Abstract
Introduction:
Psoriatic Arthritis (PsA) and Ankylosing spondylitis (AS) are chronic inflammatory diseases associated with a higher risk of cardio-metabolic comorbidities compared to the general population. Individual studies examining mortality in these patients have produced conflicting results. We performed a systematic review and meta-analysis to analyze the all-cause and cause-specific mortality in PsA and AS from the available literature.
Methods:
A comprehensive database search was performed for studies reporting all-cause or cause-specific mortality in patients with PsA and AS compared with the general population. Pooled relative risks (RRs) were calculated using random-effects model.
Results:
We included 19 studies (11 of PsA, 7 of AS, 1 of both). In PsA studies, there was no increased mortality compared to the general population (RR: 1.12, 95% CI: 0.96–1.30, 10 studies). We found a higher all-cause mortality in females (RR= 1.19, 95% CI: 1.04–1.36) but not in male (RR: 1.02, 95% CI 0.66–1.59) PsA patients. Cardiovascular, respiratory, and infection specific mortality risks were significantly higher for PsA patients (RR: 1.21, 95% CI: 1.06–1.38; RR: 3.37, 95% CI: 1.30–8.72; and 2.43, 95% CI: 1.01–5.84, respectively), but not cancer-related mortality (RR: 1.01, 95% CI: 0.91–1.11). In AS, we found a higher risk of death from all causes (RR 1.64, 95% CI: 1.49–1.80, 6 studies) and cardiovascular causes (RR 1.35, 95% CI: 1.01–1.81, 3 studies) compared to the general population. All-cause mortality was high in both males (RR 1.56, 95% CI: 1.43–1.71) and female (RR 1.85, 95% CI: 1.56–2.18) AS patients. The included AS studies did not report mortality data for non-cardiovascular causes.
Conclusion:
This systematic review and meta-analysis showed a significantly increased risk of overall mortality in AS, but not PsA. Cardiovascular-specific mortality was higher for both PsA and AS, which emphasizes the importance of early screening and management of cardiovascular risk factors.
Keywords: spondyloarthritis, psoriatic arthritis, ankylosing spondylitis, mortality, epidemiology, comorbidity
Introduction:
Spondyloarthritides (SpA), a group of chronic inflammatory diseases involving the spine, peripheral joints, and entheseal sites, affect up to 2.5% of the population [1]. Epidemiological data in SpA is mostly limited to the two common subtypes: psoriatic arthritis (PsA) and ankylosing spondylitis (AS), also known as radiographic axial SpA. The increased mortality risk from autoimmune diseases such as systemic lupus erythematosus and rheumatoid arthritis compared to the general population is well recognized [2–3]. However, the evidence for mortality risk in PsA and AS has been inconsistent, possibly because of inherent heterogeneity in the study populations and protocols, along with the limited number of observational studies performed to date. It is postulated that a higher prevalence of cardiometabolic conditions such as hypertension, dyslipidemia, diabetes, and obesity in comparison to the general population is associated with higher mortality in SpA [3–7]. Other associated comorbidities include depression, infections, malignancies, chronic obstructive and restrictive lung diseases with variable prevalence and unclear association with mortality [8–10]. Shared genetic and immunologic pathways, systemic inflammation, therapies used for SpA, and increased prevalence of risk factors such as smoking, and alcohol intake may also have a contributory role. The objective of this study was to perform a systematic review and meta-analysis combining all the available literature to date to investigate whether overall mortality is increased in PsA and AS and how associated comorbidities influence this risk. A better understanding of the overall mortality in these two most prevalent subtypes of SpA and associated comorbidities may help devise preventive and screening measures in this population and advance our knowledge of disease pathogenesis.
Materials and methods:
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. Four authors (HC, NB, KS, AD) in two pairs independently participated in the literature search, study selection, data extraction, and methodological quality assessment. Any disagreements were resolved by discussion, or by a fifth author (PK) if an agreement was not reached.
Literature search
A comprehensive search of several databases from each database’s inception to April 9, 2021, was conducted. The databases included Ovid MEDLINE® and MEDLINE® Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Ovid MEDLINE® Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, and Scopus. The search strategy was designed and conducted by an experienced librarian with input from the study’s principal investigator (Supplementary File 1). A controlled vocabulary supplemented with keywords was used to search for studies of the risk of overall and cause-specific mortality in psoriatic arthritis and ankylosing spondylitis patients. Only studies published in English were included.
Study selection
We screened the titles and abstracts that resulted from database search and then reviewed full texts of studies that were included by at least one reviewer based on the abstract and title.
We included all original reports fulfilling the following criteria: 1) published, peer-reviewed, English-language cohort studies, 2) reported all-cause or cause-specific mortality risk estimates (relative risks [RRs], crude mortality rate ratios, standardized mortality ratios [SMRs], or hazard ratios with 95% confidence intervals [CIs]), and 3) compared mortality in PsA and AS patients with the general population. For cause-specific mortality, we examined cardiovascular, respiratory, renal, accident/suicide, and malignancy. Case reports, case series, review articles, commentaries, and editorials were excluded. Bibliographies belonging to included studies, reviews, and relevant articles were screened for additional studies.
Studies that investigated only certain subgroups within PsA or AS were also excluded (e.g., studies including only HLA-B27 positive AS patients [11] or only those with vertebral fractures) [12]. We screened out duplicate publications. Full-text articles were given preference over abstracts.
Data extraction and assessment of risk of bias
We extracted information from the included studies on a standardized form. The extracted data included first author, publication year and country, representativeness of the population of PsA and AS patients (inpatient and/or outpatient), adjustments to age and sex, representativeness of comparison group, all-cause and cause-specific mortality estimates. The methodological quality (risk of bias) was evaluated using the Newcastle Ottawa Scale (NOS), (Supplementary File 2)[13]. We modified the tool so that we did not use a numeric value to represent quality, rather, we identified the factors that would increase the risk of bias in this specific context and used them to make an overall judgment about bias. We considered studies that did not adjust mortality estimates or used administrative codes for PsA diagnosis to be at high risk of bias. Overall certainty in the evidence was evaluated using the GRADE approach (Grading of Recommendations, Assessment, Development and Evaluation [14]. To assess heterogeneity, we used the I2 statistic, assuming values of 25%, 50%, and 75% to represent low, moderate, and high heterogeneity, respectively [15]. We conducted sensitivity analyses for only population-based studies and assessed the influence of individual studies on the overall effect by omitting one study at a time with overlapping data or high risk of bias. We performed subgroup analyses of older (before 2010) and newer (after 2010) studies to see if any difference could be seen in the studies in the post-biologic era. Finally, to assess the potential for publication bias, we visually inspected the funnel plots (Supplementary File 3).
Statistical analyses
All statistical analyses were conducted using RevMan 5.4 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Meta-analyses of all-cause mortality, sex-adjusted overall mortality, and cause-specific mortality were performed. We calculated relative risks (RRs) with 95% confidence intervals (CI) as the measure of association. Standardized mortality ratios (SMRs), hazard ratios, mortality rate ratios, and odds ratios were considered comparable estimates of RRs. We chose a random-effects model (DerSimonian and Laird) [16] because of potential heterogeneity among the studies. If a study provided multiple estimates, we used the multivariable-adjusted values from the best-reported model. Similarly, if mortality rates were provided for multiple time points, we included data from the last follow-up provided [17]. To maintain independence results from the same administrative data in different studies were included only in separate meta-analyses. For example, among the two studies by Ogdie et al. using the UK THIN Database, one of them reporting all-cause mortality [18] was used in overall mortality meta-analysis, and the more recent study reporting cause-specific mortality was used for separate meta-analyses [19] for cause-specific mortality. We did include the two studies from Olmsted County, MN, [20,21] for overall PsA mortality because these studies were non-overlapping and included patients from different years in both case and comparison groups.
