Abstract
Aim
There is a high demand for information on COVID-19 vaccination for patients with childhood epilepsy with centrotemporal spikes (BECTS). Patients with this condition need a stable, daily life; unfortunately, the decision of vaccination is not easy for their parents. We evaluated patients with BECTS for symptoms and seizure control after COVID-19 vaccination.
Methods
We asked the caregivers of all patients who visited our hospital to report their vaccination status, and if vaccinated, their experience in terms of adverse effects and seizure control after the second dose of the four Chinese-approved COVID-19 vaccines.
Results
Seventy-seven children had received their second COVID-19 vaccine dose: 58 of 77 (75.3%) received Sinopharm (Beijing): BBIBP-CorV (Vero cells) and 16 (20.8%) received CanSino: Ad5-nCoV. Twenty of seventy-seven (25.97%) patients with BECTS reported having side effects; all effects were mild that could be relieved themselves. For Sinopharm (Beijing): BBIBP-CorV (Vero cells), the most frequent local side effect reported by the parents was pain at the site of injection (17.24%) and systematic side effect was fatigue (15.52%). For CanSino: Ad5-nCoV, the most reported local side effect was pain at the site of injection (6.25%). All parents reported that their child’s side effects could be relieved by themselves. No patient reported status epilepticus or exacerbation of a pre-existing condition. If non-vaccinated, the cause of hesitation was explored: 40% of parents worried about inducing seizures, 19% of parents worried about vaccine side effects, 32% of parents worried about the vaccine-antiepileptic drug interactions, and 9% of parents feared for their child’s physical condition. More than 34.1% of parents accepted that the decision to get the vaccine for their child was difficult. Over 90% of parents believe that research on the safety and tolerability of vaccination would help them to make the decision.
Conclusion
Our data suggest that COVID-19 vaccination is well tolerated and safe in patients below 18 years of age having BECTS, thereby supporting the recommendation of vaccination.
Keywords: Vaccine, COVID-19, Safety, BECTS, Vaccine hesitancy
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has seriously threatened public health and safety with the recent pandemic [1]. So far, immunization has been a functional and effective way to protect human health [2], [3]. As of March 2022, 35 vaccines have been approved [4]. According to an online survey in China, 70.9% of parents are willing to vaccinate their children [5]. Six main vaccines have been approved for use in China, and they are based on different technologies: four inactivated vaccines, one protein submit vaccine, and one replicating viral vector vaccine [6]. Epilepsy is a neurological disorder that manifests as seizures [7]. Some studies have revealed that for adult patients with epilepsy (PwE), the COVID-19 vaccines are safe and tolerable, very few patients reported seizure worsening, and most side effects were mild [8], [9]. The rollout of COVID-19 vaccines has been efficient and quick. Although vaccines have been deemed safe, compared with their healthy peers, patients under 18 years of age with epilepsy need further consideration in terms of vaccine safety [11]. A long-term study showed that vaccination for children diagnosed with epilepsy has positive outcomes; however, this has not been extended to include COVID-19 vaccines [10], [11]. The caregivers of patients under 18 years of age with epilepsy have been hesitant to make the decision to vaccinate them owing to their worries regarding worsening of seizures and other side effects, as it has been difficult to weigh the pros and cons of vaccination [12]. For example, fever, a common side effect of immunization, could trigger a seizure, especially in patients with Dravet syndrome [13]. Benign childhood epilepsy with centrotemporal spikes (BECTS) is one of most common conditions and accounts for 20% of the cases of childhood epilepsies. It usually has a benign prognosis and most patients are able to live and study normally [14], [15]. However, parents who prevent their children from being vaccinated could expose their children to risk. Thus, the help of professionals is required. There has not been much research on the safety and tolerance of the COVID-19 vaccine in children diagnosed with BECTS. In the study, we investigated the safety and tolerability of the COVID-19 vaccine in patients under 18 years of age with BECTS, and we illustrate the causes underlying vaccine hesitancy. We also outline plans and detect the difficulty level of making the decision of vaccination for parents.
2. Material and methods
We designed this study to evaluate the safety and tolerability of the COVID-19 vaccines in patients under 18 with epilepsy. We recruited 120 patients under 18 years of age with BECTS who met the International League Against Epilepsy (ILAE) criteria, and invited their parents to fill the questionnaire when they visited the Department of Neurology, Anhui Provincial Children’s Hospital between October 1, 2021 and October 31, 2021. Three patients were excluded when their parents reported comorbidities. Clinical data were collected using a structured questionnaire.
The questionnaire included information regarding the social background, vaccine type and dose, epilepsy characteristics, possible side effects, and whether the seizures worsened.
We asked the caregivers of the vaccinated patients if their seizures had worsened or if they exhibited side effects. All parents were asked to report side effects that emerged within seven days after vaccination, and seizure worsening within 3 months.
We asked the caregivers of the non-vaccinated patients the reason behind their hesitance and if they had any plans of vaccinating their children.
