Abstract
Background and Aims
Cannabis withdrawal is a well‐characterized phenomenon that occurs in approximately half of regular and dependent cannabis users after abrupt cessation or significant reductions in cannabis products that contain Δ9‐tetrahydrocannabinol (THC). This review describes the diagnosis, prevalence, course and management of cannabis withdrawal and highlights opportunities for future clinical research.
Methods
Narrative review of literature.
Results
Symptom onset typically occurs 24–48 hours after cessation and most symptoms generally peak at days 2–6, with some symptoms lasting up to 3 weeks or more in heavy cannabis users. The most common features of cannabis withdrawal are anxiety, irritability, anger or aggression, disturbed sleep/dreaming, depressed mood and loss of appetite. Less common physical symptoms include chills, headaches, physical tension, sweating and stomach pain. Despite limited empirical evidence, supportive counselling and psychoeducation are the first‐line approaches in the management of cannabis withdrawal. There are no medications currently approved specifically for medically assisted withdrawal (MAW). Medications have been used to manage short‐term symptoms (e.g. anxiety, sleep, nausea). A number of promising pharmacological agents have been examined in controlled trials, but these have been underpowered and positive findings not reliably replicated. Some (e.g. cannabis agonists) are used ‘off‐label’ in clinical practice. Inpatient admission for MAW may be clinically indicated for patients who have significant comorbid mental health disorders and polysubstance use to avoid severe complications.
Conclusions
The clinical significance of cannabis withdrawal is that its symptoms may precipitate relapse to cannabis use. Complicated withdrawal may occur in people with concurrent mental health and polysubstance use.
Keywords: assessment, cannabis withdrawal syndrome, clinical management, pharmacology, prevalence, time course
DEPENDENCE, WITHDRAWAL STATES AND CLINICAL MANAGEMENT
Description
Cannabis withdrawal refers to symptoms that occur after abrupt cessation or significant reductions in the use of cannabis products containing Δ9‐tetrahydrocannabinol (THC), the main psychoactive component in cannabis. These symptoms occur most often in regular and heavy cannabis users and the most common symptoms are anxiety, irritability, anger or aggression, disturbed sleep/dreaming, depressed mood and loss of appetite. Less commonly reported physical symptoms include chills, headaches, physical tension, sweating and stomach pain [1, 2]. Cessation of short‐term cannabidiol (CBD), a non‐psychoactive cannabinoid, does not appear to result in withdrawal [3].
Symptom onset typically occurs 24–48 hours after cessation and most symptoms generally peak at days 2–6. The duration and severity of cannabis withdrawal is associated with the amount of cannabis consumed before cessation, but can vary considerably. In heavy users withdrawal symptoms can occur for up to 2–3 weeks or longer.
Prevalence
A meta‐analysis pooling studies of more than 20 000 regular and dependent cannabis users estimated that 47% of individuals reported cannabis withdrawal measured by standardized scales [4]. The prevalence in community samples was 17%, increasing to 54% in outpatient samples and 87% in inpatients [4].
The prevalence of cannabis withdrawal symptoms is higher in users with a history of daily cannabis use, concurrent cannabis and tobacco use and other substance use disorders [4].
Objectives of management
Cannabis withdrawal does not carry a high risk of severe adverse outcomes. The presence of medical or psychiatric comorbidities such as polysubstance use and dependence may result in more severe complications and symptoms of cannabis withdrawal, necessitating additional management. The clinical significance of cannabis withdrawal is that it may undermine abstinence by precipitating a relapse to cannabis use which immediately relieves these symptoms [5]. Irritability and mood effects can also negatively impact personal relationships and work productivity.
Synthetic cannabinoids (SCBs) are classed as new psychoactive substances (NPS) and are made in clandestine laboratories. Unlike cannabis, SCBs are a heterogeneous group that may contain multiple, typically synthetic compounds with broad structural diversity and are therefore not reviewed in this paper. SCBs can be 2–100 times more potent than THC [6], are more likely to result in problematic use and faster development of tolerance and potentially result in more severe withdrawal than natural cannabis [7]. Their mechanisms and effects remain poorly understood.
Cannabis dependence
Individuals with cannabis use disorder (CUD) generally experience greater severity and duration of cannabis withdrawal symptoms than those without CUD. This is most probably related to the greater frequency and quantity of their cannabis use and their heavier exposure to THC [8]. CUD is characterized by persistent cannabis use despite negative effects on the social functioning and physical or mental health of the user or the health of other individuals. Two diagnostic systems classify and define the severity of the disorder: the Diagnostic and Statistical Manual of Mental Disorders (DSM)‐5 [1] and International Classification of Diseases (ICD)‐11 [9]. CUD severity is coded in DSM‐5 as mild (presence of two to three symptoms), moderate (presence of four to five symptoms) or severe (presence of six or more symptoms). ICD‐11 classifies cannabis use into hazardous cannabis use (potential to cause harm), harmful pattern of cannabis use (causing harm) and cannabis dependence.
Neurobiological evidence
The cannabis plant contains approximately 120 cannabinoids, the most studied of which are THC and CBD. The body's own endogenous cannabinoids act as partial agonists of the body's CB1 and CB2 receptors [10, 11], as does THC [12], whereas CBD acts as an allosteric modulator of these receptors [13, 14]. The psychoactive effects of THC are underpinned by its strong affinity for CB1 receptors, which are predominantly distributed within the brain. CB1 antagonists, such as rimonabant, reduce the subjective effects of cannabis, demonstrating the role of CB1 binding in the psychoactive effects of cannabis use [15]. THC has a much lower affinity for the CB2 receptor that is predominantly found in immune cells. Conversely, CBD has a stronger affinity for the CB2 receptor, but has a relatively much lower affinity for either receptor than THC.
There are endogenous cannabinoid neurotransmitters in humans and other animals. The best‐characterized are anandamide (AEA) and 2‐arachidonoylglycerol (2‐AG). These endocannabinoids are degraded by enzymes that include fatty acid amide hydrolase (FAAH) for anandamine and monoacylglycerol lipase (MAGL) for 2‐AG. THC is metabolized by the enzymes CYP3A4 and CYP2C9 [16]. FAAH binding is reduced in chronic and recent cannabis users [17], and inhibition of FAAH using PF‐04457845 has been shown to reduce cannabis withdrawal [18].
There is good neurobiological and clinical evidence for a pharmacologically specific cannabis withdrawal syndrome. CB1 antagonists precipitate specific withdrawal symptoms in animal models of cannabis dependence [19]. In human studies, administration of CB1 agonists (THC) blocks or relieves withdrawal symptoms [20, 21, 22]. Neurobiological and clinical studies indicate that symptoms of cannabis withdrawal are consistent with the core symptoms of other substance withdrawal syndromes and reflect neurochemical changes in the limbic system [2].
Cannabis withdrawal criteria DSM‐5 and ICD‐11
Withdrawal was included as a distinct disorder and as a CUD diagnostic criterion item in DSM‐5. Cannabis withdrawal is also included in ICD‐11 (Table 1).
TABLE 1.
Cannabis withdrawal criteria: DSM‐5 and ICD‐11
| DSM‐5 Cannabis withdrawal disorder (292.0) [1] | ICD‐11 Cannabis withdrawal (6C41.4) [9] |
|---|---|
| Signs and symptoms | Description |
| Three (or more) of the following signs and symptoms develop after cessation of heavy and prolonged cannabis use (daily or almost daily use over a period of at least a few months): | …that occurs upon cessation or reduction of use of cannabis in individuals who have developed cannabis dependence or have used cannabis for a prolonged period or in large amounts |
|
Presenting features of cannabis withdrawal may include: irritability, anger or aggressive behaviour, shakiness, insomnia, restlessness, anxiety, depressed or dysphoric mood, decreased appetite and weight loss, headache, sweating or chills, abdominal cramps and muscle aches |
|
The signs or symptoms cause clinically significant distress or impairment in social, occupational or other important areas of functioning The signs or symptoms do not relate to another medical condition or better explained by another mental disorder, including intoxication or withdrawal from another substance |
Is a clinically significant cluster of symptoms, behaviours and/or physiological features, varying in degree of severity and duration |
Withdrawal severity
In most instances, cannabis withdrawal is not severe and does not have a high risk of severe adverse outcomes. Medical or psychiatric comorbidities increase the risk of severity and the requirement for additional management. Cannabis withdrawal severity can be evaluated by a clinical examination of the number and intensity of DSM‐5 or ICD‐11 cannabis withdrawal features and by administering standardized measures of cannabis withdrawal. Unlike symptoms of other substance use disorders (SUDs), DSM‐5 and ICD‐11 assess only the presence or absence of cannabis withdrawal symptoms, rather than their severity. The most clinically relevant criterion is the degree of patient distress or impairment in functioning. This can be assessed both subjectively (e.g. anxiety, sleep quality) and objectively (e.g. weight loss, fever).
The two most widely used cannabis withdrawal scales are the 16‐item marijuana withdrawal checklist (MWC [23]), a revised version of the original 22‐item scale [24], and the 19‐item cannabis withdrawal scale (CWS [25]; see Supporting information). Both the MWC and CWS were developed before DSM‐5. There is considerable overlap of features between DSM‐5 and MWC and CWS, but not complete concordance. An adaption of the MWC, the 14‐item composite withdrawal scale (WDS [26]), corresponds more closely with cannabis withdrawal symptoms described in the DSM‐5. By comparison with other substance withdrawal scales (e.g. alcohol withdrawal scale), there has been limited psychometric validation of the various cannabis withdrawal scales.