Results
Studies selected and characteristics
The study selection flowchart is depicted in Figure 1. We screened 3124 articles by title and abstract and assessed 88 full-text articles for eligibility. We ultimately included 19 studies that met our inclusion criteria: eleven for PsA, seven for AS, and one study mentioned outcomes for both PsA and AS (Tables 1,2). The studies were conducted in 10 countries: Canada, Denmark, France, Hong Kong, Korea, Norway, Sweden, Taiwan, UK, and USA [17–34]. The study period (including follow-ups) varied between 4 to 40 years. Because of possible sample overlap, we compared studies with similar country of origin and data sources. In case of overlapping data, the most recently published and largest studies from a given population were included. Among PsA studies, we found overlapping data or data from different years in a few databases, including three from Canada [22,35,36] two studies each from Denmark, [23,37] Taiwan [24,38], and Olmsted County, MN USA [31,32]. Similarly, there were two studies from the UK The Health Improvement Network (THIN) database [18,19]. Out of these studies, the following containing the most recent data were included to maintain independence: 1) for all-cause mortality, we included Ogdie et al. (2014), Dai et al. (2018), Skov et al. (2019), and Elalouf et al. (2020) [18,22–24]; 2) for cause-specific and cardiovascular mortality, we included Ogdie et al. (2017), Skov et al. (2019) and Elalouf et al. (2020) [19,22,23]. Among two AS studies from Korea that had overlapping data [25,39] we included the study by Bae et al. (2019) for all-cause mortality as it included data from more recent years. The mean age of PsA patients ranged from 43.7 to 59.5 years, and the percentage of female patients ranged from 44.0–49.6%. The mean age of AS patients was 37.0 to 70.5 years, with females representing 18.3–47.0% of patients included in the studies.
Figure 1.
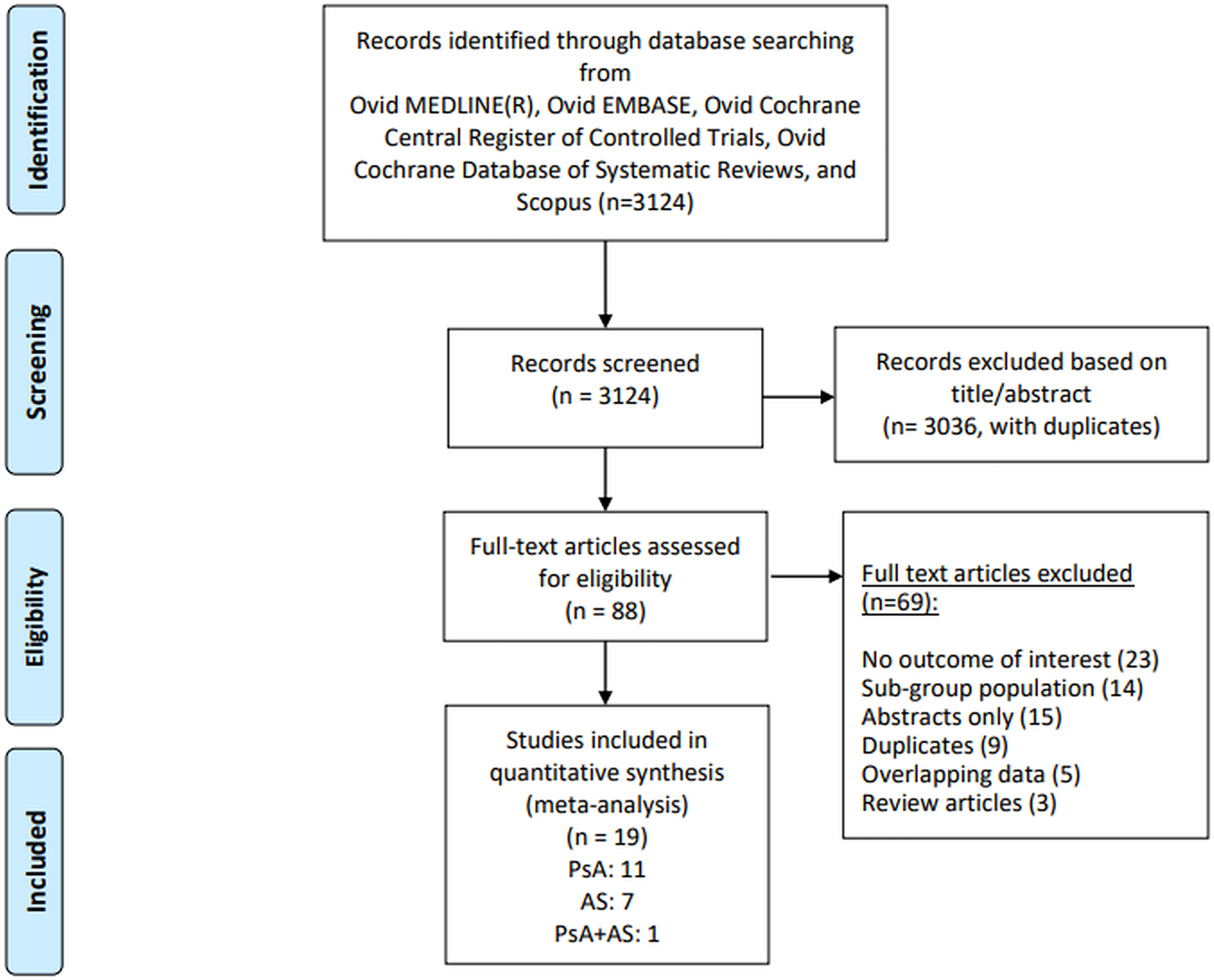
Flow chart describing systematic search and study selection process
Table 1.
