Abstract
Purpose
The COVID-19 pandemic’s impact on buprenorphine treatment for opioid use disorder among adolescents and young adults (AYAs) is unknown.
Methods
We used IQVIA Longitudinal Prescription Claims, including US AYAs aged 12–29 with at least 1 buprenorphine fill between January 2018 and August 2020, stratifying by age group and insurance. We compared buprenorphine prescriptions in March-August 2019 to March-August 2020.
Results
The monthly buprenorphine prescription rate increased 8.3% among AYAs aged 12–17 but decreased 7.5% among 18- to 24-year-olds and decreased 5.1% among 25- to 29-year-olds. In these age groups, Medicaid prescriptions did not significantly change, whereas commercial insurance prescriptions decreased 12.9% among 18- to 24-year-olds and 11.8% in 25- to 29-year-olds, and cash/other prescriptions decreased 18.7% among 18- to 24-year-olds and 19.9% in 25- to 29-year-olds (p < .001 for all).
Discussion
Buprenorphine prescriptions paid with commercial insurance or cash among young adults significantly decreased early in the pandemic, suggesting a possible unmet treatment need among this group.
Keywords: Opioid use disorder, Adolescent, Adolescent health services, Medication for addiction treatment, Medication for opioid use disorder, Insurance, Medicaid, COVID-19
Implications and Contribution.
During the early COVID-19 pandemic, buprenorphine prescriptions paid with commercial insurance or cash among young adults significantly decreased, suggesting a possible unmet need among this group. The stable Medicaid prescription rate during the early pandemic demonstrates the safety net role Medicaid has played in providing access to addiction treatment.
See Related Editorial on p.143
Drug overdose deaths increased substantially during the COVID-19 pandemic to over 104,000 deaths in the 12-month period ending in September 2021 [1], with increases disproportionally impacting people of color [2]. The death toll among adolescents and young adults (AYAs) in 2020 rose to its highest ever of 7,091 deaths among 15- to 24-year-olds, a 49% increase from 2019 [3]. AYAs are less likely than older adults to receive addiction treatment, and specifically medications for opioid use disorder (MOUD) such as buprenorphine [4,5], due to many barriers including limited availability of youth-serving addiction treatment providers [6], and misconceptions and stigma regarding the use of MOUD in youth [7]. Policy changes were implemented that helped to stabilize the overall volume of buprenorphine filled during the COVID-19 pandemic [8,9]; however, changes in buprenorphine fills among AYAs are unknown.
Methods
We used IQVIA Longitudinal Prescription Claims (LRx) anonymized patient-level prescription data to characterize buprenorphine utilization changes during the early phases of the COVID-19 pandemic among US AYAs aged 12–29 with at least 1 buprenorphine fill between January 2018 and August 2020. Buprenorphine is the most commonly prescribed MOUD for AYAs, and is the only one reliably captured in a large prescription database [10]. We stratified analyses by age group (12–17, 18–24, and 25–29 years), starting at age 12 to capture even young AYAs with OUD given that MOUD is indicated in clinical guidelines with no lower age limit [7], and extending beyond the traditionally conceptualized “transitional age adults” to include individuals through age 29 given the pandemic’s demonstrated economic upon young adults up to age 29 [11]. We stratified by insurance (Medicaid, commercial, cash/other) to evaluate whether insurance served as an effect moderator, and compared the buprenorphine prescription rate per 100,000 AYAs (using American Community Survey population data) in March-August 2019 to March-August 2020. We plotted monthly buprenorphine prescription rates per 100,000. Because age was treated as a fixed variable in each calendar year there was a prescription decrease every January when a new cohort of 12-year-olds entered the dataset; to correct for this we smoothed the rates using a mean of 6 months surrounding each month. We used Stata, version 15 (StataCorp, College Station, TX), and 2-sided t-tests with significance set at p < .05. The study was exempted from review by the Johns Hopkins Bloomberg School of Public Health Institutional Review Board.
