Abstract
Background
Chinese herbal medicines (CHMs) have been widely used in the treatment of cervicogenic dizziness (CGD) based on their empirical effectiveness and safety. Herein, we reviewed and evaluated the clinical evidence of the efficacy and safety of CHMs for CGD.
Methods
Among the relevant studies published in 11 electronic databases up to December 2021, only randomised controlled trials were included. Methodological quality was assessed using the revised Cochrane risk-of-bias tool for randomised trials, and the strength of evidence for the main outcomes was evaluated using the grading of recommendations assessment, development, and evaluation system.
Results
All 35 included randomised controlled trials with 3,862 participants were conducted with six types of modified CHM and four types of active controls. More than half of the included studies were of low quality because of the high risk of bias due to deviations from intended interventions. CHM plus active control was more effective in the treatment of CGD than active control alone. CHM plus anti-vertigo drugs, CHM plus manual therapy, CHM plus acupuncture therapy, and CHM plus manual and acupuncture therapy were all effective in treating CGD, with CHM plus manual and acupuncture therapy showing the most reliable effect. All CHMs were effective for specific patterns of CGD when administered with active controls, with Dingxuan Tang and Yiqi Congming Tang demonstrating the most reliable effects. No serious adverse events were reported in any of the included studies.
Conclusion
The current evidence suggests that CHM may enhance the treatment of CGD when combined with other treatments without serious adverse events. Further high-quality evidence is needed to draw definitive conclusions.
1. Introduction
Cervicogenic dizziness (CGD), a major cause of dizziness, is associated with a variety of symptoms, such as headache, unsteadiness, light-headedness, perception of spinning, nausea, and general disorientation, coexisting with neck pain or stiffness [1–4]. Its prevalence is estimated to be 6.4–8.5% [5–7]; however, CGD is common in older patients, especially those with cervical spine dysfunction. Therefore, there is growing apprehension that the number of patients with CGD will increase in accordance with a worldwide ageing population [8–10].
Although it is known that CGD originates from the cervical spine, its pathogenesis remains unclear [11]. Until now, the most prevalent hypothesis is that CGD is caused by disharmonic hyperactivity of the cervical mechanoreceptors located in the joints, ligaments, and muscle spindles, which occurs when the proprioceptive system of the neck is damaged due to muscular fatigue, degeneration, or trauma [10, 12–14]. In a recent review, CGD was classified according to the aetiopathological mechanisms into neural types, comprising degenerative cervical spine disorder, whiplash-associated disorder, and Barré–Liéou syndrome, and vascular types, comprising Bow Hunter's syndrome and Beauty Parlour syndrome. However, these diseases also overlap because they do not have completely distinct mechanisms [15]. Because there are no established diagnostic criteria for CGD, physicians usually diagnose CGD when the patients' symptoms are not related to other neurological or neuro-otological causes of dizziness [16, 17].
The treatment of CGD has not yet been standardised. Previous studies have explored a variety of treatments to improve the severity and frequency of dizziness by relaxing muscles and ameliorating abnormal proprioceptive sensitivity or impaired blood flow in the cervical region. Treatment strategies include physical therapy [1, 3, 7, 10, 18–22], surgery [10, 16], topical drug injection [9, 23], acupuncture therapy [24, 25], and medications, such as muscle relaxants, opioids, nonsteroidal anti-inflammatory drugs, and anxiolytics, in combination with Chinese herbal medicines (CHMs). CHMs have been widely used for CGD, either alone or in combination with other treatments, based on their empirical effectiveness to suppress pain and improve blood circulation in the human body [24, 26]. However, there has been no systematic verification of their efficacy and safety in the treatment of CGD based on clinical evidence.
Therefore, we aimed to review and evaluate the clinical evidence on the efficacy and safety of CHM as monotherapy or adjunctive therapy for CGD, which would promote evidence-based decision-making in clinical practice.
2. Methods
2.1. Study Registration
The study protocol for this systematic review was registered with the International Prospective Register of Systematic Reviews (registration number: CRD42020199222; registration date: October 27, 2020) and the Research Registry (Review Registry Unique Identifying Number: reviewregistry1036; registration date: November 19, 2020). The study protocol was published [27], and there have been no subsequent amendments that could result in a significant change in the study design. This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses statement [28]. A preprint has previously been published in Research Square (DOI: https://doi.org/10.21203/rs.3.rs-364098/v1; registration date: March 31, 2021) [29].
2.2. Data Sources and Search Strategy
One researcher (HO) comprehensively searched the following 11 electronic databases for relevant studies published up to December 2021 without language or publication status restrictions: three English databases (Medical Literature Analysis and Retrieval System Online (MEDLINE) via PubMed, Excerpta Medica Database (EMBASE) via Elsevier, and the Cochrane Central Register of Controlled Trials (CENTRAL)), six Korean databases (KoreaMed, Korean Studies Information Service System, Research Information Sharing Service, National Digital Science Library, Korean Medical Database, and Database Periodical Information Academic), one Chinese database (China National Knowledge Infrastructure), and one Japanese database (Citation Information by NII). A manual search on Google Scholar was also performed to identify additional eligible studies among those listed in the reference sections of included studies. The search strategies were tailored to the language and search systems of the databases. The search strategies used in the three English databases (MEDLINE, EMBASE, and CENTRAL) are presented in Additional file 1.
2.3. Eligibility Criteria
2.3.1. Types of Studies
All randomised controlled trials (RCTs) related to the use of CHMs for CGD were included. All other study designs, including quasi-RCTs, were excluded.
2.3.2. Participants
All patients with CGD were included as subjects in this study, with no restrictions on ethnicity, nationality, sex, age, or biological status.
2.3.3. Interventions and Comparisons
CHMs with any formulation administered orally, such as decoction, capsules, tablets, pills, and powders, were considered experimental interventions. There was no limitation on the number or combination of herbs, CHM dose, or the frequency or duration of treatment. If the composition of CHMs used in the included studies differed from the original prescription, “modified” was indicated in front of the CHM name. No treatment and placebo were considered as control interventions to determine the efficacy of CHM as monotherapy. Active controls, such as anti-vertigo drugs, manual therapy, and acupuncture therapy, were also considered as control interventions to determine the efficacy of CHM as adjunctive therapy only when CHMs were equally applied to both the experimental and control groups. Studies comparing different combinations of CHMs or CHM alone with other active controls were excluded because they could not rigorously determine the efficacy of CHMs.
2.3.4. Outcomes
The primary outcomes were as follows:
The change in the patients' overall functional score measured by validated scales (e.g., functional scale for cervical spondylosis of vertebral artery type)
The change in the patients' simple score for dizziness (e.g., the numerical rating scale)
The change in mean blood flow velocity in the vertebrobasilar artery, as evaluated using transcranial Doppler
The secondary outcomes were as follows:
The total effective rate, strictly calculated by counting only the number of patients completely cured, to exclude researcher subjectivity and improve the reliability of the results
The changes in haematological parameters, such as fibrinogen levels, endothelin, total cholesterol (TC), and calcitonin gene-related peptide (CGRP)
Adverse events
2.4. Study Selection Process
Two reviewers (HO and SS) independently screened and assessed all retrieved studies for eligibility based on the aforementioned criteria. After duplicates were removed, the titles and abstracts of the remaining studies were screened using EndNote X9 (Clarivate Analytics, London, UK). Next, the full-text review of the eligible studies was conducted for final inclusion. Any divergence in the agreement was resolved through discussion with a third researcher (EL) at each step of the study selection process.
2.5. Data Extraction
Two reviewers independently extracted data from the included studies (HO and SS) using a predefined data acquisition form. This form included four main domains: general information (title, authors, year of publication, country of the study, and study design), participants' characteristics (age, sex, diagnostic criteria, and CGD duration), intervention and comparison details (sample size; CHM formulation and prescription name; number of herbs; CHM dose; CHM daily dose; comparison, frequency, or duration of the treatment; and follow-up information), and outcomes (primary and secondary outcomes and adverse events). Any discrepancies were resolved through discussion with a third researcher (EL).
2.6. Quality Assessment
The methodological quality of the included studies was assessed using the revised Cochrane risk-of-bias tool for randomised trials [30]. The bias domain for risk-of-bias assessment included the following: (1) bias arising from the randomisation process, (2) bias due to deviations from intended interventions, (3) bias due to missing outcome data, (4) bias in the measurement of the outcome, and (5) bias in the selection of the reported result. The risk of bias was independently evaluated by two reviewers (HO and SS) as “low,” “high,” or “some concerns.” Any divergence in the agreement was resolved through discussion with other reviewers (EL and WSC). Studies evaluated as “low-risk” in all domains were defined as high-quality studies, whereas those evaluated as “high-risk” in at least one domain were defined as low-quality studies.
Subsequently, the strength of evidence for the main outcomes was evaluated using the grading of recommendations assessment, development, and evaluation system [31]. The risk of bias; inconsistency, indirectness, and imprecision of the results; and publication bias were assessed, and the quality of the evidence was graded on a four-point scale as “high,” “moderate,” “low,” or “very low.”
2.7. Data Synthesis
When the included studies were sufficiently homogenous, quantitative synthesis was performed using RevMan software (version 5.3; Cochrane, London, UK) to analyse the efficacy of CHMs in the treatment of CGD. Subgroup analyses were conducted according to (1) the comparison types and (2) the CHM prescription names. Dichotomous outcomes were pooled using risk ratios (RRs), and continuous outcomes were pooled using mean differences (MDs), or standardised mean differences (SMDs), with 95% confidence intervals (CIs).
The statistical heterogeneity among studies was assessed by computing I2 statistics. Data were pooled using a random-effects model, if the included studies had significant heterogeneity (I2 values ≥50% indicated substantial heterogeneity and I2 values ≥75% indicated considerable heterogeneity (both were considered significant)). Otherwise, a fixed-effects model was applied [32]. Sensitivity analysis was performed to increase the robustness of the results by excluding studies with a high risk of bias and outliers. If the number of studies was sufficient (n ≥ 10), a visual inspection of the funnel plot was performed to assess publication bias. Data on the safety of CHMs in the treatment of CGD were described qualitatively.
3. Results
3.1. Study Selection
A total of 8,746 studies were identified through the database searches, and 1 additional study was identified through other sources. After removing 305 duplicates, 8,442 studies were excluded by screening the titles and abstracts. Through a review of the full texts, a further 659 studies were excluded: 17 studies with unavailable full texts, 31 nonclinical studies, 21 case reports, 164 noncomparative studies, 13 nonrandomised controlled trials, 258 studies not related to CGD, 49 studies not related to eligible intervention, and 106 studies not related to the clinical question. Finally, 35 RCTs with 3,862 participants were included in the analysis (Figure 1).
Figure 1.
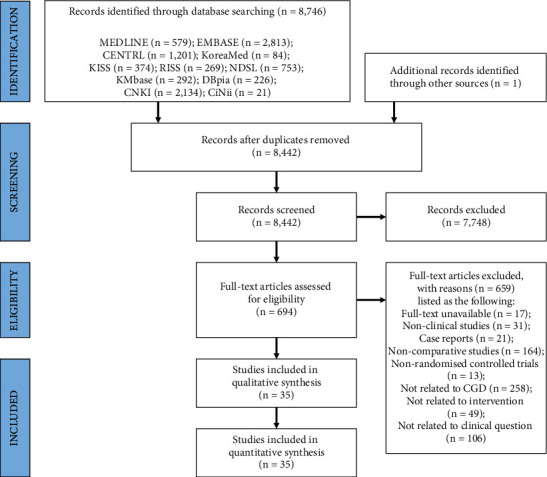
PRISMA flow diagram of the literature screening and selection process. CENTRAL, Cochrane Central Register of Controlled Trials; CGD, cervicogenic dizziness; CiNii, Citation Information by NII; CNKI, China National Knowledge Infrastructure; DBpia, Database Periodical Information Academic; EMBASE, Excerpta Medica Database; KISS, Korean Studies Information Service System; KMbase, Korean Medical Database; MEDLINE, Medical Literature Analysis and Retrieval System Online; NDSL, National Digital Science Library; PRISMA, Preferred Reporting Items for Systematic reviews and Meta-Analyses; and RISS, Research Information Sharing Service.
3.2. Study Characteristics
All included studies were RCTs conducted in China. They were classified according to the comparison types, as follows: (1) studies comparing CHMs plus anti-vertigo drugs with anti-vertigo drugs alone (n = 14), which were subdivided according to the anti-vertigo drugs used into studies using flunarizine (n = 6), betahistine (n = 5), both flunarizine and betahistine (n = 1), diphenidol (n = 1), or nimodipine (n = 1); (2) studies comparing CHMs plus manual therapy with manual therapy alone (n = 7); (3) studies comparing CHMs plus acupuncture therapy with acupuncture therapy alone (n = 13); and (4) studies comparing CHMs plus manual and acupuncture therapy with manual and acupuncture therapy alone (n = 1). None of the studies assessed the efficacy of CHM as monotherapy for CGD.
The included studies were also classified according to the CHM prescription names, as follows: (1) studies on Banxia Baizhu Tianma Tang (BBTT; n = 9), (2) studies on Buzhong Yiqi Tang (BYT; n = 2), (3) studies on Dingxuan Tang (DXT; n = 8), (4) studies on Gegen Tang (GGT; n = 7), (5) study on Gegen Jieji Tang (GJT; n = 1), and (6) studies on Yiqi Congming Tang (YCT; n = 8). All CHMs in the included studies were modified prescriptions. In summary, the studies included in this review were conducted with six types of modified CHMs (BBTT, BYT, DXT, GGT, GJT, and YCT) and four types of active controls (anti-vertigo drugs, manual therapy, acupuncture therapy, and manual and acupuncture therapy).
In addition, 10 types of outcome measurements were identified: 5 studies evaluated overall functional scores, 22 studies evaluated simple scores, 17 studies assessed the mean blood flow velocity in the vertebral arteries, 18 studies assessed the mean blood flow velocity in the basilar artery, 33 studies evaluated the total effective rate, three studies measured endothelin levels, and four studies measured CGRP, fibrinogen and TC levels. The incidence of adverse events was reported in three studies. The study characteristics and the main outcomes are summarised in Table 1.
Table 1.
