Abstract
Despite strict guidelines for coronavirus disease 2019 (COVID-19), South Korea is facing its fourth pandemic wave. In this study, by using an automated electrochemiluminescence immunoassay assay, we tracked anti-spike protein receptor-binding domain (anti-S-RBD) antibody titer from the second dose to 2 weeks after the booster dose vaccination. After the second dose, 234 participants had their anti-S-RBD antibody titers decrease over time. We also showed the booster dose (the third dose) increased antibody titer by average 14 (min–max, 2–255)-fold higher compared to the second dose among the 211-booster group participants, therefore, the booster dose could be recommended for low responders to the second dose. Our findings showed a distinct humoral response after booster doses of BNT162b2 mRNA vaccines and may provide further evidence of booster vaccination efficacy. These data will also be helpful in vaccination policy decisions that determine the need for the booster dose.
Keywords: SARS-CoV-2, COVID-19, Booster Dose, mRNA Vaccine, Healthcare Workers, Antibody Titer
Graphical Abstract
Two years have passed since the beginning of the coronavirus pandemic. Despite worldwide vaccination, many countries are experiencing a resurgence of coronavirus disease 2019 (COVID-19) due to the omicron variant (B.1.1.529) following the delta variant (B.1.617.2) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). As of 5 December 2021, over 265 million confirmed cases and nearly 5.2 million deaths have been reported globally.1 In South Korea, despite 81.2% of the population having been vaccinated since February 2021 under the national vaccine project, we are in the fourth wave of COVID-19 pandemic.2,3 The reason for this is probably because the effectiveness of the vaccine is likely to have decreased over time and novel variants have been emerging.4,5,6,7 Based on the evidences of modest difference in vaccine effectiveness with the delta variant,4,5,6,7 and the evidence of rising humoral response to the third dose of the mRNA vaccine,8,9,10,11 the FDA approved the vaccine booster dose (a third dose) for Pfizer–BioNTech’s BNT162b2 and Moderna’s mRNA-1273 mRNA vaccines.12,13 The authorization was gradually extended from immunocompromised patients to all adults.13 The authorization for a booster dose was also approved in South Korea, and more than 10% of the population was vaccinated through early December.2,3
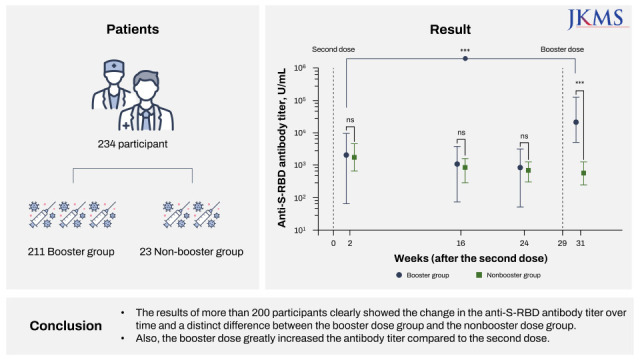
Vaccination of health care workers (HCWs) of the Boramae Medical Center, which was designated to one of the COVID-19 treatment centers, began in early March 2021, and the booster dose vaccination of Pfizer–BioNTech’s BNT162b2 also began in early November 2021. This study is a follow-up study of Kim et al.14,15 with the same cohort, which contains HCWs of the Boramae Medical Center. The booster dose was administered among the participants who wished to be additionally vaccinated at 29 weeks after the second dose. A total of five scheduled blood samplings from each participant were performed 1, 2, 16, 24, and 31 weeks after the second dose. Antibody tests for SARS-CoV-2 were performed by automated electrochemiluminescence immunoassay (ECLIA) as described in a previous study.14,15,16,17 Since the linear range of antibody titers recommended by the manufacturer is 0.4 to 250 U/mL, to ensure the concentration was within the detectable range, a manual dilution method was performed.16,17 Of the 289 participants, 234 participants were enrolled in this analysis finally; excluding 5 with a COVID-19 infection history and 50 with limited information. Among the 234 participants, 211 were administered booster doses, and the other 23 were not administered booster doses (only second dose administration). The participants’ basic information was obtained through a questionnaire. The past COVID-19 infection history was confirmed using an Elecsys Anti-SARS-CoV-2 assay (Elecsys Anti-N; Roche Diagnostics, Mannheim, Germany). The nucleocapsid (N) antigen is not a target of the BNT162b2 mRNA vaccine; therefore, it is a useful marker to determine whether the participant had been infected with SARS-CoV-2 in the past regardless the BNT16b2 mRNA vaccine administration. Demographic and basic hematologic characteristics of 234 participants are shown in Supplementary Table 1.
