Abstract
Patients with severe, COVID-related multi-organ failure often require extracorporeal life support (ECLS) such as extracorporeal membrane oxygenation (ECMO) or continuous renal replacement therapy (CRRT). ECLS can alter drug exposure via multiple mechanisms. Remdesivir (RDV) and its active metabolite GS-441524 are likely to interact with ECLS circuits, resulting in lower than expected exposures. We evaluated circuit-drug interactions in closed loop, ex vivo ECMO and CRRT circuits. We found that mean (standard deviation) recovery of RDV at 6 hours after dosing was low in both the ECMO (33.3% [2.0]) and CRRT (3.5% [0.4]) circuits. This drug loss appears to be due primarily to drug adsorption by the circuit materials and potentially due to metabolism in the blood. GS-441524 recovery at 6 hours was high in the ECMO circuit 75.8% (16.5), however, was not detectable at 6 hours in the CRRT circuit. Loss in the CRRT circuit appears to be due primarily to efficient hemodiafiltration. The extent of loss for both molecules, especially in CRRT, suggests that in patients supported with ECMO and CRRT, RDV dosing adjustments are needed.
Keywords: extracorporeal membrane oxygenation, continuous renal replacement therapy, remdesivir, COVID-19, pharmacokinetics
Introduction
Severe Acute Respiratory Syndrome Cornoavirus-2 (SARS-CoV-2) is a novel coronavirus that causes coronavirus disease-2019 (COVID-19). In severe cases, COVID-19 may lead to organ failure.1 Acute respiratory distress syndrome and acute kidney injury have rates that range from 3.4-46% and 0.5-28%, respectively, among hospitalized COVID-19 patients.2-9 These patients often require extracorporeal life support (ECLS) such as extracorporeal membrane oxygenation (ECMO) or continuous renal replacement therapy (CRRT).10-13
Although ECLS can be life-saving, mortality often exceeds 40%.14-16 The high mortality is suspected to be due, in part, to suboptimal dosing from ECLS-altered drug disposition.17 ECLS can alter drug pharmacokinetics (PK) by 1) drug adsorption by circuit components; 2) drug clearance by the circuit (e.g., hemofiltration, dialysis); and 3) physiologic alterations triggered by the circuit and underlying critical illness.18-23 Circuit-drug interactions are dependent on the physicochemical properties of the drug and circuit materials. Lipophilic and highly protein bound drugs are more likely to be adsorbed by circuit materials, while hydrophilic, low protein bound drugs are more readily cleared by hemofiltration/dialysis.23,24
Remdesivir (RDV) is the only approved therapy in adults and one of the few authorized in children by the US Food and Drug Administration for treatment of SARS-CoV-2. RDV is a prodrug with a short half-life (~1 hour) that rapidly converts to its active metabolite GS-441524 in vivo.25 RDV dosing on ECLS is unknown. Given the physicochemical properties (Table 1) of RDV and GS-441524, it is likely that RDV will be adsorbed by components of ECMO and CRRT circuits and minimally cleared by hemodiafiltration. Conversely, GS-441524 should be minimally adsorbed by circuit components but rapidly cleared by hemodiafiltration. This study quantified circuit-drug interactions between RDV and GS-441524 in isolated ECMO and CRRT ex vivo experiments.
Table 1.
Drug physiochemical properties and clearance pathways
| Drug | Charge | LogP | Plasma Protein Binding (%) |
Molecular Weight (g/mol) |
Primary Elimination Pathway |
|---|---|---|---|---|---|
| Remdesivir | Neutral | 2.01 | 88-93.6 | 602.6 | Renal |
| GS-441524 | Neutral | −0.58 | 2 | 291.26 | Renal |
Materials and Methods
Circuit Configuration
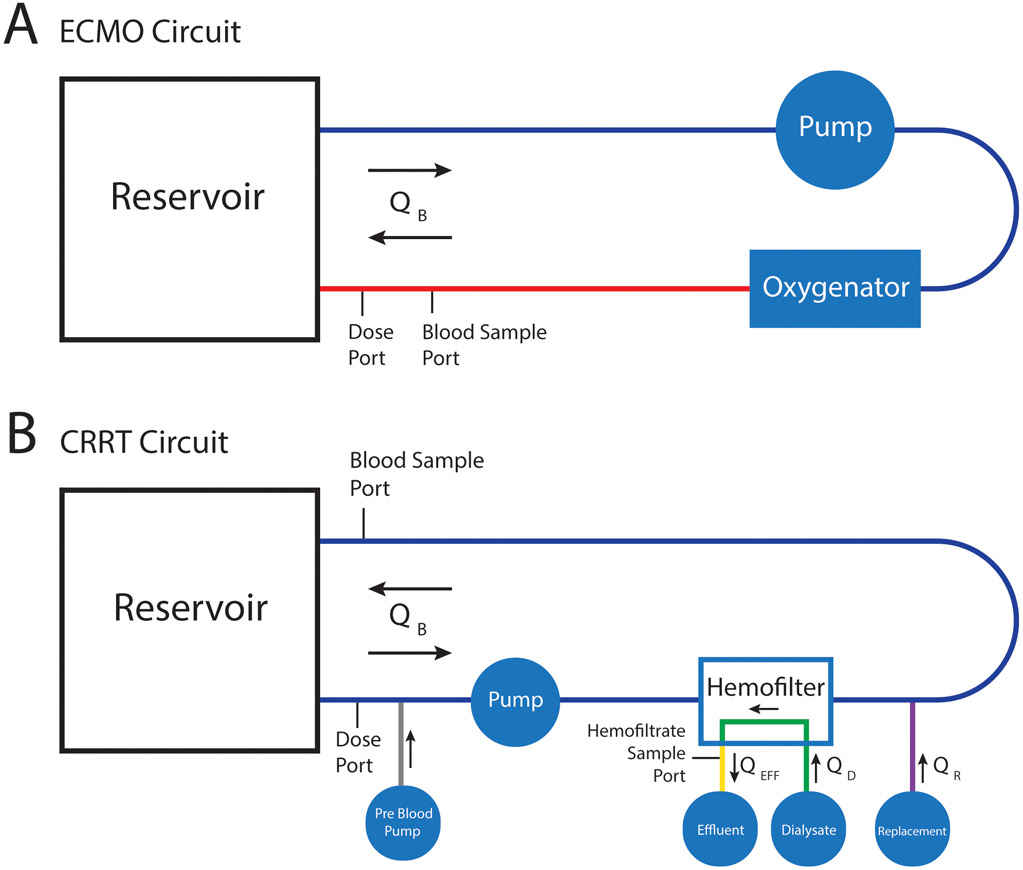
We evaluated circuit-drug interactions in closed loop, ex vivo ECMO and CRRT circuits (Table 2). ECMO circuits ran on a Rotaflow Console (Maquet, Rastatt, Germany), were constructed with a Rotaflow RF-32 Centrifugal Pump with Softline Coating (Maquet, Rastatt, Germany), Quadrox-iD Adult Oxygenator with Bioline coating (Maquet, Rastatt, Germany), ¾ inch polyvinyl chloride (PVC) tubing with Smart-X coating (LivaNova, Arvada, CO), and completed with a 1000 mL Plasma-Lyte A (Baxter Healthcare, Deerfield, IL) intravenous (IV) bag. CRRT circuits ran on a PRISMAX System (Baxter Healthcare, Deerfield, IL) with a TherMax heater (Baxter Healthcare, Deerfield, IL) and HF1000 filter set (Baxter Healthcare, Deerfield, IL) connected via a 500mL EXACTAMIX EVA bag (Baxter Healthcare, Deerfield, IL). HF1000 filter sets have an effective surface area of 1.1 m2. Each circuit configuration was run in triplicate for each drug.
Table 2.
Circuit components.
| Circuit Type | Component | Manufacturer | Model | Material |
|---|---|---|---|---|
| ECMO | Oxygenator | Maquet | Quadrox-iD Adult | Polymethylpentene hollow fibers with Biolinea coating |
| Pump | Maquet | Rotaflow RF-32 Centrifugal Pump | with Softlineb coating | |
| Tubing | LivaNova | Smart Perfusion Pack, SMA 3/8" diameter | Polyvinyl chloride with Smart-Xc coating | |
| Cannula | Medtronic | DLP™ One-Piece Pediatric Arterial Cannula, 10 Fr | Polyvinyl chloride | |
| Reservoir | Baxter | Viaflex Container, 1000 mL | Polyvinyl chloride | |
| CRRT | System | Baxter | PrisMax with TherMax heater | N/A |
| Hemofilter | Baxter | HF1000 | PolyarylEtherSulfone hollow fibers, plasticized polyvinyl chloride tubing | |
| TherMax Bag | Baxter | TherMax Blood Warmer Disposable, 27 mL | Polyurethane | |
| Reservoir | Baxter | EXACTAMIX EVA, 500 mL | Ethylene vinyl acetate |
Bioline coating: Covalently bonded recombinant human albumin and heparin.
Softline coating: Amphyphilic polymer coating.
Smart-X coating: Tribloc Copolymer (Polycaprolactone-Polydimethylsiloxane-Polycaprolactone) integrated into plastic.
ECMO Circuit Setup
ECMO circuits (Figure 1) were primed with a solution of 2 units of human red blood cells (adenine saline added leukocytes reduced [~600 mL]), 1 unit of thawed human plasma frozen within 24 hours after phlebotomy (~300 mL), and Plasma-Lyte A crystalloid (~300 mL). To limit impact on hospital blood bank supply, recently expired blood products from the American Red Cross were used. To mimic clinical practice and target physiological pH (7.2-7.5), circuits were dosed with heparin sulfate (500 units), tromethamine (2 g), sodium bicarbonate (7 mEq), calcium gluconate (650 mg), and 12.5 g human serum albumin. Additional tromethamine and CO2 were introduced as needed to maintain physiological pH.
Figure 1.
Schematic of circuit configurations by circuit type. In the ECMO circuit (A) QB represents blood flow of 1 L/min during the first 6 hours and 2 L/min during the second 6 hours of the experiment. The CRRT circuit (B) was in CVVHDF mode where QB represents blood flow of 150 mL/min during the first 6 hours and 300 mL/min during the second 6 hours of the experiment. QD represents dialysate flow that ran countercurrent at 1000 mL/h and 2000 mL/h during first and second 6 hours of experiment, respectively. In order to mimic clinical practice fluid was added to the system via the Pre Blood Pump and as replacement fluid (QR). Pre Blood Pump flows were 700 mL/h and 1400 mL/h during the first and second 6 hours of the experiment, respectively. QR were 300 mL/h and 600 mL/h during the first and second 6 hours of the experiment, respectively. This additional fluid was removed by the effluent pump (QEFF) via hemofiltration at rates of 1000 and 2000 mL/h to maintain a constant volume and prevent hemoconcentration.
Circuits were completed by 1000 mL double spiked IV bags. Blood return was directed with a 10 French arterial cannula (Medtronic, Minneapolis, MN). To quantify the impact of circuit flow, blood flow was initially set to 1 L/min prior to dose 1 (see Drug Administration below) and increased to 2 L/min at 6 hours, just before dose 2, for the remainder of the 12-hour experiment. Flow was measured using an HT110 bypass flow meter with H8XL flowsensor (Transonic, Davis, CA). Temperature was maintained at 37°C using an ECMO Water Heater (Cincinnati Sub-Zero, Cincinnati, OH) via the Quadrox-iD integrated heat exchanger.
CRRT Circuit Setup
CRRT circuits (Figure 1) were primed with a solution of 1 unit of human red blood cells (adenine saline added leukocytes reduced [~300 mL]), ~0.4 units thawed human plasma frozen within 24 hours after phlebotomy (125 mL), Plasma-Lyte A crystalloid (~150 mL), heparin sulfate (350 units), tromethamine (1.5 g), sodium bicarbonate (7 mEq), calcium gluconate (180 mg), and human serum albumin (6.25 g). Physiological pH was maintained with tromethamine.
The circuits were completed with 500mL EXACTAMIX EVA bags. PrismaSATE 4/2.5 Dialysis Solution (Baxter Healthcare, Deerfield, IL) was used for pre-blood pump, dialysis, and replacement fluids. Circuits were run in continuous venovenous hemodiafiltration (CVVHDF) mode with the following prescription: blood flow rate (QB) 150 mL/min, dialysis fluid (QD) 1000 mL/h, pre blood pump fluid 700 mL/h, replacement fluid (QR) 300 mL/h delivered after filtration, and patient fluid removal net 0 mL/h. By setting patient fluid removal to 0 mL/h, the PRISMAX system removed the extra fluid added via pre blood pump (700 mL/h) and QR (300 mL/h) via the effluent pump (QEFF). To quantify the effect of CRRT prescription on drug clearance, flow rates were doubled at 6 hours just before dose 2 for the remainder of the experiment. Blood was maintained at 37°C by a TherMax blood warmer.
Control Setup
Three standard control samples per drug were included to determine drug degradation throughout the experiment. Fifty mL of blood prime solution was drawn from the primed ECMO circuit after 5 minutes of circulation and before the start of the experiment and transferred to a PVC plastic tube (CELLTREAT, Pepperell, MA). This ensured identical composition to the circuit medium. The control samples were maintained at 37°C in a water bath.
Concentration decreases in the standard control samples prompted post hoc control experiments for RDV and GS-441524. To assess the source of loss in the control, three additional experimental conditions were studied: silanized glass to measure adsorption by the PVC tube; PVC tubing protected from light to determine light-dependent drug degradation; and a crystalloid prime solution in PVC to determine the extent of blood metabolism. All tubes except the crystalloid prime controls were filled with 30 mL of the blood prime solution as described. Crystalloid prime controls contained 30 mL of crystalloid solution: Plasma-Lyte A crystalloid (250 ml), heparin sulphate (1.75 U), sodium bicarbonate (3.5 mEq), calcium gluconate (1 g), and human serum albumin (6.25 g). Each condition was repeated in triplicate.
Drug Administration and Sample Collection
Remdesivir (Cayman Chemical, Ann Arbor, MI) or GS-441524 (Cayman Chemical, Ann Arbor, MI) were administered at time=0 minutes and time=6 hours via an arterial port in both ECMO and CRRT circuits. Drugs were dosed to achieve therapeutic concentrations of 2 mg/L for RDV and 0.2 mg/L for GS-441524.25 RDV and GS-441524 were administered into the control tubes at time=−5 minutes and placed in a rotator to mix for 5 minutes. The controls were returned to the water bath for the duration of the experiment, except during sampling. Control tubes were inverted gently 5 times before sampling.
Samples were collected at the following time points: 1, 5, 15, and 30 minutes, 1, 2, 3, 4, and 5 hours, and 5h 58 min. After dose 2 in ECMO and CRRT, additional samples were collected at: 6h 1 min, 6h 5 min, 6h 15 min, 6h 30 min, 7, 8, 9, 10, 11, 12 hours. Control experiments used a single dose run for 6 hours because there was no potential impact of flow or hemodiafiltration in isolated PVC tubes. Just before sample collection, ~3 mL of circuit blood was drawn as “waste” and returned after sample collection. For CRRT circuits, a hemofiltrate sample was collected at each time point from the tubing just prior to the effluent bag. Physiological pH of circuit blood was tested each hour with an i-STAT 1 Analyzer (Flextronics Manufacturing, Singapore) and EG6+ cartridge (Abbott, Abbott Park, IL) and adjusted as described above. Blood samples were centrifuged (3000rpm for 10 minutes at 4°C) immediately after collection. Plasma was pipetted and stored at −80°C. Hemofiltrate samples were stored at −80°C immediately following collection.
Analysis
RDV and GS-441524 concentrations were quantified using a simultaneous liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay developed at the Center for Human Toxicology at the University of Utah. Ammonium formate (1mM, pH 3.5) was added to a 200uL aliquot of plasma or hemofiltrate fortified with internal standard (6,7-dimethyl-2,3-di(2-pyridyl) quinoxaline (QX)), followed by extraction with ethyl acetate. Samples were concentrated and reconstituted in nanopure water before 30 μL was injected onto the LC-MS/MS. The LC consisted of an Accela UHPLC pump (Thermo Scientific, San Jose, CA) with a Synergi™ 2.5um Polar-RP (100mm x 2mm) analytical column (Phenomenex, Torrance, CA) maintained at 40°C with a SecurityGuard™ Polar-RP (4.0 x 3.0 mm) cartridge (Phenomenex, Torrance, CA). Compounds were eluted using a gradient of acetonitrile (A) and ammonium formate (B, 1mM, pH 3.5). The gradient started at 1% A:99% B (0 – 0.8 min), increased to 90% A:10% B (0.8 – 1.5 min), held at 90% A:10% B (1.5 – 4.5 min), then re-equilibrated prior to the next injection (4.6 – 10 min), at a constant flow rate of 0.300 mL/min. A Thermo Scientific TSQ Vantage MS/MS was operated in positive ion SRM mode, and the following transitions were monitored at the indicated collision energies (CE): 603.2→200.0 (CE=54 eV, RDV), 292.1→163.1 (CE=23 eV, GS-441524), and 312.9→78.1 (CE=45 eV, QX). A quadratic log-log regression was used to fit the calibration curve, which had a dynamic range of 1-5000 ng/mL. The assay was both precise (RDV %CV≤7.1, GS-441524 %CV≤13.4) and accurate (RDV %dev ≤ ±7.2, GS-441524 %dev ≤ ± 2.9). Samples below the limit of quantification were assigned a value of 0.
Drug recovery in plasma samples was calculated at each time point with the following equation:
where Ct is the concentration at time t and Ci is the concentration at time=5 minutes for ECMO samples and time=1 minute for CRRT and control samples.
Drug passage across the hemofilter in the CRRT system was calculated using the saturation coefficient (SA) from paired hemofiltrate and plasma samples at each time point with the following equation:
Where CH and CP are concentrations in hemofiltrate and plasma, respectively.
Statistics
To evaluate a circuit’s effect on recovery, a two-sample t-test was used. Mean recovery of circuit replicates at time=5 hours 58 minutes and time=12 hours were compared to the mean recovery in the standard control experiments at time=6 hours of the matching compound. One CRRT replicate per compound ended prematurely due to machine error. Consequently, we compared mean recovery at time=3 hours for RDV and time=5 hours for GS-441524 to mean recovery of the standard control at that time (to maintain n ≥ 3). In order to determine the source of loss in the control experiments, we compared all four control conditions of like drugs using a one-way ANOVA with post hoc comparisons using Tukey’s test.
Results
A total of 582 samples were collected (Supplemental tables 1-4). Two samples were dropped for anomalous concentration measurements (greater than two standard deviations from mean) assumed to be from a sampling or assay error, and one due to an error during collection. In the RDV CRRT circuit after the second dose, the concentration at time=6 hours 1 minute was much lower than expected for 2 of 3 circuits indicating that increased flow rates rapidly cleared RDV in the first minute. This prevented capture of the true Ci and calculation of recovery after the second dose.
Remdesivir
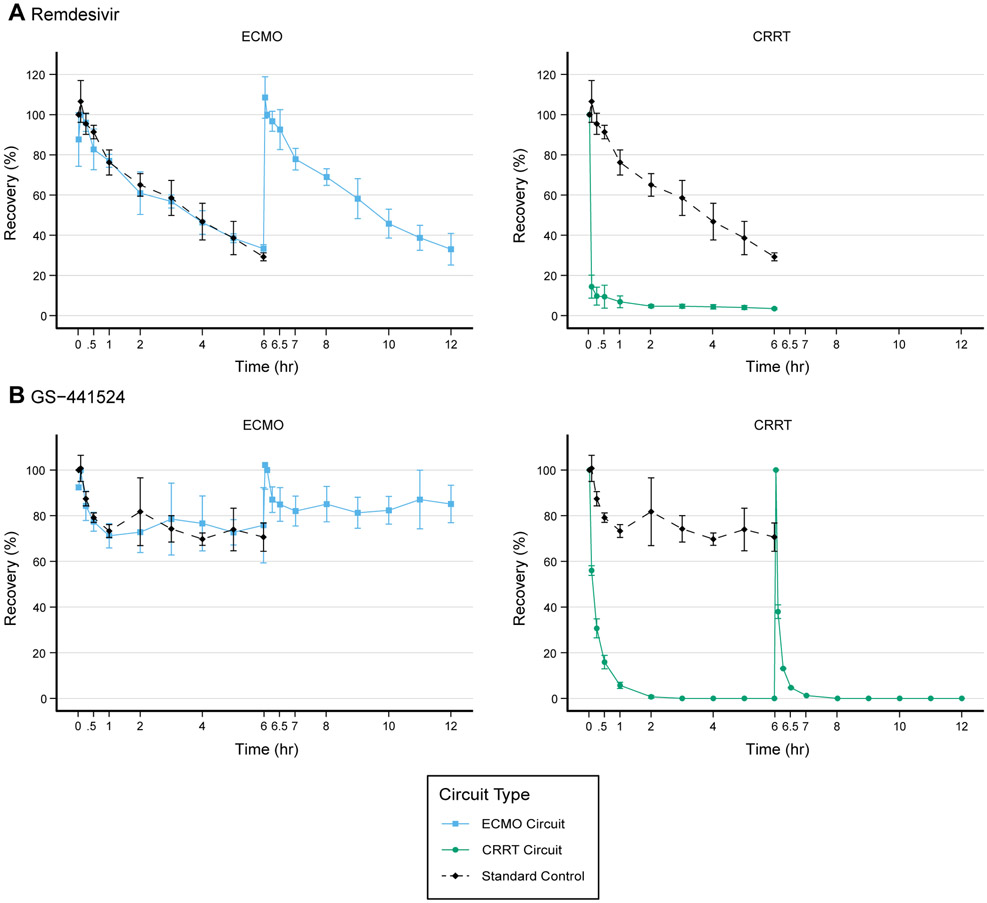
Mean (standard deviation [SD]) recovery of RDV in the ECMO circuits (n=3) was low following dose 1 at 33.3% (2.0) at 5 hours 58 minutes and dose 2 (33.0% [7.8]) at 12 hours (Figure 2). Mean recovery in the standard control (n=3) at 6 hours was 29.3% (2.0) and not significantly different from recovery in the ECMO circuit at 6 hours (P=0.07).
Figure 2.
Mean recovery (n=3) of each drug from 0-12 hours with standard deviation shown as error bars. In the remdesivir CRRT circuit after the second dose, the concentration at time=6 hours 1 minute was much lower than expected for 2 of the 3 circuits suggesting that the increased flow rates rapidly cleared remdesivir in the first minute. As such we were unable to capture the true maximum concentration for those two circuits and could not calculate recovery after the second dose. One 0-6h GS-441524 CRRT circuit ended after 5 hours due to machine error, resulting in n=2 for the 6-12h GS-441524 CRRT circuits.
Substantial loss in the CRRT circuits (n=3) occurred within minutes of dosing. Recovery in plasma following dose 1 was 14.4% (5.7) at 5 minutes and 4.7% (1.0) at 3 hours and significantly different compared to control recovery at 3 hours (P=0.008). The mean (SD) SA for remdesivir in the CRRT circuits was low at 0.006 (0.006) suggesting that remdesivir is minimally filtered/dialyzed.
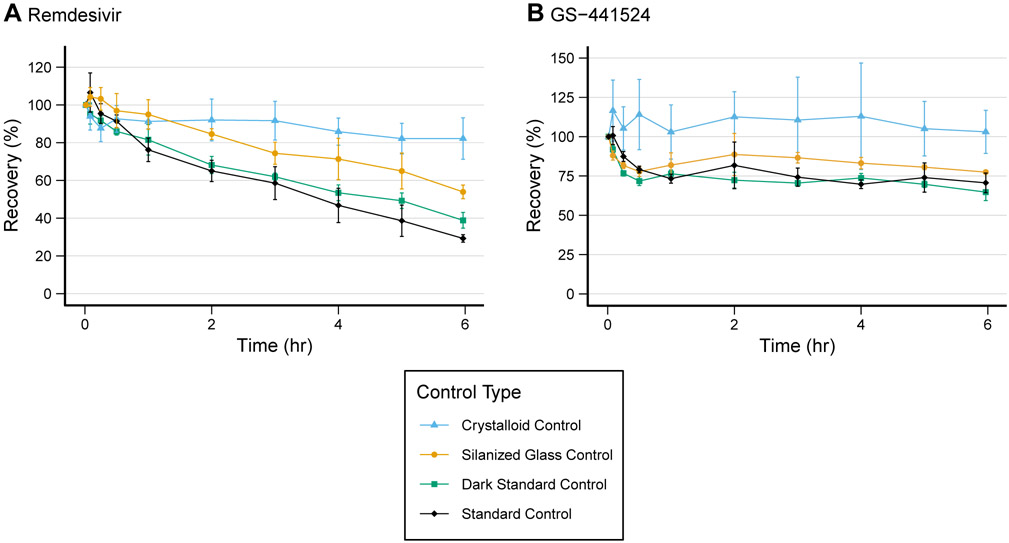
Results from the three additional control experiments are presented in Figure 3. The silanized glass and light protected controls had mean recoveries of 53.9% (3.6) and 38.8% (4.2) at 6 hours, respectively. However, the crystalloid prime control saw higher recovery at 82.2% (11) at 6 hours. Analysis with an ANOVA produced P<0.001 and an R2 of .92. We found significant differences (difference, adjusted p-value) between the crystalloid prime and standard (−52.9%, P<0.001), light protected (−43.3%, P<0.001), and silanized glass controls (−28.3%, P=0.002). A statistically significant difference was observed between the means of the standard and the silanized glass controls (24.7%, P=0.006). We were able to simultaneously quantify RDV and GS-441524 in all samples. GS-441524 was not detected at significant concentrations (<10% of RDV) in the RDV control experiments. Therefore, if there was metabolism or degradation of RDV in the blood, the primary product was not GS-441524.
Figure 3.
Recovery for each drug under four experimental control conditions. Recoveries were calculated from samples drawn over 0-6 hours as a percent of the concentration at time t=1 minute. Values are mean (n=3) with standard deviation shown as error bars.
GS-441524
Recovery of GS-441524 was higher than RDV in the ECMO circuits (Figure 2). After 5 hours 58 minutes, mean (SD) recovery was at 75.8% (16.5) and after dose 2 was 85.11% (8.2) at 12 hours. Mean recovery in the standard control (n=3) at 6 hours was 70.6% (6.2) and not significantly different from recovery in the ECMO circuit at 6 hours (p=0.7).
In CRRT circuits (n=3), recovery for GS-441524 was low at 15.9% (3.0) at 30 minutes and 0% (0) at 3 hours and significantly different from the control at 5 hours (p=0.005). After the second dose (n=2), recovery was 13.1% (0.2) at 15 minutes and 0% (0) by 2 hours post-dose. The mean (SD) SA for GS-441524 in the CRRT circuits was high at 2.3 (1.3) indicating GS-441524 is freely filtered/dialyzed.
In the additional post hoc control experiments, the mean (SD) recovery in the crystalloid primed controls was 103.1% (13.7), in the light protected was 64.7% (5.3), and silanized glass was 77.4% (1.1; Figure 3). Overall, the ANOVA analysis yielded P=0.002 and R2=.77. Again, we saw significant differences in mean recovery of −32.5 (P=0.005), −38.4 (P=0.002), and −25.6 (P=0.02) between the crystalloid prime and standard, light protected, and silanized glass controls, respectively.
Discussion
Patients with severe COVID-19-related organ failure often require ECLS. In addition, patients with chronic disease such as end-stage renal disease (ESRD) have above-average rates of severe COVID-19 infection and mortality.26-28 Although ECMO and CRRT can be life-saving technologies in patients with severe COVID-19, appropriate dosing of available drugs such as RDV in this population is unknown. Altered drug exposure places patients at risk for inappropriate dosing and therapeutic failure.29 In this study we performed ex vivo ECLS experiments to quantify the extent of RDV and GS-441524 loss in ECMO and CRRT circuits.
Substantial loss of RDV was observed in both the ECMO and CRRT circuits. The degree of loss, likely from circuit adsorption, was expected given that RDV is highly protein-bound (88-93.6%) and lipophilic (LogP 2.01). Evidence of circuit adsorption is based on three results: 1) RDV recovery in hemofiltrate was low. Loss via the hemofilter can occur through a) passive diffusion across the membrane; b) areas of stasis that trap the drug; and c) direct adsorption. Low hemofiltrate concentrations argue against passive diffusion. Stasis trapping typically results in high variability as the “trapped” drug is periodically released. The drug therefore was likely adsorbed by circuit components or permanently “trapped”, which is functionally equivalent. 2) The standard controls had recoveries comparable to the circuits, supporting drug interaction with surface materials and the prime solution as a mechanism of loss rather than flow rate, which when doubled had minimal impact on recovery. 3) RDV loss was observed in all control conditions. Light degradation did not appear to play a significant role as the recovery in those controls was not different from the standard controls. However, recovery was higher in the crystalloid and silanized glass controls pointing to surface material adsorption and RDV metabolism in blood as mechanisms of loss. These results are consistent with previous literature on circuit-drug interactions predicted by physiochemical properties 24,30,31; and esterase metabolism of RDV in blood.32
In contrast, GS-441524 was minimally extracted by ECMO circuits but highly extracted by CRRT circuits. We expect minimal adsorption but efficient hemodiafiltration based on GS-441524’s low protein binding (2%) and hydrophilicity (LogP −0.58). If adsorption played an extensive role, we would expect to see drug loss in both ECMO and CRRT circuits. Because substantial loss was only observed in CRRT, either GS-441524 interacted with a component specific to the CRRT circuit or it was cleared by hemodiafiltration. High concentrations of GS-441524 recovered in the hemofiltrate provide evidence that efficient hemodiafiltration was the primary mechanism of loss. Additionally, the post hoc control experiments suggest that some loss may be due to GS-441524 metabolism in the blood.32
Our study has a few limitations. We only evaluated one system each for the ECMO and CRRT circuits. Previous studies have shown that drug loss can vary based on circuit materials and coatings.23 Second, there was variability in the maximum concentration achieved in each circuit due to variations in circuit volumes and reconstituted drug concentrations. We do not believe this impacted the overall results as all concentrations were within therapeutic range. There was an approximately 1-fold higher concentration of albumin in the crystalloid prime control solution than that of the controls using standard control blood. This could impact protein binding of RDV in the crystalloid controls and subsequently alter the extent of loss from PVC adhesion. However, it is unlikely to change our overall conclusion, that multiple factors contribute to RDV loss in circuits and controls. Third, hemolysis is known to occur in the setting of ECLS both ex vivo and in vivo. Hemolysis can impact drug concentrations via two primary mechanisms: 1) adsorption of drug to proteins released from the cytoplasm of erythrocytes and 2) direct release of drug from cytoplasm for drugs that are highly partitioned into erythrocytes.33 Our experimental design allowed us to determine net recovery of drug but not to isolate the different factors that contributed to drug loss. Finally, a machine error during some CRRT runs caused us to prematurely end two of our circuits. While not affecting prior samples, this resulted in an n=2 for the later samples in one 0-6h RDV and GS-441524 experiment. We mitigated this by using earlier timepoints (where n=3) for statistical comparisons. We do not believe this influenced our conclusions because the observed recovery at these timepoints was either the same or within the SD of the recovery at time=5 hours 58 minutes.
Conclusion
RDV is extracted by ECMO and CRRT primarily by drug adsorption to circuit materials and potentially to drug metabolism in the blood. GS-441524 was not substantially extracted by the ECMO circuit but rapidly cleared by hemodiafiltration in the CRRT circuit. The extent of loss for both molecules, especially in CRRT, suggests that in patients supported with ECMO and CRRT, dosing adjustments are needed. Optimal dosing regimens can be predicted using ex vivo data such as these to inform an ECMO or CRRT compartment in a physiological based pharmacokinetic model.
Supplementary Material
Acknowledgements
We are grateful to Russ Telford and Emma Rudié for providing statistical and coding expertise.
Conflicts of Interest and Source Funding:
None of the authors declare any conflict of interest. Dr. Watt receives support for pediatric research from the National Institute of Child Health and Human Development (R01HD097775, R21HD104412).
References
- 1.Gupta A, Madhavan MV, Sehgal K, et al. : Extrapulmonary manifestations of COVID-19. Nat. Med 26 (7): 1017–1032, 2020. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 2.Chan L, Chaudhary K, Saha A, et al. : AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol 32 (1): 151–160, 2021. doi: 10.1681/asn.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen T, Wu D, Chen H, et al. : Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368: m1295, 2020. doi: 10.1136/bmj.m1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guan W-j, Ni Z-y, Hu Y, et al. : Clinical Characteristics of Coronavirus Disease 2019 in China. NEMJ 382 (18): 1708–1720, 2020. doi: 10.1056/nejmoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C, Wang Y, Li X, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet 395 (10223): 497–506, 2020. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richardson S, Hirsch JS, Narasimhan M, et al. : Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 323 (20): 2052, 2020. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D, Hu B, Hu C, et al. : Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus–Infected Pneumonia in Wuhan, China. JAMA 323 (11): 1061, 2020. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, et al. : Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 8 (5): 475–481, 2020. doi: 10.1016/s2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. The Lancet 395 (10229): 1054–1062, 2020. doi: 10.1016/s0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbaro RP, MacLaren G, Boonstra PS, et al. : Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. The Lancet: 1071–1078, 2020. doi: 10.1016/s0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mustafa AK, Alexander PJ, Joshi DJ, et al. : Extracorporeal Membrane Oxygenation for Patients with COVID-19 in Severe Respiratory Failure. JAMA Surg: 1–3, 2020. doi: 10.1001/jamasurg.2020.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ronco C, Reis T, Husain-Syed F: Management of acute kidney injury in patients with COVID-19. The Lancet Respir Med 8 (7): 738–742, 2020. doi: 10.1016/s2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaefi S, Brenner SK, Gupta S, et al. : Extracorporeal membrane oxygenation in patients with severe respiratory failure from COVID-19. Intensive Care Med, 2021. doi: 10.1007/s00134-020-06331-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayes LW, Oster RA, Tofil NM, Tolwani AJ: Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care 24 (3): 394–400, 2009. doi: 10.1016/j.jcrc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Ricci Z, Goldstein SL: Pediatric Continuous Renal Replacement Therapy. S. Karger AG, 2016, pp 121–130. [DOI] [PubMed] [Google Scholar]
- 16.Watson RS, Crow SS, Hartman ME, Lacroix J, Odetola FO: Epidemiology and Outcomes of Pediatric Multiple Organ Dysfunction Syndrome. Pediatr Crit Care Med 18: S4–S16, 2017. doi: 10.1097/pcc.0000000000001047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nolin TD, Aronoff GR, Fissell WH, et al. : Pharmacokinetic Assessment in Patients Receiving Continuous RRT: Perspectives from the Kidney Health Initiative. Clin J Am Soc Nephrol 10 (1): 159–164, 2015. doi: 10.2215/cjn.05630614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clewell HJ, Teeguarden J, McDonald T, et al. : Review and Evaluation of the Potential Impact of Age- and Gender-Specific Pharmacokinetic Differences on Tissue Dosimetry. Crit Rev in Toxicol 32 (5): 329–389, 2002. doi: 10.1080/20024091064264. [DOI] [PubMed] [Google Scholar]
- 19.Lorenzin A, Garzotto F, Alghisi A, et al. : CVVHD treatment with CARPEDIEM: small solute clearance at different blood and dialysate flows with three different surface area filter configurations. Pediatr Nephrol 31 (10): 1659–1665, 2016. doi: 10.1007/s00467-016-3397-2. [DOI] [PubMed] [Google Scholar]
- 20.McQueen CA: Neonatal Ontogeny of Murine Arylamine N-Acetyltransferases: Implications for Arylamine Genotoxicity. Toxicol Sci 73 (2): 279–286, 2003. doi: 10.1093/toxsci/kfg086. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura AT, Btaiche IF, Pasko DA, Jain JC, Mueller BA: In vitro clearance of trace elements via continuous renal replacement therapy. J Ren Nutr 14 (4): 214–219, 2004. doi: 10.1016/s1051-2276(04)00125-6. [DOI] [PubMed] [Google Scholar]
- 22.Watt KM, Cohen-Wolkowiez M, Williams DC, et al. : Antifungal Extraction by the Extracorporeal Membrane Oxygenation Circuit. J Extra Corpor Technol 49 (3): 150–159, 2017. [PMC free article] [PubMed] [Google Scholar]
- 23.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D: Determinants of drug absorption in different ECMO circuits. Intensive Care Med 36 (12): 2109–2116, 2010. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shekar K, Roberts JA, McDonald CI, et al. : Protein-bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: Results from an ex vivo study. Crit Care 19 (1): 1–8, 2015. doi: 10.1186/s13054-015-0891-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Humeniuk R, Mathias A, Cao H, et al. : Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin Transl Sci 13 (5): 896–906, 2020. doi: 10.1111/cts.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nopsopon T, Kittrakulrat J, Takkavatakarn K, Eiamsitrakoon T, Kanjanabuch T, Pongpirul K: Covid-19 in end-stage renal disease patients with renal replacement therapies: A systematic review and meta-analysis. PLOS Negl Trop Dis 15 (6): e0009156, 2021. doi: 10.1371/journal.pntd.0009156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sosa R, Garcia P, Cipriano EO, et al. : Coronavirus Disease 2019 in Patients With End-Stage Kidney Disease on Hemodialysis in Guatemala. Kidney Int Rep 6 (4): 1110–1117, 2021. doi: 10.1016/j.ekir.2021.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rastad H, Ejtahed H-S, Shafiee G, et al. : The risk factors associated with COVID-19-Related death among patients with end-stage renal disease. BMC Nephrol 22 (1), 2021. doi: 10.1186/s12882-020-02221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shekar K, Roberts JA, McDonald CI, et al. : Sequestration of drugs in the circuit may lead to therapeutic failure during extracorporeal membrane oxygenation. Crit Care 16 (5): R194–R194, 2012. doi: 10.1186/cc11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Böhler J, Donauer J, Keller F: Pharmacokinetic principles during continuous renal replacement therapy: Drugs and dosage. Kidney Int 56: S24–S28, 1999. doi: 10.1046/j.1523-1755.56.s.72.2.x. [DOI] [PubMed] [Google Scholar]
- 31.Lau AH, Kronfol NO: Determinants of Drug Removal by Continuous Hemofiltration. Int J Artif Organs 17 (7): 373–378, 1994. doi: 10.1177/039139889401700702. [DOI] [PubMed] [Google Scholar]
- 32.Avataneo V, de Nicolò A, Cusato J, et al. : Development and validation of a UHPLC-MS/MS method for quantification of the prodrug remdesivir and its metabolite GS-441524: a tool for clinical pharmacokinetics of SARS-CoV-2/COVID-19 and Ebola virus disease. J Antimicrob Chemother 75 (7): 1772–1777, 2020. doi: 10.1093/jac/dkaa152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams DC, Turi JL, Hornik CP, et al. : Circuit Oxygenator Contributes to Extracorporeal Membrane Oxygenation–Induced Hemolysis. ASAIO J 61 (2): 190–195, 2015. doi: 10.1097/mat.0000000000000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.