Abstract
Tumor-infiltrating lymphocytes (TILs) and programmed cell death 1 ligand 1 (PD-L1) are established prognostic and predictive biomarkers for certain breast cancer subsets. However, their association with the immune response complexity is not fully understood. Therefore, we analyzed the association between the immune cell fractions in breast cancer tissues and histologically assessed TIL (hTIL) and PD-L1 (hPD-L1). Forty-five tumor and eighteen blood samples were collected from patients with breast cancer. Total leukocyte counts, frequency of 11 immune cell populations, and PD-L1 expression in each cell fraction were evaluated by flow cytometry. TILs and PD-L1 were assessed by hematoxylin and eosin staining and immunohistochemistry, respectively. A higher hTIL score showed association with increased leukocyte infiltration, higher CD4+ and CD8+ T cell proportions, and lower natural killer and natural killer T cell proportions. PD-L1 was highly expressed in nonclassical monocytes, monocyte/macrophages, myeloid-derived suppressor cells, myeloid dendritic cells, dendritic cells, and other lineages in tumors. hPD-L1 positivity reflected PD-L1 expression accurately in these fractions, as well as increased leukocyte infiltration in tumors. These results indicate that hTILs reflect differences in the immune responses in the tumor microenvironment, and certain immune cell fractions are favorably expressed in the PD-L1 pathway in breast cancer microenvironments.
Subject terms: Cancer, Immunology, Biomarkers, Oncology
Introduction
Breast cancer is the most common malignancy among women worldwide. Although progress has been made regarding multimodal treatment comprising surgery, systemic therapy, and radiation therapy, the cure of advanced and recurrent diseases continues to be difficult1. The recent success in the clinical use of immune checkpoint inhibitors for multiple cancers has attracted attention in tumor immunology, and an improved understanding of tumor immunology may inform the development of new treatment strategies or effective use of existing therapies2.
Histologically assessed tumor-infiltrating lymphocytes (hTILs) can provide prognostic information for diverse solid tumor types and may be of value in predicting response to treatment3. In breast cancer, amid some controversy, hTIL has been detected more frequently in the triple-negative subtype or human epidermal growth factor receptor-2 (HER-2)-positive subtype than in the luminal subtype3 and is correlated with clinicopathological factors other than subtypes4,5. hTIL is associated with the prognosis of disease-free and overall survival in triple-negative and HER-2-positive subtypes4,6. Additionally, hTIL has been proposed as a predictor of response to neoadjuvant chemotherapy in all molecular subtypes7. Programmed cell death 1 ligand 1 (PD-L1) is expressed in immune cells, including T cells, B cells, macrophages, monocytes, and dendritic cells, as well as in tumor cells. PD-L1 can bind to the programmed cell death protein 1 (PD-1) expressed on activated T cells. Interactions with PD-L1 enable PD-1 signaling to counter the activation of T cells during the effector phase of the immune response8,9. Histologically assessed PD-L1 (hPD-L1) is expressed in HER-2 and triple-negative subtypes more frequently than in luminal subtypes and is correlated with poor prognoses, high histological grades, and lymphatic vessel invasions10. hPD-L1 has been established clinically as a predictor of atezolizumab efficacy in triple-negative advanced breast cancer11. Recently, different types of immune cell subsets evaluated primarily via immunohistochemistry (IHC) have been found to be associated with various clinicopathological factors or prognoses. These evaluations indicate their clinical significance in breast cancer12–28 and are summarized in Supplementary Table S1.
Therefore, hTIL and hPD-L1 have been established as biomarkers for breast cancer; recent studies have shifted their attention to the various immune cell subsets that make up hTILs. However, the complexity of the multiple types of immune cells in TIL or PD-L1-expressing cells is not yet fully understood because of the technical difficulties in detecting multiple types of immune cells in the tissues using conventional IHC. Therefore, we first used multicolor flow cytometry (FCM) to assess the multiple immune cell fractions in breast cancer tissue and blood. We then analyzed the association between hTIL and hPD-L1. By performing a systematic analysis of the immune cell composition, we aimed to show that specific immunological profiles of the breast cancer microenvironment were represented histologically by hTIL and hPD-L1.
Methods
Patients
Forty-seven tumor samples from the primary site and 19 matched blood samples were obtained from patients with breast cancer regardless of clinicopathological factors or treatment histories, except for patients with distant metastases or complete clinical responses to neoadjuvant chemotherapy. No patient in this study received irradiation or endocrine therapy before surgery. Following an amendment of the study protocol in July 2016, blood sample collection commenced; therefore, there were cases with no blood samples. Clinicopathological data, including menopausal statuses, histories of preoperative chemotherapy, histological types, invasive tumor sizes, lymph node statuses, lymphatic involvement, vascular involvement, histological grades, estrogen receptor (ER) statuses, progesterone receptor (PgR) statuses, HER-2 statuses, and Ki67 labeling indexes, were collected by reviewing case records. Histological grades were evaluated according to the method described by Robbins et al.29. ER, PgR, and HER-2 statuses were evaluated using IHC staining. The cutoff value for ER and PgR positivity was set at ≥ 10%30. The HER-2 status was determined according to the ASCO CAP guideline 201331. For reasons mentioned later, 45 tumor samples and 18 blood samples were included in the analysis. The clinicopathological characteristics of the entire cohort and the cohort with blood samples are presented in Supplementary Tables S2 and S3, respectively.
Tumor-infiltrating lymphocyte/peripheral blood mononuclear cell (PBMC) preparation
Tumor and blood samples were collected simultaneously during surgery. Tumor samples were mechanically dissociated on ice with 10% fetal bovine serum-phosphate-buffered saline (FBS-PBS), filtered using a 70-micron strainer, and washed with 10% FBS-PBS. All blood samples were collected using a collection tube containing ethylenediaminetetraacetic acid-2Na. For both the tumor and blood samples, mononuclear cell components were separated using density-gradient centrifugation with a Ficoll-Paque PLUS (Cytiva Inc., Tokyo, Japan) according to the manufacturer’s instructions. Then, they were suspended in a CELLBANKER I (Takara Bio Inc. Shiga, Japan) and stored in liquid nitrogen.
