Abstract
Chloroform-soluble material was extracted from two strains of L. pneumophila serogroup 1 following growth in continuous culture. The purified material was identified as poly-3-hydroxybutyrate (PHB) by nuclear magnetic resonance spectroscopy and by gas chromatography-mass spectrometry. PHB yields of up to 16% of cell dry weight were extracted from culture samples. The PHB was located in electron-dense intracellular inclusions, which fluoresced bright yellow when stained with the lipophilic dye Nile red. A Nile red spectrofluorometric assay provided a more accurate and reliable determination of the PHB content. PHB accumulation increased threefold during iron-limited culture and was inversely related to the concentration of iron metabolized. Chemostat-grown cells survived in a culturable state for at least 600 days when incubated at 24°C in a low-nutrient tap water environment. Nile red spectrofluorometry and flow cytometry demonstrated that PHB reserves were utilized during starvation. PHB utilization, as revealed by the decline in mean cellular fluorescence and cell complexity, correlated with loss of culturability. Fluorescence microscopy provided visual evidence of PHB utilization, with a marked reduction in the number of Nile red-stained granules during starvation. Heat shock treatment failed to resuscitate nonculturable cells. This study demonstrates that L. pneumophila accumulates significant intracellular reserves of PHB, which promote its long-term survival under conditions of starvation.
Legionella species are natural freshwater inhabitants and readily colonize artificial aquatic environments, such as cooling towers and potable-water systems (9, 18, 22, 34). The environmental persistence of legionellae is aided by their ability to adapt to a variety of different ecological niches, either as intracellular parasites of amebae, as free-living members of complex biofilm communities, or as planktonic cells (19, 40, 47, 49).
Aquatic amebae play a central role in Legionella ecology by supporting intracellular multiplication and providing protection under suboptimal growth conditions (4, 6, 29, 30). Outside the amebal host, legionellae encounter stressful environmental conditions, such as limited nutrient availability (27, 28). In vitro studies have demonstrated that Legionella pneumophila can adapt to starvation conditions and survive in a culturable state for prolonged periods without growth (42, 52). Intra-amebal growth is believed to promote this extracellular survival by inducing a stress-resistant phenotype, characterized by altered morphology and envelope composition and increased resistance to antimicrobial agents (5, 7, 8).
Intracellular energy reserves, such as poly-3-hydroxybutyrate (PHB), may also promote environmental persistence. PHB is a homopolymer of 3-hydroxybutyric acid, which some bacteria accumulate during unbalanced growth to provide an endogenous source of carbon and energy (13, 45). Indirect evidence for the occurrence of PHB in L. pneumophila has been provided by Fourier transform infrared spectroscopy of whole cells (25) and by pyrolysis mass spectrometry (MS) (51). We have demonstrated the ability of Legionella species to metabolize 3-hydroxybutyric acid and provided preliminary chemical evidence for the presence of PHB in chemostat cultures of L. pneumophila (31, 32). However, the material was not rigorously characterized and its physiological significance has not been investigated.
In this paper we describe the isolation and purification of PHB-like material from two strains of L. pneumophila and its characterization by nuclear magnetic resonance (NMR) spectroscopy and gas chromatography (GC)-MS. We have investigated the physiology of PHB formation under iron-limited and -replete conditions and have demonstrated a relationship between PHB content and the survival of L. pneumophila in low-nutrient environments.
MATERIALS AND METHODS
Strains and culture.
Two strains of L. pneumophila serogroup 1 subgroup Pontiac, an environmental strain (74/81) and a clinical isolate (Corby), were grown in tyrosine-limited chemostat culture in ACES [N-(2-acetamido)-2-aminoethanesulfonic acid]-buffered chemically defined (ABCD) medium as described previously (31). Strain Corby was also grown under iron limitation, as detailed by James et al. (28). Steady-state culture samples were harvested by centrifugation and washed with deionized water before analysis.
PHB extraction and quantitation.
PHB was extracted and purified by a modification of the method of Findlay and White (21). In brief, 0.5 g of lyophilized washed biomass was extracted with boiling chloroform (60 ml) in a Soxtec apparatus (Tekator Ltd.) for up to 8 h. After being cooled, the extract was rotary evaporated and purified to a white membrane-like solid by sequential washing with ice-cold ethanol and cold diethyl ether. The purified material was dehydrated to crotonic acid and quantitated spectrophotometrically, as described by Slepecky and Law (43). A standard curve was prepared with authentic PHB from an Alcaligenes sp. (Sigma-Aldrich Co. Ltd.).
NMR spectroscopy.
For 13C NMR, 30 mg of purified material was dissolved in 4 ml of deuteriochloroform in a 10-mm-diameter NMR tube. Proton-decoupled NMR spectra were obtained with a Varian FT80 Fourier transform spectrometer at ambient temperature and a field strength of 20 MHz. Fully coupled spectra were recorded by switching the decoupler off during acquisition and back on during the delay period. Chemical shifts were expressed relative to tetramethylsilane at 0 ppm, using deuteriochloroform as the secondary reference. 1H NMR spectra were recorded at 30°C with a Varian Unity 500-MHz spectrometer. Approximately 3 mg of sample was dissolved in 0.8 ml of deuteriochloroform in a 5-mm-diameter NMR tube. The spectral width was 5,000, and 19,776 data points were collected. Spectra were processed with the manufacturer’s software with a gaussian apodization function. The spectra were referenced to tetramethylsilane at 0 ppm via residual chloroform at 7.25 ppm.
