Abstract
Two commercial immunomagnetic separation (IMS) kits for Cryptosporidium were compared for recovery of oocysts from environmental samples. Oocyst recovery efficiencies with the Dynal and Crypto-Scan kits ranged from 62 to 100% and 34 to 74%, respectively, for seeded environmental water concentrates (turbidity of 210 to 11,480 nephelometric turbidity units). Recovery efficiencies were dependent on the mechanism of agitation during the magnetic capture procedure. An assay combining in vitro cell culture and reverse transcriptase PCR demonstrated that oocysts recovered by IMS retained their infectivity.
Waterborne Cryptosporidium parvum continues to present a significant threat to public health. To protect against further waterborne outbreaks of cryptosporidiosis, water utilities are taking additional measures to protect watershed areas, investigating new disinfection procedures, routinely monitoring sources of water, and developing new technologies for detecting C. parvum and other pathogens in water. However, the recovery efficiencies of the method currently used to detect waterborne oocysts are low and extremely variable. Recent interlaboratory evaluations of the method demonstrated recovery efficiencies ranging from 0 to 140% (15). Oocyst losses are known to occur at the filtration, standard centrifugation, and density gradient centrifugation stages of the process (18, 19). Because of the inadequacies of the method, alternative techniques have been proposed and evaluated and are routinely used outside the United States.
Since large volumes of source water (10 to 100 liters of raw water) need to be analyzed, filtration cannot be eliminated from the procedure, although alternative filtration techniques have been shown to increase recovery. Such filtration techniques include the use of flat membrane filters, vortex flow filtration, and filter capsules (9, 14, 19). In contrast, some or all of the centrifugation steps used to concentrate and purify filtered samples can be modified or replaced by alternatives. Immunomagnetic separation (IMS), with antibodies, paramagnetic particles, and magnetic concentration, provides such an alternative. IMS-captured cells are associated with a solid surface, which can be held stationary while the surrounding supernatant matrix is removed by repeated washing. Therefore, the technique effectively eliminates or greatly reduces the concentration of substances that might be inhibitory to subsequent manipulations or toxic to the target cells. IMS has been used with varying efficiencies to recover a variety of microorganisms spiked into environmental samples, although naturally occurring organisms were not recovered from unspiked samples in all investigations. The organisms used for such studies included Giardia lamblia, enteric viruses, and Helicobacter pylori from fresh water (4, 13, 23); hepatitis A virus from shellfish (11); Pseudomonas stutzeri from seawater (2); and Salmonella spp., Mycobacterium avium, and enterohemorrhagic Escherichia coli from fecal samples (8, 17, 25). IMS has also been used to recover Cryptosporidium sp. oocysts from environmental water samples. Recovery efficiencies from seeded 1-liter water concentrates ranged from 63 to 82% with a flowthrough magnetic column but were as low as 10% when a static separation system was used (24). A continuous IMS procedure, with the oocyst suspension circulating past a magnet, was reported to be useful for recovery of oocysts, but the efficiency was affected by flow rate, oocyst-paramagnetic bead contact time, and oocyst-bead ratio (22). In addition, concentration of oocysts by IMS has been used prior to PCR-based detection (10, 16). In both of these previous studies, low numbers of oocysts were detected by the final PCR but recovery efficiencies for IMS were not reported.
Although the use of IMS has been reported for recovery of C. parvum oocysts from environmental samples, different systems have been used without a thorough evaluation of the recovery efficiencies. Also, recently developed Cryptosporidium IMS kits, specifically aimed at the water industry, have become commercially available. These kits have been evaluated individually, with widely differing recovery efficiencies which ranged from 68 to 126% for one kit (Crypto-Scan [14]) and 49 to 102% for the second (Dynal [7]), with a variety of oocyst seed densities and water turbidities. However, the kits have not been compared in side-by-side evaluations. Consequently, the objective of this investigation was to evaluate IMS for recovery of C. parvum oocysts from water concentrates by comparing two commercial kits. In addition, studies were conducted to evaluate the effect of IMS on the infectivity of captured oocysts. Because IMS is an antibody-based technique and in vitro application of antibodies has been demonstrated to inhibit the infectivity of C. parvum (12, 20), it might be expected that IMS recovery of oocysts would neutralize captured oocysts. Therefore, the effect of IMS on oocyst infectivity was evaluated by means of a previously described in vitro cell culture assay combined with mRNA extraction and reverse transcriptase (RT) PCR (21).