Flow cytometry (FCM) analysis
The cryopreserved TILs and PBMCs were thawed and washed with 10% FBS-PBS. The cell suspensions were then processed for surface staining with an antibody cocktail (Supplementary Table S4) for 20 min at 4 °C. The cells were washed with PBS containing 2% FBS and resuspended in CellFix (BD Biosciences, Franklin Lakes, NJ, USA). The stained cells were detected using an LSR II Fortessa with FACS Diva software (BD Biosciences). All analyses were performed using FlowJo software (BD Biosciences). The gating strategy is shown in Supplementary Fig. S1. Immune cell fractions were classified into the following according to the definitions shown in Supplementary Table S5: leukocytes, total T cells (Total T), CD4+ T cells (CD4+ T), CD8+ T cells (CD8+T), B cells (B), monocytes/macrophages (Mo/Mφ), nonclassical monocytes (CD16+ Mo), myeloid-derived suppressor cells (MDSCs), dendritic cells (DCs), myeloid dendritic cells (mDCs), natural killer (NK), minor NK cells, and natural killer T cells (NKT). Two authors (TH and MY) verified the raw data independently to exclude input errors. Two cases with a low number of living cells (count < 1000) in the FCM analysis of the tumor tissue were excluded. A blood sample that was identical to one of the two cases was also excluded. The leukocyte density, based on the weight of the tissue fragment and number of viable CD45+ cells, was determined using a previously described method32. For the immune cell fraction, we determined both the percentages of each fraction in the leukocytes (% in leukocytes) and densities based on the weights of the tissue fragments (count/g).
Histological evaluation of tumor immunity-related biomarkers
We evaluated the histological tumor immunity-related biomarkers, as described previously33–36. Briefly, the percentages of stromal TILs were evaluated using 4-µm sections from formalin-fixed specimens stained with hematoxylin and eosin using a light microscope at × 200–400 magnification. Stromal TILs were defined as mononuclear cells localized in the stromal tissue of breast cancer. The stromal TIL count was categorized, according to the International TILs Working Group guideline, into three grades: low (0–10%), intermediate (10–40%), and high (40–90%), and scored from 0 to 2. The denominator used to determine the TIL grade was the stromal tissue area. PD-L1 expression was assessed using IHC with rabbit monoclonal anti-PD-L1 clone SP142 (prediluted; Spring Bioscience, Pleasanton, CA, USA) and the Ventana Benchmark ULTRA auto staining machine. Tumors with ≥ 1% immune cells with cytoplasmic and/or membrane PD-L1 staining were determined to be PD-L1 positive. Stromal TIL counts and PD-L1 expression were evaluated by two evaluators (CH and SK). The histologically assessed TIL and PD-L1 were described as hTIL and hPD-L1, respectively, to distinguish them from the FCM-assessed data. In one case, the hTIL and hPD-L1 could not be evaluated because the remaining tumor tissue was insufficient. Therefore, hTIL and hPD-L1 were analyzed in the remaining 44 patients.
Statistical analyses
All statistical analyses and graph drawings were performed using GraphPad Prism ver. 9.1.0 software. The normality of the FCM data was tested using the D'Agostino–Pearson normality test. The correlation analyses between groups were performed using Spearman’s rank correlation coefficient. For the comparison of the paired samples of the tumor and blood, the Wilcoxon test was used. For the comparison of the two unpaired groups, the Mann–Whitney U test was used. A Chi-square test was used to compare clinicopathological factors of the hTILs and hPD-L1. A Fisher’s exact test was used when the Chi-square test indicated a significant P-value (P < 0.05) and there were cells with a sample size of ≤ 5.
Ethical approval and participant consent
This study was conducted at Tokai University Hospital and approved by the Ethics Committee, which conforms to the provisions of the Declaration of Helsinki (Accepted project No. 16R-279). The patients were enrolled from May 2015 to April 2019, and written informed consent was obtained from all patients.
Results
Distribution of leukocyte and immune cell fraction determined by FCM
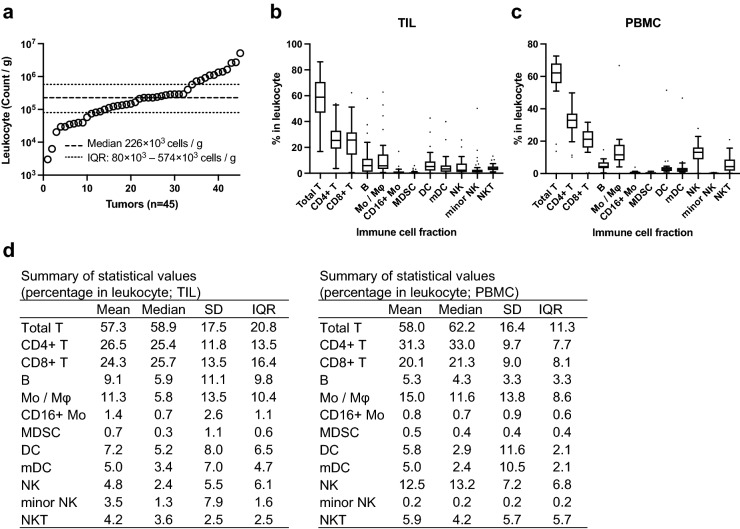
Leukocyte densities based on the weights of the tissue fragments and the number of viable CD45+ cells were determined in the whole cohort (n = 45). The mean density and interquartile range of the tumor-infiltrating leukocytes were 226 × 103 cells/g and 80 × 103 to 574 × 103 cells/g, respectively (Fig. 1a). For the immune cell fraction in the leukocytes of the tumor tissues (TILs), the main population comprised CD8+ T, CD4+ T, Mo/Mφ, and B cells (Fig. 1b, d). A similar trend was observed in the leukocyte composition in the blood (PBMC), showing that the main population comprised CD8+ T and CD4+ T cells (Fig. 1c, d).
Figure 1.
Summary of leukocytes and immune cell fractions distribution determined using flow cytometry (FCM). Tumor and blood samples were assessed using multicolor FCM, and leukocyte and 11 types of immune cell fractions in the samples were analyzed. (a) Distribution of leukocyte density (count/g) determined by FCM in 45 breast tumors. The graph shows the medians, 25th percentile, and 75th percentile. (b, c) Percentages of each immune cell fraction in tumors (n = 45) and blood samples (n = 18) showed as Tukey box plots. (d) Statistical values are summarized for the percentages of each immune cell fraction in the tumor tissues (n = 45).
