Abstract
Introduction
Atopic dermatitis (AD) is one of the most common skin diseases, and it may be associated with skin cancer risk. However, there is a controversy pertaining to whether it implies a greater or decreased risk of skin cancers. We aimed to study the relationship between AD and skin cancer risk.
Methods
PubMed and Embase databases from their inception to 4 August 2021 were systematically searched.
Results
We evaluated 16 studies involving a total of 9,638,093 participants examining the contribution of AD to skin cancers. Random-effects model was applied to estimate the overall effect sizes. The pooled analysis of 16 studies indicated that AD was significantly associated with an overall increased risk of skin cancer. Subgroup pooled analyses showed that AD was statistically associated with an increased risk of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). With regard to cohort study, AD was statistically associated with an increased risk of nonmelanoma skin cancer (NMSC), BCC, and SCC, but not melanoma risk. Sensitivity analysis revealed that excluding each study in turn did not alter the overall combined results. No publication bias existed among the studies.
Conclusion
It can be concluded that AD is associated with risk of skin cancers; however, this association still needs to be verified in well-designed, worldwide trials (especially prospective, non-Western studies). The mechanism of AD leading to skin cancer is not clear, and further research is needed to explore the possibility of a potential pathogenesis.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-022-00720-2.
Keywords: Atopic dermatitis, Basal cell carcinoma, Skin cancer
Key Summary Points
| Why carry out this study? |
| Atopic dermatitis is a common skin disease. To detect and prevent skin cancer earlier, we analyzed whether atopic dermatitis is a risk factor for skin cancer. |
| What was the hypothesis of the study? |
| Atopic dermatitis is a risk factor for skin cancer. |
| What was learned from the study? |
| Atopic dermatitis has the potential to predict increased risk of basal cell carcinoma (BCC), squamous cell carcinoma (SCC), and nonmelanoma skin cancer (NMSC). |
| How might this affect research and/or treatment in the future? |
| Although the impact of atopic dermatitis on skin cancer needs to be supported by further research, this study points to a new possibility for clinical application and future research. |
Introduction
Skin cancer and atopic dermatitis (AD) are among the major public health problems globally. Melanoma is an aggressive and deadly skin cancer. Nonmelanoma skin cancer (NMSC), such as squamous cell carcinoma (SCC) and basal cell carcinoma (BCC), are also very common forms of skin cancer. AD or eczema is a chronic recurrent inflammatory skin disease associated with epithelial, immune, and environmental factors [1, 2]. It is characterized by intense itching, breakdown of the skin barrier, and activation of the type-2-mediated immune response in the skin [3, 4]. Population-based studies showed that the prevalence rate of eczema is approximately 10.7% among children and 7.2% among adults [5]. AD not only causes serious financial burden but also seriously affects the quality of patients’ lives. For example, AD may be associated with skin cancer risk. Jensen et al. found an inverse association between AD and melanoma, and also found that patients with AD are at increased risk of BCC and SCC [6]. Hagströmer et al. found a nonsignificant risk elevation for nonmelanoma skin cancer [7].
Although many studies have focused on the association of AD with skin cancers [8–18], whether AD implies a greater or decreased risk of skin cancers is still controversial. Therefore, the aim of this study was to investigate the relationship between AD and the risk of skin cancers.
Methods
The study was conducted following the Meta-analysis of Observational Studies in Epidemiology guidelines along with the Preferred Reporting Items for Systematic Reviews and Meta-analyses standards [19]. The research is registered with INPLASY202090029. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Search Strategy and Selection Criteria
We systematically searched PubMed and Embase databases on 4 August 2021, for studies performed on the relationship between AD and skin cancers. Animal studies, case reports, reviews, and meta-analyses were excluded. Our core search keywords were “Atopic dermatitis,” “Eczema,” “Cohort, and Case–Control Studies.” The inclusion criteria were as follows: cohort and case–control studies assessing the relationship between AD and skin cancers that comprised two comparator groups, where one group had AD and the other (control) did not. Two authors (ZY and WHM) independently reviewed the titles and abstracts of the retrieved studies on the basis of the inclusion criteria. The reference lists of eligible studies or related meta-analyses were also screened to find additional pertinent studies. The quality of the studies and risk of bias were assessed according to the Newcastle–Ottawa Scale (NOS) [20]. All disagreements were resolved by discussion with the corresponding authors.