Viable C. parvum oocysts (Iowa isolate) were obtained from the Sterling Parasitology Laboratory (University of Arizona, Tucson). Different oocyst densities were obtained by dilution in sterile deionized water and were seeded into 10-ml volumes of deionized water, packed pellets of source water, or fecal samples for evaluation of recovery efficiencies. Nonviable, formalin-fixed oocysts were used for some experiments. These oocysts were resuspended in 10% formalin for 2 h and then washed with phosphate-buffered saline by centrifugation and resuspension. Diluted oocyst suspensions were enumerated (10 replicate counts) by fluorescence microscopy on 50-μl aliquots by using well slides (Meridian Diagnostics, Cincinnati, Ohio) and a fluorescein isothiocyanate-labeled anti-Cryptosporidium immunoglobulin M antibody (Waterborne, Inc., New Orleans, La.).
Packed pellets of source water samples (100 liters) were obtained by filtration and centrifugation (1). The turbidity of environmental water samples and concentrates was determined with a Hach 2100N turbidity meter (Hach Co., Loveland, Colo.). The turbidity and pH values of the original water samples at the time of collection ranged from 0.45 to 8.3 nephelometric turbidity units (NTU) and pH 7.9 to 8.5. The turbidities of the concentrates ranged from 210 to 11,480 NTU. Most of these source water concentrates did not contain Cryptosporidium oocysts, as determined by fluorescence microscopy. Two samples did contain oocysts but at levels which did not have an impact on the reported recovery efficiencies (sample C-2, one oocyst per 100 liters; sample E-4, four oocysts per 100 liters). Oocysts were seeded into a packed pellet volume equivalent to 10 liters in a total volume of 10 ml. One-gram aliquots of a Cryptosporidium-negative bovine fecal sample were also seeded with 496 ± 22 oocysts. The negative status of fecal samples was determined by IMS and immunofluorescent staining prior to seeding. The oocysts used for recovery studies in this investigation were “aged” (i.e., were 2 to 6 months old) because oocysts in environmental water samples are more likely to be aged. Use of fresh oocysts as the seed may result in overestimation of recovery efficiencies if fresh oocysts, with intact surface antigens, are bound by the antibody more readily than aged oocysts with potentially degraded antigens. However, to ensure that we were assessing the effects of IMS rather than the effects of oocyst age on oocyst infectivity, fresh oocysts (<1 month old) were used for the infectivity investigations.
Recovery of oocysts with the Dynal and Crypto-Scan IMS kits was performed according to the instructions provided by the manufacturers (Dynal, Lake Success, N.Y., and Clearwater Diagnostics, Portland, Maine, respectively). The suitability of IMS-recovered oocysts for molecular analysis was demonstrated by PCR after three cycles of freezing (−70°C) and thawing (65°C), followed by incubation (99°C) for 5 min, to lyse the oocysts. A 361-bp fragment of the C. parvum heat shock protein gene (hsp70) was amplified by using previously described primers (21). The infectivity of IMS-recovered oocysts from seeded environmental water concentrates was determined by in vitro cultivation on monolayers of the continuous human cell lines Caco-2 (ATCC HTB37) and HCT-8 (ATCC CCL244). Following IMS and with oocysts still attached to the paramagnetic beads, a conventional in vitro excystation procedure was performed (6). The oocyst-paramagnetic bead complex (OBC) was incubated in 300 μl of Hanks’ balanced salt solution (Sigma Chemical Co., St. Louis, Mo.) containing 0.7% bile and 50 μl of sodium bicarbonate (5 mM) at 37°C for 3 h, without vortexing, instead of 0.1 M HCl as used in the normal disassociation procedure. The disassociated beads were removed by magnetic capture along with unexcysted and empty oocysts, leaving a suspension of purified sporozoites. The sporozoites were resuspended in 3 ml of Eagle’s minimal essential medium (Sigma) or RPMI 1640 (Sigma) containing 0.1% bovine serum albumin and then inoculated onto Caco-2 or HCT-8 cell monolayers, respectively, and grown in single-well chamber slides (Erie Scientific, Portsmouth, N.H.) as described previously (21). Infections were detected by RT-PCR of C. parvum-specific mRNA according to previously described methods which have been used to demonstrate infectivity of 10 oocysts or fewer (21). Control RT-PCR amplifications were performed on the extracted mRNA by using primers that amplified a 600-bp fragment from mRNA transcripts of the GAPDH (glyceraldehyde-3-phosphate dehydrogenase) gene present in the human cells (Stratagene, La Jolla, Calif.). Amplification products were visualized by ethidium bromide staining of an agarose electrophoretic gel.
