Abstract
In cells of Rhodococcus opacus GM-14, GM-29, and 1CP, the contents of branched (10-methyl) fatty acids increased from 3% to 15 to 34% of the total fatty acids when the cells were grown on benzene, phenol, 4-chlorophenol, chlorobenzene, or toluene as the sole source of carbon and energy, in comparison with cells grown on fructose. In addition, the content of trans-hexadecenoic acid increased from 5% to 8 to 18% with phenol or chlorophenol as the carbon source. The 10-methyl branched fatty acid content of R. opacus GM-14 cells increased in a dose-related manner following exposure to phenol or toluene when toluene was not utilized as the growth substrate. The results suggest that 10-methyl branched fatty acids may participate in the adaptation of R. opacus to lipophilic aromatic compounds.
Aromatic hydrophobic compounds are toxic to bacteria due to their high partition into the membrane (15, 16). A change in the degree of saturation of cellular fatty acids is a well-known reaction of bacteria to the presence of membrane-active compounds (2, 8). Another adaptation response is the isomerization of cis unsaturated fatty acids to the trans form, which has been described for several Pseudomonas strains (4, 5, 11, 20).
The influence of organic solvents on the cellular fatty acid composition described above has been shown for gram-negative bacteria. The effect of aromatic compounds on the fatty acid composition of nocardioform bacteria, particularly those that are able to utilize high concentrations of such compounds, has not been studied intensively.
We studied the response of the cellular fatty acid composition of Rhodococcus strains to the presence of aromatic compounds.
Three bacterial strains were used in this study. Rhodococcus opacus GM-14 grew in mineral medium on benzene or chlorobenzene as the sole carbon and energy source when substrates were added in the liquid phase (21). New isolate GM-29 was obtained from an enrichment culture with toluene as the sole carbon source. It grew in saturated aqueous solutions of toluene and benzene when the substrates were added at amounts of up to 7 g liter−1. Strain 1CP, which degrades 4-chlorophenol and 2,4-dichlorophenol, was isolated by Gorlatov et al. (3) in 1994 and, based on phenotypic characteristics, was identified as R. erythropolis. As determined by 16S rRNA gene sequences strains GM-29 and 1CP belong to the species R. opacus. Data on 16S rRNA gene sequences are available from the EMBL database. The accession numbers are Y11892 and Y11893 for strains GM-29 and 1CP, respectively (14).
Bacteria were cultivated in 1-liter flasks with 200 ml of mineral KSN medium (21) on a gyratory shaker at 28°C. Aromatic compounds were added directly to the culture medium. The cells were grown to early stationary phase, harvested by filtration (Supor-450; 0.2-μm pore size), and washed twice with mineral medium. The fatty acids were isolated from 50 to 60 mg of wet cells by direct saponification. Fatty acid methyl esters (FAME) were analyzed by gas chromatography (GC)-mass spectrometry with an HP 6890A gas chromatograph equipped with an HP 5972A mass selective detector (Hewlett-Packard Co., Palo Alto, Calif.) and an HP-Ultra 2 cross-linked 5% phenyl methyl silicone capillary column (25 m by 0.2 mm; 0.33 μm). The oven temperature was programmed with injection and a 1-min hold at 80°C, followed by an increase to 160°C at 60°C min−1, a hold at 160°C for 28 min, and an increase at 5°C min−1 to 230°C. Individual FAME were identified by comparing their mass spectra with those in the Wiley 138K mass spectral database. The cis and trans isomers of hexadecenoic and octadecenoic acids were verified by comparison with the retention times of authentic standards from the Even Unsaturated Fatty Acid Methyl Esters Kit (Analabs, North Haven, Conn.). The fatty acid content of cells was calculated as the average of three independent cultivations; the standard deviation was less than 7%.
All three strains changed their fatty acid composition when grown on aromatic compounds as the sole sources of carbon and energy, in comparison to cells grown on fructose (Table 1).
TABLE 1.