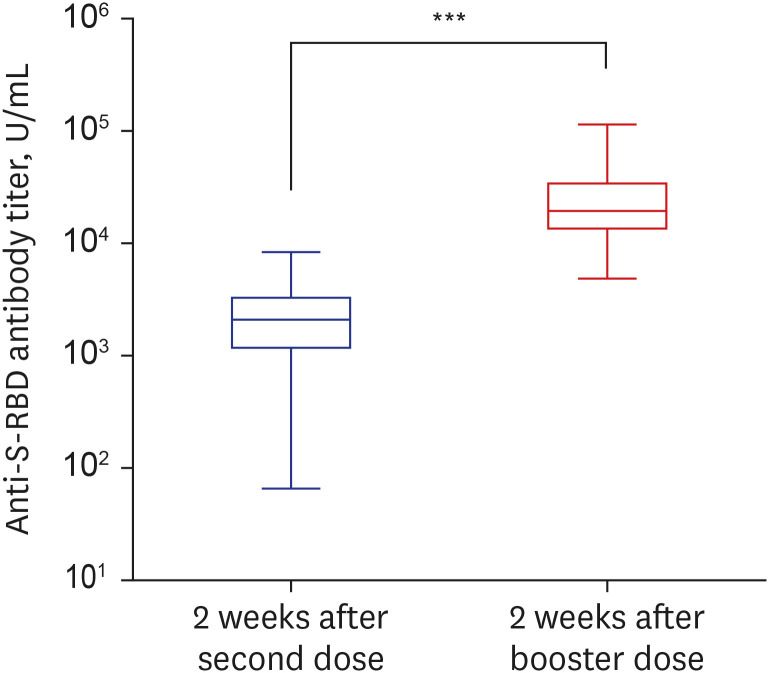
To examine the humoral immunity response of the vaccine, a quantitative Elecsys Anti-SARS-CoV-2 S assay (Elecsys Anti-S; Roche Diagnostics) using a Cobas 8000 e801 unit (Roche Diagnostics) was performed. The Elecsys Anti-S assay uses a recombinant protein representing the receptor binding domain (RBD) of the spike (S) antigen, which favors quantitative determination of high-affinity antibodies against SARS-CoV-2. Using the Elecsys Anti-S assay, anti-S-RBD antibody titers of the second dose effect and the booster dose effect were compared (Fig. 1). Among booster dose group (n = 211), the median antibody titers were 2,082 U/mL (interquartile range [IQR], 1,231–3,153 U/mL) at 2 weeks after the second dose and 21,657 U/ml (IQR, 15,687–34,218 U/mL) at 2 weeks after the booster dose (Fig. 1). The anti-S-RBD antibody titer 2 weeks after the booster dose was 14 (min–max, 2–225)-fold higher on average than it was 2 weeks after the second dose.
Fig. 1. Comparison of anti-S-RBD antibody titers at 2 weeks after the second dose and 2 weeks after the booster dose of BNT162b2 mRNA vaccination. The box plot graph shows the antibody titer of SARS-CoV-2 by log10 scale. The box bounds the IQR divided by the median, and Tukey-style whiskers extend to a maximum of 1.5 × IQR beyond the box.
S-RBD = spike protein receptor-binding domain, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, IQR = interquartile range.