We asked the caregivers of all patients to scale the level of the difficulty of making the decision to vaccinate their children. We further asked if any research would help them make the decision.
2.1. Questionnaire
The questionnaire was in English and, hence, translated into Chinese. Two independent pediatricians reviewed it and pretested it on two patients.
The following sections were included in the questionnaire:
-
–
Introduction: the purpose of the study
-
–
Informed consent (mandatory)
-
–
Social and demographic information of the caregivers and patients (patients’ age, sex, and educational background and the caregivers’ educational background)
-
–
Epilepsy-related variables (age at onset of epilepsy, seizure type, frequency and duration, medical therapy, comorbidities, history of status epilepticus in the three months preceding the vaccination).
The design of seizure frequency was based on the Revised Seizure-based Outcome Classification System (Duke) with Analysis of Relationship to HRQOL (health-related quality of life). It has three seizure categories: seizure free, ≤10 seizures per year, and >10 seizures per year [16].
-
–
Vaccine-related variables (type, dose, date of the latest dose, general vaccination side effects and their severity, duration of side effects, epilepsy-related vaccination adverse effects, seizure frequency, seizure intensity, and new seizures).
According to a study from the UK, several side effects for vaccines have been listed that can be classified under local and systemic effects. The former includes injection site redness, injection site swelling, and injection site pain, and the latter includes fever, headache, fatigue, chills and shivers, myalgia, nausea/vomiting, arthralgia, diarrhea, decreased appetite, and rash [17].
The grades of adverse effects established by the Food and Drug Administration (FDA) are as follows: Grade 1 (mild), Grade 2 (moderate), Grade 3 (severe), and Grade 4 (serious or life-threatening) [18].
We asked the caregivers of the non-vaccinated patients the reason behind their hesitance and if they had any plans of vaccinating their children.
At the end of the questionnaire, according to the Beck Anxiety Inventory, which is a four-point scale (from 0 to 3), was used and scores were sorted into none, mild, moderate, and severe [19]. The level of difficulty in making the decision for vaccination was graded as none, mild, moderate, and severe. Further, we asked whether this investigation would help them make the decision on vaccination.
2.2. Outcomes
-
1.
The primary outcome was to determine the type and duration of the side effects of the vaccine and post-vaccine risk of epilepsy worsening in patients under 18 years of age.
-
2.
The second outcome was to determine the reasons behind vaccine hesitancy among parents of patients and future plans.
-
3.
The last outcome was to explore whether this research would help parents decide whether to take the vaccine and the level of difficulty in decision-making.
2.3. Statistical analysis
Data were analyzed using IBM SPSS Statistics (Version 26) and Prism (Version 9.3.0). Descriptive statistics were used to show the demographic variables (gender, age, and educational background), epilepsy-related variables (duration time, seizure type and frequency, antiseizure drugs, and history of status epilepticus), COVID-19 vaccine-related variables (number of doses and type of vaccine, injection time), side effects of vaccination (type, duration, severity, and impaction of seizures) through frequencies, percentages, means, and standard deviations. The relationship between side effects and other variables was detected using chi-squared test (X 2), with p ≤ 0.05 considered statistically significant. Pie charts and columns were used to present the findings.
2.4. Results
-
1)
Demographic distribution of vaccinated patients and all patients
A total of 120 participants (77 vaccinated and 43 non-vaccinated) visited the hospital between October 1, 2021 and October 31, 2021. The demographic information of the cohort is shown in Table 1 . Only three parents had comorbidities such as cough, pharyngitis, and nasitis, and were excluded. Only one of them was vaccinated and did not report any side effects. A total of 117 patients were analyzed. The data of vaccinated patients are shown in Table 1; 77 (65.81%) were vaccinated, of which 2 patients (3%) were vaccinated with Anhui Zhifei Longcom ZF2001 vaccine, 58 (75%) with Sinopharm (Beijing): BBIBP-CorV (Vero cells) vaccine, 1 (1%) with Sinopharm (Wuhan) vaccine: inactivated (Vero Cells), and 16 (21%) with Sinovac: CoronaVac: Ad5-nCoV (Fig. 1 ).
-
2)
Comparison of side effects and the duration of Sinopharm (Beijing): BBIBP-CorV (Vero cells) vaccine and CanSino: Ad5-nCoV vaccine are shown in Table 3. A total of 20 (25.97%) patients reported side effects. Twenty parents (25.97%) reported that their children exhibited mild side effects after vaccination, mainly those who were vaccinated with Sinopharm (Beijing): BBIBP-CorV (Vero Cells) and CanSino: Ad5-nCoV. Of the 58 patients vaccinated with Sinopharm (Beijing): BBIBP-CorV (Vero Cells); for local side effects: pain at the injection site (17.24%), redness at the injection site (3.45%), swelling at the injection site (3.45%) and systematic side effects: headache (3.45%), dizziness (1.72%), fatigue (15.52%), nausea/vomiting (1.72%), and decreased appetite (13.79%). Of the 16 of 77 (20.78%) patients vaccinated with CanSino: Ad5-nCoV, only one (6.25%) reported pain at the injection site; as for systematic side effects, the following was observed: headache (6.25%), dizziness (12.50%), fatigue (12.50%), and decreased appetite (12.50%). As seen in Table 2 , age (T = −2.05), education of the patients (X 2 = 10.743), and the vaccine type (X 2 = 27.156) were significant factors influencing patient-related side effects (p < 0.05).