Differential diagnosis
Because many cannabis withdrawal symptoms are not specific to cannabis, a differential diagnosis needs to determine if they are better explained by withdrawal from another substance (e.g. tobacco or alcohol) or are symptoms of a comorbid mental disorder or medical condition.
Polysubstance use
People with a CUD are more likely to use other substances. In nationally representative US surveys, people with a CUD in the past 12 months were six times more likely to have an alcohol use disorder and nine times more likely to have another drug use disorder [27]. Three in four cannabis users seeking treatment for a CUD will have another SUD [1]. Because of the high prevalence of comorbid substance use and dependence, it is important to know if the patient only wants to cease cannabis use, or some or all of the substances that they use. In outpatient settings the patient may prefer to continue to use other substances. There are insufficient studies to decide whether it is better to withdraw from multiple substances sequentially or concurrently. In an inpatient setting, all non‐prescribed substances are usually stopped.
Withdrawal from other substances (whether prescribed or recreational) can produce similar symptoms to cannabis withdrawal. For example, opiate and alcohol withdrawal can produce irritability, anger or aggressive behaviour, shakiness, insomnia, restlessness, anxiety, depressed or dysphoric mood, decreased appetite and weight loss, headache, sweating or chills, abdominal cramps and muscle aches. In the case of alcohol withdrawal delirium tremens, this is a medical emergency. A key tool in a differential diagnosis is the cessation of cannabis use with 24–48 hours of onset and an improvement in symptoms after 4–7 days of abstinence, with careful assessment of other substance use and abstinence patterns.
We know little about interactions between the effects of cannabis and other drugs [28], but polysubstance use may increase the severity of withdrawal. Cannabinoid receptors are major targets, directly or indirectly, for many drugs of abuse including prescription analgesics, but interactions between these drugs are poorly understood. Additive intoxication and withdrawal effects may occur when cannabis is combined with central nervous system (CNS) depressants such as alcohol and opioids (e.g. drowsiness, ataxia) and some benzodiazepines or new psychoactive substances (NPS) [29]. Patients who co‐use tobacco and cannabis report more withdrawal symptoms than those who use cannabis without tobacco [30]. A clinical priority should be to identify which substances have been used and to manage the withdrawal symptoms of the highest‐risk substances or substance combinations.
Psychiatric comorbidity
There is a well‐documented dose–response relationship between the level of cannabis use, CUD and psychiatric comorbidity. A differential diagnosis therefore requires assessment of concurrent mental health disorders, symptoms of which may be exacerbated by or mimic cannabis withdrawal. In a national stratified Australian sample, 70% of adults who met CUD criteria in the past year had at least one other psychiatric disorder [31]. In US national surveys, having a CUD in the past 12 months is associated with increased risk of post‐traumatic stress disorder [PTSD; odds ratio (OR) = 4.3], personality disorder (OR = 4.8), mood disorders (OR = 3.8) and anxiety disorders (OR = 2.8) [27]. Heavy cannabis use increases psychosis risk, and cannabis use worsens symptoms of schizophrenia [32].
Physical comorbidities
It is beyond the scope of this review to provide a comprehensive description of physical conditions that should be considered in a differential diagnosis for cannabis withdrawal. As cannabis withdrawal symptoms can mirror other physical disorders, a comprehensive medical review with an emphasis on gastrointestinal and neurological systems is recommended.
GOLD‐STANDARD CURRENT PRACTICE
Setting and rationale for inpatient/outpatient
Inpatient admission is rarely required for uncomplicated medically assisted withdrawal (MAW). Supervised or inpatient treatment for withdrawal should be considered if the patient is a polysubstance user and has a history of complicated withdrawal from these other substances. If exacerbation of a mental health condition is likely to produce major functional impairment, or increase the risk of self‐harm, supervised inpatient MAW should also be considered. Medical oversight is recommended for patients with medical conditions that may be adversely affected by substance withdrawal. Individuals who have been unable to manage their cannabis use in an outpatient setting should be considered for inpatient support.
Time‐frame
The onset of cannabis withdrawal symptoms typically occurs 24–48 hours after cessation of use. The early phase of withdrawal is usually characterized by insomnia, irritability, decreased appetite, shakiness and, less often, sweating and chills. These early symptoms are most likely to peak at days 2–6. They improve as THC levels reduce over 7 days of abstinence. Anger and aggression and depressed mood may occur as early as 1 week into cannabis withdrawal but typically peak after 2 weeks of abstinence [33, 34] (Figure 1). Sleep disturbances may continue for several weeks or longer.
FIGURE 1.
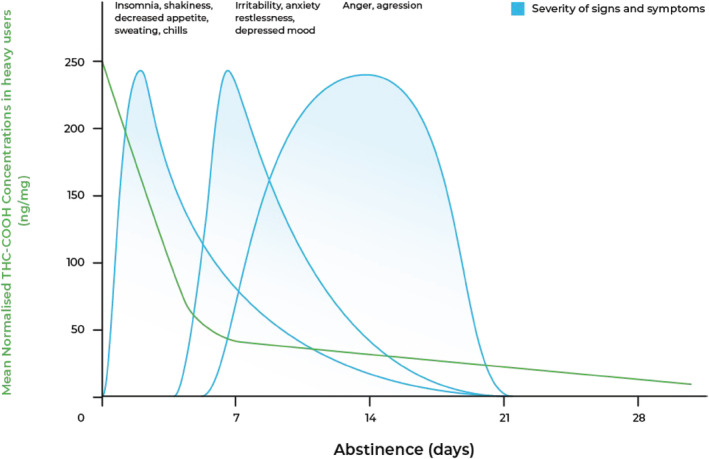
Typical course of cannabis withdrawal. Adapted from Goodwin et al. [35] and Queensland Health (2012) [34, 90]. Typical urinary tetrahydrocannabinol carboxylic acid (THC‐COOH; the main secondary metabolite of THC) levels are drawn from Goodwin et al. [35] and reflect high‐range, chronic cannabis use
THC‐COOH is generally associated with severity of the cannabis withdrawal syndrome, as measured by scores on the MWC [8]. Future research studies may determine if THC‐COOH levels can more precisely guide the management and duration of cannabis withdrawal [8]. Figure 1 displays typical reductions in mean daily creatinine‐normalized THC‐COOH levels after abstinence in heavy, chronic cannabis users [35].
There is a large amount of variation in the course and severity of cannabis withdrawal. Some patients who report low levels of cannabis use and few CUD symptoms (e.g. only two to three DSM‐5 criteria) report uncomfortable withdrawal symptoms that significantly impair their day‐to‐day functioning. There are limited empirical data on the degree to which individual differences in physical, psychiatric or metabolic factors contribute to cannabis withdrawal severity. Up to 50% of between‐individual differences in cannabis withdrawal can be attributed to genetic variation [36]. Some studies of recreational and regular cannabis users [37] and those seeking treatment [26, 38] report that females may experience more severe cannabis withdrawal symptoms than males, even when matched for cannabis use and other demographic characteristics.
Need for pre‐detoxification harm reduction
Cannabis pre‐detoxification harm reduction strategies are not well studied and there is no standard practice. As with other substances, an incremental and slow reduction in cannabis intake and/or use of lower THC products over an extended period (weeks) may reduce the probability and severity of withdrawal symptoms.
Risks/benefits
There are few risks related directly to cannabis withdrawal. The greatest risk is relapse [5]. Previously highlighted in this review is elevated risk of polysubstance use and concurrent withdrawal from higher‐risk substances. If cannabis withdrawal exacerbates depression (± suicide), anxiety and psychosis, then risks are increased and more regular monitoring and delay in detoxification may be clinically indicated. All medications carry risk of side‐effects. Consideration should be given to the potential side‐effects of using pharmacotherapies that are largely untested in this population, compared to the temporary uncomfortable withdrawal period.
Non‐pharmacological interventions
To our knowledge, there are no high‐quality studies on the most effective behavioural approaches and no studies that compare the effectiveness of behavioural and pharmacological approaches to managing withdrawal. Most pharmacological studies of withdrawal include some form of concurrent behavioural intervention, but the differential efficacy of these behavioural approaches has not been assessed (Table 2).
TABLE 2.