***P < 0.001.
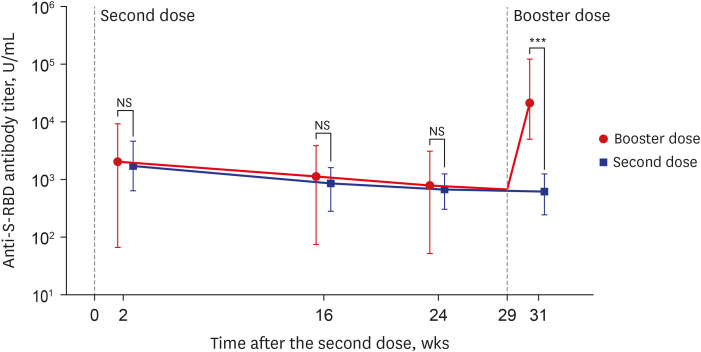
When analyzing the antibody dynamics of consecutive blood samples divided into the booster dose group and the non-booster dose (only second dose administration) group, the change in S-RBD antibody titer revealed a clear difference (Fig. 2). Both groups, the booster dose group (n = 211) and the non-booster dose group (n = 23), showed the same decreasing titer pattern before booster dose administration (24 weeks after the second dose). Based on the titers at 2 weeks after the second dose, the median reduction rates at 24 weeks after the second dose were 60.72% (IQR, 44.33–71.51%) and 62.35% (IQR, 30.88–78.75%), respectively, and there were no significant differences between the two groups (P = 0.677). However, in the booster dose group, all of these group participants showed significantly higher anti-S-RBD antibody titers after booster dose administration (2 weeks after the booster dose); the median antibody titer just before booster dose (24 weeks after the second dose, 5 weeks before the booster dose) was 868 U/mL (min–mix, 53–3,125 U/mL), and the median antibody titer just after booster dose (2 weeks after booster dose) was 21,657 U/mL (min–max 5,095–127,064 U/mL). This result indicated that the antibody titer increased by approximately 35 (min–max, 6–278)-fold due to the booster dose. The participants who were not administered booster doses (n = 23) had a further decrease in antibody titer at 31 weeks after the second dose, and the median antibody titer was 589 U/mL (min–max, 252–1,230 U/mL). Therefore, comparing the median antibody titer at 31 weeks after the second dose (2 weeks after the booster dose), there was an about 37-fold difference between the booster dose group (21,657 U/mL) and the non-booster dose group (589 U/mL, P < 0.001) (Fig. 2).
Fig. 2. Time series of anti-S-RBD antibody titers over a vaccination period of 31 weeks after the second dose (or 2 weeks after the booster dose) administration. The change of anti S-RBD antibody titer over time in the booster dose group (red line, n = 211, administration of booster [third] doses, responded to collect 5 times blood sampling and no history of SARS-CoV-2 infection), and in the second dose group (blue line, n = 23, no administration of booster [third] doses, responded to collect 5 times blood sampling and no history of SARS-CoV-2 infection). The graph shows maximum (upper boundary line), median (dot), minimum (lower boundary line) values of the antibody titer of SARS-CoV-2 by log10 scale.
S-RBD = spike protein receptor-binding domain, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, NS = no statistical significance (P > 0.05).
***P < 0.001.
In addition, based on the antibody titer, the booster dose could be recommended for low antibody production subjects. Particularly, it is encouraging that antibody production significantly increased in participants with low antibody production after the booster dose. Among the 3 low responders after the second dose (antibody titer, < 200 U/mL of more or equal to one sample during the study period), all showed a robust response after the booster dose (53 → 14,849 U/mL, 149 → 5,095 U/mL and 184 → 12,965 U/mL after the booster dose, respectively).
This study should be interpreted with the following limitations. First, plaque reduction neutralizing test (PRNT), the gold standard for assessing effective antibody quantification of viruses, was not performed.18,19 Since the PRNT is a time and labor-intensive test, and requires a high safety level as well, it is difficult to conduct easily in a general laboratory. Many studies already have reported that commercial immunoassay has strong positive correlation with the neutralizing activity test.20,21,22,23 Therefore, immunoassay such as ECLIA used in this study is frequently used for calculating viral neutralization activity. Second, this study focused on the antibody titer against the RBD of the S protein of SARS-CoV-2. It remains controversial whether these antibody titers have a clear correlation between adaptive humoral immunity (including neutralizing antibody titer) and adaptive cellular immunity (including T cell activity).23,24,25,26,27 Antibody titer alone is insufficient to represent total immunity to SARS-CoV-2 infection. In line with the difficulties of measuring the protective immunity with the vaccine, the efficacy against the dominant variant forms such as the delta variant (B.1.617.2) and the omicron variant (B.1.1.529) could not be also revealed.
