Abstract
The health problems caused by iron (Fe) and zinc (Zn) deficiency plague developing and underdeveloped countries. A vegetarian person mainly depends on cereal based diet with low quantity of Fe and Zn. Biofortification is an economical and sustainable approach to challenge the micronutrient malnutrition problem globally. Pearl millet (Pennisetum glaucum (L.) R. Br.) is one of the nutri-cereals and mostly grown under hot, dry conditions on infertile soils of low water-holding capacity, where other crops generally fail. It contains anti-nutrient compounds like phytic acid and polyphenols which reduce the mineral bioavailability because of their chelating properties. Biofortification of pearl millet is like a double-edged sword which cuts down the economic burden and simultaneously supplies required nutrition to the poor, offering a great scope for food security as well as nutritional security. With this background, this review focus on biofortification of grain Fe and Zn content in pearl millet. Genetic research on Fe and Zn uptake and accumulation in pearl millet grain is crucial in identifying the ‘bottlenecks’ in biofortification. The review also reveals the need and strategies for increasing bioavailability of Fe and Zn in humans by increasing promoters and decreasing anti-nutritional factors in pearl millet.
Keywords: Biofortification, Fe and Zn, Pearl millet, Nutritional security
Introduction
Nutritional insecurity is the major menace in the world which needs to be addressed on a priority basis. Globally, it was estimated that at least 50% of children in the age group of 6 months to 5 years and > 2 billion people are facing the deficiency of essential micronutrients and vitamins (Oh et al. 2020). Deficiencies in micronutrients mainly of iron, zinc, iodine, vitamin A and folate have a negative consequence that adds to the burden of food deficiency due to poverty (CDC 2020). Millets can play an important role in addressing the challenge of nutritional insecurity, worldwide. Millets are small-seeded crops like pearl millet (Pennisetum glaucum), foxtail millet (Setaria italica), finger millet (Eleusine coracana), kodo millet (Paspalum setaceum) and barnyard millet (Echinochlo autilis). Among all millets, pearl millet [Pennisetum glaucum (L). R. Br] has many advantages in terms of environmental challenges and nutritional security (Vadez et al. 2012).At present, India is the largest producer of pearl millet in Asia, both in terms of area and production and the second-largest producer of rice and wheat globally (FAO 2020). The production of pearl millet is 8.61 million tonnes in India and productivity is 1243 kg/ha (Directorate of Millets Development 2020; Project Coordinator Review 2020). Despite the fact that food security has been achieved, the major challenge of nutritional security remains unresolved.
Globally, iron and zinc deficiencies are the major problems and various dietary factors influencing the development of iron deficiency anaemia include low dietary iron intake and/or the intake of compounds that decreases its bioavailability. Iron deficiency may also result from a low intake of other essential nutrients, such as vitamin A, E, B12, and folate (WHO 1996). It was estimated that dietary iron (mainly heme iron) contribute 10–15% of total iron intake in meat-eating populations, but, because of its higher and more uniform absorption (estimated at 15–35%), it could contribute ≥ 40% of total absorbed iron (Hurrell et al. 2010). Iron absorption is a complex process that reflects not only the iron content in the diet but also the bioavailability of iron. Similar to iron, zinc deficiency is largely related to inadequate intake or absorption of zinc from the diet. Many times people may take sufficient zinc but still face zinc deficiency which is mainly due to a high level of inhibitors like phytate, cadmium etc. in the diet (WHO 1996).
To overcome iron and zinc deficiency, there are many strategies which include dietary diversification, food fortification, external supplementation and biofortification. The most acceptable and reliable approach is biofortification, as it is a highly cost-effective and sustainable approach to enhance the essential micronutrient in staple food grain crops (Rehman et al. 2021). Biofortification is a strategy to increase the bioavailability and the concentration of nutrients in crops through both conventional plant breeding and recombinant DNA technology (genetic engineering) approaches, which reduces the concentration of antinutrients and favours micronutrient absorption (Sperotto et al. 2017). However, like in other cereals, biofortification in pearl millet is still limited by the presence of antinutrients like phytic acid, polyphenols, tannin and oxalates. The present review aims to focus on biofortification, genetic variability and molecular approaches to increase the grain iron and zinc content in pearl millet.
Present scenario and effects of iron and zinc malnutrition
Iron deficiency is the most widespread nutritional problem- whose significance ranks 9th among all the human health risks (Stoltzfus 2003). It mainly affects the physical and mental development of children and also their ability to learn (Talpur et al. 2018). Iron deficiency leads to a serious condition called anaemia, which is also a prime cause of women’s death during childbirth (Anuradha et al. 2017). The main causes of iron deficiency include low intake of bioavailable iron, increased iron requirements as a result of rapid growth, pregnancy, menstruation, and excess blood loss caused by pathologic infections (Larocque et al. 2005; Crompton and Nesheim 2002). Among the population of the developing world including India, the most commonly followed diet is vegetarian which largely depend on cereals and legumes and has limited access to fruits, meat, eggs, milk products etc. (Puranik et al. 2017). This low nutrient diet is the main cause of micronutrient deficiency. Micronutrient deficiency mainly affects women and children. An estimated 45% of deaths of children under the age of 5 are linked to micronutrient malnutrition (Gernand et al. 2016). During early infancy, iron requirements are met by small amounts of iron contained in human milk (Joint 2004). After birth, its need increases markedly and reaches approximately 0.7–0.9 mg/day during the remainder of the first year (Joint 2004). Adolescents also have higher iron requirements, especially during periods of growth. The average adults store about 1–3 g of iron in their bodies (Joint 2004). The iron requirement in menstruating women increases to levels above average iron requirements (Abbaspour et al. 2014).
Like iron deficiency, Zinc insufficiency is accompanied by a wide range of physiological problems such as hindrance in brain development, improper growth and augmented vulnerability to infectious diseases like diarrhoea, pneumonia and low birth outcomes in pregnant women (Hambidge and Krebs 2007). It is essential for the proper development of the human body and its deficiency is ranked as the 5th major risk factor for impairment (Anuradha et al. 2017).Its deficiency is particularly prevalent in children under 5 years old as they have a comparatively huge demand for zinc to sustain development (Black et al. 2008). Zinc deficiency is also responsible for the economic burden of disease in developing countries and has a substantial impact on Gross National Product (GDP) through declining productivity, which automatically increases health care costs (Darnton-Hill et al. 2005).The World Bank, in collaboration with the WHO, developed the Disability Adjusted Life Years (DALYs) methodology and introduced DALYs as a measure for the global burden of disease (GBD) (Stein et al. 2009).
Importance of iron and zinc in the human body
Iron is an essential element for most life on earth, including human beings, by participating in a wide variety of metabolic processes, including oxygen transport, electron transport and DNA synthesis (Venkataramani2021). Iron is needed for a number of highly complex processes, e.g. the transportation of oxygen around the body (Vogt et al. 2021). It is required for the production of red blood cells (a process known as haematopoiesis), but it's also part of haemoglobin (that is the pigment of the red blood cells), binding to the oxygen and thus facilitating its transport from the lungs to all cells throughout the body (Duck and Connor 2016). Iron exists in complicated forms with protein (heme protein), as heme compounds (Hb or Mb), heme enzymes and non-heme compounds (flavin-Fe enzymes, transferrin and ferritin). The human body requires iron for the production of heme enzymes and non-heme enzymes that are essential in the transfer of electrons (cytochromes and catalase) and oxidation–reduction reactions (Rodgers and Gilreath 2019). Almost two-third of the iron is found in the Hb, 25% is found in a readily mobilizable iron store and approximately 15% is associated with myoglobin in muscle cells and a variety of enzymes involved in the oxidative metabolism (Trumbo et al. 2001). The average adult male has approximately 1000 mg of iron as stored iron, which is sufficient for about 3 years, while the average woman has only approximately 300 mg, sufficient for about 6 months (UCSF 2020). Men need 28 mg of iron per day; pregnant women need 38 mg per day while lactating and non-pregnant women need 30 mg per day (Kaur 2016).
Zinc has various physiological roles in a biological system and estimated that about 3000 proteins in the human body are zinc-dependent proteins (Krezel and Maret 2016). Its interaction with enzymes and other proteins are necessary for the structural, functional and regulatory processes in the human body.
Factors affecting iron and zinc bioavailability
Plant-based foods like grains, beans, vegetables, fruits, nuts, and seeds contain non-heme iron (Patel et al. 2017). Non-heme iron is the major fraction of total dietary iron, its absorption rate may only be 2–20% from vegetable and staple crops such as rice, wheat bran and maize (Abbaspour et al. 2014).Although absorption from the non-heme iron pool constitutes a major fraction of daily iron intake, unlike heme iron, its absorption depends on the presence of a variety of dietary components ingested simultaneously (Fleming 2005). Ascorbic acid and muscle tissue from meat, fish, and poultry (Dasa and Abera 2018) enhance non-heme iron absorption, whereas phytic acid (Armah et al. 2015), polyphenols (Ahmad et al. 2017), and soy (Milman 2020) inhibit non-heme iron absorption. The mixture of foods eaten by humans and the interplay of nutrients and non-nutrients affects non-heme iron absorption in complex ways. Pearl millet contains non-heme iron whose bioavailability is highly influenced by dietary constituents and depends on luminal dietary factors viz. enhancer and/or inhibitors of absorption (Moretti 2017).
Enhancers of iron absorption
Ascorbic acid can counteract the inhibitory effect of phytic acid, polyphenols, and calcium. Organic acid such as citric acid has been reported to potentially enhance iron absorption (Teucher et al. 2004).
Inhibitors of iron absorption
Phytic acid
Phytic acid (myoinositol-1, 2, 3, 4, 5, 6 hexakisphosphate) has a strong chelating ability and readily forms complexes with monovalent and multivalent cations of potassium, calcium, iron, zinc and magnesium, reducing their bioavailability and creating a deficit in their absorption. Cereals contain intrinsic phytase, able to degrade phytic acid, but the conditions required for the degradation to take place are not easily met in common food preparation methods (Koreissi-Dembele et al. 2013). Phytate can be enzymatically degraded before consumption (Hurrell et al. 2003). Sandberg et al. (1996) has suggested that a phytase that is activated at gut pH may be effective in increasing iron absorption when added to a meal just before consumption. The addition of dietary phytase has been shown to enhance iron bioavailability when added to a meal prior to consumption (Troesch et al. 2009).
Polyphenols and tannins
Polyphenols, similarly to phytic acid, can bind with iron and make it unavailable for absorption by inducing its precipitation or reducing its solubility. As polyphenols are a complex and diverse class of chemical compounds (flavonoids, tannic acid), it can be expected that different polyphenols from different foods affect iron absorption differently (Moretti 2017).
Calcium
Calcium is the only dietary component to inhibit both non-heme as well as heme iron absorption (Hallberg et al.1991).
