Abstract
NeuroD1 is a neuronal differentiation factor that contains a basic helix–loop–helix (bHLH) motif. Recently, NeuroD1 was found to be associated with tumorigenesis in neuroblastoma (NB) and is known to promote cell proliferation and migration in these cells. Here we found that MYCN regulates the expression of NeuroD1 in NB cells and that the downregulation of MYCN using short hairpin RNAs (shRNA) results in the inhibition of cellular proliferation in NB cells. Moreover, the phenotype induced by MYCN shRNA was rescued by the exogenous expression of NeuroD1. Chromatin immunoprecipitation (ChIP) assay showed that MYCN directly binds to the E-box element in the NeuroD1 promoter region. In addition, our evaluation of two clinical databases showed that there was a positive correlation between the expression of MYCN and NeuroD1 in NB patients, which supports our in vitro data. In conclusion, this study demonstrates that MYCN-regulated NeuroD1 expression is one of the important mechanisms underlying enhanced cellular proliferation induced by the increase in MYCN expression in NB, and our results provide an important therapeutic target for NB in the future.
Key words: Neuroblastoma, Cellular proliferation, NeuroD1, MYCN
INTRODUCTION
Neuroblastoma (NB) is the most frequently observed extracranial pediatric solid tumor, accounting for approximately 15% of all pediatric cancer deaths, and the prognosis of this tumor is poor1. NB are initially derived from the sympathetic neuron lineage of neural crest cells and can develop anywhere in the sympathetic nervous system2,3. Significant progress in the clinical treatment of NB has reduced the overall mortality rate of this disease during the last few decades4. However, the underlying molecular mechanisms and causes of NB have not yet been identified.
NeuroD1, also known as BETA2, was first reported by Naya et al. as a β-cell-specific transactivator5,6. NeuroD1 has subsequently been isolated as a neurogenic differentiation factor in both frog and mouse embryos and can convert embryonic epidermal cells into fully differentiated neurons in Xenopus models while promoting the differentiation of neural precursor cells7. NeuroD1 is highly expressed in the sensory neurons during both cochlear and vestibular development and is also found in the developing spinal ganglia, all of which originate from neural crest cells8,9. In addition, it has been recently reported that NeuroD1 plays an important role in maintaining neural precursor cells during adult neurogenesis10. Furthermore, analysis of the clinical data of human NB has revealed that increased NeuroD1 expression is closely associated with poor prognosis, suggesting that NeuroD1 could be involved in NB tumorigenesis11. Our previous study found that NeuroD1 promotes NB cell proliferation by inducing ALK expression12, which is one of the critical genes indicating predisposition for NB and contributes to unfavorable outcomes in patients. Thus far, the data suggest that NeuroD1 is an important oncogenic factor in NB, the functions of which are closely related to tumor cell motility, proliferation, and invasion13–15. However, the reason for the aberrant NeuroD1 expression in NB has not been revealed.
Transcription factor MYCN is considered as one of the most important factors for tumorigenesis in NB16. MYCN is frequently amplified in unfavorable NB and promotes its invasion, metastasis, and growth17. However, MYCN expression is also indispensable for normal development18. During neural crest development in avian embryos, MYCN is firstly expressed throughout the entire cell population, and then as development continues its expression is modified to support two stages of neural crest development: neural crest migration and differentiation19,20. It is believed that NB originates around these two periods in which the hierarchical expression of regulatory molecules is implemented to determine the appropriate fate of neural crest cells and the production of the various different nervous tissues, such as the spinal and cranial ganglia21.
It is also generally accepted that there is a close connection between tumorigenesis and normal development. Considering the roles of NeuroD1 and MYCN in neural crest development, neurogenesis, and the development of NB, we hypothesized that there is a potential correlation between NeuroD1 and MYCN activity. In this study, we found that MYCN regulates NeuroD1 expression in NB cells. Clinical data also support a strong positive correlation between the expression of MYCN and NeuroD1 in NB patients. Additionally, the exogenous expression of NeuroD1 rescued the cell growth impaired by MYCN inhibition in NB cell lines. Finally, we identified NeuroD1 as a direct transcriptional target of MYCN using chromatin immunoprecipitation (ChIP) assay and luciferase reporter assay. Taken together, these results suggest that NeuroD1 could be a potential therapeutic target in NB.
MATERIALS AND METHODS
Plasmids
Nontargeting short hairpin RNA (shRNA) and specific shRNAs against human MYCN were obtained from Sigma-Aldrich (St. Louis, MO, USA). The shRNA sequences are listed in Table 1. A human MYCN cDNA clone and a NeuroD1 cDNA were amplified from human embryonic kidney (HEK) 293 cell cDNA using attB-flanked primers and then cloned into lentivirus-based CMV-RfA-IRES2-Venus vector (RIKEN BioResource Center, Ibaraki, Japan). CMV-Venus was used as a control expression vector.
