Abstract
Osteoarthritis (OA) has a very high incidence worldwide and has become a very common joint disease in the elderly. Currently, the treatment methods for OA include surgery, drug therapy, and exercise therapy. In recent years, the treatment of certain diseases by exercise has received increasing research and attention. Proper exercise can improve the physiological function of various organs of the body. At present, the treatment of OA is usually symptomatic. Limited methods are available for the treatment of OA according to its pathogenesis, and effective intervention has not been developed to slow down the progress of OA from the molecular level. Only by clarifying the mechanism of exercise treatment of OA and the influence of different exercise intensities on OA patients can we choose the appropriate exercise prescription to prevent and treat OA. This review mainly expounds the mechanism that exercise alleviates the pathological changes of OA by affecting the degradation of the ECM, apoptosis, inflammatory response, autophagy, and changes of ncRNA, and summarizes the effects of different exercise types on OA patients. Finally, it is found that different exercise types, exercise intensity, exercise time and exercise frequency have different effects on OA patients. At the same time, suitable exercise prescriptions are recommended for OA patients.
Keywords: osteoarthritis, exercise, pathology, mechanism, therapy
Introduction
Osteoarthritis (OA) has a very high incidence worldwide, and it is strongly associated with age (van Saase et al., 1989). Most of the patients with OA are aged over 60. With the aging of the population, the disease has become a common joint disease today. Heredity, hormones, diet, obesity, smoking, and drinking can lead to OA (Felson et al., 1997). Its early symptoms mainly include pain and joint stiffness, and later secondary changes such as muscle atrophy and joint contracture occur (Bennell et al., 2012). Therefore, we advocate early intervention for patients with OA to prevent further deterioration of the disease. However, the disease treatment still encounters many shortcomings, and further research and exploration are needed. Currently, the treatment methods for OA include surgery, drug therapy, and exercise therapy (Spahn et al., 2013). In recent years, the treatment of certain diseases by exercise has received increasing research and attention. Exercise is an economical and effective treatment (Ettinger et al., 1997). Proper exercise can improve the physiological function of various organs of the body and improve the overall morphology of the body (Benedetti et al., 2018). In addition, exercise can also relieve pain (Belavy et al., 2021; Zheng et al., 2021; Peng et al., 2022; Wu et al., 2022). For OA patients, ladder treatment is generally adopted, starting with basic treatment. If it is ineffective, drug therapy or surgery can be used, and exercise therapy is one of the basic treatment methods. The effects of different exercise types on OA have been widely studied.
At present, the treatment of OA is usually symptomatic. Limited methods are available for the treatment of OA according to its pathogenesis. Exercise can alleviate OA at a molecular level. Only by clarifying the mechanism of exercise treatment of OA and the influence of different exercise intensities on normal joints and OA patients can we choose the appropriate exercise prescription to prevent and treat OA. This review mainly expounds the mechanism that exercise alleviates the pathological changes of OA by affecting the degradation of the ECM, apoptosis, inflammatory response, autophagy, and changes of ncRNA, and summarizes the effects of different exercise types on OA patients. Finally, it is found that different exercise types, exercise intensity, exercise time and exercise frequency have different effects on OA patients. At the same time, suitable exercise prescriptions are recommended for OA patients.
Pathological Change Mechanism of Osteoarthritis
For OA patients, the integrity of the entire joint tissue is damaged, including articular cartilage, subchondral bone, and synovial membrane (Loeser et al., 2012). Articular cartilage is mainly composed of the extracellular matrix (ECM), consisting of water, collagen, proteoglycans, mucopolysaccharides, type II collagen, and chondrocytes (Luo et al., 2017). With the presence of prolonged, excessive mechanical stimulation of the body, articular cartilage will suffer. In the early stage of articular cartilage injury, the concentration of growth factors in the ECM increases as chondrocytes gather in the damaged area, resulting in transient cell proliferation and ECM synthesis (Suri and Walsh, 2012). As the damage worsens, the blood supply to the articular cartilage worsens to the point that the cartilage does not have adequate access to nutrients. This condition results in cartilage cell apoptosis, ECM synthesis, and the disappearance of the articular cartilage degeneration (Eyre, 2004). The two bones constantly rub, resulting in joint pain, swelling, and function limitation. Its continuous development causes OA (Rim et al., 2020). Subchondral bone includes subchondral cancellous bone and cortical plates. X-ray and Magnetic Resonance Imaging (MRI) diagnosis can reveal the abnormal remodeling of subchondral bone and a series of changes in bonemorphology, such as bone spurs, osteophytes, and wear in OA patients (Braun and Gold, 2012). This sequence of changes may be caused by an imbalance in the production and destruction of osteoblasts and osteoclasts (Burr and Gallant, 2012). This morphological change of subchondral bone may also cause damage to the articular cartilage overlying it. Patients with OA have an exceptionally high probability of suffering from synovitis, and this condition is related to the pain and function of the knee joint during OA development (Sowers et al., 2011). The synovial membrane is the connective tissue membrane covered on the inner surface of the joint capsule, which can produce synovial fluid to reduce joint friction and the loss of cartilage and ensure the metabolism and nutrition supply of joint cartilage (Pap et al., 2020). Synovial cells mainly include synovial macrophages, fibroblast-like synovial cells, and mesenchymal stem cells. The macrophages can engulf the damaged tissue. When synovitis occurs in the body, the macrophages decompose to produce inflammatory factors, and the generation and degradation of cartilage matrix are in dynamic balance under normal conditions. Inflammatory mediators can destroy this balance, decompose chondrocytes, and aggravate synovitis (Da et al., 2007). Fibroblast-like synovial cells can produce hyaluronic acid, which is an essential component of joint synovial fluid. During OA progression, the content of hyaluronic acid is remarkably reduced, resulting in the reduction of joint function and damage to the integrity of the joint surface (Scanzello and Goldring, 2012).
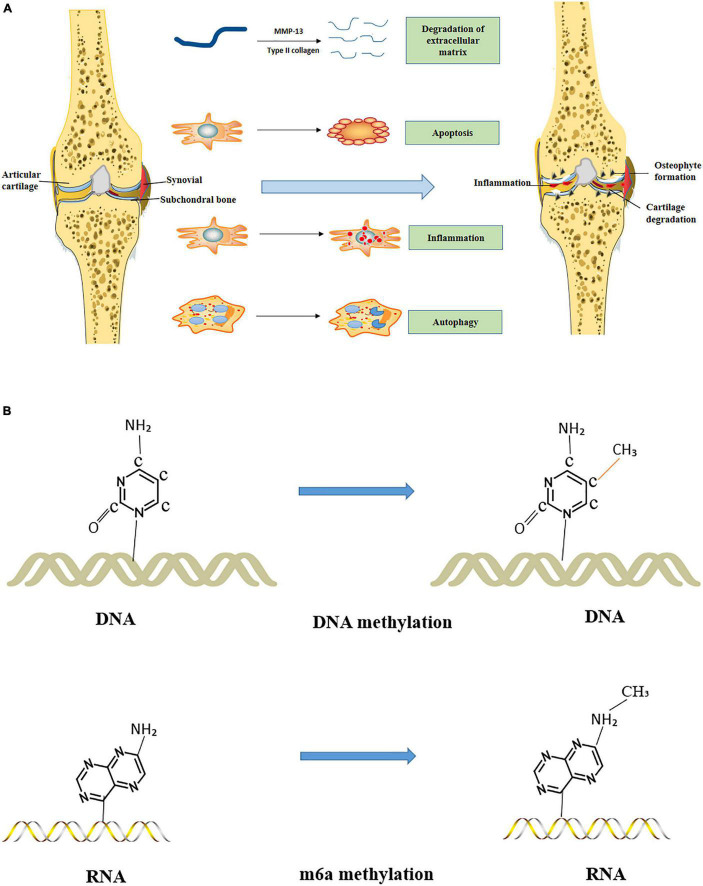
Above all, articular cartilage, synovium, and subchondral bone abnormalities are the main pathological changes of OA (Valdes and Spector, 2010). Considering that articular cartilage receives nutrients through synovial fluid, articular cartilage in patients with synovitis may undergo pathological changes. Moreover, the increase of macrophages in patients with synovitis may cause subchondral bones in OA to form osteophytes. Ayral et al. (2005) evaluated the correlation between synovitis and the severity of cartilage structure damage in OA patients through a one-year multicenter longitudinal study involving 422 patients. After arthroscopic and pain scoring, they found that compared with patients with normal synovial membrane, patients with synovitis had more severe articular cartilage lesions. The degree of deterioration after 1 year was statistically different. Yusup et al. (2015) studied 40 patients with knee OA and scored the structures such as joint cartilage, subchondral bone, and synovial membrane by using 3.0-T MRI and found that synovitis mainly occurred in the early and late stage of OA patients. The destruction of joint cartilage and a series of changes in subchondral bone could induce synovitis, and a strong correlation was observed among the three parameters. Some studies (Norrdin et al., 1998) use horse as a model to study the pathological changes of OA, in which the morphological changes of subchondral bone occur before articular cartilage injury. In other studies (Brandt et al., 1991), dog is used as the model to investigate the pathological changes of OA, in which the morphological changes of early subchondral bone coincided with an articular cartilage injury. Generally, the pathological mechanisms of articular cartilage, synovium, and subchondral bone in OA mainly include the degradation of ECM, cell proliferation, cell apoptosis, inflammatory reaction, changes of non-coding RNA, and methylation (Mobasheri et al., 2017) (Figure 1).
FIGURE 1.
Pathogenesis of Osteoarthritis (OA). (A) The degradation of ECM, apoptosis, inflammatory response, and autophagy mechanisms in OA. (B) Methylation in OA.
Degradation of Extracellular Matrix
The ECM is a complex network of large molecules such as polysaccharides and proteins around cells. This structure and special cells make up the cartilage (Theocharis et al., 2019). Many diseases are associated with changes in the composition and properties of ECM, such as OA. In healthy articular cartilage, the synthesis and metabolism of ECM should always maintain a dynamic balance to maintain homeostasis. The secretion and operation of some components of ECM are completed by articular cartilage (Rahmati et al., 2017). When the activities of synthesis and catabolism of articular cartilage are imbalanced, the details and homeostasis of ECM are also affected (Guo et al., 2002). Considering that the degradation of ECM leads to the loss of cartilage tissue, ECM also plays a role in maintaining the function of chondrocytes. The continuous degradation of ECM will induce OA.
Based on the summarizing of articles related to the pathogenesis of OA and the function of chondrocytes, Goldring and Goldring (2007) found that the degradation of ECM and decomposition of collagen could lead to the gradual loss of the shape and position of articular cartilage to induce OA. Setayeshmehr et al. (2019) summarized the functions and effects of various ECM scaffolds through summarizing and analyzing the related articles on hybrid and composite scaffolds derived from chondrocyte ECM. They concluded that the degradation of chondrocyte ECM could lead to cartilage degeneration. Malemud (2017) analyzed the role of matrix metalloproteinases in OA. They found that in the ECM of the cartilage, some essential proteins would degrade. Matrix metalloproteinases can promote or inhibit the degradation of these proteins, change the biomechanical properties of tissues, and destroy articular cartilage. In addition, based on the analysis of the action mechanism of ECM and its degrading enzymes in OA, the ECM of cartilage in OA patients can release certain fibronectin, thereby inducing the expression of related proteases and degrading the components of the ECM (Pérez-García et al., 2019).
Kapoor et al. (2011) and Theocharis et al. (2019) also found that the tissue protein would be hydrolyzed gradually during the development of OA. Then, some catabolic mediators will be produced, which will induce the expression of some related cytokines and proteases, and finally lead to ECM degradation. This situation will lead to prolonged OA. Based on the analysis of the changes of some macromolecules in the ECM of early OA patients, the content of proteins related to ECM was changed, such as collagen and cartilage protein. Hence, the degradation of ECM and even the deterioration of OA disease occurred (Lorenzo et al., 2004).
