Abstract
Rhizobitoxine is synthesized by the legume symbiont Bradyrhizobium elkanii and the plant pathogen Burkholderia andropogonis. Rhizobitoxine competitively inhibited 1-aminocyclopropane-1-carboxylate (ACC) synthase bLE-ACS2 from the tomato, a key enzyme in the pathway of ethylene biosynthesis. Based on this inhibition of ACC synthase, we have developed a new assay for rhizobitoxine.
Rhizobitoxine [2-amino-4-(2-amino-3-hydropropoxy)-trans-but-3-enoic acid] is synthesized by the legume symbiont Bradyrhizobium elkanii (3, 5, 7, 8, 11, 15) and the plant pathogen Burkholderia andropogonis (9, 10). The bacterial toxin induces foliar chlorosis of the host plants; however, the role of the toxin in symbiosis and pathogenicity remains unclear. Enzymatic studies have revealed that rhizobitoxine inhibits β-cystathionase in the methionine biosynthetic pathway (2, 12, 22).
Owens et al. (13) have demonstrated that rhizobitoxine inhibits the production of ethylene by apple tissues by measuring the incorporation of 14C from 14C-labeled methionine into ethylene. 1-Aminocyclopropane-1-carboxylase (ACC) synthase (S-adenosyl-l-methionine methylthioadenosine-lyase, EC 4.4.1.14) is the rate-limiting enzyme in the biosynthesis of ethylene from methionine in higher plants (23). Aminoethoxyvinylglycine (AVG), a structural analogue of rhizobitoxine, has been used as an inhibitor in the enzymatic studies of ACC synthase, because AVG is commercially available. Therefore, rhizobitoxine is expected to be a potent inhibitor of ACC synthase. The latter may be a target enzyme for rhizobitoxine during symbiosis between B. elkanii and the host plant, but rhizobitoxine inhibition of ACC synthase has not yet been verified. Indeed, ethylene has been suggested to be a component of the signaling pathway controlling the rhizobial infection of legumes (1, 16, 17).
Assay methods for rhizobitoxine have included the scoring of chlorosis symptoms in host plants (11), use of an amino acid analyzer (5), and the inactivation of β-cystathionase partially purified from Salmonella typhimurium (12).
The objectives of this work are to examine whether rhizobitoxine is a potent inhibitor of ACC synthase and to develop a new method for assaying rhizobitoxine by inhibition of the enzyme.
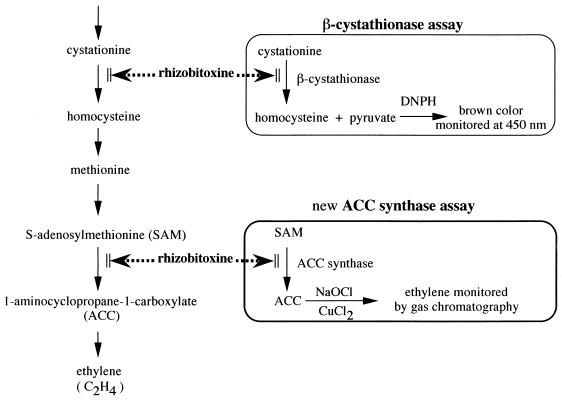
Two enzymatic assays for rhizobitoxine, the ACC synthase assay described in this work and the previously reported β-cystathionase assay (18), are schematically shown in Fig. 1. We used ACC synthase bLE-ACS2 purified from the fusion protein of cDNA from a ripe, wounded tomato pericarp as described previously (20). Rhizobitoxine and dihydrorhizobitoxine were purified from a culture of B. elkanii USDA 94 as described previously (8).
FIG. 1.
Rhizobitoxine inhibition of enzymes involved in the pathway of ethylene biosynthesis and two enzymatic assay systems for rhizobitoxine. The new ACC synthase assay is based on the inhibition of ACC synthase (tomato bLE-ACS2 expressed in E. coli) by rhizobitoxine. The β-cystathionase assay was reported previously by Ruan and Peters (18). DNPH, 2,4-dinitrophenylhydrazine.
