. coli rPvs48/45 protein. After overnight incubation, plates were washed and blocked for 2 h at room temperature (RT), with 100 μL per well of PBS-T and 5% skim milk (Gibco™, USA). Sera collected at day 60 samples were diluted at 1:300 in PBS-T and 2.5% skim milk were added to the plates and incubated for 2 h at RT. As above, sera from E. coli rPvs48/45 immunized animals were pre-adsorbed with E. coli powder to remove potential anti-E. coli antibodies. After washing, plates were incubated for 15 min with urea at concentrations ranging from 0 to 7 mol/L in duplicate wells. Then, the reaction was revealed using an alkaline phosphatase-conjugated anti-human IgG, as described above. The urea concentration resulting in 50% of the original OD values (IC50) was calculated using linear regression [28]. All data sets fit well the linear regression models (R2>0.94, data not shown).
Indirect Immunofluorescence Antibody Test (IFAT)
P. vivax infected red blood cells (iRBC) were obtained from infected patients with parasitemias ranging from 2,000-16,000 parasites/μL and 10-20% of the parasites were gametocytes. White blood cells layers were removed after blood centrifugation at 5,000 rpm for 10 min. Enriched P. vivax gametocytes obtained by 45% percoll gradient [29] were placed on masked 12-well glass microscope slides and kept at −70°C until use. Slides were thawed, and mice sera from each test and control group were pooled and incubated at 1:20 dilution in PBS-Evans blue solution for 30 min. After PBS washing, slides were incubated with fluorescein isothiocyanate (FITC) conjugated, affinity-purified anti-mouse IgG antibody at 1:100 dilution. Slides were examined under an epifluorescence microscope with a 40x objective lens.
Direct mosquito membrane-feeding assays (DMFA)
DMFA was performed to evaluate whether mouse anti-rPvs48/45 antibodies blocked ex-vivo P. vivax transmission to vector mosquitoes. Assays were carried out using P. vivax gametocyte iRBC obtained from patients from a malaria-endemic area of Colombia (Chocó) [30], supplemented with sera from immunized mice, as described before [25, 31]. Briefly, for each independent assay, gametocyte-rich P. vivax iRBC (>2,000 gametocytes/μL) were collected by arm venipuncture from individual patients in heparinized tubes and maintained at 37°C until DMFA was completed. Before mosquito feeding, blood was centrifuged, and the cell pellets were subsequently reconstituted with a pool of heat-inactivated male AB+ sera (control) (H5667 Sigma-Aldrich, Inc.) or with pooled mice test sera at a 1:16 dilution. A total of ~100 adult females of An. albimanus mosquitoes, previously subjected to overnight fasting, were fed for 15-20 minutes/cage with this mixture. Mosquito infection was evaluated by light microscopy on day seven post-feeding after midguts were stained with 2% mercurochrome to assess the number of oocysts in the mosquito dissected midguts. Forty mosquitoes were examined to determine the mean oocyst density of a group and to calculate the percent (%) inhibition of mean oocyst intensity (%TRA) as described elsewhere [23]. The DMFA was performed in duplicate for all the vaccine formulations.
Statistical analysis
Comparisons of interest were made between antibody response against the two rPvs48/45 proteins and the adjuvant formulation. A descriptive analysis was conducted to evaluate trends in humoral responses in each study group, and kinetic profiles of antibody titers were depicted for each group. Mann–Whitney test was performed to compare the antibody responses between two proteins for each adjuvant. To compare three adjuvants for each protein, Kruskal-Wallis was used, followed by Dunn’s multiple comparison test. For the DMFA analysis, a zero-inflated negative binomial model was utilized as described before [32]. All statistical tests were performed in Prism 6 (GraphPad Software) or R (version 3.5.3, R Foundation for Statistical Computing), and the p-values <0.05 were considered significant.
Results
rPvs48/45 immunogenicity
Mice immunized with either E. coli or CHO rPvs48/45 formulated in Alhydrogel (5 seroconversion in 5 mice, 5/5) or Montanide ISA-51 (5/5) seroconverted after the second immunization doses, and most of them displayed a booster response after the third immunization. However, one animal of each test group immunized with the GLA-SE formulations died during the second dose, so they were excluded from the analysis [E. coli (4/5) and Montanide ISA-51, (4/5)].
