Abstract
Transection, lesion and unit recording studies have localized rapid eye movement (REM) sleep mechanisms to the pons. Recent work has emphasized the role of pontine cholinergic cells, especially those of the pedunculopontine tegmentum (PPT). The present study differentiated REM sleep deficits associated with lesions of the PPT from other pontine regions implicated in REM sleep generation, including those with predominantly cholinergic vs non-cholinergic cells. Twelve hour polygraphic recordings were obtained in 18 cats before and 1–2 weeks after bilateral electrolytic or radio frequency lesions of either: (1) PPT, which contains the dorsolateral pontine cholinergic cell column; (2) laterodorsal tegmental nucleus (LDT), which contains the dorsomedial pontine cholinergic cell column; (3) locus ceruleus (LC), which contains mostly noradrenergic cells; or (4) subceruleus (LC alpha, peri-LC alpha and the lateral tegmental field), which also contains predominantly noncholinergic cells. There were three main findings: (1) Only lesions of PPT and subceruleus significantly affected REM sleep time. These lesions produced comparable reductions in REM sleep time but influenced REM sleep components quite differently: (ii) PPT lesions, estimated to damage 90±4% of cholinergic cells, reduced the number of REM sleep entrances and phasic events, including ponto-geniculo-occipital (PGO) spikes and rapid eye movements (REMs), but did not prevent complete atonia during REM sleep: (iii) Subceruleus lesions eliminated atonia during REM sleep. Mobility appeared to arouse the cat prematurely from REM sleep and may explain the brief duration of REM sleep epochs seen exclusively in this group. Despite the reduced amount of REM sleep, the total number of PGO spikes and REM sleep entrances increased over baseline values. Collectively, the results distinguish pontine loci regulating phasic events vs atonia. PPT lesions reduced phasic events, whereas subceruleus lesions created REM sleep without atonia. Severe REM sleep deficits after large pontine lesions, including PPT and subceruleus, might be explained by simultaneous production of both REM sleep syndromes. However, extensive loss of ACh neurons in the PPT does not disrupt REM sleep atonia.
Keywords: REM sleep, Pedunculopontine tegmentum, Miosis, Acetylcholine, Locus ceruleus complex, Norepinephrine, Cat
INTRODUCTION
The search for the substrates of rapid eye movement (REM) sleep spans three and a half decades since the serendipitous discovery of this sleep state by Aserinsky and Kleitman1. REM sleep is characterized by tonic components, including thalamocortical electroencephalographic (EEG) desynchronization and skeletal muscle atonia, and also by phasic components, notably rapid-eye-movements (REMs) and ponto-geniculo-occipital (PGO) spikes6,7,12,24,37–45,50,53,57.
Neural generators for these tonic and phasic events exist within the pons, as REM sleep signs are abolished rostral to transections at the pontomesencephalic juncture and caudal to transections at the pontomedullary juncture23,52,53. Extracellular unit, pharmacologic and lesion studies further localized REM components to the pontine tegmentum 2,3,5,8,12,14–28,31–35,37–48, 53–55,62, but an integrating mechanism for onset and maintenance of the REM sleep state remains unspecified.
Recent work suggests that pontine cholinergic cells might provide such a mechanism. For example, rats with inbred cholinergic hyperfunction have excess REM sleep47. Moreover, systemic administration or pontine infusion of cholinergic agonists induce REM sleep, whereas antagonists delay its onset2,3,41,50,59.
Cholinergic cells within the pedunculopontine tegmentum (PPT) have been implicated in the generation of phasic events21,22,31,32,37–39,42,61, particularly PGO spikes, and also project into the adjacent subceruleus, which is thought to regulate skeletal atonia during REM sleep, see e.g. refs. 14, 15, 26, 33, 38, 45. Subceruleus is predominantly non-cholinergic, but local microinjection of cholinergic agonists into this area induces atonia (see e.g. refs. 3, 41, 53), suggesting that putative atonia cells are cholinoceptive. Finally, neurotoxin lesions destroying a majority of pontine cholinergic cells, especially within PPT, also dramatically attenuated REM sleep and dissociated its components62.
Immunohistochemical studies correlating post-lesion cell loss in PPT with REM sleep dysfunction also indicated extensive damage to cholinergic and non-cholinergic cells in the medial pontine tegmentum, including the hypothesized atonia region62. Thus, REM sleep disintegration after large pontine lesions may have been affected by inclusion of dorsomedial pontine structures involved in REM sleep generation.
The present study addressed this possibility by differentiating REM sleep deficits associated with lesions of the dorsolateral vs dorsomedial pontine tegmentum. Specifically, relatively discrete, bilateral lesions of the cholinergic PPT were compared with lesions of the cholinergic laterodorsal tegmental nucleus (LDT) as well as with lesions of predominantly non-cholinergic regions, including locus ceruleus (LC) and/or subceruleus (LC alpha, peri-LC alpha and the lateral tegmental field).
MATERIALS AND METHODS
Surgery
Eighteen cats, weighing 2.0–3.0 kg, had aseptic, stereotaxic surgery under sodium pentobarbital anesthesia (35 mg/kg, i.p.) for implantation of electrodes to assess sleep-waking state patterns. All cats had jewelers’ screws threaded into the bone over motor cortex for cortical EEGs (A27, L4 and 8, or L8 and 10) and into the frontal sinus for evaluation of eye movements (electrooculogram or EOG); stainless steel wires were inserted into the nuchal musculature to register tone (electromyogram or EMG). Twelve of the cats also had tripolar leads in the lateral geniculate nucleus (LGN), bilaterally, to record PGO spikes (A6, L10, H +2.3 or 3.0)56.
Baseline 12 h recordings
Cats were chamber-adapted for at least 24 h before baseline polygraphic recordings. which were initiated between 08.00 and 10.00 h.
Lesions
Cats were re-anesthetized for bilateral radio frequency (n = 7) or electrolytic lesions (n = 11). There were two target sites: (1) the dorsolateral pontine tegmentum, especially PPT and peribrachial (PB) regions – P2: L1.5, 2.5 and 3.5 at H –1.5, –2.5 and –3.5; and P1: L2 and 3.5; H –3.0 or (2) the dorsomedial pontine tegmentum, particulary the LC complex: P1.5, 2.5; L 2.7; H –4.2. Electrolytic lesions were 3 mA for 30–40 s per coordinate, whereas radio frequency lesions were 60 s per site. Cats were allowed a 1–2 week recovery after lesions.
Post-lesion 12 h recordings
Animals were re-evaluated for sleep-waking state parameters 7 days (n = 9) or 14 days (n = 9) after lesions. 12 h recordings were obtained, as during baseline.
Other pre-and post-lesion studies
Cats underwent electrophysiological or acute epilepsy studies at least 1 week before baseline 12 h recordings and again after the conclusion of post-lesion 12 h recorclings13,50
Histology
At the end of the study, animals were given an overdose of sodium pentobarbital (50–70 mg/kg) and perfused with either 10% formalin in buffered saline or cold saline followed by cold paraformaldehyde. Brains were removed for histology. Frozen, coronal sections were obtained in all cats and stained with Cresyl violet or thionine for verification of lesion sites. Five cats in this study were also evaluated for cholinergic cell loss in PPT and LDT, using choline acetyl transferase (ChAT) immunohistochemistry, as previously reported13.
Data analysis
Dependent variables were as follows:
Sleep-waking state parameters
Waking, slow-wave-sleep (SWS) and REM sleep episodes lasting at least 1 min were identified by the criteria of Ursin and Sterman58, and the following parameters were quantified for each pre-and post-lesion 12 h recording: (i) Percent time, number of episodes and mean epoch duration (min) of each sleep or waking state; (ii) REM sleep components. Each REM sleep episode was evaluated for integrity of tonic components (EEG desynchronization and atonia) as well as phasic components. Phasic events were indexed by REMs in all cats (seconds of REM sleep with REMs) and by PGO spikes (>50 µV in REM and >75 µV in SWS) obtained in 7 cats; (iii) Percentages of active vs quiet REM sleep. Each REM sleep episode was subdivided into active or quiet REM sleep, defined by the number of seconds with vs without REMs and/or PGO spikes, respectively; (iv) REM cycles. The number and mean duration (min) of REM cycles were calculated from the midpoints of consecutive REM episodes without intervening wakefulness (see e.g. ref. 51).
Histology
Histologic localization of lesions was determined with reference to Berman’s atlas4. Parameters included site and size of lesions, as well as damage to cell populations which predominantly contain acetylcholine (ACh) or norepinephrine (NE).
(i). Lesion site and size.
Percent tissue damage was estimated by measuring the area of lesion (mm) and dividing by total area of tissue (mm) at 1 mm intervals on an anterior-posterior (AP) plane from A2 to P4. We quantified total damage at these stereotaxic co-ordinates as well as percent of tissue loss at specific pontine sites, with an emphasis on the 7 regions listed as follows.
Three cholinergic cell columns, including LDT (AP0 to P4), PPT (P1 to P2) and PB (P3 and P4), were identified with reference to the Berman atlas4 and Jones and Webster22.
Three predominantly noradrenergic sites, including LC, LC-alpha, and peri-LC alpha (AP0-P4), were identified with reference to Sakai40.
Finally, the reticular core ventral to the LC complex was identified with reference to Berman’s atlas4. This region contains few cholinergic or noradrenergic cells21 and corresponds to the parvocellular, lateral and gigantocellular tegmental fields (FIT, FTL and FTG) from AP0 through P4.
(ii). Percent damage to ACH vs NE cell regions.
Cholinergic and noradrenergic cells are interspersed throughout the dorsal pontine tegmentum in cats but are unevenly distributed within PPT, PB, LDT and LC complex (see e.g. refs. 21, 63). As a result, estimates of tissue damage do not proportionately represent the involvement of cholinergic vs noradrenergic cell areas within these structures. This problem was addressed in two ways.
First, the number of cholinergic cells (ChAT+) in PPT and LDT was determined in five cats, four with primary PPT lesions and one with a primary LDT lesion, and expressed as percent of immunoreactive cells in 3 unlesioned ‘control’ cats13.