Data Analysis
Two authors (ZY and WHM) extracted all data. When one study included more than one cohort, we pooled each cohort as an independent study. For each independent study, we recorded the following variables: first author’s last name, publication year, region in which the study was performed, type of study design, type of cancer, participants’ sex and age, sample size, and outcome measurements related to risk estimates with 95% confidence intervals (CIs) and adjustment factors.
A pooled analysis was conducted to explore the association between eczema and different cancers. The Cochrane Q and I2 statistics were used to evaluate heterogeneity [21]. When either the P-value was < 0.1 or the I2 value was > 50%, the data were considered to be heterogeneous, and a random-effects model [22] was applied to estimate the overall effect sizes. Otherwise, a fixed-effects model was used [23]. To further explore the origin of heterogeneity, we performed subgroup analyses by region, type of study design, and type of cancer. To assess the stability of our results, sensitivity analyses were conducted by excluding each study in turn to estimate the influence of each individual study on the pooled results. Beggar’s test [24] and Egger’s test [25] were used to assess potential publication bias. STATA software v12.0 (College Station, TX, USA) was used to analyze the data.
Results
Search Results and Study Characteristics
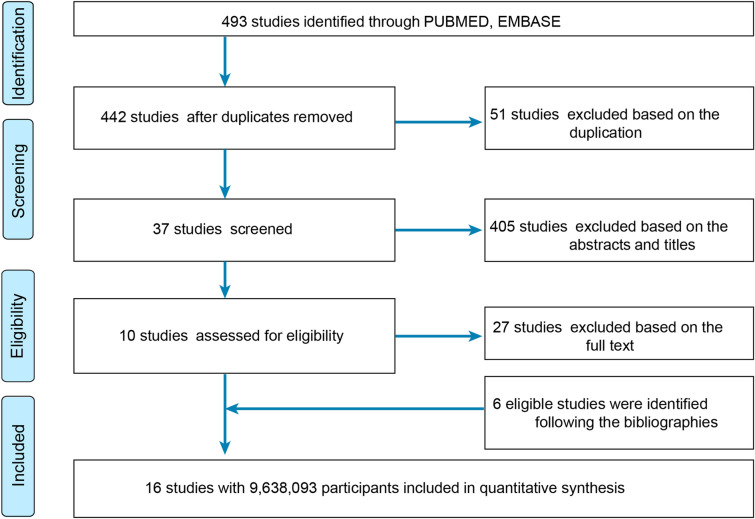
A total of 493 studies were retrieved from the PubMed and the Embase databases, and after removing 51 duplicates and further excluding 405 studies after title and abstract screening and 27 on the basis of the full article, 10 studies remained. However, six additional eligible studies were identified after screening the references of relevant studies. As a result, 16 studies [6–18, 26–28], involving a total of 9,638,093 participants, that examined the contribution of AD to skin cancers eventually fulfilled the established criteria (Fig. 1). Details on the characteristics of the studies are summarized in Table 1, and assessments of the studies are summarized in Table S1 in the Supplementary Material. Eight population-based cohort studies [6, 7, 9, 13, 18, 26–28], and eight case–control studies were included in this analysis [8, 10–12, 14–17]. Of these, one is from Finland [9], two from Sweden [7, 27], three from Denmark [6, 18, 26], four from USA [8, 10, 13, 15, 17], one from Belgium [16], one from Canada [11], one from Montenegro [12], one from Netherlands [14], and two from UK [18, 28].
Fig. 1.
Flow diagram summarizing the pooled analysis phases (i.e., identification, screening, eligibility assessment, and ultimate inclusion)
Table 1.
Characteristics of included studies
| Studies | OR | Study period | Region | Study design | Age (years) | Sex | Participants | Cancer | Statistical analysis | Adjustments | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Milán et al. [9] | 1.1 | 1976–1999 | Finland | Cohort study | ≥ 18 | Male and female | 666 | BCC | Conditional logistic regression analysis | NA | 8 |
| Ming et al. [10] | 0.85 | 1998–2001 | USA | Case–control study | 66.4 versus 60.1* | Male: 51.3% versus 39.9% | 4591 | NMSC | Multivariable logistic regression | Age, sex, topical steroid use, and ethnicity | 7 |
| Hagströmer et al. [7] | 1.5 | 1965–1999 | Sweden | Cohort study | 15.7* | Male and female | 15,666 | NMSC, melanoma | χ2 test | NA | 7 |
| Olesen et al. [26] | 2.4 | 1977–1996 | Denmark | Cohort study | NA | Male and female | 6275 | NMSC | Poisson regression methods | NA | 7 |
| Synnerstad et al. [27] | 0.49 | 1986–2004 | Sweden | Cohort study | 2.6 (5.5) to 39.3 (10.9)* | Male and female | 6280 | Melanoma | Poisson regression analysis | Age group, sex, and year | 8 |
| Arana et al. [28] | 1.74 | 1992–2006 | UK | Retrospective cohort study | All ages | Male and female | 4,518,131 | Melanoma, NMSC | Mantel–Haenszel | Age and sex | 7 |
| El-Zein et al. [11] | 0.64 | 1979–1985 | Canadian | Case–control study | 35–70 | Males | 3809 | Melanoma | Logistic regression | Age, income, respondent status, ancestry, and sports and/or outdoor activities | 6 |
| Janković et al. [12] | 4.17 | 2006–2007 | Montenegro | Case–control study | NA | Female | 200 | BCC | Multivariate logistic regression analysis | Age, sex, and marital status | 7 |
| Dyer et al. [13] | 1.54 | NA | USA | Cohort study | Median age 72 | Female 34 (3%) versus male 1097 (97%) | 1131 | BCC | Multiple logistic regression | Sex, age, education, basal cell carcinomas in prior 5 years, squamous cell carcinomas in prior 5 years, actinic keratoses at baseline, family history of skin cancer, current or former smoker | 8 |
| Jensen et al. [6] | 0.59 | 1977–2006 | Denmark | Cohort study | NA | Male and female | 31,330 | Melanoma, BCC, SCC | Byar’s approximation | NA | 8 |
| Hajdarbegovic et al. [14] | 1.05 | 2000–2010 | Netherlands | Case–control study | 57 ± 14 versus 56 ± 14* | Male and female | 353 | Melanoma | Logistic multiple regression analysis | Unadjusted OR | 7 |
| Cheng et al. [15] | 1.83 | NA | USA | Case–control study | 25–74 | Female | 1312 | SCC | Multiple logistic regression | Age, gender, and skin reaction to the first hour of sunlight during summer (blister, painful sunburn followed by peeling, mild sunburn followed by tanning, tanning with no sunburn) | 8 |
| 1.52 | NA | USA | Case–control study | 25–74 | Female | NA | BCC | Multiple logistic regression | Age, gender, and skin reaction to the first hour of sunlight during summer (blister, painful sunburn followed by peeling, mild sunburn followed by tanning, tanning with no sunburn) | 8 | |
| Marasigan et al. [16] | 0.46 | NA | Belgium | Case–control study | 57.4* for controls, 52.2* for cases | Male and female | 232 | Melanoma | Conditional logistic regression | Age, sex, sunburn sensitivity, hair color, number of moles, sunburn as juvenile, ever sunbed use, familial melanoma | 8 |
| Cho et al. [8] | 1.75 | 1996–2010 | USA | Case–control study | 69.6 (13.5) versus 69.5 (13.5)* | Female | 1179 | SCC | Logistic regression analysis | Race, smoking history, ionizing radiation exposure, corticosteroid and cyclosporine use, non-SCC skin cancers, odds ratio for SCC development | 9 |
| D'Arcy et al. [17] | 1.07 | 1992–2013 | USA | Case–control study | 66–99 | Female | 1,844,575 | Melanoma | Logistic regression analysis | Sex, age, race, calendar year of selection, and measures of socioeconomic status and healthcare utilization | 7 |
| Mansfield et al. [31] | 0.96 | 1998–2016 | UK | Cohort study | 41.1 [24.9–60.7] versus 39.8 [25.9–58.4]# | Female | 2,711,745 | Melanoma | Cox proportional hazards regression model | Sex, primary care practice, date, and age | 8 |
| 1.1 | 1998–2016 | UK | Cohort study | 41.1 [24.9–60.7] versus 39.8 [25.9–58.4]# | Female | NA | NMSC | Cox proportional hazards regression model | Sex, primary care practice, date, and age | 8 | |
| 0.64 | 1982–2016 | Denmark | Cohort study | 13.7 [1.7–21.1] versus 13.5 [1.7–20.8]# | Female | 490,618 | Melanoma | Cox proportional hazards regression model | Sex, date, and age | 8 | |
| 1.17 | 1982–2016 | Denmark | Cohort study | 13.7 [1.7–21.1] versus 13.5 [1.7–20.8]# | Female | NA | NMSC | Cox proportional hazards regression model | Sex, date, and age | 8 |
*Mean (SD); #median [IQR]
Qualitative Analysis
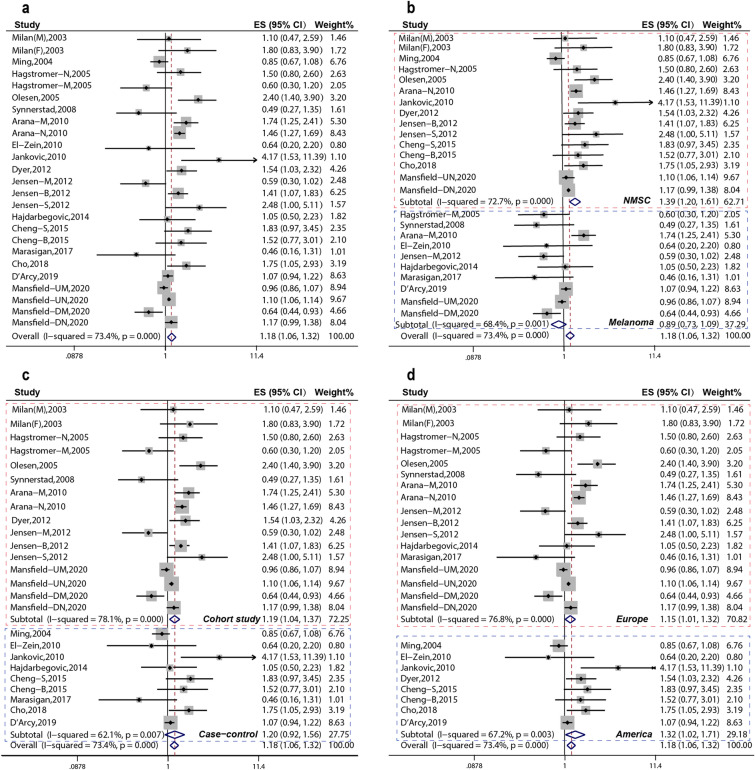
Firstly, the pooled analysis of 16 studies [6–18, 26–28] indicated that AD was significantly associated with an increased risk of overall skin cancer (OR 1.18, 95% CI 1.06–1.32); moreover, substantial heterogeneity was observed (Pheterogeneity = 0.000, I2 = 73.4%) (Fig. 2a). Subgroup pooled analyses were performed according to cancer type, study design, and region, and we found that AD was significantly associated with an increased risk of overall skin cancer in the following subgroups: NMSC subgroup: OR 1.39, 95% CI 1.20–1.61 (Fig. 2b), cohort study subgroup: OR 1.19, 95% CI 1.04–1.37 (Fig. 2c), American subgroup: OR 1.32, 95% CI 1.02–1.71 (Fig. 2d), and Europe subgroup: OR, 1.15; 95% CI, 1.01–1.32 (Fig. 2d), but not melanoma subgroup: OR 0.89, 95% CI 0.73–1.09 (Fig. 2b).
Fig. 2.
Estimated effects of AD on skin cancer risk. a Forest plot for effects of AD on skin cancer risk. b Forest plot for subgroup analysis by cancer type. c Forest plot for subgroup analysis by study design. d Forest plot for subgroup analysis by region
Secondly, according to Fig. 2b, the pooled analysis indicated that AD was significantly associated with an increased risk of NMSC (OR 1.39, 95% CI 1.20–1.61, Pheterogeneity = 0.000, I2 = 72.7%). Further subgroup analyses with regard to specific NMSC type were performed to further explore the origin of heterogeneity; we found that AD was significantly increased with an increased risk of BCC (OR 1.51, 95% CI 1.24–1.84, Pheterogeneity = 0.426, I2 = 0.0%) and SCC (OR 1.90, 95% CI 1.33–2.72, Pheterogeneity = 0.770, I2 = 0.0%) (Fig. 3a). With regard to study design, AD was significantly increased with an increased risk of NMSC in cohort study subgroup (OR 1.39, 95% CI 1.19–1.63, Pheterogeneity = 0.000, I2 = 73.2%) (Fig. 3b).
Fig. 3.
Subgroup analysis of effect of AD on skin cancer risk. a Forest plot for subgroup analysis by specific NMSC type. b Forest plot for subgroup analysis by study design. c Forest plot for subgroup analysis of cohort studies by cancer type. d Forest plot for subgroup analysis of cohort studies by region
Thirdly, according to Fig. 2c, AD was significantly associated with an increased risk of overall skin cancer in cohort subgroups (OR 1.19, 95% CI 1.04–1.37). Further subgroup analyses were performed by specific cancer type, and the pooled analysis of cohort studies indicated that AD was significantly associated with an increased risk of BCC (OR 1.45, 95% CI 1.17–1.78) and SCC (OR 2.48, 95% CI 1.10–5.61), but not melanoma (Fig. 3c). According to region, the pooled analysis of cohort studies indicated that AD was statistically associated with an increased risk of skin cancer in Europe (OR 1.17, 95% CI 1.02–1.35) and America (OR 1.54, 95% CI 1.03–2.31) (Fig. 3d).
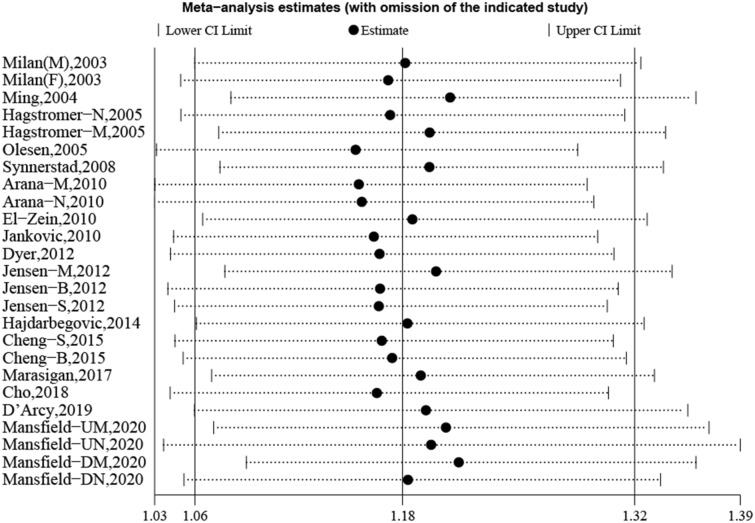
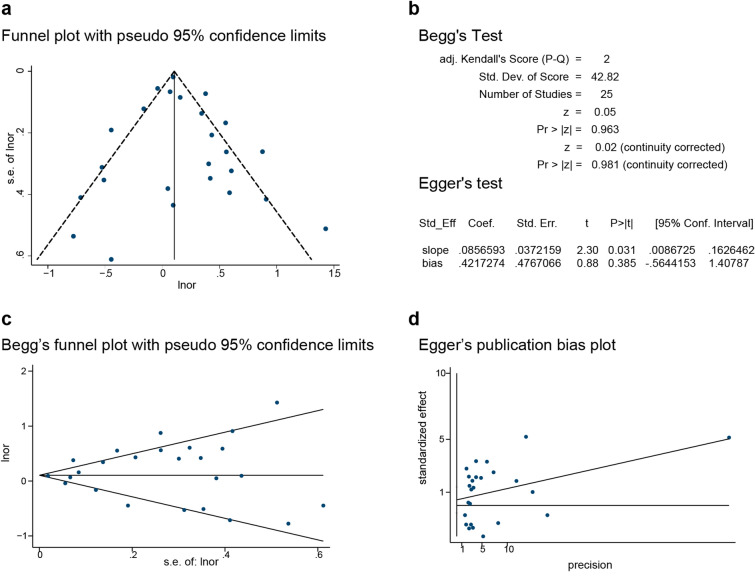
Lastly, to assess the stability of our results, sensitivity analysis was conducted, and revealed that excluding each study in turn did not alter the overall combined results (Fig. 4). Publication bias was evaluated following Beggar’s rank correlation and Egger’s linear regression tests, which indicated that no publication bias existed among the studies (Beggar’s: P > |z|= 0.981; Egger’s: P = 0.746, 95% CI −0.564 to 1.408) (Fig. 5).
Fig. 4.
Sensitivity analysis regarding the association between AD and skin cancer
Fig. 5.
Publication bias among the studies indicating the association of AD with skin cancers. a Funnel plot indicating the lack of publication bias among the studies. b–d Beggar’s and Egger’s tests indicating the lack of publication bias among the studies
Discussion
We reviewed the epidemiological evidence on the association between atopic dermatitis and skin cancer risk, and pooled this analysis. The study showed that AD was significantly associated with an increased risk of overall skin cancer. Moreover, sensitivity analysis by excluding each study in turn demonstrated stable consequence, and no publication bias existed among the included studies. Therefore, the outcome was robust and reliable, and regular skin cancer screenings are recommended for patients with AD.
Furthermore, we performed subgroup analyses to assess the association between AD and skin cancer, and to explore the origin of heterogeneity. According to cancer type, AD was associated with a significantly elevated risk of NMSC, but with a nonsignificant decreased risk of melanoma. However, the review by Karim et al. showed that allergic diseases appeared to reduce the risk for developing melanoma and NMSC [29]. Our outcomes were more credible. Possible reasons may be that our pooled analysis included some more eligible studies on the association between skin cancers and AD.
According to study design, only the pooled analyses of cohort studies demonstrated AD increasing skin cancer risk. This was more credible, because the design of cohort studies is from cause to effect, with strong ability to demonstrate causality, high quality of evidence, and better confirmation of the etiological hypothesis. According to region, AD was significantly associated with an increased risk of skin cancer in both Europe and America. The above results suggest that different study designs and regions might affect the stability of the association between AD and skin cancer risk. Unfortunately, we did not discover the origin of heterogeneity. Therefore, the results should be interpreted with caution.
Further analyses found that AD was statistically associated with an increased risk of basal cell carcinoma and squamous cell carcinoma. This conclusion was similar to that of Jensen et al. [6]. However, this result was not consistently supported by Cheng et al.’s study [15].
Unfortunately, it is unclear why skin cancer risk would be increased in patients affected by AD. One reason might be that patients with AD often receive phototherapy, and phototherapy has been linked with various skin cancers [30]. Additionally, patients with AD often require more skin-related tests associated with an increased risk of skin cancer [31]. Atopic dermatitis and other inflammatory skin diseases were often accompanied by dysregulation of human microflora involved in the regulation of skin cancer progression [32].
This study has several limitations. First, substantial heterogeneity was inevitable. Second, adjustment factors varied among different studies, and this may have contributed to some uncertainty regarding the estimates. Third, all included studies were from Europe and America, and non-Western studies are required to provide more convincing evidence.
Conclusions
In conclusion, this study demonstrated that AD was significantly associated with an increased risk of skin cancer, basal cell carcinoma, and squamous cell carcinoma. Further studies, including well-designed, worldwide trials (especially prospective, non-Western studies), are required to provide more convincing evidence. At the same time, the mechanism of AD leading to skin cancer is not clear, and further research is needed to explore the possibility of a potential link or a common pathogenesis.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This project was funded by the National Natural Science Foundation of China (no. 81760136, 81860553); the Ten-thousand Talents Program of Yunnan Province (no. YNWR-MY-2018-039); the Medical Leadership Foundation of Health and Family Planning Commission of Yunnan Province, China (no. L-201613); the Yunnan Province Clinical Research Center for Skin Immune Diseases (no. 2019ZF012); and the Yunnan Province Clinical Center for Skin Immune Diseases (no. ZX2019-03-02). The journal’s Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Dr. Yuye Li had full access to all the data in the study and takes responsibility for their integrity along with the accuracy of the analyses. Conception and design: Yun Zhu and Yuye Li. Acquisition, analysis, or interpretation of data: all named authors (Yun Zhu, Hongmei Wang, Juan He, Luhui Yang, Xiaoyan Zhou, Zhe Li, Huiling Zhou, Huadi Zhao, and Yuye Li). Drafting of the manuscript: Yun Zhu. Critical revision of the manuscript for important intellectual content: Yun Zhu and Yuye Li. Statistical analysis: Yun Zhu.
Disclosures
Yun Zhu, Hongmei Wang, Juan He, Luhui Yang, Xiaoyan Zhou, Zhe Li, Huiling Zhou, Huadi Zhao, and Yuye Li have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary material.
References
- 1.Berke R, Singh A, Guralnick M. Atopic dermatitis: an overview. Am Fam Physician. 2012;86(1):35–42. [PubMed] [Google Scholar]
- 2.Bieber T. Atopic dermatitis. N Engl J Med. 2008;358(14):1483–1494. doi: 10.1056/NEJMra074081. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Bierbrier R, Drucker AM, et al. Noncutaneous and cutaneous cancer risk in patients with atopic dermatitis: a systematic review and meta-analysis. JAMA Dermatol. 2020;156(2):158–171. doi: 10.1001/jamadermatol.2019.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vineis P, Crosignani P, Sacerdote C, et al. Haematopoietic cancer and medical history: a multicentre case control study. J Epidemiol Commun Health. 2000;54(6):431–436. doi: 10.1136/jech.54.6.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Silverberg JI, Garg NK, Paller AS, et al. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol. 2015;135(1):56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 6.Jensen AO, Svaerke C, Körmendiné Farkas D, et al. Atopic dermatitis and risk of skin cancer: a Danish nationwide cohort study (1977–2006) Am J Clin Dermatol. 2012;13(1):29–36. doi: 10.2165/11593280-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Hagströmer L, Ye W, Nyrén O, et al. Incidence of cancer among patients with atopic dermatitis. Arch Dermatol. 2005;141(9):1123–1127. doi: 10.1001/archderm.141.9.1123. [DOI] [PubMed] [Google Scholar]
- 8.Cho JM, Davis DMR, Wetter DA, et al. Association between atopic dermatitis and squamous cell carcinoma: a case–control study. Int J Dermatol. 2018;57(3):313–316. doi: 10.1111/ijd.13857. [DOI] [PubMed] [Google Scholar]
- 9.Milán T, Verkasalo PK, Kaprio J, et al. Lifestyle differences in twin pairs discordant for basal cell carcinoma of the skin. Br J Dermatol. 2003;149(1):115–123. doi: 10.1046/j.1365-2133.2003.05352.x. [DOI] [PubMed] [Google Scholar]
- 10.Ming ME, Levy R, Hoffstad O, et al. The lack of a relationship between atopic dermatitis and nonmelanoma skin cancers. J Am Acad Dermatol. 2004;50(3):357–362. doi: 10.1016/j.jaad.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 11.El-Zein M, Parent ME, Kâ K, et al. History of asthma or eczema and cancer risk among men: a population-based case-control study in Montreal, Quebec, Canada. Ann Allergy Asthma Immunol. 2010;104(5):378–384. doi: 10.1016/j.anai.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 12.Janković S, Maksimović N, Janković J, et al. Risk factors for basal cell carcinoma: results from the case–control study. Central Eur J Med. 2010;5(6):666–673. [Google Scholar]
- 13.Dyer RK, Weinstock MA, Cohen TS, et al. Predictors of basal cell carcinoma in high-risk patients in the VATTC (VA Topical Tretinoin Chemoprevention) trial. J Invest Dermatol. 2012;132(11):2544–2551. doi: 10.1038/jid.2012.227. [DOI] [PubMed] [Google Scholar]
- 14.Hajdarbegovic E, Atiq N, van der Leest R, et al. Atopic dermatitis is not a protective factor for melanoma but asthma may be. Int J Clin Oncol. 2014;19(4):708–711. doi: 10.1007/s10147-013-0589-7. [DOI] [PubMed] [Google Scholar]
- 15.Cheng J, Zens MS, Duell E, et al. History of allergy and atopic dermatitis in relation to squamous cell and basal cell carcinoma of the skin. Cancer Epidemiol Biomarkers Prev. 2015;24(4):749–754. doi: 10.1158/1055-9965.EPI-14-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marasigan V, Morren MA, Lambert J, et al. Inverse association between atopy and melanoma: a case–control study. Acta Derm Venereol. 2017;97(1):54–57. doi: 10.2340/00015555-2476. [DOI] [PubMed] [Google Scholar]
- 17.D'Arcy M, Rivera DR, Grothen A, et al. Allergies and the subsequent risk of cancer among elderly adults in the United States. Cancer Epidemiol Biomarkers Prev. 2019;28(4):741–750. doi: 10.1158/1055-9965.EPI-18-0887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansfield KE, Schmidt SAJ, Darvalics B, et al. The association between atopic eczema and cancer in England and Denmark: two cohort studies. Pharmacoepidemiol Drug Saf. 2019;28:11. [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 23.Woolf B. On estimating the relation between blood group and disease. Ann Hum Genet. 1955;19(4):251–253. doi: 10.1111/j.1469-1809.1955.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 24.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olesen AB, Engholm G, Storm HH, et al. The risk of cancer among patients previously hospitalized for atopic dermatitis. J Invest Dermatol. 2005;125(3):445–449. doi: 10.1111/j.0022-202X.2005.23839.x. [DOI] [PubMed] [Google Scholar]
- 27.Synnerstad I, Fredrikson M, Ternesten-Bratel A, et al. Low risk of melanoma in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2008;22(12):1423–1428. doi: 10.1111/j.1468-3083.2008.02888.x. [DOI] [PubMed] [Google Scholar]
- 28.Arana A, Wentworth CE, Fernández-Vidaurre C, et al. Incidence of cancer in the general population and in patients with or without atopic dermatitis in the UK. Br J Dermatol. 2010;163(5):1036–1043. doi: 10.1111/j.1365-2133.2010.09887.x. [DOI] [PubMed] [Google Scholar]
- 29.Karim AF, Westenberg LEH, Eurelings LEM, et al. The association between allergic diseases and cancer: a systematic review of the literature. Neth J Med. 2019;77(2):42–66. [PubMed] [Google Scholar]
- 30.Lam M, Zhu JW, Tadrous M, et al. Association between topical calcineurin inhibitor use and risk of cancer, including lymphoma, keratinocyte carcinoma, and melanoma: a systematic review and meta-analysis. JAMA Dermatol. 2021;157(5):549–558. doi: 10.1001/jamadermatol.2021.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mansfield KE, Schmidt SAJ, Darvalics B, et al. Association between atopic eczema and cancer in England and Denmark. JAMA Dermatol. 2020;156(10):1086–1097. doi: 10.1001/jamadermatol.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woo YR, Cho SH, Lee JD et al. The human microbiota and skin cancer. Int J Mol Sci 2022; 23(3). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article/as supplementary material.