The inadequate dietary intake of Zn (2+) and inhibitors of its absorption are the most common causative factor for its deficiency. Phytate has a strong negative effect on Zn (2+) absorption from composite meals. Cadmium, which is increasing in the environment, also inhibits Zn (2+) absorption (Lonnerdal 2000). The type of protein in a meal also affects Zn (2+) bioavailability. Casein in milk has a negative effect on its absorption (Sandstrom et al. 1983). The calcium content of the diet may, however, affect Zn (2+) absorption from phytate-containing meals. This is because calcium has a tendency to form complexes with phytate and Zn (2+) that are insoluble and consequently have an inhibitory effect on its absorption (Fordyce and Robbins 1987). Amino acids, such as histidine and methionine, and other low-molecular-weight ions, such as EDTA and organic acids (e.g. citrate), are known to have a positive effect on zinc absorption (Lonnerdal 2000). Micronutrient absorption can be increased by reducing the concentration of antinutrients (Sperotto et al. 2017). Several studies have reported the range of natural variability of minerals content in millet grain, but very limited work was done for antinutritional factors (Simwemba et al. 1984). Nowadays, a number of strategies are used to overcome the effects of these food antinutrients, including processing treatments such as milling, soaking, germination, autoclave, microwave, treatment and fermentation (Samtiya et al. 2020).
Physiological processes of metal uptake and translocation in plants
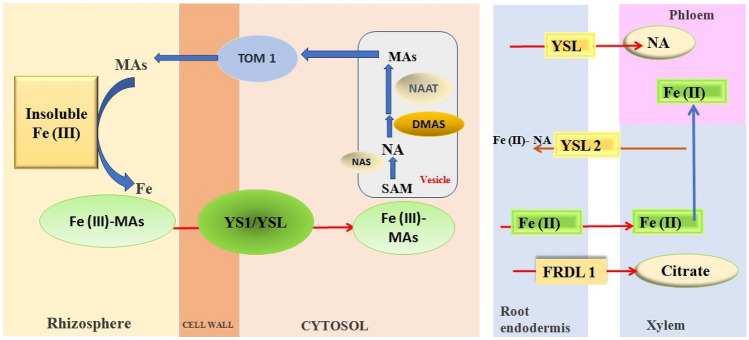
Micronutrient concentrations in plants are controlled by complex homeostatic mechanisms. Iron is slightly soluble under aerobic conditions, high-pH and calcareous soils (Ramzani et al. 2016). The mugineic acid family phytosiderophores (MAs), which are Fe (III)-solubilizing molecules, secreted from Fe-deficient graminaceous plants (Bashir et al. 2006). MAs-Fe (III) is taken up into root cells through the Yellow Stripe (YS) or Yellow Stripe-Like (YSL) transporters (Curie et al. 2001). Yellow stripe 1 (YS1) is firstly identified from maize and targeted to the plasma membrane. Eighteen putative Yellow Stripe 1 (YS1)-like genes (OsYSLs) are identified in the rice genome (Koike et al. 2004). MAs have been identified to date, all of which are synthesized through a conserved pathway from S-adenosyl-L-methionine (SAM). This pathway includes three sequential enzymatic reactions mediated by Nicotianamine Synthase (NAS), Nicotianamine Aminotransferase (NAAT), and Deoxymugineic Acid Synthase (DMAS), generating 2-Deoxymugineic Acid (DMA), and the precursor of all other MAs (Barberon et al. 2011). The NAS enzyme is localized on the membrane of the vesicles, whereas NAAT is present within the vesicles, suggesting that these vesicles are the site of MA biosynthesis. The Transporter of Mugineic Acid Family Phytosiderophores 1 (TOM1) from rice, revealing the final piece in the mechanism (Nozoye et al. 2011). After iron is transported to the root endodermis from epidermis, it needs to be transported to the above ground parts of plants through the xylem. The contents of organic acids, such as citrate, malate, and succinate, are elevated in xylem under iron deficient conditions. The transport of citrate and iron to the xylem is mediated by FRDL1 in rice, which is crucial for iron translocation (Yokosho et al. 2016). Iron is also capable of translocation in xylem in the form of Fe-nicotianamine (NA) and Fe-MAs. NA as a non-protein amino acid is produced from S-adenosyl methionine by nicotianamine synthase (NAS) (Bonneau et al. 2016). In rice, NA and 2′-deoxymugineic acid (DMA) are present in xylem exudates. Once the iron reaches the leaves, it must be unloaded to leaf cells from the apoplastic space. NA and DMA are also required for the phloem-based transport (Curie et al. 2009). In rice, OsYSL2 is likely to be involved in the translocation of Fe (II)-NA to shoots and seeds (Ishimaru et al. 2010). OsYSL9 is involved in the Fe distribution in developing seeds via Fe(II)-NA and Fe(III)-DMA form (Senoura et al. 2017). Iron mobilization and uptake in higher plants are represented in Fig. 1. Similarly, Zn (2+) is taken by the plants as divalent cation, Zn(2+) or as a Zn- phyto-siderophore complex which assists Zn (2+) to move towards root hairs (Yoneyama et al. 2015). Subsequently, Zn (2+) is radially transported across root layers to be loaded onto the vasculature in the root stele. Zn (2+) is effluxed into the xylem for long distance transport by the Heavy Metal Transporters (HMA), which localize to the plasma membrane of the root and shoot vasculature. Zn (2+) are thought to be transported in the phloem as NA chelates and, although the transporters involved in phloem loading and unloading are not fully known, they are thought to include members of the YSL group (Ajeesh et al. 2020). The schematic representation of Zn (2+) transport from soil to grain is illustrated in Fig. 2.
Fig. 1.
Schematic representation of Fe acquisition and transport in higher plants Abbreviations: DMAS deoxymugineic acid synthase, MAs mugineic acid family phytosiderophores, NA nicotianamine, NAAT nicotianamine amino transferase, NAS nicotianamine synthase, SAM S-adenosyl-l-methionine, TOM1 transporter of mugineic acid family phytosiderophores 1, YS1/YSL YELLOW STRIPE 1/YELLOW STRIPE 1–like, FRDL 1 (Ferric Reductase Defective Like 1)
Fig. 2.
Schematic representation of zinc acquisition and its transport from soil to grain Abbreviations: ZIP (ZRT- IRT-like proteins), HMA (heavy metal ATPases), MTP (metal tolerance protein), YSL (yellow-stripe-like) transporters
Why does pearl millet serve as a model crop for food and nutritional security?
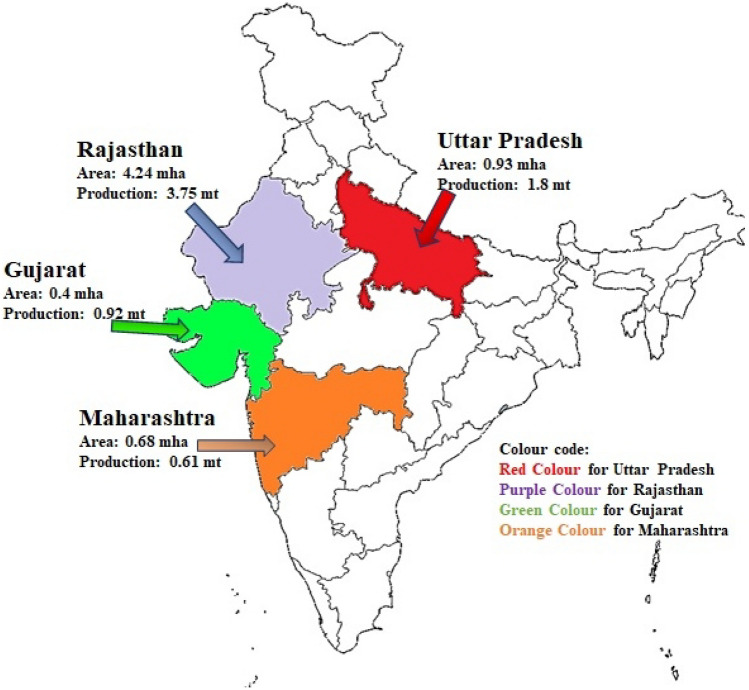
In India, the major crops are wheat, rice, maize, millets and pulses (Nelson et al. 2019). Pearl millet is less popular than wheat and rice except in some Indian states (Fig. 3). It is serving as a staple food crop in Indian states like Uttar Pradesh, Maharashtra, Gujarat and Rajasthan. The inhabitants of such states cultivate it and consume it as the main food (Basavaraj et al. 2010).
Fig. 3.
The map of India presents four major states with cultivation areas and production of pearl millet (Uttar Pradesh, Maharashtra, Gujarat and Rajasthan)
Climate-resilient: At present, the climate is changing and the water level is declining. In the coming days, it would be hard to irrigate crops but pearl millet can be grown in an enormous array of environmental conditions including repeated drought events, heat stress and meager soil fertility (Shivhare and Lata 2017). It can even grow in areas that received less rainfall, Jaisalmer and Hanumangarh, Rajasthan, India from 2006 to 2016 received less than 300 mm of annual rainfall from the average annual rainfall (Yadav et al. 2018). Hence, it provide a source of food security in a drought-prone area. It is cultivated in 26 million hectares in some of the harshest semi-arid tropical environments of South Asia and sub-Sahara Africa (All India Coordinated Research Project on Pearl Millet (AICRP-PM)). Hence, pearl millet is one of the most imperative staple crop in South Asia and sub-Sahara Africa (Stich et al. 2010).
Nutritional value: Pearl millet is a very nutritious cereal compared to other cereals. It is a principal source of protein, vitamins, fat, minerals and micronutrients like Fe (2+ , 3+), Zn (2+), Ca (2+), K (1+) etc. for millions of poor populations where it is cultivated. The total amount of Fe (2+ , 3+) in pearl millet will provide about 60% of the Estimated Average Requirement (EAR). It is highly fibrous (12 g/kg) compared to other food grains. It is also high in fat content (50 mg/kg), mainly unsaturated fatty acids with improved fat digestibility as compared to other food grains, but it has high activities of lipases that result in rapid release of fatty acids, which limits its shelf life. It is having a high amount of vitamin B complex (thiamine, riboflavin and niacin) (Satyavathi 2019). It is also rich in Vitamin A and folic acid than wheat (Satyavathi 2019). Among micro-nutrients, it is loaded with an abundance of Fe (2+ , 3+) and Zn (2+) content (Satyavathi et al. 2021). Hence, Pearl millet is a highly nutritious cereal whereas bioavailability is low, because of the presence of certain anti-nutritional factors like phytic acid, polyphenols etc. Polyphenols content was found to range from 4.91 to 7.65 g /kg whereas phytic acid content ranged from 3.54 to 8.25 g/kg (Satyavathi et al. 2017). Protein and starch digestibility of pearl millet is low because of the anti-nutrients in grain. The digestibility for protein ranged between 54.2 and 59.2%, whereas 12–18.7 mg maltose released/g for starch (Satyavathi et al. 2017). Recently, pearl millet is getting attention for its good nutritional quality in the medical field (Kaur et al. 2014). Pearl millet is a sustainable cereal with good dietary properties for diabetic patients. It has superior glycemic control over wheat and rice because of the presence of slowly digestible starch (SDS) and resistant starch (RS). The Government of India declared '2018' as "National Year of Millets" to march towards "Malnutrition Free India" by the end of the year 2022. In this regard, Indian Council of Agricultural Research (ICAR), New Delhi, India has established minimum levels of Fe (2+ , 3+) and Zn (2+) to be bred into national varieties of pearl millet. The UN Food and Agriculture Organization (FAO) also decided the year 2023 to be the “International Year of Millets”.