Second, to supplement immunohistochemical findings, we approximated damage to cholinergic vs catecholaminergic (presumably noradrenergic) cell regions from normative data employing ChAT and tyrosine hydroxylase (TB) labeling21. The procedure involved counting the number of ChAT+ vs TH+ cell areas in six structures (LDT, PPT, PB, LC, LC alpha and peri-LC alpha), as displayed upon coronal sections by Jones and Beaudet21 and Webster and Jones62. We then superimposed our lesions, counted the number of ChAT+ and TH+ cell areas affected and divided by the total number of cell areas to generate estimates of percent damage to ChAT+ vs TH+ cell areas.
This measure does not precisely reflect the number of cells lost after lesions; however, the extent of damage to areas containing cholinergic vs noradrenergic cells has been used for correlation with REM sleep parameters and yielded similar correlations to those based upon cell counts62.
Statistical analysis
Sleep-waking state parameters were subjected to 2-way analyses of variance (ANOVAs), comparing pre-post-lesion findings (repeated measure) as a function of lesion groups (non-repeated measure). Histologic variables were assessed with non-repeated measures ANOVAs. Post-hoc tests were dependent or independent t-tests for repeated vs non-repeated measures, respectively. Finally, interactions between sleep and histologic measures were assessed by Pearson Product Moment correlations.
RESULTS
Three groups of subjects were identified a posteriori, based upon pontine lesion site. The salient features of each group are described first; afterward, the population data are elaborated for sleep-waking state parameters, histologic variables and interactions between the two.
Group composition
Fig. 1 illustrates REM sleep components before and after lesions together with coronal sections through the pons in cats representative of each group:
Fig. 1.
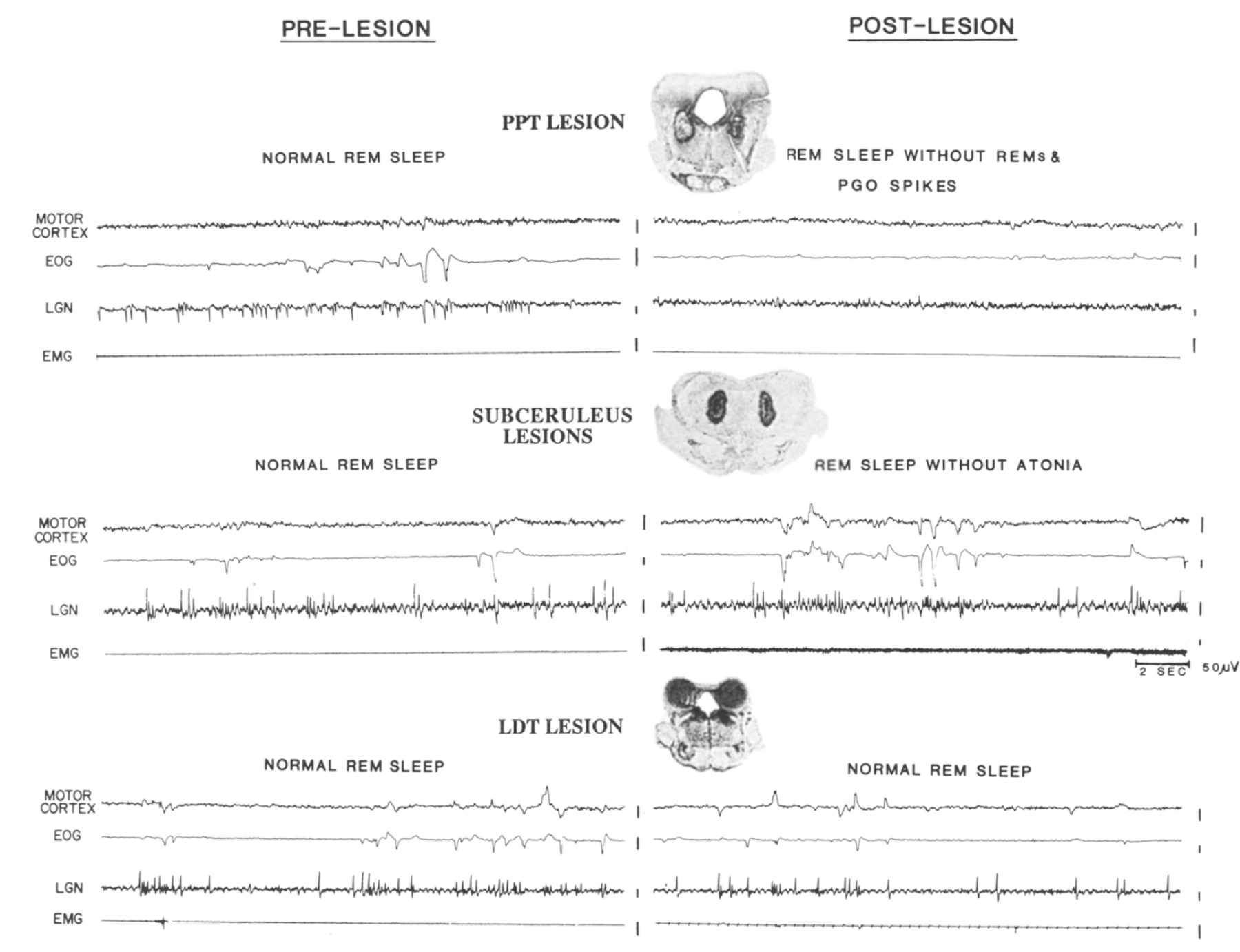
Twenty-second polygraphic tracings of REM sleep before and after lesions, together with a coronal section through the center of the pontine lesion in 1 cat per group. Each tracing displays a motor cortex electroencephalogram (EEG), an electrooculogram (EOG), an EEG from lateral geniculate nucleus (LGN) to register pontogeniculooccipital (PGO) spikes and an electromyogram (EMG). Motor cortex EEG desynchronization was intact regardless of lesion site. Top: Group 1 PPTIPB lesions, illustrated by a radio frequency lesion of the pedunculopontine tegmentum (PPT), diminshed clustered phasic events (rapid-eye-movements or REMs and PGO spikes) during REM sleep. Middle: Group 2 subceruleus lesions, illustrated by an electrolytic lesion of pen LC-alpha and the underlying reticular core, mostly the lateral tegmental field (FTL), abolished atonia during REM sleep and also seemed to increase phasic events (REMs and PGO spikes). Bottom: Group 3 LDT and/or LC lesions, illustrated by a lesion encompassing nearly all of the laterodorsal tegmental nucleus (LDT), did not affect REM sleep.
Group 1. PPT/PB lesions (n = 6).
Loss or diminution of phasic events during REM sleep accompanied lesions of PPT (Fig. 1, top). REM sleep after PPT lesions contained fewer eye movements (n = 6) and either no discernible PGO spikes (n = 1) or low amplitude spikes (n = 1), when compared to normal pre-lesion tracings. However, tonic REM sleep components, including EEG desynchronization and atonia, were completely intact after PPT/PB lesions.
Group 2. Subceruleus lesions (n = 6).
Loss of atonia during REM sleep followed lesions of subceruleus, including peri-LC alpha, LC alpha and the underlying reticular core, particularly the lateral tegmental field (Fig. 1, middle). EEG desynchronization (n = 6), PGO spikes (n = 3) and REMs (n = 6) were completely intact in the post-lesion records of all cats in this group; in fact, phasic events (REMs and/or PGO spikes) increased after subceruleus lesions.
Group 3. LDT and/or LC lesions (n = 6).
Observed polygraphic components of REM sleep were not affected by lesions primarily involving LC or LDT (Fig. 1, bottom). EEG desynchronization (n = 6), REMs (n = 6), PGO spikes (n = 3) and atonia (n = 6) were similar in pre- and post-lesion tracings.
Population data: sleep-waking states
Table I summarizes waking and SWS measures before and after lesions in the 3 lesion groups. Neither state was significantly affected by lesions, although there was a trend towards increased deep SWS percentages after subceruleus lesions (P < 0.1)
TABLE I.
Means and standard deviation for various parameters of waking and slow-wave-sleep (SWS) before and after lesions in 3 groups of cats
| Pre-lesion baseline (n = 18) | PPTIPB lesion (n = 6) | Subceruleus lesion (n = 6) | LDTILC lesion (n = 6) | |
|---|---|---|---|---|
| Wakefulness | ||||
| percent time | 41.0±5.4 | 42.1±11.8 | 44.8±8.9 | 44.2±4.2 |
| number of epochs | 32.2±7.6 | 32.2±8.4 | 30.0±9.7 | 27.6±5.6 |
| epoch duration | 9.7±3.3 | 9.2±1.3 | 11.4±2.9 | 11.5±3.2 |
| SWS | ||||
| percent time | 44.0±6.0 | 45.7±12.7 | 43.1±8.4 | 44.1±3.7 |
| number of epochs | 41.7±9.0 | 33.2±9.4 | 39.0±9.2 | 37.6±5.2 |
| epochs duration | 7.8±1.7 | 9.6±4.4 | 8.2±2.3 | 7.9±1.9 |
| Light (1) vs deep (2) SWS (% of total SWS) | ||||
| SWS 1 | 56.0±18 | 58.0±13 | 46.0±11 | 57.0±28 |
| SWS 2 | 44.0±18 | 42.0±13 | 54.0±11 | 43.0±28 |
Two-way analysis of variance compared each variable as a function of lesion group (PPT, subceruleus, LDTILC) with repeated measures over time (pre- vs post-lesion). There were no statistically significant differences in waking or SWS, but there was a trend towards increased deep SWS in the subceruleus lesion group (P < 0.01).
Fig. 2 and Table II summarize several significant differences in REM sleep measures (F = P < 0.05). Post hoc t-tests indicated no differences between groups during baseline, and pre-lesion values are combined for this reason (n = 18)
Fig. 2.