Table 1.
Demographic distribution of vaccinated patients and all patients.
| Variables | Anhui Zhifei Longcom | Sinopharm (Beijing): BBIBP-CorV (Vero Cells) Second dose N = 58 (75.3) | Sinopharm (Wuhan): Inactivated (Vero Cells) Second dose N = 1 (1.2) | Sinovac: CoronaVac: Ad5-nCoV two doses N = 16 (20.8) | All vaccinated patients | All participants | |
|---|---|---|---|---|---|---|---|
| ZF2001 Second dose N = 2 (2.6) | N = 77 | N = 117 | |||||
| Gender (N/%) | |||||||
| male | 0 | 27 (35) | 1 (1.2) | 7 (9) | 35 (45.5) | 57(48) | |
| female | 2 (2.6) | 31 (40.2) | 0 | 9 (11.7) | 42 (54.5) | 60(52) | |
| Age | |||||||
| mean/(SD) | 14.5 (0.7) | 15.01(1.52) | 14 | 14.63(1.71) | 14.91 (1.50) | 13.214 (3.03) | |
| Educational background | |||||||
| Primary school | 34 (29) | ||||||
| Junior high school | 2 (2.6) | 18 (23.4) | 1(1.3) | 7 (9) | 28 (36.4) | 33 (28) | |
| Senior high school | 33 (42.9) | 8 (10.4) | 41 (53.2) | 42 (36) | |||
| Undergraduate | 7 (9) | 1 (1.3) | 8 (10.4) | 8 (7) | |||
| Epilepsy characteristics of vaccinated patients | |||||||
| Epilepsy duration years Mean(SD) | 1.55 (2.05) | 5.79 (3.11) | 5 | 6.88 (2.68) | 7.59 (2.67) | 5.9(3.50) | |
| Number of antiepileptic drugs | |||||||
| <2 | 1 (1.30) | 44 (57.14) | 0 (0) | 10 (12.99) | 55 (71.43) | 86 (73.50) | |
| >=2 | 1 (1.30) | 14 (18.18) | 1 (1.30) | 6 (7.79) | 22 (28.57) | 31 (26.50) | |
| Type of seizures | |||||||
| Tonic-clonic seizures | 1 (1.30) | 6 (7.79) | 0 (0) | 5 (6.50) | 12 (15.58) | 22 (18.8) | |
| Focal seizures | 1 (1.30) | 5 (6.49) | 1 (1.30) | 4 (5.19) | 11 (14.29) | 24 (20.5) | |
| Focal to bilateral seizures | 0 (0) | 45 (58.44) | 0 (0) | 4 (5.19) | 49 (63.64) | 51 (43.6) | |
| Others | 0 | 0 | 0 | 0 | 9 (7.7) | ||
| Unknown | 0 (0) | 2 (2.60) | 0 (0) | 3 (3.90) | 5 (5.19) | 11 (9.4) | |
| Seizure frequency | |||||||
| None | 1 (1.30) | 4 (5.19) | 1 (1.30) | 6 (7.79) | 12 (15.58) | 34 (29) | |
| Low frequency | 1 (1.30) | 51 (66.23) | 0 (0) | 10 (12.99) | 62 (80.52) | 77 (66) | |
| High frequency | 0 (0) | 3 (3.90) | 0 (0) | 0 (0) | 3 (3.90) | 6 (5) | |
| History of status epilepticus | |||||||
| Yes | 1 (1.30) | 0 (0) | 0 | 2 (2.60) | 3 (3.90) | 4(3.40) | |
| No | 1 (1.30) | 58 (75.32) | 1 (1.30) | 14 (18.18) | 74 (96.10) | 113(96.58) | |
Fig. 1.
