Abstract
Background
The relationship between HIV infection and COVID-19 clinical outcomes remains a significant public health research problem. We aimed to determine the association of HIV comorbidity with COVID-19 mortality.
Methods
We searched PubMed, Google Scholar and World Health Organization library databases for relevant studies. All searches were conducted from 1st to 7th December 2021. Title, abstract and full text screening was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. The relative risk of mortality in HIV-infected COVID-19 patients was computed using a random-effects model. All analyses were performed using Meta and Metasens statistical packages available in R version 4.2.1 software package. The quality of included studies was assessed using the GRADE approach, Egger’s test was employed to determine the risk of bias.
Results
A total of 16 studies were included in this review. Among the COVID-19 patients with HIV infection, the mortality rate due to COVID-19 was 7.97% (4 287/53,801), and among the COVID-19 patients without HIV infection, the mortality rate due to COVID-19 was 0.69% (127, 961/18, 513, 747). In the random effects model, we found no statistically significant relative risk of mortality in HIV-infected COVID-19 patients (RR 1.07, 95% CI 0.86–1.32). The between-studies heterogeneity was substantial (I2 = 91%, P < 0.01), while the risk of publication bias was not significant.
Conclusion
Findings did not link HIV infection with an increased risk of COVID-19 mortality. Our results add to the conflicting data on the relationship between COVID-19 and HIV infection.
Keywords: COVID-19, HIV, AIDS, Mortality, Systematic review, Meta-analysis
Introduction
Four decades into the human immunodeficiency virus (HIV) epidemic, the virus persists as a significant public health challenge [1], [2]. The Joint United Nations Programme on HIV/AIDS (UNAIDS) statistics show that an estimated 37.7 million (95% confidence interval [CI] 30.2 million – 45.1 million) people were living with HIV and AIDS globally in 2020. 28.2 million were accessing antiretroviral therapy as of 30 June 2021. One and a half million people (1.0 million – 2.0 million) became newly infected with HIV in 2020 [3]. Most people living with HIV (PLHIV) are from developing countries, with sub-Saharan Africa contributing the most significant burden. Despite advances in the knowledge of HIV prevention and treatment, significant numbers of incident HIV infections and AIDS-related deaths continue to happen globally [4], with an estimated 680,000 AIDS-related deaths in 2020 alone [5]. AIDS is defined as the laboratory diagnosis of HIV infection plus either an opportunistic infection or a CD4 count of below 200/μL [1].
In March 2020, the World Health Organization (WHO) declared a severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) outbreak, with origins in China, a pandemic, and named coronavirus disease 2019 (COVID-19). Two years later, the COVID-19 pandemic remains a significant global health concern. WHO weekly epidemiological updates show that, as of 2 January 2022, the international cumulative number of cases at 288,867,634 cases, with 9,520,488 incident cases and 41,178 new deaths in the preceding seven days [6]. Despite a wide array of prevention strategies globally, including vaccination rollout, which is now at an advanced stage in many countries, the spread of the SARS-CoV-2 virus continues inexorably, making it one of the most significant global health threats of the 21st century.
The SARS-CoV-2 infection has been shown to have more adverse outcomes in people living with comorbidities. Hypertension, diabetes mellitus, chronic kidney disease, obesity, and malignancies are some of the comorbidities proven by scientific evidence to be associated with worse outcomes among COVID-19 patients than the general population [7], [8], [9], [10]. Given that PLHIV usually do experience adverse consequences when they are co-infected with another pathogen or have other comorbidities, there has been a general interest in the outcomes of COVID-19 among PLHIV [11], [12], [13]. These constitute a diverse group, including those not on highly active antiretroviral therapy (HAART), those newly on HAART, and those on HAART with virological suppression. Yet others are now on second line, third line or salvage HAART regimens after treatment failure on previous regimens. It has been hypothesised that those with complete virological suppression would not have outcomes significantly different from those of the general population, whilst those not yet on HAART or not yet virologically suppressed, or with other coexisting opportunistic infections or morbidities, would experience more adverse outcomes from COVID-19. In the context of HIV, viral suppression is defined as having less than 200 copies of HIV per millilitre of blood.
Others have theorised that because of HAART, and as a result of SARS-CoV-2 being a virus, individuals already on treatment would not be severely affected by COVID-19. As a matter of fact, at the onset of the COVID-19 pandemic, lopinavir with ritonavir, which is a part of some HAART regimens, was used to treat COVID-19 in some countries [14]. Subsequent clinical evidence refuted its effectiveness [15], [16]. In a systematic review, the pooled effectiveness of the repurposed drug for treatment of COVID-19 was noted to be insignificant for virological cure (RR 1.06, 95% CI 0.85–1.31), radiological improvement (RR 0.81 95% CI 0.62–1.05) and adverse events (RR 2.96 95% CI 0.17–38.90) [17]. Hence its use for treatment of COVID-19 is no longer advised. On the other hand, it has been argued that HIV is immunosuppressive and would therefore worsen health outcomes from COVID-19. Patients on immunosuppressive therapies such as for cancer chemotherapy and immunosuppressant drugs were also expected to suffer adverse outcomes and have higher mortality from COVID-19, but this has not been substantiated by recent evidence [18], [19]. Despite virological suppression, people on long-term HAART are at significant risk of other comorbidities, including cardiovascular, hepatic and renal disease, malignancies, and some related to the antiretroviral drugs themselves. These include chronic kidney disease in those on tenofovir-containing regimens, and anaemia on zidovudine-containing regimens [11], [20].