The antibody titers between E. coli and CHO proteins formulated in the same adjuvant were first compared. With Alhydrogel adjuvant (Figure 2A), mice immunized with the CHO protein demonstrated an antibody peak (endpoint titers of 105) on day 60. On the other hand, the E. coli Alhydrogel formulations reached a significantly lower antibody titer peak (5 x103), which decreased (102) within the following 2-4 months. Mice of subgroup B-test, immunized with GLA-SE formulations using either E. coli or CHO proteins, developed comparable antibody patterns with a peak (105) on day 60, which progressively decreased (103) over the subsequent 4 months (day 180) (Figure 2B). The CHO protein in the Montanide ISA-51 formulation (subgroup C-test), developed a high antibody response peak (105-106) that remained high for up to six months (105) (Figure 2C). In contrast, the E. coli formulation with Montanide ISA-51 induced a modest response (103) that remained low for the four-month follow-up. Comparison of mouse antibody titers elicited by the two different proteins at day 60 using Mann–Whitney tests indicated significant differences (p <0.005) in sera of animals immunized with Alhydrogel and Montanide ISA-51 formulations (Table 1). Conversely, antibody titers in the GLA-SE adjuvant groups did not show a significant difference (p=0.1). Next, antibody titers among different adjuvant groups within the same protein were compared (Figures 2D and 2E). When day 60 titers were analyzed by Dunn's multiple comparison tests (Table 1), there were no statistical differences for the CHO proteins. On the other hand, for E. coli protein, GLA-SE formulation showed significantly higher titers than Montanide and Alhydrogel formulations.
Figure 2. Antibody Titers.
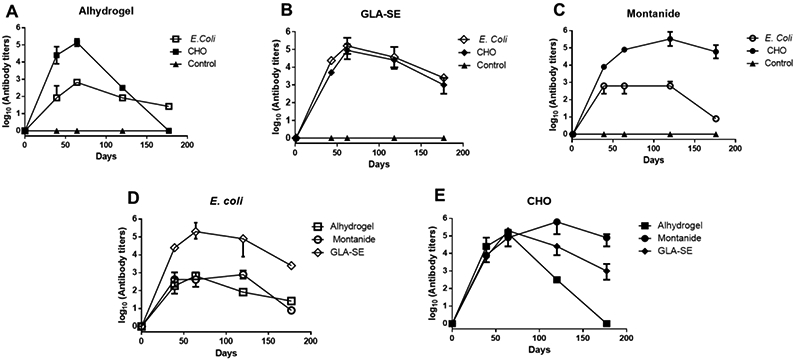
Kinetics of antibody titers in BALB/c mice upon immunization with the full-length P. vivax rPvs48/45 proteins produced E. coli (open symbols) or CHO (closed symbols) formulated with (A) Alhydrogel, (B) GLA-SE, or (C) Montanide ISA-51. Each plot has its respective control group per adjuvant (▲). Antibody titers correspond to the last dilution of the test sera in which OD405 values were above that of the cut-off. The cut-off value was defined as the mean plus 3SD of OD405 value of pooled naïve mouse sera tested at 1:300 dilution. The median +/− IQR of each group is shown. (D and E) The same data presented in 2A-2C are plotted to compare the kinetics of antibodies among adjuvant groups against either E. coli (D) or CHO-rPvs48/45 (E) proteins.
Table 1.
Immunogenicity in mice of CHO and E. coli rPvs48/45 formulated in different adjuvants at day 60.
| Adjuvant |
E. coli rPvs48/45 |
Mice response |
CHO rPvs48/45 |
Mice response |
P |
|---|---|---|---|---|---|
| Alhydrogel | 103 ± 0.00 | 5/5 | 105 ± 0.22 | 5/5 | 0.0079 |
| GLA-SE | 105 ± 0.48 | 4/4 | 105 ± 0.52 | 4/4 | 0.1000 |
| Montanide | 103 ± 0.52 | 5/5 | 105 ± 0.00 | 5/5 | 0.0079 |
Mann–Whitney test was performed with log10 (Antibody titers) (mean +/− SD) to compare the antibody response between E. coli and CHO rPvs48/45 in different adjuvants. P values, *, ≤ 0.05 were considered significant.
Dunn’s multiple comparison tests showed significant differences (p<0.05) between GLA-SE compared to the other two adjuvants for the E. coli, whereas no statistical differences were observed for the CHO protein.
