Abstract
Hepatocellular carcinoma (HCC) is one of the most common and deadly human cancers. The five-year survival rate is very low. Unfortunately, there are few efficacious therapeutic options. Until recently, Sorafenib has been the only available systemic drug for advanced HCC. However, it has very limited survival benefits and new therapies are urgently needed. In this study, we investigated the anti-HCC activity of carfilzomib, a second-generation, irreversible proteasome inhibitor, as a single agent and in combination with sorafenib. In vitro, we found that carfilzomib has moderate anticancer activity toward liver cancer cells, but strongly enhances the ability of sorafenib to suppress HCC cell growth, proliferation, migration, invasion and survival. Remarkably, the drug combination exhibits even more potent antitumor activity when tested in animal tumor models. Mechanistically, the combined treatment activates caspase-dependent and ER stress/CHOP-mediated apoptotic pathways, and suppresses epithelial-mesenchymal transition (EMT). In conclusion, our results demonstrate that the combination of carfilzomib and sorafenib has synergistic antitumor activities against HCC, providing a potential therapeutic strategy to improve the mortality and morbidity of HCC patients.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most prevalent malignancies and the third leading cause of cancer death in the world (1), particularly in China and other far eastern countries (2). Despite the overall decreased rate in cancer incident and mortality in the USA, that of HCC continues to rise due to the epidemic of obesity and non-acoholic fatty liver disease (NAFLD) (3). Surgery is currently considered as the best treatment strategy for early stage HCC, but over 70% of HCC patients are already in the advanced stage when initially diagnosed and they are no longer suitable for surgical resection (3). Unlike other malignancies such as lung or breast cancer, advanced HCC has few therapeutic options because it is highly resistant to cytotoxic chemotherapy or radiotherapy (3). Moreover, recent clinical trials indicate that advanced HCC only has a moderate response rate to immune checkpoint inhibitors (4). Therefore, there is an urgent need to develop new treatments to improve the clinical outcome of this fatal disease.
Over the past two decades, targeted therapies have significantly enhanced our ability to treat cancer. Monoclonal antibodies and small molecules targeting cancer drivers have become standard treatment regimes for solid tumors and hematologic malignancies. Sorafenib is a pan-kinase inhibitor that blocks both cancer cell proliferation and angiogenesis. Sorafenib broadly inhibits multiple oncogenic kinases, including RAF, PDGFR and VEGFR (5). Until last year’s US FDA approval of Regorafenib, a Sorafenib-like multikinase inhibitor, it has been the only clinical medicine for treating advanced HCC (6,7). However, its survival benefit is only 3–6 months in overall survival (OS) (6–8). Many patients require a dosage reduction or treatment cessation due to intolerable adverse effects (9). Furthermore, the disease relapses quickly even for the initial responders (10). For these reasons, it is necessary to improve the efficacy and durable response of sorafenib in advanced HCC patients.
Proteasome inhibitors, such as bortezomib, are molecular targeted agents that have significantly improved the overall survival (OS) of patients with multiple myeloma (MM) (11). Bortezomib binds to the catalytic site of the 26S proteasome, preventing degradation of pro-apoptotic factors and promoting programmed cell death of cancer cells (12). In recent years, many clinical trials have been conducted with bortezomib for solid tumors but had little success as a single agent (13,14). Bortezomib has also been tested in combination with chemotherapeutic agents such as docetaxel but failed to achieve desired clinical endpoints (13,14). These unsuccessful clinical trials highlight the need for high quality mechanistic studies that can help design rational drug combinations and improve the outcome of human trials.
Bortezomib was previously shown to induce HCC cell death when used in combination with a tumor-targeting TRAIL (15) or with sorafenib (16). In the latter study, when PLC/PRF/5 cells were pretreated with sorafenib, bortezomib was proposed to induce apoptosis through PP2A-dependent Akt inactivation. These observations suggest that proteasome inhibition has the potential to improve the therapeutic effect of sorafenib in HCC. Carfilzomib is a second-generation proteasome inhibitor. Unlike bortezomib that reversibly inhibits the proteasome, carfilzomib irreversibly binds to the catalytic site of the proteome, resulting in sustained proteosomal inhibition (17). In vitro studies have shown the superiority of carfilzomib over bortezomib for treatment of relapsed multiple myeloma (18). In a chemically induced rat liver cancer model, carfilzomib has preventive benefit against hepatocarcinogenesis (19). However, whether carfilzomib is active against HCC and whether it enhances the anticancer activity of sorafenib remains unresolved. In the present study, we performed a series of in vitro and in vivo experiments to evaluate the preclinical efficacy of carfilzomib alone or in combination with sorafenib. Our results demonstrate that carfilzomib has moderate anticancer activity. However, the combination of carfilzomib and sorafenib displays strong antitumor activity against HCC. Our study indicates that the combinational therapy of carfilzomib and sorafenib is a potentially useful strategy to improve the treatment outcome for HCC patients.
Materials and Methods
Cell culture, drugs and antibodies
HCC cell line Hep3B was obtained from American Type Culture Collection (ATCC, Manassas, VA), and Bel-7402 was kindly provided by Dr. Yue Li, Southern Medical University, Guangzhou, China. The cell lines have been authenticated by the Short Tandem Repeat (STR) analysis. Hep3B and Bel-7402 cells were cultured in Dulbecco’s Modified Eagle Medium (GIBCO BRL) supplemented with 10% fetal bovine serum (GIBCO BRL) at 37°C in an atmosphere of 5% CO2, and Mycoplasma contamination was routinely examined by PCR. Cells with low passage numbers (less than 20) were used in this study. Carfilzomib and sorafenib were purchased from Selleck Chemicals. Cleaved caspase-3 (#9664), cleaved caspase-7 (#8438), cleaved caspase-9 (#7237), cleaved PARP (#5625), ATF-4 (#11815), eIF2α (#5324), p-eIF2α (#3398) and GAPDH (#5174) rabbit monoclonal antibodies, anti-CHOP (#2895) mouse antibodies, HRP-conjugated anti-rabbit (#7074) and anti-mouse (#7076) secondary antibodies were purchased from Cell Signaling Technology (USA). PERK (#ab65142), p-PERK (#ab192591), CHOP (#ab11419) and anti-Ki67 antibodies (#ab15580) were purchased from Abcam. Mouse Anti-E-cadherin (#610181), N-cadherin (#610921) and β-catenin (#610154) were purchased from BD Biosciences.
Cell proliferation assay
Cell proliferation was assessed by the MTS method using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit (G3580, Promega) according to the manufacturer’s instructions. Briefly, 2,000 cells were plated into each well of 96-well plates in 200 μL culture medium and incubated overnight for attachment. On the following day, cells were treated with drug or drug vehicle for 5 days. For the MTS assay, 20 μL MTS reagent was added to each well and incubated for another 2 hours. The absorbance was measured at 490 nm with a multifunctional microplate reader.
Colony formation assay
Cells were seeded onto 6-well plates (1,000 cells/well) and cultured at 37°C with 5% CO2 overnight. The cells were then treated with drug or drug vehicle for about 2 weeks until colonies were visible. Cell culture media with different concentration of sorafenib and/or carfilzomib were changed every three days during this period. It has been suggested that free plasma sorafenib concentrations are likely to be in the sub-μM range due to extensive plasma binding (20). So we performed this assay using sorafenib concentrations of 0.1 μM or 0.5 μM. The colonies were washed with PBS twice, fixed with 4% paraformaldehyde for 15 min and stained with 0.1% crystal violet solution for 30 min. After washing with PBS, the number of colonies (> 50 cells/colony) was counted under the microscope.