General characteristics of the included studies.
| Study ID | Sample size (A : B) |
Study of the country | Mean age (range; yr) | CGD duration (range) | Intervention group (A) | Control group (B) | Treatment duration | Follow-up | Outcome | Results | AE (n) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bai [33] | 80 (40 : 40) |
China | (A) 35.6 ± 6.4 (22∼54) (B) 36.2 ± 7.2 (22∼55) |
(A) NR (1.5 days∼4 yr) (B) 1.0 ± 0.6 yr (2 days∼4 yr) |
Modified BYT + (B) | AT (1 time/day) | 20 days | NR | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) > (B)∗ (2) N.S. (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ |
NR |
| Chen [34] | 120 (60 : 60) |
China | (A) 43.81 ± 5.57 (25∼58) (B) 43.75 ± 5.61 (22∼57) |
(A) 7.65 ± 1.79 mon (2∼11 mon) (B) 7.63 ± 1.82 mon (2∼11 mon) |
Modified GGT + (B) | AD: flunarizine (10 mg bid) | 1 month | NR | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ |
NR |
| Cheng [35] | 84 (42 : 42) |
China | Modified DXT + (B) | AD: flunarizine (10 mg qd) | 2 weeks | NR | (1) SS (2) TER (3) CGRP level |
(1) (A) > (B)† (2) (A) > (B)∗ (3) (A) > (B)† |
NR | ||
| Dai [36] | 82 (41 : 41) |
China | 46.2 ± 5.1 (24∼65) | NR (3∼11 mon) | Modified YCT + (B) | AT (1 time/day) | 4 weeks | NR | (1) TER (2) Fib level (3) TC level |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ |
NR |
| Gao [37] | 106 (53 : 53) |
China | (A) 54.3 ± 5.6 (24∼62) (B) 55.4 ± 5.2 (23∼63) |
(A) 5.4 ± 0.6 yr (3.0∼10.5 yr) (B) 5.6 ± 0.5 yr (3.5∼11.0 yr) |
Modified BBTT + (B) | AD: flunarizine (5∼10 mg·qd) | 2 weeks | NR | (1) RVA-BF (2) LVA-BF (3) BA-BF (4) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ |
NR |
| Gu [38] | 70 (35 : 35) |
China | 52.17 ± 6.34 (24∼65) | 3.08 ± 0.41 mon (2∼6 mon) | Modified YCT + (B) | AT (1 time/2 day) | 4 weeks | NR | (1) SS (2) OFS (3) RVA-BF (4) LVA-BF (5) BA-BF (6) TER (7) Fib level (8) TC level |
(1) (A) > (B)† (2) (A) > (B)† (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ (6) (A) > (B)∗ (7) (A) > (B)† (8) (A) > (B)† |
NR |
| Gu [39] | 80 (40 : 40) |
China | (A) 41.9 ± 5.6 (20∼64) (B) 41.3 ± 5.3 (21∼63) |
NR | Modified BBTT + (B) | AD: flunarizine (10∼20 mg·qd) | 2∼8 weeks | NR | (1) TER | (1) (A) > (B)† | NR |
| Hu [40] | 200 (120 : 80) |
China | (A) 55.71 ± 6.93 (B) 56.43 ± 7.34 |
(A) 10.37 ± 3.23 yr (B) 10.53 ± 4.12 yr |
Modified GJT + (B) | AD: betahistine (6 mg tid) | 2 weeks | NR | (1) SS (2) TER |
(1) (A) > (B)∗ (2) (A) > (B) † |
NR |
| Huagn [41] | 98 (49 : 49) |
China | (A) 67.82 ± 5.95 (B) 65.26 ± 5.43 |
(A) 3.28 ± 0.69 yr (B) 3.34 ± 0.75 yr |
Modified GGT + (B) | AD: betahistine (6 mg·tid) | 2 weeks | NR | (1) OFS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) > (B)† (2) (A) > (B)† (3) (A) > (B)† (4) (A) > (B)† (5) (A) > (B)∗ |
NR |
| Huang [42] | 120 (60 : 60) |
China | (A) 43.63 ± 4.72 (25∼57) (B) 43.72 ± 4.54 (27∼58) |
(A) 5.70 ± 1.14 yr (4 mon∼10 yr) (B) 5.65 ± 1.21 yr (3 mon∼10 yr) |
Modified BBTT + (B) | MT: Tuina (1 time/2 days) | 1 month | NR | (1) SS (2) OFS (3) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ |
NR |
| Ji [43] | 60 (30 : 30) |
China | NR (40∼70) | NR | Modified DXT + (B) | AD: flunarizine (5 mg·qd) | 2 weeks | NR | (1) SS (2) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ |
NR |
| Ju [44] | 120 (60 : 60) |
China | (A) 67.82 ± 2.41 (60∼75) (B) 67.91 ± 2.37 (64∼74) |
(A) 5.12 ± 0.82 yr (1∼9 yr) (B) 5.30 ± 0.85 yr (1∼10 yr) |
Modified GGT + (B) | AT (6 times/week) | 4 weeks | 6 months | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ |
NR |
| Li [45] | 68 (34 : 34) |
China | (A) 52.60 ± 2.58 (25∼68) (B) 42.58 ± 2.65 (24∼65) |
(A) 3.95 ± 0.78 mon (2∼8 mon) (B) 3.92 ± 0.85 mon (1∼8 mon) |
Modified YCT + (B) | AT (1 time/day) | 4 weeks | NR | (1) SS (2) TER |
(1) (A) > (B)† (2) (A) > (B)∗ |
NR |
| Li [46] | 116 (58 : 58) |
China | (A) 42.98 ± 9.21 (33∼63) (B) 42.91 ± 9.45 (32∼62) |
(A) 5.37 ± 0.65 yr (1 mon∼10 yr) (B) 5.32 ± 0.61 yr (1 mon∼10 yr) |
Modified DXT + (B) | AD: diphenidol (tid) | 1 month | NR | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER (6) ET level (7) CGRP level |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ (6) (A) > (B)∗ (7) (A) > (B)∗ |
NR |
| Liu [47] | 126 (63 : 63) |
China | (A) 52.64 ± 8.25 (26∼68) (B) 52.47 ± 8.14 (22∼65) |
(A) 3.98 ± 1.02 yr (0.6∼5 yr) (B) 3.94 ± 1.05 yr (0.8∼6 yr) |
Modified DXT + (B) | MT: Tuina (1 time/day) | 4 weeks | NR | (1) SS (2) OFS (3) RVA-BF (4) LVA-BF (5) BA-BF (6) TER (7) ET level (8) CGRP level |
(1) (A) > (B)∗ (2) (A) > (B)† (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ (6) (A) > (B)∗ (7) (A) > (B)∗ (8) (A) > (B)† |
NR |
| Lyu [48] | 54 (27 : 27) |
China | (A) 35.24 ± 2.15 (20∼59) (B) 31.17 ± 1.53 (18∼60) |
NR | Modified BYT + (B) | AT (1 time/day) | 20 days | NR | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) > (B)∗ (2) N.S. (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ |
NR |
| Pan [49] | 100 (50 : 50) |
China | (A) 42.41 ± 5.93 (B) 40.87 ± 6.25 |
(A) 3.91 ± 0.74 mon (B) 4.18 ± 0.81 mon |
Modified BBTT + (B) | MT: Tuina (1 time/day) | 2 weeks | NR | (1) SS (2) TER (3) ET level (4) CGRP level |
(1) (A) > (B)† (2) (A) > (B)∗ (3) (A) > (B)† (4) (A) > (B)† |
Gastrointestinal discomfort (1) |
| Qin [50] | 163 (79 : 84) |
China | 54.78 ± 10.36 | NR | Modified YCT + (B) | AD: betahistine (6 mg·tid) | 2 weeks | 3 months | (1) SS | (1) (A) > (B)∗ | NR |
| Qiu [51] | 110 (55 : 55) |
China | (A) 53.8 ± 5.5 (43∼65) (B) 52.6 ± 4.7 (42∼63) |
(A) 4.5 ± 0.7 mon (1∼8 mon) (B) 4.4 ± 0.8 mon (2∼9 mon) |
Modified YCT + (B) | AT (1 time/day) | 1 month | NR | (1) SS (2) BA-BF (3) TER (4) Fib level (5) TC level |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ |
NR |
| Shang [52] | 82 (41 : 41) |
China | 40.2 ± 1.7 (31∼67) | 3.1 ± 0.5 yr (0.33∼8 yr) | Modified GGT + (B) | MT (qd) | 2 weeks | NR | (1) SS (2) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ |
NR |
| Shang [53] | 134 (67 : 67) |
China | (A) 36.21 ± 4.74 (19∼63) (B) 36.51 ± 4.43 (18∼64) |
(A) 1.35 ± 0.82 yr (2 mon∼5 yr) (B) 1.21 ± 0.78 yr (1 mon∼4 yr) |
Modified GGT + (B) | AD: nimodipine (4 mg/day) | 2 weeks | NR | (1) RVA-BF (2) LVA-BF (3) BA-BF (4) TER |
(1) (A) > (B)† (2) (A) > (B)† (3) (A) > (B)† (4) (A) > (B)∗ |
NR |
| Shen [54] | 120 (60 : 60) |
China | (A) 54.22 ± 5.31 (42∼67) (B) 54.53 ± 5.07 (43∼66) |
NR | Modified YCT + (B) | AT (1 time/day) | NR | NR | (1) SS (2) TER |
(1) (A) > (B)† (2) (A) > (B)† |
NR |
| Shi [55] | 74 (37 : 37) |
China | (A) 54.8 ± 8.9 (B) 55.6 ± 8.4 |
(A) 3.3 ± 0.9 days (B) 3.5 ± 0.6 days |
Modified DXT + (B) | AD: betahistine (12 mg·tid) | 2 weeks | NR | (1) RVA-BF (2) LVA-BF (3) BA-BF (4) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ |
NR |
| Tan [56] | 154 (77 : 77) |
China | 23.6 ± 2.5 (18∼30) | 37.6 ± 7.9 days (7∼60 days) | Modified BBTT + (B) | AD: betahistine (8 mg·bid) | 10 days | NR | (1) TER | (1) (A) > (B)† | NR |
| Wang [57] | 66 (34 : 32) |
China | (A) 35.34 ± 3.24 (20∼64) (B) 35.63 ± 2.89 (20∼65) |
(A) 3.63 ± 1.45 yr (0.2∼10 yr) (B) 3.74 ± 1.63 yr (0.8∼12 yr) |
Modified DXT + (B) | MT: Tuina (5 times/week) | 4 weeks | NR | (1) SS (2) TER |
(1) (A) > (B)† (2) (A) > (B)† |
NR |
| Wang [58] | 160 (80 : 80) |
China | 49.37 ± 7.48 (33∼78) | 3.29 ± 1.44 yr (0.5∼9.5 yr) | Modified BBTT + (B) | AD: flunarizine (5 mg·qd) | 4 weeks | NR | (1) TER | (1) (A) > (B)∗ | NR |
| Wang [59] | 86 (43 : 43) |
China | (A) 44.76 ± 3.69 (23∼67) (B) 45.01 ± 3.12 (22∼68) |
(A) 1.04 ± 0.63 yr (4 mon∼2 yr) (B) 1.13 ± 0.64 yr (3 mon∼2 yr) |
Modified YCT + (B) | AT (1 time/day) | 4 weeks | NR | (1) RVA-BF (2) LVA-BF (3) BA-BF (4) TER (5) Fib level (6) TC level |
(1) (A) > (B)† (2) (A) > (B)† (3) (A) > (B)† (4) (A) > (B)∗ (5) (A) > (B)† (6) (A) > (B)† |
NR |
| Wang [60] | 80 (40 : 40) |
China | (A) 54.23 ± 9.09 (25∼73) (B) 54.71 ± 9.91 (25∼72) |
3.29 ± 1.44 yr (7 days∼3 mon) | Modified BBTT + (B) | AT (5 times/week) | 2 weeks | NR | (1) TER | (1) (A) > (B)∗ | Abdominal pain (1) Fainting during acupuncture (1) |
| Xu [61] | 112 (56 : 56) |
China | (A) 41.12 ± 3.24 (18∼65) (B) 40.92 ± 3.38 (18∼67) |
(A) 2.67 ± 3.24 yr (1∼4 yr) (B) 2.71 ± 0.92 yr (1∼5 yr) |
Modified GGT + (B) | MT: Tuina (3 times/day) | 4 weeks | NR | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ |
NR |
| Yang [62] | 146 (73 : 73) |
China | (A) 35.72 ± 6.66 (18∼54) (B) 35.37 ± 6.51 (19∼55) |
(A) 3.14 ± 0.75 mon (1∼5 mon) (B) 3.37 ± 0.81 mon (2∼5 mon) |
Modified YCT + (B) | AT (1 time/day) | 2 weeks | NR | (1) RVA-BF (2) LVA-BF (3) BA-BF (4) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ |
NR |
| Yang [63] | 143 (73 : 70) |
China | (A) 37.4 ± 1.5 (20∼70) (B) 36.5 ± 1.2 (18∼69) |
(A) 2.4 ± 0.3 yr (0.5 mon∼8 yr) (B) 2.5 ± 0.2 yr (1 mon∼7 yr) |
Modified DXT + (B) | AD: flunarizine (10 mg·qd) and betahistine (20 mg/day) | 2 weeks | 6 months | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ (3) (A) > (B)∗ (4) (A) > (B)∗ (5) (A) > (B)∗ |
Rash (1) Gastrointestinal discomfort (1) Diarrhea (1) Fatigue (2) |
| Yao [64] | 78 (39 : 39) |
China | (A) 42.17 ± 4.35 (22∼58) (B) 42.59 ± 5.38 (23∼62) |
(A) 5.86 ± 1.35 yr (0.04∼9 yr) (B) 6.19 ± 1.34 yr (0.04∼11 yr) |
Modified BBTT + (B) | AT (1 time/5 days) | 6 weeks | NR | (1) RVA-BF (2) LVA-BF (3) BA-BF (4) TER |
(1) (A) > (B)† (2) (A) > (B)† (3) (A) > (B)† (4) (A) > (B)† |
NR |
| Zhang [65] | 290 (145: 145) |
China | (A) 57.97 ± 3.54 (47∼76) (B) 58.45 ± 3.36 (46∼76) |
(A) 2.56 ± 1.42 yr (1.5∼4.5 yr) (B) 2.85 ± 1.36 yr (1.5∼5 yr) |
Modified BBTT + (B) | AT + MT (AT: 1 time/day, MT: Tuina, 1 time/2 days) | 4 weeks | NR | (1) OFS (2) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ |
NR |
| Zhu [66] | 120 (60 : 60) |
China | (A) NR (31∼59) (B) NR (33∼58) |
(A) NR (10 days∼3 yr) (B) NR (7 days∼4 yr) |
Modified DXT + (B) | MT: Tuina (1 time/day) | 2 weeks | NR | (1) SS (2) RVA-BF (3) LVA-BF (4) BA-BF (5) TER |
(1) (A) < (B)† (2) (A) > (B)† (3) (A) > (B)† (4) (A) > (B)∗ (5) (A) > (B)∗ |
NR |
| Zhu [67] | 60 (30 : 30) |
China | (A) 45.5 ± 3.4 (20∼67) (B) 42.3 ± 2.1 (21∼65) |
(A) NR (5 days∼9 yr) (B) NR (7 days∼10 yr) |
Modified GGT + (B) | AT (1 time/day) | 2 weeks | NR | (1) SS (2) TER |
(1) (A) > (B)∗ (2) (A) > (B)∗ |
NR |
Significant differences between the two groups are indicated as follows: ∗p < 0.05 and †p < 0.01. Insignificant differences between the two groups (p > 0.05) are indicated by N.S. AD, anti-vertigo drug; AE, adverse events; AT, acupuncture therapy; BA-BF, basilar artery blood flow; BBTT, Banxia Baizhu Tianma Tang; BYT, Buzhong Yiqi Tang; CGD, cervicogenic dizziness; CGRP, calcitonin gene-related peptide; DXT, Dingxuan Tang; ET, endothelin; Fib, fibrinogen; GGT, Gegen Tang; GJT, Gegen Jieji Tang; LVA-BF, left vertebral artery blood flow; MT, manual therapy; NR, not reported; OFS, Overall functional score; RVA-BF, right vertebral artery blood flow; SS, simple score; TC, total cholesterol; TER, total effective rate; and YCT, Yiqi Congming Tang.