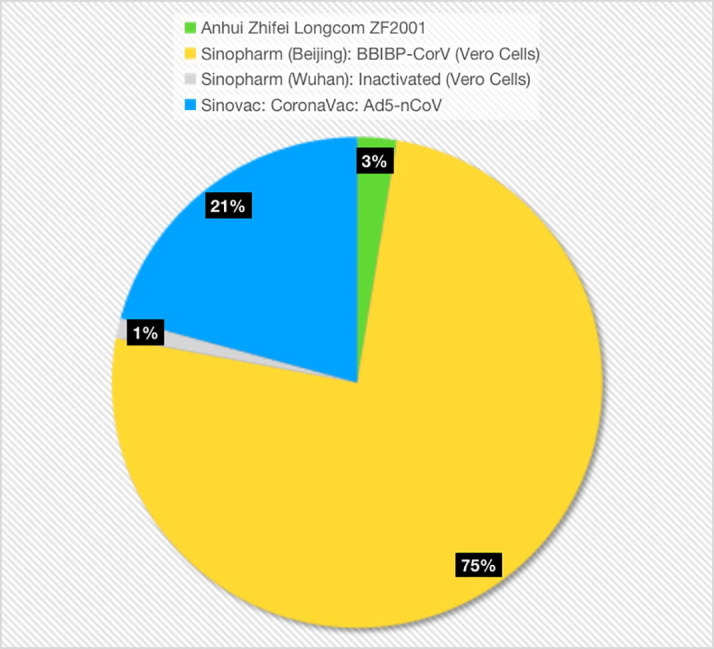
Type of vaccines; N = 77.
Table 3.
Comparison of the side effects and their duration of the Sinopharm (Beijing): BBIBP-CorV (Vero cells) and CanSino: Ad5-nCoV vaccines.
| Sinopharm (Beijing): BBIBP-CorV (Vero Cells) | CanSino: Ad5-nCoV | χ2 | p | Overall | |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 5 (33.3%) | 3 (60%) | 8 | ||
| Male | 10 (66.7%) | 2 (40%) | 12 | ||
| Age | 14.47 (1.46/12–16) | 14.80 (1.79/12–17) | 14.55 (1.50/12–17) | ||
| Local Side effects | |||||
| Pain at the injection site N (%)/duration of relief range | 10 (23%/6 ± 2.83 1–10) | 1 (2.33%/3) | 1.197 | 0.274 | 11 |
| Redness at the injection site | 2 (4.65%/4 ± 4.24 1–7) | 0 | 0.567 | 0.451 | 2 |
| Swelling at the injection site | 2 (4.65%/2 ± 1.41 1–3) | 0 | 0.567 | 0.451 | 2 |
| Systemic side effects | |||||
| Headache | 2 (4.65%/8 ± 2.83 6–10) | 1 (2.33%/5) | 0.253 | 0.615 | 3 |
| Dizziness | 1 (2.33%/10) | 2 (4.65%/5) | 3.744 | 0.053 | 3 |
| Fatigue | 9 (20.93%/6.11 ± 1.96 3–10) | 2 (4.65%/3 ± 2.83 1–5) | 0.09 | 0.764 | 11 |
| Nausea/vomiting | 1 (2.33%/7) | 0 | 0.28 | 0.597 | 1 |
| Decreased appetite | 8 (18.60%/6.13 + -2.10 3–10) | 2 (4.65%/5) | 0.018 | 0.893 | 10 |
Table 2.
Characteristics of patients who exhibited vaccine-related side effects.
| Variable | Side effect (N = 20) | Test of significance | P value |
|---|---|---|---|
| Age | T = −2.05 | 0.045 | |
| Mean ± SD | 14.55 ± 1.50 | ||
| Median (min ∼ max) | 15 (12–17) | ||
| Gender | X2 = 1.594 | 0.207 | |
| Male | 12 (60%) | ||
| Female | 8 (40%) | ||
| Children education | X2 = 10.743 | 0.010 | |
| junior high school | 10 (50%) | ||
| senior high school | 9 (45%) | ||
| undergraduate | 1 (5%) | ||
| The form of seizure | X2 = 0.703 | 0.945 | |
| Generalized seizures | 3 (15%) | ||
| Focal seizures | 3 (15%) | ||
| Generalized-focal seizures | 12 (60%) | ||
| Unknown | 2 (10%) | ||
| The time child was diagnosed with epilepsy (Months) | T = 0.900 | 0.371 | |
| Mean ± SD | 79.8 ± 34.882 | ||
| Median (min ∼ max) (Months) | 90 (0–132) | ||
| The vaccine type | X2 = 27.156 | <0.001 | |
| Sinopharm (Beijing): BBIBP-CorV (Vero Cells) two doses | 15 (75%) | ||
| CanSino: Ad5-nCoV two doses | 5 (25%) | ||
| Seizure frequency | X2 = 13.274 | 0.004 | |
| None | 4 (20%) | ||
| low frequency | 15 (75%) | ||
| high frequency | 1 (5%) | ||
Logistic regression did not detect any significant results in the relationship between side effects and other variables, such as age, gender, symptom of epilepsy, and children’s education.