Analysis of apoptosis by flow cytometry
Apoptosis was analyzed using the Annexin V-FITC/PI apoptosis kit (KGA108, KeyGEN Biotech, China) according to the manufacturer’s instruction. Briefly, cells were harvested and washed in cold phosphate-buffered saline (PBS). 500 μL binding buffer was added to each sample. Cells were mixed with annexin-V-FITC and PI solution, and incubated at room temperature for 15 min. The stained cells were analyzed by flow cytometry (Novocyte, ACEA Biosciences, China) at the fluorescence emission of 530 nm.
Transwell migration and invasion assays
Cell migration and invasion assays were performed on transwell chambers with 8-μm pore-size filters without (for migration) or with (for invasion) coated matrigel (Falcon). Cells were trypsinized and resuspended in serum-free medium. 250 μL of cell suspension (1 × 10 5 cells) was added to the upper chambers in a transwell insert, and the upper chambers were then placed into the wells of a 24-well plate. 750 μL culture medium containing 20% FBS was added to the lower chamber. After transwell inserts were cultured at 5% CO2 at 37°C for 24 h, cells on the top of the membrane were removed with a cotton swabs. Cells attached on the underside of the membrane were fixed and stained with 0.1% crystal violet. After washing with PBS, the number of cells was counted in three random microscopic fields under the microscope.
Caspase activity assay
The activity of caspase 3/7 was measured using a Caspase-Glo® 3/7 Assay kit (G8091, Promega) with a modified protocol. Briefly, the proluminescent substrate containing the DEVD sequence is cleaved by caspase 3/7, respectively. After caspase cleavage, the substrate for luciferase (aminoluciferin) was released, inducing the luciferase reaction, which was analyzed in a total volume of 200 μl in 96-well plates. 100 μl reagent was added into each well of a white-walled 96-well plate containing 100 μl of blank, control or treated cells in culture medium. The mixture was incubated at room temperature for 2 hours and measured for luminescence by a plate-reading luminometer.
Immunoblot
Equal amount of protein extracts from HCC cells or tissues were separated by 10% SDS-PAGE, and transferred onto Immobilon-P membrane (Millipore). After blocking with 5% non-fat milk in TBST (with 0.1% Tween-20), the membrane was incubated with the indicated primary antibodies overnight at 4°C, washed with TBST, incubated with secondary antibodies at room temperature for 60 min, and detected by the Western Lightning Plus-ECL Kit (Thermo, USA) according to manufacturer’s instruction.
In vivo studies and immunohistochemistry
The animal protocol was approved by the Animal Ethics Committee of Sun Yat-Sen University Cancer Center. Subcutaneous xenograft models were established and drug treatments were carried out as previously described (21–25). Four-week-old female BALB/c nude mice were used in this study. Log phase Hep3B cells were harvested and resuspended in PBS at a density of 3 × 107 cells/ml. 0.1 ml cell suspension containing 3 × 106 cells were injected subcutaneously into the right flank of each mouse. Mice were then randomly divided into four groups and were treated with drug vehicle, 4 mg/kg carfilzomib, 15 mg/kg sorafenib, and carfilzomib plus sorafenib, respectively. Carfilzomib was diluted with physiological saline and a volume of 200μL was intraperitoneally injected twice weekly. Sorafenib was resuspended in an oral vehicle containing Cremophor EL (Sigma-Aldrich), 95% ethanol and water in a ratio of 1:1:6, and a volume of 200μL was orally administrated by gavage daily. Mice in the combination group received both drugs treatment given the same as in the single treatment group concurrently. Mice in the control group received drug vehicles only.
Tumor volumes and mice weight were measured every three days. Tumor volumes were calculated using the following formula: volume (mm3) = length× width2 × 0.5. After 3 weeks of treatment, the mice were euthanized by cervical dislocation. The subcutaneous tumors were harvested. Part of the tumors was frozen in liquid nitrogen for immunoblot analysis. The remainder was fixed in 4% formalin for HE or immunohistochemistry (IHC) staining. For IHC staining, antibody against Ki-67 was used to evaluate cell proliferation rate. The Ki-67 staining score was determined using the following formula: overall scores = intensity score × percentage of positive cells. The intensity scores were graded as 0–3 (0 = negative staining, 1 = weakly positive staining, 2 = moderately positive staining and 3 = strongly positive staining). The percentage of positive cells was graded from 0 to 100%. The staining results were observed in three random microscopic fields under the microscope.
Xenograft mouse model was used to evaluate sorafenib/carfilzomib effect with or without CHOP expression. Hep3B cells expressing CHOP shRNA or control shRNA were injected subcutaneously into the athymic nude mice. After tumors were developed, mice were treated using combined sorafenib/carfilzomib as mentioned above. For IHC staining, anti-CHOP antibody was used to detect the CHOP expression in the tumor tissues. Cell proliferation was detected by Ki67 staining. Apoptotic cells in tumor tissues were detected by Terminal deoxynucleotidyl transferase-mediated dUTP-digoxigenin nick end labeling (TUNEL) assay with In Situ Cell Death Detection Kit (Roche). The cell nuclei with brown color were considered as cells undergoing programmed death.
Statistical analysis
One-way ANOVA was used to measure difference among treated groups versus control group. Unpaired t-test was used to compare the differences between each treated group to the combination group in colony formation, apoptosis assay and migration assays. The difference in tumor growth rate between the 4 different groups of nude mice was determined by repeated measures ANOVA. Results are shown as mean ± SD. A p value of less than 0.05 is considered statistically significant. All statistical analyses were conducted using the SPSS17.0 statistical software (Version 17.0 SPSS Inc.) and GraphPad Prism 6.0 (GraphPad Software).
Results
Carfilzomib has anti-HCC activity and displays synergy with sorafenib
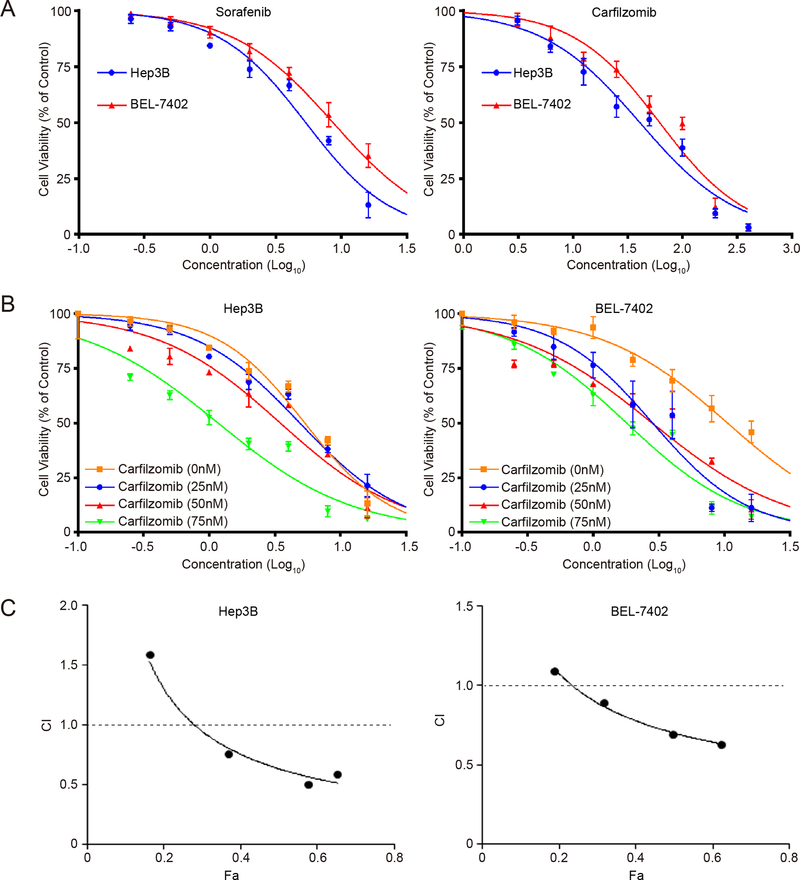
Carfilzomib is a second generation of proteasome inhibitor that can irreversibly target the proteasome. To evaluate its therapeutic potential toward liver cancer, we studied carfilzomib in Hep3B and Bel-7402, two commonly used liver cancer cell lines. Indeed, carfilzomib displays excellent anticancer activity as measured by the MTS assay, with an IC50 value at 40.80 nM and 61.95 nM, respectively, which compare favorably with sorafenib that shows an IC50 of 5.35 μM and 8.62 μM for Hep3B and Bel-7402, respectively (Fig. 1A). We further evaluated the combinational activity of carfilzomib with sorafenib. The results show that carfilzomib significantly enhances sorafenib anti-HCC activity in a dosage-dependent manner, though there is a slight variation between the two HCC cell lines (Fig. 1B). We therefore evaluated their synergism using the Chou-Talalay method with the Calcusyn software (Biosoft, Cambridge, UK). A combination index (CI) less than 1 indicates the drug combination has synergism. The results show that the combination index (CI) value can reach as low as 0.506, demonstrating a strong synergistic activity for this drug combination (Fig. 1C, Table 1).