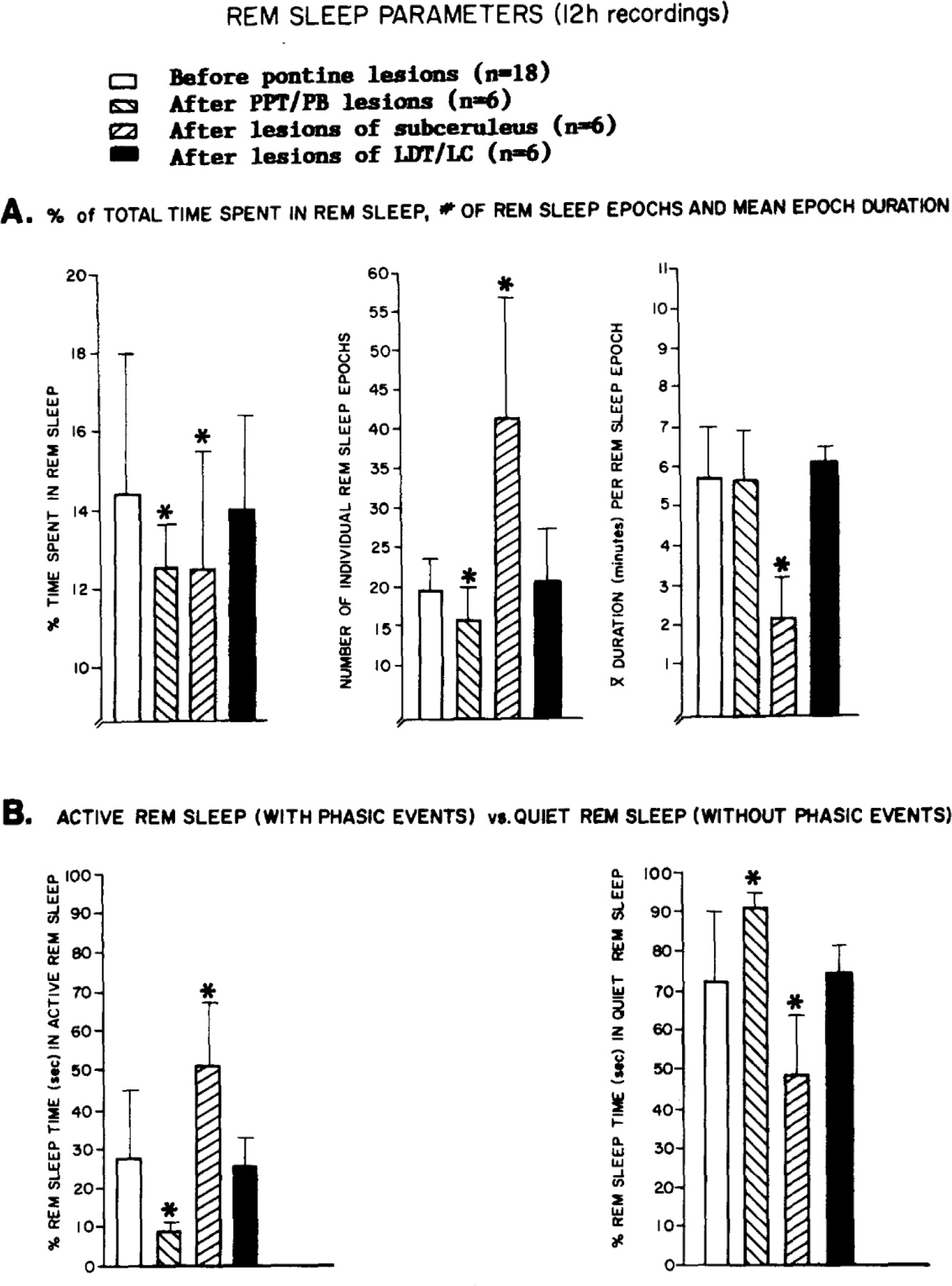
Means and standard deviations for various parameters of REM sleep before and after lesions in 3 groups of cats. Two-way analysis of variance compared each variable as a function of lesion group (PPT/PB, subceruleus or LDT/LC) with repeated measures over time (pre-vs post-lesion). A: percent of the 12 h recording time spent in REM sleep (group: F = NS; time: F = 36.3, P < 0.01; group x time: F 12.8, P < 0.01), number of REM sleep episodes (group: F = 7.3, P < 0.01; time: F =8.1, P < 0.01, Group x time: F = 17.3, P < 0.05) and mean REM sleep epoch duration (group: F = 8.9, P < 0.01; time: F = 10.5, P < 0.01; group x time: F = 8,0, P < 0.05). 13: percentages of active vs quiet REM sleep. Active REM sleep is defined by clustered phasic events, whereas quiet REM sleep does not contain clustered REMs and PGOs. Each second of REM sleep was classified as active or quiet. F tests are identical, as active and quiet REM percentages cumulate to 100% (group: F = 3.95, P < 0.05; time: F = NS; group x time: F = 8.0, P < 0.05). Post hoc t-tests indicated no difference between groups in pre-lesion measures, which are combined. There were also no differences between pre- and post-lesion REM sleep measures in the LDT/LC group. Significant dependent or independent t-tests for PPT/PB or subceruleus lesion groups are indicated by asterisks.
TABLE II.
Mean number and duration (min) ± standard deviations of REM sleep cycles during 12 h recordings obtained before (n = 18) and after lesions (n = 6 each) in 3 groups of cats
| number of REM cycles | duration (min) of REM cycles | |
|---|---|---|
| Pre-lesion baseline (n = 18) | 5.7 ± 1.8 | 22.6 ± 3.5 |
| PPT lesion (n = 6) | 2.0 ± 1.6* | 23.2 ± 7.3 |
| Subceruleus lesion (n = 6) | 12.0 ± 4.0* | 10.5 ± 3.2* |
| LDT/LC lesion (n = 6) | 5.0 ± 2.5 | 20.8 ± 8.1 |
REM sleep cycles were calculated from the midpoints of consecutive REM sleep epochs within each sleep cycle51. Two-way analysis of variance compared each variable as a function of lesion group (PPT, subceruleus, LDT/LC) with repeated measures over time (pre- vs post-lesion). F-tests were significant for the number of REM cycles (time: F = 24.0, P < 0.01; group x time: F = 10.2, P < 0.05) and their duration (time: F = 5.1, P < 0.05; group x time: F = 4.8, P < 0.05). PPT/PB lesions were associated with fewer REM cycles. Subceruleus lesions induced more REM sleep cycles with shorter durations, possibly related to the increased phasic activity in this group. Post-hoc mests:
= P < 0.05 from baseline and post-lesion LDT/LC data.
Lesions of PPT and subceruleus were accompanied by small, but statistically significant reductions in REM sleep percentages (Fig. 2A; ~20% each from baseline). However, different factors seemed to contribute to REM sleep loss in these groups.
Group 1, PPT/PB lesions.
Lesions diminished phasic REM sleep components in conjunction with significantly fewer entrances into REM sleep (fewer REM sleep episodes in Fig. 2a) and with significantly lower percentages of active REM sleep (reduced percentages of REM sleep with phasic events in Fig. 2b). However, the duration of REM epochs (Fig. 2a) and of REM cycles (Table II) was not different from baseline and accounts for the nominal reduction in total REM sleep time following PPTIPB lesions.
Group 2. Subeeruleus lesions.
Lesions eliminated atonic and produced shorter but more frequent REM cycles (Table II). Opposing effects were also detected in the duration and number of REM epidoses (Fig. 2a), resulting in the modest suppression of total REM sleep time in this group.
Subceruleus lesions also induced high density REMs and PGOs (Fig. 1, middle) and high percentages of active REM sleep (Fig. 2b). The number of PGO spikes before and after REM onset also tended to increase after subceruleus lesions, in spite of the post-lesion reduction in REM sleep time.
PGO spikes were quantified in each REM sleep epoch and in each SWS epoch preceding REM onset during 12 h recordings obtained in the 3 cats with geniculate waves. During REM sleep, PGO spikes >50 µV averaged 1552.7±656.8 per 12 h before lesions and 2668.8±1303 after subceruleus lesions (t = 35.89, P < 0.01, two-tailed). Geniculate spikes also increased during the SWS epochs that preceded REM onset, as PGO spikes >75 µV averaged 846.7 ± 351 per 12 h before lesions vs 1882.3 ± 495.4 after subceruleus lesions (t = 24.67, P < 0.05, two-tailed). Thus, the mean number of PGO spikes per 12 h increased from 2400 to 4551 after subceruleus lesions (P < 0.01, two tailed, dependent t-test), despite the decrease in REM sleep time.
Group 3. LDT/LC lesions.
LDT and/or LC lesions did not affect REM sleep parameters when compared to pre-lesion baselines (Fig. 2; Table II).
Post-lesion effects on day 7 vs 14.
Changes in REM sleep polygraphic components were similar on post-lesion days 7 and 14. EEG desynchronization was unaffected by any lesion, whereas phasic events (PPT/PB lesions) or atonia (subceruleus lesions) were either absent or diminished at both post-lesion recording intervals.
REM sleep percentages, episodes and epoch durations were slightly higher on post-lesion day 14 than day 7, but the difference was not statistically significant. For example, REM sleep percentages in the subceruleus group were 11.8 ± 5.3 at 7 days post-lesion and 12.6 ± 4.6 at 14 days post-lesion. For the PPT/PB group, REM sleep percentages were 11.7 ± 1.8 at post-lesion day 7 and 12.6 ± 1.1 on post-lesion day 14. Thus, deficits persist at least 14 days.
Population data: histology
Lesion site and size
All lesions were located in the pontomesencephalic tegmentum. Fig. 3 shows reconstructions of 2 cats per group in serial coronal sections from Al through P4. In each group, one lesion is hatched, and one is dotted. Other lesions are depicted elsewhere13,50.
Fig. 3.