Antibody avidity
The analysis of specific antibody binding to the two rPvs48/45 proteins (CHO and E. coli) formulated in different adjuvants is shown in Figure 3 (A-C). The E. coli protein induced lower avidity antibodies than the CHO protein in Alhydrogel (p = 0.0079) and GLA-SE (p = 0.0286) formulations, but no difference in Montanide ISA-51 formulations (p = 0.4127). When avidity among different formulations was compared using Dunn’s multiple comparisons, the E. coli proteins formulated in Alhydrogel and GLA-SE adjuvants displayed a significantly lower IC50 compared to Montanide ISA-51 (p<0.05) (Figure 3D), whereas the IC50 of CHO-Pvs48/45 antibodies elicited by the three different formulations displayed no significant differences (Figure 3E).
Figure 3. IC50.
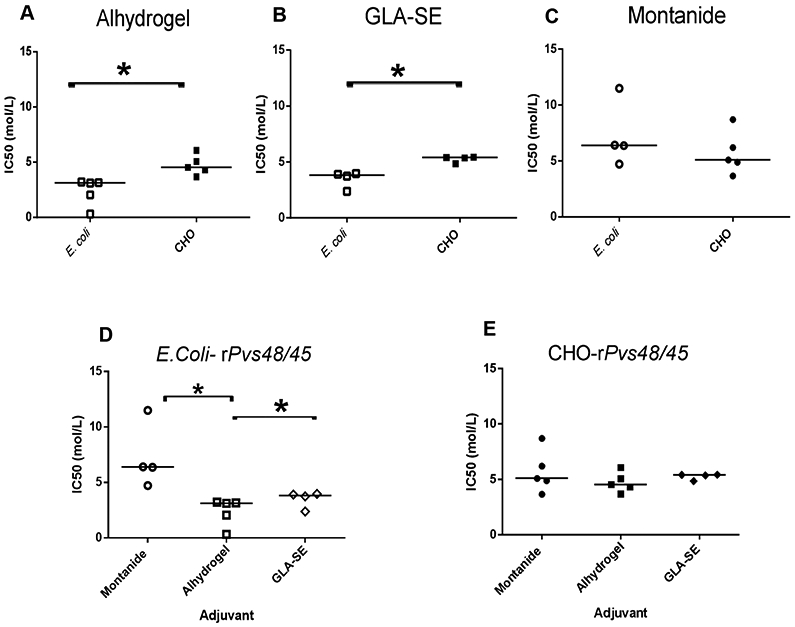
IC50 (mol/L) of sera samples from mice immunized with E. coli or CHO rPvs48/45 in each indicated adjuvant, collected at day 60 (A, B and C). Comparisons between CHO-rPvs48/45 and E. coli rPvs48/45 protein within each adjuvant are shown. Mann-Whitney analysis shows significant differences between E. coli and CHO proteins formulated in Alhydrogel (p=0.0079) and GLA-SE (p=0.0286). (D and E) comparison among adjuvants to each rPvs48/45 protein. Dunn’s multiple comparison tests showed significant differences for E. coli protein (D) (*p<0.05), but not for CHO protein (E). Bar corresponds to the median of each subgroup.
Recognition of the parasite protein in IFAT
Sera collected at day 60 of each mice group were assessed for their reactivity with the parasite protein using IFAT. The sera of mice immunized with CHO and E. coli rPvs48/45 formulated in Montanide ISA-51 showed strong reactivity with P. vivax gametocytes (Figure 4). In contrast, the sera from mice immunized with GLA-SE or Alhydrogel reacted weakly or not at all, respectively (data not shown).
Figure 4. IFAT.
Recognition of parasite protein by pools of sera of immunized mice with E. coli and CHO-rPvs48/45 formulated in Montanide ISA-51 at day 60 by IFAT. Mice serum was incubated with acetone-fixed smears containing P. vivax gametocyte enriched erythrocytic forms. Parasites seen under light (left) or epifluorescence (right) microscopy with a 40X objective lens are shown. Picture scale 238μm.