Whole-cell fatty acid compositions of R. opacus GM-14, GM-29, and 1CP grown in KSN medium with different compounds as the sole carbon sources
| Fatty acid(s) | Relative content (%) of fatty acid(s) in cells of strain:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GM-14
|
GM-29
|
1CP
|
||||||||||
| Fructosea | Phenolb | 4-Chloro-phenolc | Benzenea | Chloro-benzeneb | Fructosea | Phenolb | Benzenea | Toluenea | Fructosea | Phenolb | 4-Chloro-phenolc | |
| 9:0-13:0 | 0.4 | 1.1 | 0.8 | 2.4 | 4.1 | 0.7 | 0.8 | 3.7 | 3.6 | 0.8 | 0.7 | 0.6 |
| 14:0 | 2.6 | 2.9 | 2.7 | 5.8 | 4.8 | 3.5 | 2.5 | 4.8 | 6.4 | 3.0 | 1.9 | 2.1 |
| 15:0 | 5.9 | 12.2 | 9.4 | 18.3 | 16.6 | 3.2 | 10.5 | 18.7 | 11.0 | 3.6 | 5.2 | 7.2 |
| 16:0 | 30.1 | 23.1 | 23.1 | 27.2 | 21.6 | 31.9 | 25.0 | 20.9 | 30.8 | 31.7 | 19.7 | 20.7 |
| 17:0 | 4.8 | 7.4 | 7.1 | 10.5 | 8.6 | 1.8 | 8.6 | 8.5 | 6.5 | 3.6 | 5.0 | 7.0 |
| 18:0 | 4.4 | 2.5 | 2.8 | 4.0 | 3.6 | 1.7 | 2.7 | 2.6 | 2.4 | 3.5 | 1.3 | 2.1 |
| 19:0 | <0.5 | 0.8 | 1.6 | 2.7 | 1.7 | <0.5 | 0.8 | 1.6 | 1.3 | <0.5 | 1.3 | 1.3 |
| Minor saturated | <0.5 | NDd | 1.0 | 1.7 | 1.6 | ND | <0.5 | 1.4 | 1.0 | ND | 0.9 | 1.8 |
| Sum of straight saturated | 48.8 | 49.9 | 48.5 | 72.6 | 62.6 | 42.9 | 51.1 | 62.1 | 63.0 | 46.5 | 35.9 | 42.8 |
| 16:1 (ω 7c) | 7.9 | 2.3 | 0.9 | 1.0 | 0.9 | 15.5 | 3.9 | 1.7 | 4.5 | 8.8 | 1.3 | 1.4 |
| 16:1 (ω 7t) | 4.8 | 8.3 | 10.8 | 2.4 | 3.7 | 5.2 | 5.3 | 4.3 | 2.9 | 4.8 | 17.5 | 10.4 |
| 17:1 (ω 8c) | 13.1 | 9.8 | 4.2 | 2.4 | 2.4 | 8.5 | 7.6 | 5.0 | 5.2 | 8.9 | 5.2 | 1.9 |
| 17:1 (ω 5c) | ND | 0.8 | 1.8 | 0.1 | 0.9 | ND | 0.6 | 0.9 | <0.5 | ND | 2.9 | 2.8 |
| 18:1 (ω 9c) | 20.0 | 7.3 | 4.8 | 2.7 | 3.6 | 22.5 | 7.9 | 2.2 | 6.5 | 25.0 | 4.9 | 2.2 |
| 19:1 | 0.8 | 2.2 | 1.8 | 3.7 | 5.9 | <0.5 | 2.0 | 3.7 | 2.9 | 0.7 | 1.2 | 1.3 |
| Minor unsaturated | 1.7 | 3.5 | 3.2 | 2.1 | 2.7 | 1.3 | 1.9 | 2.7 | 3.1 | 2.1 | 2.3 | 2.9 |
| Sum of unsaturated | 48.8 | 34.2 | 27.6 | 14.3 | 20.1 | 54.2 | 29.5 | 20.4 | 25.5 | 50.3 | 35.3 | 22.9 |
| 10-Me-16:0 | <0.5 | 1.2 | 0.8 | 1.6 | 1.9 | 0.5 | 3.0 | 2.6 | 1.7 | 0.4 | 0.3 | 3.2 |
| 10-Me-17:0 | 0.9 | 6.4 | 6.4 | 3.9 | 4.1 | 0.5 | 6.9 | 7.7 | 3.5 | 0.8 | 7.1 | 8.5 |
| 10-Me-18:0 | 1.3 | 6.9 | 14.2 | 5.2 | 8.3 | 1.9 | 9.3 | 5.5 | 5.3 | 2.0 | 18.5 | 17.5 |
| 10-Me-19:0 | ND | 0.9 | 2.8 | 1.9 | 2.8 | ND | <0.5 | 1.7 | 1.0 | ND | 2.9 | 5.1 |
| Sum of branched chain | 2.4 | 15.4 | 24.1 | 12.7 | 17.1 | 2.9 | 19.6 | 17.6 | 11.5 | 3.2 | 28.8 | 34.3 |
| Total | 100 | 99.5 | 100 | 99.6 | 99.8 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
The substrate concentration was 1.0 g liter−1.