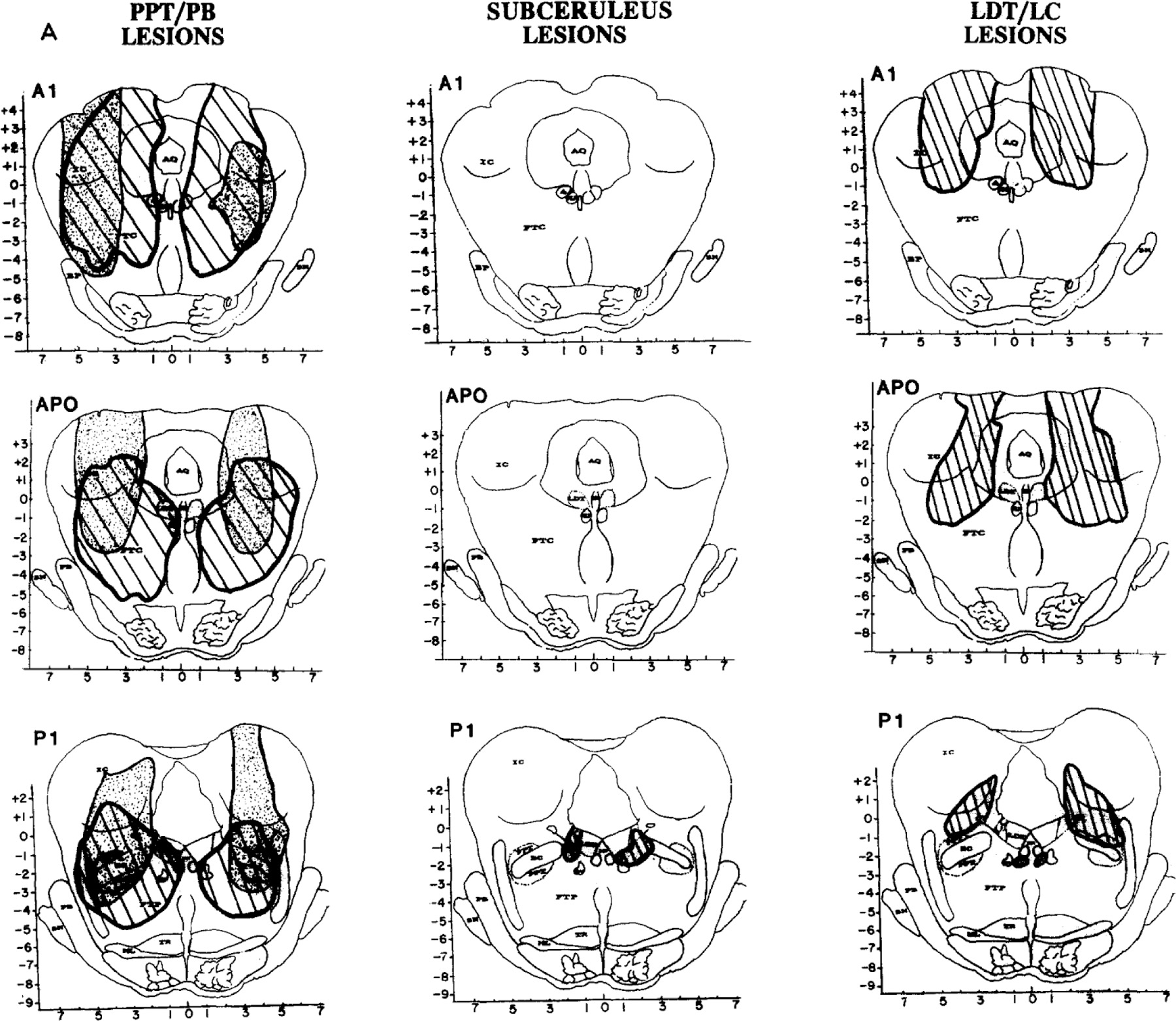
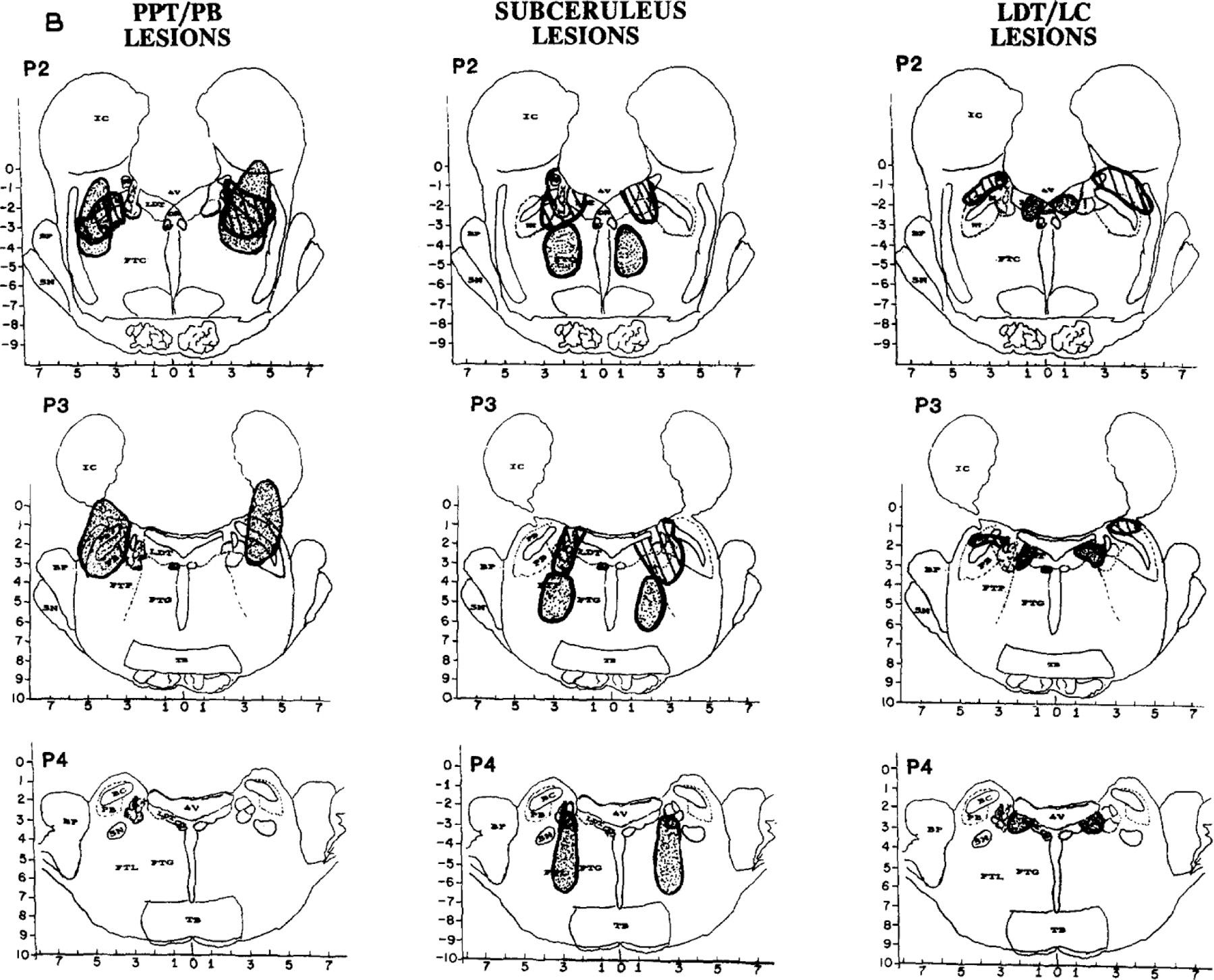
B. Reconstructions of lesions in 2 cats (one dotted and the other striped) in each of the 3 lesion groups, PPT/PB (left), subceruleus (middle) and LDT/LC (right). Coronal section and abbreviations are adapted from the Berman atlas and approximate 1 mm intervals from Al throught P4. Left panel: Group 1 PPT/PB. Lesions were larger than in the other 2 groups and involved most of the lateral pontine cholinergic cell columns, including PFT and PB, often encompassed the central tegmental field (FTC) and sometimes the parvocellular tegmental field (FTP) as well. Tissue lost posterior to P2 is illustrated in 1 cat (dotted lesion); damage to the peribrachial (PB) cholinergic columns is evident at P3. Middle panel: Group 2 subceruleus. Lesions were smaller as well as caudal and ventromedial to PPT/PB lesions. Peri-LC alpha was damaged in all 6 cats. Some lesions encompassed locus ceruleus complex (LCx; striped lesion), and others damaged peri-LC alpha together with the underlying reticular formation (dotted), mostly the lateral tegemental field (FTL) and some of the gigantocellular tegmental field (FTG). Right panel: Group 3 LDT/LC. One cat in this group had a large lateral lesion involving most of FTC and some of the LC (striped). The other lesions in the group were more medial (dotted), encompassing all or parts of the lateral dorsal tegmenturn (LDT) and/or locus ceruleus (LC).
Table III summarizes mean percent of total tissue destroyed per group and also the percent destruction at specific pontine sites from APO through P4, which contain the target sites for lesions. A blow-up of the regions containing the 3 cholinergic cell columns (LDT, PPT and PB) as well as the constituents of LC complex (LC, LC alpha and peri-LC alpha) is adjacent to Table III to facilitate identification on full coronal sections in Fig. 3.
TABLE III.