In summary, we report a consecutive decrease in the S-RBD antibody titer after the second dose and a change after the booster dose. The results of more than 200 participants clearly showed the change in the anti-S-RBD antibody titer over time and a distinct difference between the booster dose group and the non-booster dose group. Also, the booster dose greatly increased the antibody titer compared to the second dose. Our findings are first in Koreans and the results are similar to the previous evidence suggesting a pronounced humoral response after booster doses of mRNA vaccines.8,9,10,11
Currently, South Korea is facing an increasing number of confirmed COVID-19 cases and deaths. There are various opinions about booster doses, and it is also true that there was a lack of research on booster doses. We clearly show the changes in the anti-S-RBD antibody titer over time between the second dose and booster dose while maintaining a strict vaccination interval. It will also be useful for vaccination policy decisions, which determine the need for a booster dose or the interval between vaccination.
Ethics statement
This study was approved by the Institutional Review Board of the Boramae Medical Center (No. 30-2021-31) and written informed consent was obtained from all participants.
ACKNOWLEDGMENTS
The reagent kits were provided by Roche Diagnostics.
Footnotes
Funding: Participants’ payment on transportation reimbursement was supported by OHC (Osang Healthcare, Gyeonggi-do, Republic of Korea) in 2021.
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Kim N, Roh EY, Yoon JH, Park H, Shin S.
- Data curation: Roh EY, Yoon JH.
- Formal analysis: Kwon SR, Kim N, Minn D, Park S.
- Fund acquisition: Shin S.
- Investigation: Kwon SR, Kim N.
- Methodology: Kim N, Park H, Shin S.
- Software: Kwon SR, Kim N, Minn D, Park S.
- Validation: Park H, Minn D, Park S.
- Writing - original draft: Kwon SR, Kim N.
- Writing - review & editing: Kwon SR, Kim N, Shin S.
SUPPLEMENTARY MATERIAL
Demographic and hematologic characteristics of the 234 enrolled participants
References
- 1.World Health Organization. The coronavirus (COVID-19) dashboard. [Accessed December 5, 2021]. https://covid19.who.int/
- 2.Ministry of Health and Welfare. Coronavirus (COVID-19), Republic of Korea. [Accessed December 5, 2021]. http://ncov.mohw.go.kr/index.jsp .
- 3.Korea Disease and Control and Prevention Agency. COVID-19 vaccination. [Accessed December 5, 2021]. https://ncv.kdca.go.kr/
- 4.Pouwels KB, Pritchard E, Matthews PC, Stoesser N, Eyre DW, Vihta KD, et al. Effect of Delta variant on viral burden and vaccine effectiveness against new SARS-CoV-2 infections in the UK. Nat Med. 2021;27(12):2127–2135. doi: 10.1038/s41591-021-01548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez Bernal J, Andrews N, Gower C, Gallagher E, Simmons R, Thelwall S, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) Variant. N Engl J Med. 2021;385(7):585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thompson RN, Hill EM, Gog JR. SARS-CoV-2 incidence and vaccine escape. Lancet Infect Dis. 2021;21(7):913–914. doi: 10.1016/S1473-3099(21)00202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dalle Carbonare L, Valenti MT, Bisoffi Z, Piubelli C, Pizzato M, Accordini S, et al. Serology study after BTN162b2 vaccination in participants previously infected with SARS-CoV-2 in two different waves versus naïve. Commun Med. 2021;1:38. doi: 10.1038/s43856-021-00039-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ducloux D, Colladant M, Chabannes M, Yannaraki M, Courivaud C. Humoral response after 3 doses of the BNT162b2 mRNA COVID-19 vaccine in patients on hemodialysis. Kidney Int. 2021;100(3):702–704. doi: 10.1016/j.kint.2021.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu K, Choi A, Koch M, Ma L, Hill A, Nunna N, et al. Preliminary analysis of safety and immunogenicity of a SARS-CoV-2 variant vaccine booster. medRxiv. 2021 May 6; doi: 10.1101/2021.05.05.21256716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfizer. Second quarter 2021 Earnings teleconference 2021. [Accessed December 5, 2021]. https://s21.q4cdn.com/317678438/files/doc_financials/2021/q2/Q2-2021-Earnings-Charts-FINAL.pdf .