Results
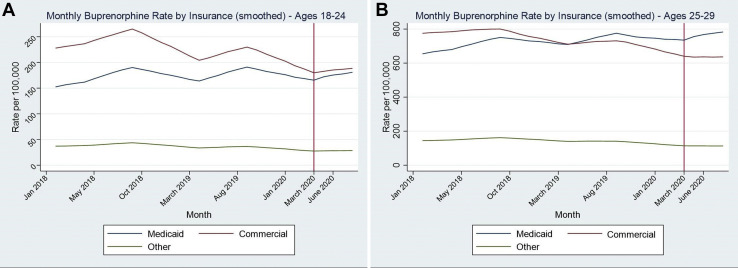
A total of 413,152 AYAs aged 12–29 filled buprenorphine prescriptions between January 2018 and August 2020; monthly prescriptions throughout this period ranged between 90,932 and 120,740. Among AYAs age 12–17, the monthly rate of buprenorphine prescriptions per 100,000 increased by 8.3% (p = .0019) from prepandemic to during pandemic, with small statistically significant increases in Medicaid and commercial insurance prescriptions, and a statistically significant decrease in prescriptions filled with cash (Table 1 ). The monthly rate decreased 7.5% during the pandemic among 18- to 24-year-olds (p = .036) and decreased 5.1% among 25- to 29-year-olds (p = .0024). Among AYAs aged 18–24 and 25–29, Medicaid prescription rates did not significantly change from pre-COVID-19 (March–August 2019) to during COVID-19 (March–August 2020). In contrast, commercial insurance prescriptions during COVID-19 decreased 12.9% among 18- to 24-year-olds and 11.8% in 25- to 29-year-olds, and cash/other prescriptions decreased 18.7% among 18- to 24-year olds and 19.9% in 25- to 29-year-olds (p < .001 for all). Declining commercial prescriptions were also observed in the smoothed monthly buprenorphine rate plot, whereas Medicaid rates remained stable (Figure 1 ).
Table 1.
Monthly rate of buprenorphine prescriptions filled per 100,000 population by insurance used for prescription claim: pre-COVID-19 (March through August 2019) versus during COVID-19 (March through August 2020)
| Rate of prescriptions for 100,000 population (95% CI) |
|||
|---|---|---|---|
| Age category | |||
| 12–17 years | 18–24 years | 25–29 years | |
| Overall rate | |||
| Pre-COVID-19 | 25.11 (24.49–25.72) | 478.14 (451.84–504.44) | 1772.90 (1734.88–1810.93) |
| During COVID-19 | 27.39 (26.12–28.66) | 444.92 (421.42–468.43) | 1686.67 (1647.08–1726.26) |
| Pr (|T| > |t|) = 0.0019 | Pr (|T| > |t|) = 0.0360 | Pr (|T| > |t|) = 0.0024 | |
| Insurance type | |||
| Medicaid | |||
| Pre-COVID-19 | 6.85 (6.62–7.07) | 172.82 (161.36–184.28) | 731.42 (703.40–759.45) |
| During COVID-19 | 7.75 (7.32–8.18) | 175.50 (164.20–186.80) | 767.57 (738.12–797.02) |
| Pr (|T| > |t|) = 0.001 | Pr (|T| > |t|) = 0.668 | Pr (|T| > |t|) = 0.045 | |
| Commercial | |||
| Pre-COVID-19 | 9.83 (9.53–10.13) | 212.77 (201.59–223.94) | 721.86 (711.53–732.19) |
| During COVID-19 | 10.70 (10.13–11.27) | 185.38 (178.80–191.95) | 636.67 (628.50–644.85) |
| Pr (|T| > |t|) = 0.006 | Pr (|T| > |t|) < 0.001 | Pr (|T| > |t|) < 0.001 | |
| Other | |||
| Pre-COVID-19 | 2.06 (1.94–2.18) | 34.68 (33.48–35.88) | 141.48 (140.31–142.65) |
| During COVID-19 | 1.87 (1.77–1.97) | 28.20 (26.79–29.61) | 113.38 (110.61–116.16) |
| Pr (|T| > |t|) = 0.009 | Pr (|T| > |t|) < 0.001 | Pr (|T| > |t|) < 0.001 | |
Figure 1.
Monthly rate of buprenorphine prescriptions by insurance status per 1,000 population before and during the COVID-19 pandemic: (A) ages 18–24 and (B) ages 25–29.
Discussion
Our findings suggest that during the early COVID-19 pandemic, buprenorphine prescriptions paid with commercial insurance or cash among young adults significantly decreased, suggesting a possible unmet treatment need among this group. The stable Medicaid prescription rate during the early pandemic demonstrates the safety net role Medicaid has played in providing access to addiction treatment. Our findings contrast with a study among adults of all ages which found no significant change in buprenorphine filled with commercial insurance [9]. It is possible that some AYAs discontinuing buprenorphine transitioned to methadone (not captured in our data); however, these transitions are likely uncommon and would not result in decreased buprenorphine in only young adults. Young adults in their 20s are more likely than older adults to have lost a job or been unemployed as a result of the COVID-19 pandemic [11], and our study demonstrates that these young adults may be more susceptible to the health consequences of these economic impacts that result in interruptions in employer-sponsored commercial insurance and financial hardship limiting individuals’ ability to pay cash for prescriptions.
Existing policies that can stabilize coverage for young adults, such as allowing young adults to stay on their parents’ commercial insurance coverage through age 26, did not appear to influence buprenorphine receipt given the same trend was observed in the 18- to 24-year-olds as well as the older 25- to 29-year olds, the majority of whom would not be eligible for parental coverage. Conversely, younger adolescents aged 12–17 with commercial insurance most likely only have insurance through their parents/guardians; the increase in commercial insurance buprenorphine claims for this age group starkly contrasts with the trend seen in young adults and could have many contributions, among which may be that their parents were less susceptible to the economic disruptions noted for young adults.
