Abstract
Myocardial injury has been reported as a complication of COVID-19. Although several mechanisms have been proposed as its cause, they are mostly based on autopsy studies, We report a 49-year-old male with COVID-19-associated myocardial injury presented like fulminant myocarditis. We performed endomyocardial biopsy on day 2 and we confirmed the presence of microthrombosis histologically. He died on day 5 due to cardiogenic shock.
Keywords: Cardiogenic shock, SARS-CoV-2, Cytokine storm, Biopsy
1. Introduction
Myocarditis has been reported as a complication of COVID-19 infection. Mostly, it has been diagnosed by cardiac magnetic resonance imaging and elevated cardiac enzymes [1,2]. We report a case with COVID-19-associated myocardial injury presented like fulminant myocarditis in whom the presence of microthrombosis was histologically confirmed by endomyocardial biopsy.
2. Case Report
2.1. Presentation
A 49-year-old male without prior medical history was admitted to an outside hospital in a shock-state. He had the first dose of COVID-19 vaccination 9 days before and had been suffered from sore throat, chill and fever for 4 days. Then, he was confirmed to be affected by COVID-19 with its antigen test on the nasopharyngeal swab one day before admission. Echocardiography showed a low left ventricular ejection fraction (<20%) and diffuse myocardial hypertrophy. Chest computed tomography revealed bilateral mild ground glass appearance, bilateral pleural effusion and pericardial effusion. He was intubated and cannulated for extracorporeal membrane oxygenation (ECMO), and was transferred to our hospital with inotropes infusion for further treatment.
On admission to our hospital, blood tests showed elevated troponin T (1.010 ng/mL, normal range ≤0.014 ng/mL), NT-proBNP (27541.0 pg/mL, normal range ≤125.0 pg/mL), creatine kinase (929 IU/L, normal range 24-195 IU/L), C-reactive protein (1.685 mg/dL, normal range ≤0.180 mg/dL) and D-dimer (3.8 µg/mL, normal range <1.0 µg/mL) while white blood cell count was 7100 (normal range 4000-8000/µL) and platelet count was 53000 (normal range 150-360/µL). Coronary angiography revealed no obstructive coronary artery disease. We administered methylprednisolone (1000 mg daily for 3 days) and gamma globulin (80 g daily for 2 days) in addition to catecholamine infusion and ECMO support. However, the hemodynamics did not improve. On day 2, we performed endomyocardial biopsy from the right ventricle. He died on day 5 due to intractable cardiogenic shock.
2.2. Histopathological findings of endomyocardial biopsy samples
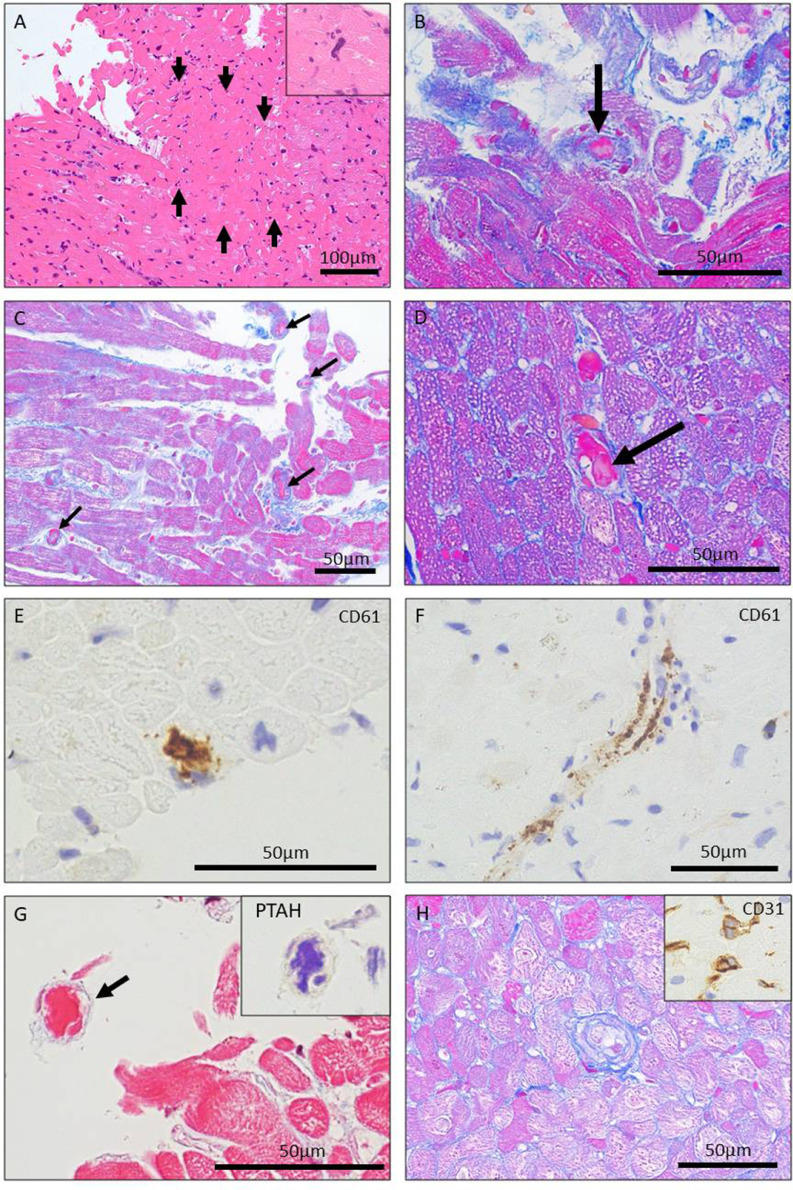
Myocardial samples were processed for light microscopy. There was mild lymphocytic infiltration and moderate to severe perivascular fibrosis with wall thickening of intramural arterioles. There was no sign of severe myocardial injury compatible with typical active myocarditis. However, ischemic changes were found with a focal coagulative necrotic area at microvascular level (approximately 0.08 mm2 in area) with losing nuclei, accompanied by microthrombi with fibrin and platelets in small vessels, which suggested microvascular thrombotic injury (Figure 1 A, B). Scattered megakaryocytes were also seen in the capillaries (inset in Figure 1A). Microthrombi was seen throughout the specimens (Figure 1C) and myocytes in non-necrotic areas often showed diffuse cytoplasmic vacuolization (Figure 1D), which also indicate ischemic insults. The presence of platelets in obstructive and non-obstructive microthrombi within the lumens of microvessels was confirmed by immunohistochemical expression of CD61 (Figure 1, E and F). Fibrin-rich microthrombi were also present as confirmed by phosphotungstic acid-hematoxylin (PTAH) stain (Figure 1G). Microvessels including intramural arterioles often showed swollen endothelial cells, a possible sign of endothelial activation (Figure 1H). A part of formalin-fixed paraffin-embedded sample was analyzed at National Institute of Infectious Diseases for multivirus real–time polymerase chain reaction (PCR) assay for comprehensive viral gene analysis [3]. There was no viral genome including SARS-CoV-2.
Figure 1.
Histology and immunohistology of endomyocardial biopsy samples. A. A focal small area of coagulative necrosis of myocytes (arrows) suggesting acute ischemic injury at microvascular level (Hematoxylin and eosin stain). The inset highlights the presence of a megakaryocyte within the capillary lumen. B. High-power view of a microthrombus with fibrin and platelets within the arteriole (arrow) in close proximity to the necrotic area in Figure 1A (Masson's trichrome stain). C. Scattered microthrombi (arrows) seen in microvessels (Masson's trichrome stain). D. High-power view of another microthrombus (arrow) accompanied by myocytes with diffuse cytoplasmic vacuolization. E and F. Immunostaining against CD61 showing platelets in obstructive and non-obstructive microthrombi in microvessels. G. Fibrin-rich microthrombus (arrow, Masson's trichrome stain) confirmed by phosphotungstic acid-hematoxylin (PTAH) stain (the inset). H.Arteriolar endothelial cell swelling is seen (Masson's trichrome stain). The inset highlights swollen endothelial cells in microvessels (immunostaining against CD31).
3. Discussion
We experienced a case with COVID-19 showing cardiogenic shock in whom microvascular thrombosis was demonstrated by endomyocardial biopsy.
Pathological studies have demonstrated that myocardial injury has been common in patients with COVID-19. The mechanisms hypothesized include direct viral invasion of the heart, immune-mediated myocarditis, stress-induced cardiomyopathy, myocardial infarction, cytokine storm and microvascular thrombi [4,5]. One report shows myocardial localization of coronavirus in a patient with COVID-19 myocarditis [6]. There may be multiple mechanisms that cause cardiac injury in patients with COVID-19.
The incidence of pathologically proven myocarditis ranges 2%–30% in autopsied patients with COVID-19 [4,5,7]. Although the present case showed a drastic clinical course compatible with severe fulminant acute myocarditis, the pathologic study demonstrated only mild myocardial inflammation without associated myocyte necrosis. Instead, we found microthrombosis in small vessels and ischemic necrotic changes. According to a recent study of systematic pathological analysis of 40 hearts from patients dying of COVID-19, 14 cases had myocardial necrosis and 9 of 14 (64%) had microthrombi in small vessels [8]. Interestingly, none had typical evidence of myocarditis as defined by the European Society of Cardiology [9]. Other recent investigations also reported the incidence of microthrombi in the autopsied hearts with COVID-19 to be high as 70% (48 of 69 cases) [10] and 80% (12 of 15 cases) [7]. The present case adds further evidence to these observations by demonstrating the presence of microthrombosis associated with acute myocardial ischemic injury in acute phase of COVID-19 by endomyocardial biopsy prior to death. Of note, in our case, there were only fresh microthrombi, and no organizing and/or organized mural thrombi which coexisted in reported autopsy cases. Microthrombosis throughout the specimens, presumably in the setting of endothelial dysfunction, must have contributed to the global myocardial ischemia causing diffuse LV systolic dysfunction, as histologically shown by coagulation necrosis and diffuse cytoplasmic vacuolization of myocytes, both of which are characteristic of acute ischemia. Patients with COVID-19 are at a high risk for thromboembolic events probably resulting from cytokine storm. Although no data on inflammatory cytokine levels were available in the present case, there could be excessive cytokine release, endothelial dysfunction and coagulopathy, as is common in COVID-19, which related to microthrombi formation in myocardial small vessels and myocardial injury [11].
Sampling errors should be born in mind in the analysis of endomyocardial biopsy specimens but failure to demonstrate viral genomes by PCR may support the above hypothesis. Moderate to severe perivascular fibrosis may suggest the presence of subclinical underlying heart disease such as hypertension but it was not apparent from his medical history. Catecholamine infusion can cause myocardial injury which is usually characterized by contraction band necrosis of single myocyte or groups of several myocytes. However, we infused catecholamine for only 1 day before endomyocardial biopsy and coagulative necrosis with losing nuclei, not contraction band necrosis, found in the present case suggested ischemic injury was the most likely explanation. Although a direct causal relationship may not be definitively established, no other causes were difficult to identify than COVID-19-associated myocardial injury. The vaccine-related associated myocarditis or myocardial injury has been reported mostly in young male recipients after the 2nd dose with most being mild illness, which is different from the present case. Rare fatal cases have also been reported including two adolescent males resembling a catecholamine-induced injury but without microthrombosis microscopically [12] and two patients in forties (one woman and one man) with histologically-proven fulminant myocarditis [13], both of which were histopathologically different from the present cases. Yet, we cannot fully deny the possibility that the vaccination itself or in combination with COVID-19 might have influenced on myocardial damage.
This is a case in summer in 2021 and the patient was possibly affected by COVID-19 delta variant. We do not know yet whether COVID-19 omicron variant causes myocardial injury in the similar manner.
4. Conclusion
Myocarditis associated with COVID-19 has been mostly diagnosed by imaging studies and its mechanisms are only speculation. We showed microthrombosis as a cause of myocardial injury which mimicked fulminant myocarditis in acute phase of COVID-19 by endomyocardial biopsy. Unfortunately, our case died on day 5 but this report may give a clue to further treatment in a patient with COVID-19 with myocardial injury.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
All authors have declared there are no conflicts of interest. There are no relationships with industry.
Acknowledgments
We thank Manabu Kobayashi, MT and Motomu Tsuji, MD, PhD for their excellent help in preparing histological biopsy samples.
References
- 1.Garot J, Amour J, Pezel T, Dermoch F, Messadaa K, Felten ML, et al. SARS-CoV-2 fulminant myocarditis. J Am Coll Cardiol Case Rep. 2020;2:1342–1346. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khatri A, Wallach F. Coronavirus disease 2019 (Covid-19) presenting as purulent fulminant myopericarditis and cardiac tamponade: a case report and literature review. Heart Lung. 2020;49:858–863. doi: 10.1016/j.hrtlng.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katano H, Kano M, Nakamura T, Kanno T, Asanuma H, Sata T. A novel real-time PCR system for simultaneous detection of human viruses in clinical samples from patients with uncertain diagnoses. J Med Virol. 2011;83:322–330. doi: 10.1002/jmv.21962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC, et al. Pathological features of COVID-19-associated myocardial injury: a multicenter cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bois MC, Boire NA, Layman AJ, Aubry MC, Alexander MP, Roden AC, et al. COVID-19-associated nonocclusive fibrin microthrombi in the heart. Circulation. 2021;143:230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, et al. Microthrombi as a major cause of cardiac injury in COVID-19. A pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 9.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. [DOI] [PubMed] [Google Scholar]
- 10.Brener MI, Hulke ML, Fukuma N, Golob S, Zilinyi RS, Zhou Z, et al. Clinico-histopathologic and single-nuclei RNA-sequencing insights into cardiac injury and microthrombi in critical COVID-19. JCI Insight. 2022;7 doi: 10.1172/jci.insight.154633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu SX, Tyagi T, Jain K, Gu VW, Lee SH, Hwa JM, et al. Thrombocytopathy and endotheliopathy: crucial contributors to COVID-19 thromboinflammation. Nat Rev Cardiol. 2021;18:194–209. doi: 10.1038/s41569-020-00469-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gill JR, Tashjian R, Duncanson E. Autopsy histopathologic cardiac findings in two adolescents following the second COVID-19 vaccine dose. Arch Pathol Lab. 2022 doi: 10.5858/arpa.2021-0435-SA. [DOI] [PubMed] [Google Scholar]
- 13.Verma AK, Lavine KJ, Lin CY. Myocarditis after Covid-19 mRNA Vaccination. N Engl J Med. 2021;385(14):1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]