Table 1.
shRNA Sequences
| shRNA Clone | shRNA Sequence (5′–3′) |
|---|---|
| Human MYCN shRNA-1 | CCGGGCCAGTATTAGACTGGAAGTTCTCGAGAACTTCCAGTCTAATACTGGCTTTTT |
| Human MYCN shRNA-2 | CCGGCGGACGAAGATGACTTCTACTCTCGAGAGTAGAAGTCATCTTCGTCCGTTTTT |
Cell Culture and Transfection
The human NB cell line SH-SY5Y was obtained from the American Type Culture Collection (Manassas, VA, USA). The human NB cell line IMR-32, CHP-134, and SK-N-AS cells were purchased from RIKEN (Tsukuba, Japan). The NB cells were cultured in RPMI supplemented with 10% heat-inactivated fetal bovine serum (FBS) under 5% CO2 at 37°C. HEK293T cells were obtained from IFOM (Milano, Italy), and the cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplied with 10% heat-inactivated FBS. For transfection experiments, the NB cells were incubated with plasmid containing lentivirus in the presence of 8 μg/ml polybrene (Sigma-Aldrich) for 24 h. The lentivirus packaging was performed as previously described11. Transfection experiments in HEK293T cells were performed with FuGENE HD (Promega, Madison, WI, USA).
Quantitative Polymerase Chain Reaction (qPCR)
Total RNA extraction and qPCR were carried out with an ISOGEN (Nippon Gene, Tokyo, Japan) kit and SYBR Green Realtime PCR Master Mix (TOYOBO) as previously described12. The primers used are listed in Table 2.
Table 2.
Primer Sequences for PCR Experiments
| Primer Name | Forward Primer Sequence (5′–3′) | Reverse Primer Sequence (5′–3′) |
|---|---|---|
| Human NeuroD1 | AAGCCATGAACGCAGAGGAG | CTGAACGAAGGAGACCAGGT |
| Human GAPDH | ATCATCCCTGCCTCTACTGG | CCCTCCGACGCCTGCTTCAC |
| Human MYCN | CGACCACAAGGCCCTCAGTA | CAGCCTTGGTGTTGGAGGAG |
| ChIP for E-box | CGGAGTCTCTAACTGGCGAC | ACTTCTCCCCACGCCTTGCT |
Western Blot Analysis
Cell lysates were prepared using radioimmunoprecipitation assay (RIPA) lysis buffer and were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (GE Healthcare, Marlborough, MA, USA). The membrane was blocked with 5% dry milk in phosphate-buffered saline (PBS) for 1 h and was then incubated with the indicated primary antibody at 4°C overnight. After washing, the membrane was incubated with horseradish peroxidase-conjugated secondary antibodies at room temperature for 1 h, and peroxidase activity was detected using ECL (GE Healthcare). Anti-MYCN (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-NeuroD1 (1:500; Cell Signaling Technology, MA, USA), and anti-β-actin (1:1,000; Sigma-Aldrich) antibodies were used.
Cellular Proliferation Assays
The infected cells were reseeded in 96-well cell culture plates and allowed to adhere overnight. At day 0, the culture medium was changed to DMEM supplemented with 2% heat-inactivated FBS. At each time point, the cell number was calculated based on their absorbance at 450 nm using a Cell Counting Kit-8 (Dojindo, Kumamoto, Japan).
ChIP Assay
ChIP assays using IMR-32 cells were carried out as previously reported20, using an anti-MYCN antibody. Primer information is listed in Table 2.
Soft Agar Assay
The six-well plate was plated with 1.5 ml of bottom agar (0.5% agar/RPMI + 10% FBS) per well. IMR-32 cells (2,500 cells per well) transfected with indicated vectors were mixed with top agar (0.33% agar/RPMI + 10% FBS) and were then plated on the bottom agar. The colonies were stained with crystal violet and counted 2 weeks later.
Luciferase Reporter Assay
The NeuroD1 promoter region (from −235 to +30) amplified from genomic DNA of HEK293 cells was cloned into pGL4.74 (Promega). pGL4.74-Mut was generated by site-directed mutagenesis replacing E-Box (CACGTG) with CGCGAC. The HEK293T cells (5 × 104 cells/well) were seeded in a 24-well plate and allowed to adhere overnight. HEK293T cells were transfected with pRL-CMV (Renilla) control reporter, pGL4.74-NeuroD1 or pGL4.74-Mut, MYCN expression vector, or control-expressing vector with FuGENE HD. Forty-eight hours after transfection, cells were lysed, and both Firefly and Renilla luciferase activities were measured with the Dual-Luciferase Reporter Assay system (Promega) according to the manufacturer’s instructions. The firefly luminescence signals were normalized by those of Renilla.
Statistical Analysis
Statistical analyses were performed using GraphPad software (GraphPad Software, San Diego, CA, USA). The p value was calculated using a Student’s t-test, and results are presented as the mean ± standard deviation (SD).