Pharmacotherapy studies for cannabis withdrawal
| Medication | Approved for use in cannabis withdrawal | Primary or Secondary outcome in study | Design and main findings | Limitation and future research |
|---|---|---|---|---|
| Cannabis agonists | ||||
| Dronabinol | No | Secondary [46, 54] |
Randomized, double‐blind 12 week outpatient study of cannabis‐dependent users [46]. Primary outcome was cannabis abstinence. Patients (n = 79) were titrated to 20 mg twice daily over the period of 1 week, or placebo (n = 77). Both groups received weekly coping skills intervention and MET Dronabinol increased treatment retention and reduced cannabis withdrawal symptoms (measured by the WDS) over time compared to placebo. Medication was well tolerated and no difference in side effects reported between groups [46] |
Replication RCTs required with cannabis withdrawal as a primary outcome. Greater than one‐third attrition among studies |
| An RCT by the same authors as [46] found no benefit from adding lofexidine (a potent α2‐adrenergic receptor agonist with moderate agonist effects) to dronabinol (n = 61) on cannabis withdrawal (measured by the WDS), compared to placebo (n = 61) in cannabis‐dependent individuals [54] | Outcomes relied on self‐report. Not possible to examine dronabinol in absence of lofexidine | |||
| Nabiximols, Nabilone |
No |
Primary [48] |
Randomized, double‐blind inpatient study of cannabis‐dependent users (mean 22.98 g/week) [48]. Patients (n = 27) completed a 6‐day regimen of nabiximols providing a maximal daily dose of 86.4 mg THC and 80 mg CBD or placebo (n = 24). Both groups received CBT‐based self‐completed work‐books during a 9‐day admission Nabiximols reduced CWS scores for the duration of treatment and improved retention relative to placebo. No serious adverse events or differences in side effects reported between groups [48] |
Larger‐scale validation RCTs required to assess the effectiveness of nabiximols in cannabis withdrawal, including dose–response data. Treatment withdrawal high (> 1/3). Baseline CWS score differed between groups |
|
Secondary [56] |
Cannabis‐dependent outpatients were randomized (double‐blind) to either as‐needed (self‐titrated) nabiximols spray (n = 20) up to 113.4 mg THC/105 mg CBD or placebo (n = 20) daily for 12 weeks. Primary outcomes were tolerability and cannabis abstinence. Secondary outcomes were cannabis use, withdrawal and craving. Both groups received MET + CBT No difference between nabiximols and placebo in withdrawal scores (MWC). Over the 12‐week study, reduction in cannabis craving (MCQ‐SF) statistically favoured nabiximols. No serious adverse events or differences in side effects reported between groups [56] |
Pilot study with small sample size. High variability in amount of nabiximols self‐administered | ||
| Primary [49] |
Cannabis‐dependent participants (n = 16) completed an 8‐week double‐blind trial consisting of four smoke‐as‐usual conditions and four cannabis abstinence conditions during which they administered self‐titrated and high dose Sativex nasal spray, and self‐titrated and frequent dose placebo spray. Primary outcomes were medication tolerability, serious adverse events, craving and withdrawal High fixed doses of Sativex were well tolerated and significantly reduced withdrawal (CWS and MWC) during abstinence relative to placebo. Cravings (MCQ) were not reduced. Self‐titrated Sativex doses were lower than fixed doses, but self‐titrated dosing also showed limited efficacy compared to placebo |
Small sample size and short duration Sativex contains approximately 1:1 ratio of THC:CBD, potentially obscuring the respective contribution of CBD and THC |
||
| No | Secondary [62] |
Cannabis‐dependent outpatients were randomized to either 2 mg/day of nabilone (n = 10), maximum dose reached after a 3‐week escalation or placebo (n = 8) for 10 weeks [62]. Primary outcomes were safety and tolerability. Cannabis craving and anxiety were secondary outcomes. Both groups received behavioural ‘medication management’ No difference between nabilone and placebo on cannabis craving (MCQ) or anxiety (BAI). No serious adverse events or differences in side effects reported between groups [62] |
Pilot study of small sample size and short duration | |
| Nabilone and varenicline | No | Secondary [60] |
Non‐treatment‐seeking tobacco and cannabis co‐users smoking at least two joints a day for at least 6 days a week were randomized to receive varenicline (n = 28) or a placebo (n = 18) in a double‐blind placebo‐controlled trial that consisted of an outpatient phase followed by an inpatient phase. During the inpatient phase all participants received nabilone and a placebo The purpose of the outpatient phase was for varenicline titration and to discontinue tobacco smoking. Participants were not instructed to discontinue cannabis. In the inpatient phase participants received 4 mg twice daily nabilone and a placebo crossed‐over across two 8‐day medication phases. In this phase participants used experimental cannabis (days 1–2), followed by abstinence (days 3–5), and a ‘relapse’ period during which participants could purchase active cannabis (days 6–8). This order—cannabis consumption, abstinence and relapse—was repeated administering the countervailing medication (nabilone or placebo) Outcomes during the outpatient phase were tobacco and other drug use and adverse events. During the inpatient phase outcomes included mood and craving measured using a VAS, sleep assessed using a wrist‐worn monitoring system and VAS, and relapse defined as the number of inhalations that participants purchased during the relapse phase The inclusion of nabilone significantly attenuated the withdrawal‐related mood and sleep symptoms, except for VAS ratings of the term ‘anxious’. Cannabis craving did not differ. Sleep onset was significantly increased and duration decreased during abstinence in participants receiving placebo in lieu of nabilone |
Assessment of cannabis withdrawal symptoms did not use standardized scales. Low, unbalanced sample size between study arms |
| Oral THC | No | Primary [51] |
Outpatients (n = 8) who used cannabis at least 25 days per month in the preceding 6 months completed a repeated‐measures laboratory study consisting of three smoking‐as‐usual conditions (A), followed by an abstinence period (B, C and D) in a ABACAD design. During the abstinence periods participants were double‐blind administered placebo, and a low (30 mg) or high (90 mg) dose of oral THC. The low THC condition always preceded the high condition for safety reasons The main outcome was withdrawal (MWC). A composite WDS was calculated from items within the MWC. Secondary outcomes consisted of cravings (MCQ), other symptomatology measured using the Brief Symptom Inventory, mood assessed using the POMS, heart rate and blood pressure Compared to smoking‐as‐usual, administration of placebo resulted in significantly increased WDS and endorsement of five items on the MWC (aggression, craving, depressed mood, irritability and sleep difficulty). The effects of THC administration were examined in these items only. Administration of the low dose of THC significantly suppressed WDS, and responding on the irritability, aggression and sleep difficulty items. The high THC dose additionally suppressed endorsement of the depressed mood and craving items |
Small sample size. It is possible that participants continued undetected low levels of cannabis use during abstinence |
| FAAH inhibitors | ||||
| PF‐04457845 | No | Primary [18] | Randomized, double‐blind study of cannabis‐dependent inpatients (5–8 days) then 3 weeks post‐discharge [18]. Cannabis withdrawal was the primary outcome during hospital admission. Male patients (n = 46) were administered PF‐04457845 4 mg once daily over the duration of the study or placebo (n = 24). No formal concurrent psychosocial treatment reported. PF‐04457845 significantly reduced cannabis withdrawal (MWSC) in early stages (days 0–2) but not later stages (days 2–4) of inpatient treatment and resulted in less cannabis use post‐discharge, compared to placebo. On average, the treatment group reported better overall sleep. There were no significant effects of interest on mood states measured using VAS. The drug group showed lower scores on a number of mood scales prior to drug administration (day 0). No serious adverse events were reported and no difference in side effects observed between groups [18] |
Requires further studies with males and females for generalizability A large‐scale multi‐centre study is under way Group differences observed in withdrawal and mood ratings prior to drug administration (day 0) |
| Atypical anti‐convulsants | ||||
| Quetiapine | No | Secondary [47] |
Randomized, double‐blind placebo‐controlled study of cannabis‐dependent individuals seeking treatment. Randomly assigned to titrate to dose target (300 mg) or maximum tolerated quetiapine (n = 66) or placebo (n = 64). Primary outcomes were measures of daily cannabis use. Secondary outcomes were weekly urinary THC concentration, craving (MCQ; MCr), withdrawal (MWC), sleep problems (MOS‐SS) and study retention.Quetiapine treatment significantly reduced cannabis withdrawal. With each week in the study, withdrawal scores decreased on average by 10.4% in the treatment and 6.5% in the placebo arm. No difference was observed in craving scores or sleep problems between the two groups. Time to dropout did not differ between groups |
Large dropout, 44% of randomized participants withdrew prior to completion of the study |
| Anti‐convulsants | ||||
| Gabapentin | No | Primary [50] |
Randomized, double‐blind study of treatment‐seeking, cannabis‐dependent outpatients [50]. Patients (n = 25) received gabapentin (1200 mg/day) for 11 weeks (followed by tapering) or placebo (n = 25). Manual‐guided, abstinence‐orientated individual counselling was provided to both groups Gabapentin significantly reduced cannabis withdrawal (MWC) and cannabis use over the course of the study, compared with placebo. Mood (BDI‐II) and sleep (PSQI) were also significantly improved. Medication was well tolerated and no difference in side effects reported between groups [50] |