Each CHM prescription was applied to a specific pattern of symptoms in traditional Chinese medicine: BBTT to the wind-phlegm type or phlegm stasis type; BYT to qi and blood deficiency type; DXT to spleen deficiency and dampness type, qi deficiency and blood stasis type, or hyperactivity of liver yang type; GGT to wind type with disharmony between ying and wei; GJT to collateral stasis type; and YCT to qi and blood deficiency type or qi deficiency and sputum silting up type. All modified CHMs included at least one-third of the original prescriptions. The duration of administration ranged from 10 days to 8 weeks, with 2- and 4-week regimens being the most frequent. The details of the CHMs prescribed in the included studies are summarised in Tables 2 and 3.
Table 2.
Details of the Chinese herbal medicines BBTT, BYT, and DXT in the included studies.
| Study ID | Gao [37] | Gu [39] | Huang [42] | Pan [49] | Tan [56] | Wang [58] | Wang [60] | Yao [64] | Zhang [65] | Bai [33] | Lyu [48] | Cheng [35] | Ji [43] | Li [46] | Liu [47] | Shi [55] | Wang [57] | Yang [63] | Zhu [66] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHM | BBTT | BYT | DXT | ||||||||||||||||
| Administration duration and frequency | 2 wks, NR | 2∼8 wks, tid | 1 mon, bid | 2 wks, bid | 10 dys, bid | 4 wks, bid | 2 wks, bid | 6 wks, bid | 4 wks, bid | 2 dys, bid | 20 dys, bid | 2 wks, bid | 2 wks, bid | 1 mon, bid | 4 wks, bid | 2 wks, bid | 4 wks, bid | 2 wks, bid | 2 wks, bid |
| Atractylodis Rhizoma Alba | 12 | 7.5 | 15 | 10 | 9 | 15 | 12 | 10 | 18 | 15 | 10 | 20 | 10 | 20 | 15 | 10 | |||
| Citri Reticulatae Pericarpium | 12 | 6 | 10 | 10 | 12 | 10 | 6 | 10 | 10 | 10 | |||||||||
| Glycyrrhizae Radix et Rhizoma | 9 | 6 | 9 | 9 | 5 | 6 | 3 | 10 | 9 | 5 | 10 | 9 | 10 | ||||||
| Citrus reticulata Blanco | 9 | 9 | 9 | 10 | |||||||||||||||
| Gastrodiae Rhizoma | 12 | 12 | 9 | 9 | 12 | 20 | 10 | 15 | 15 | 10 | 12 | 15 | 10∼15 | ||||||
| Pinelliae Tuber | 10 | 5 | 10 | 9 | 9 | 10 | 15 | 6 | 12 | 9 | 18 | 10 | |||||||
| Poria Sclerotium | 30 | 7.5 | 12 | 10 | 9 | 12 | 30 | 20 | 9 | 15 | 25 | 30 | 10 | 30 | |||||
| Poria Sclertum Cum Pini Radix | 10 | ||||||||||||||||||
| Zingiberis Rhizoma Recens | 5 | 9 | 10 | 6 | 9 | 9 | 6 | 10 | |||||||||||
| Zizyphi Fructus | 3EA | 2EA | 3EA | 3EA | 10 | 9 | |||||||||||||
| Angelicae Gigantis Radix | 10 | 10 | 15 | 10 | 15 | 10 | |||||||||||||
| Bupleuri Radix | 10 | 12 | 10 | ||||||||||||||||
| Cimicifugae Rhizoma | 10 | 6 | |||||||||||||||||
| Codonopsis Pilosulae Radix | 10 | 10 | 20 | 25 | 30 | 15 | |||||||||||||
| Astragali Radix | 30 | 60 | 30 | 20 | |||||||||||||||
| Uncariae Ramulus Cum Uncus | 10 | 12 | 15 | 30 | |||||||||||||||
| Salviae miltiorrhizae Radix | 9 | 15 | 15∼30 | ||||||||||||||||
| Polygoni Multiflori Radix | 10 | 10 | |||||||||||||||||
| Scorpio | 3 | 12 | 5 | ||||||||||||||||
| Lumbricus | 10 | 15 | |||||||||||||||||
| Paeoniae Radix | 7.5 | 10 | 10 | 10 | 10 | 10 | 15 | 10 | 30 | ||||||||||
| Cinnamomi Ramulus | 10 | 10 | |||||||||||||||||
| Puerariae Radix | 20 | 30 | 10 | 20 | 20 | 20 | 20 | 30∼60 | |||||||||||
| Osterici seu Notopterygii Radix et Ehizoma | 10 | ||||||||||||||||||
| Phellodendri Cortex | 10 | 12 | |||||||||||||||||
| Viticis Fructus | 12 | ||||||||||||||||||
| Cnidii Rhizoma | 12 | 10 | 10 | 10 | 10 | 15 | 10 | 9 | 15∼30 | ||||||||||
| Alismatis Rhizoma | 10 | 9 | 20 | 30 | 20 | 20 | |||||||||||||
| Arisaematis Rhizoma | 10 | ||||||||||||||||||
| Magnoliae Cortex | 12 | ||||||||||||||||||
| Phyllostachyos Caulis in Taeniam | 7.5 | 10 | |||||||||||||||||
| Aurantii Fructus Immaturus | 6 | ||||||||||||||||||
| Myrrha | 10 | ||||||||||||||||||
| Olibanum | 10 | ||||||||||||||||||
| Fossilia Ossis Mastodi | 30 | 30 | |||||||||||||||||
| Ostreae Testa | 30 | 30 | 30 | ||||||||||||||||
| Nelumbinis Folium | 15 | ||||||||||||||||||
| Zingiberis Rhizoma | 9 | ||||||||||||||||||
| Margaritiferae Usta Concha | 30 | ||||||||||||||||||
| Loranthi Ramulus et Folium | 12 | ||||||||||||||||||
| Chrysanthmi Flos | 10 | ||||||||||||||||||
| Batryticatus Bombyx | 6 | 10 | |||||||||||||||||
| Notoginseng Radix et Rhizoma | 6 | ||||||||||||||||||
| Carthami Flos | 10 | ||||||||||||||||||
| Persicae Semen | 10 | ||||||||||||||||||
| Aconiti Lateralis Radix Preparata | 5 | ||||||||||||||||||
| Rehmanniae Radix Preparata | 15 | ||||||||||||||||||
| Cuscutae Semen | 15 | ||||||||||||||||||
| Cistanchis Herba | 15 | ||||||||||||||||||
| Eucommiae Cortex | 15 | ||||||||||||||||||
BBTT, Banxia Baizhu Tianma Tang; BYT, Buzhong Yiqi Tang; CHM, Chinese herbal medicine; DXT, Dingxuan Tang.
Table 3.
Details of the Chinese herbal medicines GGT, GJT, and YCT in the included studies.
| Study ID | Chen [34] | Huagn [41] | Ju [44] | Shang [52] | Shang [53] | Xu [61] | Zhu [67] | Hu [40] | Dai [36] | Gu [38] | Li [45] | Qin [50] | Qiu [51] | Shen [54] | Wang [59] | Yang [62] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHM | GGT | GJT | YCT | |||||||||||||
| Administration duration and frequency | 1 mon, bid | 2 wks, bid | 4 wks, bid | 2 wks, bid | 2 wks, bid | 4 wks, bid | 2 wks, bid | 2 wks, tid | 4 wks, bid | 4 wks, tid | 4 wks, bid | 2 wks, bid | 1 mon, bid | NR, bid | 4 wks, bid | 2 wks, NR |
| Atractylodis Rhizoma Alba | 12 | 20 | NR | 12 | 12 | 20 | ||||||||||
| Glycyrrhizae Radix et Rhizoma | 6 | 10 | 6 | 6 | 6 | 10 | 6 | 10 | 6 | 13 | 13 | 10 | 6 | |||
| Citrus reticulata Blanco | 15 | NR | 15 | |||||||||||||
| Gastrodiae Rhizoma | 15 | 15 | ||||||||||||||
| Pinelliae Tuber | 12 | 11 | 11 | |||||||||||||
| Zingiberis Rhizoma Recens | 10 | 10 | 6 | 9 | 10 | |||||||||||
| Zizyphi Fructus | 15 | 3EA | 9 | 10 | ||||||||||||
| Angelicae Gigantis Radix | 15 | 15 | ||||||||||||||
| Bupleuri Radix | NR | |||||||||||||||
| Cimicifugae Rhizoma | 9 | 10 | 9 | NR | 7 | 7 | 10 | 9 | ||||||||
| Codonopsis pilosulae Radix | 20 | 15 | NR | 20 | ||||||||||||
| Ginseng Radix | 15 | 11 | 11 | 15 | ||||||||||||
| Astragali Radix | 15 | 15 | 30 | NR | 12 | 12 | 30 | 15 | ||||||||
| Salviae miltiorrhizae Radix | 30 | 12 | 14 | 14 | ||||||||||||
| Polygoni Multiflori Radix | 12 | 11 | 11 | |||||||||||||
| Scorpio | 10 | NR | ||||||||||||||
| Lumbricus | 15 | |||||||||||||||
| Paeoniae Radix | 15 | 20 | 10 | 9 | 12 | 15 | 12 | NR | 15 | 10 | 11 | 11 | 10 | |||
| Cinnamomi Ramulus | 15 | 10 | 9 | 6 | 6 | 12 | NR | |||||||||
| Puerariae Radix | 30 | 30 | 20 | 15 | 30 | 30 | 60 | NR | 12 | 15 | 10 | NR | 10 | 10 | 15 | 9 |
| Osterici seu Notopterygii Radix et Rhizoma | 10 | NR | ||||||||||||||
| Angelicae Dahuricae Radix | 10 | NR | ||||||||||||||
| Phellodendri Cortex | 3 | 8 | 8 | 3 | ||||||||||||
| Viticis Fructus | 15 | 15 | 15 | NR | 13 | 13 | 15 | 6 | ||||||||
| Cnidii Rhizoma | 15 | 10 | 10 | 9 | 9 | NR | 20 | NR | 20 | |||||||
| Alismatis Rhizoma | 10 | NR | 10 | |||||||||||||
| Ephedrae Herba | 5 | 9 | 6 | |||||||||||||
| Ostreae Testa | 30 | |||||||||||||||
| Polygalae Radix | NR | |||||||||||||||
| Ligustici Tenuissimi Rhizoma et Radix | NR | |||||||||||||||
| Batryticatus Bombyx | 6 | 6 | ||||||||||||||
| Notoginseng Radix et Rhizoma | 3 | |||||||||||||||
| Achyranthis Radix | 15 | |||||||||||||||
| Chaenomelis Fructus | 15 | |||||||||||||||
| Lycopodii Herba | 15 | |||||||||||||||
| Coicis Semen | 30 | |||||||||||||||
| Lycopi Herba | 12 | |||||||||||||||
| Eleocharitis Rhizoma | NR | |||||||||||||||
CHM, Chinese herbal medicine; GGT, Gegen Tang; GJT, Gegen Jieji Tang; NR, not reported; YCT, Yiqi Congming Tang.
3.3. Risk-of-Bias Assessment
For bias arising from the randomisation process, 18 studies were evaluated as “low-risk” because the randomisation process for the allocation sequence was clearly described. The remaining 17 studies were evaluated as “some concerns” because insufficient relevant information was provided. For bias due to deviations from intended interventions, 21 studies, most of which included manual or acupuncture therapy as active controls, were evaluated as “high-risk” because it was unclear whether blinding of participants and trial personnel had been sufficiently performed using sham-massage or sham-acupuncture. The remaining 14 studies were evaluated as “some concerns.” For bias due to missing outcome data, 30 studies were evaluated as “low-risk,” and 1 study was evaluated as “high-risk” because there were missing data (only the results of the per-protocol analysis were reported). The remaining 4 studies were evaluated as “some concerns” because insufficient relevant information was provided. For bias in the measurement of the outcome, 20 studies were evaluated as “low-risk,” and the remaining 15 studies were evaluated as “some concerns” because it was difficult to judge whether the outcome measures used in the studies were affected by the awareness of the outcome assessors. For bias in the selection of the reported result, 3 studies were evaluated as “low-risk” because there was no suspicion of deliberate nonreporting, and 3 studies were evaluated as “high-risk” because selective outcome reporting was suspected. The remaining 29 studies were evaluated as “some concerns” because there was no basis for bias assessment (e.g., study protocols). Finally, for the overall risk of bias, 23 studies assessed as “high-risk” were considered low-quality studies; 2 were considered high-quality studies; and the remaining 10 studies were evaluated as “some concerns” (Figure 2).