Functional activity of anti rPvs48/45 antibodies
Two independent ex vivo DMFA were carried out using sera of mice immunized with the different antigen formulations; i.e., a total of 6 independent DMFA were conducted. As shown in Tables 2 and S1, 4 out of 6 pooled sera of mice immunized with the different rPvs48/45 formulations significantly reduced the number of oocysts in the mosquito midgut as compared to the non-immune control sera (human AB+) (p<0.05). When E. coli or CHO proteins were compared within the same adjuvant, there was no significant difference for Alhydrogel and GLA-SE adjuvant groups. On the other hand, CHO immunization was superior to E. coli immunization with Montanide (p<0.001). Since sera from different subgroups (different adjuvants) were tested in different DMFAs, it is difficult to compare them within the same protein statistically. However, Montanide groups showed the highest %TRA for both proteins, and anti-CHO rPvs48/45 sera in Montanide ISA-51 showed the best capacity to inhibit and block the transmission of the P. vivax gametocytes (90.8%TRA).
Table 2.
Transmission blocking activity of sera from BALB/c mice sera after immunization with E. coli and CHO rPvs48/45 proteins.
| Adjuvant | Best estimates of %TRA from two assays to E. coli- rPvs48/45a |
Best estimates of %TRA from two assays to CHO-rPvs48/45a |
E. coli vs. CHO- rPvs48/45 |
||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| %TRA | (95%CI) | p-value | %TRA | (95%CI) | p-value | p-value | |
| Alhydrogel | 48.1 | (7 to 72) | 0.030 | 42.5 | (−2 to 68) | 0.057 | 0.718 |
| GLA-SE | 38.0 | (−10 to 65) | 0.111 | 61.2 | (29 to 79) | 0.002 | 0.150 |
| Montanide | 57.8 | (24 to 77) | 0.005 | 90.8 | (82 to 96) | <0.001 | <0.001 |
The best estimate of % inhibition in oocyst density (%TRA) at 95% confidence intervals (95%CI), and p-value for antisera were calculated against Normal AB+ sera using a zero-inflated negative binomial model as described previously [32]
Discussion
This study confirms the immunogenicity achieved by two rPvs48/45 recombinant proteins produced in E. coli and CHO expression systems in BALB/c mice [25]. It also indicates the recognition of the parasite antigen by the anti-protein antibodies in IFAT and their functional activity in ex vivo DMFA. Similar results had been shown in a previous pilot study conducted in a small number of mice and non-human primates using the E. coli rPvs48/45 formulated in Freund’s adjuvant and Montanide ISA-51, respectively [25]. Here we expanded the immunogenicity analyses to two other adjuvants, Alhydrogel and GLA-SE [33, 34], to select the one with the best performance for further testing in the non-human primate model and potential clinical development. It is encouraging to confirm the consistent immunogenicity and functional activity of this parasite antigen expressed in both E. coli and CHO systems, and, therefore, its potential for malaria human vaccine development [15, 25]. As shown in Figure 2, most of the formulation induced seroconversion after the second immunization in all animals, and some formulations induced antibody peaks titers of 105-106. However, different antibody patterns were observed between the distinct formulations. GLA-SE presented a similar antibody pattern with both proteins (Figure 2B), however, animals immunized with CHO-rPvs48/45 formulated in Montanide ISA-51 maintained a higher level of antibodies (~105) for the six months of follow up, whereas the titers elicited by the E. coli protein formulated in Montanide ISA-51 declined faster. This is intriguing as this adjuvant is characterized by inducing high and long-lasting antibody titers in animal models and humans [22, 35-37], suggesting an efficient B cell memory function [37, 38]. Likewise, antibody titers elicited by this protein formulated in Alhydrogel showed a high peak of antibody response on day 60 after the third immunization (~105), followed by a faster decay. The E. coli rPvs48/45 protein formulated either in Alhydrogel or Montanide ISA-51 presented a similar but lower antibody pattern than the CHO protein. In addition to these quantitative differences of specific antibodies, the different groups displayed qualitative antibody differences. Although the avidity elicited by both Montanide ISA-51 protein formulations (CHO and E. coli) were similar (p= 0.4127), the two proteins induced antibodies with differential avidity when formulated with Alhydrogel (p = 0.0079) and GLA-SE (p =0.0286), displaying a particularly lower avidity with the E. coli product. It is not surprising that the Alhydrogel formulations induced the lowest and shorter responses, and in the case of the E. coli formulation, lower avidity and no recognition of the parasite in IFAT (data not shown). Although this adjuvant is the most extensively used in humans because of its safety, low reactogenicity and good tolerance [39], it has also been observed to be comparatively a weaker adjuvant for TB vaccine candidates [40]. GLA-SE and Montanide ISA-51 adjuvants are known to induce strong cellular and humoral immune responses in animal models and humans [27, 41-43].
