Abstract
Deinococci with radiation resistance greater than that of Deinococcus radiophilus (ATCC 27603) were isolated from three commercial oyster extracts stored at 4, 20, and 30°C. During storage the number of other bacteria declined and deinococci became the predominant group in the microflora, particularly at 20°C, although at 30°C the number of deinococci as well rapidly declined. The results suggest that the natural habitat of deinococci is an aerobic environment containing a slightly elevated saline content, soluble protein, and low sugar levels.
The deinococci were originally classified as members of the genus Micrococcus when the first species, Deinococcus radiodurans R1, (previously named Micrococcus radiodurans) was isolated from cans of meat subjected to sterilizing doses of UV radiation in the megarad range (1). Brooks and Murray (2) assigned them to a new family, Deinococcaceae, because these organisms were not included in the Approved Lists of Bacterial Names (17).
Deinococcus spp. are radiation resistant, strictly aerobic, catalase-positive, gram-positive, tetrad-forming cocci. However, their natural habitat has not been well defined because all of the extant species are chemoorganotrophic, require complex media for growth, and have been isolated from a diversity of sites. These include materials, surfaces, and dust contaminated by humans and animals as well as soil, soil contaminated by animals, animals contaminated by soil (3, 7, 9–12, 14, 15), meat (5), and animal feces and sewage (8). Their desiccation and radiation resistance properties probably allow them to persist in dust and cause aerial contamination at a low level in many sites, including clean rooms. Although they are not pathogenic, their unusual radiation resistance makes them of interest.
Commercial oyster extract is a heavily salted liquid produced from oysters in eastern Asia in various ways. It contains about 20% NaCl, 65% water, 6.4% crude protein, and 11% carbohydrate/lipid and has a water activity of about 0.79 and a pH of 5.4, although there is considerable variation (3, 16). The production steps include a heating step to concentrate the extract which is used to make oyster sauce, a condiment commonly used to flavor cooked meals, such as Chinese stir-fried foods.
In a study of the growth of microorganisms in oyster extract, red colonies were often detected on the plates. In this report we show that these organisms were deinococci, revealing a new source for these radiation-resistant bacteria.
Three oyster extract samples, A, B and C, were obtained from a local oyster sauce manufacturer in Hong Kong. Their place of manufacture (outside of Hong Kong) was not revealed to us for commercial reasons. They were stored at 4°C for 115 days, 20°C for 114 days, and 30°C for 113 days. They were sampled for total aerobic bacterial (TAB) counts at appropriate times (see Fig. 1). On receipt, samples were diluted 1:10 and salinity was measured with a salinity meter (YSI model 33) at room temperature.
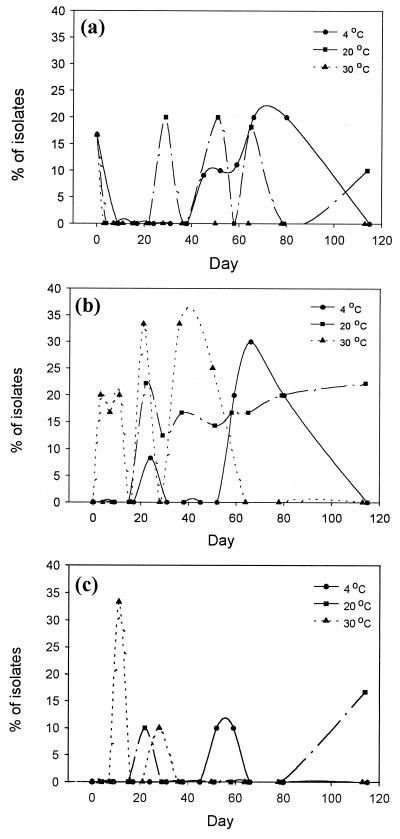
FIG. 1.
The probability of isolating deinococci from oyster extract sample A (a), sample B (b), and sample C (c) at 4, 20, and 30°C for up to 114 days.
Prepoured and dried standard plate count agar (SPCA) (Oxoid) plates were used for TAB counts by the spread plate method and for isolation and purification of Deinococcus spp.; incubation was at 25 ± 1°C for 5 days. The bacterial colonies were statistically isolated using Harrison’s disc and method (6). The number of colonies selected was the square root of the TAB count of each agar plate.
All the isolates with red colonies were catalase-positive, strictly aerobic, gram-positive nonmotile cocci up to 2 μm in diameter, in pairs and tetrads. They required 5 days at 25°C to form visible red colonies on SPCA. None produced acid from glucose in standard peptone medium, 28% reduced nitrate, 42% were weak nitrate reducers, and 30% did not reduce nitrate. UV irradiation resistance was tested as follows. Cultures were spread on SPCA plates and placed, with lids removed, under and 70 cm away from a 30-W UV germicidal lamp (Philips) in a safety cabinet for 0, 1, 2, 4, and 8 min and then incubated at 25°C for 5 days. Control cultures included Deinococcus radiophilus (ATCC 27603) (positive) and Escherichia coli (ATCC 10536) (negative). The cultures and D. radiophilus survived 8 min under the conditions described above, but the E. coli culture did not survive 30 s under the same conditions. The oyster extract cultures exhibited greater tolerance to UV radiation than the D. radiophilus culture, which grew very little after 8 min of UV exposure. The results, combined with TAB counts, were plotted and expressed as the probability of isolating deinococci during storage at the temperatures used.
The TAB counts (not shown) for all oyster extract samples showed a decline in number over time and the decline rate increased as storage temperature rose from 4 to 30°C. A similar pattern has been seen in salted butter stored over similar temperature ranges (13).
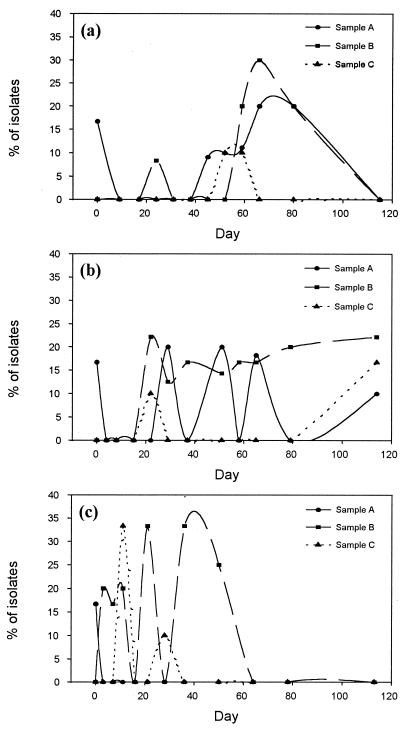
Generally, presumptive deinococci were more frequently isolated from samples A and B than from sample C (Fig. 1). At storage temperatures of 4 and 20°C, they were also more frequently isolated than at 30°C as time progressed, except for sample C (Fig. 2). It was also noted that at the lower storage temperature, more deinococci were isolated at the later stage of storage than at the earlier stage, and deinococci were less frequently isolated later at the higher storage temperature. The results indicate that deinococci were more resistant to the relatively high-saline conditions in the oyster extract than the other initial bacterial populations. At extremely high salinity, the probability of isolating deinococci was also very low; see, e.g., data for sample C (Fig. 2 and Table 1).
FIG. 2.
The probability of isolating deinococci from oyster extract samples A, B, and C after storage at 4°C (a), 20°C (b), and 30°C (c) for up to 114 days.
TABLE 1.
The salinity levels of three commercial oyster extract samples and the overall probability of isolating deinococci
| Oyster extract sample | Relative salinity, % NaCl (wt/vol) | Overall average percentage for isolation of deinococci, %a |
|---|---|---|
| A | 15 | 5.2 |
| B | 17 | 10.2 |
| C | 24 | 2.5 |
Percentage at all three storage temperatures.
The difficulty of isolating deinococcal species at the low storage temperature was mainly due to the higher proportion of other bacterial species in the oyster extract, particularly early in the storage period. This reduced the chance of isolating deinococci. Increasing the storage temperature caused the demise of other bacteria and left the more-resistant deinococci.
It was found that 20°C was the most suitable selective temperature for deinococci in oyster extract. It provided a mild selectivity pressure for eliminating other bacterial species and retaining a stable proportion of deinococci in oyster extract. However, the level of salinity in the sample must be suitable, as high salinity seemed to reduce deinococcal numbers rapidly.
The ultimate source of these organisms is not known, but given the nature of these samples, which are produced in a marine environment, it is likely that the natural habitat of these isolates is an aerobic environment containing a slightly elevated saline level, soluble protein, and low levels of sugar.
Acknowledgments
The authors are grateful to Patricia Leung for the supply and preparation of samples and to all technical staff of the Department of Biology and Chemistry of City University of Hong Kong for technical and material assistance.
REFERENCES
- 1.Anderson A W, Nordan H C, Cain R F, Parrish G, Duggan D. Studies on a radio-resistant micrococcus. I. Isolation, morphology, cultural characteristics, and resistance to gamma radiation. Food Technol. 1956;10:575–577. [Google Scholar]
- 2.Brooks B W, Murray R G E. Nomenclature for “Micrococcus radiodurans” and other radiation-resistant cocci: Deinococcaceae fam. nov. and Deinococcus gen. nov., including five species. Int J Syst Bacteriol. 1981;31:353–360. [Google Scholar]
- 3.Chan W F. M.Phil. thesis. Hong Kong, People’s Republic of China: City University of Hong Kong; 1998. . (Submitted.) [Google Scholar]
- 4.Christensen E A, Kristensen H. Radiation resistance of microorganisms from air in clean premises. Acta Pathol Microbiol Scand Sect B. 1981;89:293–301. doi: 10.1111/j.1699-0463.1981.tb00192_89b.x. [DOI] [PubMed] [Google Scholar]
- 5.Grant I R, Patterson M F. A novel radiation-resistant Deinobacter sp. isolated from irradiated pork. Lett Appl Microbiol. 1989;8:21–24. [Google Scholar]
- 6.Harrigan W F, McCance M E, editors. Laboratory methods in food and dairy microbiology. London, United Kingdom: Academic Press; 1976. [Google Scholar]
- 7.Ito H. Isolation of Micrococcus radiodurans occurring in radurized sawdust culture media of mushroom. Agric Biol Chem. 1977;41:35–41. [Google Scholar]
- 8.Ito H, Iizuka H, Takehisa M, Watanabe H. Isolation and identification of radiation-resistant cocci belonging to genus Deinococcus from sewage sludges and animal feeds. Agric Biol Chem. 1983;47:1239–1247. [Google Scholar]
- 9.Krabbenhoft K L, Anderson A W, Elliker P R. Ecology of Micrococcus radiodurans. Appl Microbiol. 1965;13:1030–1037. doi: 10.1128/am.13.6.1030-1037.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kristensen H, Christensen E A. Radiation-resistant microorganisms isolated from textiles. Acta Pathol Microbiol Scand Sect B. 1981;89:303–309. doi: 10.1111/j.1699-0463.1981.tb00193_89b.x. [DOI] [PubMed] [Google Scholar]
- 11.Murray R G E. Family II. Deinococcaceae Brooks and Murray 1981, 356VP. In: Sneath P H A, Mair N S, Sharpe M E, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 2. Baltimore, Md: Williams & Wilkins; 1986. pp. 1035–1043. [Google Scholar]
- 12.Murray R G E. Proceedings of the IVth International Symposium on Microbial Ecology. Ljubljana, Slovenia: Slovene Society for Microbiology; 1986. The biology and ecology of the Deinococcaceae; pp. 153–158. [Google Scholar]
- 13.O’Toole D K. Effect of storage temperature on microbial growth in continuously made butter. Aust J Dairy Technol. 1978;33:85–87. [Google Scholar]
- 14.Sanders S W, Maxcy R B. Patterns of cell division, DNA base compositions, and fine structures of some radiation-resistant vegetative bacteria found in food. Appl Environ Microbiol. 1979;37:159–168. doi: 10.1128/aem.37.1.159-168.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanders S W, Maxcy R B. Isolation of radiation-resistant bacteria without exposure to irradiation. Appl Environ Microbiol. 1979;38:436–439. doi: 10.1128/aem.38.3.436-439.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheng G J, Yen T H, Li C F, Chi S P S. Manufacture and quality improvement of oyster sauce. II. Manufacturing oyster sauce. Food Sci China. 1988;15:33–46. . (In Chinese.) [Google Scholar]
- 17.Sneath P H A, editor. Approved lists of bacterial names. Int J Syst Bacteriol. 1980;30:225–420. [Google Scholar]