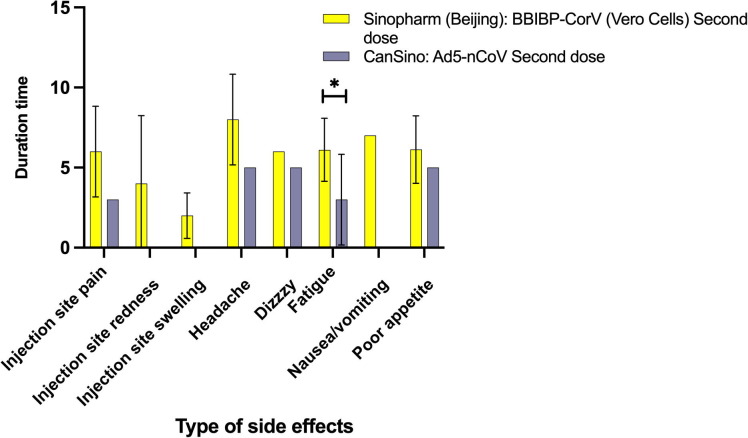
For data comparing the side effects of the second dose of the two main vaccines, Sinopharm (Beijing): BBIBP-CorV (Vero Cells) and CanSino: Ad5-nCoV, please see Table 3. In terms of duration, fatigue (p = 0.0878) was significantly different between the two vaccines (Fig. 2 and Table 3 ).
Fig. 2.
Duration of side effects with two vaccines; N = 45.
Two vaccines were compared for the duration of side effects. For children vaccinated with Sinopharm (Beijing): BBIBP-CorV (Vero Cells) second dose, the local side effects were as follows: pain at the injection site, 6 ± 2.83; redness at the injection site, 4 ± 4.24; and swelling at the injection site, 2 ± 1.41. The systemic side effects lasted as follows: headache, 8 ± 2.83; dizziness, 10; nausea, 7; and decreased appetite, 6.13 ± 2.10. For children vaccinated with CanSino: Ad5-nCoV second dose, the local side effect, pain at the injection site lasted, 3; as for systemic side effects, the duration were as follows: headache, 5; dizziness, 5; and decreased appetite, 2.
For epilepsy-related problems, only one caregiver reported a seizure longer than 5 min within 3 months after the second dose of CanSino: Ad5-nCoV.
-
3)
Non-vaccinated group (N = 43)
There are several reasons for vaccine hesitation. Forty percent of parents worried about the induction of seizures, 19% of parents worried about the side effects of the vaccine, 32% of parents worried about the interaction with antiepileptic drugs, and 9% of parents feared that the child’s physical condition was not suitable for vaccination.
There are future plans for non-vaccinated patients; 32% are on the waiting list, 49% of parents are undecided, and 19% have chosen to forego the vaccine. The X 2 test (father’s education, P = 0.311; mother’s education, P = 0.23) did not find any difference in the choice to vaccinate in terms of the parents’ educational background.
-
4)
For all participants (N = 117)
The level of difficulty in making the decision of vaccinating their children was determined. We found that 51.20% parents reported slight difficulty, and 34.1% parents reported moderate (20.5%) and severe (13.6%) difficulties. About 90.5% of parents agreed that the research would help them make the decision.
3. Ethical
Ethical approval was obtained from the research committee at Anhui Provincial Children’s Hospital, China.
4. Discussion
4.1. Side effects
The study recorded details of the second dose of four vaccines: Anhui Zhifei Longcom ZF2001; Sinopharm (Beijing): BBIBPCorV (Vero Cells); Sinopharm (Wuhan): Inactivated (Vero Cells); Sinovac: CoronaVac: Ad5-nCoV. Compared with studies that involved adults [8], [9], the results of this study showed that after immunization, the risk of seizure worsening on getting the two main vaccines is very low, proving their safety in patients under 18 with BECTS.
A total of 20 parents (25.97%) reported that their children exhibited mild side effects after the second dose of the Sinopharm (Beijing): BBIBP-CorV (Vero Cells) and CanSino: Ad5-nCoV vaccines. Out of the 58 patients vaccinated with the Sinopharm (Beijing): BBIBP-CorV (Vero Cells) vaccine, 15 (25.86%) reported side effects.The most frequent local side effect is pain at the injection site (17.24%), consistent with a study in Iran (26.9%) [20]. In terms of systematic side effects, in our study, the most frequently reported side effect is fatigue (15.52%),none of the parents reported fever after vaccination, whereas, in the study of patients with malignancy in Iran, the most frequently reported side effects in adults was fever (31.6%) and fatigue (21.6%) [20]. In clinical trials involving patients under 18 years (13–17 years old), the most common local side effect was also pain at the injection site (7.9%) and systematic side effect was fever (9.5%) [21]. This suggests that the side effects of the vaccine in children with epilepsy are not very different from those in the general population, and even in this study, no children were reported to have fever. Of the 16 of 77 (20.78%) patients vaccinated with CanSino: Ad5-nCoV vaccine, among local side effects, only one (6.25%) parent reported pain at the injection site, and as for systematic side effects, they reported headache (6.25%), dizziness (12.50%), fatigue (12.50%), and decreased appetite (12.50%). These parameters were lower than those reported by a study in Mexico, wherein pain at the injection site (48.1%), redness at the injection site (3.7%), swelling (3.7%), fatigue (50%), fever (16.7%), and headache (25.9%) were the presentations [22]. In clinical trials involving adults, the most reported local side effect was pain at the injection site (54%), and the most reported systemic side effects were fever (46%), fatigue (44%), headache (39%), and muscle pain (17%). In our study, the percentage of reported side effects was also lower than that reported in previous human trial [23]. For patients under 18 years with BECTS, the rate of parent-reported side effects was lower than that reported in previous studies. All reported mild side effects that could be alleviated by resting, which is also better than those reported by some previous studies [8], [9]. Shorter durations and milder symptoms have been reported. It indicates that in the currently approved vaccinations for Covid-19, parental concerns about side effects in children based on the child's own underlying disease are not warranted, as there has been no unexpected side effects and the occurrence rate is low.