Methanolysis and GC-MS.
A portion of the purified material (0.5 mg) was methanolyzed (1.7 ml of methanol plus 0.2 ml of concentrated HCl; 4 h at 100°C), and the resulting methyl-3-hydroxybutyric acid was recovered by chloroform extraction. GC-MS was performed with a Kratos MS80 spectrometer interfaced to a Carlo-Erba 5160 chromatograph fitted with a 25 m-by-0.2-mm BP-1 fused-silica column (SGE Ltd., Milton Keynes, United Kingdom). Helium was used as the carrier gas, and samples were introduced by split injection (split ratio, 1:30) at an injector temperature of 250°C. The column was temperature programmed from 50 to 100°C at 5°C min−1 and then to 200°C at 20°C min−1. Electron ionization (EI) spectra were recorded at an ionization energy of 70 eV. Chemical ionization (CI) mass spectra were obtained with isobutane as the reagent gas.
PHB measurement by Nile red spectrofluorometry.
The PHB contents of intact cells were measured by the Nile red (9-diethylamino-5H-benzo [α]phenoxazine-5-one; Sigma-Aldrich Co. Ltd.) spectrofluorometric assay of Degelau et al. (15) with modifications. Fluorescence was measured with a Perkin-Elmer model LS-5B luminescence spectrometer. The assay conditions were optimized by performing a number of validation experiments (data not shown). For sample analysis, formalin-treated cells (1% [vol/vol] formaldehyde for 30 min) were washed, resuspended in deionized water to a standard optical density at 540 nm (OD540) of 0.5, and stained with 1 μl of Nile red (25 mM in DMSO) ml−1 for 60 min at 25°C. The instrument was autozeroed against a sample of unstained bacteria, and fluorescence was recorded at excitation and emission wavelengths of 545 and 600 nm, respectively, with integration over a 4-s response time. The temperature was maintained at 25°C by a thermostatically controlled cuvette holder.
Survival of L. pneumophila in tap water.
Bacteria from a tyrosine-limited chemostat culture were washed three times in deionized water and resuspended in filter-sterilized tap water at a density of approximately 109 CFU ml−1. Duplicate suspensions were incubated in the dark at 24°C with gentle agitation (150 rpm). Culturability on buffered charcoal-yeast extract (BCYE) agar and the OD540 were determined at intervals as described previously (28). The total cell count was determined by phase-contrast microscopy with a Thoma counting chamber and a Leitz Dialux 20 microscope. The PHB contents of samples were monitored by the Nile red spectrofluorometric assay.
Flow cytometry.
Samples of the starved population were stained with Nile red and analyzed with a Becton Dickinson Immunocytometry Systems benchtop flow cytometer (FACScan) operated via a Macintosh computer system. Real-time data acquisition and analysis was controlled by CellQuest software, while FACSComp software was responsible for daily setup and quality control. Calibration was performed with CaliBRITE beads (Becton Dickinson Immunocytometry Systems). The results for each sample were based on the analysis of 10,000 events.
Microscopy.
Dry smears of formalin-treated cells were stained with Nile red (25 mM Nile red in DMSO, diluted 1/500 in sterile deionized water) for 30 min, rinsed, and examined by epifluorescence microscopy (Nikon). Cell morphology was monitored by differential interference contrast microscopy, as described previously (28).
Heat shock treatment of starved cells.
Samples of starved cells were incubated at 45°C for 60 min. At 10-min intervals during heating, 1-ml aliquots were removed and plated on BCYE agar to determine culturability. After 60 min of heat treatment, the suspension was incubated at 24°C for 24 h before being resampled and plated on BCYE agar.
RESULTS
PHB accumulation by strain 74/81.
A water-insoluble white membrane-like material was purified from hot-chloroform extracts of strain 74/81. Its 1H decoupled 13C NMR spectrum comprised four resonances at 169.03, 67.50, 40.69, and 19.65 ppm and was identical to the spectrum of authentic PHB from an Alcaligenes sp. In the fully coupled spectrum, the last three signals were split into a doublet, a triplet, and a quartet, respectively, confirming that they originated from methine, methylene, and methyl carbon atoms; the singlet at 169.03 ppm was attributed to a carbonyl resonance. The chemical shifts and values of the coupling constants (1JCH) were consistent with these assignments (Table 1). In the 13C NMR spectrum of free 3-hydroxybutyric acid, the carboxyl carbon was, as expected, more deshielded than the ester-linked carbonyl of the polymer and resonated 7 ppm downfield. The hydroxylated carbon, being less deshielded in the free acid, was shifted 3 ppm upfield.
TABLE 1.
Carbon 13 NMR spectrum of purified PHB from L. pneumophila
| Chemical shift (ppm) | Intensity ratio of multiplet | Coupling constant (1JCH [Hz]) |
|---|---|---|
| 169.01 | ||
| 67.50 | 1:1 | 151 |
| 40.69 | 1:2:1 | 128 |
| 19.65 | 1:3:3:1 | 126 |
A portion of the purified material was depolymerized with methanolic HCl and analyzed by GC-MS. In the EI mass spectrum, the molecular ion at m/z 118 was extremely weak and was accompanied by M + 1 and M − 1 ions. Facile elimination of water produced an ion at m/z 100, and loss of a methoxy group yielded m/z 87. The ion at m/z 74 originated via a McLafferty rearrangement, due to cleavage between C-2 and C-3, with hydrogen transfer from carbon 4 to the charged fragment (33). The presence of a hydroxyl group on carbon 3 was suggested by a characteristic fragment at m/z 103 (cleavage alpha to the hydroxylated carbon). The molecular weight was confirmed by the observation of a protonated molecule at m/z 119 in the CI spectrum, which eliminated water and methanol, giving fragments at m/z 101 and 87. Identical spectra and chromatographic data were obtained from a methanolysate of authentic PHB. Several minor components (<2% of the intensity of the methyl-3-hydroxybutyrate signal) were present in the methanolysate of L. pneumophila PHB. CI MS of the two most significant of these produced spectra characterized by protonated molecules at m/z 133 and abundant dehydration fragments (m/z 115), enabling their tentative identification as the methyl esters of isomeric hydroxy valerates.
PHB accumulation by strain Corby.
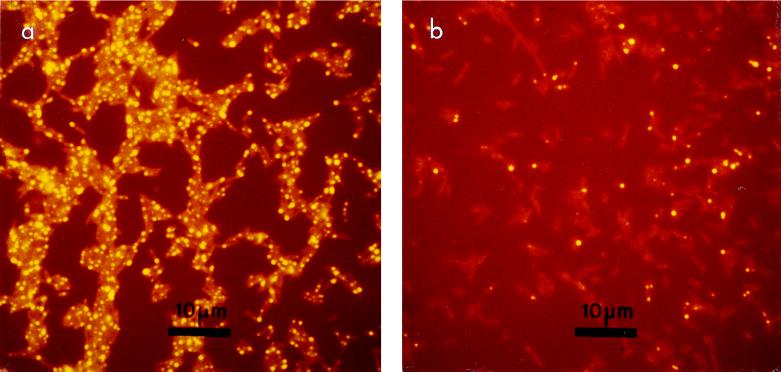
During studies with strain Corby, electron-dense inclusions were observed in electron micrographs of cells grown in iron-limited and -replete chemostat cultures. Fluorescence microscopy after staining with Nile red revealed yellow-fluorescing intracellular inclusions. Cells normally contained between one and three granules (Fig. 1a).
FIG. 1.
Fluorescence microscopy of L. pneumophila stained with Nile red. (a) Tyrosine-limited cells containing multiple yellow-fluorescent intracellular inclusions; (b) cells after 500 days of starvation, illustrating a marked decrease in granule content.
Proton NMR spectra of purified chloroform extracts from iron-replete cultures displayed three clusters of resonances. The chemical-shift values, multiplicities, and coupling constants (3JH,H) of these signals were consistent with their identification as methyl, methylene, and methine protons (Table 2). The spectra of extracts from iron-limited cells were comparable (data not shown).
TABLE 2.
1H NMR spectra of PHB purified from iron-replete cultures of L. pneumophila
| Proton | Chemical shifta (ppm) | Coupled protons | Coupling constant (3JH,H [Hz] ± 0.3 Hz) |
|---|---|---|---|
| CH | 5.24 | CH-CH2 a | 7.3 |
| CH2 a | 2.59 | CH-CH2 b | 5.5 |
| CH2 b | 2.46 | CH-CH3 | 6.0 |
| CH3 | 1.26 |
Relative to trimethylsilane at 0 ppm.
Low PHB yields, 2 to 5% of cell dry weight, were recovered from iron-limited and -replete cells by hot-chloroform extraction. Additional experiments demonstrated that the efficiency of the chloroform extraction was relatively poor, recovering less than 50% of cellular PHB during a single extraction cycle. Improved recovery of cellular PHB was achieved by performing multiple extraction cycles. Yields of up to 16% of cell dry weight were achieved after three extraction cycles.
A spectrofluorometric assay based on the lipophilic dye Nile red was used as a more accurate and less hazardous procedure for PHB determination. PHB content, as determined by exhaustive chloroform extraction and spectrophotometric measurement, was linearly correlated to Nile red fluorescence (R2 = 0.96). This relationship (described by the equation y = 1.0114x + 9.1039, where y and x represent sample fluorescence and PHB content, respectively) provided the basis for a quantitative assay to determine the PHB contents of cell samples.
Spectrofluorometric analysis of nine separate iron-limited and -replete samples demonstrated a threefold increase in PHB accumulation under iron-limited conditions, with mean PHB yields of 12 and 4% of cell dry weight for iron-limited and -replete cultures, respectively. The PHB contents of iron-limited cultures ranged between 6 and 18% of cell dry weight. These iron-limited cultures were grown with different concentrations of iron. Additional analysis revealed that the PHB yield was enhanced in cultures grown at lower concentrations of iron, with an inverse linear relationship between the concentration of iron metabolized by iron-limited cultures and PHB content (R2 = 0.95).
Survival of L. pneumophila.
L. pneumophila Corby survived in a culturable state in filter-sterilized tap water for at least 600 days (Fig. 2). Culturability declined rapidly during the first week, with the number of CFU decreasing by 30%. This initial phase was followed by a more gradual decline in culturability, with approximately 25% of the population still culturable after 280 days and 10% of the population culture positive after 380 days. Spectrofluorometric analysis of Nile red-stained samples demonstrated a decrease in fluorescence (Fig. 2). As with culturability, the most rapid decline in fluorescence occurred during the first week of starvation (33% decrease), after which there was a gradual decline in the rate of PHB utilization until the PHB was depleted. A progressive decline in the OD of the suspension, which mirrored the decrease in fluorescence, was also observed (data not shown). The changes in culturability and turbidity were not accompanied by a comparable decrease in the total cell count, which remained relatively constant after an initial decline of 20% during the first week (Fig. 2).
FIG. 2.
Survival of L. pneumophila in sterile tap water. □, Culturability on BCYE agar; ■, Nile red fluorescence; ▴, total cell count.
Figure 3 illustrates how the relationship between culturability and fluorescence changed with time. Both culturability and fluorescence declined markedly during the first week, after which there was a marked reduction in the rate at which both parameters changed. Over the next 50 days, fluorescence decreased by 40% while culturability only declined by approximately 10% (Fig. 3). After 400 days of starvation, no further decline in fluorescence was detected while culturability continued to decline independently. A repeat experiment performed with a different culture sample of the same strain produced similar results.
FIG. 3.
Relationship between culturability and Nile red fluorescence during starvation. Days zero to 5 (a), 6 to 56 (b), and 57 to 600 (c) following incubation in tap water are shown. Fluorescence and culturability data from Fig. 2 were expressed as percentages of their respective values at day zero to illustrate the relationship between PHB utilization and loss of culturability during starvation.
Flow cytometry of Nile red-stained samples confirmed a decrease in cellular fluorescence during starvation. At day 0, the fluorescence intensities of 98% of the cells were greater than 10 (within the gated region M1 [Fig. 4a]). However, after 490 days, the fluorescence intensities of approximately 90% of the population had declined to the background level of the unstained sample (Fig. 4b). There was a gradual decline in cellular fluorescence during starvation, in close agreement with the fluorometric data (data not shown). A linear relationship was detected between the decrease in culturability and mean cellular fluorescence (R2 = 0.96) when both were expressed as percentages of their respective values at day zero (data not shown).
FIG. 4.
Changes in cellular fluorescence and population distribution during starvation as detected by flow cytometry. The histogram plots (a and b) illustrate the fluorescence intensities recorded at 580 ± 25 nm for samples collected at day zero (a) and after 490 days of starvation (b). The dot plots (c and d) illustrate the relative cell complexities and size distributions (side scatter is representative of relative complexity and granularity; forward scatter is representative of relative cell length) for samples collected at day zero (c) and day 490 (d). All plots are based on the analysis of 10,000 events.
Flow cytometry also revealed changes in cell complexity and size during starvation. Initially, a broad distribution of cell complexity and size was observed (Fig. 4c). After 490 days of starvation, the mean cell complexity (y-mean) and mean cell length (x-mean) were reduced by 80 and 30%, respectively (Fig. 4d). This trend was confirmed by analysis of the gated region R2, which represents small cells with low internal complexity. The percentage of cells in this region increased from 7% at day zero to 86% after 490 days of starvation (Fig. 4c and d). A linear relationship was also observed between the decline in culturability and mean cell complexity (R2 = 0.97 [data not shown]).
Microscopic examination of the starved cells revealed changes in morphology. At day zero the majority of cells contained between one and three brightly fluorescing intracellular granules (Fig. 1a). After 500 days of starvation, a marked decrease in granule content was observed, with many cells devoid of granules (Fig. 1b). Differential interference contrast microscopy also revealed some alterations in cellular morphology in response to starvation. At day zero, most cells appeared to be intact, but after 600 days of starvation much cell debris was observed (data not shown).
A heat shock experiment was performed after 600 days of starvation to investigate whether nonculturable cells could be resuscitated. Heating cells to 45°C for up to 60 min, prior to plating them on BCYE agar, failed to increase culturability. Indeed, a 30% decline in recovery was observed after 60 min of heat shock. The suspension was also incubated at 24°C after heat treatment, and culturability was monitored after 24 h. Again, no increase in culturability was detected.
DISCUSSION
PHB formation by L. pneumophila.
We previously reported the presence of electron-dense cytoplasmic inclusions in chemostat cultures of strain 74/81 grown at different temperatures and provided preliminary chemical evidence for the presence of PHB (31). In the current study, a white solid was purified from hot-chloroform extracts of strain 74/81. Carbon 13 NMR spectroscopy provided strong evidence that this material is a polymer of 3-hydroxybutyric acid. The chemical shifts and values of the coupling constants were in close agreement with published solution values and with those obtained by cross-polarization magic-angle spinning NMR and in vivo NMR experiments (2, 3, 16, 17). Material purified from strain Corby also yielded 1H NMR spectra with coupling constants and chemical shifts consistent with PHB (17, 38). Taken together with GC-MS analysis, these data establish the structure of the purified chloroform-extractable material as that of PHB.
Intracellular granules were observed in electron micrographs for iron-limited and -replete samples, in agreement with previous reports of granule formation by L. pneumophila (11, 31, 39). These inclusions fluoresced bright yellow after being stained with Nile red, confirming their lipid nature. Nile red is a lipophilic stain which produces yellow-gold fluorescence in hydrophobic environments, and it has been used extensively for the detection of neutral and polar lipid inclusions in eukaryotic cell types (10, 23, 24). Nile blue A, a basic oxazime dye containing traces of the oxidized product Nile red, has been used to visualize PHB granules in Bacillus megaterium and Azobacter chroococcum (35).
The efficiency of hot-chloroform extraction was relatively poor, and multiple extraction cycles were required to obtain a more accurate quantitation of cellular PHB. By performing multiple extraction cycles, we demonstrated that L. pneumophila can accumulate more significant PHB reserves than was previously thought (31). Poor PHB recovery by chloroform extraction has been reported previously for studies with Alcaligenes eutrophus (37).
A Nile red spectrofluorometric assay was found to be a more accurate and reliable procedure for determining the PHB content of intact cells, in agreement with the observations of Degelau et al. (15). PHB accumulation was enhanced during iron-limited growth, consistent with the physiology of PHB formation, which is promoted during unbalanced growth when an essential nutrient other than the carbon and energy source is limiting (13, 14, 45). Furthermore, the PHB content of iron-limited cells was inversely related to the concentration of iron metabolized. This reflects the increased concentration of excess carbon source which becomes available at lower concentrations of iron and can be channelled into PHB formation.
PHB synthesis by L. pneumophila is likely to proceed via pyruvate and acetyl-coenzyme A (CoA). Acetyl-CoA acyl transferase, CoA-SH, and NADH are key regulatory elements in the PHB biosynthetic pathway. During balanced growth, CoA-SH levels are high and polymer synthesis is inhibited. However, when growth is limited by an essential nutrient other than the carbon and energy source, the NADH concentration increases, resulting in inhibition of early enzymes of the tricarboxylic acid cycle, namely, citrate synthase and isocitrate dehydrogenase. This leads to an accumulation of acetyl-CoA, which relieves the inhibition exerted by CoA-SH, leading to 3-hydroxybutyric acid formation (13, 45). Therefore, excess metabolic intermediates are channelled into PHB formation in a reductive process, which also regenerates reduced cofactors.
PHB and survival of L. pneumophila in a low-nutrient environment.
The present study demonstrates that L. pneumophila can maintain a vegetative state for prolonged periods (at least 600 days) when incubated in a low-nutrient environment. This is consistent with previous studies reporting the survival of L. pneumophila under starvation conditions (26, 42, 50, 52).
Nile red spectrofluorometry and flow cytometry demonstrated that intracellular PHB reserves were utilized during starvation. This was also demonstrated by a decline in the mean cell complexity and culture turbidity, which represented the decrease in cell refractivity due to the loss of electron-dense intracellular granules (44). Fluorescence microscopy provided visual evidence of PHB utilization, with a marked reduction in the granule content of the cells during starvation.
The response of the culture to starvation changed with time. The marked decline in fluorescence during the first week probably represents expenditure of carbon and energy reserves associated with adapting to a low-nutrient environment. The associated loss of culturability probably represents those cells that were unable to adapt to the new environment. The subsequent survival stage was characterized by a marked reduction in the rate of PHB utilization, indicating that the metabolic activity of the cells had become more closely coupled to their energy requirement for maintenance of a vegetative state. Little loss of culturability occurred during this period, indicating adaptation to starvation conditions, with PHB promoting survival. After prolonged starvation, changes in culturability became independent of PHB utilization. It is possible that extracellular nutrients released by cell death and lysis were contributing to survival at this stage. Alternatively, the spectrofluorometric assay may not have been sufficiently sensitive to detect very low rates of residual PHB utilization associated with a very low metabolic activity of the surviving cells. Consistent with the latter possibility, a linear relationship was demonstrated between the decreases in mean cellular fluorescence and culturability (both expressed as percentages), confirming that PHB utilization correlated with loss of culturability. Therefore, this study demonstrates that PHB is an important energy reserve, which supports the long-term survival of L. pneumophila in a culturable state in a low-nutrient environment. In the natural habitat, the survival of L. pneumophila will be influenced by other parameters, such as temperature and changes in nutrient availability, which may affect the rates of PHB accumulation and utilization.
Several studies have noted the formation of inclusions resembling PHB granules by L. pneumophila during intracellular growth in aquatic amebae (1, 20, 41, 48). Restricted availability of essential nutrients, such as iron, is likely to promote PHB formation by L. pneumophila inside the amebal host. Indeed, this study has demonstrated that PHB accumulation is promoted during iron-limited growth in vitro. Therefore, as well as providing protection and supporting proliferation in hostile environments, amebae may also contribute to the environmental persistence of legionellae by inducing a PHB-rich phenotype, which is physiologically more prepared for extracellular survival in low-nutrient environments.
Although this study demonstrates that PHB depletion correlates with loss of culturability, it is possible that these cells entered a viable-but-nonculturable (VBNC) state. L. pneumophila has been reported to enter a dormant state when exposed to low-nutrient environments (26, 36). Colbourne and Dennis (12) suggested that dormant L. pneumophila cells were resuscitated by heat shock treatment. However, in the present study comparable heat shock treatment failed to revive potentially nonculturable cells. A recent study by Steinert and colleagues (46), using both acridine orange staining and 16S rRNA hybridization, demonstrated that L. pneumophila entered a viable-but-nonculturable state following starvation, from which it could be resuscitated by the addition of Acanthamoeba castellanii. Therefore, in the present study it is possible that the appropriate physicochemical stimuli to induce resuscitation were not applied.
In conclusion, this study demonstrates that L. pneumophila accumulates considerable intracellular reserves of PHB, which support its long-term survival in a culturable state under starvation conditions. This endogenous energy reserve is likely to play an important role in promoting the persistence of legionellae in stressful low-nutrient environments outside the amebal host.
ACKNOWLEDGMENTS
We thank B. Mulloy, National Institute for Biological Standards and Control, United Kingdom, for performing proton NMR analysis.
This work was supported by project grant JR 121/2790 from the Department of Health, United Kingdom.
REFERENCES
- 1.Anand C M, Skinner A R, Malic A, Kurtz J B. Interaction of Legionella pneumophila and a free living amoeba (Acanthamoeba palestinensis) J Hyg. 1983;91:167–178. doi: 10.1017/s0022172400060174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnard G N, Sanders J K M. Observation of mobile poly-(β-hydroxybutyrate) in the storage granules of Methylobacterium AM1 by in vivo13C-NMR spectroscopy. FEBS Lett. 1988;231:16–18. [Google Scholar]
- 3.Barnard G N, Sanders J K M. The poly-β-hydroxybutyrate granule in vivo: a new insight based on NMR spectroscopy of whole cells. J Biol Chem. 1989;264:3286–3291. [PubMed] [Google Scholar]
- 4.Barbaree J M, Fields B S, Feeley J C, Gorman G W, Martin W T. Isolation of protozoa from water associated with a legionellosis outbreak and demonstration of intracellular multiplication of Legionella pneumophila. Appl Environ Microbiol. 1986;51:422–424. doi: 10.1128/aem.51.2.422-424.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker J, Brown M R W. Speculations on the influence of infecting phenotype on virulence and antibiotic susceptibility of Legionella pneumophila. J Antimicrob Chemother. 1995;36:7–21. doi: 10.1093/jac/36.1.7. [DOI] [PubMed] [Google Scholar]
- 6.Barker J, Brown M R W, Collier P J, Farrell I, Gilbert P. Relationship between Legionella pneumophila and Acanthamoeba polyphaga: physiological status and susceptibility to chemical inactivation. Appl Environ Microbiol. 1992;58:2420–2425. doi: 10.1128/aem.58.8.2420-2425.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barker J, Lambert P A, Brown M R W. Influence of inter-amoebic and other growth conditions on the surface properties of Legionella pneumophila. Infect Immun. 1993;61:3503–3510. doi: 10.1128/iai.61.8.3503-3510.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker J, Scaife H, Brown M R W. Intraphagocytic growth induces an antibiotic-resistant phenotype of Legionella pneumophila. Antimicrob Agents Chemother. 1995;39:2684–2688. doi: 10.1128/aac.39.12.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bartlett C L R, Kurtz J B, Hutchinson J G, Turner G C, Wright A E. Legionella in hospital and hotel water supplies. Lancet. 1983;ii:1315. doi: 10.1016/s0140-6736(83)91203-5. [DOI] [PubMed] [Google Scholar]
- 10.Brown W J, Sullivan T R, Greenspan P. Nile red staining of lysosomal phospholipid inclusions. Histochemistry. 1992;97:349–354. doi: 10.1007/BF00270037. [DOI] [PubMed] [Google Scholar]
- 11.Chandler F W, Cole R M, Hicklin M D, Blackmon J A, Callaway C S. Ultrastructure of the Legionnaires’ disease bacterium. Ann Intern Med. 1979;90:642–647. doi: 10.7326/0003-4819-90-4-642. [DOI] [PubMed] [Google Scholar]
- 12.Colbourne J S, Dennis P J. The ecology and survival of Legionella pneumophila. J Inst Water Environ Manag. 1989;3:345–350. [Google Scholar]
- 13.Dawes E A. Microbial energy reserve compounds. In: Dawes E A, editor. Microbial energetics. London, United Kingdom: Blackie; 1986. pp. 145–165. [Google Scholar]
- 14.Dawes E A, Senior P J. The role and regulation of energy reserve polymers in micro-organisms. Adv Microb Physiol. 1973;10:135–266. doi: 10.1016/s0065-2911(08)60088-0. [DOI] [PubMed] [Google Scholar]
- 15.Degelau A, Scheper T, Bailey J E, Guske C. Fluorometric measurement of poly-β-hydroxybutyrate in Alcaligenes eutrophus by flow cytometry and spectrofluorometry. Appl Microbiol Biotechnol. 1995;42:653–657. [Google Scholar]
- 16.Doi Y, Kawaguchi Y, Nakamura Y, Kunioka M. Nuclear magnetic resonance studies of poly-(3-hydroxybutyrate) and polyphosphate metabolism in Alcaligenes eutrophus. Appl Environ Microbiol. 1989;55:2932–2938. doi: 10.1128/aem.55.11.2932-2938.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doi Y, Kunioka M, Nakamura Y, Soga K. 1H and 13C NMR analysis of poly (β-hydroxybutyrate) isolated from Bacillus megaterium. Macromolecules. 1986;19:1274–1276. [Google Scholar]
- 18.Dondero T J, Jr, Rendtorff R C, Mallison G F, Weeks R M, Levy J S, Wong E W, Schaffner W. An outbreak of Legionnaires’ disease associated with a contaminated air-conditioning cooling tower. New Engl J Med. 1980;302:365–370. doi: 10.1056/NEJM198002143020703. [DOI] [PubMed] [Google Scholar]
- 19.Fields B S. The molecular ecology of legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842x(96)10041-x. [DOI] [PubMed] [Google Scholar]
- 20.Fields B S, Barbaree J M, Shotts E B, Jr, Feeley F C, Morrill W A, Sanden G N, Dykstra M J. Comparison of guinea pig and protozoan models for determining virulence of Legionella species. Infect Immun. 1986;53:553–559. doi: 10.1128/iai.53.3.553-559.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Findlay R H, White D C. Polymeric beta-hydroxyalkanoates from environmental samples and Bacillus megaterium. Appl Environ Microbiol. 1983;45:71–78. doi: 10.1128/aem.45.1.71-78.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fliermans C B, Cherry W B, Orrison L H, Smith S J, Tison D L, Pope D H. Ecological distribution of Legionella pneumophila. Appl Environ Microbiol. 1981;41:9–16. doi: 10.1128/aem.41.1.9-16.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler S D, Greenspan P. Application of Nile red, a fluorescent hydrophobic probe, for the detection of neutral lipid deposits in tissue sections: comparison with oil red O. J Histochem Cytochem. 1985;33:833–836. doi: 10.1177/33.8.4020099. [DOI] [PubMed] [Google Scholar]
- 24.Greenspan P, Mayer E P, Fowler S D. Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol. 1985;100:965–973. doi: 10.1083/jcb.100.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helm D, Labischinski H, Schallehn G, Naumann D. Classification and identification of bacteria by Fourier-transform infrared spectroscopy. J Gen Microbiol. 1991;137:69–79. doi: 10.1099/00221287-137-1-69. [DOI] [PubMed] [Google Scholar]
- 26.Hussong D, Colwell R R, O’Brien M, Weiss E, Pearson A D, Weiner R M, Burge W D. Viable Legionella pneumophila not detectable by culture on agar media. Bio/Technology. 1987;5:947–950. [Google Scholar]
- 27.James B W, Mauchline W S, Dennis P J, Keevil C W. A study of iron acquisition mechanisms of Legionella pneumophila grown in chemostat culture. Curr Microbiol. 1997;34:238–243. doi: 10.1007/s002849900176. [DOI] [PubMed] [Google Scholar]
- 28.James B W, Mauchline W S, Fitzgeorge R B, Dennis P J, Keevil C W. Influence of iron-limited continuous culture on physiology and virulence of Legionella pneumophila. Infect Immun. 1995;63:4224–4230. doi: 10.1128/iai.63.11.4224-4230.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kilvington S, Price J. Survival of Legionella pneumophila within cysts of Acanthamoeba polyphaga following chlorine exposure. J Appl Bacteriol. 1990;68:519–525. doi: 10.1111/j.1365-2672.1990.tb02904.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuchta J M, States S J, McNamara A M, Wadowsky R M, Yee R B. Susceptibility of Legionella pneumophila to chlorine in tap water. Appl Environ Microbiol. 1983;46:1134–1139. doi: 10.1128/aem.46.5.1134-1139.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mauchline W S, Araujo R, Wait R, Dowsett A B, Dennis P J, Keevil C W. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. J Gen Microbiol. 1992;138:2371–2380. doi: 10.1099/00221287-138-11-2371. [DOI] [PubMed] [Google Scholar]
- 32.Mauchline W S, Keevil C W. Development of the BIOLOG substrate utilization system for identification of Legionella spp. Appl Environ Microbiol. 1991;57:3345–3349. doi: 10.1128/aem.57.11.3345-3349.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLafferty F W, Turecek F, editors. Interpretation of mass spectra. 4th ed. Mill Valley, Calif: University Science Books; 1993. p. 72. [Google Scholar]
- 34.Miller R D, Kenepp K A. Risk assessments for Legionnaires’ disease based on routine surveillance of cooling towers for legionellae. In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 40–43. [Google Scholar]
- 35.Ostle A G, Holt J G. Nile blue A as a fluorescent stain for poly-β-hydroxybutyrate. Appl Environ Microbiol. 1982;44:238–241. doi: 10.1128/aem.44.1.238-241.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paszko-Kolva C, Shahamat M, Colwell R R. Long term survival of Legionella pneumophila serogroup 1 under low nutrient conditions and associated morphological changes. FEMS Microbiol Ecol. 1992;102:45–55. [Google Scholar]
- 37.Ramsay J A. PHA: its separation from microbial biomass and its biodegradation. In: Braunegg G, editor. Physiology, kinetics, production and use of biopolymers. Proceedings of the European Federation of Biotechnology, Graz, Austria. 1994. pp. 49–58. [Google Scholar]
- 38.Reusch R N. Biological complexes of poly-β-hydroxybutyrate. FEMS Microbiol Rev. 1992;103:119–130. doi: 10.1111/j.1574-6968.1992.tb05829.x. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers F G, Davey M R. Ultrastructure of the cell envelope layers and surface details of Legionella pneumophila. J Gen Microbiol. 1982;128:1547–1557. doi: 10.1099/00221287-128-7-1547. [DOI] [PubMed] [Google Scholar]
- 40.Rogers J, Keevil C W. Immunogold and fluorescein immunolabelling of Legionella pneumophila within an aquatic biofilm visualized using episcopic differential interference contrast microscopy. Appl Environ Microbiol. 1992;58:2326–2330. doi: 10.1128/aem.58.7.2326-2330.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowbotham T J. Current views on the relationship between amoebae, legionellae and man. Isr J Med Sci. 1986;22:678–689. [PubMed] [Google Scholar]
- 42.Skaliy P, McEachern H V. Survival of the Legionnaires’ disease bacterium in water. Ann Intern Med. 1979;90:662–663. doi: 10.7326/0003-4819-90-4-662. [DOI] [PubMed] [Google Scholar]
- 43.Slepecky R A, Law J H. A rapid spectrophotometric assay for alpha, beta-unsaturated acids and beta-hydroxy acids. Anal Chem. 1960;32:1697–1699. [Google Scholar]
- 44.Srienc F, Arnold B, Bailey J E. Characterization of intracellular accumulation of poly-β-hydroxybutyrate (PHB) in individual cells of Alcaligenes eutrophus H16 by flow cytometry. Biotech Bioeng. 1984;26:982–987. doi: 10.1002/bit.260260824. [DOI] [PubMed] [Google Scholar]
- 45.Steinbüchel A, Schlegel H G. Physiology and molecular genetics of poly(β-hydroxyalkanoic acid) synthesis in Alcaligenes eutrophus. Mol Microbiol. 1991;5:535–542. doi: 10.1111/j.1365-2958.1991.tb00725.x. [DOI] [PubMed] [Google Scholar]
- 46.Steinert M, Emödy L, Amann R, Hacker J. Resuscitation of viable but nonculturable Legionella pneumophila Philadelphia JR32 by Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:2047–2053. doi: 10.1128/aem.63.5.2047-2053.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stout J E, Yu V L, Best M G. Ecology of Legionella pneumophila within water distribution systems. Appl Environ Microbiol. 1985;49:221–228. doi: 10.1128/aem.49.1.221-228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vandenesch F, Surgot M, Bornstein N, Paucod J C, Marmet D, Isoard P, Fleurette J. Relationship between free amoebae and Legionella: studies in vitro and in vivo. Int J Med Microbiol. 1990;272:265–275. doi: 10.1016/s0934-8840(11)80027-7. [DOI] [PubMed] [Google Scholar]
- 49.Wadowsky R M, Yee R B, Mezmar L, Wing E J, Dowling J N. Hot water systems as sources of Legionella pneumophila in hospital and non-hospital plumbing fixtures. Appl Environ Microbiol. 1982;43:1104–1110. doi: 10.1128/aem.43.5.1104-1110.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang W L L, Martin B J, Cravens J, Johnson M A. Growth, survival, and resistance to the Legionnaires’ disease bacterium. Ann Intern Med. 1979;90:614–618. doi: 10.7326/0003-4819-90-4-614. [DOI] [PubMed] [Google Scholar]
- 51.Watt B E, Morgan S L, Fox A. 2-Butenoic acid, a chemical marker for poly-β-hydroxybutyrate identified by pyrolysis-gas chromatography/mass spectrometry in analyses of whole microbial cells. J Anal Appl Pyrol. 1991;19:237–249. [Google Scholar]
- 52.West A A, Rogers J, Lee J V, Keevil C W. Lack of dormancy in Legionella pneumophila? In: Barbaree J M, Breiman R F, Dufour A P, editors. Legionella: current status and emerging perspectives. Washington, D.C: American Society for Microbiology; 1993. pp. 201–203. [Google Scholar]