Abstract
Anisi stellati fructus (ASF) is the fruit of Illicium verum Hook F. (Chinese star anise), which is native to many countries, and is a significant Chinese medicinal herb. Gastric cancer (GC) is one of the major fatal types of cancers with multiple stages and a poor prognosis. The present review aims to discuss the bioactive properties of ASF and its phytocompounds against GC, with a particular insight into the molecular mechanisms and signaling pathways involved in its anti-GC mechanism. Furthermore, it highlights the potential mechanism of action of major phytocompounds of ASF against GC. Clinical studies (in vitro and in vivo) regarding the action of ASF and its major bioactive compounds such as quercetin, luteolin, kaempferol, d-limonene, and honokiol against GC were reviewed. For this review, search of literature was performed in Science, PubMed, Google Scholar, Web of Science, and Scopus related to ASF and its phytocompounds, from which only relevant studies were chosen. Major bioactive compounds of ASF and their extracts have proven to be effective against GC due to the mechanistic action of these compounds involving signaling pathways that target cancer cell apoptosis, proliferation, and tumor metastasis in GC cells. Existing reports of these compounds and their combinatory effects with other modern anticancer agents have also been reviewed. From its traditional use to its role as an anticancer agent, ASF and its bioactive phytocompounds have been observed to be effective in modern research, specifically against GC. However, further studies are required for the identification of molecular targets and pharmacokinetic potential and for the formulation of anti-GC drugs.
1. Gastric Cancer (GC): Epidemiology and Etiology
Cancer is broadly defined as the uncontrolled proliferation of cells. It is classified into several stages before being termed fatal for a living system [1]. The World Health Organization (WHO) entails several factors to be involved in its progression, including lifestyle, diet, environment, gender, genetics, and overall health [2]. According to the Global Cancer Observatory (GCO), gastric cancer (GC) ranks the fifth most common cancer to be diagnosed globally and remains the third leading cause of cancer mortality after lung and colorectal cancer, respectively (5-year survival rate > 25%), with approximately 1 in 12 (<8%) cancer-related deaths being attributable to GC [3]. The mortality risk of GC from birth till the age of 70 is more than 1% and 0.5% for males and females, respectively [4]. According to the GLOBOCON project, there were more than 100,000 new cases of GC and more than 700,000 GC-related deaths in 2018. The occurrence of GC is variable with respect to region and ethnicity and is reported to be more prevalent (more than 2 times) in males than females in developed regions. In more than five countries of the world, GC has the highest prevalence among all types of cancers in males [5]. In countries of Central, Eastern Asia, and Africa, the prevalence rate of GC is the highest, whereas the lowest rate of incidence is in Korea, with almost 4 cases per 100,000 cases for males, respectively [6].
Though GC is one of the most fatal types of cancers, it is also one of the most influential types on the basis of human behaviors and therefore is preventable [7]. Till the 1980s, it was the leading cause of cancer-related deaths until it was overshadowed by lung cancer, as the rate of incidence of the latter was on the increase than the former, particularly in developed countries. Nevertheless, GC sustains a high mortality rate worldwide attributing to life year-burden [8]. There are a variety of factors that affect its development and manifestation, which can either be genetic or environmental and triggered by the presence or abundance of pathogens in the surroundings [9]. However, drastic changes in lifestyle, profound awareness, and pathogenic eradication have caused the incidental rate of GC to substantially decline over the years [10–12]. Nevertheless, GC is a progressive form of cancer, which is comprised of multiple stages, commencing from chronic superficial gastritis, atrophic gastritis, intestinal metaplasia, and dysplasia and adenocarcinoma [13]. Apart from these stages, gastric precancerous lesions (GPLs), including intestinal metaplasia and dysplasia, are significant indicatives of GC occurrence [14]. Various reports demonstrate the yearly prevalence of GC-related intestinal metaplasia, mild and moderate dysplasia, and severe dysplasia to be 0.25, 0.6, and 6% after a 5-year period of GC diagnosis [15]. Hereditary diffuse gastric cancer (HDGC) is one of the most common forms of GC, which is associated with familial history, caused by mutations in the cadherin 1 (CDH1) gene [16]. Other changes leading to the development of cancer that occur due to long-term inflammation include lack of balance between epithelial cell differentiation and apoptosis, atrophy and achlorhydria, and gastric colonization by enteric microorganisms with nitrate reductase movement, which encourages the development of cancer-causing nitrosamines. Corpus-dominating atrophy, or the deficiency of specific glandular cell types like parietal cells, is an important step towards the initiation of cancer [17].
2. Association of Helicobacter pylori with GC
The major risk factor for GC is the bacterium Helicobacter pylori, which was discovered by Barry Marshall and Robin Warren in 1982, prior to which other factors such as lifestyle, diet, and stress were considered to be major risk factors for gastric disease [18]. H. pylori is a Gram-negative, spiral-shaped bacterium, which predominantly resides in the human stomach and is reported to colonize the human gut of immunocompromised individuals during infection [19]. It is a stomach pathogen that is responsible for stomach-related ulcers (duodenal and gastric) and cancer (gastric, mucosa-related lymphoid tissue lymphoma) in infected hosts [20]. The incidence of H. pylori has been reported to elevate the risk of stomach cancer to a fivefold ratio within a decade of infection. Moreover, more than 90% of noncardia subtypes are reported to be associated with the pathogen [21]. Polymorphisms of IL-10 and IL-17, which are associated with GC, are also involved in the interaction with H. pylori infection [22]. Histopathologically persistent gastritis, gastric decay, intestinal metaplasia, dysplasia, and malignancy are different phases which ultimately occur due to H. pylori infection, more than often leading to mortality [23]. The role of H. pylori in the initiation of GC involves direct infection and inflammation that occurs in the gastrointestinal mucosa of the host [24]. For instance, genes for the Type IV secretion system, which are reported to be essential for the transport of CagA proteins into human epithelial cells by H. pylori, were observed to be abundant in the intragastric microbiome of intestinal metaplasia patients [25]. Cag-Pathogenicity Island (cagPAI) consists of more than 30 genes that encode the Type IV secretion system and the CagA protein. Strains of H. pylori that express cagPAI are associated with pathogenesis of gastric disease, peptic ulcers, and gastric cancer [26]. CagL, another protein, aids in translocating CagA and secreting IL-8 from H. pylori. In vitro studies have reported the role of CagA in inducing tumorigenesis in AGS cells [27]. Abl and Src kinases phosphorylate CagA on tyrosine residue at 4 distinctive EPIYA motifs inside the host cell, which results in several morphological cellular changes and increased cellular migration [28]. These EPIYA motifs are significant as their phosphorylation status, and the amount is an indicator for GC [29]. Tyrosine-phosphorylated CagA activates tyrosine phosphatase in the host cell, which in turn activates ERK1/2 and C-terminal Src kinase, while the interaction between the two results in cell elongation [30]. Even in its nonphosphorylated form, CagA has several pathogenic effects by targeting various cellular components like E-cadherin, c-Met, and Grb-2 to mediate proinflammatory responses, disrupting cell to cell apical junctions, activating β-catenin, and resulting in the loss of cell polarity [31, 32].
The highly pathogenic cytotoxin secreted by H. pylori, vacuolating cytotoxin A (VacA), is associated with various genotypes and vacuolating activities. Patients infected with H. pylori strains that express VacA with s1 or m1 genotype are reported to have an elevated risk of gastric cancer. Therefore, VacA expression has been designated as a significant biomarker for the development of gastric cancer [33]. For the evasion of the bacterium from the host's immune system, genetic diversity is one of the major factors that H. pylori undertakes for the initiation of chronic inflammation and host colonization. Previous literature reported the involvement of CagA and VacA genes in the progression of neoplastic or nonneoplastic GC [34]. Moreover, H. pylori produces the enzyme urease, which releases ammonium and carbon dioxide for the neutralization of stomach acids, allowing the survival of the bacterium. Ammonia further causes alterations in the tissue structure, whereas carbon dioxide offers protection to H. pylori from host immune cells and induces angiogenesis, thus promoting GC [35]. Apart from GC, disturbance in the tissue microenvironment (TME) of the host gastric mucosa [36, 37] also involves H. Pylori infection. Furthermore, other bacterial species than H. pylori can also be attributable to carcinogenesis in the gastric mucosa [38].
Apart from bacterial pathogens, the occurrence of GC can be attributable to the infection caused by viruses, including Epstein-Barr virus (EBV), which has been reported to be associated with more than 9% of GC cases [39]. Its role in the manifestation and growth of GC has been regarded as a complex one, as many other factors are commonly associated with the incidence of this disease [40]. Like H. pylori, EBV's role in GC is also linked with genetic mutation, characterized here by the posttranscriptional genetic regulation of EBV by miRNAs [41].