Estimates of tissue damage, expressed as percent tissue loss, at 1 mm intervals from APO through P4 in 3 groups of cats (n = 6 each): PPT/PB, subceruleus and LDT/LC lesions
| TOTAL LESION | MOSTLY CHOLINKRGIC CELL REGIONS |
MOSTLY NONCHOLINKRGIC CELL REGIONS |
HISTOLOGIC RECONSTRUCTION |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PPT | PB | LDT | LC complex |
Reticular Core | LEFT PONTINE TEGMENTUM | ||||
| LC | LCa | pLCa | |||||||
| APO | |||||||||
| PPT/PB | 21.8* | --- | --- | 37.5 | --- | --- | --- | --- |
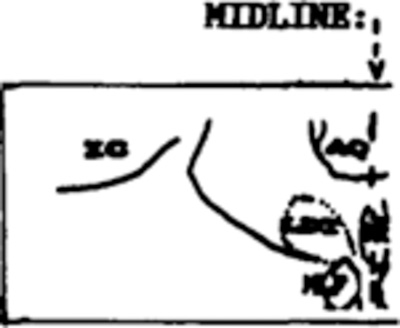
|
| SUBCERULEUS | 0.0 | --- | --- | 0.0 | --- | --- | --- | --- | |
| LDT/LC | 4.5 | --- | --- | 0.0 | --- | --- | --- | --- | |
| P1 | |||||||||
| PPT/PB | 18.1* | 80.0* | --- | 25.8 | 33.8 | --- | --- | 26.6 |

|
| SUBCERULEUS | 2.2 | 6.8 | --- | 19.2 | 8.3 | --- | --- | 23.3 | |
| LDT/LC | 5.1 | 10.0 | --- | 31.7 | 43.3 | --- | --- | 5.8 | |
| P2 | |||||||||
| PPT/PB | 6.7 | 70.8* | --- | 1.0 | 21.1 | 19.1 | 2.3 | 11.6 |
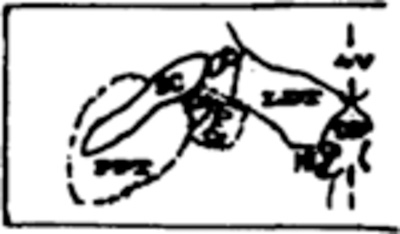
|
| SUBCERULEUS | 4.1 | 19.6 | --- | 20.8 | 29.2 | 57.5* | 53.3* | 42.8* | |
| LDT/LC | 5.0 | 10.0 | --- | 38.3 | 40.0 | 8.3 | 3.3 | 0.3 | |
| P3 | |||||||||
| PPT/PB | 12.0* | --- | 56.3 | 0.0* | 0.0 | 12.5 | 0.0 | 0.0 |
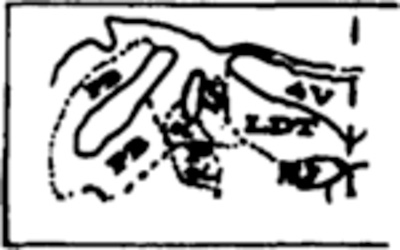
|
| SUBCERULEUS | 5.7 | --- | 9.6 | 31.7 | 33.3 | 50.0* | 66.6* | 40.0* | |
| LDT/LC | 5.2 | --- | 3.3 | 29.2 | 20.0 | 8.3 | 0.0 | 0.3 | |
| P4 | |||||||||
| PPT/PB | --- | --- | {60.0}+ | --- | --- | --- | --- | --- |
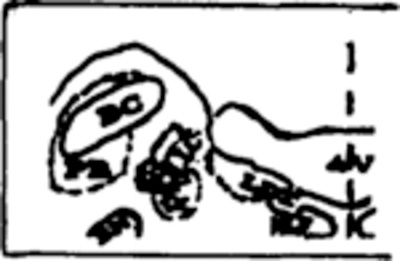
|
| SUBCERULEUS | 6.8 | --- | 5.0 | 0.0 | 30.0 | 54.2* | 81.6* | 21.6 | |
| LDT/LC | 5.6 | --- | 21.7 | 12.5 | 30.8 | 4.2 | 0.0 | 0.0 | |
| GRAND MEAN ± SD/LESION GROUPS | Total Lesion | PPT | PB | LDT | LC complex |
Reticular core | |||
| LC | LCa | pLCa | |||||||
| PPT/PB | 14.7*± 7 | 75.4*± 7 | 58.2+ ±3 | 16.1 ±19 | 18.3 ±17 | 15.8 ±5 | 1.2 ±2 | 12.7 ±13 | |
| SUBCERULIUS | 3.7 ±3 | 13.2 ±9 | 7.3 ±3 | 14.3 ±14 | 25.2 ±11 | 54.0* ±4 | 67.2* ±14 | 31.9*±11 | |
| LDT/LC | 5.1± 4 | 10.0 ± 0 | 12.5 ± 13 | 22.3 ± 16 | 33.5 ± 11 | 6.9 ± 2 | 1.1 ± 2 | 1.6 ± 3 | |
Percentages were derived from the area of lesion, in mm, divided by total mm of tissue. Mean values per group are provided for total lesion size as well as damage to the 3 predominately cholinergic cell regions, including PPT, PB and LDT, and 4 predominately non-cholinergic regions, including locus ceruleus (LC), LC alpha (LCa), peri-LC alpha (pLCa), and the underlying reticular core. A blow-up of a coronal section through the left pontine tegmentum is provided at each AP level to facilitate identification of cholinergic columns and components of locus ceruleus complex on the full coronal sections in Fig. 3. Tissue was available at all planes in all cats except the PPT/PB group, in which tissue was lost posterior to P2 in 4 cats. Since PB exists at P3 and P4, it was not possible to statistically assess total tissue loss at this site (grand mean for PB = 58%+). ANOVAs compared damage estimates per group for each histologic index. Significant group differences were obtained for total lesion size (F = 5.9, P < 0.05), PPT damage (F = 58.04, P < 0.001), LC alpha (F = 29.94, P < 0.001), peri-LC alpha (F = 20.11, P < 0.001) and the underlying reticular formation (F = 10.27, P < 0.001).
Post-hoc t-tests were statistically significant at P < 0.05 from other groups; { } includes tissue from only 1 cat; - - - structure not present at that AP, or insufficient tissue available (PPT/PB group at P4 only).
Group 1. PPT/PB lesion (n = 5 radio frequency and 1 electrolytic).
Fig. 3 (left) illustrates the 2 largest lesions, which extended from A2 or A3 rostrally (shown from Al only) to at least P2 caudally. Of the target sites, the main damage was to the dorsolateral Pontine cholinergic column, predominantly PPT.
Population data in Table III indicate that PPT/PB lesions destroyed a mean of 14.7% of total tissue per coronal section APO through P4 and were 3 to 4 times larger than in the other 2 lesion groups (t = 2.6, P < 0.05 from subceruleus group). Damage to PPT averaged 75.4% in the PPT/PB group, whereas less than 13% of PPT was damaged in the other 2 groups (e.g. t = 8.9, P < 0.01 from the subceruleus group).
Sections posterior to P2, which contain PB, were available in 2 cats, one of which is shown in Fig. 3. In this and the other cat, PB damage at P3 and P4 averaged 58.2%, when compared to less than 15% damage in the other 2 groups. Finally, damage to central and parvocellular tegmental fields (FTC, FTP) rostral to APO was prominent after some PPT lesions (Fig. 3, left).
Group 2. Subceruleus lesions (n = 5 electrolytic and 1 radio frequency).
Lesions were caudal (P1 and P4) and medial or ventromedial to those in the PPT/PB group (Fig. 3, middle). The only common lesion site in the subceruleus group was peri-LC alpha.
Two types of subceruleus lesions induced REM sleep without atonia. The extremes are illustrated in Fig. 3 (middle). One cat’s lesion (hatched) primarily involved LC complex in conjunction with maximal destruction of cholinergic cell columns, mostly in LDT, in the subceruleus group. The other cat’s lesion (dotted) was larger, caudal, and involved none of LDT, PPT, PB or LC; this cat sustained damage to peri-LC alpha, FTL and some of FTG.
The population data in Table III reveal maximal damage to LC alpha, peri-LC alpha and underlying reticular core, mostly FTL, after subceruleus lesions. Damage to each of these areas was significantly higher than in the PPT lesion group (t-tests ranged from 4.9 to 8.0, P < 0.05) and also the LDT/LC lesion group (t-tests ranged from 4.1 to 18.2, P < 0.05).
Group 3, LDT/LC lesions (n = 5 radio frequency and 1 electrolytic).
Lesions ranged in size and location. Fig. 3 (right) illustrates the largest but least localized lesion in this group (hatched lesion with extensive FTC damage) as well as the smallest but most localized lesion (dotted lesion of LDT). Table III indicates that average lesion size was intermediate between PPT and subceruleus lesion groups and that sites most damaged were LDT (~22%) and/or LC proper (~33%).
Estimated tissue loss at LDT and/or LC varied considerably between subjects and did not differ significantly from PPT or subceruleus lesion groups. However, destruction of PPT was significantly less than in the PPT lesion group, and damage to subceruleus was significantly less extensive than in the subceruleus lesion group.
Immunohistochemical estimates: ACH vs NE cell damage
ChAT immunohistochemistry
Bilateral cell counts of ChAT+ cells within PPT and LDT were made in 5 cats with lesions and compared to control data from 3 cats without lesions13. Lesions of PPT/PB (n = 4) resulted in 90 ± 4% fewer ChAT + cells within PPT (10% of controls ) vs 46 ± 32% in LDT (54% of controls). Conversely, the LDT/LC lesion resulted in 75.4% fewer ChAT+ cells within LDT (24.6% of controls) vs 59.3% in PPT (40.7% of controls).
Immunohistochemical findings correspond to estimated tissue loss at these sites. Percent of ChAT+ cell loss at PPT correlated with percent of tissue damage at PPT (n = 5, r = 0.905, P < 0.05). Similarly, ChAT+ cell loss at LDT correlated with percent of tissue damage at LDT (n = 5, r = 0.82, P < 0.05).
Damage to cholinergic vs noradrenergic cell areas.
Table IV contains estimated loss of cholinergic vs catecholaminergic cell regions in all cats, based upon normative ChAT vs TH immunohistochemical data. PPT lesions damaged 74.3% of ChAT+ areas and 64.7% of TH+ areas in PPT. Estimated damage to ChAT+ cell areas in PPT/PB correlated with actual ChAT+ cell counts in 4 cats (r = 0.91, P < 0.05). In contrast, subceruleus lesions encompassed 52.4% of TH+ cell areas and 45.6% of ChAT+ cell areas within LC complex. LDT/LC lesions encompassed >20% of ChAT+ and TH+ areas in LDT as well as 19.1% of ChAT+ and 8.6% TH+ areas in LC complex.
TABLE IV.
Estimates of damage to cholinergic or catecholaminergic cell areas in LDT, PPT and LC complex
| Lesion group | Pontine site |
||
|---|---|---|---|
| LDT | PPT | LC complex | |
| Percent of ChAT+ cell areas damaged | |||
| PPT/PB | 15.8±12 | 74.3±23* | 10.0±4 |
| Subceruleus | 16.7±16 | 18.6±13 | 45.6±18* |
| LDT/Lc | 20.4±17 | 9.0±9 | 19.1±16 |
| Percent of TH+ cell areas damaged | |||
| PPT/PB | 16.7±5 | 63.2±17* | 6.0±5 |
| Subceruleus | 11.0±4 | 28.7±12 | 52.4±24* |
| LDT/LC | 23.0±19 | 9.7±8 | 8.6±5 |
We superimposed our lesions upon normative data identifying regions containing choline acetyltransferase positive (ChAT+) and tyrosine hydroxylase positive (TH+) cell21.The percent of ChAT+ or TH+ areas lost ± standard deviations are provided for 3 groups of cats (n = 6 each) after lesions of different parts of the pontomesencephalic tegmentum. Abbreviations are as in Table III. Simple analysis of variance indicated significant differences between lesion groups in damage to ChAT+ and TH+ cells in the PPT and the LC complex. Asterisks indicate significant post-hoc f-tests. Lesions aimed at PPT damaged significantly more ChAT+ and TH+ cell areas in PPT, whereas lesions aimed at subceruleus damaged significantly more ChAT+ and TH+ cell areas in LC complex.