HarvestPlus biofortification program of Pearl millet
HarvestPlus program of the Consultative Group on International Agricultural Research (CGIAR) targeted the crops in order to improve the nutritional background of crop varieties genetically with their desired traits in different countries through a conventional breeding program. HarvestPlus breeding strategies were implemented in India at ICRISAT to improve the Fe (2+ , 3+) and Zn (2+) content in the edible part of pearl millet. It is done in two stages through initial survey and screening to know the baseline and variability present in pearl millet and then to enhance the grain Fe (2+ , 3+) through breeding approaches. The nutritionist team determined the target level of essential micronutrient that is needed to make a measurable effect on the human body (Table 1). They initially look at such variables as bioconversion and bioavailability of ingested nutrients, because micronutrient losses during cooking, storage and other processing are very high. Targets were set for children of 4–6 years old and women of reproductive age (HarvestPlus).The main goal of pearl millet biofortification is to increase Fe (2+ , 3+) (target value: 77 mg/kg) content in grain.
Table 1.
Details of the target level of iron content.
Source: HarvestPlus https://www.harvestplus.org/sites/default/files/Biofortification_Progress_Briefs_August2014_WEB_2.pdf
| Nutrition factors | Nutrient intake at current | Target levels required for optimum growth | |
|---|---|---|---|
|
Pearl millet consumption, grams/day (dry weight) |
Women | 300 g/day | 244 g/day |
| Children | 150 g/day | 72 g/day | |
| Fe retention (%) | 90% | 95% | |
| Fe absorption (%) | 5% | 7–7.5% | |
| Absorbed incremental Fe as % of EAR | 60% | 60% | |
EAR estimated average requirement
Initial phase of HarvestPlus program (2003–2008)
The first phase started with the screening of pearl millet germplasm accession for a diverse range of Fe (2+ , 3+) and Zn (2+) by ICRISAT and found a range of 30–76 mg/kg of Fe (2+ , 3+)and 25–65 mg/kg of Zn (2+). By using this data target was fixed to enhance the Fe (2+ , 3+) content in pearl millet grains in populations and hybrids (Table 2). Both micronutrients have a high correlation under additive genetic control. Potential parents and the most promising local germplasm were used to study the G × E interaction for verifying the stable genotypes. With the effort of ICRISAT and the Indian National Agricultural Research System (NARS) partners, a handful of biofortified varieties and hybrids got released by Govt. of India for cultivation as part of Harvestplus program. Rai et al. (2016) screened around 18 open-pollinated varieties (OPVs) and 122 hybrids released and/or commercialized in India. Among OPVs, ICTP 8203 released in 1988 (Fe: 67 mg/kg and Zn: 52 mg/kg) and ICMV 221 in 1993 (Fe: 61 mg/kg and Zn: 45 mg/kg) were found promising for high iron-zinc content. While, among hybrids Ajeet 38, Proagro XL 51, PAC 903 and 86M86 have been developed with Fe (2+ , 3+) content of 55–56 mg/kg and Zn (2+) content of 39–41 mg/kg. These genotypes were selected as a donor for imparting high micronutrients content and used for initial crosses.
Table 2.
Focussed biofortification program of iron pearl millet by HarvestPlus.
Source: HarvestPlus,http://www.harvestplus.org/sites/default/files/BiofortificationProgressBriefsAugust2014WEB0.pdf
| Crop | Target Country | Baseline (mg/kg) | Target increment (mg/kg) | Target level in crop (mg/kg) | Farmer benefits |
|---|---|---|---|---|---|
| Pearl millet | India, secondary countries: West Africa | 47 | + 30 | 77 | High yielding, downy mildew resistant, drought tolerant |
Second phase of HarvestPlus program (2009–2013)
The breeding line and germplasm containing more than 90 mg/kg Fe (2+ , 3+) and 60 mg/kg Zn (2+) were validated. Large-scale and high throughput screening of high Fe (2+ , 3+) lines through XRF spectrometry was carried out by ICRISAT, and helped in the development of Fe (2+ , 3+) rich OPV, Dhanshakti. Later, they concentrated on the development of hybrids. Currently, under the HarvestPlus program and other consortium programs, breeders from many institutions around the world are primarily focusing on the development of stable and high grain Fe (2+ , 3+) containing hybrids. The details of high iron varieties and hybrids are shown in Table 3 and variability studies in pearl millet for grain Fe (2+ , 3+) and Zn (2+) are shown in Table 4. Several studies reported improved lines of grain Fe (2+ , 3+) content in pearl millet germplasm beyond the HarvestPlus target level (target increment of Fe: 30 mg/kg). Huge variability existed for micronutrient content among pearl millet lines which were far better than regularly consumed cereals (wheat and rice), even after biofortification efforts (Bouis et al. 2017).
Table 3.
Details of high iron varieties/hybrids of pearl millet.
Source: ICAR- All India Coordinated Research Project on Pearl Millet, http://www.aicpmip.res.in/pearl%20millet%20hybrids%20and%20varieties.pdf
| S. nos | Hybrid/variety | Parentage | Bred at | Area of adoption | Salient features | DF | DM | GY (t/ha) | Fe (mg/kg) | Zn (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Dhanshakti (ICTP 8203 Fe10-2) | (S. O. 1146 (E) 24.04.2014) | Mahatma Phule Krishi Vidyapeeth (MPKV), Dhule | Maharashtra, Karnataka, AP, Tamil Nadu, Rajasthan, Haryana, MP, Gujarat, UP and Punjab | Early maturing bold, globular, shining slate grey colored seed resistant to downy mildew disease | 45 | 76 | 2.2 | 81 | 43 |
| 2 | HHB 299(MH 2076) |
ICMA 04888 × H 13/0001 |
Chaudhary Charan Singh Haryana Agricultural University (CCS HAU), Hisar | Rajasthan, Haryana, Gujarat, Punjab, Delhi, Maharashtra and Tamil Nadu | Medium maturing, compact panicle grayish hexagonal-shaped grain and resistant to major diseases | 50 | 81 | 3.3 | 73 | 41 |
| 3 |
AHB 1200 Fe (MH 2072 (AHB 1200) |
ICMA 98222 × AUBI 1101 |
National Agricultural Research Project (NARP), Aurangabad | Rajasthan, Haryana, Gujarat, Punjab, Delhi, Maharashtra and Tamil Nadu, AP and Telangana | Medium maturing, cylindrical panicle resistant to downy mildew and highly responsive to fertilizers | 47 | 78 | 3.2 | 77 | 39 |
| 4 |
AHB 1269Fe (MH 2185) |
ICMA 98222A1 × AUBI 1101 | National Agricultural Research Project (NARP), Aurangabad | Rajasthan, Haryana, Gujarat, Punjab, UP, Delhi, Maharashtra, Tamil Nadu, AP, Telangana and Karnataka | Medium maturing, long cylindrical type panicle, bold grain and resistant to Major diseases | 50 | 81 | 3.2 | 91 | 43 |
| 5 | HHB 311 | ICMA 02333 × H 14/003 | Chaudhary Charan Singh Haryana Agricultural University (CCS HAU), Hisar | Rajasthan, Haryana, Gujarat, MP, Punjab, Delhi, Maharashtra and Tamil Nadu, | Medium maturing, compact panicle having grey colored hexagonal-shaped grain, Highly resistant to downy mildew and other diseases | 50 | 81 | 3.2 | 83 | 39 |
| 6 | RHB 233(MH 2173) | ICMA 99444 × RIB 15176 | Sri Karan Narendra Agriculture University (SKNAU), Jobner | Rajasthan, Haryana, Gujarat, MP, Punjab, Delhi, Maharashtra and Tamil Nadu, | Medium maturing, grey globular shaped grain; Highly resistant to blast and downy mildew diseases | 49 | 80 | 3.2 | 83 | 46 |
| 7 | RHB 234(MH 2174) | ICMA 02333 × RIB 15177 | Sri Karan Narendra Agriculture University (SKNAU), Jobner | Rajasthan, Haryana, Gujarat, MP, Punjab, Delhi, Maharashtra and Tamil Nadu | Medium maturing, conical-shaped compact panicle with grayish colored hexagonal-shaped grain, Highly resistant to downy mildew | 49 | 81 | 3.2 | 84 | 4 |
DF days to flowering, DM days to maturity, GY grain Yield, Fe Iron, Zn Zinc
Table 4.