Figure 2.
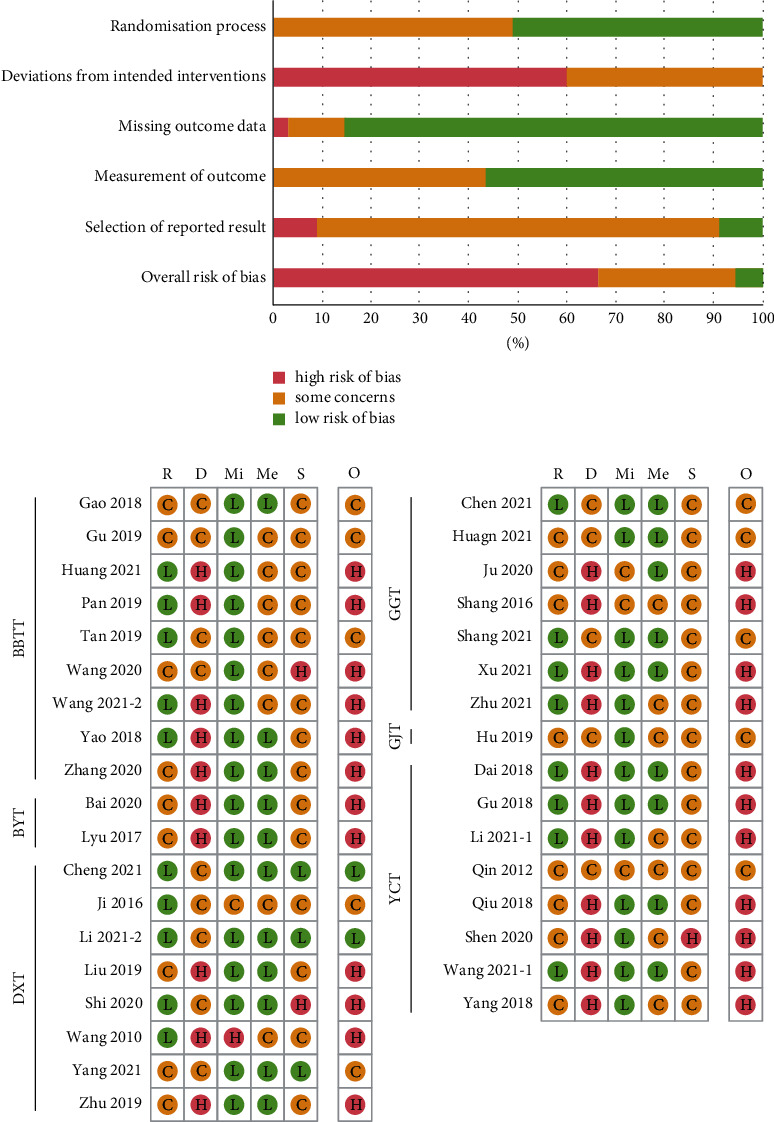
Risk of bias summary for all included studies.
The risk of bias was evaluated as “low,” “high,” or “some concerns,” represented by the following symbols: “L,” “H,” and “C,” respectively. D, bias due to deviations from intended interventions; Me, bias in the measurement of the outcome; Mi, bias due to missing outcome data; O, overall risk of bias; R, bias arising from the randomisation process; and S, bias in the selection of the reported result.
3.4. Efficacy
In the total analysis of all included studies, compared with the active controls alone, CHMs plus active controls significantly reduced the overall functional scores (five studies: SMD, 2.31 (95% CI: 1.48–3.14); I2 = 94%), endothelin (three studies: MD, 14.57 (95% CI: 6.81–22.32); I2 = 96%), fibrinogen (four studies: MD, 0.31 (95% CI: 0.12–0.50); I2 = 97%), and TC levels (four studies: MD, 0.56 (95% CI: 0.31–0.82); I2 = 71%). In addition, CHMs plus active controls significantly increased the simple scores (22 studies: SMD, 1.82 (95% CI: 1.26–2.38); I2 = 97%), the blood flow velocity in the left vertebral artery (17 studies: MD, 5.70 (95% CI: 4.18–7.22); I2 = 97%), right vertebral artery (17 studies: MD, 4.83 (95% CI: 3.37–6.29); I2 = 97%), basilar artery (18 studies: MD, 5.58 (95% CI: 4.24–6.92); I2 = 96%), CGRP levels (four studies: MD, 6.24 (95% CI: 4.37–8.11); I2 = 96%), and total effective rate (33 studies: RR, 1.55 (95% CI: 1.42–1.69); I2 = 0%).
3.4.1. CHMs plus Anti-Vertigo Drugs versus Anti-Vertigo Drugs Alone
In the subanalysis of the 14 studies using anti-vertigo drugs as active controls, compared with the anti-vertigo drugs alone, CHMs plus anti-vertigo drugs significantly reduced the overall functional scores (one study: MD, 7.80 (95% CI: 6.02–9.58)) and endothelin levels (one study: MD, 11.14 (95% CI: 9.49–12.79)). In addition, CHMs plus anti-vertigo drugs significantly increased the simple scores (seven studies: SMD, 2.45 (95% CI: 1.32–3.58); I2 = 98%), the blood flow velocity in the left vertebral artery (seven studies: MD, 5.39 (95% CI: 3.33–7.45); I2 = 98%), right vertebral artery (seven studies: MD, 5.28 (95% CI: 3.38–7.18); I2 = 97%), and basilar artery (seven studies: MD, 5.28 (95% CI: 3.97–6.59); I2 = 92%). CHMs plus anti-vertigo drugs also significantly improved the total effective rate (13 studies: RR, 1.53 (95% CI: 1.35–1.73); I2 = 21%). However, the changes in the CGRP levels (two studies: MD, 8.89 (95% CI: −0.76–18.54); I2 = 98%) did not show a significant difference between the intervention and control groups.
In the additional subanalysis of the components of anti-vertigo drug, the combination of CHMs and flunarizine significantly increased the simple scores (three studies: SMD, 2.16 (95% CI: 0.44–3.87); I2 = 97%), the blood flow velocity in the left vertebral artery (two studies: MD, 3.96 (95% CI: 1.91–6.01); I2 = 94%), right vertebral artery (two studies: MD, 4.80 (95% CI: 4.23–5.38); I2 = 0%), basilar artery (two studies: MD, 4.85 (95% CI: 4.04–5.65); I2 = 0%), CGRP levels (one study: MD, 13.89 (95% CI: 11.48–16.30)), and the total effective rate (six studies: RR, 1.48 (95% CI: 1.16–1.90); I2 = 50%). The combination of CHMs and betahistine significantly reduced the overall functional scores (one study: MD, 7.80 (95% CI: 6.02–9.58)) and increased the blood flow velocity in the left vertebral artery (two studies: MD, 8.73 (95% CI: 5.49–11.97); I2 = 94%), right vertebral artery (two studies: MD, 7.77 (95% CI: 7.17–8.37); I2 = 25%), basilar artery (two studies: MD, 5.70 (95% CI: 5.15–6.24); I2 = 0%), and the total effective rate (four studies: RR, 1.68 (95% CI: 1.27–2.23); I2 = 0%). However, the changes in the simple scores (two studies: SMD, 1.29 (95% CI: −0.34–2.91); I2 = 98%) did not show a significant difference between the intervention and control groups. The combination of CHMs with flunarizine and betahistine significantly increased the simple scores (one study: MD, 6.98 (95% CI: 6.48–7.48)), the blood flow velocity in the left vertebral artery (one study: MD, 4.59 (95% CI: 3.28–5.90)), right vertebral artery (one study: MD, 5.04 (95% CI: 3.85–6.23)), basilar artery (one study: MD, 6.92 (95% CI: 5.74–8.10)), and the total effective rate (one study: RR, 1.97 (95% CI: 1.29–3.00)). The combination of CHMs and diphenidol significantly increased the simple scores (one study: MD, 2.67 (95% CI: 2.41–2.93)), the blood flow velocity in the left vertebral artery (one study: MD, 5.51 (95% CI: 4.39–6.63)), right vertebral artery (one study: MD, 4.69 (95% CI: 3.77–5.61)), basilar artery (one study: MD, 6.23 (95% CI: 4.42–8.04)), CGRP levels (one study: MD, 4.04 (95% CI: 3.68–4.40)), and reduced endothelin levels (one study: MD, 11.14 (95% CI: 9.49–12.79)). However, the changes in the total effective rate (one study: RR, 1.40 (95% CI: 0.80–2.44)) did not show a significant difference between the intervention and control groups. The combination of CHMs and nimodipine significantly increased the blood flow velocity in the left vertebral artery (one study: MD, 2.40 (95% CI: 1.90–2.90)), right vertebral artery (one study: MD, 1.82 (95% CI: 1.35–2.29)), and basilar artery (one study: MD, 2.74 (95% CI: 2.19–3.29)). However, the changes in the total effective rate (one study: RR, 1.32 (95% CI: 0.85–2.04)) did not show a significant difference between the intervention and control groups.
3.4.2. CHMs plus Manual Therapy versus Manual Therapy Alone
In the subanalysis of the seven studies using manual therapy as an active control, compared with the manual therapy alone, CHMs plus manual therapy significantly increased the simple scores (seven studies: SMD, 1.33 (95% CI: 0.12–2.54); I2 = 98%), the blood flow velocity in the left vertebral artery (three studies: MD, 6.24 (95% CI: 1.36–11.12); I2 = 98%), right vertebral artery (three studies: MD, 5.62 (95% CI: 1.03–10.21); I2 = 98%), basilar artery (three studies: MD, 4.62 (95% CI: 0.32–8.91); I2 = 97%), and CGRP levels (two studies: MD, 4.63 (95% CI: 2.25–7.00); I2 = 93%). Furthermore, CHMs plus manual therapy significantly improved the total effective rate (six studies: RR, 1.71 (95% CI: 1.36–2.16); I2 = 0%). However, the changes in the overall functional scores (two studies: SMD, 3.17 (95% CI: −0.15–6.48); I2 = 98%) and endothelin levels (two studies: MD, 16.48 (95% CI: −0.34–33.31); I2 = 98%) did not show significant differences between the intervention and control groups.
3.4.3. CHMs plus Acupuncture Therapy versus Acupuncture Therapy Alone
In the subanalysis of the thirteen studies using acupuncture therapy as an active control, compared with the acupuncture therapy alone, CHMs plus acupuncture therapy significantly reduced the overall functional scores (one study: MD, 1.91 (95% CI: 1.37–2.45)), fibrinogen (four studies: MD, 0.31 (95% CI: 0.12–0.50); I2 = 97%), and TC levels (four studies: MD, 0.56 (95% CI: 0.31–0.82); I2 = 71%). In addition, CHMs plus acupuncture therapy significantly increased the simple scores (eight studies: SMD, 1.72 (95% CI: 1.33–2.11); I2 = 79%), the blood flow velocity in the left vertebral artery (seven studies: MD, 5.81 (95% CI: 2.92–8.70); I2 = 95%), right vertebral artery (seven studies: MD, 4.03 (95% CI: 1.05–7.01); I2 = 96%), basilar artery (eight studies: MD, 6.43 (95% CI: 2.97–9.89); I2 = 97%), and the total effective rate (thirteen studies: RR, 1.54 (95% CI: 1.32–1.78); I2 = 0%).
3.4.4. CHMs plus Manual and Acupuncture Therapy versus Manual and Acupuncture Therapy Alone
In the subanalysis of the one study using manual and acupuncture therapy as an active control, CHMs plus manual and acupuncture therapy significantly reduced the overall functional scores (one study: MD, 7.06 (95% CI: 6.27–7.85)) and improved the total effective rate (one study: RR, 1.40 (95% CI: 1.02–1.94)), compared with the active control alone.
3.4.5. BBTT plus Active Controls versus Active Controls Alone
In the subanalysis of the nine studies using BBTT as CHM, compared with the active controls alone, BBTT plus active controls significantly reduced the overall functional scores (two studies: SMD, 3.44 (95% CI: 0.69–6.20); I2 = 98%) and endothelin levels (one study: MD, 25.13 (95% CI: 21.29–28.97)) and increased the simple scores (two studies: MD, 5.15 (95% CI: 4.81–5.50); I2 = 0%), the blood flow velocity in the left vertebral artery (two studies: MD, 4.44 (95% CI: 3.18–5.69); I2 = 71%), right vertebral artery (two studies: MD, 3.85 (95% CI: 2.29–5.41); I2 = 84%), basilar artery (two studies: MD, 3.48 (95% CI: 0.04–6.92); I2 = 95%), and CGRP levels (one study: MD, 5.89 (95% CI: 4.78–7.00)). BBTT plus active controls also significantly improved the total effective rate (nine studies: RR, 1.48 (95% CI: 1.29–1.70); I2 = 33%).
3.4.6. BYT plus Active Controls versus Active Controls Alone
In the subanalysis of the two studies using BYT as CHM, compared with the acupuncture therapy alone, BYT plus acupuncture therapy significantly increased the simple scores (two studies: MD, 2.04 (95% CI: 1.35–2.72); I2 = 0%) and the blood flow velocity in the left vertebral artery (two studies: MD, 1.72 (95% CI: 0.57–2.87); I2 = 0%). However, the changes in the blood flow velocity in the basilar artery (two studies: MD, 0.43 (95% CI: −0.68–1.55); I2 = 0%) and the total effective rate (two studies: RR, 1.27 (95% CI: 0.70–2.28); I2 = 0%) did not show significant differences between the intervention and control groups. Notably, the blood flow velocity in the right vertebral artery (two studies: MD, −1.80 (95% CI: −2.88–0.72); I2 = 0%) showed a significant increase in the control group compared with the intervention group.
3.4.7. DXT plus Active Controls versus Active Controls Alone
In the subanalysis of the eight studies using DXT as CHM, compared with the active controls alone, DXT plus active controls significantly reduced the overall functional scores (one study: MD, 5.68 (95% CI: 4.36–7.00)) and endothelin levels (two studies: MD, 9.71 (95% CI: 6.61–12.81); I2 = 76%) and increased the simple scores (seven studies: SMD, 1.67 (95% CI: 0.20–3.14); I2 = 98%), the blood flow velocity in the left vertebral artery (five studies: MD, 5.13 (95% CI: 3.87–6.40); I2 = 78%), right vertebral artery (five studies: MD, 5.12 (95% CI: 3.42–6.83); I2 = 90%), basilar artery (five studies: MD, 5.14 (95% CI: 2.66–7.62); I2 = 92%), and CGRP levels (three studies: MD, 6.41 (95% CI: 4.15–8.67); I2 = 97%). Moreover, DXT plus active controls significantly improved the total effective rate (eight studies: RR, 1.61 (95% CI: 1.33–1.95); I2 = 0%).