RESULTS
NeuroD1 Expression Is Closely Associated With MYCN Expression in NB
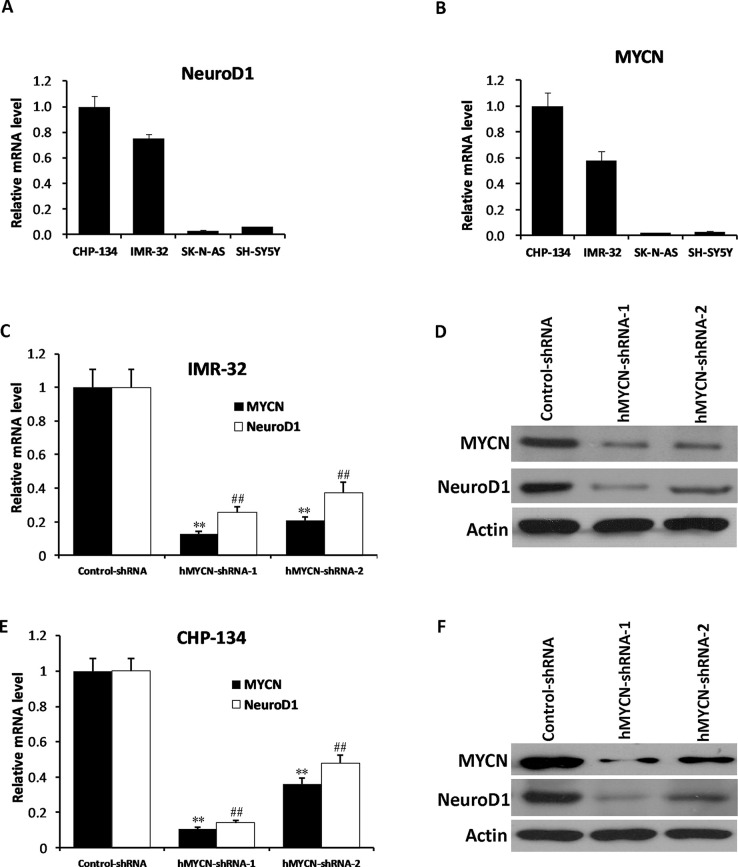
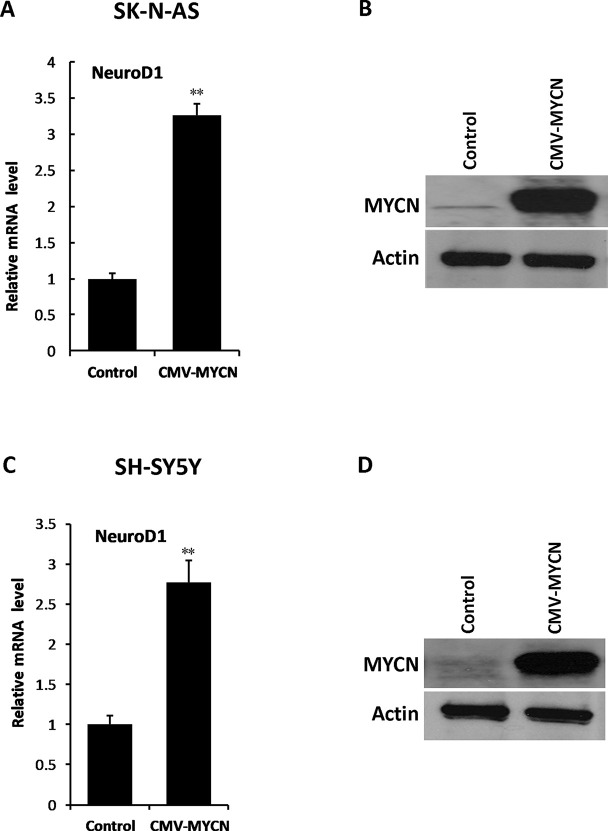
To identify the relationship between MYCN and NeuroD1, we initially examined the mRNA expression levels of MYCN and NeuroD1 in the CHP-134, IMR-32, SK-N-AS, and SH-SY5Y cell lines. The results showed that NeuroD1 was highly expressed in CHP-134 and IMR-32 cells (MYCN amplified cell lines), while it was lowly expressed in SK-N-AS and SH-SY5Y cells (non-MYCN amplified cell lines) (Fig. 1A and B), suggesting that there might be an underlying connection between these two genes in NB. In order to investigate the relationship between MYCN and NeuroD1, we performed knockdown experiments with two different shRNAs targeting MYCN. CHP-134 and IMR-32 cells were infected with control shRNA or MYCN shRNA-containing lentivirus, respectively. Twenty-four hours after infection, puromycin (final concentration: 2 μg/ml) was added to the cell culture medium for achieving the shRNA-positive cells. At day 3, total RNA and protein were extracted, and qPCR and Western blot analysis were performed, respectively. The results showed that MYCN shRNAs significantly suppressed MYCN expression at both the mRNA and protein levels in IMR-32 cells and CHP-134 cells, and the expression level of NeuroD1 was decreased as well (Fig. 1C–F). To further investigate the relationship between MYCN and NeuroD1, an MYCN-expressing vector was used to infect SK-N-AS and SH-SY5Y cells, which are nonamplified MYCN NB cell lines. Subsequently, the expression of NeuroD1 was significantly increased along with elevation of MYCN expression (Fig. 2A–D). These results suggest that NeuroD1 might be a potential downstream factor of MYCN in NB cells.