One‐third of patients completed the trial. Validation studies with larger sizes and higher retention are required |
| Topiramate | No | Secondary [64] |
Heavy adolescent and young adult cannabis users (80% met cannabis use or dependence criteria) were double‐blind randomized to topiramate (titrated over 4 weeks then stabilized at 200 mg/day for 2 weeks, n = 40) or placebo (n = 26) over 6 weeks. Both groups received MET. Primary outcomes were efficacy and feasibility in reducing cannabis use. Secondary outcome was depression scores (BDI), a symptom of cannabis withdrawal There were no group differences on depression scores. Topiramate was poorly tolerated by this young sample (15–25 years of age) and of those participants who withdrew from the topiramate condition, 67% attributed their withdrawal to adverse medication side effects (depression, anxiety, difficulty with coordination or balance, weight loss and paraesthesia) [64] |
Approximately half (59%) of patients completed the trial. Topiramate was poorly tolerated by this young patient sample. Further research should first consider safety, dose and tolerability studies across age groups |
| Mood stabilizers | ||||
| Lithium | No | Primary [53] |
Randomized, double‐blind study of treatment‐seeking, cannabis‐dependent inpatients received either oral lithium (500 mg, n = 19) or placebo (n = 22) twice daily for 8 days. Cannabis withdrawal (CWS) was a primary outcome. No concurrent formal behavioural treatment. ‘Standard withdrawal care’ reported Lithium did not significantly affect total CWS scores or treatment retention compared to placebo. No serious adverse events were reported and no difference in side effects observed between groups. Post‐withdrawal measures of mood, anxiety and quality of life did not differ between groups [53] |
As noted by authors, two‐thirds of study participants were administered nitrazepam (a benzodiazepine) for sleep problems during their inpatient stay. This may have impacted CWS scores. Attrition was high (50% in lithium group) |
| Divalproex | No | Primary [63] |
Randomized, double‐blind study of treatment‐seeking, cannabis‐dependent outpatients received either divalproex sodium (n = 13) or placebo (n = 12). Active medication was titrated to a maximum 2000 mg per day, starting at 500 mg daily for days 1–7, 1000 mg for days 8–13 and 1500 mg for weeks 2–6. Maximal dose was only provided to patients with blood levels < 50 ng/ml. Patients experiencing side effects or blood levels > 120 ng/ml had their dose lowered. Irritability, depression and anxiety (measured by SIS, HSC) were main outcomes and reflected some features of cannabis withdrawal. All patient received ‘manualized relapse prevention therapy’ There were no differences between groups on these selected withdrawal symptoms. Marijuana craving measured using a VAS reduced over the duration of the trial but did not differ between groups. There was no substantial increase in physical complaints among those individuals who initially received divalproex sodium compared to placebo. Three patients were withdrawn from divalproex for jitteriness, depression and/or abdominal cramping |
Small trial. No standardized cannabis withdraw measure. > 90% male sample |
| SSRI | ||||
| Escitalopram | No | Secondary [59] |
Randomized, double‐blind study of treatment‐seeking, cannabis‐dependent outpatients received either escitalopram (10 mg/day, n = 26) or placebo (n = 26) for 9 weeks (+ 14 weeks of follow‐up). Cannabis withdrawal (CIWA) was a secondary outcome. Depression and anxiety (measured by BDI, STAI, HAQ) were also secondary outcomes and reflected some features of cannabis withdrawal. All patient received MET+CBT [55]. Escitalopram was no different to placebo on withdrawal scores or anxiety and depression scores during withdrawal and post‐withdrawal periods. Most treatment attrition was from the escitalopram group (16 of 26). Adverse events and side‐effects not specifically reported [59] |
Small sample size. Half of patients completed the initial 9 weeks of the study |
| Bupropion | No | Secondary [52] |
Randomized, double‐blind study of treatment‐seeking, cannabis‐dependent outpatients received either nefazodone (n = 36) or bupropion‐sustained release (n = 40) or placebo (n = 30) for 13 weeks (10 weeks medication). Nefazodone maximum dosage of 600 mg per day was reached beginning at 150 mg and increasing by 150 mg every 5 days if tolerated. Bupropion‐sustained release started at 150 mg, after 3 days increasing to 300 mg per day, if tolerated. Cannabis withdrawal was a secondary outcome. Anxiety (HAM‐A), irritability (SIS) and sleep disturbances (SMHSQ) reflected some features of cannabis withdrawal. All patient received a coping skills therapy programme There were no significant differences in cannabis withdrawal symptoms between nefazodone or bupropion‐sustained release, compared to placebo. Side effects were reported by 45% of the bupropion‐sustained release group (mainly headaches and nausea) and 42% of the nefazodone group (mainly diarrhoea), compared to 27% in placebo [52] |
Approximately 50% of patients withdrew before completion of the 10‐week medication phase |
| No | Primary [55] |
Randomized, double‐blind study of heavy cannabis users meeting cannabis abuse or dependence criteria who were asked to cease cannabis for 14 days received either bupropion‐sustained release (days 1–3, 150 mg once a day; days 4–21, 150 mg twice a day, n = 10 randomized, five completed) or placebo (n = 12 randomized, four completed). Cannabis withdrawal was a primary outcome (MWC). All patients received MET [55] There was no statistically significant difference between bupropion‐sustained release and placebo on withdrawal scores. Scores on depression (BDI) and anxiety (BAI) inventories did not differ between groups. No significant difference between groups in sleep measures [55] |
Small sample size. High attrition (> 50%). No ITT analysis | |
| Non‐benzodiazepine GABA(A) receptor agonist | ||||
| Zolpidem ‐extended release | No | Secondary [57] |
Cross‐over placebo design. Heavy cannabis users (at least 25 days/month) alternated between ad libitum cannabis use (mean 0.8 g per use) and cannabis abstinence (3 days). Extended‐release zolpidem (12.5 mg) or placebo was administered at bedtime during abstinence periods. The primary outcome was sleep quality (polysomnography, respiratory function, PSQI). Cannabis withdrawal was a secondary outcome (MWC and WDS) There were no differences between extended‐release zolpidem or placebo in cannabis withdrawal scores (MWC, WDS). Some features of cannabis withdrawal induced sleep problems (i.e. REM sleep) were improved with medication but some were not (i.e. time spent in different sleep stages, sleep latency), compared to placebo [57] |
As noted by authors, short withdrawal period (3 days). Non‐randomized. Double‐blind only to those administering drugs |
| No | Primary [61] |
Counterbalanced placebo design; 11 non‐treatment‐seeking heavy cannabis users (at least three joints daily, 5 days per week) over three 8‐day inpatient phases. Participants completed three distinct medication phases always beginning with placebo on day 1 with the following drugs taken daily for 7 days: placebo, 12.5 mg zolpidem alone and 12.5 mg zolpidem with 6 mg total nabilone. Drug order was randomized. Additionally, participants consumed cannabis in the following mannger: active cannabis (5.6% THC) on day one; inactive cannabis (0% THC) on days 2‐4; active cannabis on days 5‐8 as a masure of relapse. Cannabis was provided on day 1 by the experimenter, but during study days 2‐8 the cannabis had to be purchased on a per inhalation cost. Mood (non‐standard VAS), sleep (non‐standard VAS and wrist activity monitor), food intake and body weight reflected some features of cannabis withdrawal [61] Both medications decreased withdrawal‐related disruptions in sleep, compared to placebo. Only zolpidem in combination with nabilone decreased withdrawal‐related disruptions in mood and food intake, relative to placebo [61] |
Small sample. No cannabis withdrawal scale. Mood and sleep scales not standardized. Not randomized | |
| Α2A adrenergic receptor agonists | ||||
| Guanfacine | No | Secondary [58] |
Open‐label, single‐arm pilot trial of cannabis‐dependent treatment‐seeking outpatients (n = 22) [58]. Participants were titrated to maximum tolerable or target dose over a period of 2 weeks. This was lengthened to 8 weeks due to a greater than expected number of adverse events. Primary outcomes were feasibility (retention and side‐effects). Quantity and frequency of cannabis use and withdrawal (MCW‐10) were secondary outcomes to establish efficacy No significant change in withdrawal symptom severity over the duration of the study |
Small sample size with a low retention rate (~40% of enrolled participants completed the trial) No control condition |
BAI = Beck Anxiety Inventory; BDI and BDI–II = Beck Depression Inventory; CBD = cannabidiol; CBT = cognitive behavioural therapy; CWS = cannabis withdrawal scale; HAQ = HAM‐A = Hamilton anxiety scale; Hamilton anxiety questionnaire; HSC = Hopkins symptom checklist; ITT = intention to treat; MET = motivational enhancement therapy; MCQ = marijuana craving questionnaire; MCr = marijuana craving report; MOS‐SS = medical outcomes study—sleep scale; MWC and MWSC = marijuana withdrawal (symptom) checklist; POMS = profile of mood states; PSQI = Pittsburgh sleep quality index; RCT = randomized controlled trial; REM = rapid eye movement; SIS = Snaith irritability scale; SMHSQ = St Mary's Hospital sleep questionnaire; SSRI = selective serotonin re‐uptake inhibitors; STAI = state–trait anxiety inventory; THC = delta‐9‐tetrahydrocannabinol; VAS = visual analogue scale; WDS = composite withdrawal scale.
A 12‐week single‐arm cognitive behavioural therapy (CBT) study randomly assigned 13 regular cannabis users to selective serotonin re‐uptake inhibitors (SSRI) or placebo. It found no significant differences on the Clinical Institute Withdrawal Assessment (CIWA) scores (adapted for cannabis) [39] between patients who relapsed and those who did not. Changes in CIWA scores in response to CBT were not reported nor any differences between those given SSRI or placebo [40].