Interactions between histologic and REM sleep parameters
Histologic and sleep data indicate quite different effects after lesions of PPT vs subceruleus. PPT destruction reduced phasic activity and REM entrances, whereas subceruleus damage, especially to peri-LC alpha, was accompanied by REM sleep without atonia and abbreviated REM sleep epochs as well as by frequent phasic events and REM sleep entrances.
Correlational data extend these conclusions. Pearson product moment correlations revealed several significant interactions between PPT/PB or subceruleus damage and REM sleep parameters. Table Va summarizes correlations between local tissue damage and REM sleep data, whereas Table Vb lists the correlations between estimated ACH and/or NE cell loss at these sites and REM sleep changes.
TABLE Va.
Pearson product moment correlations between percent tissue damage and REM sleep paramaters in 2 groups, PPT/PB lesions (n = 6) and subceruleus lesions (n = 6)
| REM sleep measure | Lesion size |
||||||
|---|---|---|---|---|---|---|---|
| PPT lesion group |
Subceruleus lesion group |
||||||
| PPT | LDT | Total lesion | LCa | pLCa | sub-LC corn plex | Total lesion | |
| % active REM | −0.81* | −0.43 | −0.50 | +0.95* | +0.93* | +0.01 | +0.42 |
| Number of REM episodes | −0.20 | −0.08 | +0.12 | −0.04 | −0.18 | +0.92* | +0.70 |
| REM epoch duration | −0.07 | +0.50 | −0.24 | −0.60 | −0.80* | −0.70 | −0.80* |
| % REM (time/12 h) | −0.56 | +0.09 | +0.70 | −0.93* | −0.79* | +0.10 | −0.20 |
Findings are provided for specific pontine lesion targets as well as for total lesion size from APO through P4, as depicted in Table III. Per capita PPT damage was significantly correlated with reduced phasic events (active REM sleep). Damage to subceruleus and sometimes the reticular core (mostly FTL) was significantly correlated with increased active REM sleep and the number of REM sleep episodes (positive correlation) and reduced REM sleep epoch durations and percentages (negative correlation).
= correlations 0.73 are statistically significant at P < 0.05.
TABLE Vb.
Pearson product moment correlations between estimates of ChAT+ and/or TH+ cell areas lost and REM sleep parameters in 2 groups, PPT lesions (n = 6) and subceruleus lesions (n = 6).
| REM sleep measure | ChAT + areas damaged by PPT lesion |
TH+ and ChAT + areas damaged by subcerukus lesion |
|||
|---|---|---|---|---|---|
| PPT | LDT | LCp | LCa | pLCa | |
| % active REM | −0.83* | −0.17 | +0.11 | +0.87* | +0.88* |
| Number of REM episodes | −0.17 | −0.29 | +0.26 | +0.75* | +0.31 |
| REM epoch duration | −0.06 | −0.19 | −0.24 | −0.32 | −0.78* |
| % REM (time/12 h) | −0.56 | −0.33 | −0.51 | −0.90* | −0.83* |
Abbreviations as in Table III. Damage to ChAT+ areas in PPT was significantly correlated with reduced phasic events (active REM sleep) after PPT lesions. Damage to ChAT+ and TH+ areas after subceruleus lesions (LC alpha and peri-LC alpha) was significantly correlated with increased active REM sleep and the number of REM sleep episodes (positive correlation) and reduced REM sleep epoch durations and percentages (negative correlation).
= correlations ≥0.73 are statistically significant at P < 0.05.
Group 1. PPT/PB lesions.
Increased damage to tissue and to ChAT+ areas within PPT/PB was accompanied by reduced percentages of active REM sleep (r = –0.81, P < 0.05 in Table Va and Table Vb, respectively). In contrast, damage to TH+ cell areas within PPT did not correlate significantly with any REM sleep parameter, nor did individual or combined estimates of ChAT+ and TH+ areas damaged at any other site. These results suggest that the most reliable effect of PPT lesions is the suppression of phasic events during REM sleep, potentially mediated by loss of cholinergic neurons.
Group 2. Subceruleus lesions.
Table Va indicates positive correlations between tissue damage in subceruleus and various REM sleep parameters. Damage to LC alpha and peri-LC alpha accompanied increased percentages of active REM sleep as well as reduced REM sleep time and epoch durations. Damage to the underlying reticular core correlated significantly with increased number of REM episodes (r = 0.92, P < 0.05).
Table Vb indicates that TH+ cell areas lost in LC alpha and peri-LC alpha also correlated with increased percentages of active REM sleep (r = 0.78, P < 0.05) and with reduced REM sleep time (r = –0.77, P < 0.05). In contrast, no significant correlations were obtained with ChAT + cell loss at any site after subceruleus lesions (Table Vb).
Even though ChAT+ cell areas alone did not correlate with REM sleep parameters in this group, combined TH+ and ChAT + areas lost within subceruleus yielded the highest correlations between immunohistochemical estimates and REM sleep data. Loss of TH+ and ChAT+ areas in LC alpha and peri-LC alpha accompanied increased percentages of active REM sleep as well as reduced REM sleep time and epoch durations (Table Vb).
Group 3. LDT/LC lesions.
Correlations between histologic variables and REM sleep data after lesions of LDT and/or LC are excluded from Table 5 because none was statistically significant. In fact, mean damage to either LDT or LC did not exceed 33.5% in any group, did not differentiate the 3 lesion groups and did not seem to contribute to REM sleep changes observed after PPT/PB or subceruleus lesions.
Furthermore, unintentional involvement of structures anterior to AP0, notably extensive tissue loss in FTC, was equivalent after LDT/LC or PPT/PB lesions (Fig. 3, left vs right). Since lesions of LDT, LC and/or FTC did not alter REM sleep, damage to the rostral reticular formation did not appear essential to REM sleep anomalies seen in the PPT/PB lesion group.
Other factors
Lesion procedure (radio frequency vs electrolytic) did not appear to affect the results. Site-specific tissue loss and REM sleep effects were comparable with either lesion technique and fell within one standard deviation of the mean.
Another potential confounding factor was the conduct of other experimental procedures. Electrophysiological studies (auditory evoked responses) were conducted prior to baseline sleep recordings in 7 cats, 6 with PPT/PB lesions and 1 with a primary LDT lesion13. Epilepsy studies (systemic penicillin epilepsy and electroconvulsive shock) had been conducted prior to baseline sleep recordings in 11 cats, 6 with subceruleus lesions and 5 with LDT and/or LC lesions50.
Auditory stimulation can augment REM sleep if applied during sleep10, but the tests were conducted only in the waking state in this study and never on the day of sleep recordings. The epilepsy models tested have transient effects on sleep7,49,50 and are unlikely to have persisted during the minimum 1-week interval between the last seizure test and baseline recordings50. In any case, the baseline sleep data in all 3 experimental groups corresponds to normative data for 12 h recordings in cats51, 58, and there were no differences between groups in baseline values, regardless of prior exposure to evoked potential or epilepsy trials.
Finally, the behavioral status and management of cats with pontine lesions has been described in detail elsewhere13,50. Even so, it should be mentioned that miosis (pupil diameter <2 mm in waking) was detected in all the cats with PPT/PB lesions and remains enigmatic.
DISCUSSION
Our data shed some light on the pontine mechanisms underlying REM sleep components. The PPT and the subceruleus appear to have quite different roles in the generation of discrete REM sleep polygraphic components, and neither alone suffices as a REM sleep integrating mechanism.
Present findings elaborate those of Webster and Jones62, who demonstrated dissociation of REM sleep components and substantial REM sleep loss after large neurotoxin lesions of the pontomesencephalic tegmentum. We observed virtually every REM sleep effect they reported in spite of the difference in lesion technique.
Damage to PPT was similar in the 2 studies, based upon bilateral symmetry, tissue loss and estimated immunohistochemical changes from lesion reconstructions at comparable stereotaxic coordinates62. Available immunohistochemical data indicated 90% cholinergic cell loss in PPT in our study13 vs 71% in theirs22.
Our medial pontine lesions, including LDT, LC and/or subceruleus (LC alpha, peri-LC alpha and FTL), seem to fall within the larger area damaged in the Webster and Jones study62. The present experiment differs in that smaller, lateral vs medial pontine lesions were separately placed. We found that PPT lesions suppressed PGOs and REMs and also reduced the number of REM episodes, whereas subceruleus lesions eliminated atonia during REM sleep and abbreviated REM sleep epochs.
In contrast, Webster and Jones62 observed all these REM sleep effects after their pontine lesion, possibly because it encompassed PPT and also subceruleus regions. The combined effects of these lesion sites on REM sleep components may have exacerbated REM sleep loss in their cats, which was equivalent to the sum of REM sleep decrements in our PPT and subceruleus lesion groups at comparable post-lesion intervals.
Some of the findings reported here are consistent with elements of the brainstem cholinergic hypothesis of REM sleep, particulary the role of PPT in the generation of the phasic REM sleep elements, both REMs and PGO spikes. The occurrence of phasic events in SWS heralds the onset of REM sleep; their attenuation after PPT lesions may have contributed to the reduction of REM sleep entrances and REM sleep time seen in this group.
On the other hand, EEG desynchronization and atonia during REM sleep were intact after PPT lesions, demonstrating that this region is not required to orchestrate the confluence of all REM sleep components. The duration of REM sleep epochs was also unaffected by PPT lesions, indicating that the integrity of PPT may not be essential for REM sleep maintenance either.