3.4.8. GGT plus Active Controls versus Active Controls Alone
In the subanalysis of the seven studies using GGT as CHM, compared with the active controls alone, GGT plus manual therapy significantly reduced the overall functional scores (one study: MD, 7.80 (95% CI: 6.02–9.58)) and increased the simple scores (five studies: SMD, 1.92 (95% CI: 0.99–2.85); I2 = 94%), the blood flow velocity in the left vertebral artery (five studies: MD, 7.29 (95% CI: 3.51–11.07); I2 = 99%), right vertebral artery (five studies: MD, 6.18 (95% CI: 3.12–9.24); I2 = 99%), and basilar artery (five studies: MD, 5.19 (95% CI: 3.50–6.88); I2 = 96%). Moreover, GGT plus active controls significantly improved the total effective rate (six studies: RR, 1.62 (95% CI: 1.32–1.99); I2 = 0%).
3.4.9. GJT plus Active Controls versus Active Controls Alone
In the subanalysis of the one study using GJT as CHM, compared with the betahistine alone, GJT plus betahistine significantly increased the simple scores (one study: MD, 2.00 (95% CI: 1.75–2.25)). However, the total effective rate (one study: RR, 2.19 (95% CI: 0.99–4.86)) was not significantly different between the intervention and control groups.
3.4.10. YCT plus Active Controls versus Active Controls Alone
In the subanalysis of the eight studies using YCT as CHM, compared with the active controls alone, YCT plus active controls significantly reduced the overall functional scores (one study: MD, 1.91 (95% CI: 1.37–2.45)), fibrinogen (four studies: MD, 0.31 (95% CI: 0.12–0.50); I2 = 97%) and TC levels (four studies: MD, 0.56 (95% CI: 0.31–0.82); I2 = 71%) and increased the simple scores (five studies: SMD, 1.79 (95% CI: 0.93–2.64); I2 = 94%), blood flow velocity in the left vertebral artery (three studies: MD, 7.63 (95% CI: 4.69–10.57); I2 = 80%), right vertebral artery (three studies: MD, 7.34 (95% CI: 6.02–8.66); I2 = 0%), and basilar artery (four studies: MD, 11.01 (95% CI: 4.46–17.56); I2 = 96%). Furthermore, YCT plus active controls significantly improved the total effective rate (seven studies: RR, 1.54 (95% CI: 1.28–1.84); I2 = 0%).
Summarizing the results of the subanalysis according to CHM prescription names, BBTT, DXT, GGT, and YCT showed significant treatment effects on various primary and secondary outcomes and had relatively more clinical evidence compared with the remaining CHM prescription names. GJT was investigated in only one RCT and demonstrated a significant effect on only one primary outcome (change in the simple scores), without statistically significant effects on the other outcome (total effective rate). In the two RCTs investigating BYT, there were significant effects on two primary outcomes (change in the simple scores and blood flow velocity in the left vertebral artery), while the effects on the remaining outcomes were either not significant (blow flow velocity in the basilar artery and total effective rate) or were significant in the control group (the blood velocity for the right vertebral artery). The results of the total analysis and the subanalyses of the efficacy of CHMs are shown in Table 4.
Table 4.
Summary of findings.
| Outcomes | No. of participants (RCTs) | Anticipated absolute effects (95% CI) | Relative effect (95% CI) | I 2 value | Quality of evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|---|
| Risk with control group | Risk with CHM group | ||||||
| Total analysis | |||||||
| OFS | 704 (5) | — | SMD 2.31 lower (1.48–3.14 lower) | — | 94% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| SS | 2,289 (22) | — | SMD 1.82 higher (1.26–2.38 higher) | — | 97% | ⊕○○○ Very low |
Risk of bias (−1) Publication bias (−1) Inconsistency (−2) |
| LVA-BF | 1,778 (17) | — | MD 5.70 higher (4.18–7.22 higher) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−2) Strong association (+1) |
| RVA-BF | 1,778 (17) | — | MD 4.83 higher (3.37–6.29 higher) | — | 97% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−2) Publication bias (−1) Strong association (+1) |
| BA-BF | 1,888 (18) | — | MD 5.58 higher (4.24–6.92 higher) | — | 96% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−2) Publication bias (−1) Strong association (+1) |
| TER | 3,582 (33) | 295 per 1,000 | 450 per 1,000 (419–499) | RR 1.55 (1.42–1.69) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| ET level | 342 (3) | — | MD 14.57 lower (6.81–22.32 lower) | — | 96% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−2) |
| CGRP level | 426 (4) | — | MD 6.24 higher (4.37–8.11 higher) | — | 96% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−2) |
| Fib level (vs. AT) | 348 (4) | — | MD 0.31 lower (0.12–0.50 lower) | — | 97% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−2) |
| TC level (vs. AT) | 348 (4) | — | MD 0.56 lower (0.31–0.82 lower) | — | 71% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
|
| |||||||
| Subgroup analysis according to the comparison types | |||||||
| CHM plus AD vs. AD | |||||||
| OFS (vs. betahistine) | 98 (1) | — | MD 7.80 lower (6.02–9.58 lower) | — | N/A | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
| SS | 886 (7) | — | SMD 2.45 higher (1.32–3.58 higher) | — | 98% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| SS (vs. flunarizine) | 264 (3) | — | SMD 2.16 higher (0.44–3.87 higher) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| SS (vs. betahistine) | 363 (2) | — | SMD 1.29 higher (0.34 lower–2.91 higher) | — | 98% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| SS (vs. flunarizine and betahistine) | 143 (1) | — | MD 6.98 higher (6.48–7.48 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| SS (vs. diphenidol) | 116 (1) | — | MD 2.67 higher (2.41–2.93 higher) | — | N/A | ⊕⊕⊕○ Moderate |
Imprecision (−1) |
| LVA-BF | 791 (7) | — | MD 5.39 higher (3.33–7.45 higher) | — | 98% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| LVA-BF (vs. flunarizine) | 226 (2) | — | MD 3.96 higher (1.91–6.01 higher) | — | 94% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| LVA-BF (vs. betahistine) |
172 (2) | — | MD 8.73 higher (5.49–11.97 higher) | — | 94% | ⊕○○○ Very low |
Risk of bias (−1) Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| LVA-BF (vs. flunarizine and betahistine) | 143 (1) | — | MD 4.59 higher (3.28–5.90 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| LVA-BF (vs. diphenidol) | 116 (1) | — | MD 5.51 higher (4.39–6.63 higher) | — | N/A | ⊕⊕⊕○ Moderate |
Imprecision (−1) |
| LVA-BF (vs. nimodipine) | 134 (1) | — | MD 2.40 higher (1.90–2.90 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| RVA-BF | 791 (7) | — | MD 5.28 higher (3.38–7.18 higher) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| RVA-BF (vs. flunarizine) | 226 (2) | — | MD 4.80 higher (4.23–5.38 higher) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| RVA-BF (vs. betahistine) | 172 (2) | — | MD 7.77 higher (7.17–8.37 higher) | — | 25% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| RVA-BF (vs. flunarizine and betahistine) | 143 (1) | — | MD 5.04 higher (3.85–6.23 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| RVA-BF (vs. diphenidol) | 116 (1) | — | MD 4.69 higher (3.77–5.61 higher) | — | N/A | ⊕⊕⊕○ Moderate |
Imprecision (−1) |
| RVA-BF (vs. nimodipine) | 134 (1) | — | MD 1.82 higher (1.35–2.29 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| BA-BF | 791 (7) | — | MD 5.28 higher (3.97–6.59 higher) | — | 92% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| BA-BF (vs. flunarizine) | 226 (2) | — | MD 4.85 higher (4.04–5.65 higher) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| BA–BF (vs. betahistine) | 172 (2) | — | MD 5.70 higher (5.15–6.24 higher) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| BA–BF (vs. flunarizine and betahistine) | 143 (1) | — | MD 6.92 higher (5.74–8.10 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| BA-BF (vs. diphenidol) | 116 (1) | — | MD 6.23 higher (4.42–8.04 higher) | — | N/A | ⊕⊕⊕○ Moderate |
Imprecision (−1) |
| BA-BF (vs. nimodipine) | 134 (1) | — | MD 2.74 higher (2.19–3.29 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| TER | 1,529 (13) | 311 per 1,000 | 461 per 1,000 (420–538) | RR 1.53 (1.35–1.73) | 21% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| TER (vs. flunarizine) | 610 (6) | 407 per 1,000 | 590 per 1,000 (472–773) | RR 1.48 (1.16–1.90) | 50% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TER (vs. betahistine) | 526 (4) | 206 per 1,000 | 322 per 1,000 (262–459) | RR 1.68 (1.27–2.23) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| TER (vs. flunarizine and betahistine) | 143 (1) | 286 per 1,000 | 562 per 1,000 (369–858) | RR 1.97 (1.29–3.00) | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| TER (vs. diphenidol) | 116 (1) | 259 per 1,000 | 362 per 1,000 (207–632) | RR 1.40 (0.80–2.44) | N/A | ⊕⊕○○ Low |
Imprecision (−2) |
| TER (vs. nimodipine) | 134 (1) | 328 per 1,000 | 433 per 1,000 (279–669) | RR 1.32 (0.85–2.04) | N/A | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
| ET level (vs. diphenidol) | 116 (1) | — | MD 11.14 lower (9.49–12.79 lower) | — | N/A | ⊕⊕⊕○ Moderate |
Imprecision (−1) |
| CGRP level | 200 (2) | — | MD 8.89 higher (0.76 lower–18.54 higher) | — | 98% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−2) |
| CGRP level (vs. flunarizine) | 84 (1) | — | MD 13.89 higher (11.48–16.30 higher) | — | N/A | ⊕⊕○○ Low |
Imprecision (−2) |
| CGRP level (vs. diphenidol) | 116 (1) | — | MD 4.04 higher (3.68–4.40 higher) | — | N/A | ⊕⊕⊕○ Moderate |
Imprecision (−1) |
|
| |||||||
| CHM plus MT vs. MT | |||||||
| OFS | 246 (2) | — | SMD 3.17 lower (6.48 lower–0.15 higher) | — | 98% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−2) |
| SS | 726 (7) | — | SMD 1.33 higher (0.12–2.54 higher) | — | 98% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| LVA-BF | 358 (3) | — | MD 6.24 higher (1.36–11.12 higher) | — | 98% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| RVA-BF | 358 (3) | — | MD 5.62 higher (1.03–10.21 higher) | — | 98% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| BA-BF | 358 (3) | — | MD 4.62 higher (0.32–8.91 higher) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TER | 614 (6) | 235 per 1,000 | 406 per 1,000 (320–508) | RR 1.71 (1.36–2.16) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| ET level | 226 (2) | — | MD 16.48 lower (33.31 lower–0.34 higher) | — | 98% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−2) |
| CGRP level | 226 (2) | — | MD 4.63 higher (2.25–7.00 higher) | — | 93% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
|
| |||||||
| CHM plus AT vs. AT | |||||||
| OFS | 70 (1) | — | MD 1.91 lower (1.37–2.45 lower) | — | N/A | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
| SS | 677 (8) | — | SMD 1.72 higher (1.33–2.11 higher) | — | 79% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| LVA-BF | 629 (7) | — | MD 5.81 higher (2.92–8.70 higher) | — | 95% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| RVA-BF | 629 (7) | — | MD 4.03 higher (1.05–7.01 higher) | — | 96% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| BA-BF | 739 (8) | — | MD 6.43 higher (2.97–9.89 higher) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TER | 1,149 (13) | 307 per 1,000 | 471 per 1,000 (405–546) | RR 1.54 (1.32–1.78) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
|
| |||||||
| CHM plus MT plus AT vs. MT plus AT | |||||||
| OFS | 290 (1) | — | MD 7.06 lower Risk of bias (−1) Imprecision (−1; 6.27–7.85 lower) |
— | N/A | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| TER | 290 (1) | 290 per 1,000 | 407 per 1,000 (296–563) | RR 1.40 (1.02–1.94) | N/A | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
|
| |||||||
| Subgroup analysis according to the CHM prescription names | |||||||
| BBTT plus active controls vs. active controls | |||||||
| OFS | 410 (2) | — | SMD 3.44 lower (0.69–6.20 lower) | — | 98% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| SS | 220 (2) | — | MD 5.15 higher (4.81–5.50 higher) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| LVA-BF | 184 (2) | — | MD 4.44 higher (3.18–5.69 higher) | — | 71% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| RVA-BF | 184 (2) | — | MD 3.85 higher (2.29–5.41 higher) | — | 84% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| BA-BF | 184 (2) | — | MD 3.48 higher (0.04–6.92 higher) | — | 95% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| TER | 1,168 (9) | 329 per 1,000 | 486 per 1,000 (424–559) | RR 1.48 (1.29–1.70) |
33% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| ET level | 100 (1) | — | MD 25.13 lower (21.29–28.97 lower) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| CGRP level | 100 (1) | — | MD 5.89 higher (4.78–7.00 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
|
| |||||||
| BYT plus active controls vs. active controls | |||||||
| SS | 134 (2) | — | MD 2.04 higher (1.35–2.72 higher) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| LVA-BF | 134 (2) | — | MD 1.72 higher (0.57–2.87 higher) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| RVA-BF | 134 (2) | — | MD 1.80 lower (0.72–2.88 lower) | — | 0% | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| BA-BF | 134 (2) | — | MD 0.43 higher (0.68 lower–1.55 higher) | — | 0% | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
| TER | 134 (2) | 224 per 1,000 | 284 per 1,000 (157–511) | RR 1.27 (0.70–2.28) | 0% | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
|
| |||||||
| DXT plus active controls vs. active controls | |||||||
| OFS | 126 (1) | — | MD 5.68 lower (4.36–7.00 lower) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| SS | 715 (7) | — | SMD 1.67 higher (0.20–3.14 higher) | — | 98% | ⊕⊕⊕○ Moderate |
Inconsistency (−1) |
| LVA-BF | 579 (5) | — | MD 5.13 higher (3.87–6.40 higher) | — | 78% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| RVA-BF | 579 (5) | — | MD 5.12 higher (3.42–6.83 higher) | — | 90% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| BA-BF | 579 (5) | — | MD 5.14 higher (2.66–7.62 higher) | — | 92% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TER | 789 (8) | 265 per 1,000 | 431 per 1,000 (352–517) | RR 1.61 (1.33–1.95) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| ET level | 242 (2) | — | MD 9.71 lower (6.61–12.81 lower) | — | 76% | ⊕○○○ Very low |
Risk of bias (−1) Inconsistency (−1) Imprecision (−1) |
| CGRP level | 326 (3) | — | MD 6.41 higher (4.15–8.67 higher) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
|
| |||||||
| GGT plus active controls vs. active controls | |||||||
| OFS | 98 (1) | — | MD 7.80 lower (6.02–9.58 lower) | — | N/A | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
| SS | 489 (5) | — | SMD 1.92 higher (0.99–2.85 higher) | — | 94% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| LVA-BF | 579 (5) | — | MD 7.29 higher (3.51–11.07 higher) | — | 99% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| RVA-BF | 579 (5) | — | MD 6.18 higher (3.12–9.24 higher) | — | 99% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| BA-BF | 579 (5) | — | MD 5.19 higher (3.50–6.88 higher) | — | 96% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TER | 609 (6) | 299 per 1,000 | 485 per 1,000 (395–595) | RR 1.62 (1.32–1.99) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
|
| |||||||
| GJT plus active controls vs. active controls | |||||||
| SS | 200 (1) | — | MD 2.00 higher (1.75–2.25 higher) | — | N/A | ⊕⊕○○ Low |
Risk of bias (−1) Imprecision (−1) |
| TER | 200 (1) | 88 per 1,000 | 187 per 1,000 (87–425) | RR 2.19 (0.99–4.86) | N/A | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
|
| |||||||
| YCT plus active controls vs. active controls | |||||||
| OFS | 70 (1) | — | MD 1.91 lower (1.37–2.45 lower) | — | N/A | ⊕○○○ Very low |
Risk of bias (−1) Imprecision (−2) |
| SS | 531 (5) | — | SMD 1.79 higher (0.93–2.64 higher) | — | 94% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| LVA-BF | 302 (3) | — | MD 7.63 higher (4.69–10.57 higher) | — | 80% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| RVA-BF | 302 (3) | — | MD 7.34 higher (6.02–8.66 higher) | — | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| BA-BF | 412 (4) | — | MD 11.01 higher (4.46–17.56 higher) | — | 96% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TER | 682 (7) | 328 per 1,000 | 504 per 1,000 (420–604) | RR 1.54 (1.28–1.84) | 0% | ⊕⊕⊕○ Moderate |
Risk of bias (−1) |
| Fib level | 348 (4) | — | MD 0.31 lower (0.12–0.50 lower) | — | 97% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
| TC level | 348 (4) | — | MD 0.56 lower (0.31–0.82 lower) | — | 71% | ⊕⊕○○ Low |
Risk of bias (−1) Inconsistency (−1) |
If the evidence of more than 10 studies showed MD <4 for the change in the blood flow velocity in the vertebrobasilar artery or RR >2 for the total effective rate, it was considered that there was a strong association for a treatment effect. AD, anti-vertigo drugs; AT, acupuncture therapy; BA-BF, basal artery blood flow; BBTT, Banxia Baizhu Tianma Tang; BYT, Buzhong Yiqi Tang; CHM, Chinese herbal medicine; CI, confidence interval; CGRP, calcitonin gene-related peptide; DXT, Dingxuan Tang; ET, endothelin; Fib, fibrinogen; GGT, Gegen Tang; GJT, Gegen Jieji Tang; GRADE, the grading of recommendations assessment, development, and evaluation; LVA-BF, left vertebral artery blood flow; MD, mean difference; MT, manual therapy; OFS, overall functional score; RCT, randomised controlled trial; RR, risk ratio; RVA-BF, right vertebral artery blood flow; SMD, standardised mean difference; SS, simple score; TC, total cholesterol; TER, total effective rate; YCT, Yiqi Congming Tang.