ACC synthase activity was measured by incubating (30°C, 15 min) a reaction mixture containing ACC synthase, 100 μM S-adenosylmethionine (SAM), 50 μM pyridoxal phosphate, 100 μg of bovine serum albumin, 125 mM HEPES-KOH (pH 8.5), and various concentrations of inhibitors such as rhizobitoxine in a total volume of 0.4 ml. The amount of ACC formed was determined by the methods of Lizada and Yang (4).
In practice, 50 μl of 0.8 M HEPES-KOH buffer (pH 8.9), 50 μl of 40 μM pyridoxal phosphate, 50 μl of 0.02% bovine serum albumin, 50 μl of sterile water, 50 μl of ACC synthase preparation (9 U), 100 μl of sample solution, and 50 μl of 0.8 mM SAM in 0.001 N sulfate solution were sequentially pipetted into a blood sampling vacuum tube (5 ml; TERUMO, Tokyo, Japan) on ice. After incubation (30°C, 15 min), the reaction was stopped by adding 100 μl of 20 mM CuCl2 and cooling the tube in an ice water bath. Upon the addition of 100 μl of NaOCl reagent (5% sodium hypochlorite solution–saturated NaOH solution, 1:1 [vol/vol]), to convert ACC into ethylene, a rubber stopper was immediately placed on the tube. After 5 min of incubation on ice, the gas phase (1.0 ml) was sampled and injected into a gas chromatograph (Shimadzu CG-7A) equipped with a Porapack R column (internal diameter, 2.2 mm; length, 2 m) and a flame ionization detector in order to determine ethylene concentration.
The β-cystathionase assay was carried out according to the method of Ruan and Peters (18), with the exception that the crude enzyme preparation of β-cystathionase was isolated from a 1-liter culture of Escherichia coli K-12 grown in M9 medium at 37°C for 20 h.
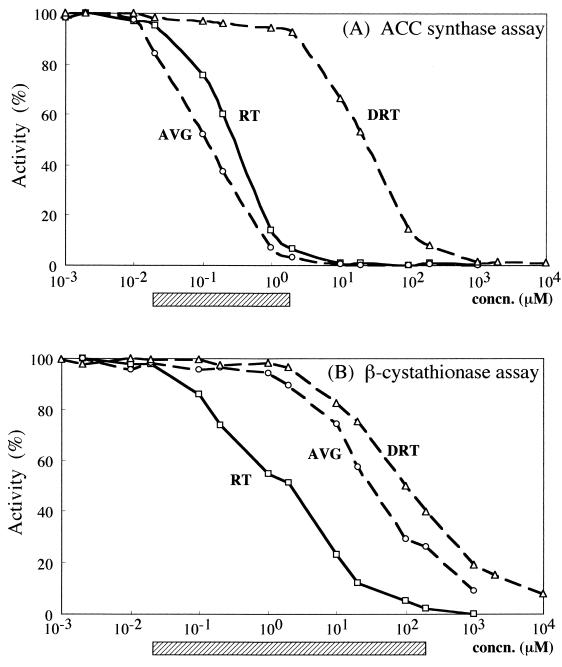
Rhizobitoxine strongly inhibited ACC synthase activity (Fig. 2A). Inhibition was detectable in the presence of 0.02 μM rhizobitoxine, and 93% of the activity was inhibited at 2 μM rhizobitoxine. AVG was approximately 1.7 times more effective than rhizobitoxine as an inhibitor of ACC synthase.
FIG. 2.
Inhibition of ACC synthase (bLE-ACS2) (A) and β-cystathionase from E. coli K-12 (B) by rhizobitoxine (RT), dihydrorhizobitoxine (DRT), and AVG. Enzymatic activities are expressed as percentages of activities in the absence of inhibitors, which were 6.28 nmol of ACC produced/h for the ACC synthase assay (A) and 50 nmol of pyruvate produced/h for the β-cystathionase assay (B). Each striped box indicates the range of rhizobitoxine concentrations that can be determined by the respective assay system. Concentrations of rhizobitoxine, AVG, and dihydrorhizobitoxine are shown in sample solutions (100 μl for the ACC synthase assay and 50 μl for the β-cystathionase assay).