Figure 1. Sorafenib and carfilzomib synergistically inhibit the proliferation of HCC cells.
(A) Hep3B and Bel-7402 cells were treated with various concentrations of sorafenib or carfilzomib for 48h. Cell proliferation was determined by the MTS assay in triplicate. Data represent mean ± SD. IC50 value of sorafenib and carfilzomib in HCC cell lines was then determined.
(B) Sorafenib inhibition of HCC cell proliferation is enhanced by carfilzomib. Hep3B and Bel-7402 cells were treated with sorafenib and various concentrations of carfilzomib for 48h. Cell proliferation was determined by the MTS assay. The assays were performed in triplicate. Data represent mean ± SD.
(C) CalcuSyn software was used to determine whether there was synergism between sorafenib and carfilzomib. Combination index (CI) of the combination of sorafenib and carfilzomib is shown. The assays were repeated in triplicate.
Table 1.
The combination index calculated by CalcuSyn software
| Sorafenib (μM) |
Carfilzomib (nM) |
Hep3B |
Bel-7402 |
||
|---|---|---|---|---|---|
| Fa | CI | Fa | CI | ||
| 1.25 | 12.5 | 0.162 | 1.589 | 0.187 | 1.091 |
| 2.5 | 25 | 0.368 | 0.757 | 0.315 | 0.895 |
| 5.0 | 50 | 0.575 | 0.506 | 0.494 | 0.692 |
| 7.5 | 100 | 0.651 | 0.591 | 0.620 | 0.632 |
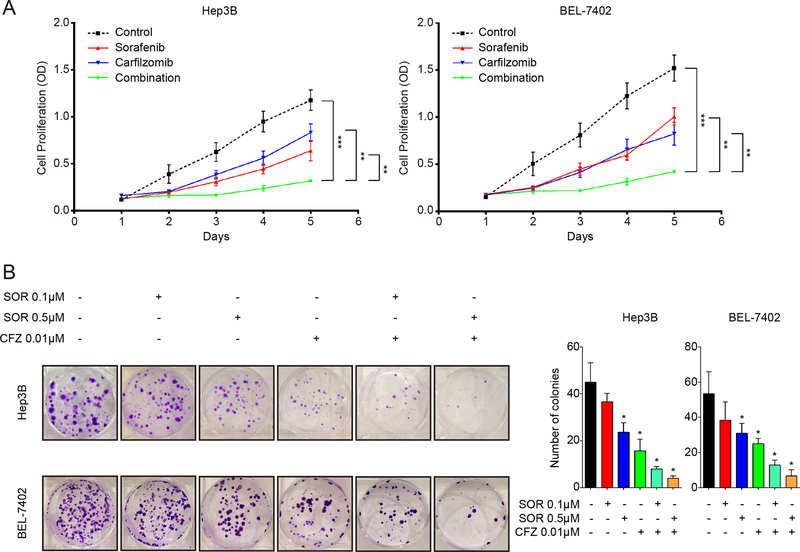
To further explore the anti-proliferation activity of sorafenib in combination with carfilzomib, we analyzed their time-dependent effect on Hep3B and Bel-7402 cells. Sorafenib and carfilzomib together exhibits much more potent and durable inhibition of HCC cell proliferation than either drug alone (p < 0.05) (Fig. 2A). When evaluated by the colony formation assay, 0.01μM carfilzomib and 0.5 μM sorafenib individually only moderately inhibit the colony formation, and 0.1 μM sorafenib alone showed no significant effect (Fig. 2B). In contrast, their combination results in far more significant reduction in the number of colonies (all p values < 0.05) (Fig. 2B). These findings demonstrate that carfilzomib has anticancer activity toward HCC and has the ability to significantly enhance the therapeutic effect of sorafenib.
Figure 2. Sorafenib and carfilzomib synergistically inhibit oncogenic growth of HCC cells.
(A) Hep3B and Bel-7402 cells were treated with sorafenib (2.5 μM) and carfilzomib (0.025 μM) individually or in combination for various times. HCC cell proliferation was determined by the MTS assay.
(B) Carfilzomib enhances the ability of sorafenib to inhibit colony formation of HCC cells. Hep3B and Bel-7402 cells were treated with sorafenib (0.1 μM or 0.5 μM) and carfilzomib (0.01 μM) individually or in combination for about two weeks. The inhibition effects were observed based on the number of colonies formed. The results shown are representative of three independent experiments. All Data were shown as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001.
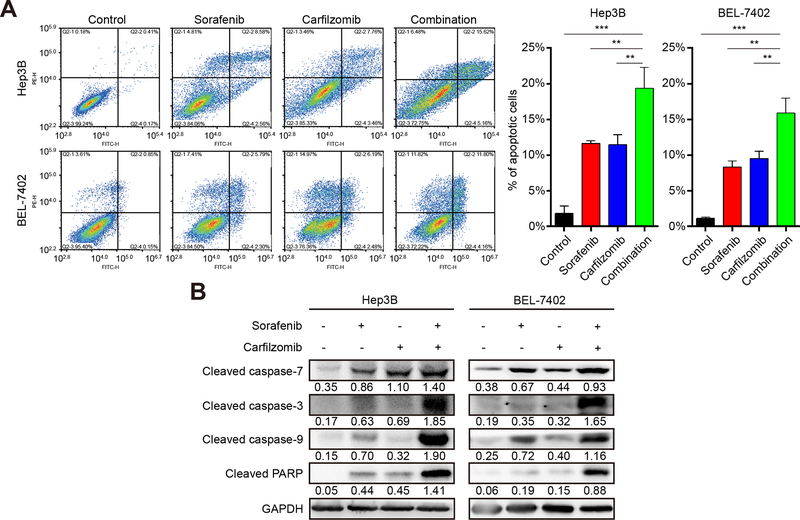
Carfilzomib promotes the apoptotic activity of sorafenib in HCC
Inducing cancer cell death is an important anticancer mechanism. We asked if carfilzomib and sorafenib have this therapeutic property. To this end, we used flow cytometry to analyze Hep3B and Bel-7402 cells treated with carfilzomib and sorafenib individually or in combination. After treatment for 24 hours, both carfilzomib and sorafenib result in significant apoptotic cell population, which is further increased when two drugs were used together (p < 0.05) (Fig. 3A). We next investigated whether the induced cell death is caspase-dependent. By immunoblot analysis, we found that in HCC cells treatment with sorafenib and carfilzomib, or in combination, leads to an elevated cleavage of caspase-3, caspase-7, caspase-9 and PARP (Fig. 3B), indicating that the two drugs cause apoptosis through a caspase-dependent manner.