Apoptosis
Apoptosis is an active gene-determined process that automatically ends life, and it is often called programmed cell death, a basic biological phenomenon of cells (Musumeci et al., 2011). Apoptosis involves endogenous and exogenous pathways, which play an essential role in maintaining the function of various tissues in the body (Elmore, 2007). A correlation has been observed between the degree of cartilage injury and apoptosis, which is a vital mechanism of cartilage injury (Hwang and Kim, 2015). Moreover, the apoptosis of OA chondrocytes is related to cartilage degradation (Kim et al., 2003). When OA occurs, it will produce matrix-degrading enzymes, leading to the degradation of the ECM and the destruction of cell homeostasis. Cell stress induces an oxidation reaction, leading to the apoptosis of chondrocytes and regulating the pathological changes of cartilage tissues (Bauer et al., 2006).
Histologically, Musumeci et al. (2011) studied the articular cartilage of normal individuals and OA patients by staining apoptotic cells. They found that the articular cartilage of OA patients changed in structure. The pro-apoptotic receptor on the cell surface could induce apoptosis through an exogenous pathway in the damaged articular cartilage. Aigner et al. (2004) summarized the incidence, induction mechanism, and morphology of apoptosis in OA patients and found that apoptosis occurred in patients with OA, but the incidence was not high. The body induced apoptosis through specific proteoglycans, signaling molecules, and other pathways to regulate the pathological changes of articular cartilage and accelerate or delay the progression of OA. Based on the synthesis of related articles on OA and apoptosis, some articles indicate that apoptosis induces OA, while other articles indicate that OA induces apoptosis. These two views are disputed, and more reports believe that these conditions affect and promote each other and have a correlation (Zamli and Sharif, 2011). Chondrocytes in the knee joints of patients with OA and the apoptosis of chondrocytes and its relationship with cartilage degradation have been studied. Notably, the findings indicate that first, in some populations, the morphology and function of cartilage are normal (Hashimoto et al., 1998). Still, many apoptotic cells appear, indicating that apoptosis may lead to degenerative changes in articular cartilage, thus inducing OA. Furthermore, the number of apoptotic articular cartilage cells in the OA population increased, and the number of apoptotic cells was related to cartilage degradation. Finally, when all subjects, including the average population and OA patients, were analyzed, a correlation was found between age and apoptosis of chondrocytes. Similarly, rabbit chondrocytes have been studied and compared with mature rabbits. Results show that the cell density of each layer of articular cartilage in rabbits decreased, and the expression level of pro-apoptotic genes increased. These studies further proved the correlation between age and chondrocyte apoptosis at the animal level and the phenomenon of apoptosis that existed in the early stage of OA (Todd Allen et al., 2004). In another study involving horses, a positive correlation was found between the severity of chondrocyte injury and apoptosis. The expression level of pro-apoptotic factors is higher in horses that often suffer from OA. Apoptosis participates in the pathogenesis of OA. Apoptosis has been observed on human femoral head cartilage (Thomas et al., 2011). In comparison with the average population, the expression of receptors and the degree of apoptosis in OA patients’ articular cartilage was much higher. In the late stages of OA, the degree of apoptosis become even worse (Héraud et al., 2000).
Inflammatory Reaction
The inflammatory reaction is a basic pathological process mainly involving a defensive response when the body is stimulated. Inflammation is involved in the pathological process of many diseases, such as cancer (Gianni et al., 2016), tendon or ligament injury (Gracey et al., 2020). As early as the middle of the 19th century, studies pointed out that inflammation was closely related to OA. Articular chondrocytes and synovial cells all expressed inflammatory mediators (Liu-Bryan, 2013). Inflammatory factors such as IL-1β and TNFα participate in the inflammatory response in articular chondrocytes and synovial cells (Malfait, 2016). The cells mainly involved in the inflammatory response are macrophages and monocytes. The degree of inflammation is related to the degree of joint dysfunction and inflammatory factors (Scanzello et al., 2011). In the early stage of OA, the expression of monocytes and inflammatory mediators increased, whereas with the continuous development of OA, the expression of monocytes and inflammatory mediators was gradually decreased (Benito et al., 2005).
Rosshirt et al. (2019) used the knee joints of 55 OA patients as the research object, described the activation state of T cells, and found that a large proportion of T cells were activated to participate in OA inflammation, which mainly affected the joint itself the most. Inflammation can promote catabolism and participate in the pathogenesis of the disease. van den Bosch (2019) found a large number of inflammatory mediators in the tissues of patients with OA. These inflammatory mediators can induce the generation of degrading enzymes in articular cartilage, leading to the degradation of the ECM and the destruction of cartilage tissue. Lieberthal et al. (2015) established a post-traumatic OA model and observed the relationship between inflammation and OA progression. They found that an inflammatory reaction occurred in the early stage of OA. In patients with, multiple inflammatory pathways were activated and produced multiple inflammatory mediators such as cytokines and chemokines, which played an essential role in the pathogenesis of OA (Scanzello, 2017a). The genomic expression profiles of OA patients have been collected and subjected to meta-analysis, and the results show that MAP kinase, NF-κB activation, and oxidative phosphorylation can induce inflammatory signals and participate in the pathological change mechanism of OA (Li et al., 2014). Macrophages are immune cells that play an essential role in the inflammatory response (Bondeson et al., 2010). Woodell-May and Sommerfeld (2020) explored the role of immune cells such as macrophages in the synovial membrane of patients with OA. They found that in OA, macrophages could release oxygen free radicals, proteases, and inflammatory factors, thus affecting the microenvironment of inflammation. In the wound healing stage, macrophages also release IL-1, IL-6, and other inflammatory factors to regulate the inflammatory response. By stimulating protease, inflammation can cause cartilage degeneration and eventually induce OA. Based on the relationship between mechanical injury and OA, mechanical damage can lead to mechanical inflammation, which activates NF-kB and inflammatory mitogen-activated protein kinase, thereby causing a series of functional problems of the join (Vincent, 2019). If the rash persists for a long time, it will affect the repair of cartilage tissue. Based on the summary of articles related to inflammation in OA (Goldring and Otero, 2011), when some risk factors inducing OA appear, the expression of pro-inflammatory factors and various related enzymes are upregulated in articular cartilage and synovial tissue through specific signaling pathways. Inflammatory elements, which are essential for cartilage damage and repair, can change adjacent joint tissues and form a vicious circle (Houard et al., 2013). The knee joint is damaged under severe mechanical stimulation, causing catabolism and stress response of chondrocytes, and finally inducing inflammation, leading to joint pain. Inflammation is one of the important mechanisms that lead to the pathological changes of joint cartilage and synovial membrane (Scanzello, 2017b).
Autophagy
During autophagy, cells degrade their damaged organelles and macromolecular substances by using lysosomes to regulate autophagy-related genes, which can achieve the renewal of organelles and is essential for cell metabolism (Guo et al., 2021). Autophagy is involved in the occurrence of OA. Through cellular autophagy, the function of damaged articular cartilage can be restored, thereby alleviating the pathological process of OA. Excess ROS will lead to cartilage degradation and inhibit the synthesis of ECM. Kongara and Karantza (2012) showed that autophagy could maintain the typical morphology of cartilage and the dynamic balance of ECM synthesis and metabolism by regulating the body’s ROS.
Barranco (2015) found that the inhibition of the Akt-mTOR signaling pathway in chondrocytes can promote the autophagy of articular chondrocytes and regulate oxidative stress (Xue et al., 2017), thus participating in the development process of OA. If autophagy is activated, the damaged mitochondria and peroxidase bodies are removed, thus inhibiting the production of reactive oxygen species in the body and protecting the articular cartilage from pathological changes. Based on the summary of the roles of autophagy in OA, in the early stage of OA, moderate autophagy contributes to the survival of chondrocytes and is essential for preventing and delaying OA (Duan et al., 2020). Li et al. (2016) found that autophagy was mainly committed in the chondrocytes of patients with OA. By regulating oxidative stress response and apoptosis, the pathological changes of the knee joint can be alleviated, and chondrocytes can be protected from various stimulations. With the growth of age, the incidence of OA increases gradually, and this phenomenon is related to autophagy. Autophagy gradually weakens with age. Therefore, the structure and function of articular cartilage decreases with age, leading to pathological changes in the knee joint (Mathew et al., 2009). Caramés et al. (2010) that in OA animal models, the expression of autophagy regulatory factors is downregulated, and the steady-state of cartilage is damaged, indicating that autophagy can protect articular cartilage and play an essential role in maintaining cartilage steady state. The decrease of autophagy will induce OA. Taking articular cartilage of OA and non-OA patients as the research object, we can find that autophagy regulates the expression of OA-related genes. In comparison with patients without OA, autophagy is expressed in the chondrocytes of OA patients at a higher level, and the manifestation of autophagy markers is also upregulated (Sasaki et al., 2012). Similarly, Chang et al. (2013) took human chondrocytes as the research object and found that many autophagy-related proteins are expressed in the articular cartilage of patients with OA. In the pathogenesis of OA, autophagy plays a role in protecting chondrocytes and promoting metabolism. However, excessive autophagy causes a large number of chondrocyte deaths.
Changes in Non-coding RNA
Non-coding RNA (ncRNA) is an RNA that does not code for protein, mainly including miRNA, lncRNA, and circRNA. These RNAs can exert biological functions at the RNA level without being translated into proteins. ncRNA plays an essential role in the pathological process of many diseases, such as cardiovascular disease (Jusic and Devaux, 2020), cancer (Karreth and Pandolfi, 2013), diabetes (Beltrami et al., 2015). ncRNA also plays a vital role in the process of OA. It can promote or inhibit cartilage formation by regulating the degradation of cell-matrix, cell proliferation, apoptosis, and inflammatory response, thereby inducing or treating OA (Hong and Reddi, 2012).
Changes in the expression levels of many miRNAs can induce pathological changes in OA. MiR-204/-211 and miR-29b-3p are common miRNAs, which are differentially expressed in patients with OA. Huang et al. (2019) established an OA mouse model and found that miR-204/-211 was missing, and Runx2 was increased. Mesenchymal progenitor cells proliferated abnormally at the same time by Micro-CT and histological determination. Akt signal North is activated, thus inducing the dysfunction of various components in the joint and finally leading to OA. Therefore, miR-204/-211 can maintain intra-articular homeostasis and ensure that articular chondrocytes and synovial cells function usually. Chen et al. (2017) used rat chondrocytes as the research object. Through luciferase reporter gene detection, they found that miR-29b-3p in patients with OA was upregulated, and miR-29b-3p ultimately led to the degeneration of articular cartilage tissue by targeting and inhibiting the expression of rat GRN mRNA. In addition, microarray technology was used to detect the expression of miRNA in chondrocytes. They found that miRNA-140, miRNA-455, miR-146a, miR-155, and miR125b differed in OA patients and the average population, thus inducing pathological changes of articular cartilage, synovial membrane, and subchondral bone (Swingler et al., 2012). lncRNA also plays a vital role in OA. Throughout the cartilage development, different lncRNA is regulated, and the regular expression of lncRNA can prevent cartilage differentiation disorders. During cartilage degeneration, other lncRNA plays various roles (Zhu et al., 2019). Ye et al. (2018) studied the articular cartilage of patients with OA. They found that the expression of ZFAS1 in OA chondrocytes was downregulated, and ZFAS1 might reduce the activity of chondrocytes and induce pathological changes of articular cartilage tissues by targeting the Wnt3a signaling pathway. Similarly, the role of circa in OA has been widely studied. Cyclic RNA plays a multi-faceted regulatory role in the progression of OA (Zhang W. et al., 2021). Chen et al. (2020) found that in OA tissues, the expression levels of circRNA-UBE2G1 and HIF-1a were significantly increased, while the expression of miR-373 was downregulated. Functional testing showed that circRNA-UBE2G1 could bind to miR-373, thereby inhibiting the interaction of miR-373 and HIF-1a, damaging the chondrocytes, and leading to a series of pathological changes. In conclusion, ncRNA is closely related to OA. The differential expression of miRNA, lncRNA and circRNA in healthy people and OA patients can lead to pathological changes in articular cartilage, synovial membrane, and subchondral bone and affect the pathological process of OA.