Study characteristics of all cause and cause-specific mortality in psoriatic arthritis
| Author, year | Geographic location | Study design (data source) | Study period (total duration in years with follow up) | Population | No. of patients | Definition of cases | No. of PsA patients | Comparison group | Mean age, years | Female (%) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| All- cause mortality | ||||||||||
| Wilson et al. 2009 [20] | Olmsted Country, USA | Population-based, retrospective cohort (Rochester epidemiology project) | 1970–1999 (29) | 18+ | 124,277 | CASPAR | 147 | General Population | 42.7 +/− 15.2 | 39 |
| Buckley et al. 2010 [29] | Bath, UK | Retrospective cohort, single center (National death registry/NHS strategic tracing service) | 1985–2007 (23) | 18+ | 453 | Moll and Wright/CASPAR | 453 | General population | 49 | 48.8 |
| Mok et al. 2011 [22] | Hong Kong, China | Population- Based, Retrospective cohort (CDARS - outpatient clinics and inpatient admissions) | 1999–2008 (9) | NR | 18627 | ICD-9 | 778 | General population | 51.7 +/− 13.2 | 46 |
| Ogdie et al. 2014 [18] | UK | Population-based, longitudinal cohort study (THIN database) | 1994–2010 (16) | 18+ | 132031 | READ Code | 8706 | General Population /Rheumatoid Arthritis | 50.54 | 48.9 |
| Juneblad et al. 2016 [28] | Västerbotten County, Sweden | Retrospective, referral cohort (National death registry) | 1995–2011 (16) | 18+ | 464 | CASPAR ICD-9 | 464 | General Population | 59.5 | 49.6 |
| Dai et al. 2018 [24] | Taiwan | Nationwide, population-based cohort (National Health Insurance) | 2000–2001 (12) | 18+ | 106701 | CASPAR, ICD-9 | 8795 | General Population, Psoriasis | 45.1 | 40.5 |
| Skov et al. 2019 [23] | Denmark | Nationwide, population-based, retrospective Cohort (National patient registry) | 1998–2014 (16) | 18+ | 21,977 | ICD-10 | 9817 | General Population | 49.6 | 48.8 |
| Elalouf et al. 2020 [22] | Toronto, Canada | Prospective, specialty clinic cohort (University of Toronto PsA clinic) | 1978–2017 (39) | 18+ | 1490 | CASPAR, ICD-9, ICD10 | 1490 | General Population | 45.5 | 44 |
| Colaco et al.2020 [17] | Ontario, Canada | Population-based, retrospective cohort (Ontario Health Insurance databases) | 2008–2016 (20) | 20+ | 192288 | Validated admistrative case definitions | 15430 | General Population | - | - |
| Karmacharya et al. 2021 [21] | Olmsted County, MN, USA | Population-based, retrospective cohort (Rochester epidemiology project) | 2000–2017 (19) | 18+ | 484 | CASPAR | 164 | General Population | 46.4 (±12.0) | 47% |
| Cardiovascular mortality | ||||||||||
| Juneblad et al. 2016 [28] | Västerbotten County, Sweden | Retrospective, referral cohort (National death registry) | 1995–2005 (16) | 18+ | 464 | CASPAR, ICD 9 | 464 | General Population | 59.5 | 49.6 |
| Ogdie et al. 2017 [19] | United Kingdom | Population-based, longitudinal cohort (THIN database) | 1994–2010 (16) | 18+ | 132031 | READ code | 8706 | General Population/ Rheumatoid Arthritis | 50.54 | 48.9 |
| Skov et al. 2019 [23] | Denmark | Population-based, retrospective cohort (National patient registry) | 1998–2014 (16) | 18+ | 21977 | ICD-10 | 9817 | General Population | 49.6 | 48.8 |
| Elalouf et al. 2020 [22] | Toronto, Canada | Prospective, specialty center cohort (University of Toronto PsA clinic) | 1978–2017 (39) | 18+ | 1490 | CASPAR, ICD-9, ICD-10 | 1490 | General Population | 45.5 | 44 |
| Colaco et al. 2020 [17] | Ontario,Canada | Population-based, retrospective Cohort (Ontario Health Insurance databases) | 2008–2016 (20) | 20+ | 192288 | Validated admistrative case definitions | 15430 | General Population | - | - |
| Respiratory disease-related mortality | ||||||||||
| Ogdie et al. 2017 [19] | United Kingdom | Population-based, longitudinal cohort (THIN database) | 1994–2010 (16) | 18+ | 132031 | READ Code | 8706 | General Population/ Rheumatoid Arthritis | 50.54 | 48.9 |
| Skov et al. 2019 [23] | Denmark | Population-based, retrospective cohort (National patient registry) | 1998–2014 (16) | 18+ | 21977 | ICD-10 | 9817 | General Population | 49.6 | 48.8 |
| Elalouf et al. 2020 [22] | Toronto, Canada | Prospective, specialty center cohort (University of Toronto PsA clinic) | 1978–2017 (39) | 18+ | 1490 | CASPAR, ICD-9, ICD-10 | 1490 | General Population | 45.5 | 44 |
| Colaco et al. 2020 [17] | Ontario, Canada | Population-based, retrospective cohort (Ontario Health Insurance databases) | 2008–2016 (20) | 20+ | 192288 | Validated admistrative case definitions | 15430 | General Population | - | - |
| Infection and malignancy related mortality | ||||||||||
| Juneblad et al. 2016 [28]* | Västerbotten County, Sweden | Retrospective, referral cohort (National death registry) | 1995–2005 (16) | 18+ | 464 | CASPAR ICD 9 | 464 | General Population | 59.5 | |
| Ogdie et al. 2017 [19] | UK | Population-based, longitudinal Cohort (THIN database) | 1994–2010 (16) | 18+ | 132031 | READ Code | 8706 | General Population/ Rheumatoid Arthritis | 50.54 | 48.9 |
| Skov et al. 2019 [23] | Denmark | Population-based, retrospective cohort (National patient registry) | 1998–2014 (16) | 18+ | 21977 | ICD-10 | 9817 | General Population | 49.6 | 48.8 |
| Elalouf et al. 2020 [22] | Toronto, Canada | Prospective, specialty center cohort (University of Toronto PsA clinic) | 1978–2017 (39) | 18+ | 1490 | CASPAR, ICD-9, ICD-10 | 1490 | General Population | 45.5 | 44 |
| Colaco et al. 2020 [17] | Ontario, Canada | Population-based, retrospective cohort (Ontario Health) | 2008–2016 (20) | 20+ | 192288 | Validated administrative case definitions | 15430 | General Population | - | - |
ICD= International Classification of Disease, CASPAR=-Classification Criteria for Psoriatic Arthritis, THIN= The Health Improvement Network, CDARS= Clinical Data Analysis and Reporting System, NR= not reported.
not included for infections related mortality
Table 2.
Study characteristics of all cause and cause-specific mortality in ankylosing spondylitis
| Author, year | Geographic location | Study design (data source) | Study period (total duration in years with follow up) | Population | No. of patients | Definition of cases | No. of AS patients | Comparison group | Mean Age (year) | Female (%) |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| All-cause mortality | ||||||||||
| Mok et al. 2011 [32] | Hong Kong | Population- Based, Retrospective cohort (CDARS - outpatient clinics and inpatient admissions) | 1999–2008 (9) | NR | 18627 | ICD -9 | 2154 | General population | 51.7 +/−13.2 | 46 |
| Bakland et al. 2011 [26] | Tromsø and Finnmark counties, Norway | Retrospective, single hospital cohort (University Hospital of Northern Norway) | 1977–2009 (32) | 18+ | 677 | Modifed New York Criteria | 677 | General Population | 23.2+/−8.5 | 24.5 |
| Wright et al. 2015 [27] | Olmsted County, MN, USA | Population-based retrospective cohort (Rochester Epidemiology Project) | 1980–2009 (31) | 18+ | 86 | Modified New York Criteria | 86 | General Population | 34.9+/–9.9 | 34.5 |
| Exarchaou et al. 2016 [34] | Sweden | Population-based, retrospective cohort (National patient registry) | 2006–2012 (12) | 18+ | 8600 | ICD-code | 8600 | General Population | 49.3 | 34.5 |
| Dregan et al. 2017 [30] | UK | Population-based, prospective cohort (UK biobank data) | 2006–2010 (4) | NR | 19082 | Clinical diagnosis | 1400 | General Population | 37 | 38 |
| Bae et al. 2019 [25] | Korea | Population-based, retrospective cohort (National health insurance database) | 2010–2014 (5) | 20+ | 12988 | Medical Expenses for Rare Complaints Code, V14.0, ICD-10 | 15,547 | General Population | 40 +/− 14.2 | 27.6 |
| Cardiovascular-related mortality | ||||||||||
| Haroon et al. 2015 [31] | Ontario, Canada | Population-based, retrospective cohort (Provincial health database; Ontario registrar database) | 1995–2011 (16) | 15+ | 108079 | ICD9-CM 720 | 21,164 | General Population | 45.6 | 47 |
| Dregan et al. 2017 [30] | UK | Population-based, prospective cohort (UK biobank data) | 2006–2010 (4) | - | 19082 | Clinical diagnosis | 1400 | General Population | 37 | 38 |
| Prati et al. 2017 [33] | France | Population-based retrospective cohort (National death registry) | 1969–2009 (40) | 18+ | 1969 | ICD-8 712.4 | 2940 | General Population | 60.1 | 22 |
ICD= International Classification of Disease, CDARS= Clinical Data Analysis and Reporting System, THIN= The Health Improvement Network, NR= not reported.