Figure 3. Carfilzomib and sorafenib induce apoptosis of HCC cells in a caspase-dependent manner.
(A) Hep3B and Bel-7402 cells were treated with carfilzomib (0.1 μM) and sorafenib (7.5 μM) individually or in combination for 24 h and analyzed for cell death by flow cytometry. The experiments were performed in triplicate and data represent mean ± SD. *p < 0.05, ** p < 0.01, ***p < 0.001.
(B) Cleaved caspase-3, cleaved caspase-7, cleaved caspase-9 and cleaved PARP were used to analyze apoptotic cell death as detected by immunoblot. GAPDH was used as a loading control. Number indicates relative abundance (arbitrary unit).
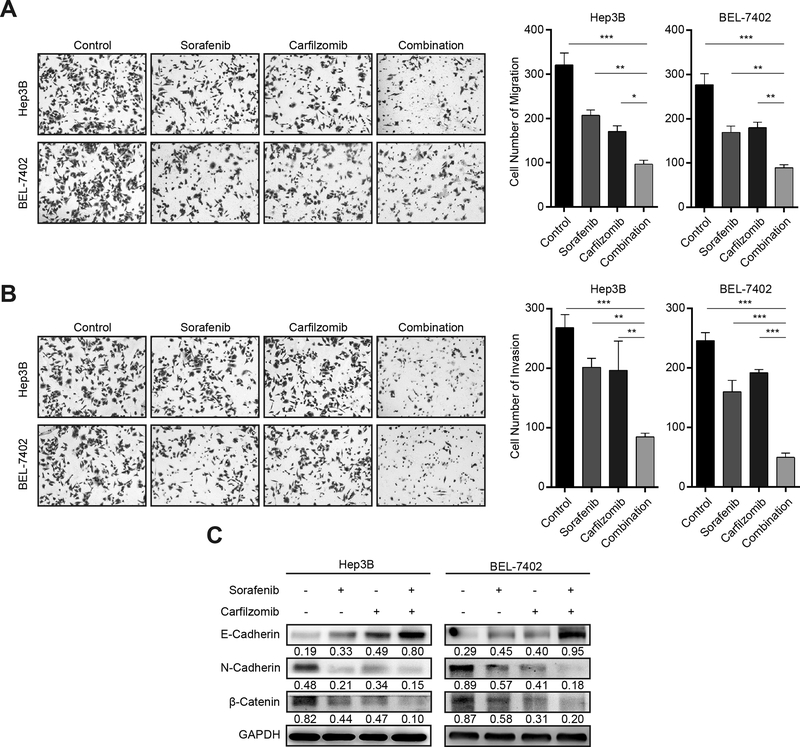
Carfilzomib and sorafenib inhibit HCC cell migration and invasion
Increased cancer cell motility and invasion are associated with liver cancer progression, which accounts for the morbidity and mortality of HCC patients. We next performed experiments to investigate the effect of carfilzomib and sorafenib on HCC cell migration and invasion. Transwell migration assay shows that each drug has moderate inhibition of HCC cell migration, but their combination has much more potently inhibitory effect (p < 0.05) (Fig. 4A). Carfilzomib and sorafenib individually only have a weak effect on HCC cell invasion (p < 0.05), but their combinational treatment, however, produces much stronger blockage of HCC cell invasion, especially for BEL-7402 cells (p < 0.05) (Fig. 4B). Taken together, these results show that the combination of carfilzomib and sorafenib markedly improves the anti-metastatic activity against HCC cells.
Figure 4. Carfilzomib and sorafenib inhibit HCC cell migration, invasion and EMT.
(A) Hep3B and Bel-7402 cells were treated with carfilzomib (0.05 μM) and sorafenib (7.5 μM) individually or in combination for 24 h. Transwell migration assays were performed to determine the migratory ability of Hep3B and Bel-7402 cells. The experiments were performed in triplicate and data represent mean ± SD. *p < 0.05, ** p < 0.01, ***p < 0.001.
(B) Hep3B and Bel-7402 cells were treated with carfilzomib (0.05 μM) and sorafenib (7.5 μM) individually or in combination for 24 h. Transwell invasion assays were performed in metrigel to determine the invasion ability of Hep3B and Bel-7402 cells. The experiments were performed in triplicate and data represent mean ± SD. *p < 0.05, ** p < 0.01, ***p < 0.001
(C) Hep3B and Bel-7402 cells were treated with carfilzomib (0.05 μM) and sorafenib (7.5 μM) individually or in combination for 24 h. The expression of the epithelial marker E-cadherin, and the mesenchymal markers N-Cadherin and β-Catenin was analyzed by immunoblot. GAPDH was used as a loading control. Number indicates relative abundance (arbitrary unit).
Epithelial to mesenchymal transition (EMT) is a biological process that converts epithelial cells to mesenchymal cells. During this process, epithelial cells lose cell-cell adhesion and gain an increased migratory and invasive ability (26). Because EMT is a major contributor to cancer cell invasiveness and metastasis, we studied how carfilzomib and sorafenib affect the EMT process in HCC cells. The results show that each drug alone moderately increases the epithelial marker E-Cadherin while decreases the expression of the mesenchymal markers N-Cadherin and β-Catenin (Fig. 4C). When the two drugs are used together, they produce much stronger inhibitory effect on EMT (Fig. 4C). These results indicate that carfilzomib and sorafenib have excellent combinational activity to block EMT in HCC cells.