Figure 1.
Knockdown of MYCN results in reduced NeuroD1 expression in neuroblastoma (NB) cell lines. (A, B) The mRNA expression levels of MYCN and NeuroD1 were detected by quantitative polymerase chain reaction (qPCR) in CHP-134, IMR-32, SK-N-AS, and SH-SY5Y cell lines. (C–F) IMR-32 and CHP-134 cells were infected with control short hairpin RNA (shRNA) and two independent MYCN shRNAs. The mRAN levels of MYCN and NeuroD1 were examined by qPCR. The results represent the mean ± standard deviation (SD) (n = 3). **p < 0.001 versus the control group (MYCN), ##p < 0.001 versus the control group (NeuroD1) (C, E). The protein levels of MYCN and NeuroD1 were examined by Western blotting. Actin was used as the loading control (D, F).
Figure 2.
Overexpression of MYCN enhances the expression of NeuroD1 in NB cell lines. (A, C) SK-N-AS cells or SH-SY5Y cells were infected with MYCN expression vector or control-expressing vector for 3 days. The qPCR analysis was performed to detect the expression level of NeuroD1. The results represent the mean ± SD (n = 3). **p < 0.001 versus the control group. (B, D) The protein level of MYCN was examined by Western blotting.
There Was a Strong Correlation Between MYCN and NeuroD1 Expression in Clinical Cases of NB
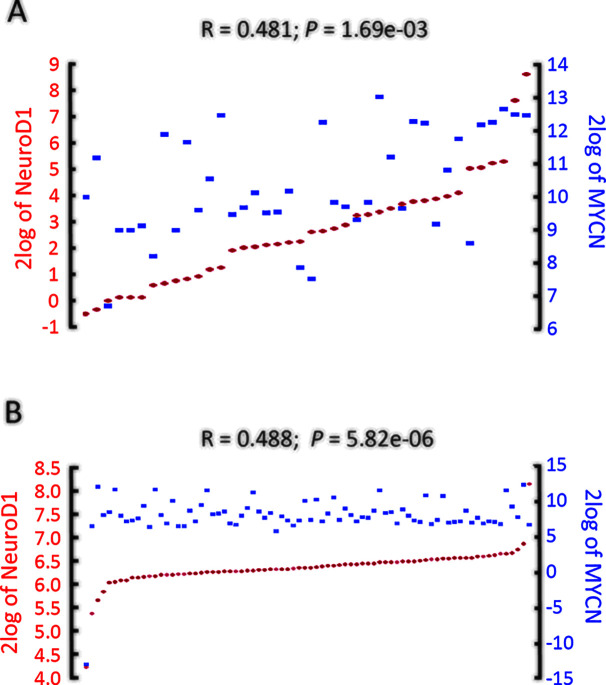
To determine whether the in vitro expression data correlated with the clinical features of human NB, we examined two datasets (Versteeg-88 and Jagannathan-100 datasets) of clinical NB tumor samples from the R2 platform, which is a publicly accessible web-based genomics analysis and visualization platform (http://r2.amc.nl), allowing researchers to work on larger datasets. In these datasets, we found that the expression of MYCN was strongly positively related to NeuroD1 expression (Versteeg-88, inss-st4, n = 40, r = 0.481, p = 1.69e−3; Jagannathan-100, inss-st4, n = 78, r = 0.488, p = 5.82e−6) (Fig. 3A and B), which is consistent with the results from the in vitro evaluations.
Figure 3.
NeuroD1 expression is tightly associated with MYCN in NB patients (http://r2.amc.nl). (A, B) MYCN expression was highly correlated with that of NeuroD1 (Versteeg-88, inss-st4, n = 40, r = 0.481, p = 1.69e−3; Jagannathan-100, inss-st4, n = 78, r = 0.488, p = 5.82e−6).