3. Treatment of GC: Conventional and Modern Methods
3.1. Conventional Methods and Chemotherapeutic Agents
Chemotherapy, radiotherapy, tumor resecting surgery, immunotherapy, and targeted therapy have all proven to be effective against GC and adenocarcinoma, which implies that a multidisciplinary approach is pertinent for the suitable selection of treatment. Chemotherapy for resectable GC is now acceptable, but classification of GC on the grounds of various molecular subtypes is significant for a more personalized, therapeutic approach. Random clinical trials prove evidence that perioperative and postoperative chemotherapy, chemoradiation, and immunotherapy are also considerable options for treatment [42]. Several cytotoxic agents are reported to be active in stages of advanced GC, including irinotecan, platinum, and taxanes. Oxaliplatin is the preferred platinum in most treatment regimens [43]. In the second-line of treatment for metastasized GC, monoclonal antibodies such as ramucirumab have proven a boost in survival of GC patients, with both its singular therapy and use in combination with paclitaxel being deemed effective. Kinase inhibitors such as lenvatinib and regorafenib have also been investigated in their use in immunotherapy in GC in East-Asian populations [44]. Nevertheless, the choice and options available for treatment are dependent on the prognosis of disease and the response of the patient to the preferred method, as these treatments come with their set of side effects.
3.2. Traditional Chinese Medicine (TCM) and GC
Over the past years, significant research in the field of cancer has resulted in scientific breakthroughs, including cancer immunotherapy, whose efficacy is dependent on the inflammation regulating the tumor microenvironment [45]. However, this approach is restricted to a slow rate of response, thus making it appropriate only for some patients [46]. This rate can be accelerated by the combination of immunotherapy with other agents, which can lead to an increase in the cure rate [47]. Traditional Chinese medicine (TCM) is a conjugative discipline, combining personalized medicine with therapeutics and cancer therapy. However, a greater part of the patients presently uses TCM as an alternate method of pain alleviation rather than the main method of treatment, despite the fact that it has been directly associated with treating major diseases such as cancer in recorded medical history [48].
Conventional treatments for diagnosed GC include tumor resection during early stages, but unfortunately, in patients with advanced stages of nonresectable tumor, the patients are advised noninvasive herbal therapy regimens meant to only alleviate their pain and increase their life expectancy [49]. Since time immemorial, plants have been used as therapeutic sources for the treatment of infections, diseases, and healthcare. Decades worth of studies have proven their efficacious potential and discovery of medicinal plant-derived drugs. The Chinese Pharmacopeia deciphers the involvement of Chinese herbs, plants, and their concoctions in addressing, managing, and curing clinical and terminal diseases. TCM includes medicinal plants and their associated constituents that can be used for therapeutic as well as theranostic purposes. They provide a wide spectrum of information about the mode of action of medicinal plants regarding managing a variety of diseases and simultaneous information about the human physiological systems [50]. Earliest reports of its use date back to 200 AD for the improvement of healthcare. Currently, traditional medicinal documentation of Ayurvedic and Chinese medicinal plants is available online in various databases. They contain comprehensive information about different plant parts, secondary metabolites, and bioactive compounds. In developing countries, people still employ traditional concoctions, which have been passed on from generations to cure diseases. This practice is now being opted by even those who reside in developed countries, where innovative research is being conducted to unlock the bioactive mechanisms effective against various diseases such as cancer [51]. Bioactive phytoconstituents that address cancer cells include alkaloids, saponins, terpenoids, tannins, and flavonoids [52]. In a similar manner, TCM has proven to be effectual in the prevention of GPL progression [53].
Traditional medicine is comprised of the comprehension of beliefs, knowledge, and information used in amalgamation with disparate therapies and other practices, which gives way to the incorporation of herbal medicines prepared from animals, minerals, and/or plant and its constituents for diagnosis, treatment, and prevention of infection and disease [54]. Therefore, in context, every geographical locale inherits its own rich knowledge of traditional medicine, which may be passed onto generations and spanning decades. In developing and developed countries, this built-up indigenous knowledge lives on to serve fundamental roles in employing localized resources like plants and herbs [55]. Therefore, this review sheds light on one of these herbs, Anisi stellati fructus, for its mechanism of action and treatment of GC.
4. An Example of TCM: ASF and Its Activity against GC
Anisi stellati fructus (ASF) is the star-shaped fruit of Illicium verum Hook F. (Chinese star anise), which belongs to the Illiciaceae family [56], but the former is reported to be a member of the Schisandraceae family [57]. I. verum is a highly regarded Chinese medicinal herb which is also mentioned in the Chinese Pharmacopeia [58]. There are various species in the genus, which demonstrate variance due to their distinctive morphology, composition, and growth habitat. I. verum is an aromatic, medium-sized tree which is grown in areas native to Jamaica, Laos, Japan, Indonesia, Philippines, and north-east and south-west Vietnam and China, respectively [56, 59], and is widely distributed in many regions of Asia and North America [60]. In various regions all over the world, it goes by different local names, including “Bādiyān” (Persian), “Badiyaan” and “Badiyaan ka phool” (Urdu), “Phoolchakri” (Hindi), “Badiane” (French), and star anise (English). In TCM, it is commonly known as Ba Jiao Hui Xiang [58, 61].
4.1. ASF in Culinary Practice
ASF is characterized by 6–8 ridged follicles that are star-shaped, woody, and wrinkled in texture. It is generally regarded as safe and nontoxic for consumption [62] and has traditionally been used as a staple spice in various cuisines, including Oriental, Indian, and Pakistani cuisines. It is a major component of the widely used five-spice powder (locally known as garam masala in the Indian subcontinent) used in the preparation of stews and curries [63]. In European countries, it is used in the preparation of alcoholic drinks along with various teas, fruit jams, and condiments [64].
4.2. Medicinal Uses of ASF
The use of ASF in treating various infections and diseases is practiced in various regions, including Asia and North America [65]. It is reported to possess antimicrobial, antiviral, and antioxidant properties [66]. In Chinese, Ayurvedic, and Unani medicine, it is reported to improve digestion and alleviate symptoms of dysentery, dyspepsia, asthma, flatulence, menstruation irregularities, colic, inflammation, bronchitis, and rheumatic diseases. Using it in a concoction for herbal teas can relieve cough and flu and can reinvigorate various organs of the human body [61].
4.3. Anticancer Properties of ASF
Though the effects of ASF and its extracts and decoctions against cancer and tumor growth have not been thoroughly established, few studies have brought them to light, providing a brief yet informative insight into its anticancer mechanism. Extracts of ASF inhibited angiogenesis in Human umbilical vein endothelial cells (HUVECs) at concentrations of 10 μg/ml, suggesting its anticancer activity [67]. Another study reported that the oral administration of ASF resulted in the decrease of metastasis in lung cancer cells with little or no cytotoxic effects [68]. In chronic myeloid leukemia (CML) cells, ASF and its combinational treatment with imatinib yielded antileukemic activity, indicating that ASF could be considered as a potential agent for CML therapy [69]. Therefore, these studies suggest that ASF possesses significant anticancer activity, which could be further elucidated by similar findings.
4.4. Chemical Constituents of ASF
The fruit of I. verum is reported to contain various alkaloids, essential oils, and tannins, with significant amounts of cis- and trans-anethole, limonene, safrole, α- and β-pinene, β-phellandrene, α-terpineol, and farnesol [70]. Flavonoids like quercetin and kaempferol and their glucosides, phenolic compounds like shikimic acid, and fatty acids such as linoleic, myristic, stearic, betunolic, and phenyl propionic acid are also reported to be active constituents of ASF [48, 71]. Furthermore, the essential oils of ASF are comprised of flavonoids, terpenes, sesquiterpenes, and lignans which are equally significant respective to their medicinal and therapeutic properties. Additional bioactive compounds which are found in ASF essential oils are myrcene, limone, linalool, luteolin, estragole, caryophyllene, γ-terpineol, and α-humulene [72]. Moreover, recent studies have reported the presence of other compounds such as β-sitosterol, α-phellandrene, β-myrcene, mairin, honokiol, cineol, and safrole [73–75].
5. Major Bioactive Compounds of ASF and Their anti-GC Effects
ASF and its compounds have been extensively reported for their anticancer activity [76, 77]. Kim et al. reported that the oral administration of ASF significantly decreased the metastasis in malignant cancer cells, which ultimately resulted in the reduction of MMP-9, MMP-13, MMP-14, uPA, and gelatinase activities by its treatment [68]. It also inhibited the activation and phosphorylation of NF-κB, AP-1, and p38 pathways, respectively, as well as suppressing tumor angiogenesis in cells. Another recent study examined the effect of ASF extract on CML cells, which demonstrated that the treatment induced cytotoxicity and proliferation inhibition in a dose-dependent manner. The combination of ASF extract with imatinib (IM) also leads to an apoptotic effect in the cells, which was not as pronounced as the singular treatment of IM on CML cells [69]. In a similar manner, many bioactive compounds of ASF (present in a major or minor amount) have been identified to be effective against GC, which are mentioned in detail in the next section.