Carfilzomib and sorafenib potently inhibit the growth of HCC xenograft tumors
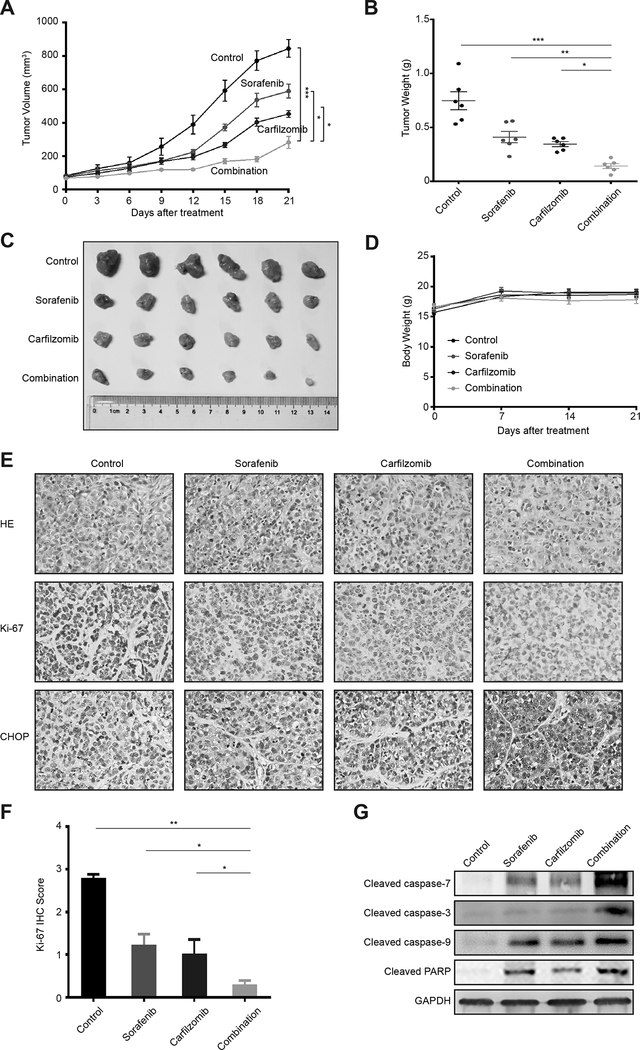
To evaluate the antitumor activity of carfilzomib and sorafenib in vivo, we established Hep3B xenograft tumors in nude mice. In this experiment, tumor bearing nude mice were randomized into four groups that were treated with vehicle control, sorafenib, carfilzomib or drug combination (n = 6 per group) for 3 weeks. Tumor volume was monitored throughout the study to evaluate the antitumor activity. As expected, sorafenib shows moderate antitumor activity (Fig. 5A). Carfilzomib has a slightly better antitumor effect than sorafenib (Fig. 5A). In contrast, the combination of sorafenib plus carfilzomib generates markedly higher antitumor activity than either drug alone, resulting in minimal tumor growth (Fig. 5A). Consistently, the drug combination group has the lowest tumor burden (Fig. 5B and 5C). Notably, all treated mice maintain similar body weight to the untreated animals and display no obvious signs of toxicity throughout the study, indicating that carfilzomib and sorafenib combination is well tolerated (Fig. 5D). Ki-67 staining of tumor sections from treated animals shows much more reduced cell proliferation in the drug combination group and individual drug groups (Fig. 5E and 5F), which is supported by the reduced mitotic cells as shown by HE staining (Fig. 5E). Moreover, caspase cleavage is higher in the drug combination group as determined by immunoblot (Fig. 5G). These data indicate that carfilzomib significantly enhances the antitumor activity of sorafenib in vivo.
Figure 5. Carfilzomib and sorafenib strongly attenuate HCC tumor growth in vivo.
(A) Xenograft tumors generated from Hep3B cells were treated with carfilzomib and sorafenib individually or in combination, or with a drug vehicle control. Growth of xenograft tumors was measured by tumor volume. Data represent mean ± SD (n = 6). Statistical analyses were performed by two-way ANOVA and sample-paired t-test. *p < 0.05, ** p < 0.01, ***p < 0.001.
(B, C) Shown are the weights and images of xenograt tumors at the end of the experiment. Data represent mean ± SD (n = 6). Statistical analyses were performed by one-way ANOVA and sample-paired t-test. *p < 0.05, ** p < 0.01, ***p < 0.001.
(D) The body weight +/− SD of mice in different treatment groups.
(E) Upper panel: Representative images of HE staining of HCC xenografted tumor sections in different treatment groups (200 × magnification); Lower panel: Representative images of Ki67 staining of HCC xenografted tumor sections in different treatment groups (200 × magnification).
(F) Quantification of IHC scores for Ki-67 staining in Hep3B xenograft tumors. Data represent mean ± SD (n = 6). Statistical analysis was performed by sample-paired t-test. *p < 0.05, ** p < 0.01, ***p < 0.001.
(G) Xenograft tumor tissues from different drug treatment groups were analyzed for cleaved caspase-3, cleaved caspase-7, cleaved caspase-9 and cleaved PARP by immunoblot. GAPDH was used as a loading control.
Carfilzomib and sorafenib trigger ER stress-mediated apoptosis through the PERK/eIF2α/ATF4/CHOP Pathway
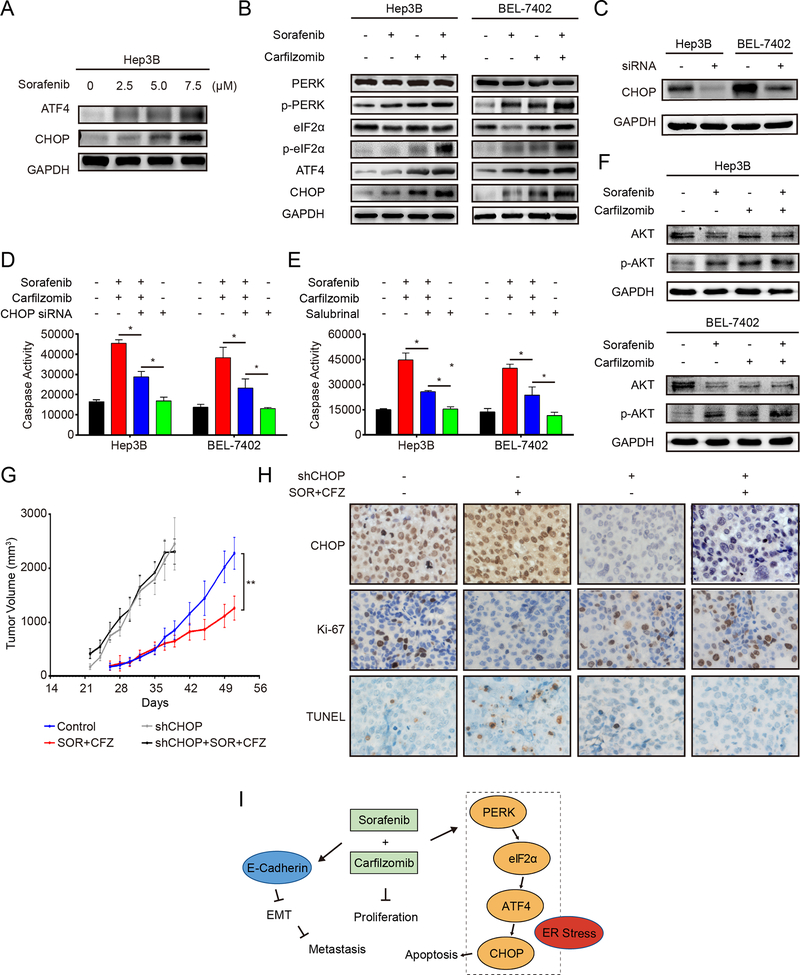
Endoplasmic reticulum (ER) stress is an important mechanism for cancer cell apoptosis (27). During ER stress, the ER stress kinase PERK is activated, which transduces the ER stress signal through phosphorylation of its downstream effectors eIF2α, and increased expression of ATF4 and CHOP. Caspase-7 is the main caspase that responds to ER stress (28). We found that caspase-7 is induced by the combinational treatment of carfilzomib and sorafenib (Fig. 3B and 5G), suggesting that ER stress is involved. These observations are consistent with the idea that proteasome inhibitor targets the ER-stress pathway (29–32). Hence, we investigated if ER stress is involved in the antitumor response by carfilzomib and sorafenib. The results show that both ATF4 and CHOP are up-regulated by sorafenib in a concentration dependent manner (Fig. 6A). When HCC cells are treated with sorafenib and carfilzomib together, the level of p-PERK and p-eIF2α is significantly increased, while total PERK and elF2α remain unchanged (Fig. 6B). Concomitantly, the expression of ATF4 and CHOP is increased (Fig. 6B). These data demonstrate that carfilzomib and sorafenib act together to induce ER-stress and ER-stress mediated apoptosis.
Figure 6. Carfilzomib and sorafenib treatments trigger ER stress-mediated apoptosis through the PERK/eIF2α/ATF4/CHOP pathway.
(A) Hep3B cells were treated with different concentrations of sorafenib (2.5, 5.0, 7.5 μM) for 24 h. The expression of ATF4 and CHOP was analyzed by immunoblot. GAPDH was used as a loading control.