Iron and zinc variability reported in earlier studies in pearl millet
| S. no | Breeding material used | Fe (mg/kg) | Zn (mg/kg) | Author reported |
|---|---|---|---|---|
| 1 |
Germplasm accessions: two sets of line x tester crosses Set 1: 89 entries (17 parents and 72 hybrids) Set 2: 28 parents and 192 hybrids Set 1 Parents: ICMB 93222, ICMB 98222, ICMB 00888, ICMB 94111, ICMB 93333, ICMB 04777, ICMB 89111, ICMB 97111, IPC 1650, IPC 843, IPC 616, IPC 774, IPC 1307, IPC 1354, IPC 390, IPC 828, IPC 1178 Set 2 Parents: 841 B, 843 B, 863 B, ICMB 88006, ICMB 91222, ICMB 92111, ICMB 95333, ICMB 96333, ICMB 99444, ICMB 00999, ICMB 02444, ICMB 03111, ICMB 04222, ICMB 04555, ICMB 04999, IPC 338, IPC 404, IPC 536, IPC 689, IPC 735, IPC 811, IPC 1254, IPC 1268, IPC 1642, ICMR 356, ICMR 06333, ICMR 06888 |
30–76 | 25–65 | Govindaraj et al. (2011) |
| 2 | Set 1: 386 Advance breeding lines, | 61–80 | 41–60 | Rai et al. (2012) |
| Set 2: 232 Population progenies | 52–135 | 40–92 | ||
| 3 |
122 Hybrids ((21 hybrids from 9 public sector research organizations, including ICRISAT; and 101 hybrids from 33 seed companies) HHB 197, ABH 999, Bio 70 GHB 719, Mahodaya 325, RBH 173, HHB 223, RBH 121, GHB 538, N 61, RHB 154, Hi Pearl 51, Shanti, GHB 744, Mukhia, GK 1135, NBH 1188, KBH 261, Bio 8494, 86M 66, Mahodaya 318, PHB 2168, JKBH 1103, NPH 1651, GHB 732, NPH 2798, Bio 8141, HHB 94, MPMH 17, RBH 177, Proagro 9444, HTBH 4201, VBBH 3028, ABH 1, NBH 1717, Bisco 5151, NBBH 411, KBH 11, NPH 3685, Ajeet 37, 86M 01, Mahodaya 331, X 7, KBH 9119, Bio 13, NBH 5464, JKBH 1096, S 368, VBBH 3087, GHB 558, Hi Pearl 138, TEJAS, Biogene 33+ , Navbharat Gavari, Mahodaya 330, Gangotry 67, HTBH 4202, RBTH 10-007, VBH 444, NBBH 908, KH 302, VBH 456, M 64, Nu 306, Kaveri Boss 65, Bisco 5141, B 2301, 86M 88, Solid 78, GK 1104, Euro 1133, MRB 204, NPH 2475, Kaveri Super Boss, Sujlam 68, Hi Pearl 91, VIRAT, Kaneri × 563, MRB 2210, TNBH 0642, KBH 287-36, NBH 4903, GK 1116, B 2195, S 361, KBH 7206, N 68, S 362, Ajeet 35, RBH 1818, Sanjivani 333, Dhamal, Proagro 9450, Nu 310, VBBH 3125, VBBH 3115, PAC 909, JKBH 676, RBTH 10-005, NBH 1024, GK 1090, Hi Pearl 130, KBH 2360, JKBH 1097, HTBH 4203, Navbharat Banas express, Saburi, PAC 931, KBH 7201, Sanjivani 2312, NBH 1134, ICMH 356, RBH 9, HTBH 4204, PAC 982, Shradha, Ajeet 39, XL 51, 86M 86, PAC 903, Ajeet 38, Sanjivani 222 |
31–61 | 32–54 | Rai et al. (2013) |
| 5 |
Parental lines Set I: 8 B-lines and 9 R-lines Set II: 16 B-lines and 12 R-lines Set I: 8 B-lines (ICMB 89111, ICMB 93222, ICMB 93333, ICMB 94111, ICMB 97111, ICMB 98222, ICMB 00888, ICMB 04777) and 9 R-lines (IPC 390, IPC 616, IPC 774, IPC 828, IPC 843, IPC 1178, IPC 1307, IPC 1354, IPC 1650) Set II: 16 B-lines (841 B, 843 B, 863 B, ICMB 88006, ICMB 91222, ICMB 92111, ICMB 95333, ICMB 96333, ICMB 99444, ICMB 00999, ICMB 02444, ICMB 03111, ICMB 04222, ICMB 04555, ICMB 04888, ICMB 04999) and 12 R-lines (IPC 338, IPC 404, IPC 536, IPC 689, IPC 735, IPC 811, IPC 1254, IPC 1268, IPC 1642, ICMR 356, ICMR 06333, ICMR 06888) |
34–102 | 34–84 | Govindaraj et al. (2013) |
|
Hybrids Set I: 72 line × tester hybrids Set II: 192 line × tester hybrids |
33–76 30–80 |
34–70 31–64 |
||
| 6 |
Parental lines: 14 B-lines (: ICMB 88004, ICMB 92111, ICMB 92888, ICMB 93222, ICMB 97111, ICMB 98222, ICMB 02555, ICMB 04888, ICMB 05555, ICMB 07555, ICMB 07777, ICMB 07999, ICMB 08222, ICMB 08333) 14 R-lines (PRP 1, PRP 2, PRP 3, PRP 4, PRP 5, PRP 6, PRP 7, PRP 8, PRP 9, IPC 616, IPC 843, IPC 1178, IPC 1354, IPC 390) |
30–77 32–82 |
27–45 29–56 |
Kanatti et al. (2014) |
| 7 | 196 Hybrids | 26–65 | 26–48 | |
| 8 | 106 RILs (F6) + 2 parents (ICMB 841-P3 × 863B-P2) + 4 checks | 28–124 | 29 -120 | Kumar et al. (2016) |
| 9 | 106 RIL population (open-pollinated) | 22–77 | 22–74 | |
| 10 | 130 Association mapping panel + two checks (ICTP 8203Fe and ICMB 98222) | 32–112 | 27–74 | Anuradha et al. (2017) |
| 11 | 317, F6-RILs | 20–131 | 18 -110 | Kumar et al. (2018) |
| 12 | 130 genotypes including one open-pollinated check variety, ICTP 8203Fe | 27–125 | 28 -86 | Anuradha et al. (2018a) |
| 13 | 130 Association mapping panel + two checks (Dhanshakti and ICMB 98222) | 23 -122 | 20- 86 | Anuradha et al. (2018b) |
| 14 | Two parents (PPMI 683 × PPMI 627) + 210 RILs (RIL 1 to RIL 210) | 36–130 | 12–29 | Singhal et al. (2018) |
| 15 | Two parents (PPMI 683 × PPMI 627) + 210 RILs (RIL 1 to RIL 210) | 36–114 | 20–106 | Singhal et al. (2021) |
The selection of germplasm lines for improved grain minerals and mapping of associated QTL will help in the selection of donor parents for developing Fe (2+ , 3+)/Zn (2+) rich hybrids or varieties. The proper knowledge of the genetic background of these micronutrients and systematic utilization of pearl millet germplasm in biofortification program is very crucial. Many pilot studies revealed the genetic basis of Fe (2+ , 3+)and Zn (2+)content in pearl millet grain (Rai et al. 2012; Govindaraj et al. 2013; Kanatti et al. 2014). The studies also interpreted the correlation between Fe (2+ , 3+) and Zn (2+), combining ability including both General Combining Ability (GCA) and Specific Combining Ability (SCA) in different crosses, gene action and broad-sense heritability (Table 5). Only a few reports are available on the exploitation of heterosis for Fe (2+ , 3+) and Zn (2+) content as the trait is predominantly governed by additive genetic variance with no better parent heterosis (Velu et al. 2011).
Table 5.
Review of the genetics of grain iron (Fe) and zinc (Zn) content in pearl millet
| Author | Objective | Material | Fe Range (mg/kg) | Zn range (mg/kg) | Correlation between Fe and Zn | Correlation of Fe and Zn with other traits | Gene action/associated marker/s or QTL | GCA and SCA | Heterosis | Genetic variability, G × E interaction (GEI) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe | Zn | ||||||||||
| Rai et al. 2012 |
a) Variability for Fe and Zn content b) Character association c) Genetics of micronutrients in grain |
a)386 advanced breeding lines b)232 early-generation progenies |
a)18–97 b)52–135 |
a) 22–69 b) 40–92 |
Highly significant positive |
a) Negative correlations between the Fe content &grain yield in 3 of the 6 trials (r = -0.39 to − 0.58) b) No correlation between the Zn content and grain yield |
Additive gene action | GCA, highly significant (p < 0.01) | – | – | – |
| Govindaraj et al. (2013) |
a) Combining ability b) Genetic variability and c) Heterosis for grain Fe and Zn |
Two sets of hybrids and their parental lines Set 1 Set 2 Parental lines |
33–76 30–80 34–102 |
34–70 31–64 34–84 |
Highly significant (p < 0.01) and positive correlation in parental lines (r = 0.90 in the set I trial and r = 0.86 in the set II trial) | – | Additive gene action |
Highly significant (p < 0.01) &positive correlation between the GCA of Fe & Zn content in parental lines (r = 0.89 in the set I trial and r = 0.78 in the set II trial) |
1) No better parent heterosis 2)set I trial: 55 out of the 72 hybrids had significant (p < 0.01) mid parent heterosis (MPH), all in the negative direction 3) 93 hybrids had significant (p < 0.01) MPH in the set II trial, of which 91 were in the negative direction |
Zn content of 37 hybrids in the set I trial& 82 hybrids in the set II trial had significant (p < 0.01) MPH, and all were in the negative direction |
1) Hybrid × environment (H × E) interaction was significant (p < 0.05) in the set I trial 2)In the set II trial, this interaction effect was highly significant (p < 0.01) |
| Kanatti et al. (2014) | a) Genetic variability b) heterosis for Fe and Zn content |
14 maintainer lines (B lines) as female parents (F), 14 restorer lines (R lines) as male parents (M), and 196 hybrids produced by F × M crosses |
Parent: 32–82 and hybrid: 26–65 | Parent: 26–65 and hybrid: 26–48 | Highly significant and high positive correlations between the Fe and Zn densities |
a) Highly significant and moderate positive correlation with grain weight (r = 0.61) in hybrids b) Negative and weak correlation with grain yield (r = 0.23) |
Additive genetic control | Significant GCA for Fe and Zn content | Fe content among the hybrids varied from 25.8 to 64.5 mg/kg, but none of the hybrids showed significant better-parent heterosis |
H × E were highly significant for all four traits, Fe content, Zn content,1000 grain weight and grain yield |
|
| Satyavathi et al. (2015) | Stability of newly developed inbred lines, derived from a RIL population for mapping high grain iron and zinc content | Twelve promising pearl millet lines | 35–98 | 40–81 | A strong correlation between grain Fe and Zn content, overall correlation r = 0.74 (p < 0.01) | – | – | – | – | The GEI was highly significant. first two principal components (PC) explained 77.14% & 85.31% of the total G x E variation for grain Fe and Zn contents respectively | |
| Kumar et al. (2016) |
a) Correlations b) Mapping quantitative trait loci controlling high iron and zinc content in self and open- pollinated grains of pearl millet |
Recombinant inbred line (RIL) |
a) Fe-Self 28–124 b) Fe-OP 22–77 |
a) Zn-Self 29–120 b) Zn-OP 22–74 |
A very strong significantly positive association between selfed [Fe] and [Zn], while correlation was moderate for trait pairs, [Fe] and Zn-OP, Fe-OP and Zn-OP, and [Zn] and Fe-OP |
a) QTLs for Fe and Zn content on LG 3 with phenotypic variation 19% and 36% respectively b) In open-pollinated seeds- two QTLs for grain Fe content on LG 3 and 5, and two QTLs for grain Zn content on LG3 & 7. The total phenotypic variance for Fe and Zn was 16 and 42% respectively |
G × E interactions were significant (at P < 0.01) for all observed traits across environments except for Fe-OP, which was Significant at P < 0.05 | ||||
| Anuradha et al. (2017) |
a) Character association b) G × E interaction in different agro- climatic zones of India |
40 Pearl millet genotypes and one check variety (Dhanshakti, G30) | 29–115 | 16–76 | Highly associated (r = 0.8, p < 0.01) | Negative correlation with grain yield | – | – | – | – |
Genotypes (G), Environmental effects (E) and GEI effects were highly significant (P < 0.01) for grain Fe and Zn content |
| Anuradha et al. (2017) | a) Association mapping of QTLs for Fe and Zn | 130lines (B lines, R lines and advanced breeding lines) + two checks, (ICTP 8203Fe and ICMB 98222) | 32–112 | 27–74 | – | – |
Xpsmp2261 (13.34% R2value), Xipes 0180 (11.40% R2-value) and Xipes 0096 (11.38% R2-value) were associated with grain Fe and Zn content |
– | – | – | – |
| Kumar et al. (2018) | a) Mapping grain iron and zinc content QTLs in an iniadi-derived immortal population of pearl millet | 317, F6-RILs | 20–131 | 18–110 | Genotypic (9.4**) and phenotypic (9.3**) associations between Fe and Zn were very strong and significantly positive | – | Three QTLs for Fe and Zn were mapped, one on LG1 and two on LG7 | – | – | – | – |
| Anuradha et al. (2018) | a) Association of agronomic traits and micronutrients in pearl millet | 130 lines including one open-pollinated check variety, ICTP 8203Fe | 27–125 | 28–86 | Grain Fe is highly associated (genotypic correlation) with Zn | Grain Fe was correlated with total phosphorus and Mn but not correlated with grain yield | Additive gene action | – | – | – | – |
| Anuradha et al. (2018) | Genetic variability for grain yield and micronutrients in the arid zone of India |
130 pearl millet genotypes including two check varieties, viz., Dhanshakti and ICMB 98222 |
23–122 | 20–86 |
Phenotypic and genotypic correlation between Fe and Zn content was highly significant and was in positive direction |
Grain Fe and Zn had a non-significant correlation with grain yield | Additive gene action | – | – | – | – |
GCA general combining ability, SCA specific combining ability, QTL quantitative trait locus, G × E interaction genotype-environment interaction
Molecular approaches for iron and zinc content in pearl millet
Detection of genes/major QTL and understanding the molecular basis of grain Fe (2+ , 3+) and Zn (2+) content will facilitate breeding for high Fe (2+ , 3+) and/or Zn (2+) in pearl millet through Marker-Assisted Selection (MAS), Marker-Assisted Backcross Breeding (MABB) and Marker-Assisted Recurrent Selection (MARS).