Methylation
Methylation is the catalyzed transfer of methyl groups from an active compound to another compound. This process can form various methyl compounds or result in chemical modification of particular proteins or nucleic acids to start methylation products (Urnov, 2002). Methylation mainly includes DNA methylation and m6a methylation (Dai et al., 2021).
DNA methylation is a chemical modification of DNA that alters genetic behavior without altering the DNA sequence. Its primary process is under the action of DNA methyltransferase. DNA methylation can cause changes in chromatin structure, DNA conformation, DNA stability, and the way DNA interacts with the protein, thereby controlling gene expression (Reynard, 2017). When CtBP1 and CtBP2 are overexpressed, the pro-inflammatory factors are increased, while the NLRP3 signaling pathway is activated to induce OA. DNA methylation in the CtBPs promoter reduces the expression level of CtBPs in OA tissues and regulate CtBP-mediated signal transduction, finally participating in the pathogenesis of OA (Sun et al., 2020). In addition, DNA methylation can participate in the pathogenesis of OA by affecting the expression of genes such as matrix metalloproteinase-3, matrix metalloproteinase-9, and type II collagen (Cui and Xu, 2018).
N6- methyladenine (m6a) is one of the most abundant chemical modifications of eukaryotic messenger RNA. m6a modifications mainly include m6a methyltransferase catalysis, m6a demethylase removal, and m6a binding protein recognition (Ma and Ji, 2020). This process is widely involved in regulating various life cycle stages such as mRNA splicing, processing, translation, and degradation. It is related to osteosarcoma, rheumatoid arthritis, osteoporosis, OA, and abnormal physiological functions (Zhang W. et al., 2020). The strange expression of m6a-related gene and protein in OA can trigger the imbalance of m6a methylation, regulate the expression of OA-related genes to participate in the occurrence and development of OA, and is closely related to the poor prognosis of patients (Yang et al., 2021). METTL3 is a methylated gene of m6a. He et al. (2021) studied mice in vivo and in vitro and induced the OA model with inflammatory stimuli such as TNF-α. The results showed that the expression of METTL3 was decreased in the OA model. Bcl2 is a downstream target gene of METTL3, and the presentation of METTL3 can promote m6A methylation of Bcl2 mRNA, thereby inhibiting chondrocyte apoptosis and autophagy. The detection of synovial tissue in patients with OA revealed that METTL3 could also induce autophagy by inhibiting ATG7 (Chen X. et al., 2021). In addition, METTL3 may participate in OA by regulating the inflammatory response (Sang et al., 2021). In summary, methylation is also an essential mechanism for pathological changes in OA. Furthermore, the epigenetic mechanism plays an essential role in the pathogenesis of OA.
Mechanism of Exercise Improving Osteoarthritis
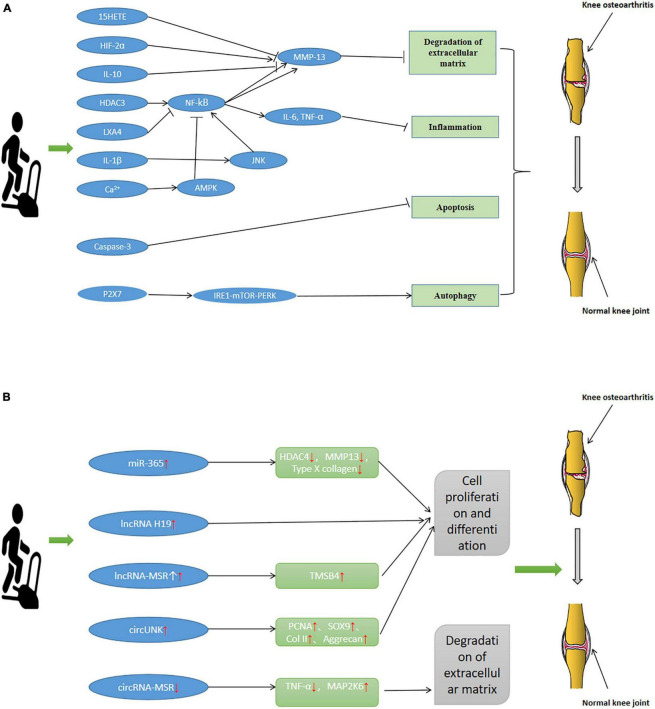
Exercise is good medicine. An increasing number of studies supports that exercise can enhance physical fitness, build up the body, and prevent and treat certain diseases, playing an increasingly important role in people’s lives (Ruegsegger and Booth, 2018). Studies (Pedersen and Saltin, 2015) have summarized the mechanism of exercise in treating 26 different conditions, providing a theoretical basis for treating diseases by exercise. The treatment of OA by exercise has attracted increasing attention and has gradually become an OA research hotspot. In the present paper, the pathological mechanism of exercise in OA treatment was summarized through the induction of relevant literature, as shown in Table 1. Exercise relieves the pathological changes of OA by affecting the degradation of the ECM, apoptosis, inflammatory response, autophagy, and changes of ncRNA. And training is used to treat OA (Figure 2).
TABLE 1.
Mechanism of exercise in the treatment of Osteoarthritis (OA).
| Researchers | Model | Exercise types | Related gene/cytokines/protein | Involved in pathways | Improved organization | Functions | Change |
| He et al., 2020 | OA mice model | Cell stretch | Irisin | Erk | Articular cartilage | Apoptosis | ↓ |
| Griffin et al., 2020 | OA mice model | Wheel-running exercise | — | — | Subchondral bone | Inflammation | ↓ |
| Lu et al., 2021 | OA rat model | Treadmill exercise | Maresin-1, MMP-13 | PI3K/AKT, NF-κB | Synovial | Inflammation | ↓ |
| Griffin et al., 2012 | OA mice model | Wheel-running exercise | KC, leptin, IL-1Ra | — | Subchondral bone, Articular cartilage | Inflammation | ↓ |
| Cormier et al., 2017 | OA rat model | Prior wheel running | — | — | Subchondral bone, Articular cartilage | Inflammation | ↓ |
| Chen et al., 2020 | OA rat model | Treadmill and wheel exercise | IL-1b, IL-6, TNF-a | JNK/NF-kB | Articular cartilage, synovial | Inflammation | ↓ |
| Rios et al., 2019 | OA rat model | Aerobic exercise | IL-6, TNF-α | — | Subchondral bone | Inflammation | ↓ |
| Li K. et al., 2021 | OA mice model | Wheel-running exercise | TLR4, MMP-13 | — | Articular cartilage | Degradation of ECM | ↓ |
| Yang et al., 2020 | OA rat model | Treadmill exercise | TRAIL | TRAIL/NF-κB/NLRP3 | Articular cartilage | Apoptosis, Inflammation | ↓ |
| Allen et al., 2017 | OA rat model | Treadmill exercise | — | — | Subchondral bone | Inflammation | ↓ |
| Zhang X. et al., 2019 | OA rat model | Treadmill exercise | Lc3B, SQSTM1 | — | Articular cartilage | Autophagy | ↑ |
| Zhang H. et al., 2019 | OA rat model | Treadmill exercise | HDAC3 | HDAC3/NF-KappaB | Articular cartilage | Inflammation | ↓ |
| Galois et al., 2004 | OA rat model | Running training | Hsp70 | — | Articular cartilage | Apoptosis | ↓ |
| Oka et al., 2019 | OA mice model | Treadmill exercise | IL-1β, MMP-13 | — | Articular cartilage | Inflammation, Degradation of ECM | ↓ |
| Blazek et al., 2016 | OA rat model | Treadmill exercise | MMP-13,Type II collagen | — | Articular cartilage | Degradation of ECM | ↓ |
| Yang et al., 2019 | OA rat model | Treadmill exercise | IL - 1β | AMPK/NF – κB | Articular cartilage | Inflammation | ↓ |
| Assis et al., 2016 | OA rat model | Aerobic exercise | IL-1β, caspase-3, MMP-13 | — | Articular cartilage | Inflammation | ↓ |
| Baur et al., 2011 | OA mice model | Running exercise | ROS, SOD2 | — | Articular cartilage | Autophagy | ↓ |
| Assis et al., 2015 | OA rat model | Treadmill exercise | muscle-specific ring- finger protein 1, atrogin-1 |
— | Articular cartilage | Degradation of ECM | ↓ |
| Yamaguchi et al., 2013 | OA rat model | Running | C2C, CPII | — | Articular cartilage | Degradation of ECM | ↓ |
| Assis et al., 2018 | OA rat model | An aerobic and an aquatic exercise | IL-10, TGF-β, collagen I and II | — | Articular cartilage | Degradation of ECM | ↓ |
| Milares et al., 2016 | OA rat model | An aquatic exercise | IL1-β, caspase-3 | — | Articular cartilage | Inflammation | ↓ |
| Szychlinska et al., 2019 | OA rat model | Physical activity | IL-6 | — | Articular cartilage | Inflammation | ↓ |
| Qian et al., 2014 | OA rat model | Passive motion | Proteoglycans, type II collagen fibers | — | Articular cartilage | Apoptosis | ↓ |
| Yang et al., 2017 | OA rat model | Treadmill exercise | LXA4 | NF-κB | Articular cartilage | Inflammation | ↓ |
| Castrogiovanni et al., 2019 | OA rat model | Physical Activity | IL-1β, IL-6, TNF-α, MMP-13 | — | synovial | Inflammation | ↓ |
| Iijima et al., 2016 | OA rat model | Treadmill walking | BMP-2, BMP-6 | — | Subchondral bone | Cartilage matrix synthesis | ↑ |
| Boudenot et al., 2014 | OA rat model | Interval training exercise | Subchondral bone | Apoptosis | ↓ | ||
| Iijima et al., 2017 | OA rat model | Treadmill exercise | BMP-2, BMP-4, BMP-6, BMP receptor 2, pSmad-5 | BMP | Articular cartilage, Subchondral bone | Inflammation, Apoptosis | ↓ |
| Oka et al., 2021 | OA mice model | Treadmill exercise | Gremlin-1, MMP-13 | — | Articular cartilage | Inflammation | ↓ |
| Vasilceac et al., 2021 | OA rat model | Resistance training | MMP-2 | — | Articular cartilage | Degradation of ECM | ↓ |
| Hong et al., 2014 | OA rat model | Treadmill exercise | IL-6, EPAS-1, MMP-13 | — | Articular cartilage | Degradation of ECM, Apoptosis | ↓ |
| Na et al., 2014 | OA rat model | Treadmill exercise | C9 | — | serum | Inflammation | ↓ |
| Cifuentes et al., 2010 | OA rat model | Impact exercise | ACAN, COL2a1, TIMP3, MMP | — | Articular cartilage | Autophagy | ↑ |
| Yang et al., 2018 | OA rat model | Treadmill exercise | LXA4 | NF-κB | Synovial | Inflammation | ↓ |
| Hsieh and Yang, 2018 | OA rat model | Swimming exercises | collagen II, MMP13 | — | Articular cartilage, Synovial | Degradation of ECM, Apoptosis | ↓ |
| Hao et al., 2021 | OA rat model | Body weight-supported treadmill training | collagen II, MMP13 | — | Articular cartilage, Subchondral bone | Degradation of ECM, Apoptosis | ↓ |
| Iijima et al., 2015 | OA rat model | Short-term gentle treadmill walking | — | — | subchondral bone | Apoptosis | ↓ |
| Al-Hashem et al., 2017 | OA rat model | Swim exercise | TBARS, TNFa, SOD | — | Articular cartilage | Inflammation | ↓ |
| Martins et al., 2019 | OA rat model | Aerobic training | MIA, IL1β, TNF | — | Articular cartilage | Inflammation | ↓ |
| Musumeci et al., 2015 | OA rat model | Treadmill exercise | lubricin | — | Articular cartilage, Synovial | Cartilage matrix synthesis | ↑ |
| Musumeci et al., 2013 | OA rat model | Treadmill exercise | caspase-3 | — | Articular cartilage | Apoptosis | ↓ |
| Tian et al., 2021 | OA rat model | Treadmill exercise | 15-HETE | PI3k-Akt | Synovial | Inflammation | ↓ |
| Fallah Mohammadi et al., 2013 | OA rat model | Treadmill exercise | IL – 1β, TNF – α | — | Articular cartilage | Inflammation | ↓ |
| Zhang J. et al., 2021 | OA rat model | Swimming exercise | caspase-3 | — | Articular cartilage | Apoptosis | ↓ |
| Li Z. et al., 2021 | OA rat model | Treadmill exercise | P2 × 7 | IRE1-mTOR-PERK | Articular cartilage | Autophagy | ↑ |
| Guan et al., 2011 | OA chicken model | mechanical stimulation | HDAC4 | — | Articular cartilage | miR-365 | ↑ |
| Zhou et al., 2021 | OA mice model | Treadmill running | — | — | Articular cartilage | lncRNA H19 | ↑ |
| Liu Q. et al., 2016 | OA cell model | mechanical stimulation | TMSB4 | — | Articular cartilage | lncRNA-MSR | ↑ |
| Liu et al., 2017 | OA cell model | mechanical stimulation | TNF-α | — | Articular cartilage | circRNA-MSR | ↓ |
| Fang et al., 2021 | OA rabbit model | running experiment | PCNA, SOX9, Col II, Aggrecan | — | Articular cartilage | circUNK | ↑ |
FIGURE 2.