The Dynal kit generated consistently higher recovery efficiencies from water concentrates, with turbidities ranging from 210 to 11,480 NTU (Table 1). With a seed density of 976 oocysts, the Dynal kit demonstrated 14.5% higher recovery than the Crypto-Scan kit, and this increased to 35.5% higher recovery when only 36 oocysts were seeded into packed pellets from 10 liters of source water. For the Crypto-Scan kit there was a significant negative correlation between oocyst recovery and water concentrate turbidity at the 95% level (r = 0.621) but not at the 99% level. Recovery efficiency was not significantly correlated with turbidity for the Dynal kit nor with oocyst seed concentration for either kit. In one instance, the Dynal kit demonstrated 100% recovery of 36 oocysts, although the actual recovery was within the range of 82 to 128% based on the mean and standard deviation of the seed enumeration for this experiment (36 ± 7.8 oocysts). Recovery efficiencies from seeded bovine fecal samples (496 ± 22 oocysts/g) were lower than from environmental water concentrates, but the highest recovery (67 ± 4.5%) was obtained with the Dynal kit. Microscopic observation of Dynal-recovered oocysts was faster than by the Crypto-Scan method due to the smaller volume of recovered oocyst suspension. Only 3 h were required to enumerate all of the Dynal-recovered oocysts from eight seeded environmental samples compared to more than 20 h for eight samples with the Crypto-Scan kit. In addition, suspensions of oocysts recovered by the Dynal procedure were contaminated with an average of 7 (n = 3) algae per 10-liter concentrate, whereas the Crypto-Scan procedure recovered an average of 334 (n = 3) algal organisms from concentrates of the same water, based on enumeration of red fluorescing algal cells with excitation and emission wavelengths of 510 to 560 nm and 590 nm, respectively.
TABLE 1.
Comparison of Dynal and Crypto-Scan IMS kits for recovery of C. parvum from 12 different seeded environmental water concentrates
| Sample | Turbidity of 10-liter concentrate (NTU) | Oocyst seed densitya | Recovery efficiency (%)b
|
|
|---|---|---|---|---|
| Dynal | Crypto-Scan | |||
| A-1 | 370 | 976 ± 47 | 93c | 79cd |
| B-1 | 320 | 273 ± 13 | 85 | 51d |
| C-1 | 280 | 273 ± 13 | 75 | 46d |
| C-2 | 370 | 273 ± 13 | 62 | 50d |
| B-2 | 280 | 273 ± 13 | 67 | 49d |
| D-1 | 210 | 36 ± 7.8 | 100 | 53d |
| E-1 | 210 | 36 ± 7.8 | 81 | 57d |
| E-2 | 540 | 142 ± 11 | 90 | 58e |
| E-3 | 830 | 284 ± 22 | 64 | 35e |
| C-3 | 1,050 | 277 ± 15 | 80c | 46ce |
| A-2 | 1,400 | 71 ± 5.6 | 86 | 35e |
| E-4 | 11,480 | 355 ± 28 | 66 | 34e |
Mean ± standard deviation (n = 10).
Based on single determinations for each of the 12 concentrate samples.
Mean of duplicate determinations.
Crypto-Scan method with no disassociation of OBC.
Crypto-Scan method with acid disassociation of OBC.