Few reports indicate that molecular studies are already underway to identify the genetic basis of micronutrients in pearl millet but very limited studies are done in molecular aspects in this crop (Table 6). Singhal et al. (2021) reported 22 QTLs for grain Fe (2+ , 3+) and Zn (2+), of which 14 were for Fe (2+ , 3+) and 8 were for Zn (2+) and also observed the huge variation among RILs. The observed phenotypic variance (R2) explained by different QTLs for grain Fe (2+ , 3+) and Zn (2+) content ranged from 2.85 (QGFe.E3.2014–2016_Q3) to 19.66% (QGFe.E1.2014–2016_Q3) and from 2.93 (QGZn.E3.2014–2016_Q3) to 25. 95% (QGZn.E1.2014–2016_Q1), respectively. They also identified the candidate genes inside the QTLs such as Ferritin gene, Al3+ transporter, K+ transporter, Zn2+ transporter and Mg2+ transporter.
Table 6.
Review of various Quantitative trait locus (QTLs) and association mapping studies carried out in reference to grain Iron (Fe) and Zinc (Zn) content in pearl millet
| Authors | Objective | Material | No. of lines evaluated | Fe range (mg/kg) | Zn range (mg/kg) | No. of markers used | No. of associated marker/s and QTLs name or linkage group (LG)/positions (cM) | Flanking markers | Phenotypic variation explained (PVE) |
|---|---|---|---|---|---|---|---|---|---|
| Kumar et al. (2016) | Mapping quantitative trait loci controlling high iron and zinc content in self and open-pollinated grains of pearl millet | F6: Recombinant inbred line (RIL) derived between ICMB 841-P3 (Low Fe-Zn) × 863B-P2 (High Fe-Zn) | 144 |
a) Fe-Self 28.4–124 b) Fe-OP 22.4–77.4 |
a) Zn-Self 28.7–119.8 b) Zn- OP 21.9–73.7 |
96 SSRs; 208 DArT |
a) In selfed seed, a single co-localized QTLs for Fe and Zn content on LG 3 (3/110) b) In open-pollinated (OP) seeds- two QTLs for grain Fe content on LG 2 (2/30) and 5 (5/118), and two QTLs for grain Zn content on LG 3 (3/110) and 7 (7/96) |
a) Fe and Zn: Xpsmp2214-Xipes0142 b) OP Fe: Xpsmp322-Xipes181 Pgpb11029-Pgpb8456 OP Zn: Xpsmp2214-Xipes0142 Xpsmp2040-Pgpb10727 |
Fe: 19% Zn:36% OP Fe:18.1%, 18.3% OP Zn: 50.1%, 19.7% |
| Anuradha et al. (2017) | Genome-wide Association mapping of QTLs for Fe and Zn | A diverse inbred panel consists of B lines, R Lines and advanced breeding lines | 130 | 32.3–111.9 | 26.6–73.7 | 250 SSRs and 17 genic markers | SSR markers were associated with both grain Fe & Zn content were present on LG 3, LG 5 and LG 7 |
Xipes 0180 (aspartic proteinase gene) Xpsmp2261 (intergenic region) Xipes 0096 (dropped) |
11.40%, 13.34% and 11.38% R2 value respectively |
| Kumar et al. (2018) | Mapping grain iron and zinc content QTLs in an iniadi-derived immortal population of pearl millet | F6- RILs derived between (ICMS 8511-S1-17–2-1–1-B-P03: Low Fe-Zn × AIMP 92901-S1-183–2-2-B-08): High Fe-Zn | 317 | 20–131 | 18–110 | 177 DArTs and 19 SSRs |
Eleven QTLs were for Fe and eight were for Zn. Three co mapped QTLs for Fe and Zn were observed, one on LG1(1/54) and two on LG7 Fe: (7/86, 7/108) Zn: (7/82,7/112) |
Fe-Zn: pgpb10531-pgpb9130 Fe: pgpb8427-pgpb13221 pgpb11938-pgpb8987 Zn: Xipes198-pgpb8427 pgpb12329-pgpb9721 |
Fe: 31.9% Zn:30.4% Fe: 12.2% 12.5% Zn: 10.2% 10.9% |
| Pujar et al. (2020) | Genome-wide association study uncovers genomic regions associated with grain iron, zinc and protein content in pearl millet | A diverse inbred panel consists of B lines, R lines and advanced breeding lines | 281 | 32–120 | 19–87 | 58,719 SNP |
18 MTAs (Marker-Trait Associations) were associated with Fe, 43 with Zn Among which 4 MTA were present on Chr-4, 5 and 7. co-segregated for Fe and Zn |
Pgl04_64673688 (unknown function) Pgl05_135500493 (Glycosyl transferase, family 1) Pgl05_144482656 (unknown function) and Pgl07_101483782 (Pentatricopeptide repeat) |
5.71%, 6.43%, 5.18% and 5.91 R2 value respectively |
| Mahendrakar et al. (2020) | Discovery and validation of candidate genes for grain iron and zinc metabolism in pearl millet [Pennisetum glaucum (L.) R. Br.] | bi-parental RIL mapping population AIMP 92,901 (high grain Fe and Zn), and ICMS 8511 (low grain Fe and Zn) | RIL | – | – | – | – |
GFeC and GZnC—PglZIP, PglNRAMP and PglFER gene families GFeC—Ferritin-like gene, PglFER1 |
– |
| Singhal et al. (2021) | Multi-environment quantitative trait loci mapping for grain iron and zinc content using bi-parental recombinant inbred line mapping population in pearl millet | F6- RILs derived between (PPMI 683 × PPMI 627) | 210 | 36–114 mg/Kg | 20–106 mg/Kg | SSRs |
a) Grain Fe- four significant common QTL (QFe2.1), (QFe3.1), (QFe5.1) and (QFe 7.1) b) Grain Zn-one common QTL at all locations on LG 3(QZn 3.2) |
a) QTL1- Ppmsb001–Ppmsb002 QTL2- IPES0142–IPES0180 QTL3- IPES0157–IPES0093 QTL4- IPES0206–IPES0015 b) IPES0142–IPES0180 |
a) QTL1-18.34, 17.98, and 15.98% on LG 2 QTL2- 14.99, 16.26, and 14.8% on LG 3 QTL3-19.66, 18.83, and 16.85% on LG 5, QTL4- 15.66, 16.61, and 14.56% on LG 7at Delhi, Dharwad and Jodhpur respectively b)14.39, 16.07, and 22.26% Delhi, Dharwad and Jodhpur respectively |
SSRs simple sequence repeat, Fe-Self (Fe content of self-pollinated grains), Fe-OP (Fe content of open-pollinated grains), DArT diversity arrays technology, SNP (single nucleotide polymorphism)
The major effective Fe (2+ , 3+) and Zn (2+) content QTL were reported by Kumar et al. (2018) using DArT and SSRs markers through the construction of a linkage map in RIL population. They reported that the grain Fe (2+ , 3+) and Zn (2+) content in the RILs ranged from 20–131 mg/kg and 18–110 mg/kg, respectively which indicated a wide range for QTL mapping. A total of 19 QTLs were identified for grain Fe (2+ , 3+) and Zn (2+) content, out of 19, 11 for Fe (2+ , 3+) and 8 for Zn (2+) were found. They further claim the reported QTL may be useful in marker-assisted selection breeding programs, genomic selection and population improvement programs for pearl millet as the phenotypic variance explained by different QTL varied from 9.0 to 31.9% for Fe (2+ , 3+) and 9.4 to 30.4% for Zn (2+).
Anuradha et al. (2017) studied association mapping for grain Fe (2+ , 3+) and Zn (2+) content using 130 pearl millet lines, genotyped with 320 DNA markers (250 SSRs and 70 genic markers) and identified three markers on trait- associated regions. Of the three associated SSRs, XPSMP 2261 was recognized in intergenic location on pseudo-molecule 5 and Xipes0810 was observed on pseudo-molecule 3. They further stressed upon that the marker, Xipes0810 may be useful in delineating high and low Fe (2+ , 3+) and Zn (2+) genotypes.
Kumar et al. (2016) identified the QTL for grain Fe(2+ , 3+) and Zn(2+) content using 106 RILs and 305 markers (96 SSR + 208 DArT) in pearl millet. They reported that grain mineral content in selfed seeds ranged from 28.4 to 124.0 mg/kg for Fe (2+ , 3+) and 28.7 to 119.8 mg/kg for Zn (2+). Grain Fe (2+ , 3+) and Zn (2+) content ranges from 22.4 to 77.4 and 21.9 to 73.7 mg/kg respectively in open-pollinated seeds. The phenotypic variation of identified QTL for Fe (2+ , 3+) and Zn (2+) on LG 3 was 19% and 36%, respectively. Similarly, two QTL for Fe (2+ , 3+) on LG 3 and LG 5 and two QTL for Zn (2+) on LG 3 and LG 7 were also reported for open-pollinated seeds with 16% and 42% phenotypic variance, respectively. They are of the opinion that QTL identified on LG3 will assist in marker-assisted selection.
Major challenges and way forward
To achieve the iron and zinc rich pearl millet, it should be capable of extracting Fe (2+ , 3+) and Zn (2+) from the soil and storing it in grain. The physiological basis for Fe (2+ , 3+) and Zn (2+) efficiency and their accumulation controlling processes in the edible part of pearl millet is not properly understood (Manwaring et al. 2016). In some crops, micronutrient fertilizers e.g. Zn2+, Ni2+, I, Co2+, Mo2+, and Se−2, have special effects on their deposition in the edible part of the plant (Bouis and Welch 2010). Whereas some other micronutrients fertilizers have a trace effect on the amount of the micronutrient accumulation, applied to soils (Dimkpa and Bindraban 2016). This is particularly a fact in the case of Fe (2 + , 3 +) as it has limited phloem sap mobility (Bouis and Welch 2010). Hence, not only the fertilizers applied to soil but also the efficiency of a plant to take up micronutrients from soil, translocation and finally stored in the edible portion is required for increasing grain Fe(2+ , 3+) and Zn (2+)content in pearl millet. Hence, more studies should be aimed to know the factors responsible for the accumulation of more Fe (2+ , 3+) and Zn (2+) in grains of pearl millet.