Histologically assessed TIL is associated with the degree of leukocyte infiltration in tumor tissue and leukocyte composition
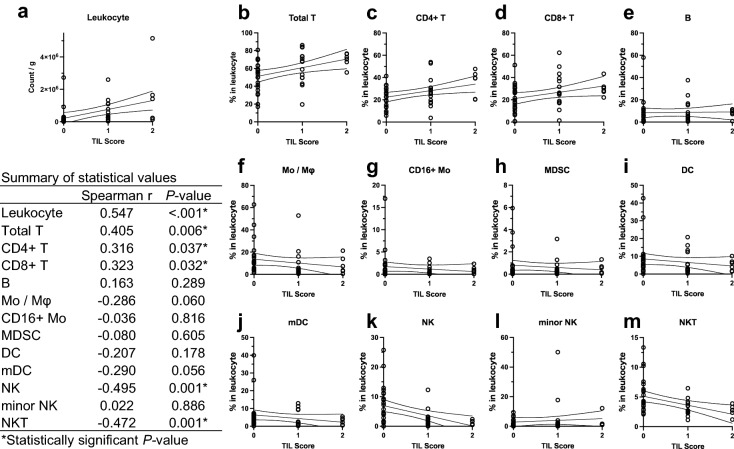
The assessment of clinicopathological characteristics using the hTIL score showed that higher hTIL scores were associated with high-grade tumors, ER negativity, higher Ki67-positive ratios, and hPD-L1 positivity (Table 1). For cases with tumor tissue samples, correlation analysis was performed for the hTIL scores and leukocyte densities (count/g) in the tumor tissues, which were strongly positively correlated with each other (Fig. 2a). Furthermore, the hTIL scores showed a positive correlation with the densities (count/g) of all immune cell fractions in the tumor tissues, except for NK (Supplementary Fig. S2). Correlation analysis was also performed for the hTIL scores and percentages of each immune cell fraction in the tumor tissues. We observed positive correlations between the hTIL scores and percentages of total T, CD4+ T, and CD8+ T (Fig. 2b–d), but negative correlations between the hTIL scores and NK and NKT (Fig. 2k, m), showing that hTIL was associated with the degree of leukocyte infiltration in tumor tissue and leukocyte composition. There were no correlations between the hTIL scores and the percentages of B, Mo/Mφ, CD16 + Mo, MDSC, DC, mDC, and minor NK cells (Fig. 2e–j, l).
Table 1.
Clinico-pathological characteristics by hTIL score.
| hTIL score 0 (N = 23) | hTIL score 1, 2 (N = 21) | p-value | hTIL score 0 (N = 23) | hTIL score 1, 2 (N = 21) | p-value | ||
|---|---|---|---|---|---|---|---|
| Menopausal status | 0.512 | Vascular invasion | 0.089 | ||||
| Unknown | 0 | 1 | Unknown | 1 | 0 | ||
| Post | 16 (69.6%) | 12 (60.0%) | Negative | 17 (77.3%) | 20 (95.2%) | ||
| Pre | 7 (30.4%) | 8 (40.0%) | Positive | 5 (22.7%) | 1 (4.8%) | ||
| Neo-adjuvant therapy | 0.155 | Histological grade | 0.011* | ||||
| Absent | 17 (73.9%) | 19 (90.5%) | Unknown | 2 | 3 | ||
| Present | 6 (26.1%) | 2 (9.5%) | Grade 3 | 2 (9.5%) | 9 (50.0%) | ||
| Histological type | 0.544 | Grade 1, 2 | 19 (90.5%) | 9 (50.0%) | |||
| IDC | 20 (87.0%) | 18 (85.7%) | ER status | 0.002* | |||
| ILC | 1 (4.3%) | 0 (0.0%) | Positive | 14 (60.9%) | 3 (14.3%) | ||
| Special | 2 (8.7%) | 3 (14.3%) | Negative | 9 (39.1%) | 18 (85.7%) | ||
| Invasive tumor size | 0.631 | PgR status | 0.269 | ||||
| Unknown | 1 | 0 | Positive | 5 (21.7%) | 2 (9.5%) | ||
| ≥ 20 mm | 19 (86.4%) | 17 (81.0%) | Negative | 18 (78.3%) | 19 (90.5%) | ||
| < 20 mm | 3 (13.6%) | 4 (19.0%) | HER2 status | 0.062 | |||
| Lymph node metastasis | 0.443 | Unknown | 0 | 1 | |||
| Unknown | 1 | 0 | Positive | 6 (26.1%) | 1 (5.0%) | ||
| Negative | 10 (45.5%) | 12 (57.1%) | Negative | 17 (73.9%) | 19 (95.0%) | ||
| Positive | 12 (54.5%) | 9 (42.9%) | Ki67 | 0.036* | |||
| Lymphatic invasion | 0.897 | ≥ 20% | 14 (60.9%) | 19 (90.5%) | |||
| Unknown | 1 | 0 | < 20% | 9 (39.1%) | 2 (9.5%) | ||
| Negative | 9 (40.9%) | 9 (42.9%) | PD-L1 | < .001* | |||
| Positive | 13 (59.1%) | 12 (57.1%) | Unknown | 0 | 0 | ||
| Positive | 7 (30.4%) | 18 (85.7%) | |||||
| Negative | 16 (69.6%) | 3 (14.3%) | |||||
*Statistically significant p-value.
Figure 2.
hTIL is associated with the degree of leukocyte infiltration in tumor tissues and leukocyte composition. For cases with tumor tissue samples, a correlation analysis was performed for (a) hTIL scores and leukocyte densities (count/g) in tumor tissues, (b–m) hTIL scores, and percentages of each immune cell fraction in the tumor tissues. The X- and Y-axes show the hTIL scores and (a) leukocyte densities (count/g) or (b–m) the percentages of the immune cell fractions in the tumor tissue, respectively. The lines in the graph indicate the regression line with a 95% confidence band. The relationship between these values was analyzed using the Spearman correlation test. Values of P < 0.05 are considered statistically significant. Statistical values are summarized in the table on the left side of the figure.