Pathological change mechanism of Osteoarthritis (OA). Exercise relieves the pathological changes of OA by affecting the degradation of the ECM, apoptosis, inflammatory response, autophagy and changes of ncRNA. (A) Mechanism of pathological changes of OA through degradation of ECM, apoptosis, inflammatory response, and autophagy. (B) Mechanism of pathological changes of OA through ncRNA.
The Role of Exercise in the Degradation of Extracellular Matrix
Exercise can delay the pathological process of OA by inhibiting the degradation of the ECM. Articular cartilage degradation is an essential pathological change of OA. Blazek et al. (2016) used OA rats as the research object. Exercise and non-exercise were used as intervention means to compare the whole gene expression of the transcriptome of articular cartilage in rats. Microarray analysis showed 644 differentially expressed genes in the articular cartilage of exercise rats. Therefore, exercise can prevent OA by changing genes related to OA’s pathogenesis and regulating the degradation and synthesis of ECM by changing metabolic pathways to alleviate the pathological changes of articular cartilage with sound prevention effects on OA. An OA rat model has been established to evaluate the expression of IL-10, TGF-β, and collagen type I and II in articular cartilage. The presentation of the above biochemical indicators increased in the articular cartilage of mice after exercise, indicating that exercise is very beneficial to articular cartilage. Moreover, considering that collagen type II is an essential component of ECM, exercise may delay the progression of OA by inhibiting the degradation of ECM and promoting cartilage synthesis (Assis et al., 2018). Vasilceac et al. (2021) conducted resistance training for eight weeks for OA rats. They analyzed whether ELISA changed the activity of MMP-2 in the tendons around the knee joint and finally found that exercise could reduce the activity of MMP-2 in the quadriceps tendon of OA rats and greatly relieve the adverse effects of OA on the knee joint. Results show that exercise may reduce the degradation of the ECM by inhibiting the activity of MMP-2 from achieving the purpose of treating OA. Exercise in different stages of the course of OA produces other effects. Type II collagen (CoII) and matrix metalloproteinase-13 (MMP-13) are essential components related to the degradation of the ECM. Hsieh and Yang (2018) established an OA rat model, in which the samples were allowed to swim in different stages of the course of OA. They found that swimming training in the early stage of OA could increase CoII. The level of MMP-13 can be reduced to maintain a balance between the degradation and anabolism of ECM and prevent cartilage damage through exercise training in the early stage of OA rather than that in the late stage.
Overall, the degradation of the ECM is the pathological change mechanism of OA. Exercise can protect articular cartilage and delay the progression of OA by inhibiting this mechanism, and it is a crucial intervention to prevent and treat OA.
The Role of Exercise in Apoptosis
Apoptosis is one of the mechanisms for the pathological changes of OA. Exercise delays the pathological process of OA by inhibiting apoptosis. Taking articular cartilage of OA rats as a research sample, Yang et al. (2020) detected the expression of relevant genes in articular cartilage after exercise through bioinformatics analysis. Results show that the levels of TRAIL, NF-κB p65, and NLRP3 in cartilage after activity were decreased. Exercise could inhibit apoptosis and prevent OA by regulating the TRAIL/NF-κB/NLRP3 signaling pathway of OA. By comparing OA rats with mild, moderate, and high-intensity training and comparing the expression of caspase three and Hsp70, we found that mild and moderate exercise could inhibit apoptosis and protect the articular cartilage (Galois et al., 2004). Qian et al. (2014) established an OA rat model, and the experimental group was subjected to passive motion. Based on the measurements of the proteoglycan content of the cartilage matrix, the number of type II collagen fibers, and apoptotic chondrocytes in the experimental and control group, the changes in biochemical signals caused by passive exercise in the early and middle stages of OA differed. In comparison with the control group, the cartilage matrix proteoglycan content and type II collagen fiber level remarkably increased three weeks after passive motion in the early stage of OA. The number of apoptotic cells was significantly reduced. However, three weeks after passive exercise in the middle stage of OA, the number of apoptotic cells was not significantly changed. Therefore, passive exercise at the early stage of OA may delay articular cartilage degeneration by inhibiting apoptosis, preventing, and treating OA. After intermittent training for OA rats, the pathological changes of their subchondral bones were assessed by immunolabeling with cleaved caspase-3 in the cortical subchondral bone. Intermittent aerobic training may prevent the OA-induced reduction in bone mineral density by reducing apoptosis (Boudenot et al., 2014). Studies (Iijima et al., 2015) have established the OA rat model and analyzed the pathological conditions of rat articular cartilage and subchondral bone before and after four weeks of exercise. They found that exercise might inhibit apoptosis and protect cartilage tissue, thus delaying OA progression. Musumeci et al. (2013) established the OA rat model to study the effect of exercise on articular cartilage. First, drugs were used to induce pathological changes in the articular cartilage. Then, exercise intervention was performed on rats to observe the morphological changes of articular cartilage. The results show that exercise might induce the expression of lubricating oil and inhibit the activity of caspase-3, thereby reducing apoptosis and delaying or even reversing pathological changes of articular cartilage. In some studies (Zhang J. et al., 2021) concerning establishing the OA rat model, the experimental group was intervened by swimming for four weeks. The morphological changes of cartilage were analyzed by hematoxylin-eosin (H&E) staining. The expression of caspase-3 was analyzed by Western blot and qPCR. The results showed that after four weeks of swimming, the abnormal morphology of articular cartilage was improved, and the level of caspase-3 protein decreased, indicating that exercise might reduce apoptosis by inhibiting the level of caspase-3 protein, thereby protecting articular cartilage and preventing and treating OA.
The Role of Exercise in Inflammatory Response
Exercise can inhibit the inflammatory response by reducing pro-inflammatory factors, thereby delaying the pathological changes of OA, which is the most common mechanism of activity in OA treatment. The inflammatory response mechanism of exercise therapy for OA has been widely studied, and many related pathways regulate the inflammatory response of OA, such as PI3k/Akt, NF-κB p65, JNK/NF-kB, HDAC3/NF-kappaB, and AMPK/NF-κB signaling pathways. Lu et al. (2021) established the OA rat model and allowed them to exercise on the treadmill. They found that after running exercise, the mice produced maresin-1, and the increased level of maresin-1 activated the PI3k/Akt pathway and inhibited the NF-κB p65 pathway, thus playing an anti-inflammatory role and delaying the pathological changes of OA. Griffin et al. (2012) established the OA mouse model by inducing a high-fat diet and then letting the mice run to observe the expression of inflammation-related factors. Finally, they found that exercise could regulate the expression of pro-inflammatory factors, promote joint health, and reduce the severity of pathological changes in joints of OA mice. To study the signal transduction of JNK/NF-kB in KOA patients, we established the 0A rat model and conducted a controlled intervention experiment. Finally, the knee joint diameter in the exercise group is lower than that in the OA group, indicating that exercise is conducive to KOA recovery. The IL-1b, IL-6, and TNF-a levels decreased, indicating that exercise can reduce the inflammatory response by regulating the JNK/NF-kB signaling pathway, which ultimately can delay the pathological changes of OA (Chen et al., 2020). Osteoarthritis rats were subjected to moderate-intensity exercise on the treadmill. The H&E staining and toluidine blue O staining were used to detect cartilage injury. The expression levels of some biochemical signals in the articular cartilage were examined via immunohistochemistry and other methods. The results showed that moderate-intensity treadmill exercise could reduce the inflammatory response and protect the articular cartilage by inhibiting the HDAC3/NF-kappaB pathway (Zhang H. et al., 2019). Similarly, Yang et al. (2019) intervened OA rats with treadmill exercise and used the articular cartilage as the research object. Observation and analysis results show that moderate-intensity exercise can reduce the sensitivity of articular cartilage and chondrocytes to inflammatory response through the AMPK/NF-κB signaling pathway, and it is an essential mechanism for reversing the pathological changes of articular cartilage. The analysis of biochemical indicators of OA rats after aerobic exercise show that the expression levels of IL-1β, caspase-3, and MMP-13 in OA rats decreased after exercise, and aerobic exercise could inhibit the inflammatory response of OA and prevent and treat the pathological changes of cartilage (Assis et al., 2016). After water sports, OA rats’ articular cartilage and inflammatory mediators were changed. After eight weeks of the experiment, the cell damage degree of the rat was relieved, and the expression levels of IL-1β and caspase-3 were reduced. Therefore, sports can regulate OA inflammatory factors and inhibit inflammatory reactions to treat OA (Milares et al., 2016).
The Role of Exercise in Autophagy
In OA, autophagy is a cellular protective response. Exercise can protect the knee joints of KOA patients by inducing autophagy, which is mainly regulated by oxidative stress. Relatively few studies have demonstrated the specific mechanism pathways through which exercise protects the knee joint through autophagy. Zhang X. et al. (2019) used sodium iodoacetate to induce OA rat model, and the experimental group was subjected to treadmill exercise for four weeks. The study found that serum IL-1β was decreased, IL-4 was increased, and the expression of type II collagen in articular cartilage was increased in rats undergoing treadmill exercise compared with those in OA rats that did not experience any activity. Therefore, treadmill exercise may protect the knee joints of OA patients by promoting the autophagy of articular cartilage. Baur et al. (2011) established an OA mouse model to study the effect of running on articular cartilage and bone of OA patients and observed the changes of biochemical signals and articular cartilage of running mice after eight weeks. They found that the content of reactive oxygen species in mice increased after exercise. Exercise can promote autophagy and protect the articular cartilage and bone of OA patients by regulating oxidative stress. Another study (Cifuentes et al., 2010) has established an OA rat model. The experimental group was exposed to treadmill exercise for 8 weeks to observe the pathological changes of articular cartilage. In comparison with OA rats that did not undergo exercise, the protein and polysaccharide contents on the surface of the articular cartilage of rats after eight weeks of the exercise was relatively high. This experiment confirmed that training can enhance the oxidative stress mechanism and protect articular cartilage. Moderate-intensity exercise plays different roles in different stages of OA progression. In the early stage of OA, the average power of movement can delay the progression of OA by promoting autophagy. In the late stage of OA, moderate-intensity exercise can over-activate purinergic receptor P2X ligand-gated ion channel 7 and increase the number of apoptotic cells, thus aggravating the progression of OA. Throughout the OA progression, the body may promote autophagy or apoptosis through the IRE1-mTOR-PERK signal axis to delay or accelerate the pathological process of OA (Li Z. et al., 2021).