All-cause and cause-specific mortality in psoriatic arthritis
All included studies were cohort studies and used the general population as a comparison group (Table 1). Among the ten included studies for PsA, seven studies were population-based studies [17,18,20,21,23,24,32]. The remaining three studies were retrospective single-center, retrospective multi-center, referral, and specialty center-based cohort studies [22,28,29] Most of the studies were from North America [17,20,21,22] and Europe [18,23,28,29] with a few studies from southeast Asia [24,32]. Six studies defined PsA based on CASPAR criteria [20,21,22,24,28,29] and the remaining four studies used administrative codes [17,19,22,32]. Mortality was defined using administrative codes and death certificates. There were 46,244 patients with PsA with a pooled RR for all-cause mortality of 1.12 (95% CI: 0.96—1.30, I2= 89%) (Figure 2A). For sex-adjusted all-cause mortality, we included four studies with 11,946 PsA patients [22,28,29,38] For males, we identified an all-cause mortality rate of 1.02 (95% CI: 0.66–1.59, I2=92%). We found a higher all-cause mortality in females, RR= 1.19 (95% CI: 1.04–1.36, I2=23%). (Supplementary File 4). The subgroup analysis comparing the more recent eight studies published after the year 2010 showed higher all-cause mortality with a pooled RR of 1.17 (95% CI:1.00–1.38, I2 =91%) compared to two studies before 2010 with a pooled RR of 0.85 (95% CI:0.65–1.11 I2 =0%) (Supplementary File 6). The all-cause mortality based on the geographical location was higher in two studies from the Asian population [24,32] with a pooled RR of 1.56 (95% CI 1.44–1.70 I2 =0) as opposed to most studies from Europe and North America that did not show an increase in overall mortality with a pooled RR 1.04 (95% CI 0.92–1.12 I2 = 7%) and 1.01 (95% CI 0.78–1.31 I2 =83%) [17,19,20–23,28,29] (Supplementary File 8).
Figure 2.
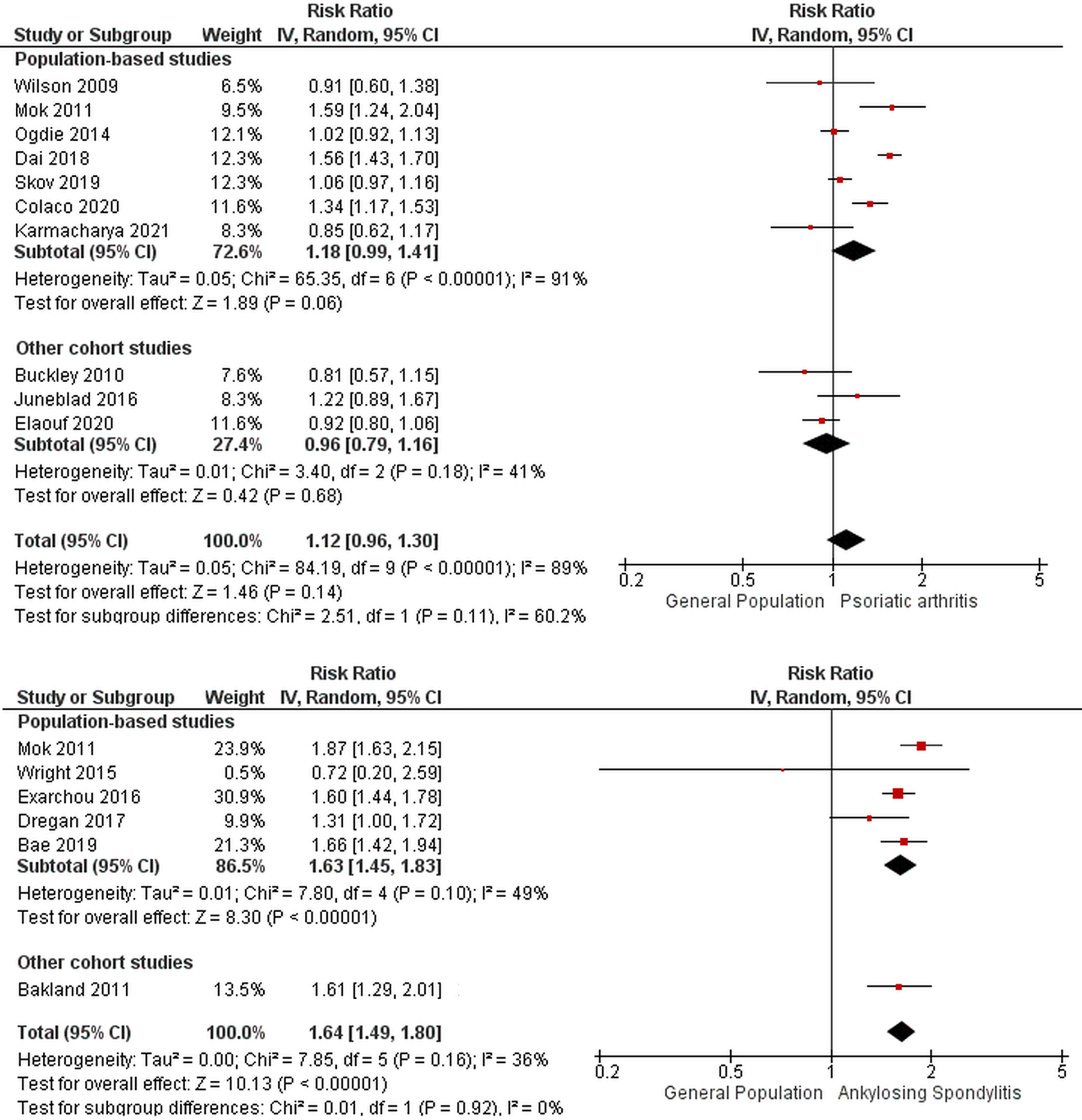
Forest plot for overall mortality in A) psoriatic arthritis B) ankylosing spondylitis
Among studies for cause-specific mortality, there were five PsA studies [17,19,22,24,28] that reported cardiovascular mortality with a total of 35,907 PsA patients (Figure 3). The pooled RR was 1.21 (95% CI: 1.06–1.38, I2= 8%). Among four studies [17,19,22,23] the pooled RR for respiratory-specific mortality was 3.37 (95% CI: 1.30–8.72, I2=97%). The mortality risk from infectious diseases in four studies was 2.43 (95% CI: 1.01–5.84, I2=87%). The risk of death from malignant causes was 1.01 (95% CI: 0.91–1.11, I2=0%) in five studies [17,19,22,23,28]. The pooled mortality rate from injury and poisoning in two studies [22,23] was 1.15 (95% CI: 0.61–2.18, I2=60%), and mortality from suicides and self-injury in the other included two studies [21,24] was 1.62 (95% CI: 0.42–6.32, I2 =78%) (Supplementary file 7).
Figure 3.