The substrate concentration was 0.5 g liter−1.
The substrate concentration was 0.1 g liter−1.
ND, not detectable.
Hexadecanoic acid was the predominant saturated fatty acid, making up 30 to 32% of the total in fructose-grown cells and 20 to 27% of the total in cells grown on the aromatic substrates. The amounts of straight-chain saturated fatty acids in cells of GM-29, 1CP, and GM-14 grown on fructose ranged from 43 to 49% of the whole-cell fatty acids. Cells of strains GM-14 and GM-29 grown on benzene, chlorobenzene, or toluene, but not those grown on phenol or 4-chlorophenol, contained >60% straight-chain saturated fatty acids. In comparison with cells grown on fructose, those grown on the aromatic substrates had two- to threefold increased contents of odd-number carbon chain saturated fatty acids, mainly C15:0 and C17:0.
The most dramatic growth substrate-dependent change was the 3- to 10-fold increase of the branched-chain (10-methyl) fatty acids in cells grown at the expense of benzene derivatives. In strain 1CP, the amount of 10-methyl branched fatty acids increased from 3.1% in cells grown on fructose to 24.8 or 34.3% in cells grown on KSN medium with phenol or 4-chlorophenol as the carbon source, respectively. In all strains, the increase in the amount of 10-methyl branched fatty acids was greatest for 10-methyl-octadecanoic acid, and it occurred at the expense of unsaturated rather than saturated fatty acids.
Cells of R. opacus GM-14, GM-29, and 1CP contained a significant amount (5%) of trans-hexadecenoic acid (16:1 ω 7t). The content of the trans isomer was higher (8 to 18%) in cells of strains GM-14 and 1CP grown on phenol and 4-chlorophenol. Isomerization of the cis to the trans form as a response to exposure to toxic compounds has been described for Pseudomonas putida, which synthesizes fatty acids by the anaerobic pathway commonly utilized by gram-negative bacteria (4, 5, 11, 20). Moreover, the occurrence of trans fatty acid isomers was reported for a few gram-negative genera and Bacillus cereus, which use the anaerobic and the aerobic routes for fatty acid biosynthesis (9, 12).
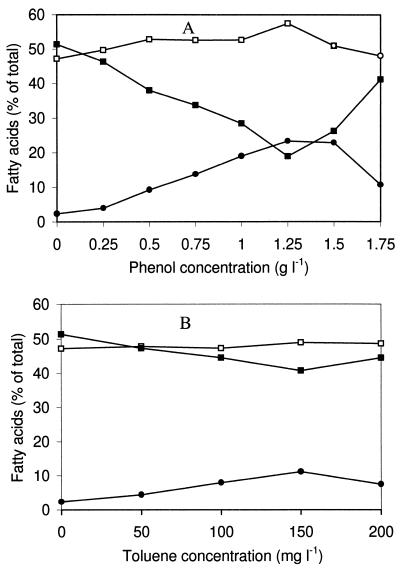
The dynamic changes in strain GM-14 fatty acid composition in response to exposure to toxic aromatic compounds are shown as an example in Fig. 1. The strain was grown in mineral medium containing fructose (1.0 g liter−1). Phenol or toluene was added in increasing concentrations to exponentially growing cultures (optical density at 540 nm [OD540], ≈0.3). The cultures were then grown for two generations and harvested.
FIG. 1.
Effects of different concentrations of phenol (A) and toluene (B) on the cellular fatty acid composition of R. opacus GM-14. Cells were grown in KSN medium containing fructose and various concentrations of phenol or toluene. Phenol or toluene added to an exponentially growing culture with an OD540 of ≈0.3. The biomass was allowed to grow for two doublings of the OD540 before harvesting. Symbols: ■, unsaturated fatty acids; □, straight-chain saturated fatty acids; •, 10-methyl branched fatty acids.