(B) Hep3B and Bel-7402 cells were treated with carfilzomib (0.1 μM) and sorafenib (7.5 μM) individually or in combination for 24 h. Activation of the PERK/eIF2α/ATF4/CHOP pathway was analyzed by the expression of various marker proteins of the pathway by immunoblot.
(C) Shown is the knockdown efficiency of CHOP by siRNA as determined by immunoblot in Hep3B and Bel-7402 cells.
(D) Knockdown of CHOP decreases the activity of caspase-3/7 induced by carfilzomib and sorafenib. Hep3B and Bel-7402 cells were transfected with CHOP siRNA for 48 h before treated with carfilzomib (0.1 μM) and sorafenib (7.5 μM) individually or in combination for another 24 h. The activity of caspase-3/7 was determined by the Caspase-Glo assay. The experiments were performed in triplicate and data represent mean ± SD. *p < 0.05.
(E) Salubrinal suppressed caspase-3/7 activities induced by carfilzomib and sorafenib. Hep3B and Bel-7402 cells were pretreated with salubrinal (20 μM) for 12 h before treated with carfilzomib (0.1 μM) and sorafenib (7.5 μM) individually or in combination for another 24 h. The activity of caspase-3/7 was determined by the Caspase-Glo assay. The experiments were performed in triplicate and data represent mean ± SD. * p < 0.05.
(F) Hep3B and Bel-7402 cells were treated with carfilzomib (0.1 μM) and sorafenib (7.5 μM) individually or in combination for 24 h. Protein levels of p-AKT and AKT were analyzed by immunoblot.
(G) Xenograft tumors generated from Hep3B cells expressing shCHOP or control shRNA were treated with combined carfilzomib/ sorafenib or a drug vehicle. Growth of xenograft tumors was measured by tumor volume. Data represent mean ± SD (n = 8). Statistical analyses were performed by two-way ANOVA and sample-paired t-test. ** p < 0.01.
(H) Representative images of IHC staining for CHOP (upper panels), Ki67 (middle panels) and TUNEL (lower panels) in HCC xenograft tumor sections in different treatment groups (400 × magnification).
(I) A working model illustrating the inhibitory mechanism of carfilzomib and sorafenib against HCC.
CHOP up-regulation is critical for ER-stress induced apoptosis (33). To ask if CHOP has a role in carfilzomib/sorafenib-induced apoptosis, we knocked down CHOP in Hep3B and Bel-7402 cells by a CHOP-specific siRNA (Fig. 6C). CHOP down-regulation abrogates caspase-3/7 cleavage (Fig. 6D). To further verify the role ER stress in carfilzomib/sorafenib-induced apoptosis, we treated HCC cells with salubrinal, a selective inhibitor of eIF2α dephosphorylation and ER-stress induced apoptosis (34). Salubrinal significantly attenuates caspase 3/7 cleavage induced by the carfilzomib and sorafenib combination (Fig. 6E). A previous study suggested that the combinational treatment with sorafenib and bortezomib enhances apoptosis in HCC cells through inhibition of AKT (16). To ask if AKT is involved in the anti-HCC effects of sorafenib and carfilzomib combination, we investigated the effect of these drugs on AKT phosphorylation, an indicator of AKT activation status. On the contrary, sorafenib and carfilzomib alone or in combination not only do not inhibit AKT phosphorylation, they actually induce robust increase in the p-AKT level (Fig. 6F). These results demonstrate that ER-stress, rather than AKT inhibition, is a major mechanism for carfilzomib/sorafenib-induced apoptotic cell death.
We further investigated the role of sorafenib/carfilzomib in ER-stress induced apoptosis using xenograft model. Subcutaneous xenograft tumors were established from Hep3B cells with stable CHOP knockdown by shRNA or a control shRNA. The growth rate of Hep3B tumors with CHOP knockdown is considerably higher than the control tumors, (Fig. 6G). While the combination of sorafenib and carfilzomib shows significant antitumor activity in the control group, it has no discernible effect toward the CHOP knockdown tumors (Fig. 6G). IHC staining shows that combinational treatment of sorafenib/carfilzomib increases CHOP expression, reduced proliferation (Ki67 staining) and elevated apoptosis (TUNEL staining) in the control tumors (Fig. 6H). In contrast, the combinational treatment has little effect on cancer cell proliferation and apoptosis in the CHOP knockdown tumors (Fig. 6H). These results indicate that carfilzomib and sorafenib together produce antitumor activity through ER stress/CHOP-mediated growth inhibition and apoptosis in vivo.
Discussion
Sorafenib has been the only available systematic drug for unresectable HCC until the recent US FDA approval of Regorafenib. Unfortunately, both Sorafenib and Regorafenib have limited clinical benefits. Developing new liver cancer drugs has proven to be especially challenging. Thus, considerable efforts have been directed at improving the treatment outcome for sorafenib. Combinational drug therapy is an useful approach to enhance their anticancer activity while minimizing the adverse effects due to reduced drug dosage of each agent. They can also reduce the potential drug resistance because each drug attacks cancer cells through distinct mechanisms. The proteasome inhibitors bortezomib and carfilzomib are FDA-approved oncology drugs for multiple myeloma. Proteasome targeting has also been recognized as a promising approach for solid tumors (35). For example, bortezomib showed modest single-agent activity in patients with relapsed or refractory advanced non-small-cell lung cancer (36). Objective clinical response for carfilzomib combined with irinotecan had 19% partial response and 6% stable disease in small cell lung cancer in a phase I clinical study (37). However, there is a strong need to improve the antitumor activity of proteasome inhibitors in order to advance them in the clinic for solid tumors. Our study demonstrates that carfilzomib and sorafenib display excellent synergistic anticancer action against HCC in vitro and in vivo. Further development of this therapeutic strategy may lead to a new treatment for HCC, and possibly other solid tumors.
Endoplasmic-reticulum stress (ER Stress), also known as unfolded protein response (UPR), happens when unfolded or misfolded proteins accumulate in the ER lumen (38). In response to ER stress, cells up-regulate molecular chaperones to restore normal protein folding and ameliorate the source of ER stress. In the event that unfolded proteins cannot be corrected, cells commit to apoptosis. The proteasome plays a critical role in clearing unfolded/misfolded proteins. Emerging evidence suggests that targeting proteasome is a promising strategy for anticancer therapy due to its ability to selectively kill malignant cells (39). Bortezomib was previously reported to induce ER-stress mediated apoptosis in multiple myeloma (MM) by increasing the expression of UPR genes BiP, CHOP and XBP-1 (29,40). In this study, we found that the combination of carfilzomib and sorafenib leads to activation of PERK, phosphorylation of eIF2α, and increased expression of ATF4 and CHOP, as well as enhanced caspase-dependent apoptosis. Thus, pharmacological inhibition of proteasome by carfilzomib enhances the ability of sorafenib to promote ER stress-dependent cell death pathway.
The combination of carfilzomib and sorafenib displays remarkably stronger inhibition of HCC cell migration and invasion than each drug individually, suggesting that they are especially useful for treatment of late stage liver tumors that are invasive and metastatic. Mechanistically, the drug combination inhibits EMT, an oncogenic process required for the metastatic colonization of HCC (41). Although sorafenib has been reported to inhibit the EMT and metastasis of HCC cells (42–44), its effect is relatively weak (Fig. 4). Here we show for the first time that carfilzomib also moderately inhibits EMT, migration and invasion of HCC cells. The combination of carfilzomib and sorafenib produces a much more robust blockage of EMT, which is consistent with the inhibitory effect on HCC cell migration and invasion.