The Role of Exercise in Non-coding RNA
Exercise can alleviate the pathological changes of OA by regulating the expression of ncRNA. The practice affect the face of miRNA, lncRNA, and circRNA in articular cartilage, synovial membrane, and subchondral bone of OA patients.
miRNA is related to maintaining the typical morphology of articular cartilage. The detection of miRNA expression in OA rats revealed that compared with the non-exercise rats, 394 differentially expressed miRNAs were detected in the exercise rats (Yang et al., 2020). The analysis of articular cartilage from different animals revealed that exercise could change the miRNA expression and improve the pathological changes of articular cartilage through a series of mechanisms. Dunn et al. (2009) analyzed the expression of miRNA in bovine articular cartilage. They found that the expression patterns of miR-221 and miR-222 in weight-bearing medial anterior condylar articular cartilage was higher than those in non-weight-bearing medial posterior condylar articular cartilage. When the articular cartilage was loaded with weight, miR-221 downregulated type II collagen and Sox9, and miR-222 downregulated HDAC4 and MMP-13, maintaining the typical morphology of articular cartilage. Guan et al. (2011) subjected chicken chondrocyte samples to mechanical stimulation to analyze the roles of relevant miRNA in the mechanical stimulation using microarray technology. Results show that mechanical stimulation could upregulate the miR-365 expression in chondrocytes. miR-365 stimulated the differentiation of chondrocytes by targeting histone deacetylase 4 (HDAC4) and finally alleviated the pathological process of cartilage tissue. In addition, the expression levels of many lncRNA changed during exercise. Zhou et al. (2021) established an OA mouse model. Mice with moderate-intensity exercise intervention had an upregulated lncRNA H19 expression, thickened articular cartilage, and resolved OA compared with mice without exercise intervention. Under the stimulation of mechanical stress, lncRNA-MSR was activated and then competitively bound to miR-152, thereby promoting the expression of TMSB4 and leading to the degradation of the ECM (Liu Q. et al., 2016). The regulatory role of circRNA during exercise is also crucial. A total of 104 circRNA were differentially expressed (44 upregulated and 60 downregulated), with increased circRNA-MSR expression, in damaged articular cartilage compared with intact articular cartilage. circRNA-MSR expression was downregulated after mechanical stress was applied to the cartilage. The downregulation of circRNA-MSR can inhibit the expression of TNF-α and promote the degradation of the extracellular chondrocyte matrix, thus enhancing the structure and function of cartilage (Liu et al., 2017). In addition, circRNA-MSR can directly target miR-643 to u-regulate MAP2K6. The downregulation of circRNA-MSR expression can promote cell proliferation and reduce cartilage damage (Jia and Wei, 2021). qRT-PCR and Western blot have been used to analyze the genes related to cartilage differentiation in cartilage tissues. The results show that the expression of circUNK was upregulated, which improved the cartilage injury in OA rabbit and promoted the expression of molecules related to cartilage differentiation, such as PCNA, SOX9, Col II, Aggrecan, and protein-polysaccharide. These molecules had sound effects on the proliferation and differentiation of articular cartilage. In conclusion, exercise or mechanical stimulation can lead to changes in the expression of ncRNA, which prevents and improves the pathological changes of articular cartilage, synovial membrane, and subchondral bone through a series of mechanisms, thereby delaying the progression of OA (Fang et al., 2021).
Effects of Different Exercise Types on Human Osteoarthritis
Many types of training can relieve pain, enhance muscle strength and improve joint stiffness (Daenen et al., 2015). For healthy people, exercise can promote good health, while for OA patients, dysfunction can be improved and diseases can be treated (Skou et al., 2018). At present, there are many types of sports for the treatment of OA, such as aerobic exercise, anti-resistance exercise, neuromuscular training, etc (Jansen et al., 2011). In addition, Chinese traditional sports such as Baduanjin, Wuqinxi, and yoga are also applied to the prevention and treatment of OA (Li et al., 2020). This paper summarizes the research on training types of OA patients by summarizing related literature, as shown in Table 2.
TABLE 2.
Different exercise types on human Osteoarthritis (OA).
| Researchers | Number of studies/subjects | Intervention studied | Exercise effect |
| Vincent et al., 2019 | N = 90 | Eccentric and Concentric Resistance Exercise | Modify function and pain symptoms |
| DeVita et al., 2018 | N = 30 | Quadriceps strength training | Increase muscle strength and improve symptomatic and functional outcomes |
| Abbott et al., 2013 | N = 206 | Manual therapy and exercise therapy | Improve knee joint function of patients |
| Lund et al., 2008 | N = 79 | A land-based exercise | Improve pain and muscle strength |
| Chen S.-M. et al., 2019 | N = 70 | Resistance Exercise | Improve muscle strength, dynamic balance and body function |
| Kabiri et al., 2018 | N = 78 | Aerobic exercise combined with resistance training | Relieve pain and improve function |
| Allen et al., 2021 | N = 345 | Stepped exercise | Improve the symptoms of the patient |
| Bennell et al., 2014b | N = 100 | Neuromuscular versus quadriceps strengthening exercise | Relieve pain and improve function |
| Ettinger et al., 1997 | N = 439 | An aerobic or a resistance exercise | Relieve pain and improve function |
| Suzuki et al., 2019 | N = 52 | Multiple exercise | Improve muscle strength and joint flexibility |
| Krauß et al., 2014 | N = 209 | Exercise Therapy | Relieve pain and improve function |
| León-Ballesteros et al., 2020 | N = 32 | Quadriceps strengthening exercise | Relieve pain |
| Rewald et al., 2020 | N = 55 | Aquatic Cycling | Relieve pain and improve function |
| Chen H. et al., 2019 | N = 171 | A home-based exercise | Relieve symptoms, increase the physical functioning, and improve quality of life |
| Alkatan et al., 2016 | N = 48 | Swimming and Cycling Training | Reduce joint pain and stiffness and improve muscle strength and functional capacity |
| Silva et al., 2008 | N = 64 | Water-based and land-based exercises | Relieve pain and improve function |
| Kuntz et al., 2018 | N = 31 | Yoga | Relieve pain and improve function |
| Huang et al., 2018 | N = 250 | Quadriceps functional exercise | Relieve pain and improve function |
| Taglietti et al., 2018 | N = 31 | Aquatic exercises | Relieve pain and improve function |
| Vincent and Vincent, 2020 | N = 88 | Resistance exercise | Relieve pain |
| Holm et al., 2021 | N = 90 | Strength training | Relieve pain |
| Waller et al., 2017 | N = 87 | High intensity aquatic resistance training | Decrease fat mass and improve walking speed |
| Fernandes et al., 2017 | N = 165 | Neuromuscular exercise | Improve the quality of life of patients |
| Ferraz et al., 2018 | N = 48 | Resistance Training | Relieve pain and increase muscle strength |
| Hsu et al., 2021 | N = 66 | Resistance Training | The body composition, blood biochemistry and lower limb function of patients were improved. |
| Devrimsel et al., 2019 | N = 60 | Neuromuscular electrical stimulation | Relieve pain and improve function |
| Hinman et al., 2007 | N = 71 | Aquatic physical therapy | Relieve pain and improve physical function, strength, and quality of life |
| Xiao et al., 2021 | N = 68 | A Wuqinxi exercise | Relieve pain and improve function |
| Yuenyongviwat et al., 2020 | N = 86 | Hip abductor strengthening exercises | Expedite improvement of less pain, symptoms, activity in daily living and quality of life |
| Rabe et al., 2018 | N = 42 | The Combined Application of Neuromuscular Electrical Stimulation and Volitional Contractions | Alleviate pain and improve physical performance |
| Lai et al., 2018 | N = 40 | Strength exercise | Improve the knee flexion proprioception |
| Steinhilber et al., 2017 | N = 210 | Tübingen exercise therapy | Increase muscle strength |
| Kuptniratsaikul et al., 2019 | N = 80 | Underwater treadmill exercise | Relieve pain and improve function |
| Oliveira et al., 2012 | N = 100 | Quadriceps strengthening exercises | Improve pain, function, and stiffness |
| Wang et al., 2007 | N = 38 | Aquatic exercise | Improve knee and hip flexibility, strength and aerobic fitness |
| Nery et al., 2021 | N = 60 | A progressive resistance exercise | Relieve pain and improve function |
| Wang et al., 2016 | N = 39 | Whole-body vibration training with quadriceps strengthening exercise | Improve symptoms, physical function and spatiotemporal parameters |
| Pisters et al., 2010 | N = 200 | Behavioral graded activity | Increase the patient’ s exercise compliance and physical activity |
| An et al., 2013 | N = 28 | Baduanjin | Relieve pain and improve function |
| Trans et al., 2009 | N = 52 | Whole body vibration | Increase muscle strength and proprioception |
| Sevick et al., 2000 | N = 439 | Resistance training | Improve physical function |
| Ojoawo et al., 2016 | N = 45 | Proprioceptive exercises | Relieve pain and joint stiffness |
| Villadsen et al., 2014 | N = 165 | Neuromuscular exercise | Relieve pain and improve activity of daily living |
| Wang et al., 2021 | N = 128 | Quadriceps strengthening exercises | Relieve pain, increase physical function, improve self-efficacy and improve quality of life. |
| Jorge et al., 2015 | N = 29 | Progressive resistance exercise | Relieve pain, improve function and improve quality of life. |
| Penninx et al., 2002 | N = 439 | Aerobic and resistance exercise | Relieve pain and improve walking speed. |
| Simão et al., 2012 | N = 32 | Whole-body vibration training | Improve patients’ self-perception of pain, balance, gait quality and inflammatory markers. |
| Lun et al., 2015 | N = 72 | Hip and leg strengthening exercise | Improve pain, function, and quality of life |
| Maurer et al., 1999 | N = 113 | Isokinetic quadriceps exercise | Relieve pain and improve function |
| Bruce-Brand et al., 2012 | N = 41 | Resistance training and neuromuscular electrical stimulation | Improve function, disability and pain |
| Hiyama et al., 2012 | N = 40 | Walking exercise | Improve executive function and dual-task performance |
| Foley et al., 2003 | N = 105 | A hydrotherapy resistance exercise with a gym based resistance exercise | Improve muscle strength and function. |
| Tsauo et al., 2008 | N = 60 | Sensorimotor training | Improve the patients’ proprioception in the knee joints and their self-reported function |
| Topp et al., 2002 | N = 102 | Dynamic or isometric resistance training | Improve functional ability and reduce knee joint pain |
| Munukka et al., 2016 | N = 87 | Aquatic resistance training | Improve cardiorespiratory fitness |
| Samut et al., 2015 | N = 42 | Isokinetic and aerobic exercise training | Enhance muscle strength and functional ability |
| Wang et al., 2011 | N = 84 | Aquatic exercises and land-based exercises | Relieve pain, improve knee joint range of motion and quality of life |
| Chen P.-Y. et al., 2021 | N = 68 | Tai chi exercise | Enhance physical function |
| Song et al., 2003 | N = 72 | Tai chi exercise | Improve symptoms, balance, and physical functioning. |
Conclusion and Outlook
At present, the incidence of OA is very high, and its pathogenesis remains unclear. The treatment of OA by exercise has received increasing research attention. Not all types of sports used can alleviate OA. The result of different types, intensity, and time of sports on OA differs.
Some studies believe that exercise does not affect OA. For example, Rios et al. (2020) intervened in KOA rats with aerobic exercise for 12 weeks and measured bone mineral density and knee joint injury. The results showed that training did not affect the common knee injury, accelerate the progression of OA, and alleviate the pathological changes of OA. Other studies believe that exercise accelerates the progression of OA. For example, Liu S.-S. et al. (2016) established a rat model of exercise-induced OA. They analyzed and compared the expression levels of various related mRNA, protein, and pathways in the standard and injury group of exercise-induced OA by using RT-qPCR, Western blot analysis, and immunohistochemical staining. The results showed that excessive pressure would lead to abnormal expression of many biochemical signals and abnormal activation of the Wnt/β-catenin pathway, leading to induced OA. Exercise has different effects on healthy and damaged articular cartilage (Rojas-Ortega et al., 2015). Notably, for articular cartilage injury, if high-intensity practice is continued, the activity of catabolic proteins is more vital than that of anabolic proteins. The body’s metabolism is imbalanced, thus efficiently inducing OA. Twelve weeks of high-intensity treadmill training in rats will increase the levels of some apoptosis-related proteins and inflammation-related factors, such as caspase-3, IL-1α, and TNF-α, which is the precursor of OA (Franciozi et al., 2013). The results indicate that high-intensity exercise may lead to the occurrence and development of OA. In addition, moderate exercise can delay the pathological process of OA. For example, a daily external force has been used to compress OA mice under low or medium intensity, and the pathological changes of articular cartilage were observed in the second and sixth weeks. Moreover, low- and medium-intensity compression can reduce the degeneration of articular cartilage and osteophytes of subchondral bone in mice and is very beneficial to the damaged joints, making it an effective method for the treatment of OA (Holyoak et al., 2019).