Strain GM-14 responded to increasing concentrations of phenol in the medium by replacing cis-unsaturated fatty acids with 10-methyl branched saturated fatty acids (Fig. 1A). There was little change in the proportions of saturated straight-chain fatty acids at phenol concentrations permissive for cell growth. While growing on fructose, the cells also used phenol. However, when phenol was added to the medium at concentrations of 0.5 to 1.25 g liter−1, the amount of phenol utilized by the cells remained constant at 0.3 to 0.4 g liter−1. This was verified by GC analysis.
The effect of toluene on the fatty acid composition of R. opacus GM-14 is shown in Fig. 1B. We chose this strain for display because it was not able to metabolize toluene (21) and the fatty acid changes therefore should reflect the response to the toxicity of the solvent. The cellular fatty acid composition changed with toluene in the growth medium similarly as with phenol: a dose-related increase in the cellular content of 10-methyl fatty acids was observed.
In summary, our study showed major changes in the whole-cell fatty acid compositions associated with the adaptation of R. opacus to the presence of aromatic solvents. Compared with cells grown in mineral medium on fructose, the cellular contents of 10-methyl branched fatty acids of the three R. opacus strains were 3- to 10-fold higher during growth on toxic aromatic compounds as sole carbon sources. Moreover, dose-related increases in the levels of cellular 10-methyl branched fatty acids were observed as a response to an increasing concentration of phenol or toluene in the medium, independently of the ability to use toluene.
Pimelobacter sp. has been reported to increase the content of 10-methylheptanoic and 10-methyloctanoic acids while growing on pyridine as the sole carbon source (13). An increase of cellular 10-methyl branched fatty acids was also observed as a response to an increased temperature in Mycobacterium phlei (18, 19).
The physiological role of 10-methyl branched fatty acids that occur in bacteria belonging to the genera Nocardia, Gordona, Rhodococcus, Mycobacterium, Dietzia, and Tsukamurella is unresolved, and the localization of 10-methyl branched fatty acids in the lipids of Rhodococcus has not been described. Mycobacterial lipids have been studied intensively, and there is evidence that 10-methyl octadecanoic (tuberculostearic), palmitic, and stearic acids are located in cell envelope lipids, mainly lipoarabinomannan and ornithine-amide lipid (1, 7, 10, 17). It is known that lipoarabinomannan is one of the major components of the cell envelope, and it traverses the cell wall of a mycobacterium. Moreover, tuberculostearic acid and palmitate are major acyl groups of the phosphatidylinositol moiety which anchors lipoarabinomannan to the cytoplasmic membrane (6, 7). Based on this, we suggest that an increasing amount of lipoarabinomannan may be involved in the protection of actinomycete cells against disruption of the membrane-cell wall structure.
Acknowledgments
We thank Raimo Mikkola for advice about GC-mass spectrometry. We are grateful to L. A. Golovleva for donation of R. erythropolis 1CP.
This work was financially supported by the Academy of Finland (M.S.S.) and the Helsinki University Fund for Center of Excellence (M.S.S.).