Because of the prospects for the Pvs48/45 for further vaccine development, it is relevant that besides reactivity with the recombinant proteins, antibodies recognized the parasite antigen in IFAT (Figure 4) and induced significant TRA (50-90%) in the ex-vivo DMFA (Table 2). Similar results have been observed with human anti-rPvs48/45 IgG affinity-purified from individuals from endemic areas [15], indicating a consistent immune response under natural and experimental conditions. As described before Pvs48/45 is a highly conserved protein [44] and although we did not characterize the parasites used here for the DMFA and those of previous studies [15] the consistent TRA in the multiple assays performed confirm the importance of this parasite antigen as vaccine candidate.
Altogether, the results obtained in this and previous studies are very encouraging to develop Pvs48/45 as a vaccine candidate for human use. First, the antigenicity and immunogenicity displayed by the protein are very promising. Second, both affinity purified anti-Pvs48/45 antibody from human sera and animal antibodies raised against the Pvs48/45 recombinant proteins have shown TRA activity [15].
Ongoing studies are evaluating the response of non-human primates with CHO-Pvs48/45 in Montanide ISA-51 formulation to confirm its immunogenicity in an animal model closer to humans. However, further research is needed toward the goal of developing this vaccine candidate in a clinical phase, focusing on a better understanding of the differences between the two recombinant products; defining the protein fragment target of protective TB antibodies; establishing the potential use of Pvs48/45 cross-reactivity and functional activity for the development of a multi-species vaccine.
Supplementary Material
Acknowledgments
The authors are grateful for the participation of communities from malaria-endemic regions of Quibdo, Ismael Roldan Hospital, and point-of-care in Tutunendo, (Quibdo), and also thank staff members of the Caucaseco SRC (M. Pardo, AF. Chamorro, F. Meneses and LF. Falla).
Funding
This study was sponsored by NIH/NIAID 1R01AI121237-01 and in part by the Intramural Research Program of NIAID, NIH.
Myriam Arevalo-Herrera reports financial support was provided by NIH, NIAID.
Footnotes
Ethics approval and consent to participate
The Animal Ethical Committee of Universidad del Valle approved the mouse study protocol. Animals were housed and handled following the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The protocol and Informed Consent to obtain human P. vivax-infected blood samples were approved by the Institutional Review Board (IRB) at the Centro Internacional de Vacunas (CECIV, Cali-Colombia) (code CECIV 1506-2017).
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request
Competing Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCE
- [1].Dhiman S. Are malaria elimination efforts on right track? An analysis of gains achieved and challenges ahead. Infect Dis Poverty. 2019;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Feachem RGA, Chen I, Akbari O, Bertozzi-Villa A, Bhatt S, Binka F, et al. Malaria eradication within a generation: ambitious, achievable, and necessary. Lancet. 2019;394:1056–112. [DOI] [PubMed] [Google Scholar]
- [3].WHO. Malaria progress report. Geneva: WHO; 2020. [Google Scholar]
- [4].WHO. Tackling antimalarial drug resistance In: www.who.int/docs/default-source/malaria/drug-resistance/who-ucn-gmp-2020-07... editor.2020.