Duration of fatigue (p = 0.0878) in patients was significantly different between the two vaccines. The duration fatigue after administering the second dose of CanSino: Ad5-nCoV (3 + −2.83) was significantly shorter than that on administering the second dose of Sinopharm (Beijing): BBIBP-CorV (Vero Cells) (6.11 + −1.96). Logistic regression showed no significant correlation between variables and whether side effects occurred. In a study by German researchers [9], it was found that the risk of experiencing general side effects was correlated to sex, age, early onset of epilepsy, and psychiatric comorbidities. It may be a coincidence, and thus further studies are required to confirm these conclusions.
Vaccination-associated seizures are an important factor affecting decision-making regarding vaccination [11].We collected data of 77 vaccinated patients under 18 years of age with BECTS; the frequency of seizures and the occurrence of status epilepticus (SE) in patients were determined: 12 of them did not have a seizure, 62 suffered low-frequency seizures, (≤10 seizures per year), three suffered high-frequency seizures (>10 seizures per year), and three suffered seizures that lasted for over 5 mins during the past year. However, none reported increased frequency and duration of new seizures or the appearance of new forms of seizures after receiving the Covid-19 vaccine. Only one patient suffered a seizure that lasted longer than 5 min within 3 months after taking the second dose of CanSino: Ad5-nCoV. Patient characteristics are as follows: 14-year-old female, seizure type was focal seizure, and she was taking an antiepileptic drug. She did not suffer seizures in the past year before vaccination. However, we learned from the patient’s caregivers that she was not taking the medication regularly in recent days, although she was taking medications for over a year as prescribed. There is no evidence that post-vaccination seizures in children with epilepsy who are not taking their medication are related to vaccination. Previous studies have not found neurological side effects in adult patients with multiple sclerosis after BBIBP-CorV (Sinopharm) COVID-19 vaccination; to be specific, the risk of seizure is low [24].
4.2. Future plan for non-vaccinated children
In terms of permitting vaccination for children, we found that the decision to vaccinate was not influenced by the parents’ or caregivers’ educational background compared with that in previous research [25]. Among parents of children with epilepsy, the decision to have their children vaccinated is a matter of weighing the pros and cons, and professional advice may more likely influence their choice versus their level of education.
4.3. Cause of hesitation
Reasons for vaccine refusal have been listed in the questionnaire. According to some previous studies [8], [9], [17], we let parents tick and fill in the blanks in the questionnaire. In the responses, 40% of parents exhibited concerns about the induction of seizures, 19% of parents were worried about vaccine side effects, 32% of parents were worried about antiepileptic drug-vaccine interactions, and 9% of parents feared for their children’s physical conditions, such as having recent seizures, which was consistent with previous research. The most common hesitation identified in the study was induction of seizures. Moreover, PwE likely refuse vaccination and ask for help from professionals due to their specific conditions [26].
Further, a parent worried that the vaccine might influence child development. To solve this problem, long-term research for children is needed. One parent worried that their child would be discriminated against for being unvaccinated. For children with BECTS and their parents, their illnesses brought a lot of pressure. A study conducted in Southern China revealed that parents of children with epilepsy showed severe symptoms of anxiety and depression [27]. As parents, they fear the risks associated with vaccines, such as side effects or seizure worsening, while at the same time wanting their children to be integrated into normal society and to be immunized against risky and prevalent diseases [28]. Approximately 34.1% of parents believed that their decision-making was moderately or severely influenced by the disorder, and they needed consultation with a professional to decide. At the end of the questionnaire, we asked parents whether this research would help them make the decision to vaccinate, and 90.5% of parents chose “yes.” Further, several parents wrote that they were waiting for more research on the safety and tolerability of vaccination for patients under 18 years of age with BECTS.
5. Limitations
-
1.
Since parents were reporting their children’s side effects, there may have been recall bias. A specialized and targeted questionnaire needs to be designed that can be completed by a competent research group.
-
2.
The sample size was moderate, and the participants were older than 12 years. More research is needed after vaccinating children under 12 years of age for comparison studies.
-
3.
This questionnaire was retrospective and based on parents’ subjective recollection without objective laboratory tests and other indicators. We can collect and analyze blood specimens, immunological data, or EEGs of participants in future studies.