Several studies have now been published on the association between HIV infection and COVID-19 [21], [22], [23], [24], [25], [26]. Ssentongo et al. found that HIV-positive persons had a significantly higher risk of SARS-CoV-2 infection [risk ratio (RR) 1.24, 95% CI (1.05–1.46)] and mortality from COVID-19 (RR 1.78, 95% CI 1.21–2.60) than HIV-negative individuals [22]. The pooled overall results in a review conducted by Dong et al. suggested that PLHIV had a higher risk of mortality from COVID-19 than those without HIV infection (odds ratio [OR] = 1.252, 95% CI 1.027–1.524) [25]. Both reviews were limited in publication bias among included studies due to the preponderance of publications on hospitalised patients with more severe disease. Contrasting evidence by Ahmad et al. that showed that PLHIV are not at greater risk than the general population [24]. Lee et al. also demonstrated PLHIV were not found to be at higher risk for adverse outcomes of COVID-19 [26]. Given the conflicting evidence on the association between HIV comorbidity and COVID-19 mortality, the questions on this association remain unanswered. To answer the ongoing questions, we undertook a systematic review and meta analysis to determine the association of HIV/AIDS comorbidity with COVID-19 mortality.
Methodology
Study design
This systematic review is registered in PROSPERO (registration number: CRD42022308959). This systematic review was conducted and reported following the reporting guideline provided in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P) statement [27]. The Centre for Reviews and Dissemination (CRD) guidance for undertaking systematic reviews in healthcare [28], and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines for design and implementation [29].
Study selection
Search strategy
The search strategy used medical subject heading (MeSH) and text word searches (Supplementary File 1). Two reviewers (IC and MM) searched PubMed, Google Scholar and World Health Organization library databases for relevant studies. To estimate the risk of pre-existing HIV/AIDS comorbidity on coronavirus disease 2019 (COVID-19) mortality. The [PEO] framework informed the development of the search strategy to ensure that the boundaries of the research question were clearly defined:
Search # 1 – Participants (population): We included studies reporting an association between HIV and mortality due to COVID-19, regardless of age or geographical location.
Search # 2 – Exposure: HIV comorbidity was defined as a patient with confirmed HIV infection prior to a positive COVID-19 test.
Search # 3 – Condition or outcome(s) of interest: The primary outcome was the mortality in patients with COVID-19 with HIV/AIDS comorbidity compared to patients with COVID-19 without HIV comorbidity.
All searches were conducted between 1st and 7th December 2021.
Inclusion and exclusion criteria
Inclusion and exclusion criteria were developed a priori in an iterative process after preliminary searches during study protocol development. Briefly, studies were eligible if they were (i) reporting factors associated with COVID—19 mortality (ii) reporting COVID-19 mortality among individuals with HIV/AIDS comorbidity with risk estimates (RR or OR) (iii) original, quantitative study design published in English.
Selecting studies
The study selection was a multi-step process that involved two reviewers. Firstly, IC and MM searched for eligible titles by analysing the titles of articles found through the search strategies’ used in the above mentioned electronic bibliographic databases guided by the study eligibility criteria. Articles with relevant titles were then exported into Convidence (https://www.covidence.org/). Convidence is an online tool for managing systematic reviews. The abstracts of all identified articles were assessed by two independent reviewers (IC and MM). The abstracts that did not meet the study eligibility criteria were excluded. Differences between reviewers at this stage were settled through reaching a consensus among reviewers. Following the abstract screening stage, full-text articles were retrieved where studies met the inclusion criteria or where there was ambiguity to be screened in greater depth by reference to the full-text assessment for eligibility. Thus, further establishing the retrieved studies’ eligibility. This stage was conducted like the abstract screening stage by two independent reviewers (PCI and NT). Studies that did not meet the inclusion criteria were excluded. A secondary search of the reference list of all the included studies was done after the full-text screening; the two reviewers carried it out for any articles that could not have been detected during the database search. At this stage, discrepancies in study selection were resolved by engaging a third reviewer (TD).
Data extraction
After the two independent reviewers’ full-text screening stage, the data extraction was performed on the included articles by two independent reviewers (NT and TD). The reviewers extracted data from the included studies separately and in-duplicate to detect inter-rater errors and reduce data errors and bias. A piloted data abstraction form was employed to extract relevant information from included studies. The tool collected information on the author, country, study aim, study design, sample size, proportion of HIV positive and HIV negative, number of cases (death) and non cases and the quality score of the study. The final data abstraction tool is available on Supplementary File 1.
Assessment of study quality and risk of bias
The GRADE approach was employed to rate the quality of the body of evidence for included studies [30]. The five GRADE considerations (bias risk of the trials, consistency of effect, imprecision, indirectness, and publication bias) we employed to assess the quality of the body of evidence. Studies were graded as very low, low, moderate and high quality. These grades corresponded to the level of confidence that the true estimate lied in the presented values. For instance, very low study quality meant very little confidence in the estimate: the true estimate is likely to be substantially different from the estimate presented in the study. High quality meant the reviewers were very confident that the true prevalence/incidence lies close to that of the estimate.T wo reviewers assessed methodological quality (MM and IC). Discrepancies were resolved by a third reviewer (TD).
The I2 statistics for statistical significance of heterogeneity and its CI (i.e., the percentage of variance not due to studies-wide sampling error) was used to calculate the magnitude of the heterogeneity between studies included in all meta-analyses. The following I2 cut-offs for low, moderate and high heterogeneity will be used to report the degree of heterogeneity, as per the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions [31]: 1—between 0% and 40% = might not be important; 2–30–60% = may represent moderate heterogeneity; 3–50–90% = may represent substantial heterogeneity; and 4–75–100% = considerable heterogeneity.
Data synthesis and statistical analysis
The primary outcome of interest was the association between HIV comorbidity and COVID-19 mortality. The Q and I2 were calculated in to assess heterogeneity. Meta-biases were evaluated using funnel plots to detect potential reporting biases and small-study effects [31] and complemented with the Egger regression test [32]. All analyses were performed using Meta and Metasens statistical packages available in R version 4.2.1 software package using the command functions described by Balduzzi et al. [33].
Results
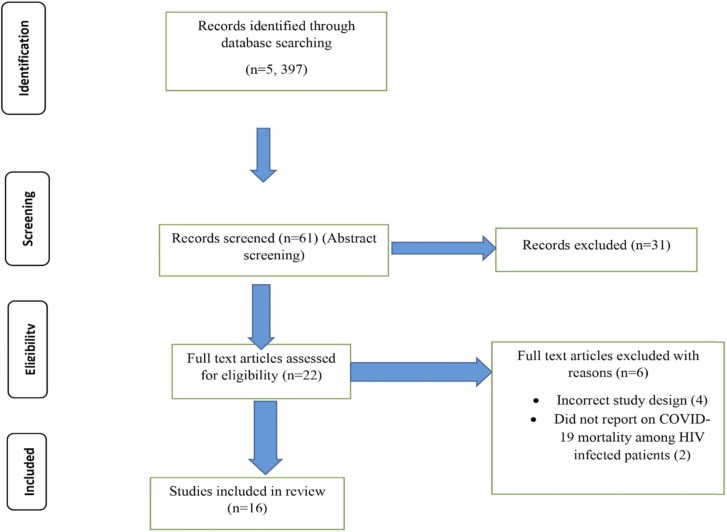
Our initial search yielded 5397 articles. Following title screening, 74 articles were eligible for inclusion in abstract screening. These articles were imported into Convidence and 13 duplicates removed, leaving 61 articles included in the abstract screening. Of these, 39 articles were excluded, leaving 22 for full-text screening. Of the 22 full-text articles screened [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], six [50], [51], [52], [53], [54], [55] were excluded, leaving 16 included in this review. More details are presented on Fig. 1 (PRISMA Flow Diagram). The majority (10) were conducted in the United States of America [38], [39], [41], [42], [43], [44], [45], [47], [49], two from the United Kingdom [34], [37], two from South Africa [36], [40], one from Spain [46] and one from China [48]. 13 studies were graded to be of high quality and three studies [44], [46], [47] were graded as moderate quality. More details are presented in Supplementary File 2.
Fig. 1.
PRISMA flow diagram.
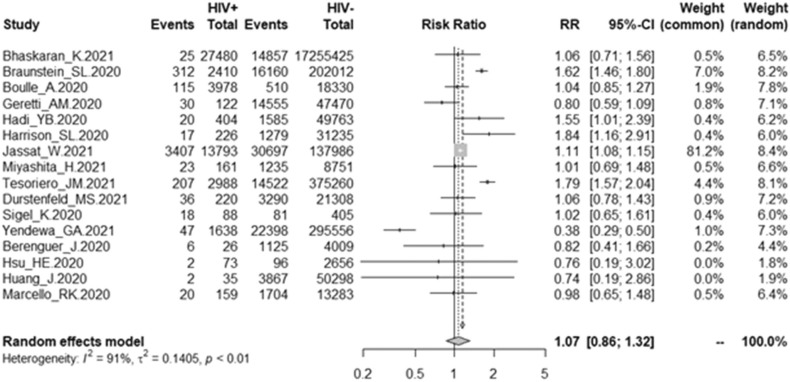
Among the COVID-19 patients with HIV infection, the mortality rate due to COVID-19 was 7.97% (4 287/53,801), and among the COVID-19 patients without HIV infection, the mortality rate due to COVID-19 was 0.69% (127, 961/18, 513, 747). In this study, in the random effects model, the pooled overall effect size (OR) was 1.07 (95% CI 0.86 – 1.32). More details are presented in Fig. 2. Among the 16 studies, substantial heterogeneity was found (I2 = 91%, P < 0.01).
Fig. 2.
Forest plot of association of HIV coinfection and mortality due to COVID-19.
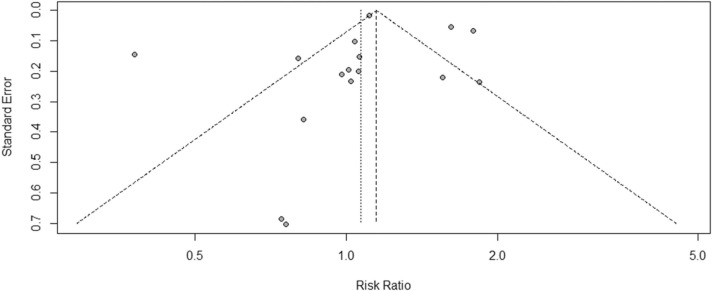
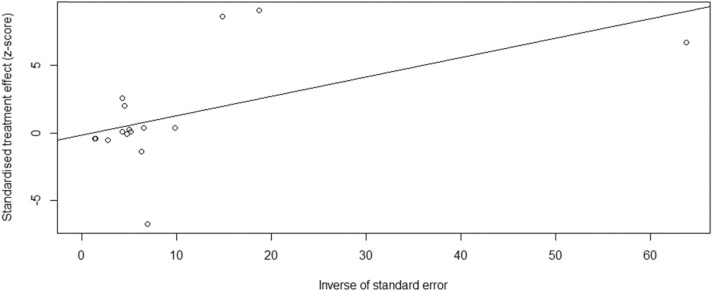
Although the funnel plot analysis appeared asymmetrical ( Fig. 3), the Egger test revealed no publication bias for the publications (t = −0.15, P = 0.88). Fig. 4 shows a linear regression graph to visualise the data presented in Fig. 3. An Egger’s regression test p-value of> 0.1 implied no statistically significant small-study effects.
Fig. 3.
Funnel plot of the included studies in this meta-analysis.
Fig. 4.
Presentation of findings for assessing and accounting for small-study effects.
In the sub group analysis, the pooled overall effect size (OR) show no significant association for studies conducted in the United States OR 1.13 (CI 0.82–1.55) and Europe OR 0.88 (95% CI 0.70–1.11). There was a significant association for studies conducted in South Africa OR 1.11 (95% CI 1.08–1.14). More details are presented in Supplementary File 3.
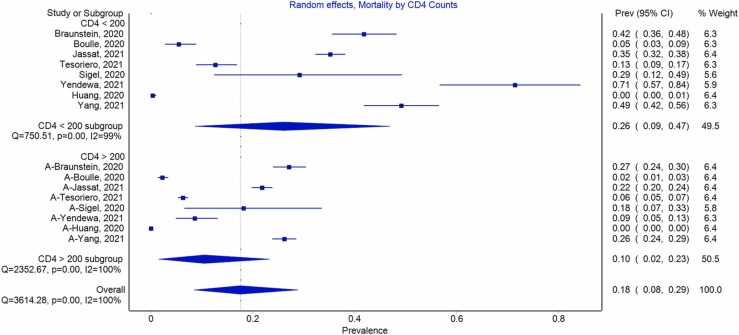
Additional sub group analysis was conducted to determine the mortality rate due to COVID-19 among HIV positive patients with CD4 < 200 and those with CD4 > 200. Among the COVID-19 patients with HIV infection and CD4 < 200, the mortality rate due to COVID-19 was 26% (CI 9–47), and among the COVID-19 patients with HIV infection and CD4 > 200, the mortality rate due to COVID-19 was 18% (CI 8–29). More details are presented in Fig. 5.
Fig. 5.
Mortality rate due to COVID-19 among HIV positive patients with CD4 < 200 and CD4 > 200.
Discussion
HIV infection has remained a significant global health challenge [56]. Due to the high numbers of PLHIV, HIV and SARS-CoV-2 co- infection is likely to have to occur with increased frequency globally. Previous experiences have shown that co-infection of PLHIV with other pathogens such as hepatitis C virus [57], [58] and mycobacterium tuberculosis suffer adverse outcomes including higher risk of mortality [59]. We aimed to assess the association between HIV and SARS-CoV-2 co- infection with mortality. Our findings revealed no statistically significant COVID-19 mortality between HIV positive and HIV negative individuals. However, when sub grouped by disease severity, a higher mortality rate was reported in HIV infected individuals with CD4 < 200 compared to their CD4 > 200 counterparts.
In this systematic review with meta-analysis of the association between HIV and SARS-CoV-2, we retrieved fewer studies from SSA where the problem is most significant, making the lack of literature the greatest caveat of this review. The study is dominated by studies conducted in US, with only two studies conducted in SSA. These two regions have very different HIV epidemics that might influence the outcomes of this study. Clinicians and public health specialists from the resource-limited settings of Africa must prioritise the use of the data available to them to carry out analyses, including retrospective analyses, to derive important evidence beneficial to local populations. Nevertheless, we found no statistically significant relative risk of mortality in HIV-infected COVID-19 patients in the random-effects model (RR 1.07, 95% CI 0.86–1.32).
The findings from this study are contrary to widespread expectations, where it is believed that PLHIV is at greater risk of mortality from COVID-19 than the general population [25], [60]. Notably, the term PLHIV encompasses a very heterogeneous group of people ranging from the newly diagnosed to those who have been on antiretroviral therapy for prolonged periods, since the widespread availability of HAART almost two decades ago. From the subgroup analysis, higher rates of COVID-19 mortality were reported among HIV infected individuals with a CD4 < 200 compared to their CD4 > 200 counterparts. This aligned with earlier reviews that revealed that PLHIV with well-controlled disease are not at risk of poorer COVID-19 disease outcomes than the general population [23], [24]. More informative analyses would stratify patients according to characteristics such as being on HAART or not, length of time on HAART, HAART regimens, virological suppression and presence or absence of other comorbidities. The data available for this meta-analysis did not permit for this level of granularity.
In a subgroup analysis, there were no statistically significant associations between being HIV positive and mortality from COVID-19 in Europe and America, whereas a statistically significant association was noted in studies in South Africa, where PLHIV were 11% more likely to die from COVID-19 (RR 1.11, 95% CI 1.08–1.14). Whilst health care systems in Europe and America are advanced, South Africa is among the most advanced in terms of healthcare in SSA, being a middle-income country. Therefore, these differences cannot be completely attributable to availability of quality care. Other explanatory variable such as health-seeking behaviours, adherence to anti-retroviral treatment and degrees of virological suppression would be worth exploring. Additionally, this review did not take into account the participants’ COVID-19 vaccination status. It is known that COVID-19 vaccines are protective from death, severe morbidity and hospitalisation [61], and countries in Europe and America are now at advanced stages with their COVID-19 vaccination programmes, whereas South Africa still lags behind significantly, with less than 40% of its population fully vaccinated. Increasing vaccine availability, fighting vaccine hesitancy and significantly improving vaccine uptake among PLHIV is as important as it in the general population. Consequently, clear policies regarding COVID-19 vaccination in HIV-infected people are important. At the time of introduction of the COVID-19 vaccines, there was confusion in many places regarding vaccination for PLHIV, and circulating falsehoods advising against such vaccination did not make this any better. Though unsubstantiated, the circulating falsehoods and lack of clear policy couples with pre-existing fear, religious objections and other drivers of vaccine hesitancy in Africa could have resulted in lower uptake of vaccines among PLHIV in sub-Saharan Africa, including in South Africa where vaccine hesitancy is widespread [62].
The included studies that reported COVID-19 related mortality among PLHIV were observational this is a limitation. Several confounders and effect modifiers such as age, gender, comorbidities, obesity, smoking, country of study, patient’s socioeconomic indices of multiple deprivation and access to care were not accounted for in these studies, which might have affected study findings. Geographic and economic variations could have also affected the results significantly. Of particular relevance would be that low-income countries of SSA, which bear the brunt of HIV disease, were underrepresented in this systematic review, with overrepresentation of the USA, where the level and access to healthcare is very different from SSA. In SSA, where the public health sectors are fragile, presentation to care is late, and treatment is suboptimal, mortality from COVID-19, including HIV positive cohorts, could be higher [50], [63]. This highlights that there is a great need for studies from the SSA region to critical comprehensively inform this important critical topic. Finally, substantial heterogeneity was observed in the included studies.
Conclusion
The results of this systematic review indicate that there is no significant difference in COVID-19 mortality rate between PLHIV and the general population. Our results indicate that PLHIV have the same risk of COVID-19 mortality as the general population. This could be attributed to a wide coverage of ART and community viral load suppression, that help to reduce risk of immunosuppression among PLHIV. However, the majority of the research studies included in this review were in high-income countries and limited studies on sub-Sahara Africa, where the HIV scourge is most marked. Our findings add to the conflicting data on the relationship between COVID-19 and HIV infection, and further research, particularly in high HIV burden settings like SSA, is warranted.
Ethics approval and consent to participate
Not applicable.
Funding
This research received no external funding.
CRediT authorship contribution statement
Tafadzwa Dzinamarira: Conceptualization, Methodology, Validation, Writing – original draft. Grant Murewanhema: Writing – original draft. Itai Chitungo: Investigation. Bernard Ngara: Methodology, Software, Formal analysis. Sphamandla Josias Nkambule: Methodology. Roda Madziva: Writing – review & editing. Helena Herrera: Writing – review & editing. Solomon Mukwenha: Writing – review & editing. Diego F. Cuadros: Writing – review & editing. Patrick Gad Iradukunda: Investigation. Moreblessing Mashora: Investigation. Nigel Tungwarara: Investigation. Gallican Nshogoza Rwibasira: Writing – review & editing. Godfrey Musuka: Conceptualization.
Competing interests
The authors declare no competing interests.
Acknowledgements
Not applicable.
Prospero registration number
CRD42022308959.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jiph.2022.05.006.
Appendix A. Supplimentary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Dzinamarira T., Kamanzi C., Mashamba-Thompson T.P. Key stakeholders’ perspectives on implementation and scale up of HIV self-testing in Rwanda. Diagnostics. 2020;10(4):194. doi: 10.3390/diagnostics10040194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iradukunda P.G., Pierre G., Muhozi V., Denhere K., Dzinamarira T. Knowledge, attitude, and practice towards COVID-19 among people living with HIV/AIDS in Kigali, Rwanda. J Community Health. 2021;46(2):245–250. doi: 10.1007/s10900-020-00938-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.UNAIDS. AIDSInfo: global data on HIV epidemiology and response; 2020. Available from: 〈http://aidsinfo.unaids.org/〉. [Accessed 2 January 2022].
- 4.Rwibasira G.N., Malamba S.S., Musengimana G., Nkunda R.C., Omolo J., Remera E., et al. Recent infections among individuals with a new HIV diagnosis in Rwanda, 2018–2020. Plos One. 2021;16(11) doi: 10.1371/journal.pone.0259708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNAIDS. Global AIDS update 2021. Available from: 〈https://www.unaids.org/sites/default/files/media_asset/2021-global-aids-update_en.pdf〉. [Accessed 2 January 2022].
- 6.WHO. Coronavirus (COVID-19) dashboard; 2022. Available from: 〈https://covid19.who.int/〉 [Accessed 7 January 2022].
- 7.Osibogun A., Balogun M., Abayomi A., Idris J., Kuyinu Y., Odukoya O., et al. Outcomes of COVID-19 patients with comorbidities in southwest Nigeria. PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanyaolu A., Okorie C., Marinkovic A., Patidar R., Younis K., Desai P., et al. Comorbidity and its impact on patients with COVID-19. SN Compr Clin Med. 2020:1–8. doi: 10.1007/s42399-020-00363-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaikh F.S., Aldhafferi N., Buker A., Alqahtani A., Dey S., Abdulhamid S., et al. Comorbidities and risk factors for severe outcomes in COVID-19 patients in Saudi Arabia: a retrospective cohort study. J Multidiscip Healthc. 2021;14:2169. doi: 10.2147/JMDH.S317884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alali AS, Alshehri AO, Assiri A, Khan S, Alkathiri MA, Almohammed OA, et al. Demographics, comorbidities, and outcomes among young and middle-aged COVID-19 patients in Saudi Arabia. Saudi Pharm J; 2021. [DOI] [PMC free article] [PubMed]
- 11.Lorenc A., Ananthavarathan P., Lorigan J., Banarsee R., Jowata M., Brook G. The prevalence of comorbidities among people living with HIV in Brent: a diverse London Borough. Lond J Prim Care. 2014;6(4):84–90. doi: 10.1080/17571472.2014.11493422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachdev D., Mara E., Hsu L., Scheer S., Rutherford G., Enanoria W., et al. COVID-19 susceptibility and outcomes among people living with HIV in San Francisco. J Acquir Immune Defic Syndr. 2021;86(1):19. doi: 10.1097/QAI.0000000000002531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shalev N., Scherer M., LaSota E.D., Antoniou P., Yin M.T., Zucker J., et al. Clinical characteristics and outcomes in people living with HIV hospitalized for COVID-19. Clin Infect Dis Publ Infect Dis Soc Am. 2020 doi: 10.1093/cid/ciaa635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meini S., Pagotto A., Longo B., Vendramin I., Pecori D., Tascini C. Role of Lopinavir/Ritonavir in the treatment of Covid-19: a review of current evidence, guideline recommendations, and perspectives. J Clin Med. 2020;9(7):2050. doi: 10.3390/jcm9072050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doggrell S.A. Does lopinavir measure up in the treatment of COVID-19? Expert Opin Investig Drugs. 2020;29(8):793–796. doi: 10.1080/13543784.2020.1777277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. New Engl J Med; 2020. [DOI] [PMC free article] [PubMed]
- 17.Patel T.K., Patel P.B., Barvaliya M., Saurabh M.K., Bhalla H.L., Khosla P.P. Efficacy and safety of Lopinavir-Ritonavir in COVID-19: a systematic review of randomized controlled trials. J Infect Public Health. 2021 doi: 10.1016/j.jiph.2021.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akama-Garren E.H., Li J.X. Prior immunosuppressive therapy is associated with mortality in COVID-19 patients: a retrospective study of 835 patients. J Med Virol. 2021;93(10):5768–5776. doi: 10.1002/jmv.27105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andersen KM, Bates BA, Rashidi ES, Olex AL, Mannon RB, Patel RC, et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol; 2021. [DOI] [PMC free article] [PubMed]
- 20.Pourcher V., Gourmelen J., Bureau I., Bouee S. Comorbidities in people living with HIV: an epidemiologic and economic analysis using a claims database in France. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirzaei H., McFarland W., Karamouzian M., Sharifi H. COVID-19 among people living with HIV: a systematic review. AIDS Behav. 2021;25(1):85–92. doi: 10.1007/s10461-020-02983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ssentongo P., Heilbrunn E.S., Ssentongo A.E., Advani S., Chinchilli V.M., Nunez J.J., et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep. 2021;11(1):1–12. doi: 10.1038/s41598-021-85359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cooper T.J., Woodward B., Alom S., Harky A. Coronavirus disease 2019 (COVID‐19) outcomes in HIV/AIDS patients: a systematic review. HIV Med. 2020;21(9):567–577. doi: 10.1111/hiv.12911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.SeyedAlinaghi S., Karimi A., MohsseniPour M., Barzegary A., Mirghaderi S.P., Fakhfouri A., et al. The clinical outcomes of COVID‐19 in HIV‐positive patients: a systematic review of current evidence. Immun Inflamm Dis. 2021;9(4):1160–1185. doi: 10.1002/iid3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Y., Li Z., Ding S., Liu S., Tang Z., Jia L., et al. HIV infection and risk of COVID-19 mortality: a meta-analysis. Medicine. 2021;100(26) doi: 10.1097/MD.0000000000026573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee K.W., Yap S.F., Ngeow Y.F., Lye M.S. COVID-19 in people living with HIV: a systematic review and meta-analysis. Int J Environ Res Public Health. 2021;18(7) doi: 10.3390/ijerph18073554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shamseer L., Moher D., Clarke M., Ghersi D., Liberati A., Petticrew M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015:349. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 28.Akers J, Aguiar-Ibáñez R, Baba-Akbari A. Systematic reviews: CRD's guidance for undertaking reviews in health care; 2009.
- 29.Stroup D.F., Berlin J.A., Morton S.C., Olkin I., Williamson G.D., Rennie D., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 30.Iorio A., Spencer F.A., Falavigna M., Alba C., Lang E., Burnand B., et al. Use of GRADE for assessment of evidence about prognosis: rating confidence in estimates of event rates in broad categories of patients. BMJ. 2015:350. doi: 10.1136/bmj.h870. [DOI] [PubMed] [Google Scholar]
- 31.Higgins J.P., Deeks J.J. Cochrane handbook for systematic reviews of interventions: cochrane book series. 2008. Selecting studies and collecting data; pp. 151–185. [Google Scholar]
- 32.Sterne J.A., Sutton A.J., Ioannidis J.P., Terrin N., Jones D.R., Lau J., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011:343. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 33.Balduzzi S., Rücker G., Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22(4):153–160. doi: 10.1136/ebmental-2019-300117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhaskaran K., Rentsch C.T., MacKenna B., Schultze A., Mehrkar A., Bates C.J., et al. HIV infection and COVID-19 death: a population-based cohort analysis of UK primary care data and linked national death registrations within the OpenSAFELY platform. Lancet HIV. 2021;8(1):e24–e32. doi: 10.1016/S2352-3018(20)30305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braunstein S.L., Lazar R., Wahnich A., Daskalakis D.C., Blackstock O.J. Coronavirus disease 2019 (COVID-19) infection among people with human immunodeficiency virus in New York City: a population-level analysis of linked surveillance data. Clin Infect Dis. 2021;72(12):e1021–e1029. doi: 10.1093/cid/ciaa1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Risk Factors for Coronavirus Disease 2019 (COVID-19) Death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis. 2021;73(7):e2005–e2015. doi: 10.1093/cid/ciaa1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geretti A.M., Stockdale A.J., Kelly S.H., Cevik M., Collins S., Waters L., et al. Outcomes of coronavirus disease 2019 (COVID-19) related hospitalization among people with human immunodeficiency virus (HIV) in the ISARIC World Health Organization (WHO) clinical characterization protocol (UK): a prospective observational study. Clin Infect Dis. 2021;73(7):e2095–e2106. doi: 10.1093/cid/ciaa1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hadi Y.B., Naqvi S.F.Z., Kupec J.T., Sarwari A.R. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS. 2020;34(13):F3–F8. doi: 10.1097/QAD.0000000000002666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harrison S.L., Fazio-Eynullayeva E., Lane D.A., Underhill P., Lip G.Y.H. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9) doi: 10.1371/journal.pmed.1003321. e1003321-e1003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jassat W., Cohen C., Tempia S., Masha M., Goldstein S., Kufa T., et al. Risk factors for COVID-19-related in-hospital mortality in a high HIV and tuberculosis prevalence setting in South Africa: a cohort study. Lancet HIV. 2021;8(9):e554–e567. doi: 10.1016/S2352-3018(21)00151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miyashita H., Kuno T. Prognosis of coronavirus disease 2019 (COVID-19) in patients with HIV infection in New York City. HIV Med. 2021;22(1):e1–e2. doi: 10.1111/hiv.12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tesoriero J.M., Swain C.-A.E., Pierce J.L., Zamboni L., Wu M., Holtgrave D.R., et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open. 2021;4(2) doi: 10.1001/jamanetworkopen.2020.37069. e2037069-e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Durstenfeld MS, Sun K, Ma Y, Rodriguez F, Secemsky EA, Parikh RV, et al. Impact of HIV Infection on COVID-19 outcomes among hospitalized adults in the U.S. medRxiv: the preprint server for health sciences; 2021. 21254938.
- 44.Sigel K., Swartz T., Golden E., Paranjpe I., Somani S., Richter F., et al. Coronavirus 2019 and people living with human immunodeficiency virus: outcomes for hospitalized patients in New York City. Clin Infect Dis. 2020;71(11):2933–2938. doi: 10.1093/cid/ciaa880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yendewa G.A., Perez J.A., Schlick K., Tribout H., McComsey G.A. Clinical features and outcomes of coronavirus disease 2019 among people with human immunodeficiency virus in the United States: a multicenter study from a large global health research network (TriNetX) Open Forum Infect Dis. 2021;8(7) doi: 10.1093/ofid/ofab272. ofab272-ofab272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berenguer J., Ryan P., Rodríguez-Baño J., Jarrín I., Carratalà J., Pachón J., et al. Characteristics and predictors of death among 4035 consecutively hospitalized patients with COVID-19 in Spain. Clin Microbiol Infect. 2020;26(11):1525–1536. doi: 10.1016/j.cmi.2020.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu H.E., Ashe E.M., Silverstein M., Hofman M., Lange S.J., Razzaghi H., et al. Race/ethnicity, underlying medical conditions, homelessness, and hospitalization status of adult patients with COVID-19 at an Urban Safety-Net Medical Center - Boston, Massachusetts, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(27):864–869. doi: 10.15585/mmwr.mm6927a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang J., Xie N., Hu X., Yan H., Ding J., Liu P., et al. Epidemiological, virological and serological features of coronavirus disease 2019 (COVID-19) cases in people living with human immunodeficiency virus in Wuhan: a population-based cohort study. Clin Infect Dis. 2021;73(7):e2086–e2094. doi: 10.1093/cid/ciaa1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalyanaraman Marcello R., Dolle J., Grami S., Adule R., Li Z., Tatem K., et al. Characteristics and outcomes of COVID-19 patients in New York City’s public hospital system. PLoS One. 2020;15(12) doi: 10.1371/journal.pone.0243027. e0243027-e0243027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Madhi S.A., Nel J. Epidemiology of severe COVID-19 from South Africa. Lancet HIV. 2021;8(9):e524–e526. doi: 10.1016/S2352-3018(21)00183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El-Solh A.A., Meduri U.G., Lawson Y., Carter M., Mergenhagen K.A. Clinical course and outcome of COVID-19 acute respiratory distress syndrome: data from a National Repository. J Intensive Care Med. 2021;36(6):664–672. doi: 10.1177/0885066621994476. [DOI] [PubMed] [Google Scholar]
- 52.Kabarriti R., Brodin N.P., Maron M.I., Guha C., Kalnicki S., Garg M.K., et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an Urban Medical Center in New York. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19795. e2019795-e2019795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ho H.-E., Peluso M.J., Margus C., Matias Lopes J.P., He C., Gaisa M.M., et al. Clinical outcomes and immunologic characteristics of coronavirus disease 2019 in people with human immunodeficiency virus. J Infect Dis. 2021;223(3):403–408. doi: 10.1093/infdis/jiaa380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gudipati S., Brar I., Murray S., McKinnon J.E., Yared N., Markowitz N. Descriptive analysis of patients living with HIV affected by COVID-19. J Acquir Immune Defic Syndr. 2020;85(2):123–126. doi: 10.1097/QAI.0000000000002450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Meyerowitz E.A., Kim A.Y., Ard K.L., Basgoz N., Chu J.T., Hurtado R.M., et al. Disproportionate burden of coronavirus disease 2019 among racial minorities and those in congregate settings among a large cohort of people with HIV. AIDS. 2020;34(12):1781. doi: 10.1097/QAD.0000000000002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dzinamarira T., Muvunyi C.M., Kamanzi C., Mashamba-Thompson T.P. HIV self-testing in Rwanda: awareness and acceptability among male clinic attendees in Kigali, Rwanda: a cross-sectional survey. Heliyon. 2020;6(3) doi: 10.1016/j.heliyon.2020.e03515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klein M.B., Rockstroh J.K., Wittkop L. Effect of coinfection with hepatitis C virus on survival of individuals with HIV-1 infection. Curr Opin HIV AIDS. 2016;11(5):521–526. doi: 10.1097/COH.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 58.Mayor A.M., Gomez M.A., Fernandez D.M., Rios-Olivares E., Thomas J.C., Hunter R.F. Morbidity and mortality profile of human immunodeficiency virus-infected patients with and without hepatitis C co-infection. Am J Trop Med Hyg. 2006;74(2):239–245. [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson K.B., Guest J.L., Rimland D. Hepatitis C virus coinfection increases mortality in HIV-infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA Cohort Study. Clin Infect Dis. 2004;39(10):1507–1513. doi: 10.1086/425360. [DOI] [PubMed] [Google Scholar]
- 60.Hariyanto T.I., Rosalind J., Christian K., Kurniawan A. Human immunodeficiency virus and mortality from coronavirus disease 2019: a systematic review and meta-analysis. S Afr J HIV Med. 2021;22(1):1220. doi: 10.4102/sajhivmed.v22i1.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matrajt L., Eaton J., Leung T., Brown E.R. Vaccine optimization for COVID-19: who to vaccinate first? Sci Adv. 2021;7(6):eabf1374. doi: 10.1126/sciadv.abf1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dzinamarira T., Nachipo B., Phiri B., Musuka G. COVID-19 vaccine roll-out in South Africa and Zimbabwe: urgent need to address community preparedness, fears and hesitancy. Vaccines. 2021;9(3):250. doi: 10.3390/vaccines9030250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dzinamarira T, Mukwenha S, Mukandavire Z, Cuadros DF, Murewanhema G, Madziva R, et al. Insights from Zimbabwe's SARS-CoV-2 genomic surveillance. Lancet Glob Health; 2021. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material