5.1. Quercetin
Quercetin (3,3′,4′,5,7-pentahydroxyavone) is a flavonoid which is abundantly found in many foods and plants, with many properties attributed to the compound, including antioxidative, anti-inflammatory, antimicrobial, and anticancer activities, respectively [78, 79]. The treatment of quercetin in cancer cells has been reported to induce apoptotic, antiulcer, and chemopreventive effects [80]. Furthermore, quercetin facilitates the prevention of mucosal damage in gastric ulcer formation, which is also said to be associated with its antibacterial action against H. pylori [81]. Various studies have reported the effectiveness of quercetin against cancer proliferation and angiogenesis. Its effect on gastric cancer apoptosis was revealed through Bax, BCL-2, and caspase analyses [82]. The regulation of P450 enzyme expression also resulted in the activation of procarcinogens by quercetin. Its treatment has also been affiliated with the enhancement of DNA repair and the elimination of carcinogens and actively proliferating cancer cells [83]. Quercetin also mediates apoptosis by the expression of various proteins such as mitogen-activated protein kinases (MAPK), phosphatidylinositide 3-kinases (PI3K), and protein kinase C (PKC) through regulating the expression of the BCL-2 family [84]. In two studies, it was demonstrated that quercetin and its combinative treatment with other cancer-inhibiting agents induce autophagy in GC cells via the negative regulation of the Akt-mTOR signaling pathway, which leads to the overall inhibition of cellular proliferation [85]. The administration of quercetin also leads to the reduction in the invasion and migration of GC cells through downregulating the expression of uPAR and uPA proteins, respectively [86]. In GC cell lines, the treatment of quercetin is demonstrated to reduce the progression of cellular growth in the cell cycle phases [87]. The combinative treatment of quercetin with curcumin resulted in the decrease of Akt and ERK phosphorylation, which suggested cellular apoptosis via the mitochondrial pathway in GC cells [88]. In in vivo models, the treatment of quercetin stimulated the generation of nitric oxide synthase (nNOS), which reacted with ROS for the inhibition of cellular proliferation of cells of the gastric mucosa, which were previously treated with ethanol [89]. Other in vivo studies depicted the administration of oral quercetin to reduce COX-2, Twist1, and ITGβ6 levels [90]. Furthermore, the downregulation of angiogenesis-related factors (VEGFA and VEGFR-2) also suggested the effectivity of quercetin against GC cells [91]. In the AGS cell line, quercetin leads to the reduction and increase in the expression of antiapoptosis (MCL-2, BCL-2, and BCL-x) and proapoptosis (Bad, Bax, and Bid) related proteins, respectively [92]. The blocking of the phosphoinositide 3-kinase- (PI3K-) Akt pathway is one of the mechanisms through which quercetin is reported to inhibit the mitochondrial pathways leading to the progression of GC, which was reported in many studies (Figure 1) [84, 93]. Recent in silico analyses have employed the network pharmacology approach for understanding the mechanism of quercetin and its involvement in molecular pathways against GC [94, 95].
Figure 1.
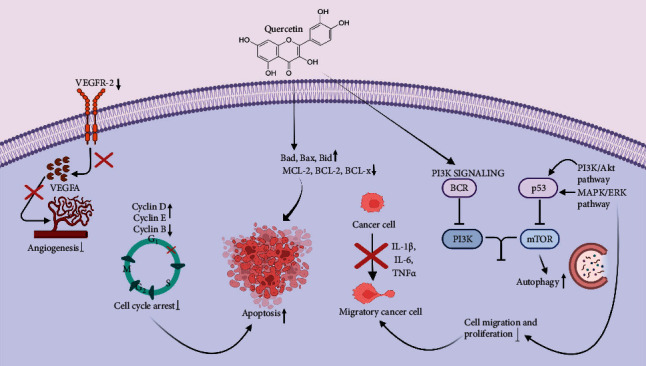
Anticancer effect of quercetin against GC.
5.2. Luteolin
Luteolin (3′,4′,5,7-tetrahydroxyflavone) is another well-known flavonoid that has been widely reported to be effective against the progression of several types of cancers, including GC [96]. In a study, luteolin was reported to inhibit the proliferation of GC cells through suppressing the Notch signaling pathway [97]. The administration of luteolin leads to the reduction in cell viability and induction of cell cycle arrest, as well as apoptosis in GC cell lines [98]. The treatment of GC cell lines with luteolin alone induced apoptosis, while its combinatory treatment together with cisplatin resulted in the down and upregulation of CDC2, CDC25C, Cyclin-B1, and p21/cip1, respectively, leading to the effective inhibition of cell growth [96]. Lu et al. reported that luteolin was attributable to the downregulation of c-Met, MMP9, and Ki-67 in GC cells while promoting the induction of apoptosis via activating apoptotic proteins (CAS3 and PARP1), thus suggesting that luteolin can target Akt/ERK signaling pathway for its anti-GC effect [97].
BCL-2 is an apoptosis regulating protein that is characteristically found to be overexpressed in various cancers [98]. In a study, new findings demonstrated luteolin to downregulate the expression of the protein via the upregulation of miR-34a, thus aiding in inhibiting cellular growth in GC [99]. The suppression of phosphorylation of MAPK, AKT, and PI3K signaling pathway, as well as the induction of apoptosis in GC cells, was also observed through the regulation of CAS3, CAS9, and Bax/BCL-2 ratio by luteolin [100]. It is reported that treating GC cells with luteolin leads to the inhibition of STAT3 phosphorylation, reducing the growth of tumors in vivo [101]. Notch signaling pathway is reported to be associated with cellular angiogenesis and the regulation of AKT, MMP-9, and NF-κB signaling pathways [102], the latter of which in turn regulates VEGF expression in various human tissues [103]. In GC, luteolin impedes the expression of VEGF via the downregulation of the Notch1 pathway, the study findings of which have been proven previously by two studies [97, 104]. Additionally, treating H. pylori-infected GC cells with luteolin resulted in the induction of IL-8 and NF-κB at both protein and mRNA levels, respectively [105]. Furthermore, two recent studies have investigated the combinatory synergistic effect of luteolin with oxaliplatin in GC cells. The combined treatment demonstrated the positive effect of both agents on the inhibition of cellular proliferation, activation of CytC/caspase signaling, and the induction of cell cycle arrest (GC/M phase) and cellular apoptosis [106] (Figure 2).
Figure 2.
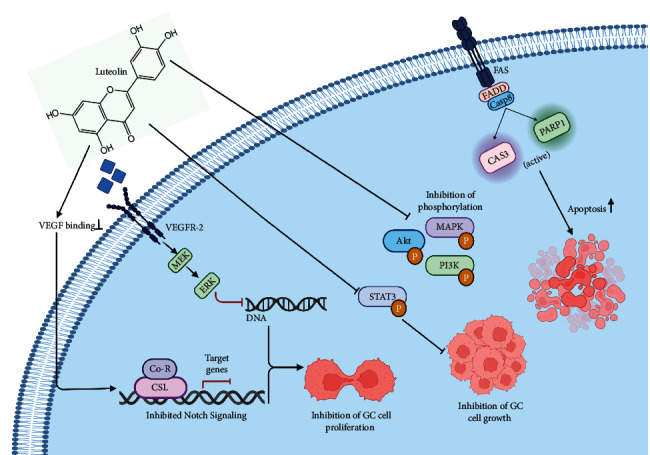
Anticancer mechanisms of luteolin against GC.
5.3. Kaempferol
Kaempferol is a flavonoid compound that is abundantly found in various plants (edible and medicinal). Its various biological activities also include the anti-inflammatory property, which is apparently useful in inhibiting the expression of several proinflammatory cytokines, NF-κB, STAT1, and AP-1 [107, 108]. Therefore, kaempferol has been investigated and reported to be effective in many types of cancers, including GC [109, 110]. In a study conducted in Spain, kaempferol consumption reduced the risk of GC, while an in vivo study reported the inhibition of cancerous cell growth in GC xenografts, thereby suggesting the antiproliferative and metastasis-inhibiting ability of the flavonoid [111, 112]. Li et al. examined the effect of kaempferol against injury to the gastric mucosa, which was induced by ethanol [113]. The treatment with kaempferol demonstrated its protective effect by facilitating the inhibition of MPO and proinflammatory cytokine levels, as well as improving NO production in cells. In AGS cells, kaempferol leads to a decrease in the expression of IL8, IL-1β, and TNF-α. Moreover, its anti-inflammatory effect was observed by the suppression of the translocation of CagA and VacA proteins of H. pylori in GC cells [114]. Kaempferol is also reported to induce autophagy and apoptosis in GC cells via activating the IRE1-JNK-CHOP signaling and AMPKα/ULK1 pathway and by positively regulating ER stress in GC cells [111, 115] (Figure 3). A recent study also shed light on the mode of action of the compound against GC through a network pharmacology approach [116]. Shrestha et al. reported the reduction in G9a expression, as well as inhibition of mTOR signaling and cellular proliferation in GC cells after kaempferol treatment [117].
Figure 3.
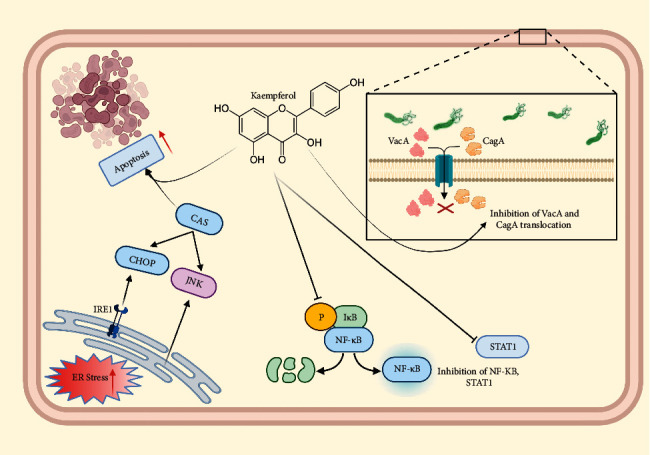
Mode of action of kaempferol against GC.