-
4.
We intend to evaluate special patient groups to detect the safety and tolerability of vaccination. For example, in patients with myoclonic epilepsy, such as Dravet syndrome [29], who easily manifest seizures after a fever, are at high risk, it is meaningful to detect the safety and tolerability of the vaccine [30]. In the future, subtypes of epilepsy can be evaluated and more subjective variables can be used to determine the influence.
6. Conclusions
The COVID-19 vaccine for patients under 18 years of age with benign childhood epilepsy with centrotemporal spikes is safe and tolerable. The side effects were mild, could be relieved within 10 days, and the risk of seizure worsening was minimal. Research on vaccine safety would help patients and their caretakers decide whether the vaccine should be administered to their children.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgments
Acknowledgements
The support of the Department of Neurology, Anhui Provincial Children’s Hospital, Hefei, China, is gratefully acknowledged.
Conflicts of interest
The authors declare that they have no conflicts of interest in connection with this work.
References
- 1.Yang Y., Peng F., Wang R., Guan K., Jiang T., Xu G., et al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold M., Dugdale S., Woodman R.J., Mccaul K.A. Use of the Australian Childhood Immunisation Register for vaccine safety data linkage. Vaccine. 2010;28:4308–4311. doi: 10.1016/j.vaccine.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 3.Gostin L.O., Salmon D.A., Larson H.J. Mandating COVID-19 vaccines. JAMA. 2021;325:532. doi: 10.1001/jama.2020.26553. [DOI] [PubMed] [Google Scholar]
- 4.COVID19 Vaccine Tracker. COVID. https://covid19.trackvaccines.org/; 2022 [accessed March 9 2022].
- 5.Yang J., Zhang T., Qi W., Zhang X., Jia M., Leng Z., et al. COVID-19 vaccination in Chinese children: a cross-sectional study on the cognition, psychological anxiety state and the willingness toward vaccination. Human Vacc Immunother. 2022;18(1):1–7. doi: 10.1080/21645515.2021.1949950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.China. COVID. https://covid19.trackvaccines.org/country/china/; 2021 [accessed December 13 2021].
- 7.Pruna D., Balestri P., Zamponi N., Grosso S., Gobbi G., Romeo A., et al. Epilepsy and vaccinations: Italian guidelines. Epilepsia. 2013;54:13–22. doi: 10.1111/epi.12306. [DOI] [PubMed] [Google Scholar]
- 8.Massoud F., Ahmad S.F., Hassan A.M., Alexander K., Al-Hashel J., Arabi M. Safety and tolerability of the novel 2019 coronavirus disease (COVID-19) vaccines among people with epilepsy (PwE): A cross-sectional study. Seizure. 2021;92:2–9. doi: 10.1016/j.seizure.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wrede R.V., Pukropski J., Moskau-Hartmann S., Surges R., Baumgartner T. COVID-19 vaccination in patients with epilepsy: First experiences in a German tertiary epilepsy center. Epilepsy Behav. 2021;122 doi: 10.1016/j.yebeh.2021.108160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zou X., Cao B. COVID-19 vaccines for children younger than 12 years: are we ready? Lancet Infect Dis. 2021;21:1614–1615. doi: 10.1016/s1473-3099(21)00384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Spiczak S., Helbig I., Drechsel-Baeuerle U., Muhle H., van Baalen A., van Kempen M.J., et al. A retrospective population-based study on seizures related to childhood vaccination. Epilepsia. 2011;52(8):1506–1512. doi: 10.1111/j.1528-1167.2011.03134.x. [DOI] [PubMed] [Google Scholar]
- 12.Righolt C.H., Pabla G., Donelle J., Brna P., Deeks S.L., Wilson S.E., et al. Vaccine coverage among children with epilepsy in two Canadian provinces: A Canadian immunization research network study. Vaccine. 2021;39(15):2117–2123. doi: 10.1016/j.vaccine.2021.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Top K.A., Brna P., Ye L., Smith B. Risk of seizures after immunization in children with epilepsy: A risk interval analysis. BMC Pediatr. 2018;18 doi: 10.1186/s12887-018-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guerrini R, Pellacani S, Benign childhood focal epilepsies. Epilepsia, 2012;53,9–18. https://doi.org/10.1111/j.1528-1167.2012.03609. [DOI] [PubMed]
- 15.Verrotti A., Casciato S., Spalice A., Carotenuto M., Striano P., Parisi P., et al. Coexistence of childhood absence epilepsy and benign epilepsy with centrotemporal spikes: A case series. Eur J Paediatr Neurol. 2017;21(3):570–575. doi: 10.1016/j.ejpn.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Vickrey B.G., Hays R.D., Engel J., Spritzer K., Rogers W.H., Rausch R., et al. Outcome assessment for epilepsy surgery: The impact of measuring health-related quality of life. Ann Neurol. 1995;37(2):158–166. doi: 10.1002/ana.410370205. [DOI] [PubMed] [Google Scholar]