- [5].Ikeda M, Kaneko M, Tachibana SI, Balikagala B, Sakurai-Yatsushiro M, Yatsushiro S, et al. Artemisinin-Resistant Plasmodium falciparum with High Survival Rates, Uganda, 2014-2016. Emerg Infect Dis. 2018;24:718–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Oboh MA, Ndiaye D, Antony HA, Badiane AS, Singh US, Ali NA, et al. Status of Artemisinin Resistance in Malaria Parasite Plasmodium falciparum from Molecular Analyses of the Kelch13 Gene in Southwestern Nigeria. Biomed Res Int. 2018;2018:2305062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Peters W Drug resistance in malaria. Recenti Prog Med. 1990;81:749–53. [PubMed] [Google Scholar]
- [8].Ranson H, Lissenden N. Insecticide Resistance in African Anopheles Mosquitoes: A Worsening Situation that Needs Urgent Action to Maintain Malaria Control. Trends Parasitol. 2016;32:187–96. [DOI] [PubMed] [Google Scholar]
- [9].Constenla D. Assessing the economic benefits of vaccines based on the health investment life course framework: a review of a broader approach to evaluate malaria vaccination. Vaccine. 2015;33:1527–40. [DOI] [PubMed] [Google Scholar]
- [10].Ndungu FM, Mwacharo J, Wambua J, Njuguna P, Marsh K, Drakeley C, et al. A sevenyear study on the effect of the pre-erythrocytic malaria vaccine candidate RTS,S/AS01 E on blood stage immunity in young Kenyan children. Wellcome Open Res. 2019;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Agnandji ST, Fernandes JF, Bache EB, Ramharter M. Clinical development of RTS,S/AS malaria vaccine: a systematic review of clinical Phase I-III trials. Future Microbiol. 2015;10:1553–78. [DOI] [PubMed] [Google Scholar]
- [12].Coelho CH, Rappuoli R, Hotez PJ, Duffy PE. Transmission-Blocking Vaccines for Malaria: Time to Talk about Vaccine Introduction. Trends Parasitol. 2019;35:483–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ishino T, Tsuboi T. Progress toward a transmission-blocking vaccine against malaria. Lancet Infect Dis. 2018;18:927–8. [DOI] [PubMed] [Google Scholar]
- [14].Sinden RE. Developing transmission-blocking strategies for malaria control. PLoS Pathog. 2017;13:e1006336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arévalo-Herrera M, Miura K, Cespedes N, Echeverry C, Solano E, Castellanos A, et al. Immunoreactivity of sera from low to moderate malaria-endemic areas against Plasmodium vivax rPvs48/45 proteins produced in Escherichia coli and Chinese hamster ovary systems. Front Immunol. 2020. [Google Scholar]
- [16].Boudin C, Diop A, Gaye A, Gadiaga L, Gouagna C, Safeukui I, et al. Plasmodium falciparum transmission blocking immunity in three areas with perennial or seasonal endemicity and different levels of transmission. Am J Trop Med Hyg. 2005;73:1090–5. [PubMed] [Google Scholar]
- [17].Wu Y, Sinden RE, Churcher TS, Tsuboi T, Yusibov V. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol. 2015;89:109–52. [DOI] [PubMed] [Google Scholar]
- [18].Menon V, Kapulu MC, Taylor I, Jewell K, Li Y, Hill F, et al. Assessment of Antibodies Induced by Multivalent Transmission-Blocking Malaria Vaccines. Front Immunol. 2017;8:1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine. 2018;36:5865–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sagara I, Healy SA, Assadou MH, Gabriel EE, Kone M, Sissoko K, et al. Safety and immunogenicity of Pfs25H-EPA/Alhydrogel, a transmission-blocking vaccine against Plasmodium falciparum: a randomised, double-blind, comparator-controlled, dose-escalation study in healthy Malian adults. Lancet Infect Dis. 2018;18:969–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Talaat KR, Ellis RD, Hurd J, Hentrich A, Gabriel E, Hynes NA, et al. Safety and Immunogenicity of Pfs25-EPA/Alhydrogel(R), a Transmission Blocking Vaccine against Plasmodium falciparum: An Open Label Study in Malaria Naive Adults. PLoS One. 2016;11:e0163144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu Y, Ellis RD, Shaffer D, Fontes E, Malkin EM, Mahanty S, et al. Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51. PLoS One. 2008;3:e2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Miura K, Swihart BJ, Deng B, Zhou L, Pham TP, Diouf A, et al. Transmission-blocking activity is determined by transmission-reducing activity and number of control oocysts in Plasmodium falciparum standard membrane-feeding assay. Vaccine. 2016;34:4145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–64. [DOI] [PubMed] [Google Scholar]