5.4. Honokiol
Honokiol (3,5-di-(2-propenyl)-1,1-biphenyl-2,2-diol) is a small, biphenolic lignan. It has been reported to suppress Akt and NF-κB activation, which in turn results in the phosphorylation and degradation of IκBα, respectively [118]. It is also reported to mediate the suppression of STAT3 activity previously induced by IL-6, where the activated form of the former has been associated with many cancer cells [119]. The treatment with honokiol has been reported to be effective against GC, where it was attributable to the induction of apoptosis and downregulation of COX-2 and PPAR-γ in GC cells [120]. The significant anticancer activity was observed to be correlated with GRP94 levels, which were found to be reduced after administration of honokiol in mice in a dose-dependent manner [121]. Therefore, it can potentially serve as a promising anticancer therapeutic target for several pathways [122]. Liu et al. observed that honokiol increases SHP-1 activity that subsequently leads to the deactivation of the STAT3 pathway, thereby suggesting that honokiol actively inhibits cellular angiogenesis and proliferation of GC cells [123]. In a study, treatment with honokiol revoked the down- and upregulation of E-cadherin and TPL2, respectively, the latter of which was observed to be subsequently associated with decreased growth and vascular density in vivo [124]. This mechanism of action, combined with the inhibition of epithelial-to-mesenchymal transition as well as regulation of apoptosis (induced by ER stress), serves a key role in the therapeutic action of honokiol against GC. In a recent study, the anticancer properties of honokiol were attributable to its ability to downregulate PPAR-γ activity, as well as the expressions of CDC25C, CDC2, and Cyclin B1, which aid in inducing ER stress which in turn decreases vascular density [125].
5.5. D-Limonene
D-Limonene is a monoterpene compound that has been reported to possess anticancer activities against different cancers [126, 127]. Lu et al. evaluated that d-limonene can inhibit the proliferation of GC cancer cells via the induction of apoptosis in cancer cells [128]. It has also been attributable to strong antioxidant activity resulting in the inhibition of H2O2-induced CAS3, CAS9, and p38/MAPK activation, as well as a decline in the BCL-2/Bax ratio, thereby indicating that it could offer protection against oxidative stress [129]. Another study evaluated that oral administration of limonene (≥400 mg/kg) in mice led to a reduction in tumor mass weight [128]. The combination of d-limonene with berberine and its singular treatment on GC cell line resulted in the increased expression of CAS3 and ROS, reduced expression of BCL-2, and cell cycle arrest, indicating that d-limonene causes apoptosis via the regulation of the mitochondrial pathway [130–132].
6. Bioactivity of ASF Compounds against H. pylori
Helicobacter pylori is often recognized to have a strong correlation with the occurrence of gastric diseases, including GC. Many bioactive compounds have been associated with the anti-H. pylori activity and other protective effects, which subsequently promote good gastric health and protection from several diseases. In a study, luteolin was observed to inhibit the activity of the arylamine N-acetyltransferase (NAT) enzyme, which is responsible for the N-acetylation of PABA and AF in H. pylori [133]. Another study demonstrated the protective effect of quercetin against H. pylori in the corpus mucosa [134]. Though the main mechanism of action of quercetin is not clear, it is reported to be associated with the declining activity of urease, as well as its ability to chelate iron, which is a major cofactor imperative for H. pylori growth. Treatment of quercetin in H. pylori-infected animals is reported to reduce the process of inflammation and bacterial count in the gastric mucosa [135].
β-Caryophyllene is a naturally occurring bicyclic sesquiterpene that is widely found in many medicinal plants and is reported to possess many protective abilities, including antibacterial and anti-inflammatory properties. In a study, β-caryophyllene demonstrated significant gastroprotective activity in an ethanol-induced gastric ulcer model, reducing the lesions by more than 70% [136]. A recent study by Shim et al. revealed that the treatment of H. pylori-caused gastrointestinal disease with β-caryophyllene demonstrated remarkable improvement in physiological symptoms and subsequently resulted in IL-1β decrease in serum [137]. Furthermore, the anti-H. pylori action of similar compounds has also been well-reported in many studies [114, 138, 139].
7. Future Perspectives and Conclusions
Medicinal herbs such as ASF have been traditionally used for the treatment of several ailments. These herbs are deemed indispensable as therapeutic candidates for treating various diseases such as cancer. The protective effects of ASF and its compounds against GC are majorly related to modulating major hallmarks of cancer, such as the inhibition of cellular proliferation, angiogenesis, inducing apoptosis as well as the suppression of cell migration, and the promotion of immune cell secretion against GC cells (Table 1). Furthermore, the ability to use ASF and its compounds in combination with other anticancer agents and/or as adjuvants in cancer immunotherapy is also an exciting and encouraging field of study, as results have been promising in recent investigations. These properties could provoke great economic interest for their pharmaceutical applications when the compounds are extracted, as compared to their laboratory synthesis. In this regard, future studies and phytochemical analyses of ASF and its bioactive compounds may result in the discovery of novel anti-GC targets and may also lead to improvements in their chemical synthesis. This approach has been further elucidated in studies of single, isolated compounds, such as quercetin, where its standalone efficacy against GC is observed to be far greater than the herb on the whole. Therefore, these phytocompounds pose a superior anti-GC potential, which could be elucidated in further in vitro and in vivo studies and clinical trials.
Table 1.
The anticancer effect of various bioactive compounds of ASF against gastric cancer in vitro.
| Compound | Cell line | Concentration used | Effect on protein/pathway (s) | References |
|---|---|---|---|---|
| Quercetin | AGS and MKN28 | 10–160 μM | Inhibit Akt-mTOR pathway | [85] |
| AGS | Quercetin alone (6.25, 12.5, 25, 50, and 100 μM) With SN-38 (5-25 nM) |
Downregulate VEGFA and VEGFR-2 ↓ COX-2, Twist1, and ITGβ6 |
[91] | |
| SNU719 and MKN74 | N/A | Inhibit EBNA-1 and LMP-2 proteins ↑ Cleaved CAS3, CAS9, and PARP Induce p53, Bax, and Puma |
[90] | |
| BGC-823 | 5, 30, 60, 90, and 120 μmol/L | Induce CAS3, Bcl-2, and Bax ↓ Bcl-2/Bax ratio ↑ CAS3 expression |
[82] | |
| BGC823 and AGS | 10 μM | ↓ Cell migration and invasion ↓ uPA and uPAR expression ↓ MMP2 and MMP9 activity inhibit Pak1-Limk1-cofilin, NF-κB, PKC-δ, and ERK1/2 signaling, and AMPKα activation |
[86] | |
| GCSC | 20–100 μM | Inhibit (PI3K)-Akt signaling | [84] | |
| HGC-27, NUGC-2, MKN-7, and MKN-28 | 70 μM (IC50-32–55 μM) | Cell cycle arrest (Gi to S phase) | [87] | |
|
| ||||
| Luteolin | AGS | 50 μM (24 h) 80 μM (48 and 72 h) IC50 29.6 ± 3.8 (48 h) and 23.5 ± 2.4 μM (72 h) |
↓ CDC2, cyclin B1, and CDC25C levels ↑ Apoptosis, CAS3, CAS6, CAS9, Bax, and p53 ↓ BCL-2 |
[96] |
| CRL-1739 | 30 μM | Induce IL-8 expression ↑ NF-κB mRNA expression |
[105] | |
| MKN45 and SGC7901 | 20 μM (24 h) 40 μM (48 h) 80 μM (72 h) |
↑ Cleaved CAS3 and PARP; induce apoptosis Downregulate MMP9 expression and c-Met/Akt/ERK signaling |
[97] | |
| MKN45 and BGC823 | 40 μM | ↑ Apoptosis Inhibit GC cell proliferation, cyclin D1, cyclin E, BCL-2, MMP2, MMP9, N-cadherin, and vimentin Induce p21, Bax, E-cadherin expression, Notch1, PI3K, AKT, mTOR, ERK, STAT3, and p38 signaling pathway |
[98] | |
| BGC-823 | 0–60 μM (48 h) | ↑ Cleaved CAS9 and CAS3 ↓ p-PI3K, p-AKT and p-mTOR, and p-ERK1/2 |
[100] | |
| MFC | Luteolin alone (20 μM) and/or oxaliplatin (5 μM (24 h)) | Downregulate ERK1/2 phosphorylation and activation Combined treatment induced cell cycle arrest (G2/M phase) Induce apoptosis |
[106] | |
| SGC-7901 | 40 μM (24 h) | Combined treatment inhibited proliferation Induce ERK1/2 phosphorylation, JNK, and P38 MAPK signal transduction Inhibit PI3K/AKT and ERK1/2 MAPK intracellular signaling Induce apoptosis |
[99] | |
| MKN28, SGC7901, and GSE-1 | 60 μM | Cell cycle arrest (G2/M phase) ↓ Cyclin B1, CDK1 and CDC25C, COX-2, p-AKT, and p-ERK ↑ Cleaved CAS3, CAS9, PARP |
[101] | |
|
| ||||
| Kaempferol | AGS, SNU-216, NCI–N87, SNU-638, and MKN-74 | 25 μM, 50 μM, and 100 μM (24 h) | Activate IRE1-JNK-CHOP signaling pathway Induce apoptosis LC3-I to LC3-II conversion Downregulate p62 |
[112] |
| Rh30 | 25 or 50 μM kaempferol and quercetin (24 h) | Induce apoptotic markers (cleaved PARP and CAS3) Inhibit cell growth, survival, migration, and invasion by blocking mTOR signaling |
[117] | |
|
| ||||
| D-Limonene | HLEC | 125–1800 μM | ↓ H2O2-induced ROS generation and BCL-2/Bax ratio Inhibit CAS3, CAS9 activation, and p38 MAPK phosphorylation |
[129] |
| MGC803 | 80 μM (24–48 h) | ↓ Mitochondrial transmembrane potential (DCm) ↑ CAS3 expression ↓ BCL-2 expression |
[131] | |
|
| ||||
| Honokiol | AGS, MKN45, N87, and SCM-1 | 20 and 50 mM | Induce apoptosis Activate 15-LOX-1 expression Regulate PPAR-g and COX-2 pathway |
[120] |
| AGS, MKN45, and SCM-1 | 20 mM IC50–AGS (20 mM) and MKN45 and SCM-1 (40 mM) |
Induce SHP-1 activity and STAT-3 dephosphorylation Activate ER stress and calpain-II regulation |
[123] | |
| AGS and MKN45 | 20 μM | Inhibit TGFβ1- or MNNG-induced EMT | [124] | |
| AGS, N87, MKN45, and SCM-1 | 5–40 μM | ↓ Glucose-regulated protein (GRP94) | [121] | |
Acknowledgments
All authors gratefully acknowledge the University of Lahore, Pakistan, for providing the funds for this study. This study was funded by the University of Lahore, Pakistan.