Despite limited evidence, standard clinical practice typically includes psychoeducation on the course and symptoms of withdrawal, coping with craving exercises, nutrition, hydration, physical exercise and sleep hygiene. It can also include motivational approaches and coping skills training [41]. Skills training in CBT such as relaxation approaches, pleasant activity scheduling, managing stress/mood/anger and goal‐setting may be of clinical benefit.
Physical exercise has been associated with improved scores in the Marijuana Craving Questionnaire—short form (MCQ‐SF) in a pilot study (n = 10) of non‐treatment‐seeking, cannabis‐dependent adults [42]. Standard sleep hygiene protocols [43] and CBT‐insomnia (CBT‐I [44]) may improve sleep in cannabis withdrawal, but this has not been well studied [45].
Medication of choice
Cannabis withdrawal pharmacotherapy
There are no medications approved to manage the cannabis withdrawal syndrome. Research on MAW has increased during the past 15 years, but remains less developed than for other drugs of abuse.
Nineteen placebo‐controlled studies (17 Clinical; two experimental) and one open‐label, non‐placebo controlled trial have been reported (Table 2). Sixteen were randomized designs. Fewer than half (n = 9) report cannabis withdrawal (or individual cannabis withdrawal symptoms) as a primary outcome and only three recruited more than 50 participants in the medication arm, two of which found a significant benefit from medication over placebo (dronabinol [46], quetiapine [47]). Attrition among the 17 clinical studies was typically greater than a third of the sample.
In the studies that used standardized measures of the cannabis withdrawal syndrome, six reported greater improvement in those given a cannabinoid medication than placebo (dronabinol [46], quetiapine [47], nabiximols [48, 49], gabapentin [50], oral THC [51]). Ten studies did not. This included trials of bupropion [52], FAAH inhibitor PF‐04457845 for the majority of the withdrawal period [18], lithium [53], dronabinol and lofexidine [54], bupropion [55], nabiximols [56], extended‐release zolpidem [57], guanfacine [58], escitalopram [59] and nabilone [60]. Some medications produced greater reductions in individual symptoms of cannabis withdrawal (e.g. zolpidem extended‐release improved some features of sleep [61], nabilone reduced craving and anxiety [62]) than placebo. This was not true in all cases (e.g. divalproex did not reduce irritability, depression and anxiety [63] and topiramate did not improve mood [64]).
The research on medications for MAW in treatment‐seeking, cannabis‐using populations is limited by the small number and low quality of studies. Larger replication studies are required to test the efficacy of agents that have shown promising results in small studies. MAW largely treats its symptoms with agents known to reduce them, but many of these drugs have not been well studied in people with CUD and their off‐label use is of uncertain efficacy and safety.
Medication use in clinical practice
No medications have been shown to be effective in MAW in randomized controlled trials (Table 2). In clinical practice, short‐term symptomatic medications have been used for (a) non‐specific general cannabis withdrawal syndrome features and (b) for specific withdrawal symptoms (e.g. sleep, nausea, anxiety, appetite stimulation) to improve patient comfort and retention during withdrawal (Table 3). As with all prescribing, a comprehensive medical, medicine and drug and alcohol use history should guide the use and doses of these medications. All medications have side‐effects and these need to be balanced against potential benefits from their unknown efficacy in this population.
TABLE 3.
Examples of medication used in clinical practice
| General withdrawal features (off‐label) | Medications |
|---|---|
|
Anxiety and agitation |
|
|
Severe tremors |
|
|
Nausea/stomach pain |
|
| Psychotic symptoms/hallucinations |
|
|
Sleep disturbances |
|
May test positive to cannabinoids in drug‐testing.
COMPLEX CASES
The case presented in this review reflects a common, complex presentation.
Polysubstance use is common in cannabis users, with alcohol use disorder (OR = 6.0) and nicotine use disorder (OR = 6.2) the most widely co‐used substances [27]. Psychiatric comorbidity is also common, with the two most common mental health problems (besides substance use disorders) mood and anxiety disorders [65, 66]. In cannabis use disorder the OR of having any mood disorder is 3.8 and for any anxiety disorder is 2.8 [27].
Presentation
A 48‐year‐old male presented to a primary care provider with stomach cramps, headache and elevated anxiety symptoms that have prevented him from working for 48 hours. He stated that while he has been off work, he has heard infrequent but multiple unfamiliar voices telling him that he is going to lose his job. The patient reports that he has been having relationship problems with his partner of 15 years, primarily due to his substance use. He ceased all substance use 60 hours prior to the assessment.
Psychosocial history
The patient is currently on sick leave but has a history of stable employment. He is married with no children. There are financial concerns due to time off work.
Medical history: he reports stomach cramps, headache, irritability/anger, loss of appetite, some chills and sweating. Previous history of hypertension, disturbed sleep, mild–moderate sleep apnoea; body mass index (BMI) is 35.
Psychiatric history: currently reports elevated anxiety and a history and previous diagnosis of unipolar depression. Denies history of schizophrenia or psychotic disorders. [Clinician query: cannabis withdrawal‐induced psychosis.] Denies thoughts of self‐harm.
Medication: escitalopram 20 mg (non‐compliant), captopril 25 mg (non‐compliant), continuous positive airway pressure (CPAP) therapy (non‐compliant).
Substance use history
The patient reports daily use of cannabis (average 2 g per day, or approximately eight joints), nicotine 15 ‘tailor‐made’ manufactured cigarettes per day plus ‘spins’ loose tobacco with high THC content cannabis plant ‘buds’. He reports infrequent use of alcohol (average three standard drinks peer week) and non‐prescribed prescription opioids (oxycodone 10 mg when available, typically one tablet per fortnight). The patient states his longest drug‐free period occurred 15 years ago for 6 months after meeting his now partner (aged 33 years). He scores 6 on the Alcohol Use Disorders Identification Test (AUDIT) [67], where ≥ 16 is suggestive of alcohol‐related problems, 13 on the Severity of Dependence Scale (SDS) [68], where ≥ 3 of 15 is indicative of cannabis‐related problems and 10 (range = 0–10) on the Fagerström Test for Nicotine Dependence (FTND [69]), indicating a high level of dependence. He reports no history of significant substance withdrawal.
Withdrawal assessment
Cannabis is commonly used with tobacco [70], and in treatment‐seeking cannabis users approximately two‐thirds also use tobacco [71]. Tobacco withdrawal symptoms overlap with cannabis withdrawal and may have a similar intensity and time‐course [72, 73]. Table 4 outlines the withdrawal features observed in this complex case and possible management. Nicotine replacement therapy (NRT) may be considered during the withdrawal period and post‐detoxification if the patient desires to quit nicotine.
TABLE 4.
Complex case: observed and self‐report symptoms and possible management
| Observed and self‐report symptoms | Cannabis | Nicotine | Possible management options |
|---|---|---|---|
| Withdrawal scale scores |
CWS [25] = 140 (range = 0–190) |
(observer range = 0–16) |
Main management approach for cannabis withdrawal syndrome: cannabinoid agonist (e.g. nabilone, off‐label) |
| Nausea, abdominal cramps, muscle aches | ++ | + |
Metoclopramide Non‐opioid analgesia Consider dronabinol (specific for nausea and general for cannabis withdrawal) |
| Headache | ++ | ++ |
Non‐opioid analgesia |
| Insomnia | ++ | ++ |
Sleep hygiene, CBT‐I Zolpidem, diazepam |
| Anxiety | ++ | ++ |
Supportive counselling Diazepam Recommence escitalopram |
| Psychosis (query cannabis withdrawal‐induced) | ++ |
– |
Quetiapine |
| Irritability | + | + |
Psychoeducation Diazepam |
| Aggressive behaviour | + | + |
Psychoeducation Diazepam |
| Restlessness | + | + |
Diazepam |
| Sweating and chills | + | + |
Supportive management |
| Decreased appetite | + | – |
Nutrition support Consider dronabinol (specific for appetite and general for cannabis withdrawal) |
++ Strong withdrawal feature present; + withdrawal feature present; − withdrawal features not present; CBT‐I = CBT‐insomnia.
The decision to recommend inpatient admission or outpatient withdrawal in this presentation relies upon an accurate assessment of the brief psychotic episodes and their potential impact on the patient's functioning. Withdrawal from other substance use that may increase risk of adverse outcomes (e.g. alcohol other CNS depressants, opioids) is not present. The patient denies suicidal thoughts, but reports dysthymic mood that is typical for him. He states that his cannabis use is heavier at night in an attempt to improve his sleep, and ceasing use has impaired sleep quality. He reports that his partner of 15 years is highly supportive of his cessation attempt and committed to a substance‐free relationship.
The patient reported a preference for home detoxification. He attended with his partner, who reports that they can monitor him closely over the next 5 days. The patient consented to attending primary care appointments daily over the next week. The main management approach to consider is a cannabinoid agonist (e.g. nabilone, off‐label) that could be slowly titrated upwards. If probable cannabis‐related withdrawal psychosis persists, inpatient withdrawal is indicated. Quetiapine (atypical antipsychotic) was introduced in this consultation for review over the following 5 days.
The patient and his partner were provided with psychoeducation on: the course and symptoms of withdrawal, exercises to cope with craving, nutrition, hydration, physical exercise and sleep hygiene. Basic skills in relaxation approaches and managing anger and aggressive behaviour were reviewed in the consultation. Admission to a CBT‐based outpatient relapse prevention programme was recommended and a referral provided.