- [25].Arevalo-Herrera M, Vallejo AF, Rubiano K, Solarte Y, Marin C, Castellanos A, et al. Recombinant Pvs48/45 antigen expressed in E. coli generates antibodies that block malaria transmission in Anopheles albimanus mosquitoes. PLoS One. 2015;10:e0119335. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [26].Ritacco FV, Wu Y, Khetan A. Cell culture media for recombinant protein expression in Chinese hamster ovary (CHO) cells: History, key components, and optimization strategies. Biotechnol Prog. 2018;34:1407–26. [DOI] [PubMed] [Google Scholar]
- [27].Cespedes N, Arevalo-Herrera M, Felger I, Reed S, Kajava AV, Corradin G, et al. Antigenicity and immunogenicity of a novel chimeric peptide antigen based on the P. vivax circumsporozoite protein. Vaccine. 2013;31:4923–30. [DOI] [PubMed] [Google Scholar]
- [28].Miura K, Orcutt AC, Muratova OV, Miller LH, Saul A, Long CA. Development and characterization of a standardized ELISA including a reference serum on each plate to detect antibodies induced by experimental malaria vaccines. Vaccine. 2008;26:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vera O, Brelas de Brito P, Albrecht L, Martins-Campos KM, Pimenta PF, Monteiro WM, et al. Purification Methodology for Viable and Infective Plasmodium vivax Gametocytes That Is Compatible with Transmission-Blocking Assays. Antimicrob Agents Chemother. 2015;59:6638–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Castellanos A, Chaparro-Narvaez P, Morales-Plaza CD, Alzate A, Padilla J, Arevalo M, et al. Malaria in gold-mining areas in Colombia. Mem Inst Oswaldo Cruz. 2016;111:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vallejo AF, Garcia J, Amado-Garavito AB, Arevalo-Herrera M, Herrera S. Plasmodium vivax gametocyte infectivity in sub-microscopic infections. Malar J. 2016;15:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miura K, Swihart BJ, Fay MP, Kumpitak C, Kiattibutr K, Sattabongkot J, et al. Evaluation and modeling of direct membrane-feeding assay with Plasmodium vivax to support development of transmission blocking vaccines. Sci Rep. 2020;10:12569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9:287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Singh M, O'Hagan DT. Recent advances in vaccine adjuvants. Pharm Res. 2002;19:715–28. [DOI] [PubMed] [Google Scholar]
- [35].Kumar R, Ledet G, Graves R, Datta D, Robinson S, Bansal GP, et al. Potent Functional Immunogenicity of Plasmodium falciparum Transmission-Blocking Antigen (Pfs25) Delivered with Nanoemulsion and Porous Polymeric Nanoparticles. Pharm Res. 2015;32:3827–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Collins WE, Barnwell JW, Sullivan JS, Nace D, Williams T, Bounngaseng A, et al. Assessment of transmission-blocking activity of candidate Pvs25 vaccine using gametocytes from chimpanzees. Am J Trop Med Hyg. 2006;74:215–21. [PubMed] [Google Scholar]
- [37].Herrera S, Fernandez OL, Vera O, Cardenas W, Ramirez O, Palacios R, et al. Phase I safety and immunogenicity trial of Plasmodium vivax CS derived long synthetic peptides adjuvanted with montanide ISA 720 or montanide ISA 51. Am J Trop Med Hyg. 2011;84:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].van Doorn E, Liu H, Huckriede A, Hak E. Safety and tolerability evaluation of the use of Montanide ISA51 as vaccine adjuvant: A systematic review. Hum Vaccin Immunother. 2016;12:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kurella S, Manocha M, Sabhnani L, Thomas B, Rao DN. New age adjuvants and delivery systems for subunit vaccines. Indian J Clin Biochem. 2000;15:83–100. [Google Scholar]
- [40].Kumar R, Angov E, Kumar N. Potent malaria transmission-blocking antibody responses elicited by Plasmodium falciparum Pfs25 expressed in Escherichia coli after successful protein refolding. Infect Immun. 2014;82:1453–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Arevalo-Herrera M, Solarte Y, Zamora F, Mendez F, Yasnot MF, Rocha L, et al. Plasmodium vivax: transmission-blocking immunity in a malaria-endemic area of Colombia. Am J Trop Med Hyg. 2005;73:38–43. [DOI] [PubMed] [Google Scholar]
- [42].Chowdhury DR, Angov E, Kariuki T, Kumar N. A potent malaria transmission blocking vaccine based on codon harmonized full length Pfs48/45 expressed in Escherichia coli. PLoS One. 2009;4:e6352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Coler RN, Baldwin SL, Shaverdian N, Bertholet S, Reed SJ, Raman VS, et al. A synthetic adjuvant to enhance and expand immune responses to influenza vaccines. PLoS One. 2010;5:e13677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vallejo AF, Martinez NL, Tobon A, Alger J, Lacerda MV, Kajava AV, et al. Global genetic diversity of the Plasmodium vivax transmission-blocking vaccine candidate Pvs48/45. Malar J. 2016;15:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.