At present, many exercises have therapeutic effects on OA, such as aerobic exercise, strength training, swimming, neuromuscular exercise, proprioceptive training, and balance training. Different types of motion produce other effects. Aerobic exercise is the most widely used in OA patients, and it can reduce the expression of IL-1β, caspase-3 and MMP-13 and prevent cartilage degradation in KOA rats (Assis et al., 2016). Aerobic exercise may be the best training method to reduce pain and improve body function (Goh et al., 2019). However, aerobic exercise with different intensities has different effects on OA patients with varying degrees of injury. Low-intensity aerobic exercise had a better therapeutic effect in patients with severe OA (Messier et al., 2021), while high-intensity aerobic exercise had a better therapeutic effect in patients with mild OA (Multanen et al., 2017). In addition, strength training could reduce the activity of MMP-2 in the quadriceps tendon of the OA rat model (Vasilceac et al., 2021), which was the most effective in improving muscle strength, and neuromuscular training was the best training method to relieve OA pain (Ageberg and Roos, 2015). Swimming intervention in the early stage of OA can alleviate the stiffness of the knee joint and is better than land exercise (Lund et al., 2008; Hsieh and Yang, 2018; Munukka et al., 2020). Land exercise can be performed after the patients’ joints have a certain degree of flexibility. Land exercise is better than water exercise in improving pain and enhancing function (Escalante et al., 2011). Many traditional techniques are being applied more and more in OA, such as Baduanjin (An et al., 2008), tai chi chuan (Zhang Z. et al., 2020), Wuqinxi (Xiao and Li, 2021), and yoga (Kuntz et al., 2018). They can improve the physical function of OA patients and have a significant impact on the psychological status of OA patients. In addition, new intervention methods such as virtual reality and sports games have been gradually used to improve people’s physical and psychological conditions (Sadeghi and Jehu, 2022). These sports have excellent development prospects in the treatment of OA.
Although many types of exercise can be used to prevent or treat OA, our most recommended method is to formulate a unique exercise prescription for each patient with OA based on the FITT (Frequency, Intensity, Time, and Type) principle. According to the exercise prescription, a study (de Rooij et al., 2017) formulated a personalized rehabilitation program for 126 OA patients for 20 weeks. It was finally found that personalized exercise therapy could effectively improve the OA of the knee joint and the body function of the patients. Exercise prescription recommendations for patients with OA are as follows (Bennell et al., 2014a): (1) Exercise frequency: It is recommended that in the initial stage, the patient had better exercise at least 12 times within three months to master the skills and ensure compliance, and then the frequency was gradually increased and maintained to two to three times a week, and a week of moderate-intensity exercise lasting 150 to 300 min was accumulated; (2) Exercise intensity: Moderate intensity exercise is recommended. Namely, the heart rate is 120–150 beats/min, and the oxygen consumption during exercise is 50–70% of the maximum oxygen consumption; (3) Exercise time: The recommended exercise time is 30–60 min per day. The exercise time was gradually increased from a short time to 30–60 min/d; (4) Exercise type: generally, aerobic exercise and strength training are recommended. Aerobic exercise such as swimming, Tai Ji Chuan, unfavorable for mountaineering, climbing stairs and other excessive weight-bearing movements, strength training have equal length, such as Zhang or isokinetic resistance movement, etc., recommend the weight-bearing exercise is given priority to; (5) Precautions: Pain relief can be obtained only after 8–11 weeks of exercise therapy. Continuous exercise for more than 12 weeks is generally recommended to relieve decreased muscle strength and muscle atrophy associated with OA. Patients should also receive regular education during training to improve their self-management awareness, compliance and exercise efficacy. It is normal to feel some discomfort or pain during exercise. Ice application is required at the joints for 15–20 min after training. If severe pain occurs during exercise or swelling aggravates the next day, you should timely adjust the amount of activity. Different types of patients may have other exercise prescriptions. Patients with higher obesity are more suitable for aquatic exercise. For patients with upper limb OA, emphasis should be placed on improving the affected joints’ range of motion and flexibility. For patients with lower limb OA, emphasis should be placed on improving patients’ muscle strength and body stability. Therefore, we should formulate individualized exercise prescriptions according to the degree of lesion and needs of OA patients.
This review describes the pathological change mechanism of OA and the molecular mechanism of exercise in the treatment of OA. By using RT-qPCR, Western blot analysis, and immunohistochemical staining, we have found that the expression levels of related mRNA, protein, and pathways were changed in OA patients after exercise. This review also summarized animal experiments related to exercise and OA and the role of different exercise types in human OA, described the research progress of exercise in the prevention and treatment of OA, and provided a basis for exercise intervention to treat OA in the future. Exercise, as an intervention means, has a great development prospect in the treatment of OA.
Author Contributions
X-AZ and X-QW: conceptualization, project administration, and funding acquisition. HK, X-AZ, and X-QW: writing – review and editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Funding
This study was supported by the Innovative Talents Support Program for Universities of Liaoning Province, No. WR2019024 and Shanghai Frontiers Science Research Base of Exercise and Metabolic Health.
References
- Abbott J. H., Robertson M. C., Chapple C., Pinto D., Wright A. A., Leon de la Barra S., et al. (2013). Manual therapy, exercise therapy, or both, in addition to usual care, for osteoarthritis of the hip or knee: a randomized controlled trial. 1: clinical effectiveness. Osteoarthritis Cartilage 21 525–534. 10.1016/j.joca.2012.12.014 [DOI] [PubMed] [Google Scholar]
- Ageberg E., Roos E. M. (2015). Neuromuscular exercise as treatment of degenerative knee disease. Exerc. Sport Sci. Rev. 43 14–22. 10.1249/JES.0000000000000030 [DOI] [PubMed] [Google Scholar]
- Aigner T., Kim H. A., Roach H. I. (2004). Apoptosis in osteoarthritis. Rheum. Dis. Clin. North Am. 30 639–653. [DOI] [PubMed] [Google Scholar]
- Al-Hashem F., El Karib A. O., Bin-Jaliah I., Dallak M., Sakr H. F., Eid R. A., et al. (2017). Exercise protects against insulin-dependent diabetes-induced osteoarthritis in rats: a scanning electron microscopy study. Ultrastruct. Pathol. 41 252–257. 10.1080/01913123.2017.1313346 [DOI] [PubMed] [Google Scholar]
- Alkatan M., Baker J. R., Machin D. R., Park W., Akkari A. S., Pasha E. P., et al. (2016). Improved function and reduced pain after swimming and cycling training in patients with osteoarthritis. J. Rheumatol. 43 666–672. 10.3899/jrheum.151110 [DOI] [PubMed] [Google Scholar]
- Allen J., Imbert I., Havelin J., Henderson T., Stevenson G., Liaw L., et al. (2017). Effects of treadmill exercise on advanced osteoarthritis pain in rats. Arthritis Rheumatol. 69 1407–1417. 10.1002/art.40101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen K. D., Woolson S., Hoenig H. M., Bongiorni D., Byrd J., Caves K., et al. (2021). Stepped exercise program for patients with knee osteoarthritis: a randomized controlled trial. Ann. Intern. Med. 174 298–307. 10.7326/M20-4447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- An B., Dai K., Zhu Z., Wang Y., Hao Y., Tang T., et al. (2008). Baduanjin alleviates the symptoms of knee osteoarthritis. J. Altern. Complement. Med. 14 167–174. 10.1089/acm.2007.0600 [DOI] [PubMed] [Google Scholar]
- An B.-C., Wang Y., Jiang X., Lu H.-S., Fang Z.-Y., Wang Y., et al. (2013). Effects of Baduanjin () exercise on knee osteoarthritis: a one-year study. Chin. J. Integr. Med. 19 143–148. 10.1007/s11655-012-1211-y [DOI] [PubMed] [Google Scholar]
- Assis L., Almeida T., Milares L. P., dos Passos N., Araújo B., Bublitz C., et al. (2015). Musculoskeletal atrophy in an experimental model of knee osteoarthritis: the effects of exercise training and low-level laser therapy. Am. J. Phys. Med. Rehabil. 94 609–616. 10.1097/PHM.0000000000000219 [DOI] [PubMed] [Google Scholar]
- Assis L., Milares L. P., Almeida T., Tim C., Magri A., Fernandes K. R., et al. (2016). Aerobic exercise training and low-level laser therapy modulate inflammatory response and degenerative process in an experimental model of knee osteoarthritis in rats. Osteoarthritis Cartilage 24 169–177. 10.1016/j.joca.2015.07.020 [DOI] [PubMed] [Google Scholar]
- Assis L., Tim C., Magri A., Fernandes K. R., Vassão P. G., Renno A. C. M. (2018). Interleukin-10 and collagen type II immunoexpression are modulated by photobiomodulation associated to aerobic and aquatic exercises in an experimental model of osteoarthritis. Lasers Med. Sci. 33 1875–1882. 10.1007/s10103-018-2541-6 [DOI] [PubMed] [Google Scholar]
- Ayral X., Pickering E. H., Woodworth T. G., Mackillop N., Dougados M. (2005). Synovitis: a potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis – results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthritis Cartilage 13 361–367. [DOI] [PubMed] [Google Scholar]
- Barranco C. (2015). Osteoarthritis: activate autophagy to prevent cartilage degeneration? Nat. Rev. Rheumatol. 11:127. 10.1038/nrrheum.2015.12 [DOI] [PubMed] [Google Scholar]
- Bauer D. C., Hunter D. J., Abramson S. B., Attur M., Corr M., Felson D., et al. (2006). Classification of osteoarthritis biomarkers: a proposed approach. Osteoarthritis Cartilage 14 723–727. [DOI] [PubMed] [Google Scholar]
- Baur A., Henkel J., Bloch W., Treiber N., Scharffetter-Kochanek K., Brüggemann G.-P., et al. (2011). Effect of exercise on bone and articular cartilage in heterozygous manganese superoxide dismutase (SOD2) deficient mice. Free Radic. Res. 45 550–558. 10.3109/10715762.2011.555483 [DOI] [PubMed] [Google Scholar]
- Belavy D. L., Van Oosterwijck J., Clarkson M., Dhondt E., Mundell N. L., Miller C. T., et al. (2021). Pain sensitivity is reduced by exercise training: evidence from a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 120 100–108. 10.1016/j.neubiorev.2020.11.012 [DOI] [PubMed] [Google Scholar]
- Beltrami C., Angelini T. G., Emanueli C. (2015). Noncoding RNAs in diabetes vascular complications. J. Mol. Cell. Cardiol. 89(Pt A), 42–50. 10.1016/j.yjmcc.2014.12.014 [DOI] [PubMed] [Google Scholar]