The Inhibition of Cell Growth Following MYCN Knockdown Can Be Restored by the Exogenous NeuroD1 Expression in NB
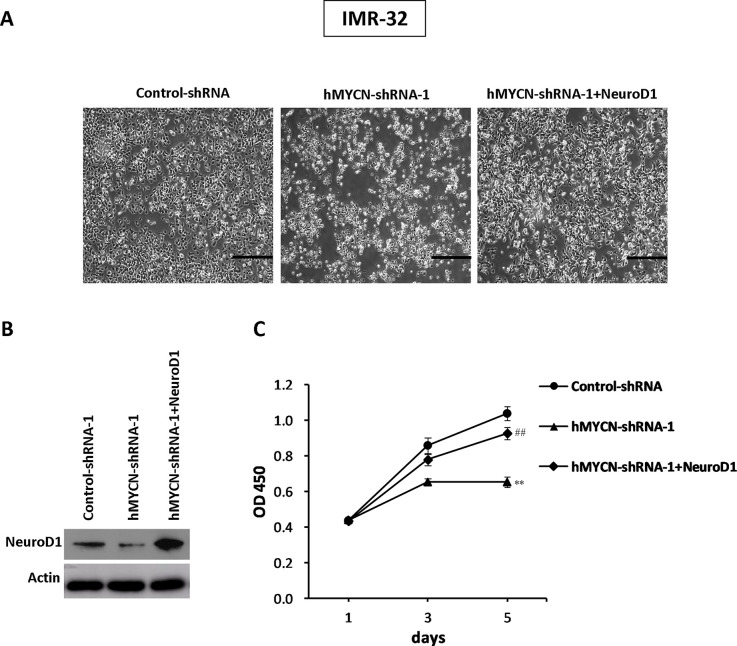
We further investigated whether NeuroD1 could rescue the phenotype induced by MYCN knockdown in NB cells. IMR-32 cells were infected with control shRNA, MYCN shRNA-1, or MYCN shRNA-1 plus NeuroD1-expressing vector containing lentivirus, and then the cell proliferation assays were performed at indicated time points. The results showed that the cells treated with MYCN shRNA showed inhibited cell growth, while exogenous expression of NeuroD1 was sufficient to restore cellular proliferation in these cells (Fig. 4A–C). Moreover, we then conducted a soft agar colony formation assay (Fig. 5A). As expected, the decreased efficiency of cell colony formation by MYCN shRNA treatment was successfully rescued by exogenous expression of NeuroD1 (Fig. 5B and C), suggesting that NeuroD1 could be a significant downstream factor in MYCN signaling pathway, making it critical to the characteristic increase in cellular proliferation associated with NB.
Figure 4.
Overexpression of NeuroD1 can restore the efficiency of cell growth in response to MYCN inhibition in NB cell lines. (A) IMR-32 cells were infected with control shRNA, MYCN shRNA-1, or MYCN shRNA-1 plus NeuroD1-expressing vector containing lentivirus. The images were captured at day 5 after infection. (B) The protein level of NeuroD1 was examined by Western blotting. (C) Cell counting kit 8 (CCK-8) assay was performed to detect cellular proliferation. The results represent the mean ± SD (n = 3). **p < 0.001 versus the control group; ##p < 0.001 versus the MYCN shRNA-1 group.
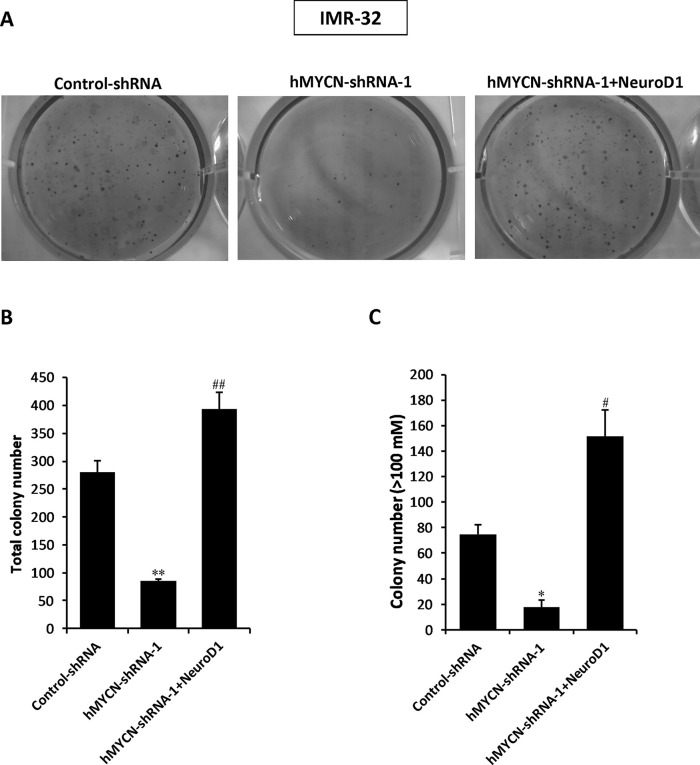
Figure 5.
Overexpression of NeuroD1 restores cell colony formation following MYCN knockdown in NB cell lines. (A) Soft agar colony formation assays were performed using IMR-32 cells infected with control shRNA, MYCN shRNA-1, or MYCN shRNA-1 plus NeuroD1-expressing vector containing lentivirus. (B, C) The total colony number and colony number (>100 mM) were counted and compared to evaluate cell colony-forming capacity. The results represent the mean ± SD (n = 3). *p < 0.01, **p < 0.001 versus the control shRNA group. #p < 0.01, ##p < 0.001 versus MYCN shRNA-1 group.
MYCN Regulates NeuroD1 Expression by Directly Binding to its Promoter Region
MYCN is a basic helix–loop–helix (bHLH) transcription factor that binds to E-box elements in the promoter regions of its target genes promoting various physiological functions such as cell cycle progression, apoptosis, and cellular transformation22. The presence of a canonical (CACGTG) E-box site is generally considered as an indication of MYCN regulation region, as the E-box is a high-affinity binding site for the MYCN protein. To evaluate whether MYCN directly binds to the NeuroD1 gene, we analyzed the sequence of the NeuroD1 promoter and found a CACGTG motif located within its promoter (Fig. 6A). We then performed a ChIP assay to check whether MYCN protein binds to the potential regulatory region in the human NeuroD1 gene. As shown in Figure 6B and C, the amount of amplified product precipitated by the anti-MYCN antibody was significantly increased in both IMR-32 and CHP-134 cells compared with those precipitated by the IgG antibody, suggesting that MYCN could bind to the promoter region of NeuroD1. To confirm functional regulation of the NeuroD1 promoter by MYCN protein, we carried out a luciferase reporter assay. In Figure 6D, the luciferase activity driven by the NeuroD1 promoter with the intact E-box was significantly higher than that with the mutated E-box. These results suggested that NeuroD1 was directly targeted by MYCN in NB, at least at this genomic site.