To develop the high grain yielding, Fe (2+ , 3+) and Zn (2+) dense pearl millet, breeders should have Fe (2+ , 3+) and Zn (2+) rich lines and access to molecular labs with skilled labour to identify Fe (2+ , 3+) and Zn (2+) efficient lines in early segregating generations. In this area, the improvement is very limited and the development of highly nutritious varieties is a challenge.
The bioavailability of a nutrient is defined as the proportion of the ingested micronutrient that is absorbed and used for normal body functions (Gharibzahedi and Jafari 2017). Many factors could affect this process, but the prominent ones are the original profile of food, processing of food, and digestion efficiency. Nutritional composition is the mix of macronutrients and micronutrients within a product, but their absorption efficiency in the body depends on the ability of food to be digested easily. Food that is absorbed easily maintains or improves health and energy status by providing appropriate macro-and micronutrients in balanced form and is thus called healthy food. Neglected or underused crops such as minor millets and pseudo-cereals were part of the common diet of ancient cultures but slowly, after the Green Revolution, the higher availability and accessibility of rice, wheat, and maize overtook these neglected crops and started providing > 60% of the calorific intake through these three crops only, thus starting to create a nutrient-imbalanced diet (Rodríguez et al. 2020). Hurrell and Egli (2010) reported improved iron absorption with the phytate-to-iron ratio of < 1:1 and preferably < 0.4:1 in plain cereal and legume-based meals, or < 6:1 in composite meals including vegetables with added enhancers like ascorbic acid and meat. In the case of zinc, its absorption in a diet based on unrefined cereals was estimated at 18–28% when the phytate/zinc ratio was > 18. Another study revealed that feeding young children with biofortified iron and zinc in pearl millet as a major food, fulfilled the dietary requirement for these micronutrients (Kodkany et al. 2013). Tako et al. (2015) evaluated pearl millet with high Fe (2+ , 3+) content enriched with high absorbable Fe (2+ , 3+) but its absorption is limited because of high polyphenols concentration. The objective of this study was to compare the capacity of biofortified iron and conventional pearl millet lines. Pearl millet lines with low iron ("DG-9444") (26 µg/g) and high iron (ICTP-8203 Fe) (85 µg/g) were investigated, and the high iron line exhibited greater absorbable iron after an in vitro comparison. The presence of high polyphenolic content and phytic acid that inhibits iron absorption was indicated by a low in vitro value. This study concludes that a high iron diet can transfer more absorbable Fe (2+ , 3+) by increased Hb and these observations are beneficial as these polyphenols compounds can be modified during breeding in order to improve the bioavailability of dietary iron. Sihag et al. (2016) studied the in vivo bioavailability of iron by weaning food developed from iron and vitamin A fortified pearl millet and also examined the effect of vitamin A on iron bioavailability in males Wistar albino rats. They provided an iron + vitamin A fortified diet to anaemic rats and found increased iron bioavailability, and liver iron returned to normal levels after 30 days, indicating a promoter role of vitamin A in intestinal iron absorption.
An increase in micronutrient contents in the pearl millet is an initial step in making it a rich source of nutrients for humans. Micronutrients in pearl millet are less bioavailable to humans due to the presence of anti-nutrients in plants that hinder the absorption of these micronutrients in human body (Bouis and Welch 2010). In general, pearl millet grains have very less bioavailability of Fe (2+ , 3+) and Zn (2+) i.e. ~ 5% of the total Fe (2+ , 3+) and ~ 25% of the total Zn (2+) present in the grain is thought to be bioavailable. By using a biofortification strategy, it is possible to greatly improve the availability of Fe (2+ , 3+) (~ 5 to 20%) and Zn (2+) genetically. Phytate and certain poly-phenolics present naturally in pearl millet act as antinutrients that reduce Fe (2+ , 3+) and Zn (2+) bioavailability. The high Fe (2+ , 3+) food can deliver more absorbable Fe (2+ , 3+) as evidenced by the amplified Hb (Bouis and Welch 2010). But some Fe (2+ , 3+) fortified varieties are also have elevated polyphenolic content, which reduces Fe (2+ , 3+) bioavailability. So the polyphenols represent prospective targets which can perhaps be modified during the breeding to improve dietary Fe (2+ , 3+) bioavailability. Hence, the anti-nutrients profiles must be carefully evaluated to further improve the nutritional benefits of this crop.
Nutritionists must have accessible resources like Fe (2+ , 3+) and Zn (2+) rich pearl millet lines, strategies to calculate the bio-accessibility and previous assessment of the efficacy of biofortified crop with Fe (2+ , 3+) and Zn (2+). Studies to know about the factors responsible for increasing/decreasing bioavailability of Fe (2+ , 3+) and Zn (2+) especially while using biofortified pearl millet is essential for driving off hidden hunger.
By virtue of its benefiting features and ecological sustainable concerns to health, a conventional but less acknowledged crop such as pearl millet provides a good option for the biofortification program. A chief priority from the breeder’s point of view is to utilize genetic diversity in pearl millet gene pools for high Fe (2+ , 3+) and Zn (2+) content. Further, these variations can be used for advance next-generation sequencing technology that generates more markers for characterization of genomics assisted breeding and marker-trait associations. After the implementation of such approaches, it will be simpler to find the genetic profile of these traits in other millets by comparative mapping. Other agronomic traits that govern Fe (2+ , 3+) and Zn (2+) content and related markers that are tightly linked to governing genes can be an efficient approach to develop pearl millet varieties with high Fe (2+ , 3+) and Zn (2+) content by conventional or modern breeding strategies and transformation methods. For instance, before arbitrarily improving grain Fe (2+ , 3+) and Zn (2+) content, the future should aim for improvement in the efficiency of mobilization, transportation and storage of Fe (2+ , 3+) and Zn (2+) content in more biologically available forms in plant.
Conclusion
To march towards "Micronutrient malnutrition free India" millets to be included in daily diet which is a rich source of micronutrients. Among all micronutrients, Fe (2+ , 3+) and Zn (2+) which plays a vital role in hidden hunger are more densely packed in the grains of pearl millet. Further biofortification of pearl millet with Fe (2+ , 3+) and Zn (2+) can address the mania very quickly. Seven biofortified varieties of pearl millet have been released in India since 2013. Their fast multiplication and extension in farmer fields is the need of the hour to alleviate the malnutrition among society. In a way, soil or foliar supplementation with micronutrient fertilizer is a practical option but not sustainable and economically feasible for resource-poor farmers particularly in semi-arid and arid regions where pearl millet is a prime crop. Hence, biofortification in pearl millet comes out to be a popular, cost-effective and long-lasting approach among underdeveloped and developing nations. Many previous studies indicated a large and useful genetic variation for grain Fe (2+ , 3+) and Zn (2+) content in pearl millet which can be exploited intensively under pearl millet biofortification program. Also, a significant and relevant relationship between grain Fe (2+ , 3+) and Zn (2+) content with other agronomic traits have been revealed in many studies. Exploiting the germplasm variability HarvestPlus program with the help of ICRISAT targeted pearl millet for biofortification by screening the germplasm and they also released cultivars for further breeding purposes. In future, designing breeding programs for making micronutrients available at needed proportion, more attention should be paid to the association between Fe (2+ , 3+) and Zn (2+) content, with the increase in promoters and reduction in anti-nutritional elements which hinder micronutrient bioavailability.
Acknowledgements
We sincerely acknowledge fellowship obtained from CRP on biofortification (ICAR funding), Division of Genetics, IARI, New Delhi. TS also thank Amity University Noida for accepting her as a Ph.D. scholar, and to pursue doctoral degree program at IARI, New Delhi.
Author’s contributions
TS performed the investigation and wrote the manuscript. CTS and SPS involved in the conceptualization, supervision and editing of manuscript. MM, NA, MSS, CB and NS involved in the editing of manuscript.
Funding
This work was supported by ICAR funded project ICAR plan CRP Biofortification, India.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Code availability
Not Applicable.
Declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Human and animal rights statements
No animal studies are presented in this manuscript. No human studies are presented in this manuscript. No potentially identifiable human images or data is presented in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
C. Tara Satyavathi, Email: csatyavathi@gmail.com
S. P. Singh, Email: spsingh_gen@iari.res.in
C. Bharadwaj, Email: bharadwaj_gen@iari.res.in
References
- Abbaspour N, Hurrell R, Kelishadi R. Review on iron and its importance for human health. J Res Med Sci. 2014;19:164. [PMC free article] [PubMed] [Google Scholar]
- Ahmad Fuzi SF, Koller D, Bruggraber S, Pereira DI, Dainty JR, Mushtaq S. A 1-h time interval between a meal containing iron and consumption of tea attenuates the inhibitory effects on iron absorption: a controlled trial in a cohort of healthy UK women using a stable iron isotope. Am J Clin Nutr. 2017;106:1413–1421. doi: 10.3945/ajcn.117.161364. [DOI] [PubMed] [Google Scholar]
- Ajeesh Krishna TP, Maharajan T, Victor Roch G, Ignacimuthu S, Antony Ceasar S. Structure, function, regulation and phylogenetic relationship of ZIP family transporters of plants. Front Plant Sci. 2020;11:662. doi: 10.3389/fpls.2020.00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AICRP-PM (All India Coordinated Research Project on Pearl Millet). http://www.Aicpmip.res.in/publication .html
- Anuradha N, Satyavathi CT, Bharadwaj C, Nepolean T, Sankar SM, Singh SP, Meena MC, Singhal T, Srivastava RK. Deciphering genomic regions for high grain iron and zinc content using association mapping in pearl millet. Front Plant Sci. 2017;8:412. doi: 10.3389/fpls.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuradha N, Satyavathi CT, Bharadwaj C, Sankar M, Pathy L. Association of agronomic traits and micronutrients in pearl millet. Int J Chem Stud. 2018;6:181–184. [Google Scholar]
- Anuradha N, Satyavathi CT, Bharadwaj C, Sankar M, Singh SP, Pathy TL. Pearl millet genetic variability for grain yield and micronutrients in the arid zone of India. J Pharmacog Phytochem. 2018;7:875–878. [Google Scholar]
- Armah SM, Boy E, Chen D, Candal P, Reddy MB. Regular consumption of a high-phytate diet reduces the inhibitory effect of phytate on nonheme-iron absorption in women with suboptimal iron stores. J Nutr. 2015;145:1735–1739. doi: 10.3945/jn.114.209957. [DOI] [PubMed] [Google Scholar]
- Barberon M, Zelazny E, Robert S, Conéjéro G, Curie C, Friml J, Vert G. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proc Natl Acad Sci USA. 2011;108:E450–E458. doi: 10.1073/pnas.1100659108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavaraj G, Rao PP, Bhagavatula S, Ahmed W (2010) Availability and utilization of pearl millet in India. SAT ejournal 8. http://oar.icrisat.org/id/eprint/96
- Bashir K, Inoue H, Nagasaka S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. Cloning and characterization of deoxymugineic acid synthase genes from graminaceous plants. J Biol Chem. 2006;281:32395–32402. doi: 10.1074/jbc.M604133200. [DOI] [PubMed] [Google Scholar]
- Black RE, Allen LH, Bhutta ZA, Caulfield LE, De Onis M, Ezzati M, Mathers C, Rivera J. Maternal and child undernutrition study group. Maternal and child undernutrition: global and regional exposures and health consequences. The Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- Bonneau J, Baumann U, Beasley J, Li Y, Johnson AA. Identification and molecular characterization of the nicotianamine synthase gene family in bread wheat. Plant Biotechnol J. 2016;14:2228–2239. doi: 10.1111/pbi.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouis HE, Saltzman A. Improving nutrition through biofortification: a review of evidence from HarvestPlus, 2003 through 2016. Glob Food Sec. 2017;12:49–58. doi: 10.1016/j.gfs.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouis HE, Welch RM. Biofortification—a sustainable agricultural strategy for reducing micronutrient malnutrition in the global south. Crop Sci. 2010;50:S–20. doi: 10.2135/cropsci2009.09.0531. [DOI] [Google Scholar]
- Center for disease control and prevention (CDC). https://www.cdc.gov/immpact/ micronutrients/index.html. Accessed July 12, 2020
- Crompton DW, Nesheim MC. Nutritional impact of intestinal helminthiasis during the human life cycle. Annu Rev Nutr. 2002;22:35–59. doi: 10.1146/annurev.nutr.22.120501.134539. [DOI] [PubMed] [Google Scholar]
- Curie C, Panaviene Z, Loulergue C, Dellaporta SL, Briat JF, Walker EL. Maize yellow stripe1 encodes a membrane protein directly involved in Fe (III) uptake. Nature. 2001;409(6818):346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S. Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot. 2009;103:1–1. doi: 10.1093/aob/mcn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnton-Hill I, Webb P, Harvey PW, Hunt JM, Dalmiya N, Chopra M, Ball MJ, Bloem MW, De Benoist B. Micronutrient deficiencies and gender: social and economic costs. Am J Clin Nutr. 2005;81:1198S–S1205. doi: 10.1093/ajcn/81.5.1198. [DOI] [PubMed] [Google Scholar]
- Dasa F, Abera T. Factors affecting iron absorption and mitigation mechanisms: a review. Int J Agric Sci Food Technol. 2018;4:24–30. [Google Scholar]
- Dimkpa CO, Bindraban PS. Fortification of micronutrients for efficient agronomic production: a review. Agron Sustain Dev. 2016;36:7. doi: 10.1007/s13593-015-0346-6. [DOI] [Google Scholar]
- Directorate of Millets Development (2020) Project Coordinator Review, 2020
- Duck KA, Connor JR. Iron uptake and transport across physiological barriers. Biometals. 2016;29:573–591. doi: 10.1007/s10534-016-9952-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RE. Advances in understanding the molecular basis for the regulation of dietary iron absorption. Curr Opin Gastroenterol. 2005;21:201–206. doi: 10.1097/01.mog.0000153362.98276.db. [DOI] [PubMed] [Google Scholar]
- Food and agriculture organization of the united nation (FAO). http://www.fao.org/india/fao-in-india/india-at-a-glance. Accessed Apr, 2020
- Fordyce F, Robbins E. Phytate× calcium/zinc molar ratios: are they predictive of zinc bioavailability? J Food Sci. 1987;52:440–444. doi: 10.1111/j.1365-2621.1987.tb06634.x. [DOI] [Google Scholar]
- Gernand AD, Schulze KJ, Stewart CP, West KP, Jr, Christian P. Micronutrient deficiencies in pregnancy worldwide: health effects and prevention. Nat Rev Endocrinol. 2016;12:274. doi: 10.1038/2Fnrendo.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharibzahedi SM, Jafari SM. The importance of minerals in human nutrition: bioavailability, food fortification, processing effects and nanoencapsulation. Trends Food Sci Technol. 2017;62:119–132. doi: 10.1016/j.tifs.2017.02.017. [DOI] [Google Scholar]
- Govindaraj M, Selvi B, Rajarathinam S, Sumathi P (2011) Genetic variability and heritability of grain yield components and grain mineral concentration in India's pearl millet (Pennisetum glaucum (L) R. Br.) accessions. Afr J Food Agric Nutr Dev 11(3)
- Govindaraj M, Rai KN, Shanmugasundaram P, Dwivedi SL, Sahrawat KL, Muthaiah AR, Rao AS. Combining ability and heterosis for grain iron and zinc densities in pearl millet. Crop Sci. 2013;53:507–517. doi: 10.2135/cropsci2012.08.0477. [DOI] [Google Scholar]
- Hallberg L, Brune M, Erlandsson M, Sandberg AS, Rossander-Hulten L. Calcium: effect of different amounts on nonheme-and heme-iron absorption in humans. Am J ClinNutr. 1991;53:112–119. doi: 10.1093/ajcn/53.1.112. [DOI] [PubMed] [Google Scholar]
- Hambidge KM, Krebs NF. Zinc deficiency: a special challenge. J Nutr. 2007;137:1101–1105. doi: 10.1093/jn/137.4.1101. [DOI] [PubMed] [Google Scholar]
- HarvestPlus. www.harvestplus.org. Accessed Jan 27 2020
- Hurrell R, Egli I. Iron bioavailability and dietary reference values. Am J Clin Nutr. 2010;91:1461S–S1467. doi: 10.3945/ajcn.2010.28674F. [DOI] [PubMed] [Google Scholar]
- Hurrell RF, Reddy MB, Juillerat MA, Cook JD. Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. Am J ClinNutr. 2003;77:1213–1219. doi: 10.1093/ajcn/77.5.1213. [DOI] [PubMed] [Google Scholar]
- Ishimaru Y, Masuda H, Bashir K, Inoue H, Tsukamoto T, Takahashi M, Nakanishi H, Aoki N, Hirose T, Ohsugi R, Nishizawa NK. Rice metal-nicotianamine transporter, OsYSL2, is required for the long-distance transport of iron and manganese. Plant J. 2010;62:379–390. doi: 10.1111/j.1365-313X.2010.04158.x. [DOI] [PubMed] [Google Scholar]
- Joint FA. Vitamin and mineral requirements in human nutrition. New York: Diamond Pocket Book (P) Ltd.; 2004. [Google Scholar]
- Kanatti A, Rai KN, Radhika K, Govindaraj M, Sahrawat KL, Rao AS. Grain iron and zinc density in pearl millet: combining ability, heterosis and association with grain yield and grain size. Springerplus. 2014;3:763. doi: 10.1186/2193-1801-3-763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S (2016) Iron deficiency anemia (IDA): a review. Int J Sci Res 5:1999–2003. https://www.researchgate.net/profile/Sukhdeep-Kaur19/publication/323689439_Iron_DeficiencyAnemiaIDAAReview/links/5c6a33374585156b57030612/Iron-Deficiency-Anemia-IDA-A-Review.pdf
- Kaur KD, Jha A, Sabikhi L, Singh AK. Significance of coarse cereals in health and nutrition: a review. J Food Sci Technol. 2014;51:1429–1441. doi: 10.1007/s13197-011-0612-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodkany BS, Bellad RM, Mahantshetti NS, Westcott JE, Krebs NF, Kemp JF, Hambidge KM. Biofortification of pearl millet with iron and zinc in a randomized controlled trial increases absorption of these minerals above physiologic requirements in young children. J Nutr. 2013;143:1489–1493. doi: 10.3945/jn.113.176677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike S, Inoue H, Mizuno D, Takahashi M, Nakanishi H, Mori S, Nishizawa NK. OsYSL2 is a rice metal-nicotianamine transporter that is regulated by iron and expressed in the phloem. Plant J. 2004;39:415–424. doi: 10.1111/j.1365-313X.2004.02146.x. [DOI] [PubMed] [Google Scholar]
- Koréissi-Dembélé Y, Fanou-Fogny N, Moretti D, Schuth S, Dossa RA, Egli I, Zimmermann MB, Brouwer ID. Dephytinisation with intrinsic wheat phytase and iron fortification significantly increase iron absorption from fonio (Digitariaexilis) meals in West African women. PLoS ONE. 2013;8:e70613. doi: 10.1371/journal.pone.0070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krężel A, Maret W. The biological inorganic chemistry of zinc ions. Arch Biochem Biophys. 2016;611:3–19. doi: 10.1016/j.abb.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Hash CT, Thirunavukkarasu N, Singh G, Rajaram V, Rathore A, Senapathy S, Mahendrakar MD, Yadav RS, Srivastava RK. Mapping quantitative trait loci controlling high iron and zinc content in self and open pollinated grains of pearl millet [Pennisetumglaucum (L.) R. Br.] Front Plant Sci. 2016;7:1636. doi: 10.3389/fpls.2016.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Hash CT, Nepolean T, Mahendrakar MD, Satyavathi CT, Singh G, Rathore A, Yadav RS, Gupta R, Srivastava RK. Mapping grain iron and zinc content quantitative trait loci in an iniadi-derived immortal population of pearl millet. Genes. 2018;9:248. doi: 10.3390/genes9050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocque R, Casapia M, Gotuzzo E, Gyorkos TW. Relationship between intensity of soil-transmitted helminth infections and anemia during pregnancy. Am J Trop Med Hyg. 2005;73:783–9. doi: 10.4269/ajtmh.2005.73.783. [DOI] [PubMed] [Google Scholar]
- Lonnerdal B. Dietary factors influencing zinc absorption. J Nutr. 2000;130:1378S–S1383. doi: 10.1093/jn/130.5.1378S. [DOI] [PubMed] [Google Scholar]