Losses were determined at various stages of the oocyst recovery process for both methods. For Dynal IMS, 3.1 to 4.6% of oocysts were not captured during the initial separation, 0.6 to 3% of oocysts remained in the microcentrifuge tube following the second capture, and 2 to 4% of oocysts remained attached to the paramagnetic beads following acid disassociation. Other researchers have reported that up to 8% of captured oocysts remained attached to Dynal beads following acid disassociation (5). The protocol provided with the original Crypto-Scan kit described resuspension of the OBC in 400 μl of deionized water (without disassociation) and division of the suspension over multiple wells for fluorescent staining and microscopic observation. However, this increased the microscopic observation time eightfold compared to the procedure for the Dynal kit (Crypto-Scan, eight 50-μl wells; Dynal, one 50-μl well). Also, oocysts recovered with the Crypto-Scan kit were more difficult to observe because of the high density of paramagnetic particles, which masked oocysts and defracted fluorescence emission. Therefore, the efficiency of acid disassociation was evaluated for Crypto-Scan particles. By using the same disassociation procedure as that described for Dynal beads, it was determined that 33% of oocysts remained attached to the Crypto-Scan particles. In addition, up to 17% of oocysts were not captured by the initial Crypto-Scan magnetic capture.
During the latter stages of this evaluation, the Crypto-Scan kit was redeveloped by the manufacturer to accommodate disassociation of the OBC in 0.1 M HCl. Recovery efficiencies for the redeveloped kit averaged 42% for a range of seeded source water concentrates (Table 1). Disassociation of the OBC obtained with the redeveloped kit required resuspension and vortexing in two successive 50-μl volumes of 0.1 M HCl. The first disassociation released 18 to 40% of oocysts, while the second disassociation released a further 10 to 18% of oocysts. To determine whether the lower recovery efficiencies obtained with Crypto-Scan were caused by the paramagnetic beads or the assay format, the two kits were compared in terms of their respective and each other’s procedures. The Crypto-Scan beads recovered an average of 42% (n = 2) of oocysts with the Crypto-Scan procedure compared to 83.5% (n = 2) of oocysts with the Dynal procedure. The Dynal paramagnetic beads demonstrated 100% recovery efficiency when assayed by the Dynal procedure, but only 52% of oocysts were captured by the Crypto-Scan assay format (n = 2). Therefore, the Crypto-Scan beads performed almost as well as the Dynal beads, and the magnetic properties of the beads and the affinity or avidity of the anti-Cryptosporidium antibodies were similar. The lower recovery efficiencies obtained with the Crypto-Scan kit resulted primarily from the format of the recovery procedure. The 90° vertical agitation used with the Dynal magnet ensured that particulate matter and debris in the sample stayed in solution in the glass tube while the paramagnetic beads were being captured by the magnet. Thus, the oocysts were separated from the rest of the sample. The flat, round magnet used with the Crypto-Scan kit necessitated a horizontal, 360° rotary action (40 to 70 rpm) to capture paramagnetic beads in a plastic petri dish. Unfortunately, the rotary action concentrated most of the sample debris in the center of the petri dish along with the OBC. Therefore, the oocysts were not effectively separated from the sample debris. Although a higher concentration of paramagnetic beads was used for the Crypto-Scan method, the larger size of the Dynal beads made a 4.5-fold-larger surface area available for immunomagnetic capture of the target.
A recent study by the manufacturers of the Dynal kit reported an average recovery of 66% for 2 × 104 oocysts seeded into environmental water concentrates (5,000 to 10,000 NTU) and 83% for eight oocysts seeded into the same sample (7). The lowest oocyst density used in our investigation was 36 ± 7.8, seeded into a water concentrate with a turbidity of 210 NTU, from which we obtained 81 to 100% recovery. Nevertheless, the recovery efficiencies obtained in this investigation are at least equal to, and generally higher than, those reported by the kit’s manufacturer (7). In contrast to the lower recoveries obtained with the Crypto-Scan kit in this investigation, a recent report demonstrated recovery efficiencies as high as 100% for 106 oocysts seeded into very turbid (4,600-NTU) river water concentrates (14). Although the Dynal kit produced consistently superior results in this investigation, it did demonstrate variable recovery efficiencies with seeded environmental water concentrates (62 to 100%), which indicates that there is a need for continued development of IMS methods.