3.5. Safety
Three of the thirty-five included studies reported adverse events. There was one case of gastrointestinal discomfort in the BBTT plus manual therapy group; one case of abdominal pain; one case of fainting during acupuncture therapy in the BBTT plus acupuncture therapy group; one case of rash, diarrhea, and gastrointestinal discomfort each, and two cases of fatigue in the DXT plus anti-vertigo drugs (flunarizine and betahistine) group. All reported adverse events were mild and transient and were evaluated as “not serious” (Table 1).
3.6. Quality of Evidence
In the comparison of CHMs plus active controls versus active controls alone, the quality of evidence for the primary outcomes ranged from “very low” to “low.” For the secondary outcomes, the quality of evidence for the total effective rate was graded as “moderate,” while that for the other outcomes was graded as “very low” or “low.” The overall quality of evidence in the total analysis was graded as “low.” In the subanalysis based on the type of active control, the overall quality of evidence was graded as “moderate” for CHMs plus manual and acupuncture therapy and as “low” for CHMs plus any other active control (anti-vertigo drugs, manual therapy, or acupuncture therapy). In the subanalysis based on the CHM prescription name, the overall quality of evidence was evaluated as “low” for all CHM prescriptions. However, its quantitative and qualitative levels were highest for DXT and YCT and lowest for BYT and GJT, respectively. The main reason for the downgrade was the high risk of bias in the included studies, the imprecision of the results due to the small sample size, and the inconsistency of the results due to the high heterogeneity among them (Table 4).
3.7. Sensitivity Analysis
For the outcomes with considerable heterogeneity among studies, we performed sensitivity analysis and adjusted the quality of evidence based on the results. After heterogeneity was eliminated by removing one to two outliers considered to have a high risk for selection and reporting biases, the quality of evidence for the efficacy of CHMs for CGD was similar to that obtained before the sensitivity analysis. Therefore, the findings in this systematic review are considered robust to the decisions made in the process of obtaining them (Table 5).
Table 5.
Adjusted quality of evidence derived by sensitivity analysis.
| Outcomes | Before SA | After SA | |||
|---|---|---|---|---|---|
| Anticipated absoluteeffects (95% CI) | I 2 value | Anticipated absolute effects (95% CI) | I 2 value | Adjusted quality of evidence (GRADE) | |
| Total analysis | |||||
| OFS | SMD 2.31 (1.48–3.14) | 94% | SMD 1.81 (1.61–2.00) | 49% | ⊕⊕⊕○ Moderate |
| TC level (vs. AT) | MD 0.56 (0.31–0.82) | 71% | MD 0.43 (0.27–0.60) | 0% | ⊕⊕⊕○ Moderate |
|
| |||||
| Subgroup analysis according to the comparison types | |||||
| CHM plus AD vs. AD | |||||
| BA-BF | MD 5.28 (3.97–6.59) | 92% | MD 5.65 (5.24–6.06) | 48% | ⊕⊕⊕○ Moderate |
|
| |||||
| CHM plus MT vs. MT | |||||
| LVA-BF | MD 6.24 (1.36–11.12) | 98% | MD 3.81 (2.84–4.79) | 0% | ⊕⊕○○ Low |
| RVA-BF | MD 5.62 (1.03–10.21) | 98% | MD 3.48 (2.52–4.44) | 0% | ⊕⊕○○ Low |
| BA-BF | MD 4.62 (0.32–8.91) | 97% | MD 6.67 (4.73–8.62) | 43% | ⊕⊕○○ Low |
|
| |||||
| CHM plus AT vs. AT | |||||
| RVA-BF | MD 4.03 (1.05–7.01) | 96% | MD 7.28 (6.33–8.22) | 0% | ⊕⊕⊕○ Moderate |
|
| |||||
| Subgroup analysis according to the CHM prescription names | |||||
| DXT plus active controls vs. active controls | |||||
| LVA-BF | MD 5.13 (3.87–6.40) | 78% | MD 4.56 (3.92–5.20) | 48% | ⊕⊕○○ Low |
| RVA-BF | MD 5.12 (3.42–6.83) | 90% | MD 4.33 (3.75–4.91) | 41% | ⊕⊕○○ Low |
| BA-BF | MD 5.14 (2.66–7.62) | 92% | MD 6.45 (5.62–7.28) | 0% | ⊕⊕○○ Low |
| CGRP level | MD 6.41 (4.15–8.67) | 97% | MD 3.87 (3.57–4.17) | 66% | ⊕⊕○○ Low |
|
| |||||
| GGT plus active controls vs. active controls | |||||
| SS | SMD 1.92 (0.99–2.85) | 94% | SMD 1.39 (1.16–1.62) | 74% | ⊕⊕○○ Low |
| LVA-BF | MD 7.29 (3.51–11.07) | 99% | MD 10.33 (9.76–10.90) | 0% | ⊕⊕⊕○ Moderate |
| BA–BF | MD 5.19 (3.50–6.88) | 96% | MD 5.46 (5.00–5.93) | 45% | ⊕⊕⊕○ Moderate |
|
| |||||
| YCT plus active controls vs. active controls | |||||
| SS | SMD 1.79 (0.93–2.64) | 94% | SMD 2.13 (1.87–2.38) | 0% | ⊕⊕⊕○ Moderate |
| LVA-BF | MD 7.63 (4.69–10.57) | 80% | MD 3.47 (3.19–3.75) | 0% | ⊕⊕○○ Low |
AD, anti-vertigo drugs; AT, acupuncture therapy; BA-BF, basal artery blood flow; CHM, Chinese herbal medicine; CI, confidence interval; CGRP, calcitonin gene-related peptide; DXT, Dingxuan Tang; GGT, Gegen Tang; GRADE, the grading of recommendations assessment, development, and evaluation; LVA-BF, left vertebral artery blood flow; MD, mean difference; MT, manual therapy; OFS, overall functional score; RVA-BF, right vertebral artery blood flow; SA, sensitivity analysis; SMD, standardised mean difference; SS, simple score; TC, total cholesterol; YCT, Yiqi Congming Tang.
3.8. Publication Bias
For seven outcomes included in more than ten studies, we examined publication bias using funnel plot analysis. For the comparisons of CHMs plus active controls, anti-vertigo drugs, or acupuncture therapy versus active controls, anti-vertigo drugs, or acupuncture therapy alone, respectively, the funnel plots of the total effective rate were symmetrical for all (Figures 3–5). Conversely, for the comparison of CHMs plus active controls versus active controls, the funnel plots of the simple scores and the blood flow velocity in the vertebrobasilar arteries showed asymmetry. In the funnel plot of the blood flow velocity in the left vertebral artery, the asymmetry was presumed to be due to considerable heterogeneity. The asymmetry for the remaining outcomes suggested potential publication bias; thus, there may be negative results not published in the literature (Figures 6–9).
Figure 3.
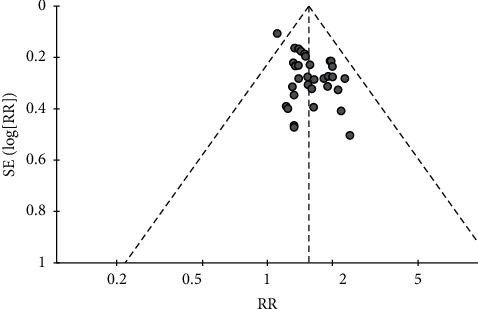
Funnel plot of the effects of CHMs plus active controls on the total effective rate.
Figure 4.
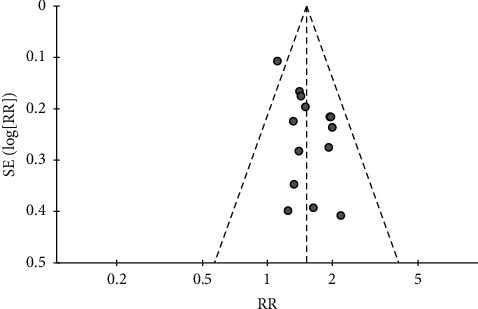
Funnel plot of the effects of CHMs plus anti-vertigo drugs on the total effective rate.
Figure 5.
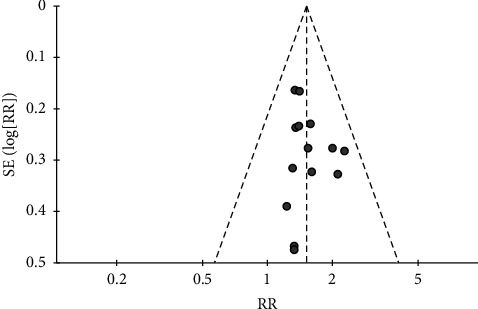
Funnel plot of the effects of CHMs plus acupuncture therapy on the total effective rate.
Figure 6.
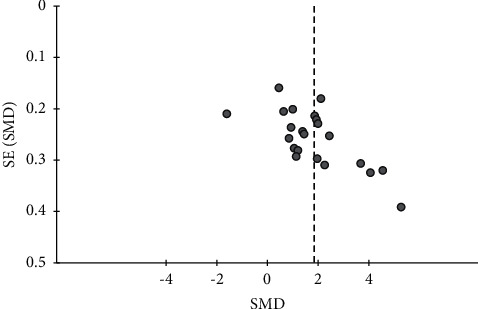
Funnel plot of the effects of CHMs plus active controls on the simple scores.
Figure 7.
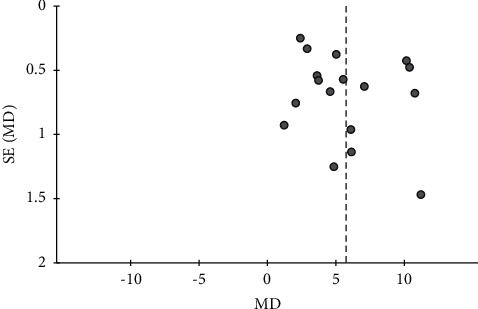
Funnel plot of the effects of CHMs plus active controls on the blood flow velocity in the left vertebral artery.
Figure 8.
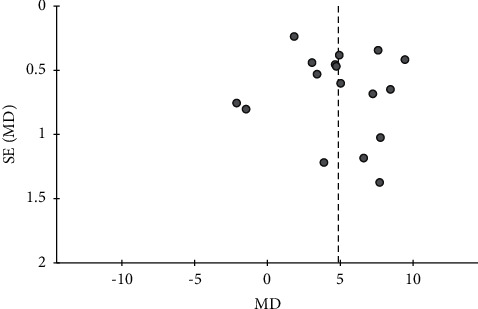
Funnel plot of the effects of CHMs plus active controls on the blood flow velocity in the right vertebral artery.