POST‐DETOXIFICATION PROGNOSIS
In prospective clinical studies in adults [74] and adolescents [5, 75, 76] with CUD the presence and severity of cannabis withdrawal does not predict cannabis use after completion of MAW. However, Davis et al. [5] found that adolescents who met DSM‐5 criteria for cannabis withdrawal relapsed to cannabis use sooner than those who did not meet DSM‐5 criteria. More studies with larger patient numbers are required to confirm these findings. Regular cannabis users subjectively report that withdrawal symptoms reduce their desire to abstain from cannabis [20, 75].
RELAPSE PREVENTION
No pharmacological approaches have been approved to prevent risk of relapse to CUD during or after MAW [77]. Psychosocial‐based interventions are the first‐line treatment [77]. The two most effective stand‐alone behavioural interventions are cognitive–behavioural therapy (CBT) and motivational enhancement therapy (MET). Both have similar efficacy [78, 79, 80, 81, 82, 83]. There is some evidence that combined CBT and MET produces better outcomes than either approach alone [77, 81].
Abstinence‐based contingency management (CM) using incentives to motivate and sustain cessation is an effective adjunct to CBT and MET increases rates of sustained abstinence [77, 81]. In the short term (up to 12–14 weeks), combined MET and CBT doubles abstinence rates and reduces consumption by an average of 25% in those who continue to use cannabis, compared to non‐active treatment [81]. There is less evidence on efficacy in the longer term (> 9 months) after treatment. Other behavioural interventions for CUD that have been investigated include social support counselling (SS), drug education counselling (DE), relapse prevention (RP), mindfulness meditation (MM) and 12‐step mutual help groups. None of these interventions have enough data to support use at this time [77, 81].
Sustained cannabis abstinence is the most effective treatment goal for patients who have a CUD and who have completed MAW [77]. However, many patients enter treatment with moderation goals and clinicians must adapt their approaches to work effectively with these patients. Reduced use is a common outcome in outpatient studies, but how long these reductions are maintained and whether reduced use improves psychosocial functioning remains unclear. To retain patients with a moderation goal in a therapeutic relationship and reduce the risk of future cannabis related‐problems, low‐risk guidelines have been endorsed by a number of health jurisdictions (e.g. [84]) and health experts [85] (see Table 5).
TABLE 5.
Low‐risk cannabis consumption guidelines
| Using lower THC content products |
| Adopting methods other than inhalation (if inhaling, avoid ‘deep inhalation’) |
| Refraining from daily or near‐daily or binging on cannabis use |
| Where available, using legal and quality‐controlled cannabis products and devices |
| If cognitive performance is impaired, temporarily suspending or substantially reducing intensity of use (e.g. frequency/potency) |
| Abstaining while pregnant or breastfeeding |
| Avoiding cannabis while driving, using machinery or engaged in other high‐risk activities |
| Exercising caution in combining other psychoactive substances with cannabis use |
| Avoiding (or adjusting) use in the presence of psychosis, other psychiatric comorbidities and/or a history of substance use disorders |
Most cannabis users also use other substances [27, 28]. Meta‐analyses demonstrate that psychosocial treatments for polysubstance use have weak efficacy compared to single‐substance psychosocial treatments [86]. There are insufficient studies to recommend either treating multiple substances sequentially or concurrently. However, a recent meta‐analysis found that combined tobacco and/or cannabis interventions had a modest effect on reducing cannabis but not tobacco. These combined interventions did not increase tobacco or cannabis cessation rates [87].
NEW DEVELOPMENTS
The management of substance withdrawal typically includes pharmacological agents which reduce clinically significant withdrawal symptoms. Cannabis does not have any approved medications for MAW, despite a well‐recognized and clinically significant withdrawal profile. A number of novel agents have been examined with some promising results (Table 2). This is an important avenue of future research, with some agents showing early efficacy. For this reason, cannabis agonists have been cautiously used ‘off‐label’ for cannabis withdrawal.
The mainstay of cannabis withdrawal management has been psychosocial education, supportive counselling and behavioural therapies. Despite the wide use of these approaches in clinical practice, few empirical studies have been conducted. These approaches need to be evaluated in controlled research settings. Given the heterogeneity of cannabis withdrawal features and substantial individual variations between patients, new developments in the management of cannabis use disorder and withdrawal are likely to include more targeted behavioural approaches (e.g. [77, 88]). The provision of increased on‐line and digital approaches to assist patients in managing cannabis withdrawal may improve accessibility and reduce costs, compared to face‐to‐face health services.
The legalization of non‐medical cannabis use in a number of high‐income countries has reduced cannabis prices and increased sales of high‐potency cannabis products in these jurisdictions [89]. The cannabis industry is lobbying to reduce cannabis taxes, opposing restrictions on maximum THC levels and promoting the sale of high‐potency cannabis such as cannabis edibles, oils, extracts and waxes. Although the effects of these changes have not yet been formally evaluated, increased use of high‐potency cannabis is likely to increase the risks for CUD and the severity of withdrawal [77]. Public health messaging should include independent information and advice on the risks of using higher‐potency cannabis.
DECLARATION OF INTERESTS
J.P.C., D.S., A.J.B. and W.D.H. declare no competing interests. B.LeF. has obtained funding from Pfizer (GRAND Awards, including salary support) for investigator‐initiated projects. B.LeF. has some in‐kind donation of cannabis product from Aurora and medication donation from Pfizer and Bioprojet and was provided a coil for TMS study from Brainsway. B.LeF. has obtained industry funding from Canopy (through research grants handled by CAMH or University of Toronto), Bioprojet, ACS and Alkermes. B.LeF. has received in kind donations of nabiximols from GW Pharma for past studies funded by CIHR and NIH. He has been a consultant for Shionogi.
AUTHOR CONTRIBUTIONS
Jason Connor: Conceptualization; project administration; supervision. Daniel Stjepanović: Conceptualization; data curation. Alan Budney: Conceptualization. Bernard Le Foll: Conceptualization; data curation. Wayne Hall: Conceptualization; supervision.
Supporting information
Data S1. Supporting Information
ACKNOWLEDGEMENTS
The Australian National Centre for Youth Substance Use Research (J.P.C., W.D.H., D.S.) is supported by funding from the Australian Government provided under the Commonwealth Drug and Alcohol Program grant. B.LeF. is supported by CAMH, a clinician–scientist award from the department of Family and Community Medicine of the University of Toronto and a Chair in Addiction Psychiatry from the department of Psychiatry of University of Toronto. A.J.B. is supported in part by US National Institute on Drug Abuse (NIDA) grants P30DA029926, T32DA037202 and R01DA015186. The funding bodies had no role in the study design, collection, analysis or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication. We would like to thank Professor John B. Saunders for his expert review and feedback on previous versions of this manuscript.
Connor JP, Stjepanović D, Budney AJ, Le Foll B, Hall WD. Clinical management of cannabis withdrawal. Addiction. 2022;117:2075–2095. 10.1111/add.15743
Funding information None.