- 17.Menni C., Klaser K., May A., Polidori L., Capdevila J., Louca P., et al. Vaccine side-effects and SARS-COV-2 infection after vaccination in users of the COVID symptom study app in the UK: A prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/s1473-3099(21)00224-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norquist J.M., Khawaja S.S., Kurian C., Mast T.C., Liaw K.-L., Robertson M.N., et al. Adaptation of a previously validated vaccination report card for use in adult vaccine clinical trials to align with the 2007 FDA toxicity grading scale guidance. Hum Vacc Immunother. 2012;8(9):1208–1212. doi: 10.4161/hv.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fydrich T., Dowdall D., Chambless D.L. Reliability and validity of the Beck Anxiety Inventory. J Anxiety Disord. 1992;6:55–61. doi: 10.1016/0887-6185(92)90026-4. [DOI] [Google Scholar]
- 20.Ariamanesh M, Porouhan P, PeyroShabany B, Fazilat-Panah D, Dehghani M, Nabavifard M, et al. Immunogenicity and safety of the inactivated SARS-COV-2 vaccine (BBIBP-CorV) in patients with malignancy. Cancer Invest 2021. https://doi.org/10.1101/2021.09.02.21262760. [DOI] [PMC free article] [PubMed]
- 21.Xia S.L., Zhang Y.T., Wang Y.X., Wang H., Yang Y.K., Gao G.F., et al. Safety and immunogenicity of an inactivated COVID-19 vaccine, BBIBP-CorV, in people younger than 18 years: A randomised, double-blind, controlled, phase 1/2 trial. Lancet Infect Dis. 2022;22(2):196–208. doi: 10.1016/s1473-3099(21)00462-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guzmán-Martínez O., Guardado K., de Guevara E.L., Navarro S., Hernández C., Zenteno-Cuevas R., et al. IGG antibodies generation and side effects caused by ad5-ncov vaccine (Cansino Biologics) and BNT162B2 vaccine (Pfizer/BioNTech) among Mexican population. Vaccines. 2021;9(9):999. doi: 10.3390/vaccines9090999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu F.-C., Li Y.-H., Guan X.-H., Hou L.-H., Wang W.-J., Li J.-X., et al. Safety, tolerability, and immunogenicity of a recombinant adenovirus type-5 vectored COVID-19 vaccine: A dose-escalation, open-label, non-randomised, first-in-human trial. The Lancet. 2020;395:1845–1854. doi: 10.1016/s0140-6736(20)31208-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Etemadifar M., Abhari A.P., Nouri H., Sigari A.A., Piran Daliyeh S.M., Maracy M.R., et al. Self-reported safety of the BBIBP-corv (Sinopharm) COVID-19 vaccine among Iranian people with multiple sclerosis. Hum Vacc Immunother. 2021 doi: 10.1101/2021.10.17.21265114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldman R.D., Staubli G., Cotanda C.P., Brown J.C., Hoeffe J., Seiler M., et al. Factors associated with parents’ willingness to enroll their children in trials for covid-19 vaccination. Hum Vacc Immunother. 2021;17:1607–1611. doi: 10.1080/21645515.2020.1834325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asadi-Pooya A.A., Barzegar Z., Sadeghian S., Nezafat A., Shahisavandi M., Nabavizadeh S.A. Covid-19 vaccine hesitancy among patients with epilepsy or other chronic conditions. Disaster Med Public. 2021:1–3. doi: 10.1017/dmp.2021.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang H., Feng Y., Zhu Z., Qiao Z., Xiao B., Feng L. Evaluation of anxiety, depression, and sleep quality among parents of children with epilepsy in southern China. Epilepsy Behav. 2020;112 doi: 10.1016/j.yebeh.2020.107340. [DOI] [PubMed] [Google Scholar]
- 28.Top K.A., Righolt C.H., Hawken S., Donelle J., Pabla G., Brna P., et al. Adverse events following immunization among children with epilepsy. Pediatr Infect Dis J. 2020;39(5):454–459. doi: 10.1097/inf.0000000000002553. [DOI] [PubMed] [Google Scholar]
- 29.Verbeek N.E., van der Maas N.A.T., Sonsma A.C.M., Ippel E., Vermeer-de Bondt P.E., Hagebeuk E., et al. Effect of vaccinations on seizure risk and disease course in Dravet syndrome. Neurology. 2015;85(7):596–603. doi: 10.1212/wnl.0000000000001855. [DOI] [PubMed] [Google Scholar]
- 30.Tro-Baumann B., von Spiczak S., Lotte J., Bast T., Haberlandt E., Sassen R., et al. A retrospective study of the relation between vaccination and occurrence of seizures in Dravet syndrome. Epilepsia. 2011;52(1):175–178. doi: 10.1111/j.1528-1167.2010.02885.x. [DOI] [PubMed] [Google Scholar]