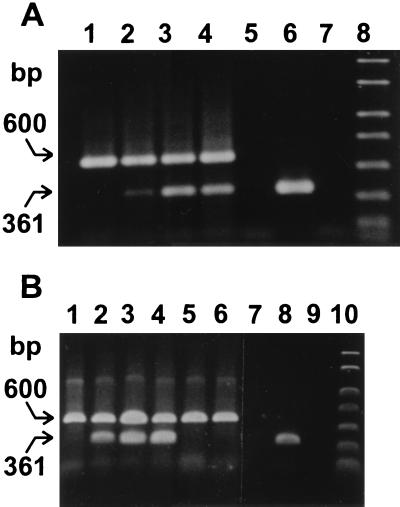
An important aspect of recovery of oocysts from environmental samples is the ability to determine whether they are infectious. An infectivity assay is important for the water industry, as it provides water utilities with a tool to assess the public health risk posed by waterborne C. parvum and to determine the efficacy of disinfection practices. Therefore, it is essential that methods used to recover oocysts do not have deleterious effects on oocyst viability or infectivity. IMS is an antibody-based technique, but it has been reported that in vitro application of antibodies inhibited the infectivity of C. parvum. In one study, antisporozoite monoclonal antibodies attached to 100% of viable sporozoites and neutralized their in vivo infectivity (20). A specific anti-C. parvum antibody was also shown to significantly reduce the infectivity of C. parvum oocysts for in vitro cultures of MDBK and Caco-2 cell lines (12). Consequently, the effect of IMS recovery on oocyst infectivity was evaluated in the present investigation. The infectivity of IMS-recovered oocysts was determined by an assay combining in vitro cell culture with mRNA extraction and C. parvum-specific RT-PCR (21). The OBCs obtained by the Dynal kit from an environmental water concentrate seeded with 142 to 568 oocysts were treated with an in vitro excystation procedure, and the paramagnetic beads, unexcysted oocysts, and empty oocysts were then removed by magnetic separation. Excystation efficiencies were always greater than 50% with this procedure. The resulting sporozoite suspensions were inoculated onto confluent cell monolayers. C. parvum-specific hsp70 mRNA transcripts were detected by RT-PCR amplification of mRNA extracted from the infected HCT-8 cells (361-bp amplicon [Fig. 1A]) and Caco-2 cells (Fig. 1B). The paramagnetic beads did not appear to inhibit either the in vitro excystation or the infectivity of released sporozoites. The same RT-PCR assay was used to demonstrate that oocysts recovered by an in-house IMS method using MACS paramagnetic particles (Miltenyi Biotec, Inc., Auburn, Calif.) and indirect antibodies were also infectious on Caco-2 cells (Fig. 1B). Negative controls comprising uninfected cell monolayers demonstrated that 361-bp amplicons were specifically amplified from C. parvum-infected cells. In addition, inoculation of cell monolayers with formalin-fixed oocysts demonstrated that amplicons were derived from active infections rather than from uninfective oocysts remaining on the monolayer following the 2-h postinfection wash. This was also confirmed by the absence of amplicons from inoculated cell monolayers which did not support infection (Fig. 1B). These results clearly demonstrated that IMS-recovered C. parvum oocysts retained the ability to infect human cell cultures grown in vitro.
FIG. 1.
Detection of infectious C. parvum recovered by IMS. (A) HCT-8 cells were infected with oocysts recovered by Dynal IMS from a 10-liter environmental water concentrate seeded as follows: lane 1, no oocysts; lane 2, 142 ± 11 oocysts; lane 3, 284 ± 22 oocysts; lane 4, 568 ± 44 oocysts. Other lanes: 5, RT negative control; 6, C. parvum DNA positive control; 7, PCR negative control; 8, molecular size markers. A C. parvum-specific 361-bp fragment was amplified from hsp70 gene transcripts by RT-PCR. The 600-bp amplicon was amplified from mRNA transcripts of the GAPDH housekeeping gene. (B) Caco-2 cells were infected with oocysts recovered from 10 ml of reagent water by Dynal (lane 3) and MACS (lane 4) IMS. Other lanes: 1, uninfected Caco-2 cells; 2, Caco-2 cells infected with oocysts not exposed to IMS; 5, monolayer inoculated with formalin-fixed oocysts; 6, 105 oocysts inoculated onto cells which did not support infection; 7, RT negative control; 8, C. parvum DNA positive control; 9, PCR negative control; 10, molecular size markers.
Although the Dynal and Crypto-Scan kits are the only complete Cryptosporidium IMS kits commercially available, in-house methods are readily formulated. We have used a variety of different types of paramagnetic beads and antibodies to obtain oocyst recovery efficiencies of up to 94% with biotin-labeled antibodies and streptavidin-coated paramagnetic beads (results not shown). Our observations demonstrate that the efficiency of an IMS system is not simply a function of the properties of the paramagnetic particles employed. Biomag paramagnetic particles have 18-fold greater magnetic susceptibility than do Dynal beads (3) and are approximately one-fifth the size of Dynal beads. This combination of small diameter (which leads to reduced drag during separation) and high magnetic susceptibility suggests that Biomag particles should be superior to Dynal beads. Nevertheless, the Dynal beads demonstrated higher recovery efficiencies than an in-house method using Biomag particles (unpublished observations).
The results of this study clearly demonstrate that IMS is a practical method for recovering Cryptosporidium oocysts from environmental samples and that captured oocysts retain their infectivity. However, different commercial IMS kits exhibit widely varying performance characteristics.
Acknowledgments
We thank Joseph Crabb (Immucell) for providing samples of the original Crypto-Scan IMS kit, Dynal for providing some of the IMS magnets, Andrew Campbell (Dynal) for technical advice, and Peggy Kimball (Metropolitan) for technical editing of the manuscript.
This work was supported by the U.S. Environmental Protection Agency (award no. R825146-01-0) and the U.S. Department of Agriculture (award no. 96-35102-3875).
REFERENCES
- 1.American Society for Testing and Materials. ASTM standards 11.01. Philadelphia, Pa: American Society for Testing and Materials; 1991. Proposed test method for Giardia cysts and Cryptosporidium oocysts in low-turbidity water by fluorescent antibody procedure; pp. 925–935. [Google Scholar]
- 2.Bard D G, Ward B B. A species-specific bacterial productivity method using immunomagnetic separation and radiotracer experiments. J Microbiol Methods. 1997;28:207–219. [Google Scholar]
- 3.Baselt D R, Lee G U, Hansen K M, Chrisey L A, Colton R J. A high-sensitivity micromachined biosensor. Proc IEEE. 1997;85:672–680. [Google Scholar]
- 4.Bifulco J M, Schaeffer F W. Antibody-magnetite method for selective concentration of Giardia lamblia cysts from water samples. Appl Environ Microbiol. 1993;59:772–776. doi: 10.1128/aem.59.3.772-776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukhari Z, McCuin R, Clancy J, Jonas A, Matheson Z, Fricker C. Proceedings of the Water Quality Technology Conference 1997. Denver, Colo: American Water Works Association; 1997. Using immunomagnetic separation for detecting Cryptosporidium; pp. 5B4.1–5. [Google Scholar]
- 6.Campbell A T, Robertson L J, Smith H V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell A T, Gron B, Johnsen S E. Immunomagnetic separation of Cryptosporidium oocysts from high turbidity water sample concentrates. In: Fricker C R, Clancy J L, Rochelle P A, editors. International symposium on waterborne Cryptosporidium. Denver, Colo: American Water Works Association; 1997. pp. 91–96. [Google Scholar]
- 8.Chapman P A, Cerdan Malo A T, Siddons C A, Harkin M. Use of commercial enzyme immunoassays and immunomagnetic separation systems for detecting Escherichia coli O157 in bovine fecal samples. Appl Environ Microbiol. 1997;63:2549–2553. doi: 10.1128/aem.63.7.2549-2553.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clancy J L, Hargy T M, Schaub S. Improved sampling methods for the recovery of Giardia and Cryptosporidium from source and treated water. In: Fricker C R, Clancy J L, Rochelle P A, editors. International symposium on waterborne Cryptosporidium. Denver, Colo: American Water Works Association; 1997. pp. 79–85. [Google Scholar]
- 10.Deng M Q, Cliver D O, Mariam T W. Immunomagnetic capture PCR to detect viable Cryptosporidium parvum oocysts from environmental samples. Appl Environ Microbiol. 1997;63:3134–3138. doi: 10.1128/aem.63.8.3134-3138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng M Y, Day S P, Cliver D O. Detection of hepatitis A virus in environmental samples by antigen-capture PCR. Appl Environ Microbiol. 1994;60:1927–1933. doi: 10.1128/aem.60.6.1927-1933.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle P S, Crabb J, Petersen C. Anti-Cryptosporidium antibodies inhibit infectivity in vitro and in vivo. Infect Immun. 1993;61:4079–4084. doi: 10.1128/iai.61.10.4079-4084.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enroth H, Engstrand L. Immunomagnetic separation and PCR for detection of Helicobacter pylori in water and stool specimens. J Clin Microbiol. 1995;33:2162–2165. doi: 10.1128/jcm.33.8.2162-2165.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fricker C R, Jonas A, Crabb J, Turner N, Smith H V. The concentration and separation of Cryptosporidium oocysts and Giardia cysts using vortex flow filtration and immunomagnetic separation. In: Fricker C R, Clancy J L, Rochelle P A, editors. International symposium on waterborne Cryptosporidium. Denver, Colo: American Water Works Association; 1997. pp. 1–8. [Google Scholar]
- 15.Jakubowski W, Boutros S, Faber W, Fayer R, Ghiorse W, LeChevallier M, Rose J, Schaub S, Singh A, Stewart M. Environmental methods for Cryptosporidium. J Am Water Works Assoc. 1996;88(9):107–121. [Google Scholar]
- 16.Johnson D W, Pieniazek N J, Griffin D W, Misener L, Rose J B. Development of a PCR protocol for sensitive detection of Cryptosporidium oocysts in water samples. Appl Environ Microbiol. 1995;61:3849–3855. doi: 10.1128/aem.61.11.3849-3855.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kongmuang U, Luk J M C, Lindberg A A. Comparison of three stool-processing methods for detection of Salmonella serogroups B, C2, and D by PCR. J Clin Microbiol. 1994;32:3072–3074. doi: 10.1128/jcm.32.12.3072-3074.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LeChevallier M W, Norton W D, Siegel J E, Abbaszadegan M. Evaluation of the immunofluorescence procedure for detection of Giardia cysts and Cryptosporidium oocysts in water. Appl Environ Microbiol. 1995;61:690–697. doi: 10.1128/aem.61.2.690-697.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nieminski E C, Schaefer III F W, Ongerth J E. Comparison of two methods for detection of Giardia cysts and Cryptosporidium oocysts in water. Appl Environ Microbiol. 1995;61:1714–1719. doi: 10.1128/aem.61.5.1714-1719.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perryman L E, Riggs M W, Mason P H, Fayer R. Kinetics of Cryptosporidium parvum sporozoite neutralization by monoclonal antibodies, immune bovine serum, and immune bovine colostrum. Infect Immun. 1990;58:257–259. doi: 10.1128/iai.58.1.257-259.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rochelle P A, Ferguson D M, Handojo T J, De Leon R, Stewart M H, Wolfe R L. An assay combining cell culture with reverse transcriptase PCR to detect and determine the infectivity of waterborne Cryptosporidium parvum. Appl Environ Microbiol. 1997;63:2029–2037. doi: 10.1128/aem.63.5.2029-2037.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossomando E F, Hesla M A, Olson E, Thomas F, Gilbert D C, Herlihy J J. Proceedings of the Water Quality Technology Conference 1995. New Orleans, La: American Water Works Association; 1995. “Continuous” immunomagnetic capture of Cryptosporidium and Giardia from surface water; pp. 847–881. [Google Scholar]
- 23.Schwab K J, De Leon R, Sobsey M D. Immunoaffinity concentration and purification of waterborne enteric viruses for detection by reverse transcriptase PCR. Appl Environ Microbiol. 1996;62:2086–2094. doi: 10.1128/aem.62.6.2086-2094.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whitmore T N. Rapid techniques for recovery of Cryptosporidium. In: Betts W B, Casemore D, Fricker C, Smith H, Watkins J, editors. Protozoan parasites and water. Cambridge, United Kingdom: The Royal Society of Chemistry; 1995. pp. 139–142. [Google Scholar]
- 25.Zhongming L, Bai G H, Fordham von Reyn C, Marino P, Brennan M J, Gine N, Morris S L. Rapid detection of Mycobacterium avium in stool samples from AIDS patients by immunomagnetic PCR. J Clin Microbiol. 1996;34:1903–1907. doi: 10.1128/jcm.34.8.1903-1907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]