- Benedetti M. G., Furlini G., Zati A., Letizia Mauro G. (2018). The effectiveness of physical exercise on bone density in osteoporotic patients. Biomed Res. Int. 2018:4840531. 10.1155/2018/4840531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito M. J., Veale D. J., FitzGerald O., van den Berg W. B., Bresnihan B. (2005). Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 64 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennell K. L., Dobson F., Hinman R. S. (2014a). Exercise in osteoarthritis: moving from prescription to adherence. Best Pract. Res. Clin. Rheumatol. 28 93–117. 10.1016/j.berh.2014.01.009 [DOI] [PubMed] [Google Scholar]
- Bennell K. L., Kyriakides M., Metcalf B., Egerton T., Wrigley T. V., Hodges P. W., et al. (2014b). Neuromuscular versus quadriceps strengthening exercise in patients with medial knee osteoarthritis and varus malalignment: a randomized controlled trial. Arthritis Rheumatol. 66 950–959. 10.1002/art.38317 [DOI] [PubMed] [Google Scholar]
- Bennell K. L., Hunter D. J., Hinman R. S. (2012). Management of osteoarthritis of the knee. BMJ 345:e4934. 10.1136/bmj.e4934 [DOI] [PubMed] [Google Scholar]
- Blazek A. D., Nam J., Gupta R., Pradhan M., Perera P., Weisleder N. L., et al. (2016). Exercise- driven metabolic pathways in healthy cartilage. Osteoarthritis Cartilage 24 1210–1222. 10.1016/j.joca.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondeson J., Blom A. B., Wainwright S., Hughes C., Caterson B., van den Berg W. B. (2010). The role of synovial macrophages and macrophage-produced mediators in driving inflammatory and destructive responses in osteoarthritis. Arthritis Rheum. 62 647–657. 10.1002/art.27290 [DOI] [PubMed] [Google Scholar]
- Boudenot A., Presle N., Uzbekov R., Toumi H., Pallu S., Lespessailles E. (2014). Effect of interval-training exercise on subchondral bone in a chemically-induced osteoarthritis model. Osteoarthritis Cartilage 22 1176–1185. 10.1016/j.joca.2014.05.020 [DOI] [PubMed] [Google Scholar]
- Brandt K. D., Braunstein E. M., Visco D. M., O’Connor B., Heck D., Albrecht M. (1991). Anterior (cranial) cruciate ligament transection in the dog: a bona fide model of osteoarthritis, not merely of cartilage injury and repair. J. Rheumatol. 18 436–446. [PubMed] [Google Scholar]
- Braun H. J., Gold G. E. (2012). Diagnosis of osteoarthritis: imaging. Bone 51 278–288. 10.1016/j.bone.2011.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Brand R. A., Walls R. J., Ong J. C., Emerson B. S., O’Byrne J. M., Moyna N. M. (2012). Effects of home-based resistance training and neuromuscular electrical stimulation in knee osteoarthritis: a randomized controlled trial. BMC Musculoskelet. Disord. 13:118. 10.1186/1471-2474-13-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr D. B., Gallant M. A. (2012). Bone remodelling in osteoarthritis. Nat. Rev. Rheumatol. 8 665–673. 10.1038/nrrheum.2012.130 [DOI] [PubMed] [Google Scholar]
- Caramés B., Taniguchi N., Otsuki S., Blanco F. J., Lotz M. (2010). Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 62 791–801. 10.1002/art.27305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castrogiovanni P., Di Rosa M., Ravalli S., Castorina A., Guglielmino C., Imbesi R., et al. (2019). Moderate physical activity as a prevention method for knee osteoarthritis and the role of Synoviocytes as Biological Key. Int. J. Mol. Sci. 20:511. 10.3390/ijms20030511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J., Wang W., Zhang H., Hu Y., Wang M., Yin Z. (2013). The dual role of autophagy in chondrocyte responses in the pathogenesis of articular cartilage degeneration in osteoarthritis. Int. J. Mol. Med. 32 1311–1318. 10.3892/ijmm.2013.1520 [DOI] [PubMed] [Google Scholar]
- Chen H., Zheng X., Huang H., Liu C., Wan Q., Shang S. (2019). The effects of a home-based exercise intervention on elderly patients with knee osteoarthritis: a quasi-experimental study. BMC Musculoskelet. Disord. 20:160. 10.1186/s12891-019-2521-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.-M., Shen F.-C., Chen J.-F., Chang W.-D., Chang N.-J. (2019). Effects of resistance exercise on glycated hemoglobin and functional performance in older patients with comorbid diabetes mellitus and knee osteoarthritis: a randomized trial. Int. J. Environ. Res. Public Health 17:224. 10.3390/ijerph17010224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Li Q., Wang J., Jin S., Zheng H., Lin J., et al. (2017). MiR-29b-3p promotes chondrocyte apoptosis and facilitates the occurrence and development of osteoarthritis by targeting PGRN. J. Cell. Mol. Med. 21 3347–3359. 10.1111/jcmm.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Lou Y., Pan Z., Cao X., Zhang L., Zhu C., et al. (2020). Treadmill and wheel exercise protect against JNK/NF-κB induced inflammation in experimental models of knee osteoarthritis. Biochem. Biophys. Res. Commun. 523 117–122. 10.1016/j.bbrc.2019.12.014 [DOI] [PubMed] [Google Scholar]
- Chen X., Gong W., Shao X., Shi T., Zhang L., Dong J., et al. (2021). METTL3-mediated mA modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann. Rheum. Dis. 81 87–99. 10.1136/annrheumdis-2021-221091 [DOI] [PubMed] [Google Scholar]
- Chen P.-Y., Song C.-Y., Yen H.-Y., Lin P.-C., Chen S.-R., Lu L.-H., et al. (2021). Impacts of tai chi exercise on functional fitness in community-dwelling older adults with mild degenerative knee osteoarthritis: a randomized controlled clinical trial. BMC Geriatr. 21:449. 10.1186/s12877-021-02390-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cifuentes D. J., Rocha L. G., Silva L. A., Brito A. C., Rueff-Barroso C. R., Porto L. C., et al. (2010). Decrease in oxidative stress and histological changes induced by physical exercise calibrated in rats with osteoarthritis induced by monosodium iodoacetate. Osteoarthritis Cartilage 18 1088–1095. 10.1016/j.joca.2010.04.004 [DOI] [PubMed] [Google Scholar]
- Cormier J., Cone K., Lanpher J., Kinens A., Henderson T., Liaw L., et al. (2017). Exercise reverses pain-related weight asymmetry and differentially modulates trabecular bone microarchitecture in a rat model of osteoarthritis. Life Sci. 180 51–59. 10.1016/j.lfs.2017.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui D., Xu X. (2018). DNA Methyltransferases, DNA methylation, and age-associated cognitive function. Int. J. Mol. Sci. 19:1315. 10.3390/ijms19051315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da R.-R., Qin Y., Baeten D., Zhang Y. (2007). B cell clonal expansion and somatic hypermutation of Ig variable heavy chain genes in the synovial membrane of patients with osteoarthritis. J. Immunol. 178 557–565. [DOI] [PubMed] [Google Scholar]
- Daenen L., Varkey E., Kellmann M., Nijs J. (2015). Exercise, not to exercise, or how to exercise in patients with chronic pain? Applying science to practice. Clin. J. Pain 31 108–114. 10.1097/AJP.0000000000000099 [DOI] [PubMed] [Google Scholar]
- Dai X., Ren T., Zhang Y., Nan N. (2021). Methylation multiplicity and its clinical values in cancer. Expert Rev. Mol. Med. 23:e2. 10.1017/erm.2021.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij M., van der Leeden M., Cheung J., van der Esch M., Häkkinen A., Haverkamp D., et al. (2017). Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: a randomized controlled trial. Arthritis Care Res. 69 807–816. 10.1002/acr.23013 [DOI] [PubMed] [Google Scholar]
- DeVita P., Aaboe J., Bartholdy C., Leonardis J. M., Bliddal H., Henriksen M. (2018). Quadriceps-strengthening exercise and quadriceps and knee biomechanics during walking in knee osteoarthritis: a two-centre randomized controlled trial. Clin. Biomech. 59 199–206. 10.1016/j.clinbiomech.2018.09.016 [DOI] [PubMed] [Google Scholar]
- Devrimsel G., Metin Y., Serdaroglu Beyazal M. (2019). Short-term effects of neuromuscular electrical stimulation and ultrasound therapies on muscle architecture and functional capacity in knee osteoarthritis: a randomized study. Clin. Rehabil. 33 418–427. 10.1177/0269215518817807 [DOI] [PubMed] [Google Scholar]
- Duan R., Xie H., Liu Z.-Z. (2020). The role of autophagy in osteoarthritis. Front. Cell Dev. Biol. 8:608388. 10.3389/fcell.2020.608388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn W., DuRaine G., Reddi A. H. (2009). Profiling microRNA expression in bovine articular cartilage and implications for mechanotransduction. Arthritis Rheum. 60 2333–2339. 10.1002/art.24678 [DOI] [PubMed] [Google Scholar]
- Elmore S. (2007). Apoptosis: a review of programmed cell death. Toxicol. Pathol. 35 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante Y., García-Hermoso A., Saavedra J. M. (2011). Effects of exercise on functional aerobic capacity in lower limb osteoarthritis: a systematic review. J. Sci. Med. Sport 14 190–198. 10.1016/j.jsams.2010.10.004 [DOI] [PubMed] [Google Scholar]
- Ettinger W. H., Burns R., Messier S. P., Applegate W., Rejeski W. J., Morgan T., et al. (1997). A randomized trial comparing aerobic exercise and resistance exercise with a health education program in older adults with knee osteoarthritis. The Fitness Arthritis and Seniors Trial (FAST). JAMA 277 25–31. [PubMed] [Google Scholar]
- Eyre D. R. (2004). Collagens and cartilage matrix homeostasis. Clin. Orthop. Relat. Res. 427(Suppl.), S118–S122. [DOI] [PubMed] [Google Scholar]
- Fallah Mohammadi M., Hajizadeh Moghaddam A., Mirkarimpur H. (2013). The effects of a moderate exercise program on knee osteoarthritis in male wistar rats. Iran. J. Basic Med. Sci. 16 683–688. [PMC free article] [PubMed] [Google Scholar]
- Fang L., Lin L., Lv Y., Huang Z., Lin X., Wang X., et al. (2021). The mechanism of aerobic exercise combined with glucosamine therapy and circUNK in improving knee osteoarthritis in rabbits. Life Sci. 275:119375. 10.1016/j.lfs.2021.119375 [DOI] [PubMed] [Google Scholar]
- Felson D. T., Zhang Y., Hannan M. T., Naimark A., Weissman B., Aliabadi P., et al. (1997). Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 40 728–733. [DOI] [PubMed] [Google Scholar]
- Fernandes L., Roos E. M., Overgaard S., Villadsen A., Søgaard R. (2017). Supervised neuromuscular exercise prior to hip and knee replacement: 12-month clinical effect and cost-utility analysis alongside a randomised controlled trial. BMC Musculoskelet. Disord. 18:5. 10.1186/s12891-016-1369-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz R. B., Gualano B., Rodrigues R., Kurimori C. O., Fuller R., Lima F. R., et al. (2018). Benefits of resistance training with blood flow restriction in knee osteoarthritis. Med. Sci. Sports Exerc. 50 897–905. 10.1249/MSS.0000000000001530 [DOI] [PubMed] [Google Scholar]
- Foley A., Halbert J., Hewitt T., Crotty M. (2003). Does hydrotherapy improve strength and physical function in patients with osteoarthritis–a randomised controlled trial comparing a gym based and a hydrotherapy based strengthening programme. Ann. Rheum. Dis. 62 1162–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franciozi C. E. S., Tarini V. A. F., Reginato R. D., Gonçalves P. R. S., Medeiros V. P., Ferretti M., et al. (2013). Gradual strenuous running regimen predisposes to osteoarthritis due to cartilage cell death and altered levels of glycosaminoglycans. Osteoarthritis Cartilage 21 965–972. 10.1016/j.joca.2013.04.007 [DOI] [PubMed] [Google Scholar]
- Galois L., Etienne S., Grossin L., Watrin-Pinzano A., Cournil-Henrionnet C., Loeuille D., et al. (2004). Dose-response relationship for exercise on severity of experimental osteoarthritis in rats: a pilot study. Osteoarthritis Cartilage 12 779–786. [DOI] [PubMed] [Google Scholar]