Data Availability
No data were used to support this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
All authors contributed equally to this work.
References
- 1.Clurman B. E., Roberts J. M. Cell cycle and cancer. Journal of the National Cancer Institute . 1995;87(20):1499–1501. doi: 10.1093/jnci/87.20.1499. [DOI] [PubMed] [Google Scholar]
- 2.Surh Y.-J. Cancer chemoprevention with dietary phytochemicals. Nature Reviews Cancer . 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 3.Yamaoka Y. How to eliminate gastric cancer-related death worldwide? Nature Reviews Clinical Oncology . 2018;15(7):407–408. doi: 10.1038/s41571-018-0029-8. [DOI] [PubMed] [Google Scholar]
- 4.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians . 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Balakrishnan M., George R., Sharma A., Graham D. Y. Changing trends in stomach cancer throughout the world. Current Gastroenterology Reports . 2017;19(8):36–55. doi: 10.1007/s11894-017-0575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawla P., Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Przegla̜d Gastroenterologiczny . 2019;14(1):26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang F., Shen X. Current prevalence status of gastric cancer and recent studies on the roles of circular RNAs and methods used to investigate circular RNAs. Cellular and Molecular Biology Letters . 2019;24(1):53–70. doi: 10.1186/s11658-019-0178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thrift A. P., El-Serag H. B. Burden of gastric cancer. Clinical Gastroenterology and Hepatology . 2020;18(3):534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yusefi A. R., Bagheri Lankarani K., Bastani P., Radinmanesh M., Kavosi Z. Risk factors for gastric cancer: a systematic review. Asian Pacific Journal of Cancer Prevention: APJCP . 2018;19:591–603. doi: 10.22034/APJCP.2018.19.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bode A. M., Dong Z., Wang H. Cancer prevention and control: alarming challenges in China. National Science Review . 2016;3(1):117–127. doi: 10.1093/nsr/nwv054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li W. Q., Zhang J. Y., Ma J. L., et al. Effects of Helicobacter pylori treatment and vitamin and garlic supplementation on gastric cancer incidence and mortality: follow-up of a randomized intervention trial. The BMJ . 2019;366:p. 366. doi: 10.1136/bmj.l5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L., Chen L., Gui Z.-X., Liu S., Wei Z.-J., Xu A.-M. Preventable lifestyle and eating habits associated with gastric adenocarcinoma: a case-control study. Journal of Cancer . 2020;11(5):1231–1239. doi: 10.7150/jca.39023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa P. A human model of gastric carcinogenesis. Cancer Research . 1988;48:3554–3560. [PubMed] [Google Scholar]
- 14.Marques-Silva L., Areia M., Elvas L., Dinis-Ribeiro M. Prevalence of gastric precancerous conditions: a systematic review and meta-analysis. European Journal of Gastroenterology and Hepatology . 2014;26(4):378–387. doi: 10.1097/meg.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 15.Yang L., Li J., Hu Z., et al. A systematic review of the mechanisms underlying treatment of gastric precancerous lesions by traditional Chinese medicine. Evidence-Based Complementary and Alternative Medicine . 2020;2020:12. doi: 10.1155/2020/9154738.9154738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinheiro H., Oliveira C., Seruca R., Carneiro F. Hereditary diffuse gastric cancer - pathophysiology and clinical management. Best Practice and Research: Clinical Gastroenterology . 2014;28(6):1055–1068. doi: 10.1016/j.bpg.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Fox J. G., Wang T. C. Inflammation, atrophy, and gastric cancer. Journal of Clinical Investigation . 2007;117(1):60–69. doi: 10.1172/jci30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed N. 23 years of the discovery of Helicobacter pylori: is the debate over? Annals of Clinical Microbiology and Antimicrobials . 2005;4(1):p. 17. doi: 10.1186/1476-0711-4-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X.-Y., Zhang P.-Y. Gastric cancer: somatic genetics as a guide to therapy. Journal of Medical Genetics . 2016;54(5):305–312. doi: 10.1136/jmedgenet-2016-104171. [DOI] [PubMed] [Google Scholar]
- 20.Yamaoka Y. Mechanisms of disease: Helicobacter pylori virulence factors. Nature Reviews Gastroenterology and Hepatology . 2010;7(11):629–641. doi: 10.1038/nrgastro.2010.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham D. Y. Helicobacter pylori update: gastric cancer, reliable therapy, and possible benefits. Gastroenterology . 2015;148(4):719–731. doi: 10.1053/j.gastro.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng Y.-C., Huang L.-R., Lin C.-L., et al. Association between proton pump inhibitors use and risk of gastric cancer in patients with GERD. Gut . 2019;68(2):374–376. doi: 10.1136/gutjnl-2018-316057. [DOI] [PubMed] [Google Scholar]
- 23.Correa P. Helicobacter pylori and gastric carcinogenesis. The American Journal of Surgical Pathology . 1995;19:S37–S43. [PubMed] [Google Scholar]
- 24.Khatoon J., Rai R. P., Prasad K. N. Role of Helicobacter pylori in gastric cancer: updates. World Journal of Gastrointestinal Oncology . 2016;8(2):147–158. doi: 10.4251/wjgo.v8.i2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park C. H., Lee A. R., Lee Y. R., Eun C. S., Lee S. K., Han D. S. Evaluation of gastric microbiome and metagenomic function in patients with intestinal metaplasia using 16S rRNA gene sequencing. Helicobacter . 2019;24 doi: 10.1111/hel.12547.e12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wroblewski L. E., Peek R. M., Jr. Pathogenic enablers - toxic relationships in the stomach. Nature Reviews Gastroenterology & Hepatology . 2016;13(6):317–318. doi: 10.1038/nrgastro.2016.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li N., Feng Y., Hu Y., et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. Journal of Experimental & Clinical Cancer Research . 2018;37(1):280–295. doi: 10.1186/s13046-018-0962-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stein M., Bagnoli F., Halenbeck R., Rappuoli R., Fantl W. J., Covacci A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Molecular Microbiology . 2002;43(4):971–980. doi: 10.1046/j.1365-2958.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 29.Basso D., Zambon C. F., Letley D. P., et al. Clinical relevance of Helicobacter pylori cagA and vacA gene polymorphisms. Gastroenterology . 2008;135(1):91–99. doi: 10.1053/j.gastro.2008.03.041. [DOI] [PubMed] [Google Scholar]
- 30.Higashi H., Tsutsumi R., Muto S., et al. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science . 2002;295(5555):683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 31.Murata-Kamiya N., Kurashima Y., Teishikata Y., et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the β-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene . 2007;26(32):4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X.-Y., Zhang P.-Y., Aboul-Soud M. A. M. From inflammation to gastric cancer: role of Helicobacter pylori. Oncology Letters . 2017;13(2):543–548. doi: 10.3892/ol.2016.5506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Capparelli R., Iannelli D. Epigenetics and Helicobacter pylori. International Journal of Molecular Sciences . 2022;23(3):1759–1769. doi: 10.3390/ijms23031759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang W. L., Yeh Y. C., Sheu B. S. The impacts of H. pylori virulence factors on the development of gastroduodenal diseases. Journal of Biomedical Science . 2018;25:68–69. doi: 10.1186/s12929-018-0466-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Olivera-Severo D., Uberti A. F., Marques M. S., et al. A new role for Helicobacter pylori urease: contributions to angiogenesis. Frontiers in Microbiology . 2017;8:1–11. doi: 10.3389/fmicb.2017.01883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baj J., Brzozowska K., Forma A., Maani A., Sitarz E., Portincasa P. Immunological aspects of the tumor microenvironment and epithelial-mesenchymal transition in gastric carcinogenesis. International Journal of Molecular Sciences . 2020a;21(7):2544–2565. doi: 10.3390/ijms21072544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baj J., Korona-Głowniak I., Forma A., et al. Mechanisms of the epithelial-mesenchymal transition and tumor microenvironment in Helicobacter pylori-induced gastric cancer. Cells . 2020;9(4):1055–1078. doi: 10.3390/cells9041055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sung J. J. Y., Coker O. O., Chu E., et al. Gastric microbes associated with gastric inflammation, atrophy and intestinal metaplasia 1 year after Helicobacter pylori eradication. Gut . 2020;69(9):1572–1581. doi: 10.1136/gutjnl-2019-319826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khan G., Hashim M. J. Global burden of deaths from Epstein-Barr virus attributable malignancies 1990-2010. Infectious Agents and Cancer . 2014;9(1):38–48. doi: 10.1186/1750-9378-9-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsao S. W., Tsang C. M., To K. F., Lo K. W. The role of Epstein-Barr virus in epithelial malignancies. The Journal of Pathology . 2015;235(2):323–333. doi: 10.1002/path.4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y., Lu L., Wu X., et al. The multifaceted role of long non-coding RNA in gastric cancer: current status and future perspectives. International Journal of Biological Sciences . 2021;17(11):2737–2755. doi: 10.7150/ijbs.61410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thiels C. A., Ikoma N., Fournier K., et al. Repeat staging laparoscopy for gastric cancer after preoperative therapy. Journal of Surgical Oncology . 2018;118(1):61–67. doi: 10.1002/jso.25094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cunningham D., Starling N., Rao S., et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. New England Journal of Medicine . 2008;358(1):36–46. doi: 10.1056/nejmoa073149. [DOI] [PubMed] [Google Scholar]
- 44.Joshi S. S., Badgwell B. D. Current treatment and recent progress in gastric cancer. CA: A Cancer Journal for Clinicians . 2021;71(3):264–279. doi: 10.3322/caac.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fukumura D., Kloepper J., Amoozgar Z., Duda D. G., Jain R. K. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nature Reviews Clinical Oncology . 2018;15(5):325–340. doi: 10.1038/nrclinonc.2018.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Barrueto L., Caminero F., Cash L., Makris C., Lamichhane P., Deshmukh R. R. Resistance to checkpoint inhibition in cancer immunotherapy. Translational Oncology . 2020;13(3) doi: 10.1016/j.tranon.2019.12.010.100738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swart M., Verbrugge I., Beltman J. B. Combination approaches with immune-checkpoint blockade in cancer therapy. Frontiers in Oncology . 2016;6:233–249. doi: 10.3389/fonc.2016.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu S. H., Chen P. S., Huang C. C., et al. Unlocking the mystery of the therapeutic effects of Chinese medicine on cancer. Frontiers in Pharmacology . 2021;11:1–9. doi: 10.3389/fphar.2020.601785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Machlowska J., Baj J., Sitarz M., Maciejewski R., Sitarz R. Gastric cancer: epidemiology, risk factors, classification, genomic characteristics and treatment strategies. International Journal of Molecular Sciences . 2020;21:311–318. doi: 10.3390/ijms21114012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu A.-P., Jia H. W., Xiao C., Lu Q. P. Theory of traditional Chinese medicine and therapeutic method of diseases. World Journal of Gastroenterology . 2004;10(13):1854–1856. doi: 10.3748/wjg.v10.i13.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J., Li J., Cao J., Jiang W. Antifungal activities of neem (Azadirachta indica) seed kernel extracts on postharvest diseases in fruits. African Journal of Microbiological Research . 2010;4(11):1100–1104. [Google Scholar]
- 52.Fridlender M., Kapulnik Y., Koltai H. Plant derived substances with anti-cancer activity: from folklore to practice. Frontiers in Plant Science . 2015;6:799–807. doi: 10.3389/fpls.2015.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zeng J., Yan R., Pan H., et al. Weipixiao attenuate early angiogenesis in rats with gastric precancerous lesions. BMC Complementary and Alternative Medicine . 2018;18:250–314. doi: 10.1186/s12906-018-2309-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balick M. J., Cox A. Plants that heal; people and culture: the science of ethno botany. Scientific American Library . 1996;73 [Google Scholar]
- 55.Eshete M. A., Molla E. L. Cultural significance of medicinal plants in healing human ailments among Guji semi-pastoralist people, Suro Barguda district, Ethiopia. Journal of Ethnobiology and Ethnomedicine . 2021;17:1–18. doi: 10.1186/s13002-021-00487-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang G.-W., Hu W.-T., Huang B.-K., Qin L.-P. Illicium verum: a review on its botany, traditional use, chemistry and pharmacology. Journal of Ethnopharmacology . 2011;136(1):10–20. doi: 10.1016/j.jep.2011.04.051. [DOI] [PubMed] [Google Scholar]
- 57.Angiosperm Phylogeny Group, Chase M. W., Christenhusz M. J., et al. An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG IV. Botanical Journal of the Linnean Society . 2016;181:1–20. [Google Scholar]
- 58.Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China . Beijing, China: China Chemical Industry Press; 2005. [Google Scholar]
- 59.Peng W., Lin Z., Wang L., Chang J., Gu F., Zhu X. Molecular characteristics of Illicium verum extractives to activate acquired immune response. Saudi Journal of Biological Sciences . 2016;23(3):348–352. doi: 10.1016/j.sjbs.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang X., Meng X., Wu J., Huang L., Chen S. Global ecological regionalization of 15 Illicium species: nature sources of shikimic acid. Chinese Medicine . 2018;13(1):31–41. doi: 10.1186/s13020-018-0186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boota T., Rehman R., Mushtaq A., Kazerooni E. G. Star anise: a review on benefits, biological activities and potential uses. International Journal of Chemical and Biochemical Sciences . 2018;14:110–114. [Google Scholar]
- 62.Techen N., Pan Z., Scheffler B., Khan I. Detection of Illicium anisatumas adulterant of Illicium verum. Planta Medica . 2009;75(04):392–395. doi: 10.1055/s-0028-1112219. [DOI] [PubMed] [Google Scholar]
- 63.Chempakan B., Balaji S. Star anise. In: Chempakam B., Parthasarathy V. A., Zachariach T. J., editors. Chemistry of Spices . King’s Lynn, UK: CABI Biddles Ltd; 2008. pp. 607–610. [Google Scholar]
- 64.Zhang Y., Ji H., Yu J. Aromatic constituents and their changes of Illicium verum processed by different heating methods. Industrial Crops and Products . 2018;118:362–366. doi: 10.1016/j.indcrop.2018.04.003. [DOI] [Google Scholar]
- 65.Li X., Xu Y. Unraveling the molecular mechanisms of Fructus Anisi Stellati as a remedy for infantile colic by network pharmacology. Evidence-Based Complementary and Alternative Medicine . 2020;2020:9. doi: 10.1155/2020/9210304.9210304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.George C. K. Star anise. In: Peter K. V., editor. Handbook of Herbs and Spices” . 2nd. Cambridge, UK: Woodhead Publishing; 2012. [DOI] [Google Scholar]
- 67.Nam N.-H., Kim H.-M., Bae K.-H., Ahn B.-Z. Inhibitory effects of vietnamese medicinal plants on tube-like formation of human umbilical venous cells. Phytotherapy Research . 2003;17(2):107–111. doi: 10.1002/ptr.934. [DOI] [PubMed] [Google Scholar]
- 68.Kim A., Im M., Ma J. Y. Anisi stellati fructus extract attenuates the in vitro and in vivo metastatic and angiogenic potential of malignant cancer cells by downregulating proteolytic activity and pro-angiogenic factors. International Journal of Oncology . 2014;45(5):1937–1948. doi: 10.3892/ijo.2014.2606. [DOI] [PubMed] [Google Scholar]
- 69.Kim Y. S., Suh S. Y., Ahn Y. T., et al. Systemic pharmacological approach to identification and experimental verification of the effect of Anisi stellati fructus extract on chronic myeloid leukemia cells. Evidence-Based Complementary and Alternative Medicine . 2019;2019:20. doi: 10.1155/2019/6959764.6959764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chouksey D., Sharma P., Pawar R. Biological activities and chemical constituents of Illicium verum hook fruits (Chinese star anise) Der Pharmacia Sinica . 2010;1:1–10. [Google Scholar]
- 71.Natural Product Activity and Species Source Database. Anisi stellati fructus. 2022. https://bidd.group/NPASS/organism.php?org_id=NPO29374 .
- 72.Luís Â., Sousa S., Wackerlig J., et al. Star anise (Illicium verum Hook. F.) essential oil: antioxidant properties and antibacterial activity against Acinetobacter baumannii. Flavour and Fragrance Journal . 2019;34:260–270. [Google Scholar]
- 73.Rashid M. A., Zuberi R. H. Pharmacognostical studies for standardisation of a medicinal spice, the fruit of Illicium verum Hook. F. PharmaTutor . 2016;4:36–41. [Google Scholar]
- 74.Patra J. K., Das G., Bose S., et al. Star anise (Illicium verum): chemical compounds, antiviral properties, and clinical relevance. Phytotherapy Research . 2020;6614:1–20. doi: 10.1002/ptr.6614. [DOI] [PubMed] [Google Scholar]
- 75.Sharafan M., Jafernik K., Ekiert H., et al. Illicium verum (Star anise) and trans-anethole as valuable raw materials for medicinal and cosmetic applications. Molecules . 2022;27(3):650–665. doi: 10.3390/molecules27030650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Mofleh I. A. Spices, herbal xenobiotics and the stomach: friends or foes? World Journal of Gastroenterology . 2010;16:2710–2719. doi: 10.3748/wjg.v16.i22.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asif M., Yehya A. H. S., Al-Mansoub M. A., et al. Anticancer attributes of Illicium verum essential oils against colon cancer. South African Journal of Botany . 2016;103:156–161. doi: 10.1016/j.sajb.2015.08.017. [DOI] [Google Scholar]
- 78.Chen C., Zhou J., Ji C. Quercetin: a potential drug to reverse multidrug resistance. Life Sciences . 2010;87(11-12):333–338. doi: 10.1016/j.lfs.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Erboga M., Aktas C., Erboga Z. F., Donmez Y. B., Gurel A. Quercetin ameliorates methotrexate-induced renal damage, apoptosis and oxidative stress in rats. Renal Failure . 2015;37(9):1492–1497. doi: 10.3109/0886022x.2015.1074521. [DOI] [PubMed] [Google Scholar]
- 80.Ranganathan S., Halagowder D., Sivasithambaram N. D. Quercetin suppresses twist to induce apoptosis in MCF-7 breast cancer cells. PLoS One . 2015;10(10) doi: 10.1371/journal.pone.0141370.e0141370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kahraman A., Erkasap N., Köken T., Serteser M., Aktepe F., Erkasap S. The antioxidative and antihistaminic properties of quercetin in ethanol-induced gastric lesions. Toxicology . 2003;183(1–3):133–142. doi: 10.1016/s0300-483x(02)00514-0. [DOI] [PubMed] [Google Scholar]
- 82.Wang P., Zhang K., Zhang Q., et al. Effects of quercetin on the apoptosis of the human gastric carcinoma cells. Toxicology in Vitro . 2012;26(2):221–228. doi: 10.1016/j.tiv.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 83.Ekström A. M., Serafini M., Nyrén O., Wolk A., Bosetti C., Bellocco R. Dietary quercetin intake and risk of gastric cancer: results from a population-based study in Sweden. Annals of Oncology: Official Journal of the European Society for Medical Oncology . 2011;22(2):438–443. doi: 10.1093/annonc/mdq390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Shen X., Si Y., Wang Z., Wang J., Guo Y., Zhang X. Quercetin inhibits the growth of human gastric cancer stem cells by inducing mitochondrial-dependent apoptosis through the inhibition of PI3K/Akt signaling. International Journal of Molecular Medicine . 2016;38(2):619–626. doi: 10.3892/ijmm.2016.2625. [DOI] [PubMed] [Google Scholar]
- 85.Wang K., Liu R., Li J., et al. Quercetin induces protective autophagy in gastric cancer cells: involvement of Akt-mTOR- and hypoxia-induced factor 1α-mediated signaling. Autophagy . 2011;7(9):966–978. doi: 10.4161/auto.7.9.15863. [DOI] [PubMed] [Google Scholar]
- 86.Li H., Chen C. Quercetin has antimetastatic effects on gastric cancer cells via the interruption of uPA/uPAR function by modulating NF-κb, PKC-δ, ERK1/2, and AMPKα. Integrative Cancer Therapies . 2017;17(2):511–523. doi: 10.1177/1534735417696702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yoshida M., Sakai T., Hosokawa N., et al. The effect of quercetin on cell cycle progression and growth of human gastric cancer cells. FEBS Letters . 1990;260(1):10–13. doi: 10.1016/0014-5793(90)80053-l. [DOI] [PubMed] [Google Scholar]
- 88.Zhang J.-Y., Lin M.-T., Zhou M.-J., et al. Combinational treatment of curcumin and quercetin against gastric cancer MGC-803 cells in vitro. Molecules . 2015;20(6):11524–11534. doi: 10.3390/molecules200611524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu J.-L., Du J., Fan L.-L., Liu X.-Y., Gu L., Ge Y.-B. Effects of quercetin on hyper-proliferation of gastric mucosal cells in rats treated with chronic oral ethanol through the reactive oxygen species-nitric oxide pathway. World Journal of Gastroenterology . 2008;14(20):3242–3248. doi: 10.3748/wjg.14.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lee H., Lee S., Shin Y., Cho M., Kang H., Cho H. Anti-cancer effect of quercetin in xenograft models with EBV-associated human gastric carcinoma. Molecules . 2016;21(10):1286–1296. doi: 10.3390/molecules21101286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lei C.-S., Hou Y.-C., Pai M.-H., Lin M.-T., Yeh S.-L. Effects of quercetin combined with anticancer drugs on metastasis-associated factors of gastric cancer cells: in vitro and in vivo studies. Journal of Nutritional Biochemistry . 2018;51:105–113. doi: 10.1016/j.jnutbio.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 92.Shang H.-S., Lu H.-F., Lee C.-H., et al. Quercetin induced cell apoptosis and altered gene expression in AGS human gastric cancer cells. Environmental Toxicology . 2018;33(11):1168–1181. doi: 10.1002/tox.22623. [DOI] [PubMed] [Google Scholar]
- 93.Pan H.-C., Jiang Q., Yu Y., Mei J.-P., Cui Y.-K., Zhao W.-J. Quercetin promotes cell apoptosis and inhibits the expression of MMP-9 and fibronectin via the AKT and ERK signalling pathways in human glioma cells. Neurochemistry International . 2015;80:60–71. doi: 10.1016/j.neuint.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 94.Almatroodi S. A., Alsahli M. A., Almatroudi A., et al. Potential therapeutic targets of quercetin, a plant flavonol, and its role in the therapy of various types of cancer through the modulation of various cell signaling pathways. Molecules . 2021;26(5):1315–1348. doi: 10.3390/molecules26051315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang L., Hu Z., Zhu J., et al. Systematic elucidation of the mechanism of quercetin against gastric cancer via network pharmacology approach. BioMed Research International . 2020;2020:11. doi: 10.1155/2020/3860213.3860213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu B., Zhang Q., Shen W., Zhu J. Anti-proliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Molecular and Cellular Biochemistry . 2008;313(1-2):125–132. doi: 10.1007/s11010-008-9749-x. [DOI] [PubMed] [Google Scholar]
- 97.Lu J., Li G., He K., et al. Luteolin exerts a marked antitumor effect in cMet-overexpressing patient-derived tumor xenograft models of gastric cancer. Journal of Translational Medicine . 2015;13(1):42–53. doi: 10.1186/s12967-015-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tomek M., Akiyama T., Dass C. R. Role of Bcl-2 in tumour cell survival and implications for pharmacotherapy. Journal of Pharmacy and Pharmacology . 2012;64(12):1695–1702. doi: 10.1111/j.2042-7158.2012.01526.x. [DOI] [PubMed] [Google Scholar]
- 99.Wu H., Huang M., Liu Y., Shu Y., Liu P. Luteolin induces apoptosis by up-regulating miR-34a in human gastric cancer cells. Technology in Cancer Research and Treatment . 2015;14(6):747–755. doi: 10.7785/tcrt.2012.500434. [DOI] [PubMed] [Google Scholar]
- 100.Lu X., Li Y., Li X., Aisa H. A. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer. Oncology Letters . 2017;14(2):1993–2000. doi: 10.3892/ol.2017.6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Song S., Su Z., Xu H., et al. Luteolin selectively kills STAT3 highly activated gastric cancer cells through enhancing the binding of STAT3 to SHP-1. Cell Death Discovery . 2017;8(2) doi: 10.1038/cddis.2017.38.e2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ruan Z.-B., Fu X.-L., Li W., Ye J., Wang R.-Z., Zhu L. Effect of notch1,2,3 genes silicing on NF-κB signaling pathway of macrophages in patients with atherosclerosis. Biomedicine and Pharmacotherapy . 2016;84:666–673. doi: 10.1016/j.biopha.2016.09.078. [DOI] [PubMed] [Google Scholar]
- 103.Hu L., Zang M.-D., Wang H.-X., et al. Biglycan stimulates VEGF expression in endothelial cells by activating the TLR signaling pathway. Molecular Oncology . 2016;10(9):1473–1484. doi: 10.1016/j.molonc.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Imran M., Rauf A., Abu-Izneid T., et al. Luteolin, a flavonoid, as an anticancer agent: a review. Biomedicine and Pharmacotherapy . 2019;112 doi: 10.1016/j.biopha.2019.108612.108710 [DOI] [PubMed] [Google Scholar]
- 105.Borzym-Kluczyk M., Leszczyńska K. Luteolin alters MUC1 extracellular domain, sT antigen, ADAM-17, IL-8, IL-10 and NF-κB expression in Helicobacter pylori-infected gastric cancer CRL-1739 cells: a preliminary study. Biomedical Reports . 2021;14(2):19–27. doi: 10.3892/br.2020.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma J., Chen X., Zhu X., et al. Luteolin potentiates low dose oxaliplatin induced inhibitory effects on cell proliferation in gastric cancer by inducing G2/M cell cycle arrest and apoptosis. Oncology Letters . 2022;23:16–28. doi: 10.3892/ol.2021.13134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lee S.-H., Kim Y.-J., Kwon S.-H., et al. Inhibitory effects of flavonoids on TNF-alpha-induced IL-8 gene expression in HEK 293 cells. BMB Reports . 2009;42(5):265–270. doi: 10.5483/bmbrep.2009.42.5.265. [DOI] [PubMed] [Google Scholar]
- 108.Chen S., Ma J., Yang L., et al. Anti-glioblastoma activity of kaempferol via programmed cell death induction: involvement of autophagy and pyroptosis. Frontiers in Bioengineering and Biotechnology . 2020;8:1–10. doi: 10.3389/fbioe.2020.614419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liao W., Chen L., Ma X., Jiao R., Li X., Wang Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. European Journal of Medicinal Chemistry . 2016;114:24–32. doi: 10.1016/j.ejmech.2016.02.045. [DOI] [PubMed] [Google Scholar]
- 110.Świeca M., Herok A., Piwowarczyk K., et al. Potentially bioaccessible phenolics from mung bean and adzuki bean sprouts enriched with probiotic-antioxidant properties and effect on the motility and survival of AGS human gastric carcinoma cells. Molecules . 2020;25(13):2963–2975. doi: 10.3390/molecules25132963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Garcia-Closas R., Gonzalez C. A., Agudo A., Riboli E. Intake of specific carotenoids and flavonoids and the risk of gastric cancer in Spain. Cancer Causes and Control . 1999;10(1):71–75. doi: 10.1023/a:1008867108960. [DOI] [PubMed] [Google Scholar]
- 112.Kim T. W., Lee S. Y., Kim M., Cheon C., Ko S. G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death & Disease . 2018;9(9):875–914. doi: 10.1038/s41419-018-0930-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li Q., Hu X., Xuan Y., et al. Kaempferol protects ethanol-induced gastric ulcers in mice via pro-inflammatory cytokines and NO. Acta Biochimica et Biophysica Sinica . 2018;50(3):246–253. doi: 10.1093/abbs/gmy002. [DOI] [PubMed] [Google Scholar]
- 114.Yeon M. J., Lee M. H., Kim D. H., et al. Anti-inflammatory effects of kaempferol on Helicobacter pylori-induced inflammation. Bioscience Biotechnology and Biochemistry . 2019;83(1):166–173. doi: 10.1080/09168451.2018.1528140. [DOI] [PubMed] [Google Scholar]
- 115.Bhosale P. B., Ha S. E., Vetrivel P., Kim H. H., Kim S. M., Kim G. S. Functions of polyphenols and its anticancer properties in biomedical research: a narrative review. Translational Cancer Research . 2020;9(12):7619–7631. doi: 10.21037/tcr-20-2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang L., Li H., Yang M., et al. Exploration in the mechanism of kaempferol for the treatment of gastric cancer based on network pharmacology. BioMed Research International . 2020;2020:11. doi: 10.1155/2020/5891016.5891016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shrestha R., Mohankumar K., Martin G., et al. Flavonoids kaempferol and quercetin are nuclear receptor 4A1 (NR4A1, Nur77) ligands and inhibit rhabdomyosarcoma cell and tumor growth. Journal of Experimental Clincal Cancer Research . 2021;40(392):1–17. doi: 10.1186/s13046-021-02199-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grivennikov S. I., Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Reviews . 2010;21(1):11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rajendran P., Li F., Shanmugam M. K., et al. Honokiol inhibits signal transducer and activator of transcription-3 signaling, proliferation, and survival of hepatocellular carcinoma cells via the protein tyrosine phosphatase SHP-1. Journal of Cellular Physiology . 2012;227(5):2184–2195. doi: 10.1002/jcp.22954. [DOI] [PubMed] [Google Scholar]
- 120.Liu S. H., Shen C. C., Yi Y. C., et al. Honokiol inhibits gastric tumourigenesis by activation of 15-lipoxygenase-1 and consequent inhibition of peroxisome proliferator-activated receptor-gamma and COX-2-dependent signals. British Journal of Pharmacology . 2010;160:1963–1972. doi: 10.1111/j.1476-5381.2010.00804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sheu M. L., Liu S. H., Lan K. H. Honokiol induces calpain-mediated glucose-regulated protein-94 cleavage and apoptosis in human gastric cancer cells and reduces tumor growth. PLoS One . 2007;2(10) doi: 10.1371/journal.pone.0001096.e1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arora S., Singh S., Piazza G. A., Contreras C. M., Panyam J., Singh A. P. Honokiol: a novel natural agent for cancer prevention and therapy. Current Molecular Medicine . 2012;12(10):1244–1252. doi: 10.2174/156652412803833508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu S. H., Wang K. B., Lan K. H., et al. Calpain/SHP-1 interaction by honokiol dampening peritoneal dissemination of gastric cancer in nu/nu mice. PLoS One . 2012;7(8) doi: 10.1371/journal.pone.0043711.e43711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pan H.-C., Lai D.-W., Lan K.-H., et al. Honokiol thwarts gastric tumor growth and peritoneal dissemination by inhibiting Tpl2 in an orthotopic model. Carcinogenesis . 2013;34(11):2568–2579. doi: 10.1093/carcin/bgt243. [DOI] [PubMed] [Google Scholar]
- 125.Rauf A., Patel S., Imran M., et al. Honokiol: an anticancer lignan. Biomedicine and Pharmacotherapy . 2018;107:555–562. doi: 10.1016/j.biopha.2018.08.054. [DOI] [PubMed] [Google Scholar]
- 126.Kaji I., Tatsuta M., Iishi H., Baba M., Inoue A., Kasugai H. Inhibition by d-limonene of experimental hepatocarcinogenesis in sprague-dawley rats does not involve p21ras plasma membrane association. International Journal of Cancer . 2001;93(3):441–444. doi: 10.1002/ijc.1353. [DOI] [PubMed] [Google Scholar]
- 127.Guyton K. Z., Kensler T. W. Prevention of liver cancer. Current Oncology Reports . 2002;4(6):464–470. doi: 10.1007/s11912-002-0057-4. [DOI] [PubMed] [Google Scholar]
- 128.Lu X.-G., Zhan L. B., Feng B. A., Qu M. Y., Yu L. H., Xie J. H. Inhibition of growth and metastasis of human gastric cancer implanted in nude mice by d-limonene. World Journal of Gastroenterology . 2004;10(14):2140–2144. doi: 10.3748/wjg.v10.i14.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bai J., Zheng Y., Wang G., Liu P. Protective effect of d-limonene against oxidative stress-induced cell damage in human lens epithelial cells via the p38 pathway. Oxidative Medicine and Cellular Longevity . 2016;2016:12. doi: 10.1155/2016/5962832.5962832 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 130.Yu X., Lin H., Wang Y., et al. D-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets and Therapy . 2018;11:1833–1847. doi: 10.2147/ott.s155716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhang X.-Z., Wang L., Liu D.-W., Tang G.-Y., Zhang H.-Y. Synergistic inhibitory effect of berberine and d-limonene on human gastric carcinoma cell line MGC803. Journal of Medicinal Food . 2014;17(9):955–962. doi: 10.1089/jmf.2013.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhou J., Azrad M., Kong L. Effect of limonene on cancer development in rodent models: a systematic review. Frontiers in Sustainable Food Systems . 2021;5:1–11. doi: 10.3389/fsufs.2021.725077. [DOI] [Google Scholar]
- 133.Chung J. G., Hsia T. C., Kuo H. M., et al. Inhibitory actions of luteolin on the growth and arylamine N-acetyltransferase activity in strains of Helicobacter pylori from ulcer patients. Toxicology in Vitro . 2001;15(3):191–198. doi: 10.1016/s0887-2333(01)00015-7. [DOI] [PubMed] [Google Scholar]
- 134.González-Segovia R., Quintanar J. L., Salinas E., Ceballos-Salazar R., Aviles-Jiménez F., Torres-López J. Effect of the flavonoid quercetin on inflammation and lipid peroxidation induced by Helicobacter pylori in gastric mucosa of Guinea pig. Journal of Gastroenterology . 2008;43(6):441–447. doi: 10.1007/s00535-008-2184-7. [DOI] [PubMed] [Google Scholar]
- 135.Haghi A., Azimi H., Rahimi R. A comprehensive review on pharmacotherapeutics of three phytochemicals, curcumin, quercetin, and allicin, in the treatment of gastric cancer. Journal of Gastrointestinal Cancer . 2017;48(4):314–320. doi: 10.1007/s12029-017-9997-7. [DOI] [PubMed] [Google Scholar]
- 136.Lemos M., Santin J. R., Mizuno C. S., et al. Copaifera langsdorffii: evaluation of potential gastroprotective of extract and isolated compounds obtained from leaves. Revista Brasileira de Farmacognosia . 2015;25(3):238–245. doi: 10.1016/j.bjp.2015.05.005. [DOI] [Google Scholar]
- 137.Shim H. I., Song D. J., Shin C. M., et al. Inhibitory effects of β-caryophyllene on Helicobacter pylori infection: a randomized double-blind, placebo-controlled study. Korean Journal of Gastroenterology . 2019;74(4):199–204. doi: 10.4166/kjg.2019.74.4.199. [DOI] [PubMed] [Google Scholar]
- 138.González A., Salillas S., Velázquez-Campoy A., et al. Identifying potential novel drugs against Helicobacter pylori by targeting the essential response regulator HsrA. Scientific Reports . 2019;9(1):11294–11313. doi: 10.1038/s41598-019-47746-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trung H. T., Huynh H. T. T., Thuy L. N. T., Van Minh H. N., Nguyen M. N. T., Thi M. N. L. Growth-inhibiting, bactericidal, antibiofilm, and urease inhibitory activities of Hibiscus rosa sinensis L. flower constituents toward antibiotic sensitive-and resistant-strains of Helicobacter pylori. ACS Omega . 2020;5(32):20080–20089. doi: 10.1021/acsomega.0c01640. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were used to support this study.