- 11.Barda N, Dagan N, Cohen C, Hernán MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet. 2021;398(10316):2093–2100. doi: 10.1016/S0140-6736(21)02249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals. [Accessed December 5, 2021]. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-additional-vaccine-dose-certain-immunocompromised .
- 13.Center for Disease Control and Prevention. Grading of Recommendation, Assessment, Development, and Evaluation (GRADE): Pfizer-BioNTech, Moderna, and Janssen COVID-19 booster dose. [Accessed December 5, 2021]. https://www.cdc.gov/vaccines/acip/recs/grade/covid-19-booster-doses.html/
- 14.Kim N, Minn D, Park S, Roh EY, Yoon JH, Park H, et al. Positivity of SARS-CoV-2 antibodies among Korean healthy healthcare workers 1 and 2 weeks after second dose of Pfizer-BioNTech vaccination. J Korean Med Sci. 2021;36(21):e158. doi: 10.3346/jkms.2021.36.e158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim N, Shin S, Minn D, Park S, An D, Park JH, et al. SARS-CoV-2 infectivity and antibody titer reduction for 6 months after second dose of BNT162b2 mRNA vaccine in healthcare workers: a prospective cohort study. J Infect Dis. 2022:jiac035. doi: 10.1093/infdis/jiac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elecsys® anti-SARS-CoV-2 S. Insert (material number 09289267190 and 09289275190) [Accessed December 5]. https://diagnostics.roche.com/global/en/products/params/elecsys-anti-sars-cov-2-s.html .
- 17.Higgins V, Fabros A, Kulasingam V. Quantitative measurement of anti-SARS-CoV-2 antibodies: analytical and clinical evaluation. J Clin Microbiol. 2021;59(4):e03149-20. doi: 10.1128/JCM.03149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eyal O, Olshevsky U, Lustig S, Paran N, Halevy M, Schneider P, et al. Development of a tissue-culture-based enzyme-immunoassay method for the quantitation of anti-vaccinia-neutralizing antibodies in human sera. J Virol Methods. 2005;130(1-2):15–21. doi: 10.1016/j.jviromet.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Bewley KR, Coombes NS, Gagnon L, McInroy L, Baker N, Shaik I, et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat Protoc. 2021;16(6):3114–3140. doi: 10.1038/s41596-021-00536-y. [DOI] [PubMed] [Google Scholar]
- 20.Padoan A, Bonfante F, Pagliari M, Bortolami A, Negrini D, Zuin S, et al. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine. 2020;62:103101. doi: 10.1016/j.ebiom.2020.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grenache DG, Ye C, Bradfute SB. Correlation of SARS-CoV-2 Neutralizing Antibodies to an Automated Chemiluminescent Serological Immunoassay. J Appl Lab Med. 2021;6(2):491–495. doi: 10.1093/jalm/jfaa195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cristiano A, Pieri M, Sarubbi S, Pelagalli M, Calugi G, Tomassetti F, et al. Evaluation of serological anti-SARS-CoV-2 chemiluminescent immunoassays correlated to live virus neutralization test, for the detection of anti-RBD antibodies as a relevant alternative in COVID-19 large-scale neutralizing activity monitoring. Clin Immunol. 2022;234:108918. doi: 10.1016/j.clim.2021.108918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kohmer N, Westhaus S, Rühl C, Ciesek S, Rabenau HF. Brief clinical evaluation of six high-throughput SARS-CoV-2 IgG antibody assays. J Clin Virol. 2020;129:104480. doi: 10.1016/j.jcv.2020.104480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586(7830):594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhang LX, Miao SY, Qin ZH, Wu JP, Chen HY, Sun HB, et al. Preliminary analysis of B- and T-cell responses to SARS-CoV-2. Mol Diagn Ther. 2020;24(5):601–609. doi: 10.1007/s40291-020-00486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes CO, Jette CA, Abernathy ME, Dam KA, Esswein SR, Gristick HB, et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588(7839):682–687. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Criscuolo E, Diotti RA, Strollo M, Rolla S, Ambrosi A, Locatelli M, et al. Weak correlation between antibody titers and neutralizing activity in sera from SARS-CoV-2 infected subjects. J Med Virol. 2021;93(4):2160–2167. doi: 10.1002/jmv.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographic and hematologic characteristics of the 234 enrolled participants