- Gianni L., Pienkowski T., Im Y.-H., Tseng L.-M., Liu M.-C., Lluch A., et al. (2016). 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 17 791–800. 10.1016/S1470-2045(16)00163-7 [DOI] [PubMed] [Google Scholar]
- Goh S.-L., Persson M. S. M., Stocks J., Hou Y., Welton N. J., Lin J., et al. (2019). Relative efficacy of different exercises for pain, function, performance and quality of life in knee and hip osteoarthritis: systematic review and network meta-analysis. Sports Med. 49 743–761. 10.1007/s40279-019-01082-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring M. B., Goldring S. R. (2007). Osteoarthritis. J. Cell. Physiol. 213 626–634. [DOI] [PubMed] [Google Scholar]
- Goldring M. B., Otero M. (2011). Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 23 471–478. 10.1097/BOR.0b013e328349c2b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracey E., Burssens A., Cambré I., Schett G., Lories R., McInnes I. B., et al. (2020). Tendon and ligament mechanical loading in the pathogenesis of inflammatory arthritis. Nat. Rev. Rheumatol. 16 193–207. 10.1038/s41584-019-0364-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin T. M., Batushansky A., Hudson J., Lopes E. B. P. (2020). Correlation network analysis shows divergent effects of a long-term, high-fat diet and exercise on early stage osteoarthritis phenotypes in mice. J. Sport Health Sci. 9 119–131. 10.1016/j.jshs.2019.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin T. M., Huebner J. L., Kraus V. B., Yan Z., Guilak F. (2012). Induction of osteoarthritis and metabolic inflammation by a very high-fat diet in mice: effects of short-term exercise. Arthritis Rheum. 64 443–453. 10.1002/art.33332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y.-J., Yang X., Wei L., Chen Q. (2011). MiR-365: a mechanosensitive microRNA stimulates chondrocyte differentiation through targeting histone deacetylase 4. FASEB J. 25 4457–4466. 10.1096/fj.11-185132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J., Chung U.-I., Kondo H., Bringhurst F. R., Kronenberg H. M. (2002). The PTH/PTHrP receptor can delay chondrocyte hypertrophy in vivo without activating phospholipase C. Dev. Cell 3 183–194. [DOI] [PubMed] [Google Scholar]
- Guo Y.-F., Su T., Yang M., Li C.-J., Guo Q., Xiao Y., et al. (2021). The role of autophagy in bone homeostasis. J. Cell. Physiol. 236 4152–4173. 10.1002/jcp.30111 [DOI] [PubMed] [Google Scholar]
- Hao X., Wang S., Zhang J., Xu T. (2021). Effects of body weight-supported treadmill training on cartilage-subchondral bone unit in the rat model of posttraumatic osteoarthritis. J. Orthop. Res. 39 1227–1235. 10.1002/jor.24791 [DOI] [PubMed] [Google Scholar]
- Hashimoto S., Ochs R. L., Komiya S., Lotz M. (1998). Linkage of chondrocyte apoptosis and cartilage degradation in human osteoarthritis. Arthritis Rheum. 41 1632–1638. [DOI] [PubMed] [Google Scholar]
- He Y., Wang W., Xu X., Yang B., Yu X., Wu Y., et al. (2021). Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through mediating Bcl2 stability via Ythdf1-mediated mA modification. Bone 154:116182. 10.1016/j.bone.2021.116182 [DOI] [PubMed] [Google Scholar]
- He Z., Li H., Han X., Zhou F., Du J., Yang Y., et al. (2020). Irisin inhibits osteocyte apoptosis by activating the Erk signaling pathway in vitro and attenuates ALCT-induced osteoarthritis in mice. Bone 141:115573. 10.1016/j.bone.2020.115573 [DOI] [PubMed] [Google Scholar]
- Héraud F., Héraud A., Harmand M. F. (2000). Apoptosis in normal and osteoarthritic human articular cartilage. Ann. Rheum. Dis. 59 959–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinman R. S., Heywood S. E., Day A. R. (2007). Aquatic physical therapy for hip and knee osteoarthritis: results of a single-blind randomized controlled trial. Phys. Ther. 87 32–43. [DOI] [PubMed] [Google Scholar]
- Hiyama Y., Yamada M., Kitagawa A., Tei N., Okada S. (2012). A four-week walking exercise programme in patients with knee osteoarthritis improves the ability of dual-task performance: a randomized controlled trial. Clin. Rehabil. 26 403–412. 10.1177/0269215511421028 [DOI] [PubMed] [Google Scholar]
- Holm P. M., Petersen K. K., Wernbom M., Schrøder H. M., Arendt-Nielsen L., Skou S. T. (2021). Strength training in addition to neuromuscular exercise and education in individuals with knee osteoarthritis-the effects on pain and sensitization. Eur. J. Pain 25 1898–1911. 10.1002/ejp.1796 [DOI] [PubMed] [Google Scholar]
- Holyoak D. T., Chlebek C., Kim M. J., Wright T. M., Otero M., van der Meulen M. C. H. (2019). Low-level cyclic tibial compression attenuates early osteoarthritis progression after joint injury in mice. Osteoarthritis Cartilage 27 1526–1536. 10.1016/j.joca.2019.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong E., Reddi A. H. (2012). MicroRNAs in chondrogenesis, articular cartilage, and osteoarthritis: implications for tissue engineering. Tissue Eng. Part B Rev. 18 445–453. 10.1089/ten.TEB.2012.0116 [DOI] [PubMed] [Google Scholar]
- Hong Y., Kim H., Lee Y., Lee S., Kim K., Jin Y., et al. (2014). Salutary effects of melatonin combined with treadmill exercise on cartilage damage. J. Pineal Res. 57 53–66. 10.1111/jpi.12143 [DOI] [PubMed] [Google Scholar]
- Houard X., Goldring M. B., Berenbaum F. (2013). Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr. Rheumatol. Rep. 15:375. 10.1007/s11926-013-0375-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh Y.-L., Yang C.-C. (2018). Early intervention of swimming exercises attenuate articular cartilage destruction in a rat model of anterior cruciate ligament and meniscus knee injuries. Life Sci. 212 267–274. 10.1016/j.lfs.2018.10.013 [DOI] [PubMed] [Google Scholar]
- Hsu Y.-I., Chen Y.-C., Lee C.-L., Chang N.-J. (2021). Effects of diet control and telemedicine-based resistance exercise intervention on patients with obesity and knee osteoarthritis: a randomized control trial. Int. J. Environ. Res. Public Health 18:7744. 10.3390/ijerph18157744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Zhao L., Fan Y., Liao L., Ma P. X., Xiao G., et al. (2019). The microRNAs miR-204 and miR-211 maintain joint homeostasis and protect against osteoarthritis progression. Nat. Commun. 10:2876. 10.1038/s41467-019-10753-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Guo B., Xu F., Zhao J. (2018). Effects of quadriceps functional exercise with isometric contraction in the treatment of knee osteoarthritis. Int. J. Rheum. Dis. 21 952–959. 10.1111/1756-185X.13082 [DOI] [PubMed] [Google Scholar]
- Hwang H. S., Kim H. A. (2015). Chondrocyte apoptosis in the pathogenesis of osteoarthritis. Int. J. Mol. Sci. 16 26035–26054. 10.3390/ijms161125943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iijima H., Aoyama T., Ito A., Tajino J., Yamaguchi S., Nagai M., et al. (2016). Exercise intervention increases expression of bone morphogenetic proteins and prevents the progression of cartilage-subchondral bone lesions in a post-traumatic rat knee model. Osteoarthritis Cartilage 24 1092–1102. 10.1016/j.joca.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Iijima H., Aoyama T., Ito A., Yamaguchi S., Nagai M., Tajino J., et al. (2015). Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthritis Cartilage 23 1563–1574. 10.1016/j.joca.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Iijima H., Ito A., Nagai M., Tajino J., Yamaguchi S., Kiyan W., et al. (2017). Physiological exercise loading suppresses post-traumatic osteoarthritis progression via an increase in bone morphogenetic proteins expression in an experimental rat knee model. Osteoarthritis Cartilage 25 964–975. 10.1016/j.joca.2016.12.008 [DOI] [PubMed] [Google Scholar]
- Jansen M. J., Viechtbauer W., Lenssen A. F., Hendriks E. J. M., de Bie R. A. (2011). Strength training alone, exercise therapy alone, and exercise therapy with passive manual mobilisation each reduce pain and disability in people with knee osteoarthritis: a systematic review. J. Physiother. 57 11–20. 10.1016/S1836-9553(11)70002-9 [DOI] [PubMed] [Google Scholar]
- Jia Z., Wei Q.-J. (2021). CircRNA-MSR Regulates LPS-Induced C28/I2 Chondrocyte Injury through miR-643/MAP2K6 Signaling Pathway. Cartilage 13(2_Suppl), 785S–795S. 10.1177/19476035211044826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge R. T. B., Souza M. C. D., Chiari A., Jones A., Fernandes A. D. R. C., Lombardi Júnior I., et al. (2015). Progressive resistance exercise in women with osteoarthritis of the knee: a randomized controlled trial. Clin. Rehabil. 29 234–243. 10.1177/0269215514540920 [DOI] [PubMed] [Google Scholar]
- Jusic A., Devaux Y. (2020). Mitochondrial noncoding RNA-regulatory network in cardiovascular disease. Basic Res. Cardiol. 115:23. 10.1007/s00395-020-0783-5 [DOI] [PubMed] [Google Scholar]
- Kabiri S., Halabchi F., Angoorani H., Yekaninejad S. (2018). Comparison of three modes of aerobic exercise combined with resistance training on the pain and function of patients with knee osteoarthritis: a randomized controlled trial. Phys. Ther. Sport 32 22–28. 10.1016/j.ptsp.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.-P., Fahmi H. (2011). Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 7 33–42. 10.1038/nrrheum.2010.196 [DOI] [PubMed] [Google Scholar]
- Karreth F. A., Pandolfi P. P. (2013). ceRNA cross-talk in cancer: When ce-bling rivalries go awry. Cancer Discov. 3 1113–1121. 10.1158/2159-8290.CD-13-0202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D. Y., Taylor H. W., Moore R. M., Paulsen D. B., Cho D. Y. (2003). Articular chondrocyte apoptosis in equine osteoarthritis. Vet. J. 166 52–57. [DOI] [PubMed] [Google Scholar]
- Kongara S., Karantza V. (2012). The interplay between autophagy and ROS in tumorigenesis. Front. Oncol. 2:171. 10.3389/fonc.2012.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauß I., Steinhilber B., Haupt G., Miller R., Martus P., Janßen P. (2014). Exercise therapy in hip osteoarthritis–a randomized controlled trial. Dtsch. Arztebl. Int. 111 592–599. 10.3238/arztebl.2014.0592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntz A. B., Chopp-Hurley J. N., Brenneman E. C., Karampatos S., Wiebenga E. G., Adachi J. D., et al. (2018). Efficacy of a biomechanically-based yoga exercise program in knee osteoarthritis: a randomized controlled trial. PLoS One 13:e0195653. 10.1371/journal.pone.0195653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuptniratsaikul V., Kittichaikarn C., Suntornpiyapan P., Kovintaset K., Inthibal S. (2019). Is four-week underwater treadmill exercise regimen compared to home exercise efficacious for pain relief and functional improvement in obese patients with knee osteoarthritis? A randomized controlled trial. Clin. Rehabil. 33 85–93. 10.1177/0269215518792041 [DOI] [PubMed] [Google Scholar]
- Lai Z., Zhang Y., Lee S., Wang L. (2018). Effects of strength exercise on the knee and ankle proprioception of individuals with knee osteoarthritis. Res. Sports Med. 26 138–146. 10.1080/15438627.2018.1431541 [DOI] [PubMed] [Google Scholar]
- León-Ballesteros S., Espinosa-Morales R., Clark-Peralta P., Gómez-Pineda A. G., Guadarrama-Becerril J. H. (2020). Kinesiotape and quadriceps strengthening with elastic band in women with knee osteoarthritis and overweight or obesity. A randomized clinical trial. Reumatol. