Abstract
Purpose
To assess the safety of inactivated coronavirus 2019 disease (COVID-19) vaccine in tuberous sclerosis complex (TSC) patients with epilepsy.
Methods
All patients with epilepsy were selected from Efficacy and Safety of Sirolimus in Pediatric Patients with Tuberous Sclerosis (ESOSPIT) project and younger than 17 years old. The patients were treated with mTOR inhibitors (rapamycin). A total of 44 patients who completed the two-dose inactivated COVID-19 vaccine between July 7, 2021, and January 1, 2022, were enrolled.
Results
The median age of seizure onset was 23 months. About two-thirds of patients have focal seizures. Thirty-three patients use antiseizure medications. The mean duration of rapamycin treatment was 55.59 ± 18.42 months. Adverse reactions within 28 days after injection occurred in 11 patients (25%), all were under 12 years old. Injection site pain was the most reported event (20.45%), which was mild in severity and improved within one day. All patients had no seizure-related changes after vaccination.
Conclusion
This study shows that the inactivated COVID-19 vaccine was well tolerated and safe in TSC patients with epilepsy, as well as for those treated with mTOR inhibitors.
Keywords: COVID-19 vaccine, Tuberous sclerosis complex, Epilepsy, mTOR inhibitor, Rapamycin
1. Introduction
The World Health Organization (WHO) announced that as of March 2022, the number of confirmed coronavirus 2019 disease (COVID-19) cases had reached more than 438 million worldwide. Children of all ages are susceptible to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Although most infected children are mild or asymptomatic, the multisystem inflammatory syndrome is still reported [1]. Infected children can also cause severe community transmission.
Vaccination and multiple preventive measures are the most effective ways to curb the spread of SARS-CoV-2 quickly. More than 140 candidate vaccines exist. CoronaVac and BBIBP-CorV (Beijing Institute of Biological Products, Beijing, China) are safe for children and adolescents over three years old [2,3]. In June 2021, inactivated COVID-19 vaccine was recommended for children over three years old in China. The Pfizer/BioNTech vaccine is recommended for children over five years old in the United States and the United Kingdom. However, the hesitation of most parents to vaccinate due to concerns regarding the safety and adverse reaction of COVID-19 vaccines is a major global public health problem [4].
Tuberous sclerosis complex (TSC) is one of the most common rare diseases that affect multiple organ systems with an incidence rate of approximately 1/6000 [5]. Epilepsy develops in 80–90% of patients with TSC and often begins in the first year of life [5]. The safety of COVID-19 vaccines is the most concern for patients with epilepsy. mTOR inhibitor, such as rapamycin, is approved to treat TSC [5]. Rapamycin has an immunosuppressive effect that is widely used in autoimmune diseases and organ transplantation. mTOR inhibitors reduce the replication of SARS-CoV-2 and become a potential drug for the treatment of COVID-19 [6]. It has been reported that seven patients with TSC on mTOR inhibitors had COVID-19 vaccinated without adverse reaction [7].
We assessed the safety of inactivated COVID-19 vaccine in pediatric TSC patients with epilepsy.
2. Material and methods
All patients were selected from the Efficacy and Safety of Sirolimus in Pediatric Patients with Tuberous Sclerosis (ESOSPIT) project of the Chinese Clinical Trial Registry (No. ChiCTR-OOB-15006535). We investigated children and adolescents younger than 17 years old with epilepsy who were diagnosed with TSC according to the 2012 International TSC Diagnostic Criteria [8]. The patients were administered 1 mg/(m2•d) rapamycin (Huadong Medicine Co., Ltd. of Hangzhou), and dose was adjusted to attain a blood concentration of 5–10 ng/mL. The Ethics Committee of the Chinese PLA General Hospital (No. S2013-028-01) approved this study.
The patients were voluntarily injected with inactivated COVID-19 vaccine in accordance with the local vaccine supply. Two-dose vaccines were administered at an interval of 3–8 weeks. The inactivated COVID-19 vaccines were CoronaVac and BBIBP-CorV. The patients who had completed two-dose vaccines were included. Telephone follow-up was performed at 7 and 28 days after injection to collect information on seizures, medication, vaccine manufacturer, and adverse reactions after injection. Seizures were classified according to the latest International League Against Epilepsy (ILAE) guidelines [9]. Seizure frequencies were divided into four categories, ranging from once every day, once every month, at least once per year, and seizure-free over 1 year.
Adverse reactions related to inactivated COVID-19 vaccine included (a) injection site adverse reactions, such as pain, swelling, induration, erythema, and pruritus, and (b) systematic adverse reactions, such as fever, cough, headache, anorexia, vomiting, acute allergic, and seizure-related changes.
3. Results
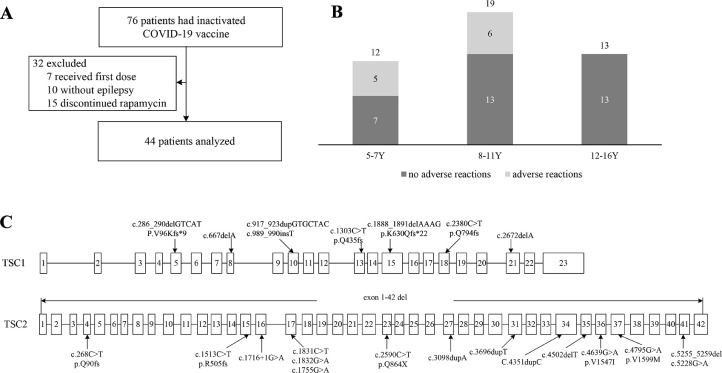
A total of 76 patients with TSC received inactivated COVID-19 vaccine between July 7, 2021, and January 1, 2022. Seven patients who did not receive the second dose of vaccine due to the short time from the first dose were excluded. Ten patients without seizures were excluded. Fifteen patients were excluded due to the discontinuation of rapamycin. Forty-four patients were eventually enrolled in the study (Fig. 1A ).
Fig. 1.
A: Flow diagram of the study patients. B: Age distribution of patients and incidence of adverse reactions at different ages. The three bars show three ages 5–7 years old, 8–11 years old, and 12–16 years old. Dark indicates the number of people with no adverse reactions and gray indicates the number of people with adverse reactions. Y: years. C: The distribution of TSC1 and TSC2 variants sites.
The age of the patients ranged from five to fifteen years with a mean of 9.62 ± 2.89 years (Table 1 ). The distribution of different age groups is shown in Fig. 1B. The male-to-female ratio was 1.16:1. The age of seizure onset ranged from one day to ten years with a median of 23 months. About two-thirds of patients have focal seizures. Two patients had seizures once per year. Four patients had seizures once every month. The other patients were seizure-free for more than a year. Ten patients did not use antiseizure medications (ASMs), 31 patients used less than two ASMs, and three patients used three ASMs (Table 1). The duration of rapamycin use ranged from 20 to 108 months with a mean of 55.59 ± 18.42 months.
Table 1.
Clinical characteristics of 44 patients.
| Number | Percentage | Number | Percentage | ||
|---|---|---|---|---|---|
| Age, years, mean±SD | 9.62 ± 2.89 | – | Number of antiseizure medications | ||
| Age of seizure onset, months | 23 (9.25, 48) | – | 0 | 10 | 22.73 |
| Gender, male | 29 | 65.91 | 1–2 | 31 | 70.45 |
| Seizure classification | ≥3 | 3 | 6.82 | ||
| Focal onset | 29 | 65.91 | Genetic variants | ||
| Generalized onset | 15 | 34.09 | TSC1 | 8 | 18.18 |
| Seizure frequency ranking | TSC2 | 17 | 38.64 | ||
| Once every day | 0 | 0 | No variants | 6 | 13.64 |
| Once every month | 4 | 9.09 | Not perform | 13 | 29.54 |
| Once per year | 2 | 4.55 | Hypopigmentation | 42 | 95.45 |
| Over 1 year seizure free | 38 | 86.36 | Shagreen patches | 19 | 43.18 |
| Time of rapamycin treatment, months | 55.59 ± 18.42 | – | Angiofibroma | 13 | 29.55 |
| Heart diseases | 5 | 11.36 | |||
| Renal diseases | 8 | 18.18 | |||
A total of 31 patients underwent genetic testing. Eight patients had TSC1 variants (NM_000368.4) and 17 patients had TSC2 variants (NM_000548.3). No TSC1 or TSC2 variants were found in the other six patients. Among the 25 patients with gene variants, seven had missense variants, six had nonsense variants, and 12 had frameshift variants. One patient had two TSC2 missense variants (c.4639G > A, c.4795G > A). The distribution of gene variants is shown in Fig. 1C.
Eleven of the 44 patients (25%) who received two-dose of inactivated COVID-19 vaccines had adverse reactions. The adverse reactions were all mild in severity. Injection site pain, which improved within one day, occurred in 9 patients (20.45%). One patient had injection site swelling. One patient had a fever (38 °C) four days after vaccination. No one had seizures within 28 days after vaccination.
In the 5–7 years old group, three patients had injection site pain, and one patient had injection site swelling. One patient had a fever. In the 8–11 years old group, six patients had injection site pain. In children older than 12 years, no one had adverse reactions (Fig. 1B).
Of the 18 patients who received two-dose of CoronaVac vaccines, two had injection site pain, and one had injection site swelling. Four of the 18 patients who received two-dose of BBIBP-CorV vaccines had injection site pain. Of the 8 patients who received two-dose of vaccines from different manufacturers, three had injection site pain, and one had a fever.
4. Discussion
This is the first report that inactivated COVID-19 vaccine is safe in TSC patients with epilepsy. All patients with epilepsy did not have seizure-related changes after vaccination in our study. It was reported that no evidence suggests worsening seizures after COVID-19 vaccination in adults [10]. Adverse reactions occurred in 25% of our patients. Similar to that in healthy children who received inactivated COVID-19 vaccine, the most common adverse reaction in our patients was the injection site pain. Adverse reactions after CoronaVac injection occurred in 29% of children and adolescents aged 3–17 years, and injection site pain, which was mild and moderate in severity, was the most common adverse reaction and occurred within seven days after injection [2]. The most common adverse reaction reported of BBIBP-CorV was also injection site pain, which mostly occurred in children under the age of 12 [3].
Our study showed that inactivated COVID-19 vaccine is safe for children and adolescents using rapamycin. As we all know, rapamycin has an immunosuppressive effect. SARS-CoV-2 infection occurred in an adult with TSC on mTOR inhibitor without serious adverse consequences [7]. Our patients who used rapamycin did not show increasing infection after vaccination. Recently, mTOR inhibitors have been found to have an immunomodulatory effect. Rapamycin treatment markedly enhanced the survival of CD8+T cells after infection and the recall response of CD8+T cells to secondary infection [11]. Low-dose mTOR inhibitor therapy in 264 healthy elderly people for 6 weeks not only enhanced the immune function and decreased infection rates but also up-regulated antiviral gene expression [12]. For other patients treated with mTOR inhibitors, such as autoimmune diseases, the safety of COVID-19 vaccines still needs further evaluation.
Homologous schedules are considered to be standard practice for each COVID-19 vaccine. However, the WHO supports a flexible approach to homologous versus heterologous vaccination schedules. Our eight patients who received inactivated vaccines from two manufacturers had no serious adverse reactions. About two-thirds of the population worldwide needs to be immunized to stop the spread of COVID-19. Further effort should be made to increase vaccination rates to prevent the spread of COVID-19.
5. Conclusion
Inactivated COVID-19 vaccine was well tolerated and safe in pediatric TSC patients with epilepsy, as well as for those treated with mTOR inhibitors. Mild adverse reactions occurred in 25% of the patients. The most common adverse reaction was injection site pain. All patients had no seizure-related changes after vaccination. Additional efforts should be made to increase vaccination rates to prevent the spread of COVID-19.
CRediT authorship contribution statement
Qian Lu: Visualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. Yang-Yang Wang: Data curation, Writing – review & editing. Qiu-Hong Wang: Data curation, Writing – review & editing. Li-Na Tang: Data curation, Formal analysis. Xiao-Yan Yang: Data curation, Writing – review & editing. Shuo Dun: Data curation, Writing – review & editing. Li-Ping Zou: Visualization, Formal analysis, Writing – review & editing, Data curation.
Declaration of Competing Interest
The authors declare that they have no competing interests.
Acknowledgments
Acknowledgment
We sincerely acknowledge the support of all of the participants who contributed to this study.
Funding/support
This study was funded by the National Key Research Program of China (Grant No. 2016YC1000700).
Availability of data
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
- 1.Alsohime F., Temsah M.H., Al-Nemri A.M., Somily A.M., Al-Subaie S. COVID-19 infection prevalence in pediatric population: etiology, clinical presentation, and outcome. J Infect Public Health. 2020;13:1791–1796. doi: 10.1016/j.jiph.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Han B., Song Y., Li C., Yang W., Ma Q., Jiang Z., Li M., Lian X., Jiao W., Wang L., Shu Q., Wu Z., Zhao Y., Li Q., Gao Q. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy children and adolescents: a double-blind, randomised, controlled, phase 1/2 clinical trial. Lancet Infect Dis. 2021;21:1645–1653. doi: 10.1016/S1473-3099(21)00319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xia S., Zhang Y., Wang Y., Wang H., Yang Y., Gao G.F., Tan W., Wu G., Xu M., Lou Z., Huang W., Xu W., Huang B., Wang H., Wang W., Zhang W., Li N., Xie Z., Ding L., You W., Zhao Y., Yang X., Liu Y., Wang Q., Huang L., Yang Y., Xu G., Luo B., Wang W., Liu P., Guo W., Yang X. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. 2021;21:39–51. doi: 10.1016/S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lazarus J.V., Ratzan S.C., Palayew A., Gostin L.O., Larson H.J., Rabin K., Kimball S., El-Mohandes A. A global survey of potential acceptance of a COVID-19 vaccine. Nat Med. 2021;27:225–228. doi: 10.1038/s41591-020-1124-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Henske E.P., Jóźwiak S., Kingswood J.C., Sampson J.R., Thiele E.A. Tuberous sclerosis complex. Nat Rev Dis Prim. 2016;2:16035. doi: 10.1038/nrdp.2016.35. [DOI] [PubMed] [Google Scholar]
- 6.Mullen P.J., Garcia G., Purkayastha A., Matulionis N., Schmid E.W., Momcilovic M., Sen C., Langerman J., Ramaiah A., Shackelford D.B., Damoiseaux R., French S.W., Plath K., Gomperts B.N., Arumugaswami V., Christofk H.R. SARS-CoV-2 infection rewires host cell metabolism and is potentially susceptible to mTORC1 inhibition. Nat Commun. 2021;12:1876. doi: 10.1038/s41467-021-22166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moloney P.B., Delanty N. Stick or twist: everolimus for seizures in tuberous sclerosis complex during the COVID-19 pandemic. Seizure. 2021;91:271–272. doi: 10.1016/j.seizure.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger D.A., Northrup H. Tuberous sclerosis complex surveillance and management: recommendations of the 2012 international tuberous sclerosis complex consensus conference. Pediatr Neurol. 2013;49:255–265. doi: 10.1016/j.pediatrneurol.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheffer I.E., Berkovic S., Capovilla G., Connolly M.B., French J., Guilhoto L., Hirsch E., Jain S., Mathern G.W., Moshé S.L., Nordli D.R., Perucca E., Tomson T., Wiebe S., Zhang Y.H., Zuberi S.M. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L., Zhang Q., Xiao J., Zhang Y., Peng W., Han X., Chen S., Yang D., Sander J.W., Zhou D., Xiong W. COVID-19 vaccine take-up rate and safety in adults with epilepsy: data from a multicenter study in China. Epilepsia. 2022;63:244–251. doi: 10.1111/epi.17138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollizzi K.N., Patel C.H., Sun I.H., Oh M.H., Waickman A.T., Wen J., Delgoffe G.M., Powell J.D. mTORC1 and mTORC2 selectively regulate CD8⁺ T cell differentiation. J Clin Investig. 2015;125:2090–2108. doi: 10.1172/JCI77746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mannick J.B., Morris M., Hockey H.P., Roma G., Beibel M., Kulmatycki K., Watkins M., Shavlakadze T., Zhou W., Quinn D., Glass D.J., Klickstein L.B. TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci Transl Med. 2018;10:1564. doi: 10.1126/scitranslmed.aaq1564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, upon reasonable request.