There are several new observations in our study with carfilzomib/sorafenib compared with the previous study with bortezomib/sorafenib. First, the previous study used sorafenib pretreatment to prevent AKT activation by bortezomib. In our study, however, carfilzomib, sorafenib and their combination all activate AKT. This observation is consistent with the notion that blockage of ERK pathway leads to feedback activation of the PI3K-AKT pathway (45). Second, carfilzomib/sorafenib strongly inhibits the epithelial-to-mesenchymal transition (EMT), and migration/invasion of HCC cells, which was not observed in the previous study. Third, carfilzomib/sorafenib triggers ER stress and ER stress-induced activation of apoptosis, which was not observed in the previous study. Finally, Bortezomib is a first-generation proteasome inhibitor that reversibly inhibits both the chymotrypsin-like (CT-L) and caspase-like (C-L) activities of the proteasome, and also inhibits the serine proteases chymase, dipeptidyl peptidase II, HtrA2/Omi, and cathepsins A and G (46–48). It is limited clinically due to acquired resistance and severe toxicities, including peripheral neuropathy (46,49–54). Moreover, recent studies indicate that these adverse events are due to off-target, nonproteasomal effects of bortezomib (46,55). In contrast, carfilzomib is highly selective for the CT-L activity. Compared with bortezomib, carfilzomib has higher affinity to proteasome and lower off-target toxicity. It does not exhibit inhibitory activity against the multiple serine proteases targeted by bortezomib, and has a markedly reduced rate of peripheral neuropathy in patients (46). For these reasons, it is important to explore the combinational therapy of carfilzomib and sorafenib in liver cancer.
In conclusion, the present study shows that the combinational treatment with carfilzomib and sorafenib synergistically inhibit HCC cells proliferation, migration and invasion. Moreover, they induce HCC cells to undergo apoptosis through ER stress and activation of the PERK/eIF2α/ATF4/CHOP pathway (Fig. 6I). Because carfilzomib and sorafenib are both FDA-approved oncology drugs, this combinational therapy is readily translatable into the clinic, improving the mortality and morbidity of liver cancer patients.
Acknowledgments
This work was supported by the National Institutes of Health R01 grant CA123391 (X.F. Zheng), and the National Natural Science Foundation of China (81372564, 81730081 and 81772635) (XX Li and HY Wang), Guangdong Natural Science Foundation for Distinguished Young Scholar (No: 2015A030306047) (XX Li). We thank Ju-Deng Zeng for technical assistance.
Footnotes
Conflicts of Interest
The authors declare no known conflicts of interest.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians 2015;65(2):87–108 doi 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA: a cancer journal for clinicians 2016;66(2):115–32 doi 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB. Hepatocellular Carcinoma. New England Journal of Medicine 2011;365(12):1118–27 doi 10.1056/NEJMra1001683. [DOI] [PubMed] [Google Scholar]
- 4.Kudo M Immune Checkpoint Blockade in Hepatocellular Carcinoma: 2017 Update. Liver Cancer 2016;6(1):1–12 doi 10.1159/000449342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, et al. BAY 43–9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer research 2004;64(19):7099–109 doi 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 6.Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. The Lancet Oncology 2009;10(1):25–34 doi 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 7.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. The New England journal of medicine 2008;359(4):378–90 doi 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 8.Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, Lacava J, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. Jama 2010;304(19):2154–60 doi 10.1001/jama.2010.1672. [DOI] [PubMed] [Google Scholar]
- 9.Sohn W, Paik YH, Cho JY, Lim HY, Ahn JM, Sinn DH, et al. Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. Journal of hepatology 2015;62(5):1112–21 doi 10.1016/j.jhep.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Lencioni R, Llovet JM, Han G, Tak WY, Yang J, Guglielmi A, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. Journal of hepatology 2016;64(5):1090–8 doi 10.1016/j.jhep.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Palumbo A, Chanan-Khan A, Weisel K, Nooka AK, Masszi T, Beksac M, et al. Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. The New England journal of medicine 2016;375(8):754–66 doi 10.1056/NEJMoa1606038. [DOI] [PubMed] [Google Scholar]
- 12.Bonvini P, Zorzi E, Basso G, Rosolen A. Bortezomib-mediated 26S proteasome inhibition causes cell-cycle arrest and induces apoptosis in CD-30+ anaplastic large cell lymphoma. Leukemia 2007;21(4):838–42 doi 10.1038/sj.leu.2404528. [DOI] [PubMed] [Google Scholar]
- 13.Caravita T, de Fabritiis P, Palumbo A, Amadori S, Boccadoro M. Bortezomib: efficacy comparisons in solid tumors and hematologic malignancies. Nat Clin Prac Oncol 2006;3(7):374–87. [DOI] [PubMed] [Google Scholar]
- 14.Huang Z, Wu Y, Zhou X, Xu J, Zhu W, Shu Y, et al. Efficacy of therapy with bortezomib in solid tumors: a review based on 32 clinical trials. Future oncology (London, England) 2014;10(10):1795–807 doi 10.2217/fon.14.30. [DOI] [PubMed] [Google Scholar]
- 15.Wahl K, Siegemund M, Lehner F, Vondran F, Nussler A, Langer F, et al. Increased apoptosis induction in hepatocellular carcinoma by a novel tumor-targeted TRAIL fusion protein combined with bortezomib. Hepatology 2013;57(2):625–36 doi 10.1002/hep.26082. [DOI] [PubMed] [Google Scholar]
- 16.Chen KF, Yu HC, Liu TH, Lee SS, Chen PJ, Cheng AL. Synergistic interactions between sorafenib and bortezomib in hepatocellular carcinoma involve PP2A-dependent Akt inactivation. Journal of hepatology 2010;52(1):88–95 doi 10.1016/j.jhep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 17.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nat Rev Clin Oncol 2017;advance online publication doi 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM, et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 2007;110(9):3281–90 doi 10.1182/blood-2007-01-065888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mansour MA, Aljoufi MA, Al-Hosaini K, Al-Rikabi AC, Nagi MN. A possible antineoplastic potential of selective, irreversible proteasome inhibitor, carfilzomib on chemically induced hepatocarcinogenesis in rats. J Biochem Mol Toxicol 2014;28(9):400–6 doi 10.1002/jbt.21577. [DOI] [PubMed] [Google Scholar]
- 20.Smith MA, Houghton P. A proposal regarding reporting of in vitro testing results. Clin Cancer Res 2013;19(11):2828–33 doi 10.1158/1078-0432.CCR-13-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Tsang CK, Wang S, Li XX, Yang Y, Fu L, et al. MAF1 suppresses AKT-mTOR signaling and liver cancer through activation of PTEN transcription. Hepatology (Baltimore, Md) 2016;63(6):1928–42 doi 10.1002/hep.28507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas JD, Zheng XFS. Golgi apparatus location and regulation of mTOR is regulated by novel Golgi recruiting protein GRP. FASEB J 2012;26(1_MeetingAbstracts):799.4-.22042224 [Google Scholar]