REFERENCES
- 1.Daffé M, Draper P. The envelope layers of mycobacteria with reference to their pathogenicity. Adv Microb Physiol. 1998;39:131–203. doi: 10.1016/s0065-2911(08)60016-8. [DOI] [PubMed] [Google Scholar]
- 2.Diefenbach R, Heipieper H-J, Keweloh H. The conversion of cis into trans unsaturated fatty acids in Pseudomonas putida P8: evidence for a role in the regulation of membrane fluidity. Appl Microbiol Biotechnol. 1992;38:382–387. [Google Scholar]
- 3.Gorlatov S N, Mal’tseva O V, Shevchenko V I, Golovleva L A. Degradation of chlorophenols by a culture of Rhodococcus erythropolis. Mikrobiologiya. 1989;58:802–806. . (In Russian.) [Google Scholar]
- 4.Heipieper H-J, de Bont J A M. Adaptation of Pseudomonas putida S12 to ethanol and toluene at the level of fatty acid composition of membranes. Appl Environ Microbiol. 1994;60:4440–4444. doi: 10.1128/aem.60.12.4440-4444.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heipieper H-J, Diefenbach R, Keweloh H. Conversion of cis unsaturated fatty acids to trans, a possible mechanism for the protection of phenol-degrading Pseudomonas putida P8 from substrate toxicity. Appl Environ Microbiol. 1992;58:1847–1852. doi: 10.1128/aem.58.6.1847-1852.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter S W H, Brennan P J. Evidence for the presence of a phosphatidylinositol anchor on the lipoarabinomannan and lipomannan of Mycobacterium tuberculosis. J Biol Chem. 1990;265:9272–9279. [PubMed] [Google Scholar]
- 7.Hunter S W H, Gaylord H, Brennan P J. Structure and antigenicity of the phosphorylated lipopolysaccharide antigens from the leprosy and tubercle bacilli. J Biol Chem. 1986;261:12345–12351. [PubMed] [Google Scholar]
- 8.Keweloh H, Diefenbach R, Rehm H J. Increase of phenol tolerance of Escherichia coli by alterations of the fatty acid composition of the membrane lipids. Arch Microbiol. 1991;157:49–53. doi: 10.1007/BF00245334. [DOI] [PubMed] [Google Scholar]
- 9.Keweloh H, Heipieper H J. Trans unsaturated fatty acids in bacteria. Lipids. 1996;31:129–137. doi: 10.1007/BF02522611. [DOI] [PubMed] [Google Scholar]
- 10.Lanéelle M-A, Promé D, Lanéelle G, Promé J-C. Ornithine lipid of Mycobacterium tuberculosis: its distribution in some slow- and fast-growing mycobacteria. J Gen Microbiol. 1990;136:773–778. doi: 10.1099/00221287-136-4-773. [DOI] [PubMed] [Google Scholar]
- 11.Pinkart H C, Wolfram J W, Rogers R, White D C. Cell envelope changes in solvent-tolerant and solvent-sensitive Pseudomonas putida strains following exposure to o-xylene. Appl Environ Microbiol. 1996;62:1129–1132. doi: 10.1128/aem.62.3.1129-1132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirttijärvi T S M, Graeffe T H, Salkinoja-Salonen M S. Bacterial contaminants in liquid packaging boards: assessment of potential for food spoilage. J Appl Bacteriol. 1996;81:445–458. doi: 10.1111/j.1365-2672.1996.tb03532.x. [DOI] [PubMed] [Google Scholar]
- 13.Rhee S-K, Lee G M, Kimand Y B, Lee S-T. Effect of pyridine on the fatty acid composition of Pimelobacter sp. FEMS Microbiol Lett. 1996;141:139–143. [Google Scholar]
- 14.Salkinoja-Salonen M S. Direct submission to EMBL database. 1997. [Google Scholar]
- 15.Sikkema J, de Bont J A M, Poolman B. Interactions of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 16.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugden E A, Samagh B S, Bundle D R, Duncan J R. Lipoarabinomannan and lipid-free arabinomannan antigens of Mycobacterium paratuberculosis. Infect Immun. 1987;55:762–770. doi: 10.1128/iai.55.3.762-770.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suutari M, Laakso S. Effect of growth temperature on the fatty acid composition of Mycobacterium phlei. Arch Microbiol. 1993;159:119–123. doi: 10.1007/BF00250270. [DOI] [PubMed] [Google Scholar]
- 19.Toriyama S, Yano I, Masui M, Kusunose E, Kusunose M, Akimori N. Regulation of cell wall mycolic acid biosynthesis in acid-fast bacteria. I. Temperature-induced changes in mycolic acid molecular species and related compounds in Mycobacterium phlei. J Biochem. 1980;88:211–221. [PubMed] [Google Scholar]
- 20.Weber F J, Isken S, de Bont J A M. Cis/trans isomerization of fatty acids as a defense mechanism of Pseudomonas putida strains to toxic concentrations of toluene. Microbiology. 1994;140:2013–2017. doi: 10.1099/13500872-140-8-2013. [DOI] [PubMed] [Google Scholar]
- 21.Zaitsev G M, Uotila J S, Tsitko I V, Lobanok A G, Salkinoja-Salonen M S. Utilization of halogenated benzenes, phenols, and benzoates by Rhodococcus opacus GM-14. Appl Environ Microbiol. 1995;61:4191–4201. doi: 10.1128/aem.61.12.4191-4201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]