Figure 6.
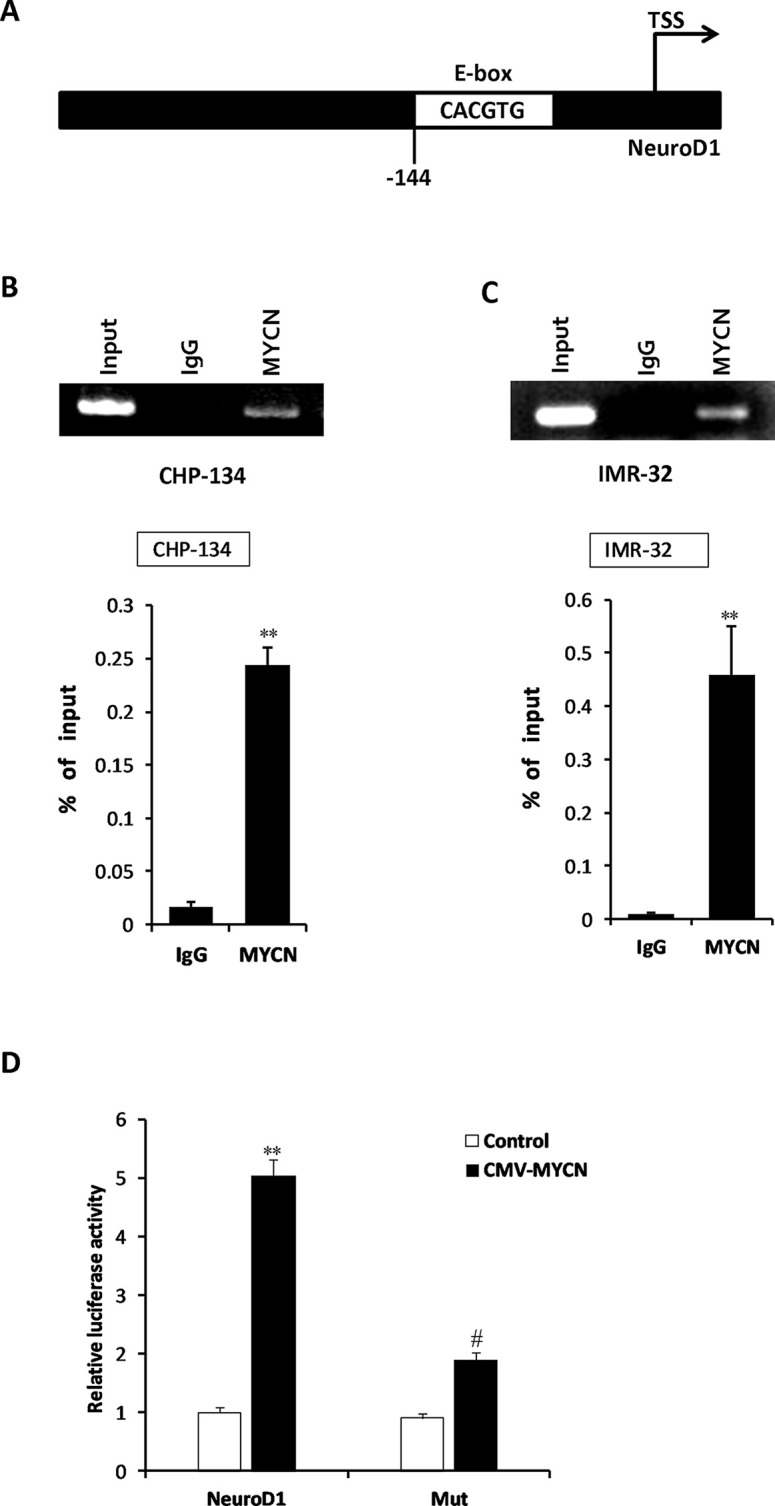
MYCN directly binds to the promoter regions of NeuroD1. (A) Schematic describing the MYCN responsive E-box (CACGTG) motif within the NeuroD1 gene promoter. TSS represents the transcription start site. (B, C) Chromatin immunoprecipitation (ChIP) assays were performed by using anti-MYCN antibodies in IMR-32 and CHP-134 cells, and an IgG antibody was used as the control. PCR was then performed to examine the bound DNA fragments. The results represent the mean ± SD (n = 3). **p < 0.001 versus the IgG group. (D) Human embryonic kidney (HEK) 293T cells were cotransfected with pRL-CMV (Renilla) control reporter vector, pGL4.74-NeuroD1 or pGL4.74-mut construct, MYCN expression vector, or control-expressing vector. The luciferase activity was measured and normalized by Renilla luminescence signals. The results represent the mean ± SD (n = 3). **p < 0.001 versus the control-expressing vector plus pGL4.74-NeuroD1-expressing group; #p < 0.01 versus the CMV-MYCN plus pGL4.74-NeuroD1-expressing group.
DISCUSSION
Since the discovery of NeuroD1 in 1995, it has continued to capture extensive interest, and its various functions have been gradually reviewed. To date, there have been two seemingly conflicting findings regarding the functions of NeuroD1. Firstly, the expression of NeuroD1 is involved in the development of the nervous and endocrine systems5,9. As a bHLH transcription factor, NeuroD1 plays an important role in embryonic neurogenesis of the central and peripheral nervous systems, where a lack of NeuroD1 results in a decrease in the number of cerebellar granule cells, sensory neuronal cells, and newborn neurons in the adult hippocampus and olfactory bulb3,8,23. During neurogenesis, NeuroD1 is involved in promoting neural progenitor cell self-renewal and differentiation3. Secondly, NeuroD1 is also considered an oncogene that promotes cellular proliferation and motility and is associated with poor prognosis in NB11. In addition, knockdown of NeuroD1 causes growth suppression in tumor spheres, which are considered to be tumor-initiating cells24, suggesting that the function of NeuroD1 is critically linked to maintaining the stemness properties of NB cells.
In approximately 25% of human NB, the amplification of the MYCN oncogene and the overexpression of MYCN protein are closely related to the aggressive progression of tumors and poor prognosis in patients25,26. As a member of the Myc family of basic helix–loop zipper transcription factors, MYCN has a variety of physiological and pathological functions associated with the occurrence and development of NB. It has been reported that targeted MYCN overexpression in the sympathetic neurons can initiate NB in mouse models27. The transcriptional induction of the MYCN target genes is the key mechanism underlying the excessive growth of MYCN-amplified NB. However, despite many achievements, the molecular mechanism underlying the link between MYCN and the aggressive progression of NB has not been fully elucidated. In this study, we demonstrated that MYCN directly targets NeuroD1 and induces the expression of NeuroD1, which may contribute to the increase in NeuroD1 expression in NB.
A comprehensive understanding of peripheral nervous system development, especially sympathetic neuron development, is required to understand the underlying molecular mechanisms supporting NB tumorigenesis3. During the progression of the peripheral nervous system, neural crest cells, which experience both migration and differentiation, give rise to various types of neuronal cells regulated by multiple bHLH transcription factors in a cascading manner28. The expression of MYCN promotes ventral migration and neuronal differentiation in neural crest cells19,20, while activation of NeuroD1 is necessary for developing sensory neurons that also originate from neural crest cells8,9. As both of these genes are essential for peripheral neurogenesis, further in vivo studies focusing on the links between these two genes are required. These studies may highlight NeuroD1 as an important therapeutic target downstream of MYCN and provide a potential clue for investigating tumorigenesis in NB.
ACKNOWLEDGMENT
This work was supported by the Liaoning Provincial Natural Science Foundation of China (2019-ZD-0323 and 2020-MS-309).
Footnotes
The authors declare no conflicts of interest.
REFERENCES
- 1. Johnsen JI, Dyberg C, Wickström M. 2019. Neuroblastoma—A neural crest derived embryonal malignancy. Front Mol Neurosci. 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benish BM. 1975. Letter: The neurocristopathies: A unifying concept of disease arising in neural crest development. Hum Pathol. 6(1):128. [DOI] [PubMed] [Google Scholar]
- 3. Nakagawara A, Ohira M. 2004. Comprehensive genomics linking between neural development and cancer: Neuroblastoma as a model. Cancer Lett. 204:213–224 [DOI] [PubMed] [Google Scholar]
- 4. Newman EA, Abdessalam S, Aldrink JH, Austin M, Heaton TE, Bruny J, Ehrlich P, Dasgupta R, Baertschiger RM, Lautz TB. 2019. Update on neuroblastoma. J Pediatr Surg. 54(3):383–389. [DOI] [PubMed] [Google Scholar]
- 5. Mutoh H, Fung BP, Naya FJ, Tsai MJ, Nishitani J, Leiter AB. 1997. The basic helix–loop–helix transcription factor BETA2/NeuroD is expressed in mammalian enteroendocrine cells and activates secretin gene expression. Proc Natl Acad Sci USA 94:3560–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Naya FJ, Stellrecht CM, Tsai MJ. 1995. Tissue-specific regulation of the insulin gene by a novel basic helix–loop–helix transcription factor. Genes Dev. 9:1009–1019. [DOI] [PubMed] [Google Scholar]
- 7. Lee JE, Hollenberg SM, Snider L, Turner D, Lipnick N, Weintraub H. 1995. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix–loop–helix protein. Science 268:836–844. [DOI] [PubMed] [Google Scholar]
- 8. Kim WY, Fritzsch B, Serls A, Bakel LA, Huang EJ, Reichardt LF, Barth DS, Lee JE. 2001. NeuroDnull mice are deaf due to a severe loss of the inner ear sensory neurons during development. Development 128:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu M, Pereira FA, Price SD, Chu MJ, Shope C, Himes D, Eatock RA, Brownell WE, Lysakowski A, Tsai MJ. 2000. Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev. 14:2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao Z, Ure K, Ables JL, Lagace DC, Nave K-A, Goebbels S, Eisch A J, Hsieh J. 2009. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci. 12:1090–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang P, Kishida S, Cao D, Tonami Y, Ping M, Nakaguro M, Koide N, Takeuchi I, Onishi A, Kadomatsu K. 2011. The neuronal differentiation factor NeuroD1 downregulates the neuronal repellent factor Slit2 expression and promotes cell motility and tumor formation of neuroblastoma. Cancer Res. 71:2938–2948. [DOI] [PubMed] [Google Scholar]
- 12. Lu F, Kishida S, Mu P, Huang P, Cao D, Tsubota S, Kadomatsu K. 2015. NeuroD1 promotes neuroblastoma cell growth by inducing the expression of ALK. Cancer Sci. 106:390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang J, Sui Y, Li Q, Zhao Y, Dong X, Yang J, Liang Z, Han Y, Tang Y, Ma J. 2020. Effective inhibition of MYC-amplified group 3 medulloblastoma by FACT-targeted curaxin drug CBL0137. Cell Death Dis. 11(12):1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Osborne JK, Larsen JE, Gonzales JX, Shames DS, Sato M, Wistuba II, Girard L, Minna JD, Cobb MH. 2013. NeuroD1 regulation of migration accompanies the differential sensitivity of neuroendocrine carcinomas to TrkB inhibition. Oncogenesis 2(8):e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boulay G, Awad ME, Riggi N, Archer TC, Iyer S, Boonseng WE, Rossetti NE, Naigles B, Rengarajan S, Volorio A, Kim JC, Mesirov JP, Tamayo P, Pomeroy SL, Aryee MJ, Rivera MN. 2017. OTX2 activity at distal regulatory elements shapes the chromatin landscape of group 3 medulloblastoma. Cancer Discov. 7:288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huang M, Weiss WA. 2013. Neuroblastoma and MYCN. Cold Spring Harb Perspect Med. 3(10):a014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otte J, Dyberg C, Pepich A, Johnsen JI. 2021. MYCN function in neuroblastoma development. Front Oncol. 10:624079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zimmerman KA, Yancopoulos GD, Collum RG, Smith RK, Kohl NE, Denis KA, Nau MM, Witte ON, Toran-Allerand D, Gee CE, Minna JD, Atl FW. 1986. Differential expression of myc family genes during murine development. Nature 319(6056):780–783. [DOI] [PubMed] [Google Scholar]
- 19. Wakamatsu Y, Watanabe Y, Nakamura H, Kondoh H. 1997. Regulation of the neural crest cell fate by N-myc: Promotion of ventral migration and neuronal differentiation. Development 124(10):1953–1962. [DOI] [PubMed] [Google Scholar]
- 20. Stanton BR, Perkins AS, Tessarollo L, Sassoon DA, Parada LF. 1992. Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 6(12A):2235–2247. [DOI] [PubMed] [Google Scholar]
- 21. Howard MJ. 2005. Mechanisms and perspectives on differentiation of autonomic neurons. Dev Biol. 277:271–286. [DOI] [PubMed] [Google Scholar]
- 22. Guccione E, Martinato F, Finocchiaro G, Luzi L, Tizzoni L, Dall’ Olio V, Zardo G, Nervi C, Bernard L, Amati B. 2006. Myc-binding-site recognition in the human genome is determined by chromatin context. Nat Cell Biol. 8:764–770. [DOI] [PubMed] [Google Scholar]
- 23. Miyata T, Maeda T, Lee JE. 1999. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 13:1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hansford LM, McKee AE, Zhang L, George RE, Gerstle JT, Thorner PS, Smith KM, Look AT, Yeger H, Miller FD, Irwin MS, Thiele CJ, Kaplan DR. 2007. Neuroblastoma cells isolated from bone marrow metastases contain a naturally enriched tumor-initiating cell. Cancer Res. 67:11234–11243. [DOI] [PubMed] [Google Scholar]
- 25. Brodeur GM. 2003. Neuroblastoma: Biological insights into a clinical 646 enigma. Nat Rev Cancer 3:203–216. [DOI] [PubMed] [Google Scholar]
- 26. Brodeur GM, Seeger RC, Schwab M, Varmus HE, Bishop JM. 1984. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224:1121–1124. [DOI] [PubMed] [Google Scholar]
- 27. Weiss WA, Aldape K, Mohapatra G, Feuerstein BG, Bishop JM. 1997. Targeted expression of MYCN causes neuroblastoma in transgenic mice. EMBO J. 16:2985–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jan YN, Jan LY. 1993. HLH proteins, fly neurogenesis, and vertebrate myogenesis. Cell 75:827–830. [DOI] [PubMed] [Google Scholar]