REFERENCES
- 1. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 2. Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–8. [DOI] [PubMed] [Google Scholar]
- 3. Taylor L, Crockett J, Tayo B, Checketts D, Sommerville K. Abrupt withdrawal of cannabidiol (CBD): a randomized trial. Epilepsy Behav. 2020;104:106938. [DOI] [PubMed] [Google Scholar]
- 4. Bahji A, Stephenson C, Tyo R, Hawken ER, Seitz DP. Prevalence of cannabis withdrawal symptoms among people with regular or dependent use of cannabinoids: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3:e202370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Davis JP, Smith DC, Morphew JW, Lei X, Zhang S. Cannabis withdrawal, posttreatment abstinence, and days to first cannabis use among emerging adults in substance use treatment: a prospective study. J Drug Issues. 2016;46:64–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Castaneto MS, Gorelick DA, Desrosiers NA, Hartman RL, Pirard S, Huestis MA. Synthetic cannabinoids: epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Craft S, Ferris JA, Barratt MJ, Maier LJ, Lynskey MT, Winstock AR, et al. Clinical withdrawal symptom profile of synthetic cannabinoid receptor agonists and comparison of effects with high potency cannabis. Psychopharmacology. 2021. Available at: 10.1007/s00213-021-05945-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Claus BB, Specka M, McAnally H, Scherbaum N, Schifano F, Bonnet U. Is the urine cannabinoid level measured via a commercial point‐of‐care semiquantitative immunoassay a cannabis withdrawal syndrome severity predictor? Front Psychiatry. 2020;11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. World Health Organization (WHO) . International Classification of Diseases for Mortality and Morbidity Statistics, 11th revision [internet]. Geneva, Switzerland: WHO; 2018. [Google Scholar]
- 10. Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocannabinoids. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, editorsPhytocannabinoids [internet], Progress in the Chemistry of Organic Natural Products 103 Cham: Springer International Publishing; 2017. p. 61–101 Available at: 10.1007/978-3-319-45541-9_3 [DOI] [PubMed] [Google Scholar]
- 11. Morales P, Hurst DP, Reggio PH. Molecular targets of the phytocannabinoids: a complex picture. In: Kinghorn AD, Falk H, Gibbons S, Kobayashi J, editorsPhytocannabinoids, Progress in the Chemistry of Organic Natural Products 103 Cham: Springer International Publishing; 2017. p. 103–31. Available at: 10.1007/978-3-319-45541-9_4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bow EW, Rimaldi JM. The structure–function relationships of classical cannabinoids: CB1/CB2 modulation. Perspect Med Chem. 2016;8:17–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laprairie RB, Bagher AM, Kelly MEM, Denovan‐Wright EM. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor: Negative allosteric modulation of CB1 by cannabidiol. Br J Pharmacol. 2015;172:4790–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Martínez‐Pinilla E, Varani K, Reyes‐Resina I, Angelats E, Vincenzi F, Ferreiro‐Vera C, et al. Binding and signaling studies disclose a potential allosteric site for cannabidiol in cannabinoid CB2 receptors. Front Pharmacol. 2017;23(8):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huestis MA, Gorelick DA, Heishman SJ, Preston KL, Nelson RA, Moolchan ET, et al. Blockade of effects of smoked marijuana by the CB1‐selective cannabinoid receptor antagonist SR141716. Arch Gen Psychiatry. 2001;58(4):322–28. [DOI] [PubMed] [Google Scholar]
- 16. Cox EJ, Maharao N, Patilea‐Vrana G, Unadkat JD, Rettie AE, McCune JS, et al. A marijuana‐drug interaction primer: precipitants, pharmacology, and pharmacokinetics. Pharmacol Ther. 2019;201:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boileau I, Mansouri E, Williams B, Le Foll B, Rusjan P, Mizrahi R, et al. Fatty acid amide hydrolase binding in brain of cannabis users: imaging with the novel radiotracer [11C]CURB. Biol Psychiatry. 2016;80:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. D'Souza DC, Cortes‐Briones J, Creatura G, Bluez G, Thurnauer H, Deaso E, et al. Efficacy and safety of a fatty acid amide hydrolase inhibitor (PF‐04457845) in the treatment of cannabis withdrawal and dependence in men: a double‐blind, placebo‐controlled, parallel group, phase 2a single‐site randomised controlled trial. Lancet Psychiatry. 2019;6:35–45. [DOI] [PubMed] [Google Scholar]
- 19. Lichtman AH, Martin BR. Marijuana withdrawal syndrome in the animal model. J Clin Pharmacol. 2002;42:20S–7S. [DOI] [PubMed] [Google Scholar]
- 20. Budney AJ. Review of the validity and significance of cannabis withdrawal syndrome. Am J Psychiatry. 2004;161:1967–77. [DOI] [PubMed] [Google Scholar]
- 21. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following oral THC administration to humans. Psychopharmacology. 1999;141:385–94. [DOI] [PubMed] [Google Scholar]
- 22. Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. [DOI] [PubMed] [Google Scholar]
- 23. Budney AJ, Moore BA, Vandrey RG, Hughes JR. The time course and significance of cannabis withdrawal. J Abnorm Psychol. 2003;112:393–402. [DOI] [PubMed] [Google Scholar]
- 24. Budney AJ, Novy PL, Hughes JR. Marijuana withdrawal among adults seeking treatment for marijuana dependence. Addiction. 1999;94:1311–22. [DOI] [PubMed] [Google Scholar]
- 25. Allsop DJ, Norberg MM, Copeland J, Fu S, Budney AJ. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 2011;119:123–9. [DOI] [PubMed] [Google Scholar]
- 26. Herrmann ES, Weerts EM, Vandrey R. Sex differences in cannabis withdrawal symptoms among treatment‐seeking cannabis users. Exp Clin Psychopharmacol. 2015;23:415–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hasin DS, Kerridge BT, Saha TD, Huang B, Pickering R, Smith SM, et al. Prevalence and correlates of DSM‐5 cannabis use disorder, 2012–2013: findings from the National Epidemiologic Survey on Alcohol and Related Conditions—III. Am J Psychiatry. 2016;173:588–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Connor JP, Feeney GFX, Kelly AB, Saunders JB. Polysubstance Use. In: The SAGE Handbook of Drug and Alcohol Studies. London, UK: SAGE Publications Ltd; 2016. p. 283–305. [Google Scholar]
- 29. Antoniou T, Bodkin J, Ho JM‐W. Drug interactions with cannabinoids. Can Med Assoc J. 2020;192:E206–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peters EN, Budney AJ, Carroll KM. Clinical correlates of co‐occurring cannabis and tobacco use: a systematic review: cannabis–tobacco clinical correlates. Addiction. 2012;107:1404–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Teesson M, Slade T, Swift W, Mills K, Memedovic S, Mewton L, et al. Prevalence, correlates and comorbidity of DSM‐IV cannabis use and cannabis use disorders in Australia. Aust NZ J Psychiatry. 2012;46:1182–92. [DOI] [PubMed] [Google Scholar]
- 32. Hasan A, von Keller R, Friemel CM, Hall W, Schneider M, Koethe D, et al. Cannabis use and psychosis: a review of reviews. Eur Arch Psychiatry Clin Neurosci. 2020;270:403–12. [DOI] [PubMed] [Google Scholar]
- 33. Lerner A, Klein M. Dependence, withdrawal and rebound of CNS drugs: an update and regulatory considerations for new drugs development. Brain Commun. 2019;1(1):fcz025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Queensland Health Queensland Alcohol and Drug Withdrawal Clinical Practice Guidelines [internet]. Herston, QLD: Mental Health, Alcohol and Other Drugs Directorate; 2012, p. 128. Available at: https://insight.qld.edu.au/shop/queensland-alcohol-and-drug-withdrawal-clinical-practice-guidelines-queensland-health-2012 accessed 10 August 2021.
- 35. Goodwin RS, Darwin WD, Chiang CN, Shih M, Li S‐H, Huestis MA. Urinary elimination of 11‐nor‐9‐carboxy‐Δ9‐tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J Anal Toxicol. 2008;32:562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Verweij KJH, Agrawal A, Nat NO, Creemers HE, Huizink AC, Martin NG, et al. A genetic perspective on the proposed inclusion of cannabis withdrawal in DSM‐5. Psychol Med. 2013;43:1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bedillion MF, Ansell EB. Differences in cannabis withdrawal symptoms for men and women over 21 days. Ann Behav Med. 2020;54:S385–5. [Google Scholar]
- 38. Bonnet U, Preuss U. The cannabis withdrawal syndrome: current insights. Subst Abuse Rehabil. 2017;8:9–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA‐Ar). Addiction. 1989;84:1353–7. [DOI] [PubMed] [Google Scholar]
- 40. Weinstein A, Miller H, Tal E, Avi IB, Herman I, Bar‐Hamburger R, et al. Treatment of cannabis withdrawal syndrome using cognitive–behavioral therapy and relapse prevention for cannabis dependence. J Group Addict Recov. 2010;5:240–63. [Google Scholar]
- 41. Steinberg KL, Roffman RA, Carroll KM, McRee B, Babor TF, Miller M, et al. Brief Counseling for Marijuana Dependence: A Manual for Treating Adults. Report No.: HHS Publication no. (SMA) 12‐4211. Rockville, MD: Center for Substance Abuse Treatment, Substance Abuse and Mental Health Services Administration; 2005. p. 208. [Google Scholar]
- 42. Buchowski MS, Meade NN, Charboneau E, Park S, Dietrich MS, Cowan RL, et al. Aerobic exercise training reduces cannabis craving and use in non‐treatment seeking cannabis‐dependent adults. PLOS ONE. 2011;6:e17465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Posner D, Gehrman PR. Sleep hygiene. In: Behavioral Treatments for Sleep Disorders [internet]. London, UK: Elsevier; 2011. [cited 2021 Aug 12], pp. 31–43. Available at: https://linkinghub.elsevier.com/retrieve/pii/B9780123815224000031 accessed 12 August 2021. [Google Scholar]
- 44. Trauer JM, Qian MY, Doyle JS, Rajaratnam SMW, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta‐analysis. Ann Intern Med. 2015;163:191–204. [DOI] [PubMed] [Google Scholar]
- 45. Shahzadi M, Abbas Q. Individualised cognitive behaviour therapy in patients of substance use disorders: three case studies. J Pak Med Assoc. 2020;70:1657–60. [DOI] [PubMed] [Google Scholar]