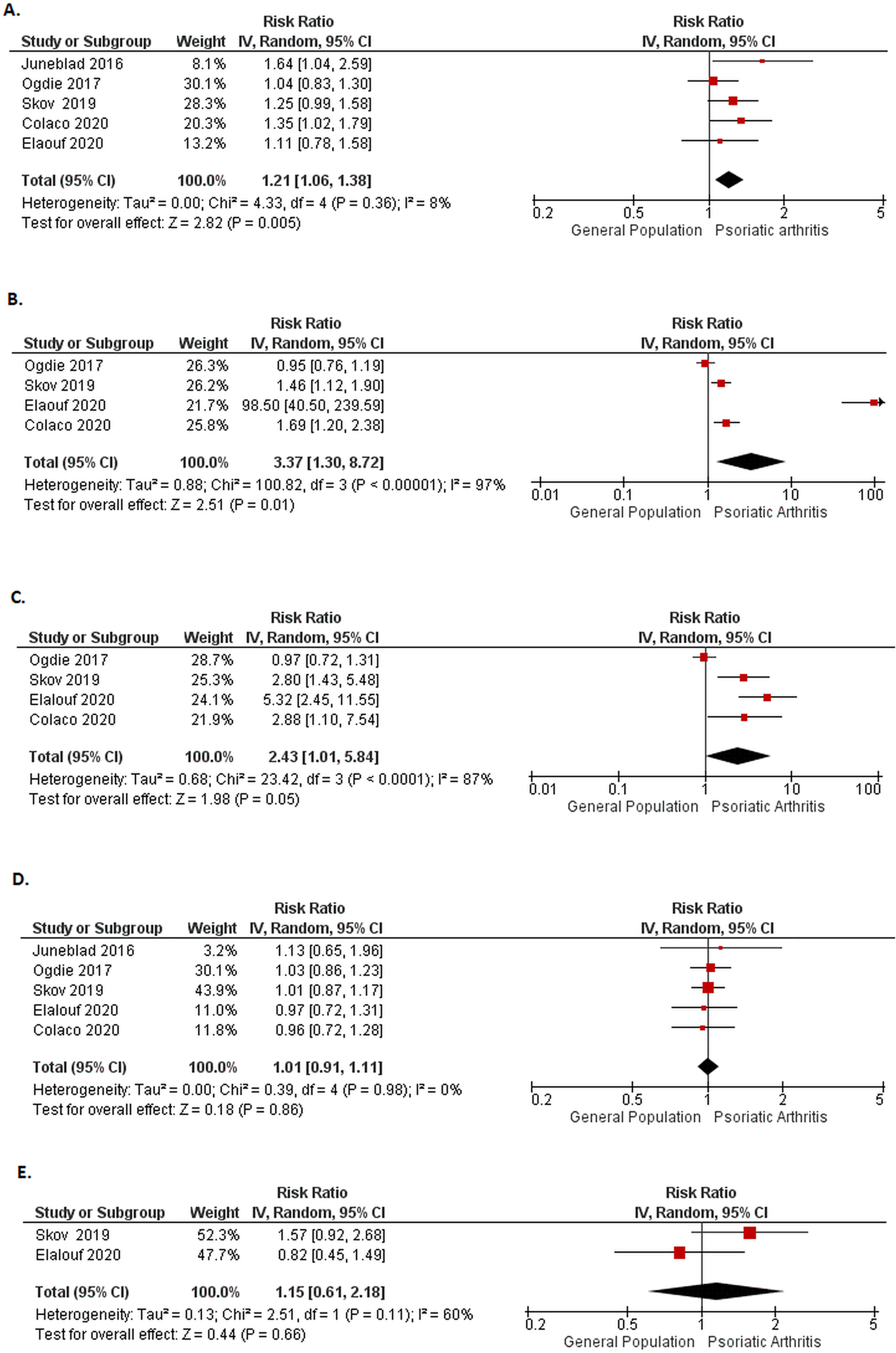
Forest plot for cause-specific mortality in psoriatic arthritis A) cardiovascular B) respiratory C) infections D) malignancy
All-cause and cause-specific mortality in ankylosing spondylitis
Among the six included AS studies for all-cause mortality, five were population-based [25,27,30,32,34] and the remaining one study was specialty clinic-based [26]. Most of the studies were from Europe [26,30,34] with two studies from Southeast Asia [25,32] and one study from North America [27]. Three studies defined AS based on administrative codes [25,32,34], two studies used Modified New York Criteria for AS [28,29], and the remaining one study defined it as per clinical diagnosis [30]. Mortality was defined using administrative codes and death certificates. The total number of AS patients was 28,464 in these studies (Table 2). The pooled RR for all-cause mortality was 1.64 (95% CI :1.49–1.80, I2= 36%) (Figure 2B). For sex-adjusted all-cause mortality, pooled RR for males was 1.56 (95% CI: 1.43–1.71, I2=0%) and for females was 1.85 (95% CI: 1.56–2.18, I2=0%) in the three studies providing mortality data stratified by sex [25,26,34] (Supplementary File 5). The results were consistent among all included studies from Asia and European countries [25,26,30,32,34]. One small study from North America did not show increase in overall mortality [27] (Supplementary File 9). The pooled RR for cardiovascular mortality in AS patients from three studies [28,29,31] was 1.35 (95% CI 1.01–1.81, I2=83%) (Figure 4).
Figure 4.

Forest plot for cause-specific cardiovascular mortality in ankylosing spondylitis
Sensitivity analysis, publication bias, and risk of bias
Sensitivity analysis, publication bias, and risk of bias of included studies were assessed if more than five studies were included for a given analysis. Sensitivity analysis performed with only population-based studies for PsA and AS (Figure 2), and subgroup analysis performed for studies in PsA published before and after 2010 did not show any difference in results. All the included AS studies were published after 2010.Sensitivity analyses performed replacing the included studies with those excluded due to overlapping data, also showed no significant change in the pooled estimates. As there were only a few studies included in each analysis, funnel plot was difficult to interpret, but no publication bias was identified visually (Supplementary file 3). For PsA studies, five studies included were judged to have low risk of bias with six studies moderate risk of bias. For AS study quality, five studies were judged to have low risk and three studies moderate risk of bias (Supplementary file 2A&B). All the included studies were observational; therefore, the certainty in evidence evaluated using the GRADE approach methodology was low to moderate [14] (Supplementary file 2C).
Discussion
This systematic review did not show a significantly increased risk of all-cause mortality in patients with PsA compared to the general population. However, mortality was slightly higher in the female PsA patients but not in males. There was an increased risk of death from cardiovascular, respiratory, and infectious causes. No increase in malignancy-related deaths was seen in PsA. For AS, there was an increased risk of all-cause mortality compared to the general population, and higher mortality was seen in both males and females. Similar to PsA, higher cardiovascular mortality was noted in AS.
Studies on overall mortality in PsA and AS show conflicting results, even among the included population-based studies. Most population-based studies [17,18,20,21,23,24] do not show increased overall mortality in PsA (Figure 2A). However, there are differences among study results from these population-based studies over time. Recent studies from the Denmark nationwide cohort [23], University of Toronto cohort [22], UK THIN database [19], and Olmsted County, MN, USA [21] did not show higher overall mortality in PsA. On the contrary, earlier studies from the first two cohorts had shown higher mortality in PsA compared to the general population but were limited by considerably smaller population size [35,37]. Moreover, longitudinal data from the University of Toronto cohort showed that mortality decreased over four decades of follow-up [36]. In contrast, two other recent population-based studies from the Taiwan National Health Insurance by Dai et al. [24] and Ontario Health Insurance Plan by Colaco et al. [17] showed excess mortality risk in PsA patients. Furthermore, the latter study did not report any change in relative excess mortality from 1996 to 2016. While it seems plausible that early recognition and improved management over time might underlie improved mortality seen in some studies, not all studies have confirmed this trend. This is further elucidated by our subgroup analysis of studies published after 2010, which also showed heterogeneous mortality risk ratios, but an overall estimate showing increase in all-cause mortality [17,19,21–24,28] (Supplementary File 6). Furthermore, study results vary among population-based studies from various geographic locations (Supplementary file 8). While no higher mortality was noted in the PsA population from Europe and North America, two population-based studies from Asia showed higher mortality [24,32]. The difference in results across geographical locations suggest a likely genetic and environmental influence.
Among AS studies, most population-based studies showed an increased risk of overall mortality [25,32,34]. In contrast, two consecutive population-based studies from Olmsted County, MN, USA had similar survivorship compared to the general population [27,40]. The studies from Olmsted County included a limited number of patients and a shorter median follow-up (8.7 years) compared to most other studies, which might have led to differences in these populations in mortality rates. Additionally, a large population-based, prospective study from the UK biobank also did not show significantly higher mortality in AS [30]. Among two studies from Korean National Insurance Database [25,39], we included the more recent study by Bae et al. that showed significantly higher mortality in AS compared to the general population [36]. The more recent study had considerably larger size (total Korean population in contrast to a stratified sample of the population in the older study) reaching statistical significance, while the smaller study only showed a trend towards increased mortality. Substituting the Bae et al. study with the smaller study [39] did not change our final results. No clear geographical differences in mortality were noted in AS (supplemental file 9). Unlike conflicting trends in mortality of PsA [17,36], mortality rates for AS patients seem to be stable from 1969 to 2009 [33] (more recent trend data were not available).