Some findings do suggest a role for the subceruleus region, especially peri-LC alpha, in REM sleep maintenance. This region is sufficient for generation of atonia, as proposed by Morrison14,15 and also Sakai38,41,43, since every cat with a significant subceruleus lesion had REM sleep without atonia. Damage to the underlying reticular formation, including FTG and particularly FTL, also accompanied loss of atonia and suppression of REM sleep, as noted by many investigators (see e.g. refs. 5, 12, 44). The integrity of the pontine atonia system could be important for sustained REM sleep epochs, as the presence of tone and mobility after subceruleus lesions seemed to arouse the cats shortly after REM sleep onset.
The reduction in REM epoch duration after subceruleus lesions was mitigated by frequent REM sleep entrances and cycles. The reduction in REM sleep time accompanying brief REM epochs may have elevated REM sleep ‘pressure’, thus increasing the number REM sleep episodes. Alternatively, the increased number of phasic events observed after subceruleus lesions might also trigger more REM sleep entrances.
Activation of both serotonergic cells in dorsal raphe and noradrenergic cells in LC complex has been implicated in suppression of phasic events16,17,41,55. Our lesion did not encroach upon the dorsal raphe but did damage the LC complex. In fact, increased phasic activity was significantly correlated with subceruleus damage, where noradrenergic cells predominate22,36. The increase in REM sleep episodes after subceruleus lesions is consistent with findings that NE agonists suppress REM sleep and that NE antagonists or intracerebral 6-hydroxydopamine can increase REM sleep8,9,18,30,35. Moreover, NE cells in these regions progressively cease firing in the REM sleep transition and are silent during REM sleep16,17,39,41.
The above facts suggest that damage to noradrenergic cells in subceruleus might release phasic events during REM sleep. This observation, together with evidence that phasic events are mediated by cholinergic activation in PPT and pb2,32,37,38,42, point to an interaction between NE and ACh in the regulation of these events. This interpretation is consistent with our estimates of immunohistochemical changes in subceruleus and PPT as well as with the ACh-NE interaction hypotheses of several investigators16,17,27,38,41,42,54.
While pontine noradrenergic cells can modulate REM sleep, they are not essential for its recurrence (see e.g. ref. 54). Our data also corroborate this conclusion, as post-lesion REM sleep changes were correlated with damage to NE cell areas only in the subceruleus lesion group. Moreover, the highest correlations were obtained by combining estimates of NE and ACH cell areas lost in subceruleus and relate best to total tissue damage in this region.
Perhaps our most surprising finding is that PPT lesions which destroyed 75% of the tissue and cholinergic cell areas in that region did not eliminate atonia during REM sleep. This result seems to conflict with evidence that ACh has an important role in atonia. Application of cholinergic agonists into the subceruleus, especially LC alpha and peri-LC alpha, reliably induces atonia2,3,29,40, 48,53, and several investigators have identified cholinergic projections from PPT, PB and LDT to subceruleus that might mediate this effect33,45.
Some of these cholinergic fibers traverse and others terminate in LC alpha, peri-LC alpha, FTL and FTG. Consequently, disruption of cholinergic terminals or fibers of passage cannot be excluded as a factor in the REM sleep deficits after subceruleus lesions, particularly since the radio frequency and electrolytic lesion procedures used here do not selectively damage cell bodies. On the other hand, this complication does not explain why lesions sufficient to destroy 85–95% of the ChAT+ cells in PPT and as much as 76.5% of ChAT+ cells in LDT never prevented complete atonia during REM sleep.
Accordingly, the cholinoceptive properties of atonia cells may not be essential for induction of atonia in REM sleep. This conclusion is consistent with findings that systemic cholinergic antagonists, such as atropine, do not affect atonia in REM sleep, even at dosages producing dramatic signs of cholinergic inactivation and dissociation of other REM sleep components50. Moreover, Lai and Siegel observed that glutamate can induce atonia following microinjection into the cholinoceptive peri-LCa as well as into non-cholinoceptive regions in the nucleus magnocellularis29. Finally, Webster and Jones62 found no significant correlation between loss of cholinergic cells in the pontomesencephalic tegmentum and loss of atonia.
A main drawback to either PPT or subceruleus as a critical REM sleep mechanism is the discrepancy between pharmacologic and lesion effects on EEG desynchronization during REM sleep. Local infusion of cholinergic agonists into subceruleus or PPT reliably induces EEG desynchronization (see e.g. refs. 2, 3), yet EEG desynchronization during REM sleep persisted after lesions of either region in our study. Webster and Jones62 also reported intact EEG desynchronization after lesions encompassing PPT and subceruleus.
It is possible that remaining cholinergic and/or noradrenergic cells in the LDT, PB and LC complex continue to generate REM sleep EEG desynchronization. However, Webster and Jones’ lesion also incorporated most of the cholinergic and noradrenergic cells in these regions without eliminating forebrain EEG desynchronization62.
In conclusion, a synergistic relation between cholinergic and noradrenergic cells of the pontomesencephalic tegmentum might explain some of the REM sleep changes after lesions of the subceruleus and/or PPT. However, it seems likely that non-cholinergic and non-adrenergic cells are also involved.
Acknowledgements:
Supported by the Veterans Administration and by PHS Grants NS25629, HL41370, MH43811 and MH42903. Cats with PPT lesions provided by Jean Harrison, Ph.D. and Jennifer Buchwald, Ph.D., Department of Physiology, Mental Retardation Research Center & Brain Research Institute, UCLA School of Medicine, Los Angeles, CA 90024, U.S.A.
REFERENCES
- 1.Aserinsky E and Kleitman N, Regularly occurring periods of eye motility, and concomitant phenomena during sleep, Science, 118 (1953) 273–274. [DOI] [PubMed] [Google Scholar]
- 2.Baghdoyan HA, Monaco AR, Rodrigo-Angulo ML, Assens F, McCarley RW and Hobson JA, Microinjection of neostigmine into the pontine reticular formation of cats enhances desynchronized sleep signs, J. Pharmacol. Exp. Ther, 321 (1984) 173–180. [PubMed] [Google Scholar]
- 3.Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW and Hobson JA, Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem sites, Brain Research, 306 (1984) 39–52. [DOI] [PubMed] [Google Scholar]
- 4.Berman AL, The Brain Stem of the Cat. A Cytoarchitectonic Atlas with Stereotaxic Coordinates University of Wisconsin Press, Madison, 1982. [Google Scholar]
- 5.Carli G and Zanchetti A, A study of pontine lesions suppressing deep sleep in the cat, Arch. Ital. Biol, 103 (1965) 751–788. [PubMed] [Google Scholar]
- 6.Chase M and Morales F, The control of motoneurons during sleep. In Kryger MH, Roth T and Dement WC (Eds.), Principles and Practice of Sleep Medicine, Saunders, Philadelphia, 1989, pp. 74–85. [Google Scholar]
- 7.Cohen HB, Thomas J and Dement WC, Sleep stages, REM deprivation and electroconvulsive threshold in the cat, Brain Research, 19 (1967) 317–317. [DOI] [PubMed] [Google Scholar]
- 8.DeSarro GB, Ascioti C, Froio F, Libri V and Nistico G, Evidence that locus coeruleus is the site where clonidine and drugs acting at alphal-and alpha2-adenoceptors affect sleep and arousal mechanisms, Br. J. Pharmacol, 90 (1987) 675–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Draskoczy PR and Schildkraut JJ, Effects of 6-hydroxydo-pamine on sleep in the rat, Nature, Vol. 233 (1971) 425–427. [DOI] [PubMed] [Google Scholar]
- 10.Drucker-Colin DR, Bemal-Pedraza J, Fernandez-Canoino F and Morrison AR, Increasing PGO spike density by auditory stimulation increases the duration and decreases the latency of rapid eye movement (REM) sleep, Brain Research, 278 (1983) 308–312. [DOI] [PubMed] [Google Scholar]
- 11.Elam M, Svensson T and Thoren P, Locus coeruleus neurons and sympathetic nerves: activation by cutaneous sensory afferents, Brain Research, 366 (1986) 254–261. [DOI] [PubMed] [Google Scholar]
- 12.Friedman L and Jones BE, Computer graphics analysis of sleep-wakefulness state changes after pontine lesions, Brain Res. Bull, 13 (1984) 53–68. [DOI] [PubMed] [Google Scholar]
- 13.Harrison JB, Woolf NJ and Buckwald JS, Cholinergic neurons of the feline pontomesencephalic tegmentum. I. Essential role in ‘Wave A’ generation, Brain Research, 520 (1990) 43–54. [DOI] [PubMed] [Google Scholar]
- 14.Hendricks JC, Morrison AR and Mann GL, Different behaviors during paradoxical sleep without atonia depend on pontine lesion site, Brain Research, 239 (1982) 81–105. [DOI] [PubMed] [Google Scholar]
- 15.Henley K and Morrison AR, A re-evaluation of the effects of lesions of the pontine tegmentum and locus coeruleus on phenomena of paradoxical sleep in the cat, Acta Neurobiol. Exp, 34 (1974) 215–232. [PubMed] [Google Scholar]
- 16.Hobson JA, McCarley RW and Wyzinski PW, Sleep cycle oscillation: reciprocal discharge by two brain stem neuronal groups, Science, 289 (1975) 55–58. [DOI] [PubMed] [Google Scholar]
- 17.Hobson JA, Lydic R and Baghdoyan HA, Evolving concept of sleep cycle generation: from brain centers to neuronal populations, Behav. Brain Sci, 9 (1986) 371–448. [Google Scholar]