Figure 9.
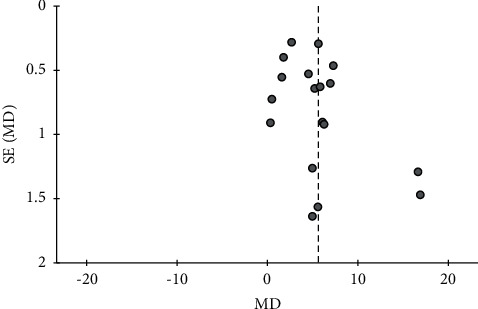
Funnel plot of the effects of CHMs plus active controls on the blood flow velocity in the basilar artery.
4. Discussion
4.1. Summary of Findings
In this study, we reviewed and evaluated the available clinical evidence on the efficacy and safety of CHM as monotherapy or adjunctive therapy in the treatment of CGD to promote evidence-based decision-making in clinical practice. As none of the 35 included RCTs [33–67] assessed the efficacy of CHM as monotherapy for CGD, we evaluated its efficacy as adjunctive therapy in combination with other active controls. The included studies were conducted with 6 types of modified CHMs and 4 types of active controls. In the risk-of-bias assessment, more than half of the included studies were considered to be of low quality because of the high risk of bias due to deviations from intended interventions. The results of the efficacy analyses of CHMs plus active controls indicated the following. First, CHMs plus active controls were more effective in treating CGD than active controls alone (the duration of administration ranged from 10 days to 8 weeks). Second, CHMs plus anti-vertigo drugs (flunarizine/betahistine/flunarizine and betahistine/diphenidol/nimodipine), CHMs plus manual therapy, CHMs plus acupuncture therapy, and CHMs plus manual and acupuncture therapy were all effective in treating CGD. Among all, CHMs plus manual and acupuncture therapy showed the most reliable effect. Third, BBTT, BYT, DXT, GGT, GJT, and YCT were effective for specific patterns in patients with CGD, when administered with active controls. Among the CHM prescriptions, DXT and YCT exhibited the most reliable effects, when combined with active controls. Regarding the safety of CHMs plus active controls in the treatment of CGD, no serious adverse events were reported in any of the included studies.
4.2. Implications for Clinical Practice
In traditional Chinese medicine, CHMs are prescribed to match the specific pattern of the patients' signs and symptoms. It is reasonable to select and prescribe the most appropriate CHM for a specific pattern in each patient with CGD, as opposed to consistently prescribing one CHM to all patients with CGD, even if it is the most evidence-based prescription for CGD. Thus, although DXT and YCT had the highest level of clinical evidence for the treatment effect on CGD in this review, it may be more effective to use other CHMs for specific patterns in some patients with CGD. In traditional Chinese medicine, wind, fire, phlegm, blood stasis, and deficiency are considered the main pathogenetic factors for CGD [25]. DXT is usually prescribed for CGD syndromes of spleen deficiency and dampness, qi deficiency and blood stasis, or hyperactivity of liver yang. DXT has the effect of removing a pathogenic mass as the original prescription, and it can be prescribed for both deficiency and excess syndromes by modification of the original prescription. CHMs can be modified for better efficacy and fewer side effects [68]. In cases of combined excess and deficiency syndromes, such as spleen deficiency and dampness type or qi deficiency and blood stasis type, DXT was modified by the addition of herbs that have effects on invigorating the qi and spleen (Codonopsis pilosulae Radix and Atractylodis Rhizoma Alba), regulating qi-flowing (Citri Reticulatae Pericarpium), enriching the blood (Angelicae Gigantis Radix), and soothing the nerves (Fossilia Ossis Mastodi), but with subtraction of other herbs from the original prescription, which have effects on suppressing hyperactive liver for calming endogenous wind (Uncariae Ramulus Cum Uncis and Scorpio) and promoting blood circulation while removing blood stasis (Salviae miltiorrhizae Radix) [43, 47]. Conversely, in cases of excess syndrome only, such as hyperactivity of liver yang type, DXT was modified by adding Puerariae Radix, which has the effect of dispelling wind-heat [57, 66]. For the combination of DXT and other treatments, quantitative clinical evidence has been reported for the use of DXT with manual therapy [47, 57, 66]. Both YCT and BYT are usually prescribed for CGD syndromes of qi and blood deficiency, while YCT is also used for the sputum silting up type. The clinical evidence for YCT is better than that for BYT because the latter showed low precision for outcomes. For the combination of YCT and other treatments, the majority of quantitative clinical evidence was reported for the use of YCT with acupuncture therapy [36, 38, 45, 51, 54, 59, 62]. BBTT, which has the effect of dispelling pathogenic wind and eliminating phlegm, is usually prescribed for CGD syndromes of wind-phlegm or phlegm stasis [37, 39, 56]. GGT is usually prescribed for CGD syndromes of wind with disharmony between ying and wei [52]. Both BBTT and GGT were used with various active controls and showed reliable treatment effects. GJT was used for the collateral stasis type with betahistine [40]. Through this review, we gain a clue about the relationship between specific patterns of CGD and CHM prescriptions; however, it remains unknown which CHM prescription is most effective for specific patterns of CGD because all included studies used only one CHM prescription with one specific pattern of CGD. Furthermore, studies are needed to confirm which CHM prescription is most effective for specific patterns of CGD.
4.3. Implications for Research
In this review, we identified fibrinogen, endothelin, TC, and CGRP as haematological parameters used in clinical studies on CGD. Endothelin and CGRP were used as indicators to determine the efficacy of CHMs plus anti-vertigo drugs and CHMs plus manual therapy. Fibrinogen and TC were used to determine the efficacy of CHMs plus acupuncture therapy. Endothelin is an endogenous vasoconstrictor that reduces the perfusion of brain tissues by constricting the blood vessels in the brain [69, 70]. CGRP is a vasodilator, mainly distributed in the central nervous system [71]. In a previous study, endothelin and CGRP were reported as important factors affecting the development of CGD with vertebrobasilar arteriospasm [72]. Fibrinogen also promotes the formation of artherosclerotic plaques [73], and TC accelerates atherosclerosis and causes lipid metabolism disorders [74]. In summary, control of endothelin and CGRP levels improves the prognosis of patients with CGD, and evaluation of fibrinogen and TC levels helps predict CGD progression. Therefore, it is recommended to use them as outcomes when conducting further clinical trials of CGD.
This research is valuable as the first systematic review to comprehensively evaluate the efficacy and safety of CHMs in treating CGD, to guide clinicians in selecting and prescribing suitable CHMs for specific patterns of CGD based on evidence-based decision-making. Furthermore, it provides knowledge of which treatments will be effective in combination with CHMs. This review may contribute to the development of effective strategies for the treatment and management of an increasing number of patients with CGD due to population ageing. Nonetheless, further high-quality evidence from rigorously conducted clinical studies, preferably conducted outside China, is required to support the clinical recommendations regarding the use of CHMs for CGD. In addition, placebo-controlled RCTs are needed to evaluate the efficacy of CHMs as monotherapy for CGD. Furthermore, experimental studies of the mechanism of action and the dose-response relationship of CHMs are necessary to determine the optimal dose.
5. Limitations
This review has some limitations. First, some of the major Chinese databases, such as Wanfang and VIP, were not included in the search process. Additionally, grey literature was not considered. Thus, there is a possibility that relevant studies were omitted. Second, the quality of the included RCTs was generally poor, in particular, because of the high risk of bias due to deviations from intended interventions. Third, most meta-analyses showed high heterogeneity among studies. Fourth, potential publication bias could not be ruled out because the assessment of publication bias was not conducted in the meta-analyses in which the number of included studies was less than 10, and all RCTs were conducted in China and published in Chinese. Fifth, there is the possibility of attrition bias because few studies presented dropout or withdrawal statistics. Sixth, it is unknown whether the treatment effect of CHMs plus active controls was maintained after completion of the intervention because most studies did not perform follow-up assessments. Finally, the safety of CHMs in patients with CGD remains unknown because few studies clearly reported that there were no adverse events.
6. Conclusions
Current evidence suggests that CHMs may have the potential to enhance the treatment effect on CGD when combined with other treatments without serious adverse events. As the overall quality of the studies included in this review was generally low, additional high-quality evidence is needed to draw definitive conclusions.
Acknowledgments
HO was supported by a scholarship from Kyung Hee University. SS and EL were supported by a grant from the National Research Foundation of Korea funded by the Korean Government (grant number: 2020R1F1A1068808). EL and WSC were supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant number: HI20C1405).
Abbreviations
- BBTT:
Banxia Baizhu Tianma Tang
- BYT:
Buzhong Yiqi Tang
- CENTRAL:
Cochrane central register of controlled trials
- CGD:
Cervicogenic dizziness
- CHM:
Chinese herbal medicine
- CGRP:
Calcitonin gene-related peptide
- CI:
Confidence interval
- DXT:
Dingxuan Tang
- EMBASE:
Excerpta medica database
- GGT:
Gegen Tang
- GJT:
Gegen Jieji Tang
- MD:
Mean difference
- MEDLINE:
Medical literature analysis and retrieval system online
- RCT:
Randomised controlled trial
- RR:
Risk ratio
- SA:
Sensitivity analysis
- SMD:
Standardised mean difference
- TC:
Total cholesterol
- YCT:
Yiqi Congming Tang.
Data Availability
All data generated or analysed during this study are included in this article (and its supplementary information files).
Disclosure
The funding bodies had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
HO was responsible for the conceptualisation of the study, funding acquisition, methodology, drafting the manuscript, and critical revision of the manuscript for important intellectual content. EL was responsible for funding acquisition, study supervision, and critical revision of the manuscript for important intellectual content. WSC was responsible for funding acquisition and critical revision of the manuscript for important intellectual content. SS was responsible for the methodology and critical revision of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Supplementary Materials
Supplementary materials are given in the .docx file format. Appendix A. Search strategies used in English databases. Description of data: search strategies used in three English databases (MEDLINE, EMBASE, and CENTRAL).