Beyond population-based studies, studies from hospital-based registries and specialty clinics might include patients with more severe disease, leading to selection bias [32]. In PsA, a large proportion of patients in the general population have mild disease and may be less likely to be managed by a specialist. As seen with psoriasis, the severity of PsA may be associated with increased mortality that has not been clearly demonstrated in studies of PsA to date [41]. Supporting this notion, a recent study from the British Society for Rheumatology Biologics Register showed significantly higher mortality (SMR 1.56; 95% CI: 1.12–2.11) in PsA patients on biologics (which likely represents severe PsA patients) compared to the general population [42]. Therefore, population-based cohort studies may provide estimates closer to true mortality rates.
While most studies reported SMRs comparing mortality in PsA to the national estimates, only a few used an internal comparison group [18,24,25,30,31]. SMRs might overestimate the risk compared to an internal comparison group, which could better approximate the true effect [43]. Lastly, while most studies provided age and sex-adjusted mortality rates, only a few studies reported mortality rates among males and females separately. Our review noted higher mortality in female PsA patients but not in males (supplementary file 4), unlike in the general population where females have lower mortality [44]. This differential effect was not seen in AS (supplementary file 5).
Our meta-analysis also examined cause-specific mortality in SpA and noted an increased risk of cardiovascular deaths in both PsA and AS. While both are associated with increased cardio-metabolic disease compared to the general population, data for cardiovascular mortality in SpA is conflicting, unlike data in rheumatoid arthritis [45]. Among the included studies for PsA, only one study by Juneblad et al. [28] had shown a significantly increased mortality risk in this group (mortality risk was numerically higher but not statistically significant in other studies). Interestingly, a 2013 systematic review that examined cardiovascular mortality in PsA that included earlier studies from some of the included databases had not shown increased cardiovascular mortality in PsA [46]. Two of the four newer studies published following this systematic review have shown significantly increased cardiovascular mortality [17,28].
Amongst the three included population-based studies for cardiovascular mortality in AS, two studies reported higher cardiovascular mortality [30,31]. Haroon et al. found increased vascular mortality (both cardiovascular and cerebrovascular) compared to population controls in their study from Ontario administrative data. Significant risk factors for vascular death in the study were age, male sex, lower income, dementia, chronic kidney disease, peripheral vascular disease, and lack of nonsteroidal anti-inflammatory drug exposure and statins in those over 65 years [31]. Similarly, Dregan et al. also demonstrated a greater risk of cardiometabolic events in AS compared to the general population [30]. The third study by Prati et al. showed increased deaths from cardiovascular causes in a younger French AS population aged 25–64 years, but results were not statistically significant [33].
Beyond cardiovascular causes, our meta-analysis showed increased mortality from respiratory diseases in PsA (Figure 3). In a study by Wong et al., pneumonia was a major cause of death along with respiratory arrest secondary to underlying COPD in PsA [35]. A more recent study from this University of Toronto cohort (included in our meta-analysis), Denmark, and Ontario, Canada [17,22,23] also showed similar results. However, a study from the UK THIN database [19] did not show an increase in mortality related to respiratory disease. Increased prevalence of risk factors for lung disease such as smoking and obesity, and the association of asthma, chronic obstructive lung disease, obstructive sleep apnea, and rarely interstitial lung disease with psoriatic disease have been described. [34–35]. While these factors could account for increased respiratory mortality, the included studies did not specifically report the characteristics of respiratory illnesses associated with increased mortality.
Our review also found a higher risk of death from infectious causes in PsA compared to the general population [17,19,22,23]. Most biologic registry studies show higher risk of serious infections in PsA with biologics, although the risk does not seem to be as high as that seen in rheumatoid arthritis (RA) [49]. Whether the risk of serious infections is associated with longer disease duration or severity of the underlying disease is unclear.
Our review did not show an increased risk of mortality from malignant causes in PsA [17,20,22 23,28,31,38] (Figure 3). This is consistent with the literature showing only a higher incidence of nonmelanoma skin cancer in PsA, but no association with hematologic and solid organ malignancies unlike RA [50]. Similarly, the risk of malignancies with traditional immunosuppressive agents or biologics in PsA, if any seems to be quite small [50]. Suicide and poisoning-related deaths were not observed to be higher in the studies from the University of Toronto and Denmark, [22,23] with only one study from the UK THIN database [20] showing higher mortality from self-injury (Supplementary file 7). This further highlights the importance of managing mental health in PsA, although more studies are needed to address this association. Our review found limited studies on cause-specific mortality in AS beyond cardiovascular diseases.
The strengths of our meta-analyses include an extensive review of the literature to date, including only cohort studies, the general population as a comparison group, and overall low to medium risk of bias in the included studies. However, we do note some limitations. First, our study included observational studies with retrospective cohorts, including specialty center, and registry-based studies. Studies with hospital-based SpA diagnoses could have contributed to an overestimation of mortality rates [22,28,30]. However, we performed a sensitivity analysis of only population-based studies, which did not show any difference in outcomes. Second, most studies used death certificates as the method of ascertaining the cause of death, which may not be complete as the diagnosis of SpA is not invariably documented on the death certificate [31]. Finally, worldwide disease synopsis studies make implicit assumptions that the diseases studied are diagnosed at similar stages across health systems, have similar disease severity, homogeneous disease domain involvement, comorbid disease prevalence, treatment access, and similar disease monitoring. It is quite plausible that these diseases and their complications are different across populations, given the heterogeneity of the genetic prevalence of risk that we now recognize exists. Future population-wide genetic analysis will hopefully shed light on this.
Conclusion
Our meta-analysis showed a significantly increased risk of overall mortality in AS, but not PsA. Interestingly, mortality risk in PsA was higher in females, but not males. There was a significantly increased risk of death from cardiovascular causes in PsA and AS. PsA patients also had increased mortality related to respiratory and infectious causes compared to the general population. These findings emphasize the importance of early comorbidity screening and management to potentially mitigate the mortality risk in SpA.
Supplementary Material
Supplementary File 1. Search strategy for the systematic review process
Supplementary File 2. A) Risk of bias of the included psoriatic arthritis studies based on New-Castle Ottawa scale for observational studies, B) Risk of bias of the included ankylosing spondylitis studies based on New-Castle Ottawa scale for observational studies, C) GRADE quality assessment of mortality in psoriatic arthritis and ankylosing spondylitis
Supplementary File 3. Funnel plot of included studies looking at overall mortality in A) psoriatic arthritis B) ankylosing spondylitis
Supplementary File 5. Forest plot for sex-stratified mortality in ankylosing spondylitis A) males B) females
Supplementary File 4. Forest plot for sex-stratified mortality in psoriatic arthritis A) males B) females
Supplementary File 6. Subgroup analysis of all-cause mortality in PsA studies published A) before B) after the year 2010.
Supplementary File 7. Forest plot mortality related to intentional self-harm/ suicide & injury and poisoning.
Supplementary File 9. Overall Mortality in AS by geographical location
Supplementary File 8. Overall mortality in PsA by geographical location
KEY POINTS.
Overall mortality in Psoriatic Arthritis (PsA) is not higher compared to the general population but there is higher risk of cardiovascular mortality in PsA.
Both overall mortality and cardiovascular specific mortality is higher in patients with Ankylosing Spondylitis (AS) compared to the general population.
The findings from this meta-analysis emphasize the importance of early screening and management of cardiovascular risk factors in patients with spondyloarthritis.
Acknowledgment
We would like to thank librarian Larry Prokop, Mayo Clinic, for help with the comprehensive search strategy.
Funding:
Dr. Karmacharya is supported by T32 AR56950 and T32AR059039 grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Spondyloarthritis Research and Treatment Network (SPARTAN) fellowship pilot grant, Assessment of SpondyloArthritis international Society (ASAS), and Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) Pilot Research Grant.
References
- 1.Stolwijk C, Boonen A, van Tubergen A, Reveille JD. Epidemiology of spondyloarthritis. Rheum Dis Clin North Am. 2012;38(3):441–476. doi: 10.1016/j.rdc.2012.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurkovich M, Vostretsova K, Chen W, Avina-Zubieta JA. Overall and cause-specific mortality in patients with systemic lupus erythematosus: a meta-analysis of observational studies. Arthritis Care Res (Hoboken). 2014;66:608–616. [DOI] [PubMed] [Google Scholar]
- 3.Dadoun S, Zeboulon-Ktorza N, Combescure C, et al. Mortality in rheumatoid arthritis over the last fifty years: systematic review and meta-analysis. Joint Bone Spine. 2013;80:29–33. [DOI] [PubMed] [Google Scholar]
- 4.Chou CH, Lin MC, Peng CL, Wu YC, Sung FC, Kao CH, et al. A nationwide population-based retrospective cohort study: increased risk of acute coronary syndrome in patients with ankylosing spondylitis. Scand J Rheumatol. 2014;43:132–6. [DOI] [PubMed] [Google Scholar]
- 5.Karmacharya P, Ogdie A, Eder L. Psoriatic arthritis and the association with cardiometabolic disease: a narrative review. Ther Adv Musculoskelet Dis. 2021. Mar 2;13:1759720X21998279. doi: 10.1177/1759720X21998279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Horst-Bruinsma IE, Nurmohamed MT, Landewé RB. Comorbidities in patients with spondyloarthritis. Rheum Dis Clin North Am. 2012. Aug;38(3):523–38. doi: 10.1016/j.rdc.2012.08.010. Epub 2012 Sep 14. [DOI] [PubMed] [Google Scholar]
- 7.Mathieu S, Soubrier M Cardiovascular events in ankylosing spondylitis: a 2018 meta-analysis Annals of the Rheumatic Diseases 2019;78:e57. [DOI] [PubMed] [Google Scholar]
- 8.Sun LM, Muo CH, Liang JA, Chang SN, Sung FC, Kao CH. Increased risk of cancer for patients with ankylosing spondylitis: a nationwide population-based retrospective cohort study. Scand J Rheumatol. 2014;43(4):301–6. doi: 10.3109/03009742.2013.863969. Epub 2014 Feb 24. [DOI] [PubMed] [Google Scholar]
- 9.Kanathur N, Lee-Chiong T. Pulmonary manifestations of ankylosing spondylitis. Clin Chest Med. 2010. Sep;31(3):547–54. doi: 10.1016/j.ccm.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Minozzi S, Bonovas S, Lytras T, Pecoraro V, González-Lorenzo M, Bastiampillai AJ, Gabrielli EM, Lonati AC, Moja L, Cinquini M, Marino V, Matucci A, Milano GM, Tocci G, Scarpa R, Goletti D, Cantini F. Risk of infections using anti-TNF agents in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis: a systematic review and meta-analysis. Expert Opin Drug [DOI] [PubMed] [Google Scholar]
- 11.Walsh JA, Zhou X, et al. (2015). “Mortality in American Veterans with the HLA-B27 gene.” Journal of Rheumatology 42(4): 638–644. [DOI] [PubMed] [Google Scholar]
- 12.Ognjenovic M, Raymond WD, Inderjeeth CA, Keen HI, Preen DB, Nossent JC. The Risk and Consequences of Vertebral Fracture in Patients with Ankylosing Spondylitis: A Population-based Data Linkage Study. J Rheumatol. 2020. Nov 1;47(11):1629–1636. doi: 10.3899/jrheum.190675. Epub 2020 Feb 15. [DOI] [PubMed] [Google Scholar]
- 13.Wells George & Shea Beverley & O’Connell J. (2014). The Newcastle-Ottawa Scale (NOS) for Assessing The Quality of Nonrandomised Studies in Meta-analyses. Ottawa Health Research Institute Web site. 7. [Google Scholar]
- 14.Murad MH. Clinical Practice Guidelines: A Primer on Development and Dissemination. Mayo Clin Proc. 2017;92(3):423–33. [DOI] [PubMed] [Google Scholar]
- 15.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986. Sep;7(3):177–88 [DOI] [PubMed] [Google Scholar]
- 17.Colaco K, Widdifield J, Luo J, Rosen CF, Alhusayen R, Paterson JM, Campbell W, Tu K, Bernatsky S, Gladman DD, Eder L. Trends in mortality and cause-specific mortality among patients with psoriasis and psoriatic arthritis in Ontario, Canada. J Am Acad Dermatol. 2021. May;84(5):1302–1309. doi: 10.1016/j.jaad.2020.10.031. Epub 2020 Oct 21. [DOI] [PubMed] [Google Scholar]
- 18.Ogdie A, Haynes K, Troxel AB, et al. Risk of mortality in patients with psoriatic arthritis, rheumatoid arthritis and psoriasis: a longitudinal cohort study. Ann Rheum Dis. 2014;73(1):149–153. doi: 10.1136/annrheumdis-2012-202424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogdie A, Maliha S, et al. (2017). “Cause-specific mortality in patients with psoriatic arthritis and rheumatoid arthritis.” Rheumatology 56(6): 907–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson FC, Icen M, Crowson CS, McEvoy MT, Gabriel SE, Kremers HM Time trends in epidemiology and characteristics of psoriatic arthritis over 3 decades: a population based study. J Rheumatol 2009;36:361–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karmacharya P, Crowson CS, Bekele D, Achenbach SJ, Davis JM 3rd, Ogdie A, Duarte-García A, Ernste FC, Maradit-Kremers H, Tollefson MM, Wright K. The Epidemiology of Psoriatic Arthritis over 5 Decades: A Population-Based Study. Arthritis Rheumatol. 2021. Mar 28. doi: 10.1002/art.41741. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elalouf O, Muntyanu A, et al. (2020). “Mortality in psoriatic arthritis: Risk, causes of death, predictors for death.” Seminars in Arthritis & Rheumatism 50(4): 571–575. [DOI] [PubMed] [Google Scholar]
- 23.Skov L, Thomsen SF, et al. (2019). “Cause-specific mortality in patients with psoriasis and psoriatic arthritis.” British Journal of Dermatology 180(1): 100–107. [DOI] [PubMed] [Google Scholar]
- 24.Dai Y-X, Hsu M-C, et al. (2018). “The Risk of Mortality among Psoriatic Patients with Varying Severity: A Nationwide Population-Based Cohort Study in Taiwan.” International Journal of Environmental Research & Public Health [Electronic Resource] 15(12): 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae KH, Hong JB, et al. (2019). “Association of Congestive Heart Failure and Death with Ankylosing Spondylitis: A Nationwide Longitudinal Cohort Study in Korea.” Journal of Korean Neurosurgical Society 62(2): 217–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakland G, Gran JT, Nossent JC. Increased mortality in ankylosing spondylitis is related to disease activity. Ann Rheum Dis. 2011. Nov;70(11):1921–5. doi: 10.1136/ard.2011.151191. Epub 2011 Jul 21. [DOI] [PubMed] [Google Scholar]
- 27.Wright KA, Crowson CS, Michet CJ and Matteson EL (2015), Time Trends in Incidence, Clinical Features, and Cardiovascular Disease in Ankylosing Spondylitis Over Three Decades: A Population-Based Study. Arthritis Care & Research, 67: 836–841. 10.1002/acr.22512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juneblad K, Rantapaa-Dahlqvist S, et al. (2016). “Disease Activity and Increased Risk of Cardiovascular Death among Patients with Psoriatic Arthritis.” Journal of Rheumatology 43(12): [DOI] [PubMed] [Google Scholar]
- 29.Buckley Cavill C, Taylor G, Kay H, Waldron N, Korendowych E. Mortality in psoriatic arthritis — a single-center study from the UK. J Rheumatol 2010;37:2141–4. Saf. 2016. Dec;15(sup1):11–34. doi: [DOI] [PubMed] [Google Scholar]
- 30.Dregan A, Chowienczyk P, et al. (2017). “Cardiovascular and type 2 diabetes morbidity and all-cause mortality among diverse chronic inflammatory disorders.” Heart 103(23): 1867–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haroon NN, Paterson JM, Li P, Inman RD, Haroon N. Patients With Ankylosing Spondylitis Have Increased Cardiovascular and Cerebrovascular Mortality: A Population-Based Study. Ann Intern Med. 2015. Sep 15;163(6):409–16. doi: 10.7326/M14-2470. [DOI] [PubMed] [Google Scholar]
- 32.Mok CC, Kwok CL, Ho LY, Chan PT, Yip SF. Life expectancy, standardized mortality ratios, and causes of death in six rheumatic diseases in Hong Kong, China. Arthritis Rheum. 2011. May;63(5):1182–9. doi: 10.1002/art.30277. [DOI] [PubMed] [Google Scholar]
- 33.Prati C, Puyraveau M, Guillot X, Verhoeven F, Wendling D. Deaths Associated with Ankylosing Spondylitis in France from 1969 to 2009. J Rheumatol. 2017. May;44(5):594–598. doi: 10.3899/jrheum.160942. Epub 2017 Mar 15. [DOI] [PubMed] [Google Scholar]
- 34.Exarchou S, Lie E, et al. (2016). “Mortality in ankylosing spondylitis: results from a nationwide population-based study.” Annals of the Rheumatic Diseases 75(8): 1466–1472. [DOI] [PubMed] [Google Scholar]
- 35.Wong K, Gladman DD, Husted J, Long JA, Farewell VT. Mortality studies in psoriatic arthritis: results from a single outpatient clinic. I. Causes and risk of death. Arthritis Rheum. 1997. Oct;40(10):1868–72. [DOI] [PubMed] [Google Scholar]
- 36.Ali Y, Tom BDM, Schentag CT, Farewell VT, Gladman DD. Improved survival in psoriatic arthritis with calendar time. Arthritis Rheum. 2007. Aug;56(8):2708–14. [DOI] [PubMed] [Google Scholar]
- 37.Ahlehoff O, Gislason GH, Charlot M, Jorgensen CH, Lindhardsen J, Olesen JB, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011. Aug;270(2):147–57. [DOI] [PubMed] [Google Scholar]
- 38.Lee M-S, Yeh Y-C, et al. (2017). “All-Cause and Cause-Specific Mortality in Patients with Psoriasis in Taiwan: A Nationwide Population-Based Study.” Journal of Investigative Dermatology 137(7): 1468–1473 [DOI] [PubMed] [Google Scholar]
- 39.Lee JS, Oh BL, Lee HY, Song YW, Lee EY (2018) Comorbidity, disability, and healthcare expenditure of ankylosing spondylitis in Korea: A population-based study. PLOS ONE 13(2): e0192524. 10.1371/journal.pone.0192524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carter ET, McKenna CH, Brian DD, Kurland LT. Epidemiology of Ankylosing spondylitis in Rochester, Minnesota, 1935–1973. Arthritis Rheum. 1979. Apr;22(4):365–70. doi: 10.1002/art.1780220408. [DOI] [PubMed] [Google Scholar]
- 41.Dhana A, Yen H, Yen H, Cho E. All-cause and cause-specific mortality in psoriasis: A systematic review and meta-analysis. J Am Acad Dermatol. 2019. May;80(5):1332–1343. doi: 10.1016/j.jaad.2018.12.037. Epub 2018 Dec 24. [DOI] [PubMed] [Google Scholar]
- 42.Fagerli KM, Kearsley-Fleet L, Mercer LK, Watson K, Packham J, Symmons DPM, Hyrich KL. Malignancy and mortality rates in patients with severe psoriatic arthritis requiring tumour-necrosis factor alpha inhibition: results from the British Society for Rheumatology Biologics Register. Rheumatology (Oxford). 2019. Jan 1;58(1):80–85. doi: 10.1093/rheumatology/key241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Card TR, Solaymani-Dodaran M, Hubbard R, Logan RFA, West J. Is an internal comparison better than using national data when estimating mortality in longitudinal studies? J Epidemiol Community Health. 2006. Sep;60(9):819–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh-Manoux A, Guéguen A, Ferrie J, et al. Gender differences in the association between morbidity and mortality among middle-aged men and women. Am J Public Health. 2008;98(12):2251–2257. doi: 10.2105/AJPH.2006.107912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sokka T, Abelson B, Pincus T. Mortality in rheumatoid arthritis: 2008 update. Clin Exp Rheumatol. 2008. Sep-Oct;26(5 Suppl 51):S35–61. [PubMed] [Google Scholar]
- 46.Horreau C, Pouplard C, Brenaut E, Barnetche T, Misery L, Cribier B, Jullien D, Aractingi S, Aubin F, Joly P, Le Maître M, Ortonne J-P, Paul C and Richard M-A (2013), Cardiovascular morbidity and mortality in psoriasis and psoriatic arthritis: a systematic literature review. J Eur Acad Dermatol Venereol, 27: 12–29. 10.1111/jdv.12163analysis, Journal of Dermatologicall Treatment, 27:4, 316–321, DOI: . [DOI] [PubMed] [Google Scholar]
- 47.Ungprasert Patompong, Srivali Narat & Thongprayoon Charat (2016) Association between psoriasis and chronic obstructive pulmonary disease: A systematic review and meta-analysis, Journal of Dermatological Treatment, 27:4, 316–321, DOI: 10.3109/09546634.2015.1107180 [DOI] [PubMed] [Google Scholar]
- 48.Bargagli E, Bellisai F, Mazzei MA et al. Interstitial lung disease associated with psoriatic arthritis: a new disease entity?. Intern Emerg Med (2020). 10.1007/s11739-020-02451-8 [DOI] [PubMed] [Google Scholar]
- 49.Christensen IE, Lillegraven S, Mielnik P, Bakland G, Loli L, Sexton J, Uhlig T, Kvien TK, Provan SA. Serious infections in patients with rheumatoid arthritis and psoriatic arthritis treated with tumour necrosis factor inhibitors: data from register linkage of the NOR-DMARD study. Ann Rheum Dis. 2021. Oct 8:annrheumdis-2021–221007. doi: 10.1136/annrheumdis-2021-221007. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karmacharya P, Shahukhal R, Ogdie A. Risk of Malignancy in Spondyloarthritis: A Systematic Review. Rheum Dis Clin North Am. 2020. Aug;46(3):463–511 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 1. Search strategy for the systematic review process
Supplementary File 2. A) Risk of bias of the included psoriatic arthritis studies based on New-Castle Ottawa scale for observational studies, B) Risk of bias of the included ankylosing spondylitis studies based on New-Castle Ottawa scale for observational studies, C) GRADE quality assessment of mortality in psoriatic arthritis and ankylosing spondylitis
Supplementary File 3. Funnel plot of included studies looking at overall mortality in A) psoriatic arthritis B) ankylosing spondylitis
Supplementary File 5. Forest plot for sex-stratified mortality in ankylosing spondylitis A) males B) females
Supplementary File 4. Forest plot for sex-stratified mortality in psoriatic arthritis A) males B) females
Supplementary File 6. Subgroup analysis of all-cause mortality in PsA studies published A) before B) after the year 2010.
Supplementary File 7. Forest plot mortality related to intentional self-harm/ suicide & injury and poisoning.
Supplementary File 9. Overall Mortality in AS by geographical location
Supplementary File 8. Overall mortality in PsA by geographical location