- 23.Tsang CK, Chen M, Cheng X, Qi Y, Chen Y, Das I, et al. SOD1 Phosphorylation by mTORC1 Couples Nutrient Sensing and Redox Regulation. Molecular Cell 2018;70(3):502–15.e8 doi 10.1016/j.molcel.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu T-J, Wang X, Zhang Y, Meng L, Kerrigan John E, Burley Stephen K, et al. Identification of a Non-Gatekeeper Hot Spot for Drug-Resistant Mutations in mTOR Kinase. Cell Reports 2015;11(3):446–59 doi 10.1016/j.celrep.2015.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H, Li X, Yang Y, Zhang Y, Wang HY, Zheng XFS. Significance and Mechanism of Androgen Receptor (AR) Overexpression and AR-mTOR Crosstalk in Hepatocellular Carcinoma. Hepatology (Baltimore, Md) 2017. doi 10.1002/hep.29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139(5):871–90 doi 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Kim I, Xu W, Reed JC. Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 2008;7(12):1013–30. [DOI] [PubMed] [Google Scholar]
- 28.Lee AS. Glucose-regulated proteins in cancer: molecular mechanisms and therapeutic potential. Nature reviews Cancer 2014;14(4):263–76 doi 10.1038/nrc3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obeng EA, Carlson LM, Gutman DM, Harrington WJ Jr., Lee KP, Boise LH. Proteasome inhibitors induce a terminal unfolded protein response in multiple myeloma cells. Blood 2006;107(12):4907–16 doi 10.1182/blood-2005-08-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fels DR, Ye J, Segan AT, Kridel SJ, Spiotto M, Olson M, et al. Preferential cytotoxicity of bortezomib toward hypoxic tumor cells via overactivation of endoplasmic reticulum stress pathways. Cancer research 2008;68(22):9323–30 doi 10.1158/0008-5472.CAN-08-2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nawrocki ST, Carew JS, Pino MS, Highshaw RA, Dunner K, Jr., Huang P, et al. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer research 2005;65(24):11658–66 doi 10.1158/0008-5472.CAN-05-2370. [DOI] [PubMed] [Google Scholar]
- 32.Ruckrich T, Kraus M, Gogel J, Beck A, Ovaa H, Verdoes M, et al. Characterization of the ubiquitin-proteasome system in bortezomib-adapted cells. Leukemia 2009;23(6):1098–105 doi 10.1038/leu.2009.8. [DOI] [PubMed] [Google Scholar]
- 33.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, et al. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes & Development 2004;18(24):3066–77 doi 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyce M, Bryant KF, Jousse C, Long K, Harding HP, Scheuner D, et al. A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science (New York, NY) 2005;307(5711):935–9 doi 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 35.Manasanch EE, Orlowski RZ. Proteasome inhibitors in cancer therapy. Nature Reviews Clinical Oncology 2017;14:417 doi 10.1038/nrclinonc.2016.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fanucchi MP, Fossella FV, Belt R, Natale R, Fidias P, Carbone DP, et al. Randomized phase II study of bortezomib alone and bortezomib in combination with docetaxel in previously treated advanced non-small-cell lung cancer. J Clin Oncol 2006;24(31):5025–33 doi 10.1200/JCO.2006.06.1853. [DOI] [PubMed] [Google Scholar]
- 37.Arnold SM, Chansky K, Leggas M, Thompson MA, Villano JL, Hamm J, et al. Phase 1b trial of proteasome inhibitor carfilzomib with irinotecan in lung cancer and other irinotecan-sensitive malignancies that have progressed on prior therapy (Onyx IST reference number: CAR-IST-553). Invest New Drugs 2017;35(5):608–15 doi 10.1007/s10637-017-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science (New York, NY) 2011;334(6059):1081–6 doi 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 39.Clarke Hanna J, Chambers Joseph E, Liniker E, Marciniak Stefan J. Endoplasmic Reticulum Stress in Malignancy. Cancer Cell;25(5):563–73 doi 10.1016/j.ccr.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 40.Dong H, Chen L, Chen X, Gu H, Gao G, Gao Y, et al. Dysregulation of unfolded protein response partially underlies proapoptotic activity of bortezomib in multiple myeloma cells. Leukemia & lymphoma 2009;50(6):974–84 doi 10.1080/10428190902895780. [DOI] [PubMed] [Google Scholar]
- 41.Giannelli G, Koudelkova P, Dituri F, Mikulits W. Role of epithelial to mesenchymal transition in hepatocellular carcinoma. Journal of hepatology 2016;65(4):798–808 doi 10.1016/j.jhep.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Feng YX, Wang T, Deng YZ, Yang P, Li JJ, Guan DX, et al. Sorafenib suppresses postsurgical recurrence and metastasis of hepatocellular carcinoma in an orthotopic mouse model. Hepatology 2011;53(2):483–92 doi 10.1002/hep.24075. [DOI] [PubMed] [Google Scholar]
- 43.Deng YR, Liu WB, Lian ZX, Li X, Hou X. Sorafenib inhibits macrophage-mediated epithelial-mesenchymal transition in hepatocellular carcinoma. Oncotarget 2016;7(25):38292–305 doi 10.18632/oncotarget.9438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen YL, Lv J, Ye XL, Sun MY, Xu Q, Liu CH, et al. Sorafenib inhibits transforming growth factor beta1-mediated epithelial-mesenchymal transition and apoptosis in mouse hepatocytes. Hepatology 2011;53(5):1708–18 doi 10.1002/hep.24254. [DOI] [PubMed] [Google Scholar]
- 45.Turke AB, Song Y, Costa C, Cook R, Arteaga CL, Asara JM, et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer research 2012;72(13):3228–37 doi 10.1158/0008-5472.CAN-11-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zang Y, Thomas SM, Chan ET, Kirk CJ, Freilino ML, DeLancey HM, et al. Carfilzomib and ONX 0912 inhibit cell survival and tumor growth of head and neck cancer and their activities are enhanced by suppression of Mcl-1 or autophagy. Clinical cancer research : an official journal of the American Association for Cancer Research 2012;18(20):5639–49 doi 10.1158/1078-0432.CCR-12-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Groll M, Ditzel L, Lowe J, Stock D, Bochtler M, Bartunik HD, et al. Structure of 20S proteasome from yeast at 2.4 A resolution. Nature 1997;386(6624):463–71 doi 10.1038/386463a0. [DOI] [PubMed] [Google Scholar]
- 48.Orlowski M, Wilk S. Catalytic activities of the 20 S proteasome, a multicatalytic proteinase complex. Archives of biochemistry and biophysics 2000;383(1):1–16 doi 10.1006/abbi.2000.2036. [DOI] [PubMed] [Google Scholar]
- 49.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. The New England journal of medicine 2003;348(26):2609–17 doi 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 50.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, et al. Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. The New England journal of medicine 2005;352(24):2487–98 doi 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 51.Orlowski RZ, Nagler A, Sonneveld P, Blade J, Hajek R, Spencer A, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2007;25(25):3892–901 doi 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 52.O’Connor OA, Wright J, Moskowitz C, Muzzy J, MacGregor-Cortelli B, Stubblefield M, et al. Phase II clinical experience with the novel proteasome inhibitor bortezomib in patients with indolent non-Hodgkin’s lymphoma and mantle cell lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2005;23(4):676–84 doi 10.1200/JCO.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 53.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2006;24(19):3113–20 doi 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 54.Lonial S, Waller EK, Richardson PG, Jagannath S, Orlowski RZ, Giver CR, et al. Risk factors and kinetics of thrombocytopenia associated with bortezomib for relapsed, refractory multiple myeloma. Blood 2005;106(12):3777–84 doi 10.1182/blood-2005-03-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arastu-Kapur S, Anderl JL, Kraus M, Parlati F, Shenk KD, Lee SJ, et al. Nonproteasomal targets of the proteasome inhibitors bortezomib and carfilzomib: a link to clinical adverse events. Clinical cancer research : an official journal of the American Association for Cancer Research 2011;17(9):2734–43 doi 10.1158/1078-0432.CCR-10-1950. [DOI] [PubMed] [Google Scholar]