References
- 1.Reid S. A., Rivett D. A. Manual therapy treatment of cervicogenic dizziness: a systematic review. Manual Therapy . 2005;10(1):4–13. doi: 10.1016/j.math.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 2.Hain T. C. Cervicogenic causes of vertigo. Current Opinion in Neurology . 2015;28(1):69–73. doi: 10.1097/wco.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 3.Yaseen K., Hendrick P., Ismail A., Felemban M., Alshehri M. A. The effectiveness of manual therapy in treating cervicogenic dizziness: a systematic review. Journal of Physical Therapy Science . 2018;30(1):96–102. doi: 10.1589/jpts.30.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson-Harvey A., Hain T. C. Symptoms in cervical vertigo. Laryngoscope Investigative Otolaryngology . 2019;4(1):109–115. doi: 10.1002/lio2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ardç F. N., Topuz B., Kara C. O. Impact of multiple etiology on dizziness handicap. Otology & Neurotology . 2006;27(5):676–680. doi: 10.1097/01.mao.0000226292.49789.c9. [DOI] [PubMed] [Google Scholar]
- 6.Lüscher M., Theilgaard S., Edholm B. Prevalence and characteristics of diagnostic groups amongst 1034 patients seen in ENT practices for dizziness. Journal of Laryngology and Otology . 2014;128(2):128–133. doi: 10.1017/s0022215114000188. [DOI] [PubMed] [Google Scholar]
- 7.Reid S. A., Callister R., Snodgrass S. J., Katekar M. G., Rivett D. A. Manual therapy for cervicogenic dizziness: long-term outcomes of a randomised trial. Manual Therapy . 2015;20(1):148–156. doi: 10.1016/j.math.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Kim T., Kim K. T., Ko H. Clinical analysis on the positional vertigo patients treated in an oriental medical center. Korean Journal of Internal Medicine . 2011;32:371–386. [Google Scholar]
- 9.Kim J. Y., Kim W. H., Lee J. S., Oh H. M., Kim D. W., Choi D. J. The diagnosis and treatment of the cervical vertigo. Korean Journal of Otorhinolaryngology-Head and Neck Surgery . 2015;58 doi: 10.3342/kjorl-hns.2015.58.3.177. [DOI] [Google Scholar]
- 10.Peng B., Li Y. Pathogenesis, diagnosis, and treatment of cervical vertigo. Pain Physician . 2015;18(4):E583–E595. doi: 10.36076/ppj.2015/18/e583. [DOI] [PubMed] [Google Scholar]
- 11.Yenigun A., Ustun M. E., Tugrul S., Dogan R., Ozturan O. Classification of vertebral artery loop formation and association with cervicogenic dizziness. Journal of Laryngology and Otology . 2016;130(12):1115–1119. doi: 10.1017/s0022215116009117. [DOI] [PubMed] [Google Scholar]
- 12.Furman J. M., Cass S. P. Balance Disorders: A Case-Study Approach . Philadelphia, PA, USA: F A Davis; 1996. [Google Scholar]
- 13.Grgić V. Cervicogenic proprioceptive vertigo: etiopathogenesis, clinical manifestations, diagnosis and therapy with special emphasis on manual therapy. Lijecnicki Vjesnik . 2006;128:288–295. [PubMed] [Google Scholar]
- 14.Magnusson M., Malmström E.-M. The conundrum of cervicogenic dizziness. Handbook of Clinical Neurology . 2016;137:365–369. doi: 10.1016/b978-0-444-63437-5.00026-1. [DOI] [PubMed] [Google Scholar]
- 15.Devaraja K. Approach to cervicogenic dizziness: a comprehensive review of its aetiopathology and management. European Archives of Oto-Rhino-Laryngology . 2018;275(10):2421–2433. doi: 10.1007/s00405-018-5088-z. [DOI] [PubMed] [Google Scholar]
- 16.Wrisley D. M., Sparto P. J., Whitney S. L., Furman J. M. Cervicogenic dizziness: a review of diagnosis and treatment. Journal of Orthopaedic and Sports Physical Therapy . 2000;30(12):755–766. doi: 10.2519/jospt.2000.30.12.755. [DOI] [PubMed] [Google Scholar]
- 17.Knapstad M. K., Nordahl S. H. G., Goplen F. K. Clinical characteristics in patients with cervicogenic dizziness: a systematic review. Health Science Reports . 2019;2(9):p. e134. doi: 10.1002/hsr2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karlberg M., Magnusson M., Eva-Maj M., Agneta M., Moritz U. Postural and symptomatic improvement after physiotherapy in patients with dizziness of suspected cervical origin. Archives of Physical Medicine and Rehabilitation . 1996;77(9):874–882. doi: 10.1016/s0003-9993(96)90273-7. [DOI] [PubMed] [Google Scholar]
- 19.Reid S. A., Rivett D. A., Katekar M. G., Callister R. Sustained natural apophyseal glides (SNAGs) are an effective treatment for cervicogenic dizziness. Manual Therapy . 2008;13(4):357–366. doi: 10.1016/j.math.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 20.Lystad R. P., Bell G., Bonnevie-Svendsen M., Carter C. V. Manual therapy with and without vestibular rehabilitation for cervicogenic dizziness: a systematic review. Chiropractic and Manual Therapies . 2011;19(1):p. 21. doi: 10.1186/2045-709x-19-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moustafa I. M., Diab A. A., Harrison D. E. The effect of normalizing the sagittal cervical configuration on dizziness, neck pain, and cervicocephalic kinesthetic sensibility: a 1-year randomized controlled study. European Journal of Physical and Rehabilitation Medicine . 2017;53(1):57–71. doi: 10.23736/s1973-9087.16.04179-4. [DOI] [PubMed] [Google Scholar]
- 22.Shin S.-H., Min K.-J., Kim E.-B., Ha W.-B., Ko Y.-S. Chuna manual therapy alone for cervicogenic dizziness: a systematic review. Journal of Korean Medicine Rehabilitation . 2019;29(1):1–6. doi: 10.18325/jkmr.2019.29.1.1. [DOI] [Google Scholar]
- 23.Kim Y., Cho S.-H. Pharmacopuncture for cervicogenic dizziness. Journal of Pharmacopuncture . 2018;21(4):241–248. doi: 10.3831/kpi.2018.21.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heikkilä H., Johansson M., Wenngren B.-I. Effects of acupuncture, cervical manipulation and NSAID therapy on dizziness and impaired head repositioning of suspected cervical origin: a pilot study. Manual Therapy . 2000;5(3):151–157. doi: 10.1054/math.2000.0357. [DOI] [PubMed] [Google Scholar]
- 25.Hou Z., Xu S., Li Q., et al. The efficacy of acupuncture for the treatment of cervical vertigo: a systematic review and meta-analysis. Evidence-Based Complementary and Alternative Medicine . 2017;2017:13. doi: 10.1155/2017/7597363.7597363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi S. Importance of cervicogenic general dizziness. Journal of Rural Medicine . 2018;13(1):48–56. doi: 10.2185/jrm.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh H., Shin S., Lee E. Herbal medicine for cervicogenic dizziness. Medicine . 2020;99(51) doi: 10.1097/md.0000000000023852.e23852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moher D., Liberati A., Tetzlaff J., Altman D. G. PRISMA group: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine . 2009;6(7) doi: 10.1371/journal.pmed.1000097.e1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oh H., Shin S., Lee E., Chung W. S. Herbal medicine for cervicogenic dizziness: a systematic review and meta-analysis. 2021. https://www.researchsquare.com/article/rs-364098/v1 . [DOI] [PMC free article] [PubMed]
- 30.Sterne J. A. C., Savović J., Page M. J., et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ . 2019;366 doi: 10.1136/bmj.l4898.l4898 [DOI] [PubMed] [Google Scholar]
- 31.Balshem H., Helfand M., Schünemann H. J., et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology . 2011;64(4):401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 32.Borenstein M., Hedges L. V., Higgins J. P. T., Rothstein H. R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Research Synthesis Methods . 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 33.Bai R. Clinical observation on the treatment of cervical vertigo by acupuncture combined with Buzhong Yiqi decoction. Chinese Journal of Ethnomedicine and Ethnopharmacy . 2020;20:113–116. [Google Scholar]
- 34.Chen J. Efficacy of Danqi Gegen decoction combined with flunarizine hydrochloride in the treatment of cervical vertigo patients with qi deficiency and blood stasis type. Journal of Chengde Medical University . 2021;6:494–496. [Google Scholar]
- 35.Cheng B., He S., Wang M., He Y. Effect of Yiqi Dingxuan decoction on NPY and IL-1β in patients with cervical vertigo treated with flunarizine hydrochloride capsule. Chinese Archives of Traditional Chinese Medicine . 2021;10:101–103. [Google Scholar]
- 36.Dai J. Acupuncture and moxibustion combined with modified Yiqi Congming decoction for treatment of qi and blood deficiency type cervical clinical observation of vertigo. Biped Health . 2018;2:184–186. [Google Scholar]
- 37.Gao E., Chen Y., Chi Z., Wang M. The clinical effect of western medicine combined with Bonxia Baizhu Tianma decoction in the treatment of cervical vertigo. Chinese Journal of Ethnomedicine and Ethnopharmacy . 2018;27:98–99. [Google Scholar]
- 38.Gu W., Min J. Clinical study on effect of Yiqi Wenzhong acupuncture and moxibustion treatment combined with Yuqicongming decoction in treatment of qi and blood deficiency type cervical vertigo. Acta Chinese Medicine . 2018;33:1359–1363. [Google Scholar]
- 39.Gu C. Evaluation of clinical efficacy of western medicine combined with Banxia Baizhu Tianma decoction on patients with cervical vertigo. Heilongjiang Journal of Traditional Chinese Medicine . 2019;6:25–26. [Google Scholar]
- 40.Hu X., Li L., Li J., Wang J. Clinical effect of Gegen Jieji capsule in treating cervical vertigo of collateral stasis type. Clinical Research in Practice . 2019;20:92–93. [Google Scholar]
- 41.Huagn T., Li X., Huang W. Observation on the curative effect of modified gegen decoction combined with acupuncture on cervical vertigo. Journal of Baotou Medical College . 2021;6:87–89. [Google Scholar]
- 42.Huang X., Hua Z., Feng Z., Liao K. Clinical observation on the treatment of cervical vertigo with the addition and subtraction of Banxia Baizhu Tianma decoction and Longshi Zhenggu massage. Chinese and Foreign Medical Research . 2021;2:1–3. [Google Scholar]
- 43.Ji C., Cheng L. Clinical observation on Dingxuan decoction in treating 30 cases of cervical vertigo. Human Journal of Traditional Chineses Medicine . 2016;32:44–45. [Google Scholar]
- 44.Ju J., Wang C. Clinical effect of Guizhi Gegen decoction combined with acupuncture in treatment of cervical vertigo in the aged and its effect on prognosis. Chinese Archives of Traditional Chinese Medicine . 2020;11:154–158. [Google Scholar]
- 45.Li A., Liu S., Liu H. The efficacy of the Yiqi Congming decoction plus acupuncture on the Qixue Kuixu type cervical vertigo. Clinical Journal of Chinese Medicine . 2021;5:113–115. [Google Scholar]
- 46.Li B., Chen L., Wang D. Effect of electroacupuncture combined with guizhi gegen decoction on cervical vertigo and its influence on TCD of vertebrobasilar artery, blood rheology indexes, and quality of life. Evidence-Based Complementary and Alternative Medicine . 2021;2021:7. doi: 10.1155/2021/2676485.2676485 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Liu S., Yuan X. A clinical study on cervical vertigo by acupoint massage combined with Yiqi Dingxuan decoction. Guiding Journal of Traditional Chinese Medicine and Pharmacology . 2019;25:111–114. [Google Scholar]
- 48.Lyu Y., Li W. Clinical study on treatment of cervical vertigo with Buzhong Yiqi tang combined with acupuncture. Acta Chinese Medicine . 2017;32:1515–1518. [Google Scholar]
- 49.Pan M., Zhang J., Rong B., Li J. Clinical study on treatment of patients with cervical vertigo with Banxia Baizhu Tianma decoction combined with acupoint manipulation massage. Journal of Basic Chinese Medicine . 2019;25:1285–1288. [Google Scholar]
- 50.Qin X., Wang J., Yang S., Lu A. Effective estimation research on cervical vertigo patients with qi deficiency and sputum silting up which were treated by Yiqi Congming decoction of Yan’s. Journal of Sichuan Traditional Chinese Medicine . 2012;30:73–75. [Google Scholar]
- 51.Qiu K., Ren Y. Effect of modified Yiqi Congming decoction combined with acupuncture on qi and blood deficiency type of cervical vertigo. Clinical Research in Practice . 2018;29:130–131. [Google Scholar]
- 52.Shang G., Ren L. Clinical observation of Guizhi Gegen decoction on cervical dizziness. Cardiovascular Disease Journal of Integrated Traditional Chinese and Western Medicine . 2016;4:179–180. [Google Scholar]
- 53.Shang Y., Hao S., Chen A. Clinical study of modified gegen decoction combined with nimodipine in the treatment of cervical vertigo. Journal of Practical Traditional Chinese Medicine . 2021;3:398–399. [Google Scholar]
- 54.Shen A. Clinical study of modified gegen decoction combined with nimodipine in the treatment of cervical vertigo. Inner Mongolia Journal of Traditional Chinese Medicine . 2020;12:120–121. [Google Scholar]
- 55.Shi D. Rongnao Dingxuan decoction combined with betahistine in the treatment of 37 cases of cervical vertigo. Chinese Journal of Traditional Medical Science and Technology . 2020;5:825–826. [Google Scholar]
- 56.Tan Y., Peng Z. Treatment of cervical vertigo with Banxia Baizhu Tianma decoction combined with western medicine. Chinese Journal of Modern Drug Application . 2019;13:159–160. [Google Scholar]
- 57.Wang J., Cao B. Clinical study of integration therapy of Tuina and Dingxuan decoction on cervical vertigo. Liaoning Journal of Traditional Chinese Medicine . 2010;37:294–296. [Google Scholar]
- 58.Wang Y. Clinical efficacy analysis of Banxia Baizhu Tianma decoction in the treatment of cervical vertigo. Guide of China Medicine . 2020;5:p. 178. [Google Scholar]
- 59.Wang Z. Clinical observation on treatment of cervical vertigo with modified Yiqi Congming decoction and acupuncture. Guangming Journal of Chinese Medicine . 2021;9:1452–1454. [Google Scholar]
- 60.Wang Q., Meng C., Chen Z. Clinical observation of Banxia Baizhu Tianma decoction combined with acupuncture and moxibustion in the treatment of cervical vertigo of phlegm turbidity type. China Practical Medicine . 2021;18:167–169. [Google Scholar]
- 61.Xu L., Wu R. Clinical study of Guizhi Gegen decoction combined with exercise therapy on cervical vertigo. Research of Integrated Traditional Chinese and Western Medicine . 2021;2:89–91. [Google Scholar]
- 62.Yang X. The clinical observation of 73 cases of vertigo with the combination of acupuncture and Yiqi and smart decoction. Chinese Journal of Ethnomedicine and Ethnopharmacy . 2018;27:96–98. [Google Scholar]
- 63.Yang X., Zhu C., Lian W. Clinical observation of Dingxuan decoction on the treatment of patients with cervical vertigo. Inner Mongolia Journal of Traditional Chinese Medicine . 2021;10:7–10. [Google Scholar]
- 64.Yao G., Chen R., Li Q., Deng H., Huang G. Therapeutic analysis of small needle knife combined with Banxia Baizhu Tianma decoction in the treatment of cervical vertigo. Heilongjiang Journal of Traditional Chinese Medicine . 2018;6:127–128. [Google Scholar]
- 65.Zhang Y., Wei X. Clinical effect of Banxia Baizhu Tianma decoction combined with acupuncture and massage in the treatment of cervical vertigo. Clinical Research Practice . 2020;23:149–151. [Google Scholar]
- 66.Zhu K. 60 Cases of cervical vertigo treated by Shujing Dingxuan decoction combined with cervical traction and massage. Traditional Chinese Medical Research . 2019;32:21–23. [Google Scholar]
- 67.Zhu J. Clinical efficacy of modified Puerariae decoction combined with acupuncture and moxibustion on patients with cervical vertigo. China Modern Doctor . 2021;14:151–154. [Google Scholar]
- 68.Iris F. F. B., Sissi W. G. Herbal Medicine: Biomolecular and Clinical Aspects . 2nd. Boca Raton, FI, USA: CRC Press; 2011. [PubMed] [Google Scholar]
- 69.Liang F., Wei D., Wang Y., Huang K., Huo Q. Research on the effect of Jingfutang on the level of plasma ET-1 and CGRP in cervical vertigo patients. Modern Journal of Integrated Traditional Chinese and Western Medicine . 2013;22:343–345. [Google Scholar]
- 70.Kowalczyk A., Kleniewska P., Kolodziejczyk M., Skibska B., Goraca A. The role of endothelin-1 and endothelin receptor antagonists in inflammatory response and sepsis. Archivum Immunologiae et Therapiae Experimentalis . 2015;63(1):41–52. doi: 10.1007/s00005-014-0310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu S., Su L. Effect of Xuanyunning on plasma ET and CGRP in patients with cervical vertigo. Chinese General Practice . 2010;13:199–200. [Google Scholar]
- 72.Wei J., Xu J., Zhang S. Relationship among plasma endothelin, calcitonin gene-related peptide and blood flow rate of bilateral vertebral arteries in patients with cervical vertigo. Journal of Nan Jing Medical University . 2008;22(3):168–171. doi: 10.1016/s1007-4376(08)60058-1. [DOI] [Google Scholar]
- 73.Levenson J., Giral P., Razavian M., Gariepy J., Simon A. Fibrinogen and silent atherosclerosis in subjects with cardiovascular risk factors. Arteriosclerosis, Thrombosis, and Vascular Biology . 1995;15(9):1263–1268. doi: 10.1161/01.atv.15.9.1263. [DOI] [PubMed] [Google Scholar]
- 74.Wang H. H., Garruti G., Liu M., Portincasa P., Wang D. Q.-H. Cholesterol and lipoprotein metabolism and atherosclerosis: recent advances in reverse cholesterol transport. Annals of Hepatology . 2017;16:S27–S42. doi: 10.5604/01.3001.0010.5495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary materials are given in the .docx file format. Appendix A. Search strategies used in English databases. Description of data: search strategies used in three English databases (MEDLINE, EMBASE, and CENTRAL).
Data Availability Statement
All data generated or analysed during this study are included in this article (and its supplementary information files).