Dihydrorhizobitoxine [O-(2-amino-3-hydroxypropyl) homoserine] was thought to be a possible precursor of rhizobitoxine (14, 19) and was always detected in soybean nodules inoculated with a rhizobitoxine-producing B. elkanii strain (5, 6). However, dihydrorhizobitoxine was approximately 100-fold less potent as an inhibitor of ACC synthase than rhizobitoxine (Fig. 2A), indicating that inhibition of ACC synthase depended mainly on rhizobitoxine even when dihydrorhizobitoxine was mixed with rhizobitoxine in a sample solution.
Rhizobitoxine inhibition of β-cystathionase isolated from E. coli K-12 (Fig. 2B) was observed at concentrations as low as 0.1 μM, and 95% inhibition occurred at about 100 μM rhizobitoxine. The E. coli K-12 enzyme appeared to be three times less sensitive to rhizobitoxine than β-cystathionase from S. typhimurium (18). Considering the goal of developing an assay for rhizobitoxine, we note that the rhizobitoxine inhibition of the ACC synthase showed a sharper response than that of the β-cystathionase from E. coli (Fig. 2).
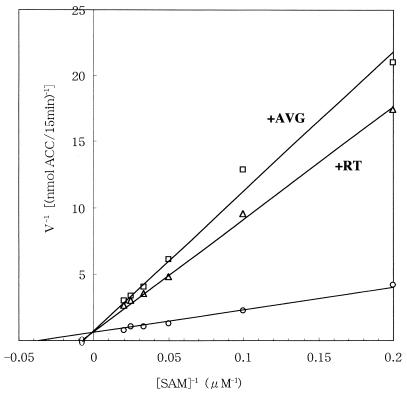
Figure 3 shows double-reciprocal plots of ACC synthase (bLE-ACS2) activity at various SAM concentrations (5 to 50 μM) in the absence or presence of 0.1 μM rhizobitoxine and 0.1 μM AVG. Inhibition of ACC synthase by rhizobitoxine and AVG was competitive with respect to SAM. The Km for SAM was calculated to be 29 μM, which corresponded to the value in previous reports (20, 21). On the other hand, an inhibition constant (Ki) for rhizobitoxine was very low (0.025 μM), indicating that rhizobitoxine is a strong inhibitor of ACC synthase bLE-ACS2 in terms of an inhibition constant. The Ki for AVG was also significantly lower (Ki = 0.019 μM) than those in previous reports (Ki = 0.25 to 2.9 μM) (21), which might be due to differences between dimeric and monomeric molecular forms of ACC synthase (21).
FIG. 3.
Inhibition of ACC synthase activity by AVG and rhizobitoxine (RT). ACC synthase was incubated with SAM (5 to 50 μM)–5 μM pyridoxal phosphate–0.1 M HEPES-KOH (pH 8.5) in the absence (○) or presence of 0.1 μM AVG (□) or 0.1 μM rhizobitoxine (▵) for 15 min at 30°C. From the Lineweaver-Burk plot, the Km for SAM was 29 μM, and the Ki values for AVG and rhizobitoxine were 0.019 and 0.025 μM, respectively.
Bradyrhizobium- and soybean-derived β-cystathionases were reportedly less sensitive to rhizobitoxine than β-cystathionase from Enterobacteriaceae, including E. coli (22). For example, β-cystathionase from the rhizobitoxine-sensitive soybean cultivar Forrest and from the rhizobitoxine-producing B. elkanii strain USDA 94 had inhibition constants (Ki = 1,048 and 22 μM, respectively) significantly higher than that of β-cystathionase from E. coli DH52 (Ki = 3 μM). However, rhizobitoxine strongly inhibited ACC synthase bLE-ACS2 activity with a low Ki (Ki = 0.025 μM) (Fig. 2 and 3). Therefore, it is possible that ACC synthase is a target enzyme of rhizobitoxine, although the enzyme source, bLE-ACS2, was not derived from legumes. One could argue that rhizobitoxine-induced chlorosis and necrosis in leaves, and necrosis in roots and nodules, of host plants are due to inhibition of ACC synthase rather than β-cystathionase.
Using the ACC synthase assay, we determined the rhizobitoxine level in a stationary-phase culture (8 days) of B. elkanii USDA94 growth in Tris-YMRT medium (8). When the culture supernatant and 10- and 100-fold dilutions (with the medium) thereof were assayed, the two dilutions yielded the same values for rhizobitoxine concentrations in the original culture supernatant (Table 1). This indicated the utility of the ACC synthase assay for rhizobitoxine.
TABLE 1.
Determination of rhizobitoxine concentration in culture supernatant of B. elkanii USDA 94
| Culture and dilu-tiona | Ethylene pro-duced from ACC (nl/ml) | ACC produced (pmol/15 min)b | Relative activity (%) | Rhizobitoxine concn (μM) of original supernatant |
|---|---|---|---|---|
| Control | 7.06 | 1,570 | 100 | |
| USDA 94 | ||||
| 100 | 4.62 | 1,030 | 65 | 17 |
| 10 | 0.56 | 126 | 8.0 | 17 |
| 1 | 0.092 | 20.4 | 1.3 | NCc |
The control was Tris-YMRT medium, and culture supernatant of B. elkanii USDA 94 was diluted with this medium (8).
In an enzyme reaction mixture at 30°C during 15 min.
NC, not calculated because the activity was beyond the calibration curve for rhizobitoxine determination.
Rhizobitoxine is synthesized not only by the symbiotic bacterium B. elkanii (3, 5, 7, 8, 11, 15) but also by the broad-host-range plant pathogen B. andropogonis (9, 10). Moreover, rhizobitoxine-analogous AVG and methoxyvinylglycine have been known to be produced by a Streptomyces sp. and Pseudomonas aeruginosa (19). These compounds are considered to be potent inhibitors of ACC synthase. Indeed, AVG was comparable to rhizobitoxine in terms of ACC synthase inhibition (Fig. 2 and 3). These facts give rise to the interesting idea that both symbiotic and pathogenic bacteria might produce ACC synthase-inhibiting compounds which are structurally and enzymologically similar to rhizobitoxine so as to control the ethylene-induced plant responses that would prevent a successful infection. This new ACC synthase assay will be also useful for surveying these compounds synthesized by plant-associated bacteria as well as for rhizobitoxine determination.
Acknowledgments
This work was supported in part by a grant from the Joint Research Program of the Institute of Genetic Ecology, Tohoku University (981002).
REFERENCES
- 1.Fearn J C, Larue T A. Ethylene inhibitors restore nodulation to Sym5 mutants of Pisum sativum L. cultivar Sparkle. Plant Physiol (Rockville) 1991;96:239–244. doi: 10.1104/pp.96.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giovanelli J, Owens L D, Mudd S H. Mechanism of inhibition of spinach β-cystathionase by rhizobitoxine. Biochim Biophys Acta. 1971;227:671–684. doi: 10.1016/0005-2744(71)90016-7. [DOI] [PubMed] [Google Scholar]
- 3.Kuykendall L D, Saxena B, Devine T E, Udell S E. Genetic diversity in Bradyrhizobium japonicum Jordan 1982 and a proposal for Bradyrhizobium elkanii sp. nov. Can J Microbiol. 1992;38:501–505. [Google Scholar]
- 4.Lizada M C C, Yang S F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylate synthase protein in wounded tomato fruit tissue. Anal Biochem. 1979;100:140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- 5.Minamisawa K, Kume N. Determination of rhizobitoxine and dihydrorhizobitoxine in soybean plants by amino acid analyzer. Soil Sci Plant Nutr. 1987;33:645–649. [Google Scholar]
- 6.Minamisawa K. Comparison of extracellular polysaccharide composition, rhizobitoxine production, and hydrogenase phenotype among various strains of Bradyrhizobium japonicum. Plant Cell Physiol. 1989;30:877–884. [Google Scholar]
- 7.Minamisawa K. Division of rhizobitoxine-producing and hydrogenase positive strains of Bradyrhizobium japonicum by nifDKE sequence divergence. Plant Cell Physiol. 1990;31:81–89. [Google Scholar]
- 8.Minamisawa K, Fukai K, Asami T. Rhizobitoxine inhibition of hydrogenase synthesis in free-living Bradyrhizobium japonicum. J Bacteriol. 1990;172:4505–4509. doi: 10.1128/jb.172.8.4505-4509.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitchell R E, Frey E J, Benn M K. Rhizobitoxine and 1-threohydroxythreonine production by the plant pathogen Pseudomonas andropogonis. Phytochemistry. 1986;25:2711–2715. [Google Scholar]
- 10.Mitchell R E, Frey E J. Rhizobitoxine and hydroxythreonine production by Pseudomonas andropogonis strains, and the implications to plant disease. Physiol Mol Plant Pathol. 1988;32:355–341. [Google Scholar]
- 11.Owens L D, Wright D A. Rhizobial-induced chlorosis in soybeans: isolation, production in nodules, and varietal specificity of the toxin. Plant Physiol (Rockville) 1965;40:927–930. doi: 10.1104/pp.40.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Owens L D, Guggenheim S, Hilton J L. An inhibitor of β-cystathionase in Salmonella typhimurium. Biochim Biophys Acta. 1968;158:219–225. doi: 10.1016/0304-4165(68)90134-7. [DOI] [PubMed] [Google Scholar]
- 13.Owens L D, Liebermann M, Kunishi A. Inhibition of ethylene production by rhizobitoxine. Plant Physiol (Rockville) 1971;48:1–4. doi: 10.1104/pp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Owens L D, Thompson J F, Fennessy P V. Dihydrorhizobitoxine, a new ether amino-acid from Rhizobium japonicum. J Chem Soc Chem Commun. 1972;1972:715. [Google Scholar]
- 15.Owens L D, Thompson J F, Pitcher R G, Williams T. Structure of rhizobitoxine, an antimetabolic enol-ether amino-acid from Rhizobium japonicum. J Chem Soc Chem Commun. 1972;1972:714. [Google Scholar]
- 16.Penmetsa R V, Cook D R. A legume ethylene-insensitive mutant hyperinfected by its rhizobial symbiont. Science. 1997;275:527–530. doi: 10.1126/science.275.5299.527. [DOI] [PubMed] [Google Scholar]
- 17.Peters N K, Crist-Estes D K. Nodule formation is stimulated by the ethylene inhibitor aminoethoxyvinylglycine. Plant Physiol (Rockville) 1989;91:690–693. doi: 10.1104/pp.91.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruan X, Peters N K. Rapid and sensitive assay for the phytotoxin rhizobitoxine. Appl Environ Microbiol. 1991;57:2097–2100. doi: 10.1128/aem.57.7.2097-2100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan X, Zhang C, Peters N K. Bradyrhizobium japonicum rhizobitoxine genes and putative enzyme functions: expression requires a translational frameshift. Proc Natl Acad Sci USA. 1993;90:2641–2645. doi: 10.1073/pnas.90.7.2641. . (Author’s correction, 90:12055.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh S, Mori H, Imaseki H. Monomeric and dimeric forms and the mechanism-based inactivation of 1-aminocyclopropane-1-carboxylate synthase. Plant Cell Physiol. 1993;34:753–760. [Google Scholar]
- 21.Satoh S, Shang F Y. S-Adenosylmethionine-dependent inactivation and radiolabeling of 1-aminocyclopropane-1-carboxylate synthase isolated from tomato. Plant Physiol (Rockville) 1988;88:109–114. doi: 10.1104/pp.88.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiong K, Fuhrmann J J. Comparison of rhizobitoxine-induced inhibition of β-cystathionase from different bradyrhizobia and soybean genotypes. Plant Soil. 1996;186:53–61. [Google Scholar]
- 23.Yang S F, Hoffman N E. Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol. 1984;35:155–189. [Google Scholar]