- 18.Jacobs BL and Jones BE, The role of central monoamine and acetylcholine systems in sleep-wakefulness states: mediation or modulation. In Butcher LL (Ed.), Cholinergic-Monoam-inergic Interactions in the Brain, Academic Press, New York, 1978, pp. 271–290. [Google Scholar]
- 19.Jones BE, Elimination of paradoxical sleep by lesions of the pontine gigantocellular tegmental field in the cat, Neurosci. Lett, 13 (1979) 285–293. [DOI] [PubMed] [Google Scholar]
- 20.Jones BE, Harper ST and Halaris AE, Effects of locus coeruleus lesions upon cerebral monoamine content, sleep wake fulness states and the response to amphetamine in the cat, Brain Research, 124 (1977) 473–496. [DOI] [PubMed] [Google Scholar]
- 21.Jones BE and Beaudet A, Distribution of acetylcholine and catecholamine neurons in the cat brainstem: a choline acetyl-transferase and tyrosine hydroxylase immunohistochemical study, J. Comp. Neurol, 261 (1987) 15–32. [DOI] [PubMed] [Google Scholar]
- 22.Jones BE and Webster HH, Neurotoxic lesions of the dorsolateral pontomesencephalic tegmentum-cholinergic cell area in the cat. I. Effects upon the cholinergic innervation of the brain, Brain Research, 458 (1988) 13–32. [DOI] [PubMed] [Google Scholar]
- 23.Jouvet M, Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique. Arch. Ital. Biol, 100 (1962) 125–206. [PubMed] [Google Scholar]
- 24.Jouvet M, Neurophysiology of the states of sleep, Physiol. Rev, 47 (1967) 117–177. [DOI] [PubMed] [Google Scholar]
- 25.Jouvet M, The role of monoamines and acetylcholine containing neurons in the regulation of the sleep-waking cycle, Ergeb Physiol, Biol. Chem. Exp. Pharmacol, 64 (1972) 166–308. [DOI] [PubMed] [Google Scholar]
- 26.Jouvet M and Delorme F, Locus coeruleus et sommeil para-doxique, C. R. Soc. Biol, 159 (1965) 895–899. [Google Scholar]
- 27.Karczmar AG, Longo VG and Scotti de Carolis A, A pharmacological model of paradoxical sleep: the role of cholinergic and monoamine systems, Physiol. Behav, 5 (1970) 175–182. [DOI] [PubMed] [Google Scholar]
- 28.Kitsikis A and Steriade M, Immediate behavioral effects of kianic acid injections into the midbrain reticular core, Behav. Brain Res, 3 (1981) 361–380. [DOI] [PubMed] [Google Scholar]
- 29.Lai YY and Siegel JS, Medullary regions mediating atonia, J. Neurosci, 8 (1988) 4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lagguzzi RF, Adrien J, Bourgoin S and Hamon M, Effects of intraventricular injection of 6-hydroxydopamine in the developing kitten. I. On the sleep-waking cycles, Brain Research, 160 (1979) 445–459. [DOI] [PubMed] [Google Scholar]
- 31.Laurent JP and Guerrero FA, Reversible suppression of pontogeniculo-occipital waves by localized cooling during paradoxical sleep in cats, Exp. Neurol, 49 (1975) 356–369. [DOI] [PubMed] [Google Scholar]
- 32.McCarley RW, Nelson JP and Hobson JA, Ponto-geniculo-occipital (PGO) burst neurons: correlational evidence for neuronal generators of PGO waves, Science, 20 (1978) 269–272. [DOI] [PubMed] [Google Scholar]
- 33.Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K and McCarley RW Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular field in the cat, Brain Research, 951 (1988) 397–402. [DOI] [PubMed] [Google Scholar]
- 34.Monmaur P and Delacour J, Effects de la lesion bilaterale du tegmentum pontique dorsolateral sur l’activite theta hippocampique au cours du sommeil paradoxal chez le rat. C.R. Seances Acad. Sci, 286 (1978) 761–764. [PubMed] [Google Scholar]
- 35.Nakamura S, Sakaguchi T, Kimura F and Aoki F, The role of alpha-adrenoceptor-mediated collateral excitation in the regulation of the electrical activity of locus coeruleus neurons, Neuroscience, 27 (1988) 921–929. [DOI] [PubMed] [Google Scholar]
- 36.Reiner P, Correlational analysis of central noradrenergic neuronal activity and sympathetic tone in behaving cats, Brain Research, 378 (1986) 86–96. [DOI] [PubMed] [Google Scholar]
- 37.Saito H, Sakai K and Jouvet M, Discharge patterns of the nucleus parabrachialis lateralis neurons of the cat during sleep and waking, Brain Research, 134 (1977) 59–72. [DOI] [PubMed] [Google Scholar]
- 38.Sakai K, Some anatomical and physiological properties of ponto-mesencephalic tegmental neurons with special reference to the PGO waves and postural atonia during paradoxical sleep in the cat. In Hobson JA and Brazier MA (Eds.), The Reticular Formation Revisited, Raven Press, New York, 1980, pp. 427–447. [Google Scholar]
- 39.Sakai K, Anatomical and physiological basis of paradoxical sleep. In McGinty DJ et al. (Eds.), Brain Mechanisms of Sleep, Raven Press, New York, 1985, pp. 111–137. [Google Scholar]
- 40.Sakai K, Neurons responsible for paradoxical sleep. In Wauquier A, Gaillard JM, Monti JM and Radulovacki M (Eds.), Sleep: Neurotransmitters and Neuromodulators, Raven Press, New York: (1985). [Google Scholar]
- 41.Sakai K, Executive mechanisms of paradoxical sleep, Arch. Ital. Biol, 126 (1988) 239–257. [PubMed] [Google Scholar]
- 42.Sakai K and Jouvet M, Brain stem PGO-on cells projecting directly to the cat dorsal lateral geniculate nucleus, Brain Research, 194 (1980) 500–505. [DOI] [PubMed] [Google Scholar]
- 43.Sakai K, Saste JP, Salvert D, Touret M, Tohyama M and Jouvet M, Tegmentoreticular projections with special reference to muscular atonia during paradoxical sleep in the cat: an HRP study, Brain Research, 176 (1979) 233–254. [DOI] [PubMed] [Google Scholar]
- 44.Sastre JP, Sakai K and Jouvet M, Are the gigantocellular tegmental field neurons responsible for paradoxical sleep?, Brain Research, 229 (1981) 147–161. [DOI] [PubMed] [Google Scholar]
- 45.Shiromani PJ, Armstrong DM and Gillin JC, Cholinergic neurons from the dorsolateral pons project to the medial pons: a WGA-HRP and choline acetyltransferase immunohistochemical study, Neurosci. Lett, 95 (1988) 19–23. [DOI] [PubMed] [Google Scholar]
- 46.Shiromani P, Armstrong D, Bruce G, Hersch L, Groves P and Gillin C, Relation of pontine choline acetyltransferase in immunoreactive neurons with cells which increase discharge during REM sleep, Brain Res. Bull, 18 (1987) 447–455. [DOI] [PubMed] [Google Scholar]
- 47.Shiromani PJ, Overstreet DL, Levy D, Goodrich CA, Campbell SS and Gillin JC, Increased REM sleep in rats selectivity bred for cholinergic hyperactivity, Neuropsychopharmacology, 1 (1988) 127–133. [DOI] [PubMed] [Google Scholar]
- 48.Shiromani PJ, Siegel JM, Tomaszewski KS and McGinty DJ, Alterations in blood pressure and REM sleep after pontine carbachol microinfusion, Exp. Neurol, 91 (1986) 285–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shouse MN, Differences between two feline epilepsy models in sleep and waking state disorders, state dependency of seizures and seizure susceptibility: amygdala kindling interferes with penicillin epilepsy, Epilepsia, 28 (1987) 399–408. [DOI] [PubMed] [Google Scholar]
- 50.Shouse MN, Siegel JM, Wu MF, Szymusiak R and Morrison AR, Mechanisms of seizure suppression during rapid-eye-movement (REM) sleep in cats, Brain Research, 505 (1989) 271–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shouse MN and Sterman MB, Sleep and kindling. II. Effects of generalized seizure induction, Exp. Neurol, 71 (1981) 563–580. [DOI] [PubMed] [Google Scholar]
- 52.Siegel JM, Pontomedullary interactions in the generation of REM sleep. In McGinty DJ, Drucker-Colin R, Morrison AR and Parmeggiani PL (Eds.), Brain Mechanisms of Sleep, Raven Press, New York, 1985, pp. 157–174. [Google Scholar]
- 53.Siegel JM, Brain stem mechanisms generating REM sleep. In Kryger MH, Roth T and Dement WC (Eds.), Principles and Practice of Sleep Medicine, Saunders, Philadelphia, 1989, pp. 86–120. [Google Scholar]
- 54.Siegel JM and Rogawski MA, A function for REM sleep: regulation of noradrenergic receptor sensitivity, Brain Res. Rev, 13 (1988) 213–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon RP, Gershon MD and Brooks DC, The role of the raphe nuclei in the generation of ponto-geniculo-occipital wave activity, Brain Research, 58 (1973) 313–330. [DOI] [PubMed] [Google Scholar]
- 56.Snider RS and Niemer WT, A Stereotaxic Atlas of the Cat Brain, University of Chicago Press, Chicago, 1961. [Google Scholar]
- 57.Steriade M and Hobson JA, Neuronal activity during the sleep-waking cycle, Prog. Neurobiol, 6 (1976) 155–376. [PubMed] [Google Scholar]
- 58.Ursin R and Sterman MB, A Manual for Standardized Scoring of Sleep and Waking States in the Adult Cat Brain Information Service/Brain Research Institute, 1981. [Google Scholar]
- 59.Velasco M and Velasco F, Brain stem regulation of cortical and motor excitability: effects on experimental focal motor seizures. In Sterman MB, Shouse MN and Passouant P (Eds.), Sleep and Epilepsy, Academic Press, New York, 1982, pp. 53–61. [Google Scholar]
- 60.Villablanca J, Behavioral and polygraphic study of ‘sleep’ and ‘wakefulness’ in chronic decerebrate cats, Electroenceph. Clin. Neurophysiol, 21 (1966) 562–577. [DOI] [PubMed] [Google Scholar]
- 61.Vincent SR and Reiner PB, The immunohistochemical localization of choline acetyltransferase in the cat brain, Brain Res. Bull, 18 (1987) 381–415. [DOI] [PubMed] [Google Scholar]
- 62.Webster HH and Jones BE, Neurotoxic lesions of the dorsolateral pontine tegmentum-cholinergic cell area in the cat. II. Effects upon sleep-waking states, Brain Research, (1989). [DOI] [PubMed]
- 63.Woolf NJ, Harrison JB and Buckwald JS, Cholinergic neurons of the feline pontomesencephalic tegmentum II. Anatomical projections, Brain Research, 520 (1990) 55–72. [DOI] [PubMed] [Google Scholar]