- 46. Levin FR, Mariani JJ, Brooks DJ, Pavlicova M, Cheng W, Nunes EV. Dronabinol for the treatment of cannabis dependence: a randomized, double‐blind, placebo‐controlled trial. Drug Alcohol Depend. 2011;116:142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mariani JJ, Pavlicova M, Jean Choi C, Basaraba C, Carpenter KM, Mahony AL, et al. Quetiapine treatment for cannabis use disorder. Drug Alcohol Depend. 2021;218:108366. [DOI] [PubMed] [Google Scholar]
- 48. Allsop DJ, Copeland J, Lintzeris N, Dunlop AJ, Montebello M, Sadler C, et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry. 2014;71:281–91. [DOI] [PubMed] [Google Scholar]
- 49. Trigo JM, Lagzdins D, Rehm J, Selby P, Gamaleddin I, Fischer B, et al. Effects of fixed or self‐titrated dosages of Sativex on cannabis withdrawal and cravings. Drug Alcohol Depend. 2016;161:298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mason BJ, Crean R, Goodell V, Light JM, Quello S, Shadan F, et al. A proof‐of‐concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis‐dependent adults. Neuropsychopharmacology. 2012;37:1689–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Budney AJ, Vandrey RG, Hughes JR, Moore BA, Bahrenburg B. Oral delta‐9‐tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 2007;86:22–9. [DOI] [PubMed] [Google Scholar]
- 52. Carpenter KM, McDowell D, Brooks DJ, Cheng WY, Levin FR. A preliminary trial: double‐blind comparison of nefazodone, bupropion‐sr, and placebo in the treatment of cannabis dependence. Am J Addict. 2009;18:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Johnston J, Lintzeris N, Allsop DJ, Suraev A, Booth J, Carson DS, et al. Lithium carbonate in the management of cannabis withdrawal: a randomized placebo‐controlled trial in an inpatient setting. Psychopharmacology. 2014;231:4623–36. [DOI] [PubMed] [Google Scholar]
- 54. Levin FR, Mariani JJ, Pavlicova M, Brooks D, Glass A, Mahony A, et al. Dronabinol and lofexidine for cannabis use disorder: a randomized, double‐blind, placebo‐controlled trial. Drug Alcohol Depend. 2016;159:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Penetar DM, Looby AR, Ryan ET, Maywalt MA, Lukas SE. Bupropion reduces some of the symptoms of marihuana withdrawal in chronic marihuana users: a pilot study. Subst Abuse. 2012;6:SART.S9706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Trigo JM, Soliman A, Quilty LC, Fischer B, Rehm J, Selby P, et al. Nabiximols combined with motivational enhancement/cognitive behavioral therapy for the treatment of cannabis dependence: a pilot randomized clinical trial. PLOS ONE. 2018;31(13):e0190768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vandrey R, Smith MT, McCann UD, Budney AJ, Curran EM. Sleep disturbance and the effects of extended‐release zolpidem during cannabis withdrawal. Drug Alcohol Depend. 2011;117:38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dakwar E, Mahony A, Choi CJ, Pavlicova M, Brooks D, Mariani JP, et al. Guanfacine extended‐release for cannabis use disorder: a pilot feasibility trial. Am J Drug Alcohol Abuse. 2020;46:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Weinstein AM, Miller H, Bluvstein I, Rapoport E, Schreiber S, Bar‐Hamburger R, et al. Treatment of cannabis dependence using escitalopram in combination with cognitive–behavior therapy: a double‐blind placebo‐controlled study. Am J Drug Alcohol Abuse. 2014;40:16–22. [DOI] [PubMed] [Google Scholar]
- 60. Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Varenicline and nabilone in tobacco and cannabis co‐users: effects on tobacco abstinence, withdrawal and a laboratory model of cannabis relapse. Addict Biol. 2019;24:765–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Herrmann ES, Cooper ZD, Bedi G, Ramesh D, Reed SC, Comer SD, et al. Effects of zolpidem alone and in combination with nabilone on cannabis withdrawal and a laboratory model of relapse in cannabis users. Psychopharmacology. 2016;233:2469–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hill KP, Palastro MD, Gruber SA, Fitzmaurice GM, Greenfield SF, Lukas SE, et al. Nabilone pharmacotherapy for cannabis dependence: a randomized, controlled pilot study. Am J Addict. 2017;26:795–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Levin FR, McDowell D, Evans SM, Nunes E, Akerele E, Donovan S, et al. Pharmacotherapy for marijuana dependence: a double‐blind, placebo‐controlled pilot study of divalproex sodium. Am J Addict. 2004;13:21–32. [DOI] [PubMed] [Google Scholar]
- 64. Miranda R, Treloar H, Blanchard A, Justus A, Monti PM, Chun T, et al. Topiramate and motivational enhancement therapy for cannabis use among youth: a randomized placebo‐controlled pilot study: topiramate and cannabis use. Addict Biol. 2017;22:779–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rehm J, Shield KD. Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep. 2019;21:1–7. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization . Depression and Other Common Mental Disorders: Global Health Estimates [internet]. Geneva: World Health Organization; 2017. Available at: https://www.who.int/publications/i/item/depression-global-health-estimates accessed 21 September 2021. [Google Scholar]
- 67. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction. 1993;88:791–804. [DOI] [PubMed] [Google Scholar]
- 68. Swift W, Copeland J, Hall W. Choosing a diagnostic cut‐off for cannabis dependence. Addiction. 1998;93:1681–92. [DOI] [PubMed] [Google Scholar]
- 69. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom K‐O. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Addiction. 1991;86:1119–27. [DOI] [PubMed] [Google Scholar]
- 70. Connor JP, Gullo MJ, White A, Kelly AB. Polysubstance use: diagnostic challenges, patterns of use and health. Curr Opinion Psychiatry. 2014;27:269–75. [DOI] [PubMed] [Google Scholar]
- 71. Connor JP, Gullo MJ, Chan G, Young RMCD, Hall WD, Feeney GFX. Polysubstance use in cannabis users referred for treatment: drug use profiles, psychiatric comorbidity and cannabis‐related beliefs. Front Psychiatry. 2013;4:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z. Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat. 2008;35:362–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vandrey RG, Budney AJ, Moore BA, Vandrey RG, Budney AJ, Moore BA, et al. A cross‐study comparison of cannabis and tobacco withdrawal. Am J Addict. 2005;14:54–63. [DOI] [PubMed] [Google Scholar]
- 74. Arendt M, Rosenberg R, Foldager L, Sher L, Munk‐Jørgensen P. Withdrawal symptoms do not predict relapse among subjects treated for cannabis dependence. Am J Addict. 2007;16:461–7. [DOI] [PubMed] [Google Scholar]
- 75. Chung T, Martin CS, Cornelius JR, Clark DB. Cannabis withdrawal predicts severity of cannabis involvement at 1‐year follow‐up among treated adolescents. Addiction. 2008;103:787–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Greene MC, Kelly JF. The prevalence of cannabis withdrawal and its influence on adolescents’ treatment response and outcomes: a 12‐month prospective investigation. J Addict Med. 2014;8:359–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Connor JP, Stjepanović D, Le Foll B, Hoch E, Budney AJ, Hall WD. Cannabis use and cannabis use disorder. Nat Rev Dis Primers. 2021;7:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Cooper K, Chatters R, Kaltenthaler E, Wong R. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol Assess. 2015;19:1–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Danovitch I, Gorelick DA. State of the art treatments for cannabis dependence. Psychiatr Clin North Am. 2012;35:309–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Davis ML, Powers MB, Handelsman P, Medina JL, Zvolensky M, Smits JA. Behavioral therapies for treatment‐seeking cannabis users: a meta‐analysis of randomized controlled trials. Eval Health Prof. 2015;38:94–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Gates PJ, Sabioni P, Copeland J, Le Foll B, Gowing L. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;CD005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lévesque A, Le Foll B. When and how to treat possible cannabis use disorder. Med Clin North Am. 2018;102:6676–81. [DOI] [PubMed] [Google Scholar]
- 83. Sabioni P, Le Foll B. Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. Focus. 2019;17:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, et al. Lower‐risk cannabis use guidelines: a comprehensive update of evidence and recommendations. Am J Public Health. 2017;107:e1–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Fischer B, Robinson T, Bullen C, Curran V, Jutras‐Aswad D, Medina‐Mora ME, et al. Lower‐Risk Cannabis Use Guidelines (LRCUG) for reducing health harms from non‐medical cannabis use: a comprehensive evidence and recommendations update. Int J Drug Policy. 2021;103381. [DOI] [PubMed] [Google Scholar]
- 86. Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, Otto MW. A meta‐analytic review of psychosocial interventions for substance use disorders. Am J Psychiatry. 2008;165:179–87. [DOI] [PubMed] [Google Scholar]
- 87. Walsh H, McNeill A, Purssell E, Duaso M. A systematic review and Bayesian meta‐analysis of interventions which target or assess co‐use of tobacco and cannabis in single‐ or multi‐substance interventions. Addiction. 2020;115:1800–14. [DOI] [PubMed] [Google Scholar]
- 88. Gullo MJ, Papinczak ZE, Feeney GFX, Young RM, Connor JP. Precision mental health care for cannabis use disorder: utility of a biosocial cognitive theory to inform treatment. Front Psychiatry. 2021;12:643107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Smart R, Caulkins JP, Kilmer B, Davenport S, Midgette G. Variation in cannabis potency and prices in a newly legal market: evidence from 30 million cannabis sales in Washington State: legal cannabis potency and price variation. Addiction. 2017;112:2167–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Centre for Alcohol and Other Drugs Drug and Alcohol Withdrawal Clinical Practice Guidelines [internet]. Sydney, NSW: NSW Department of Health; 2008 Jul p. 102. Available at: https://www1.health.nsw.gov.au/pds/ActivePDSDocuments/GL2008_011.pdf accessed 10 August 2021.
- 91. Hughes JR. Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry. 1986;43:289–94. [DOI] [PubMed] [Google Scholar]
- 92. Hughes J, Hatsukami DK. Errors in using tobacco withdrawal scale. Tob Control. 1998;7:92–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting Information