- Mahendrakar MD, Parveda M, Kishor PK, Srivastava RK. Discovery and validation of candidate genes for grain iron and zinc metabolism in pearl millet [Pennisetum glaucum (L.) R. Br.] Sci Rep. 2020;10:1–6. doi: 10.1038/s41598-020-73241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manwaring HR, Bligh HF, Yadav R. The challenges and opportunities associated with biofortification of pearl millet (Pennisetum glaucum) with elevated levels of grain iron and zinc. Front Plant Sci. 2016;7:1944. doi: 10.3389/fpls.2016.01944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman NT. A review of nutrients and compounds, which promote or inhibit intestinal iron absorption: making a platform for dietary measures that can reduce iron uptake in patients with genetic haemochromatosis. J Nutr Metab. 2020 doi: 10.1155/2020/7373498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti D (2017) Plant-based diets and iron status. In: Vegetarian and plant-based diets in health and disease prevention. Academic Press, pp 715–727. 10.1016/B978-0-12-803968-7.00039-3
- Nelson AR, Ravichandran K, Antony U. The impact of the Green Revolution on indigenous crops of India. J Ethn Foods. 2019;6:1. doi: 10.1186/s42779-019-0011-9. [DOI] [Google Scholar]
- Nozoye T, Nagasaka S, Kobayashi T, Takahashi M, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK. Phytosiderophore efflux transporters are crucial for iron acquisition in graminaceous plants. J Biol Chem. 2011;286:5446–5454. doi: 10.1074/jbc.M110.180026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh C, Keats EC, Bhutta ZA. Vitamin and mineral supplementation during pregnancy on maternal, birth, child health and development outcomes in low-and middle-income countries: a systematic review and meta-analysis. Nutrients. 2020;12:491. doi: 10.3390/nu12020491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel H, Chandra S, Alexander S, Soble J, Williams KA. Plant-based nutrition: an essential component of cardiovascular disease prevention and management. Curr Cardiol Rep. 2017;19:1. doi: 10.1007/s11886-017-0909-z. [DOI] [PubMed] [Google Scholar]
- Pujar M, Gangaprasad S, Govindaraj M, Gangurde SS, Kanatti A, Kudapa H. Genome-wide association study uncovers genomic regions associated with grain iron, zinc and protein content in pearl millet. Sci Rep. 2020;10:1–5. doi: 10.1038/s41598-020-76230-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puranik S, Kam J, Sahu PP, Yadav R, Srivastava RK, Ojulong H, Yadav R. Harnessing finger millet to combat calcium deficiency in humans: challenges and prospects. Front Plant Sci. 2017;8:1311. doi: 10.3389/fpls.2017.01311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai KN, Govindaraj M, Rao AS. Genetic enhancement of grain iron and zinc content in pearl millet. Qual Assur Saf Crops Foods. 2012;4:119–125. doi: 10.1111/j.1757-837X.2012.00135.x. [DOI] [Google Scholar]
- Rai KN, Yadav OP, Rajpurohit BS, Patil HT, Govindaraj M, Khairwal IS, Rao AS. Breeding pearl millet cultivars for high iron density with zinc density as an associated trait. J SAT Agric Res. 2013;11:1–7. [Google Scholar]
- Rai KN, Yadav OP, Govindaraj M, Pfeiffer WH, Yadav HP, Rajpurohit BS, Patil HT, Kanatti A, Rathore A, Rao AS, Shivade H. Grain iron and zinc densities in released and commercial cultivars of pearl millet (Pennisetumglaucum) Indian J Agric Sci. 2016;86:291–6. [Google Scholar]
- Ramzani PM, Khalid M, Naveed M, Irum A, Khan WU, Kausar S (2016) Iron biofortification of cereals grown under calcareous soils: problems and solutions. In: Soil science: agricultural and environmental prospectives. Springer, Cham, pp 231–258. 10.1007/978-3-319-34451-5_10
- Rehman AU, Masood S, Khan NU, Abbasi ME, Hussain Z, Ali I. Molecular basis of Iron Biofortification in crop plants; A step towards sustainability. Plant Breed. 2021;140:12–22. doi: 10.1111/pbr.12886. [DOI] [Google Scholar]
- Rodgers GM, Gilreath JA. The role of intravenous iron in the treatment of anemia associated with cancer and chemotherapy. Acta Haematol. 2019;142:13–20. doi: 10.1159/000496967. [DOI] [PubMed] [Google Scholar]
- Rodríguez JP, Rahman H, Thushar S, Singh RK. Healthy and resilient cereals and pseudo-cereals for marginal agriculture: molecular advances for improving nutrient bioavailability. Front Genet. 2020;11:49. doi: 10.3389/fgene.2020.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samtiya M, Aluko RE, Dhewa T. Plant food anti-nutritional factors and their reduction strategies: an overview. Food Prod Process Nutr. 2020;2:1–4. doi: 10.1186/s43014-020-0020-5. [DOI] [Google Scholar]
- Sandberg AS, Hulthén LR, Türk M. Dietary Aspergillus nigerphytase increases iron absorption in humans. J Nutr. 1996;126:476–480. doi: 10.1093/jn/126.2.476. [DOI] [PubMed] [Google Scholar]
- Sandström B, Cederblad Å, Lönnerdal BO. Zinc absorption from human milk, cow's milk, and infant formulas. Am J Dis Child. 1983;137:726–729. doi: 10.1001/archpedi.1983.02140340010002. [DOI] [PubMed] [Google Scholar]
- Satyavathi CT (2019) Project coordinator review. In: ICAR-AICRP on pearl millet. Agriculture University, Jodhpur, pp 1–2
- Satyavathi CT, Sankar SM, Singh SP, Bhowmick P, Bhat J, Singh O, Anuradha N. Stability analysis of grain iron and zinc content in pearl millet (Pennisetumglaucum (L.) R. Br) Int J Trop Agric. 2015;33:1387–94. [Google Scholar]
- Satyavathi CT, Praveen S, Mazumdar S, Chugh LK, Kawatra A (2017) Enhancing demand of pearl millet as super grain-current status and way forward. In: ICAR-All India Coordinated Research Project on Pearl Millet, Jodhpur
- Satyavathi CT, Ambawat S, Khandelwal V, Govindaraj M and Neeraja CN (2021) Micronutrient Rich Pearl Millet for Nutritionally Secure India
- Senoura T, Sakashita E, Kobayashi T, Takahashi M, Aung MS, Masuda H, Nakanishi H, Nishizawa NK. The iron-chelate transporter OsYSL9 plays a role in iron distribution in developing rice grains. Plant Mol Bio. 2017;95:375–87. doi: 10.1007/s11103-017-0656-y. [DOI] [PubMed] [Google Scholar]
- Shivhare R, Lata C. Exploration of genetic and genomic resources for abiotic and biotic stress tolerance in pearl millet. Front Plant Sci. 2017;7:2069. doi: 10.3389/fpls.2016.02069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sihag MK, Sharma V, Goyal A, Arora S, Kapila R. In vivo assessment of iron bioavailability from fortified pearl millet based weaning food. J Sci Food Agric. 2016;96:4410–4415. doi: 10.1002/jsfa.7651. [DOI] [PubMed] [Google Scholar]
- Simwemba CG, Hoseney RC, Varriano-Marston E, Zeleznak K. Certain B vitamin and phytic acid contents of pearl millet [Pennisetumamericanum (L.) Leeke] J Agric Food Chem. 1984;32:31–34. doi: 10.1021/jf00121a008. [DOI] [PubMed] [Google Scholar]
- Singhal T, Satyavathi CT, Kumar A, Sankar SM, Singh SP, Bharadwaj C, Aravind J, Anuradha N, Meena MC, Singh N. Genotype× environment interaction and genetic association of grain iron and zinc content with other agronomic traits in RIL population of pearl millet. Crop Pasture Sci. 2018;69:1092–1102. doi: 10.1071/CP18306. [DOI] [Google Scholar]
- Singhal T, Satyavathi CT, Singh SP, Kumar A, Sankar SM, Bhardwaj C, Mallik M, Bhat J, Anuradha N, Singh N. Multi-environment quantitative trait loci mapping for grain iron and zinc content using bi-parental recombinant inbred line mapping population in pearl millet. Front Plant Sci. 2021;12:744. doi: 10.3389/fpls.2021.659789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperotto RA, Ricachenevsky FK. Common bean Fe biofortification using model species' lessons. Front Plant Sci. 2017;8:2187. doi: 10.3389/fpls.2017.02187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein AJ, Qaim M, Nestel P (2009) Zinc deficiency and DALYs in India: Impact assessment and economic analyses. Handbook of disease burdens and quality of life measures. Springer, Heidelberg Also http://www.springer.com/medicine/book/978-0-387-78665-0. http://www.ajstein.de/cv/Stein2010_Springer_ZnD.pdf
- Stich B, Haussmann BI, Pasam R, Bhosale S, Hash CT, Melchinger AE, Parzies HK. Patterns of molecular and phenotypic diversity in pearl millet [Pennisetumglaucum (L.) R. Br] from West and Central Africa and their relation to geographical and environmental parameters. BMC Plant Biol. 2010;10:216. doi: 10.1186/1471-2229-10-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus RJ. Iron deficiency: global prevalence and consequences. Food Nutr Bull. 2003;24(4_supp_1):S99–103. doi: 10.1177/15648265030244S106. [DOI] [PubMed] [Google Scholar]
- Tako E, Reed SM, Budiman J, Hart JJ, Glahn RP. Higher iron pearl millet (Pennisetumglaucum L.) provides more absorbable iron that is limited by increased polyphenolic content. Nutrition. 2015;14:11. doi: 10.1186/1475-2891-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talpur S, Afridi HI, Kazi TG, Talpur FN. Interaction of lead with calcium, iron, and zinc in the biological samples of malnourished children. Biol Trace Elem Res. 2018;183:209–217. doi: 10.1007/s12011-017-1141-9. [DOI] [PubMed] [Google Scholar]
- Teucher O, Cori. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J VitamNutr Res. 2004;74:403–419. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- Troesch B, Egli I, Zeder C, Hurrell RF, De Pee S, Zimmermann MB. Optimization of a phytase-containing micronutrient powder with low amounts of highly bioavailable iron for in home fortification of complementary foods. Am J Clin Nutr. 2009;89:539–544. doi: 10.3945/ajcn.2008.27026. [DOI] [PubMed] [Google Scholar]
- Trumbo P, Yates AA, Schlicker S, Poos M. Dietary reference intakes: vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. J Am Diet Assoc. 2001;101:294–301. doi: 10.1016/S0002-8223(01)00078-5. [DOI] [PubMed] [Google Scholar]
- University of California San Francisco (UCSF) health. https://www.ucsfhealth.org/education/hemoglobinand functions of iron .Accessed Jan 27, 2020
- Vadez V, Hash T, Kholova J. II. 1.5 Phenotyping pearl millet for adaptation to drought. Front Physiol. 2012;3:386. doi: 10.3389/fphys.2012.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velu G, Rai KN, Muralidharan V, Longvah T, Crossa J. Gene effects and heterosis for grain iron and zinc density in pearl millet (Pennisetumglaucum (L.) R. Br) Euphytica. 2011;180:251–9. doi: 10.1007/s10681-011-0387-0. [DOI] [Google Scholar]
- Venkataramani V. Iron homeostasis and metabolism: two sides of a coin. Ferroptosis Mech Dis. 2021;25:40. doi: 10.1007/978-3-030-62026-4_3. [DOI] [PubMed] [Google Scholar]
- Vogt AC, Arsiwala T, Mohsen M, Vogel M, Manolova V, Bachmann MF. On iron metabolism and its regulation. Int J Mol Sci. 2021;22:4591. doi: 10.3390/ijms22094591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1996) Zinc. In: Trace elements in human nutrition and health. World Health Organization, Geneva
- Yadav SK, Nath S, Gautam S. Analysis of rainfall variability in western Rajasthan, India. J Pharmacogn Phytochem. 2018;7:1592. [Google Scholar]
- Yokosho K, Yamaji N, Ma JF. OsFRDL1 expressed in nodes is required for distribution of iron to grains in rice. J Exp Bot. 2016;67:5485–5494. doi: 10.1093/jxb/erw314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneyama T, Ishikawa S, Fujimaki S. Route and regulation of zinc, cadmium, and iron transport in rice plants (Oryza sativa L.) during vegetative growth and grain filling: metal transporters, metal speciation, grain Cd reduction and Zn and Fe biofortification. Int J Mol Sci. 2015;16:19111–29. doi: 10.3390/ijms160819111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Not Applicable.