Histologically assessed PD-L1 expression is associated with leukocyte infiltration in tumor tissue and reflects PD-L1 expression in certain immune cell fractions
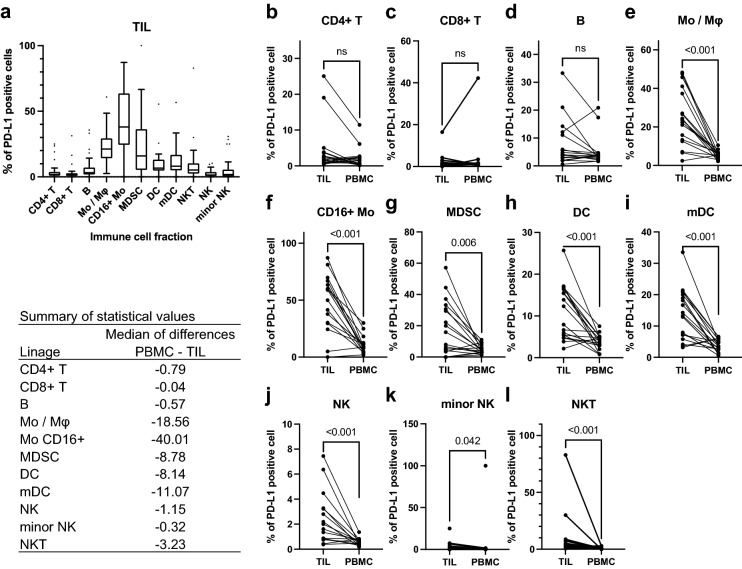
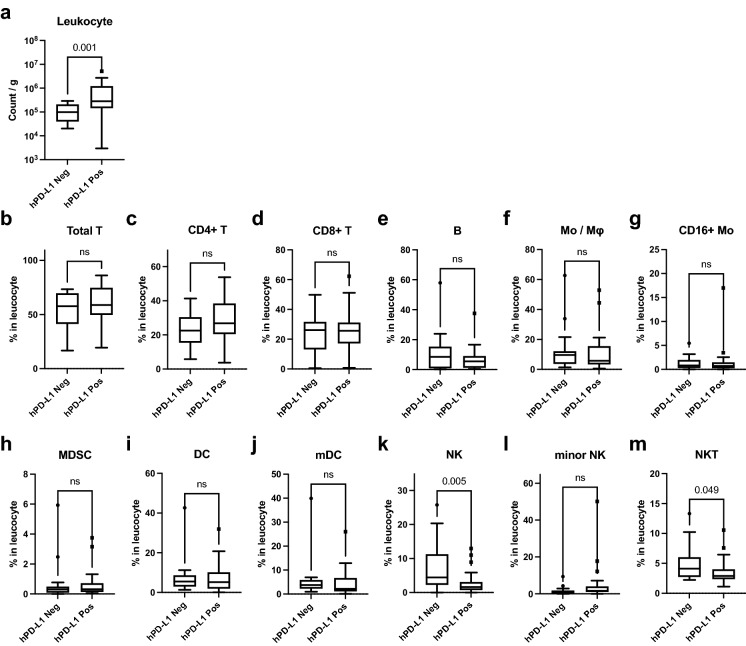
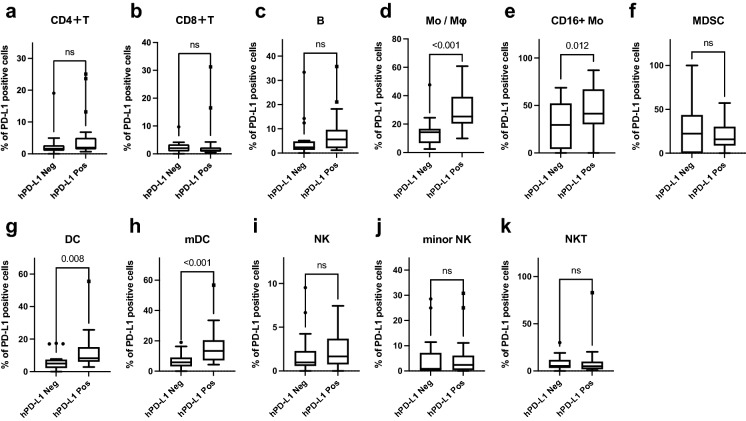
The percentages of the PD-L1-positive cells in each immune cell fraction were determined for the tumor tissues (TILs) and blood (PBMC) using FCM. The percentages of the PD-L1-positive cells were high in CD16+ Mo, Mo/Mφ, MDSC, mDC, DC populations (Fig. 3a). For cases with matched samples of blood and tumor tissue, the percentages of the PD-L1-positive cells in each immune cell fraction of the tumor tissues and blood were compared. The percentages of the PD-L1 positive cells were significantly higher in tumor tissues than in blood for all lineages except for the lymphoid fractions (Fig. 3b–l). Next, we investigated the relationship between hPD-L1 and the immunological profiles of the tumor tissues. Comparisons between the leukocyte densities (count/g) in the tumor tissues of the hPD-L1-negative and -positive cases showed that hPD-L1 positivity was associated with increased leukocyte infiltrations in tumor tissues (Fig. 4a). Similarly, hPD-L1 showed a positive correlation with the densities (count/g) of all immune cell fractions in the tumor tissues, except for B and NK cell fractions (Supplementary Fig. S3). With regard to the immune cell composition in the tumor tissues, although hPD-L1 positivity was associated with a lower percentage of NK and NKT, it was not correlated with the percentages of other lineages (Fig. 4b–m). For tumor tissue samples, percentages of the PD-L1 positive cells in each immune cell fraction in the hPD-L1-positive and -negative cases were compared. We found that hPD-L1 positivity showed a positive association with the percentages of the PD-L1 positive cells in some of the immune cell fractions, including Mo/Mφ, CD16+ Mo, DC, and mDC, but not with the other lineages (Fig. 5a–k). These data suggest that hPD-L1 expression reflects leukocyte infiltration in the tumor tissues and PD-L1 expression in certain immune cell fractions.
Figure 3.
PD-L1 is preferentially expressed in part of the immune cell fraction, and its expression is higher in tumor-infiltrating leukocytes than in blood. The percentages of PD-L1-positive cells of each immune cell fraction were determined in tumor tissues (TIL) and blood (PBMC). (a) The percentages of PD-L1-positive cells in each immune cell fraction present in tumor tissue are shown in Tukey box plots. (b–l) For the cases with matched samples of blood and tumor tissue, the percentages of PD-L1-positive cells of each immune cell fraction were compared in tumor tissue (TIL) and blood (PBMC) using the Wilcoxon test. Values of P < 0.05 are considered statistically significant. The actual P-values are shown in the graphs. The medians of the differences are summarized in the table on the left side of the figure.
Figure 4.
hPD-L1 positivity is associated with increased leukocyte infiltrations in the tumor tissue and partly with the immune cell composition. For cases with tumor tissue samples, (a) leukocyte densities (count/g) and (b–m) the percentages of the immune cell fractions in the tumor tissues were compared between the hPD-L1-negative cases and hPD-L1-positive cases using the Mann–Whitney U test. The data are presented using Tukey box plots. Values of P < 0.05 were considered statistically significant. The actual P-values are shown in the graphs.
Figure 5.
hPD-L1 positivity is associated with percentages of the PD-L1 positive cells in certain immune cell fractions. (a–k) For cases with tumor tissue samples, the percentages of PD-L1-positive cells in each immune cell fraction of the tumor tissues in the hPD-L1-negative and hPD-L1-positive cases were compared using the Mann–Whitney U test. The data are presented using Tukey box plots. Values of P < 0.05 are considered statistically significant. The actual P-values are presented in the graphs.
Discussion
In this study, we evaluated multiple immune cell fractions in both breast cancer tissues and matched blood samples. In the comprehensive analysis of the association between each immune cell fraction with hTIL and hPD-L1, we demonstrated for the first time that these biomarkers reflect not only the degree of immune cell infiltration in the tumor but also the proportion of a particular immune cell subset. We also showed that hPD-L1 reflects PD-L1 expression in certain immune cell fractions, including Mo/Mφ, CD16+ Mo, DC, and mDC.
To the best of our knowledge, most of the analyses of the immune cell compositions of breast cancer tissues using a multicolor FCM had 10 colors or less32,37, and there were only two studies with more than 11 colors38,39. Although the reactivities of the labeled antibodies were not always the same, and a direct comparison was not possible, a similar distribution of the leukocyte infiltrations was observed in the tumor tissue in our study and a previous study with a distribution median of 218 CD45+ TIL/mg of tumor tissue (interquartile range: 85–445 CD45+ TIL/mg)32. Although there are very few reports of systematic examinations of leukocyte compositions in breast cancer tissue, studies have reported the ratio of total T to be 86% (mean)32 or 75% (median)38 of the leukocytes (CD45+ cells) in breast cancer tissues, suggesting that T cells account for the majority of TILs40. In our study, the proportion of total T cells in the leukocytes in the tumors was 57.3% (mean), which was slightly lower than that in previous reports, probably owing to the difference in the antibody used and gating strategy. In three previous studies, the proportions of CD19+ B cells in CD45+ TIL were found to be 8% (mean), 4.58% (median), and approximately 10% (mean), respectively32,37,38, which were similar to our results. With regard to other lineages, the findings of a previous study showed that the proportions of CD14+/CD40+/CD163+ M2 macrophages were 0.06% (median), those of CD11b+/CD15+/HLA-DR-MDSCs were 1.19% (median), and those of CD56+ NK were 2.33% (median)38. However, there were only a few studies, and the definitions of each lineage did not match those in our study; therefore, valid comparisons could not be made. No comparable reports were found for the remaining lineages.
For cases with matched samples of blood and tumor tissue, we exploratorily analyzed the association between the immune cell composition of blood and breast cancer tissues. Immune composition of the blood partially correlated with that of tumor tissues (Supplementary Fig. S4a–l), and the percentages of the immune cell fractions in tumor tissues and blood presented certain differences (Supplementary Fig. S5a–l). However, phenotypes, such as memory and naïve T cells, which have a different composition in blood and tissue, were not analyzed in this study. Therefore, the relationship between immune cell composition in blood and breast cancer tissue cannot be thoroughly discussed with our current data, and the implications of these results will be considered in future studies. Although we have investigated the relationship between blood immune cell composition and hTIL and hPD-L1, no significant associations were observed (Supplementary Fig. S6 and S7). Similarly, no significant associations were identified between hPD-L1 and FCM-assessed PD-L1 positive ratios in the immune cell fraction of blood (Supplementary Fig. S8).
Although IHC has been used for histological evaluation of TIL in previous studies, the target antigens or clones of antibodies used in these studies are diverse, as it is not standardized nor routinary in clinical settings3,36. In addition, TIL evaluation by hematoxylin and eosin staining has been recommended by the International TILs Working Group to assess tumor-immunological status in clinical practice. However, the kind of immunological tumor status reflected by this simple index (hTIL) has not been fully investigated yet. Thus, one of the primary purposes of this study was to investigate the significance of hTIL assessed according to the mentioned guideline. Although there was a significant difference between the subtypes, hTIL was correlated with certain clinicopathological factors, including subtypes3–5, prognoses, and responses to chemotherapy4,6,7, in breast cancer. Numerous studies have also reported that ER-positive breast cancer is the least immune-infiltrated subtype, which is consistent with our results2,5. However, there are certain controversies regarding other clinicopathological factors, and results vary among different studies2,4,5,41. No studies have systematically assessed the relationship between hTIL and the immune cell fraction using FCM. In our study, hTIL assessed following the guidelines of the International TILs Working Group showed a positive correlation with the infiltration (count/g) of all immune cell fractions in tumor tissue except for NK (Supplementary Fig. S2). Moreover, cases with a higher hTIL score have a higher percentage of the lymphocyte fraction but a lower fraction of NK and NKT (Fig. 2). These data suggest that when TIL increases in tumor tissue, lymphocytic fraction shows a prominent increase, and the percentages of cells with a relatively small increase such as NK and NKT are decreased.
As mentioned previously, PD-L1 plays a significant role in immune tolerance mechanisms8,9, and its expression is suggested to reflect ongoing (or active) immune responses in addition to immunosuppression via the PD-1/PD-L1 pathway39. hPD-L1 was shown to correlate with certain clinicopathological factors, including subtypes10. It is also a clinically approved predictive marker for atezolizumab in triple-negative advanced breast cancer11. In the present study, hPD-L1 positivity was associated with ER negativity and relatively high hTIL scores but no other factors, probably owing to the small cohort size (Supplementary Table S6). Although PD-L1 expression in multiple types of immune cells or tumor cells has been reported8,9, there is no consensus as to which immune cell fraction is responsible for the substantial function of the PD-L1 pathway in breast cancer. The findings of only one report that evaluated PD-L1 expression in CD4 + T, CD8 + T, and B cells showed that the overall proportion of the PD-L1-positive TILs was very low and could only be detected in a small number of tumors39. In the present study, we found that PD-L1 was preferentially expressed in CD16 + Mo, Mo/Mφ, MDSC, mDC, DC, and the percentages of the PD-L1 positive cells of these lineages were significantly higher in tumor tissues than in blood, suggesting that these fractions are involved primarily in the PD-L1 pathway in breast cancer tissue. Additionally, we found that hPD-L1-positive tumors exhibited increased leukocyte infiltration in tumor tissues, and hPD-L1 reflected PD-L1 expression in Mo/Mφ, CD16+ Mo, DC, and mDCs. These results suggested that hPD-L1 expression can indicate the activation status of the immune tolerance mechanism that occurs in certain immune cell fractions such as CD16+ Mo, Mo/Mφ, MDSC, mDC, and DC in response to increased immune cell infiltration, mainly effector cells that secrete interferon-gamma to induce PD-L1 expression on various cells, into the breast cancer microenvironment. The significance of hPD-L1 expression in tumor cells has been previously reported9,33,42. In this study, the hPD-L1 expression in immune cells was determined using PD-L1 antibody clone SP142, following the clinically accepted diagnostic criteria11. Accordingly, tumors with 1% or more immune cells with PD-L1 staining were determined to be hPD-L1 positive, which was identified only in 2 out of 44 cases (4.5%). Although this frequency agrees with that reported in previous reports33, the small case number makes it difficult to perform a meaningful statistical analysis. Therefore, to gain insights into the significance of PD-L1 expression in tumor cells, future studies involving a large number of cases should be conducted.
This study has several limitations. The number of patients enrolled was relatively small. A pilot study empirically found that the number of cells required for FCM was not sufficient in cases of ER-positive breast cancer, especially in cases with lower Ki67s. Cases of small tumor sizes and post-NAC with complete pathological responses were excluded owing to technical problems in collecting the tumor tissues. Therefore, there was an inevitable bias in the enrollment of the cases; it differed from the general breast cancer cohort in terms of larger invasive tumor sizes, more ER-negative cases, and higher Ki67 cases (Supplementary Table S2). Although, as mentioned above, the significance of the TIL is suggested to vary between subtypes, a subgroup analysis could not be performed because of the small sample size. In future studies, the inclusion of more samples and more detailed analyses are recommended. Furthermore, the FCM data contained outliers; however, we could not compare the values because of a lack of suitable reports; hence, the biological reliability of the outliers cannot be ruled out. Therefore, all analyses were performed without the omission of outliers. However, to ensure the reliability of our analyses, we identified the outliers using the ROUT method, excluded them, and reperformed all statistical analyses. The results did not differ much (Supplementary Tables S7–S17) compared with those obtained by including the outliers, indicating the reliability of the results.
Conclusions
A comprehensive analysis of the immune cell fractions revealed the immunological profiles of breast cancer tissue represented by hTIL or hPD-L1. Our findings indicated that hTIL reflected the amount of immune cell infiltration, as well as the proportion of a particular immune cell subset. Immune cell fractions, such as CD16 + Mo, Mo/Mφ, MDSC, mDC, DC, were preferentially involved in the PD-L1 pathway in breast cancer microenvironments. Additionally, we demonstrate that hPD-L1 represented the PD-L1 expression in these immune cell fractions. Collectively, our findings provide a basic understanding of the immune response in the breast cancer microenvironment and contribute to the development of tumor immunology.
Supplementary Information
Acknowledgements
We would like to thank Editage (www.editage.com) for English language editing. This study was supported by the Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (Project No. 18K16266; to NN), and the 2020 Tokai University School of Medicine Research Aid (to TH). This work was also supported by Chugai Pharmaceutical Co., Ltd.
Author contributions
T.H. analyzed and interpreted the data and wrote the manuscript. M.T., B.T., T.O., and N.N. collected the samples. The following authors performed the experiment: H.K. (FCM), S.K. and M.Y. (processing of the MCF data), N.K., C.H., and S.K. (the histological assessment). K.H. processed the samples for the FCM and supervised the writing of the manuscript. N.N., T.I., S.K., and N.H. developed the concept, designed the study, and supervised the writing of the manuscript. All the authors read and approved the final version of this manuscript.
Data availability
The datasets generated and/or analyzed during the current study are available upon reasonable request to the corresponding author.
Competing interests
Hiroshi Kagamu has an advisory role in ImmuniT Research Inc. Naoki Harada received a salary from Chugai Pharmaceutical. Shigehisa Kitano received honoraria from Ono Pharmaceutical Co., Bristol-Myers Squibb Co., Ltd., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., and MSD Co., Ltd. Hiroshi Kagamu received honoraria from AstraZeneca K.K., Ono Pharmaceutical Co., Ltd., and Bristol-Myers Squibb Co., Ltd. Naoki Niikura received honoraria from AstraZeneca K.K., Daiichi Sankyo Co., Ltd., Pfizer Japan Inc., Eisai Co., Ltd., and Nippon Kayaku Co., Ltd. Shigehisa Kitano received research funding from Astellas Pharma Inc., Gilead Sciences Inc., Eisai Co., Ltd., Regeneron Pharmaceuticals Inc., Boehringer Ingelheim GmgH, Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Takara Bio Inc., PACT Pharma, Inc., Chugai Pharmaceutical Co., Ltd., and MSD Co., Ltd. Naoki Niikura received research funding from Chugai Pharmaceutical Co., Ltd. The remaining authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-11578-x.
References
- 1.Harbeck N, Gnant M. Breast cancer. Lancet (London, England) 2017;389:1134–1150. doi: 10.1016/s0140-6736(16)31891-8. [DOI] [PubMed] [Google Scholar]
- 2.Dieci MV, Griguolo G, Miglietta F, Guarneri V. The immune system and hormone-receptor positive breast cancer: Is it really a dead end? Cancer Treat. Rev. 2016;46:9–19. doi: 10.1016/j.ctrv.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 3.Hendry S, Salgado R, Gevaert T, Russell PA, John T, Thapa B, Christie M, van de Vijver K, Estrada MV, Gonzalez-Ericsson PI, Sanders M, Solomon B, Solinas C, Van den Eynden G, Allory Y, Preusser M, Hainfellner J, Pruneri G, Vingiani A, Demaria S, Symmans F, Nuciforo P, Comerma L, Thompson EA, Lakhani S, Kim SR, Schnitt S, Colpaert C, Sotiriou C, Scherer SJ, Ignatiadis M, Badve S, Pierce RH, Viale G, Sirtaine N, Penault-Llorca F, Sugie T, Fineberg S, Paik S, Srinivasan A, Richardson A, Wang Y, Chmielik E, Brock J, Johnson DB, Balko J, Wienert S, Bossuyt V, Michiels S, Ternes N, Burchardi N, Luen SJ, Savas P, Klauschen F, Watson PH, Nelson BH, Criscitiello C, O'Toole S, Larsimont D, de Wind R, Curigliano G, André F, Lacroix-Triki M, van de Vijver M, Rojo F, Floris G, Bedri S, Sparano J, Rimm D, Nielsen T, Kos Z, Hewitt S, Singh B, Farshid G, Loibl S, Allison KH, Tung N, Adams S, Willard-Gallo K, Horlings HM, Gandhi L, Moreira A, Hirsch F, Dieci MV, Urbanowicz M, Brcic I, Korski K, Gaire F, Koeppen H, Lo A, Giltnane J, Rebelatto MC, Steele KE, Zha J, Emancipator K, Juco JW, Denkert C, Reis-Filho J, Loi S, Fox SB. Assessing tumor-infiltrating lymphocytes in solid tumors: A practical review for pathologists and proposal for a standardized method from the International Immunooncology Biomarkers Working Group: Part 1: assessing the host immune response, TILs in invasive breast carcinoma and ductal carcinoma in situ, metastatic tumor deposits and areas for further research. Adv. Anat. Pathol. 2017;24:235–251. doi: 10.1097/pap.0000000000000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu X, Zhang Z, Wang Z, Wu P, Qiu F, Huang J. Prognostic and predictive value of tumor-infiltrating lymphocytes in breast cancer: A systematic review and meta-analysis. Clin. Transl. Oncol. 2016;18:497–506. doi: 10.1007/s12094-015-1391-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solinas C, Carbognin L, De Silva P, Criscitiello C, Lambertini M. Tumor-infiltrating lymphocytes in breast cancer according to tumor subtype: Current state of the art. Breast (Edinburgh, Scotland) 2017;35:142–150. doi: 10.1016/j.breast.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 6.Mao Y, Qu Q, Chen X, Huang O, Wu J, Shen K. The prognostic value of tumor-infiltrating lymphocytes in breast cancer: A systematic review and meta-analysis. PLoS ONE. 2016;11:e0152500. doi: 10.1371/journal.pone.0152500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Denkert C, von Minckwitz G, Darb-Esfahani S, Ingold Heppner B, Klauschen F, Furlanetto J, Pfitzner B, Huober J, Schmitt W, Blohmer JU, Kümmel S, Engels K, Lederer B, Schneeweiss A, Hartmann A, Jakisch C, Untch M, Hanusch C, Weber K, Loibl S. Abstract S1–09: Evaluation of tumor-infiltrating lymphocytes (TILs) as predictive and prognostic biomarker in different subtypes of breast cancer treated with neoadjuvant therapy—A metaanalysis of 3771 patients. Can. Res. 2017;77:S1–09. doi: 10.1158/1538-7445.SABCS16-S1-09. [DOI] [Google Scholar]
- 8.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cimino-Mathews A, Thompson E, Taube JM, Ye X, Lu Y, Meeker A, Xu H, Sharma R, Lecksell K, Cornish TC, Cuka N, Argani P, Emens LA. PD-L1 (B7–H1) expression and the immune tumor microenvironment in primary and metastatic breast carcinomas. Hum. Pathol. 2016;47:52–63. doi: 10.1016/j.humpath.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li X, Li M, Lian Z, Zhu H, Kong L, Wang P, Yu J. Prognostic role of programmed death ligand-1 expression in breast cancer: A systematic review and meta-analysis. Target Oncol. 2016;11:753–761. doi: 10.1007/s11523-016-0451-8. [DOI] [PubMed] [Google Scholar]
- 11.Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, Dieras V, Hegg R, Im SA, Shaw Wright G, Henschel V, Molinero L, Chui SY, Funke R, Husain A, Winer EP, Loi S, Emens LA. Atezolizumab and Nab-paclitaxel in advanced triple-negative breast cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 12.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Grainge MJ, Lee AH, Ellis IO, Green AR. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J. Clin. Oncol. 2011;29:1949–1955. doi: 10.1200/jco.2010.30.5037. [DOI] [PubMed] [Google Scholar]
- 13.Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, Bowden SJ, Twelves C, Bartlett JMS, Mahmoud SMA, Rakha E, Ellis IO, Liu S, Gao D, Nielsen TO, Pharoah PDP, Caldas C. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann. Oncol. 2014;25:1536–1543. doi: 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- 14.Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: A systematic review and meta-analysis. BMC Cancer. 2020;20:179. doi: 10.1186/s12885-020-6668-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnellhardt S, Erber R, Büttner-Herold M, Rosahl MC, Ott OJ, Strnad V, Beckmann MW, King L, Hartmann A, Fietkau R, Distel L. Tumour-infiltrating inflammatory cells in early breast cancer: An underrated prognostic and predictive factor? Int. J. Mol. Sci. 2020 doi: 10.3390/ijms21218238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burugu S, Asleh-Aburaya K, Nielsen TO. Immune infiltrates in the breast cancer microenvironment: Detection, characterization and clinical implication. Breast Cancer. 2017;24:3–15. doi: 10.1007/s12282-016-0698-z. [DOI] [PubMed] [Google Scholar]
- 17.Kim ST, Jeong H, Woo OH, Seo JH, Kim A, Lee ES, Shin SW, Kim YH, Kim JS, Park KH. Tumor-infiltrating lymphocytes, tumor characteristics, and recurrence in patients with early breast cancer. Am. J. Clin. Oncol. 2013;36:224–231. doi: 10.1097/COC.0b013e3182467d90. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Foulkes WD, Leung S, Gao D, Lau S, Kos Z, Nielsen TO. Prognostic significance of FOXP3+ tumor-infiltrating lymphocytes in breast cancer depends on estrogen receptor and human epidermal growth factor receptor-2 expression status and concurrent cytotoxic T-cell infiltration. Breast Cancer Res. 2014;16:432. doi: 10.1186/s13058-014-0432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mahmoud SM, Paish EC, Powe DG, Macmillan RD, Lee AH, Ellis IO, Green AR. An evaluation of the clinical significance of FOXP3+ infiltrating cells in human breast cancer. Breast Cancer Res. Treat. 2011;127:99–108. doi: 10.1007/s10549-010-0987-8. [DOI] [PubMed] [Google Scholar]
- 20.Gu-Trantien C, Loi S, Garaud S, Equeter C, Libin M, de Wind A, Ravoet M, Le Buanec H, Sibille C, Manfouo-Foutsop G, Veys I, Haibe-Kains B, Singhal SK, Michiels S, Rothé F, Salgado R, Duvillier H, Ignatiadis M, Desmedt C, Bron D, Larsimont D, Piccart M, Sotiriou C, Willard-Gallo K. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013;123:2873–2892. doi: 10.1172/jci67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. The prognostic significance of B lymphocytes in invasive carcinoma of the breast. Breast Cancer Res. Treat. 2012;132:545–553. doi: 10.1007/s10549-011-1620-1. [DOI] [PubMed] [Google Scholar]
- 22.Qiu SQ, Waaijer SJH, Zwager MC, de Vries EGE, van der Vegt B, Schröder CP. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018;70:178–189. doi: 10.1016/j.ctrv.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, Zhao YW, Wei YQ. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li F, Zhao Y, Wei L, Li S, Liu J. Tumor-infiltrating Treg, MDSC, and IDO expression associated with outcomes of neoadjuvant chemotherapy of breast cancer. Cancer Biol. Ther. 2018;19:695–705. doi: 10.1080/15384047.2018.1450116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de la Cruz-Merino L, Barco-Sánchez A, Henao Carrasco F, Nogales Fernández E, Vallejo Benítez A, Brugal Molina J, Martínez Peinado A, Grueso López A, Ruiz Borrego M, Manuel C, de Villena M, Sánchez-Margalet V, Nieto-García A, Alba Conejo E, Casares Lagar N, Ibáñez Martínez J. New insights into the role of the immune microenvironment in breast carcinoma. Clin. Dev. Immunol. 2013;2013:785317. doi: 10.1155/2013/785317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee H, Lee HJ, Song IH, Bang WS, Heo SH, Gong G, Park IA. CD11c-positive dendritic cells in triple-negative breast cancer. In Vivo. 2018;32:1561–1569. doi: 10.21873/invivo.11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuge T, Yamakawa M, Tsukamoto M. Infiltrating dendritic/Langerhans cells in primary breast cancer. Breast Cancer Res. Treat. 2000;59:141–152. doi: 10.1023/a:1006396216933. [DOI] [PubMed] [Google Scholar]
- 28.Rathore AS, Goel MM, Makker A, Kumar S, Srivastava AN. Is the tumor infiltrating natural killer cell (NK-TILs) count in infiltrating ductal carcinoma of breast prognostically significant? Asian Pac. J. Cancer Prev. 2014;15:3757–3761. doi: 10.7314/apjcp.2014.15.8.3757. [DOI] [PubMed] [Google Scholar]
- 29.Robbins P, Pinder S, de Klerk N, Dawkins H, Harvey J, Sterrett G, Ellis I, Elston C. Histological grading of breast carcinomas: A study of interobserver agreement. Hum. Pathol. 1995;26:873–879. doi: 10.1016/0046-8177(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 30.Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998;11:155–168. [PubMed] [Google Scholar]
- 31.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/jco.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 32.Buisseret L, Garaud S, de Wind A, Van den Eynden G, Boisson A, Solinas C, Gu-Trantien C, Naveaux C, Lodewyckx JN, Duvillier H, Craciun L, Veys I, Larsimont D, Piccart-Gebhart M, Stagg J, Sotiriou C, Willard-Gallo K. Tumor-infiltrating lymphocyte composition, organization and PD-1/PD-L1 expression are linked in breast cancer. Oncoimmunology. 2017;6:e1257452. doi: 10.1080/2162402x.2016.1257452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurozumi S, Inoue K, Matsumoto H, Fujii T, Horiguchi J, Oyama T, Kurosumi M, Shirabe K. Clinicopathological values of PD-L1 expression in HER2-positive breast cancer. Sci. Rep. 2019;9:16662. doi: 10.1038/s41598-019-52944-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurozumi S, Kaira K, Matsumoto H, Hirakata T, Yokobori T, Inoue K, Horiguchi J, Katayama A, Koshi H, Shimizu A, Oyama T, Sloan EK, Kurosumi M, Fujii T, Shirabe K. β(2)-Adrenergic receptor expression is associated with biomarkers of tumor immunity and predicts poor prognosis in estrogen receptor-negative breast cancer. Breast Cancer Res. Treat. 2019;177:603–610. doi: 10.1007/s10549-019-05341-6. [DOI] [PubMed] [Google Scholar]
- 35.Kurozumi S, Matsumoto H, Kurosumi M, Inoue K, Fujii T, Horiguchi J, Shirabe K, Oyama T, Kuwano H. Prognostic significance of tumour-infiltrating lymphocytes for oestrogen receptor-negative breast cancer without lymph node metastasis. Oncol. Lett. 2019;17:2647–2656. doi: 10.3892/ol.2019.9938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salgado R, Denkert C, Demaria S, Sirtaine N, Klauschen F, Pruneri G, Wienert S, Van den Eynden G, Baehner FL, Penault-Llorca F, Perez EA, Thompson EA, Symmans WF, Richardson AL, Brock J, Criscitiello C, Bailey H, Ignatiadis M, Floris G, Sparano J, Kos Z, Nielsen T, Rimm DL, Allison KH, Reis-Filho JS, Loibl S, Sotiriou C, Viale G, Badve S, Adams S, Willard-Gallo K, Loi S. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: Recommendations by an International TILs Working Group 2014. Ann. Oncol. 2015;26:259–271. doi: 10.1093/annonc/mdu450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garaud S, Buisseret L, Solinas C, Gu-Trantien C, de Wind A, Van den Eynden G, Naveaux C, Lodewyckx JN, Boisson A, Duvillier H, Craciun L, Ameye L, Veys I, Paesmans M, Larsimont D, Piccart-Gebhart M, Willard-Gallo K. Tumor infiltrating B-cells signal functional humoral immune responses in breast cancer. JCI Insight. 2019 doi: 10.1172/jci.insight.129641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Duechler M, Peczek L, Zuk K, Zalesna I, Jeziorski A, Czyz M. The heterogeneous immune microenvironment in breast cancer is affected by hypoxia-related genes. Immunobiology. 2014;219:158–165. doi: 10.1016/j.imbio.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 39.Solinas C, Garaud S, De Silva P, Boisson A, Van den Eynden G, de Wind A, Risso P, Rodrigues Vitória J, Richard F, Migliori E, Noël G, Duvillier H, Craciun L, Veys I, Awada A, Detours V, Larsimont D, Piccart-Gebhart M, Willard-Gallo K. Immune checkpoint molecules on tumor-infiltrating lymphocytes and their association with tertiary lymphoid structures in human breast cancer. Front. Immunol. 2017;8:1412. doi: 10.3389/fimmu.2017.01412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitford P, Mallon EA, George WD, Campbell AM. Flow cytometric analysis of tumour infiltrating lymphocytes in breast cancer. Br. J. Cancer. 1990;62:971–975. doi: 10.1038/bjc.1990.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stanton SE, Disis ML. Clinical significance of tumor-infiltrating lymphocytes in breast cancer. J. Immunother. Cancer. 2016;4:59. doi: 10.1186/s40425-016-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Y, Wang CL, Xian J, Ye Q, Qin X, Tan YW, Cao YD. Positive correlation between programmed death ligand-1 and p53 in triple-negative breast cancer. Onco. Targets. Ther. 2019;12:7193–7201. doi: 10.2147/ott.S209484. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available upon reasonable request to the corresponding author.