Limitations of this study include the absence of data regarding buprenorphine dispensed through opioid treatment programs, though this would be unlikely to impact adolescents under 18 years of age since very few of them use these programs. We also did not have data identifying whether a young adult was a primary subscriber or dependent in a commercial insurance plan, or whether buprenorphine was prescribed in the context of in-person versus expanded telehealth services aided by removal of regulatory barriers during the early pandemic.
As our study reports on early trends in the COVID-19 pandemic, further research is needed to examine subsequent changes. Worsening trends in overdose deaths underscore that there is likely continued unmet need for services [1,3]. Further research is also needed to assess the impact of the pandemic upon AYA access to other MOUD (methadone, naltrexone) and within marginalized groups disproportionally affected by the pandemic [2,12], the impact of disruptions in MOUD access on health disparities, overdose, and other adverse outcomes among AYAs, and the potential mitigating impact of policy changes regarding MOUD prescribing among AYAs.
Footnotes
Conflicts of interest: Dr. Alexander is past Chair and a current member of FDA’s Peripheral and Central Nervous System Advisory Committee; is a cofounding Principal and equity holder in Monument Analytics, a health care consultancy whose clients include the life sciences industry as well as plaintiffs in opioid litigation; and is a past member of OptumRx’s National P&T Committee. This arrangement has been reviewed and approved by Johns Hopkins University in accordance with its conflict-of-interest policies. No other disclosures were reported.
Funding Sources
Drs. Alinsky, Stein, and Saloner were supported by the RAND-USC Schaeffer Opioid Policy Tools and Information Center (OPTIC) (P50DA046351). Data acquisition was supported by the IQVIA Institute Human Data Science Research Collaborative. The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
References
- 1.Ahmad F., Rossen L., Sutton P. National Center for Health Statistics; Hyattsville, MD: 2022. Provisional drug overdose death counts. [Google Scholar]
- 2.Panchal N., Garfield R., Cox C., Artiga S. Kaiser Family Foundation; San Francisco, CA: 2021. Substance use issues are worsening alongside access to care.https://www.kff.org/policy-watch/substance-use-issues-are-worsening-alongside-access-to-care/ Available at: [Google Scholar]
- 3.Hedegaard H., Minino A., Spencer M., Warner M. Drug overdose deaths in the United States, 1999–2020. NCHS Data Brief. 2021:1–8. [PubMed] [Google Scholar]
- 4.Hadland S.E., Bagley S.M., Rodean J., et al. Receipt of timely addiction treatment and association of early medication treatment with retention in care among youths with opioid use disorder. JAMA Pediatr. 2018;172:1029–1037. doi: 10.1001/jamapediatrics.2018.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alinsky R.H., Zima B.T., Rodean J., et al. Receipt of addiction treatment after opioid overdose among medicaid-enrolled adolescents and young adults. JAMA Pediatr. 2020;174 doi: 10.1001/jamapediatrics.2019.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alinsky R.H., Hadland S.E., Matson P.A., et al. Adolescent-serving addiction treatment facilities in the United States and the availability of medications for opioid use disorder. J Adolesc Health. 2020;67:542–549. doi: 10.1016/j.jadohealth.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.The Society for Adolescent Health and Medicine Medication for adolescents and young adults with opioid use disorder. J Adolesc Health. 2021;68:632–636. doi: 10.1016/j.jadohealth.2020.12.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Currie J.M., Schnell M.K., Schwandt H., Zhang J. Prescribing of opioid analgesics and buprenorphine for opioid use disorder during the COVID-19 pandemic + supplemental content. JAMA Netw Open. 2021;4:216147. doi: 10.1001/jamanetworkopen.2021.6147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen T.D., Gupta S., Ziedan E., et al. Assessment of filled buprenorphine prescriptions for opioid use disorder during the coronavirus disease 2019 pandemic. JAMA Intern Med. 2021;181:562–565. doi: 10.1001/jamainternmed.2020.7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hadland S.E., Frank Wharam J.W., Schuster M.A., et al. Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001-2014. JAMA Pediatr. 2017;171:747–755. doi: 10.1001/jamapediatrics.2017.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pew Research Center . Pew Research Center; Washington, DC: 2020. Economic fallout from COVID-19 continues to hit lower-income Americans the hardest. [Google Scholar]
- 12.Rosales R., Janssen T., Yermash J., et al. Persons from racial and ethnic minority groups receiving medication for opioid use disorder experienced increased difficulty accessing harm reduction services during COVID-19. J Subst Abuse Treat. 2022;132:740–5472. doi: 10.1016/j.jsat.2021.108648. [DOI] [PMC free article] [PubMed] [Google Scholar]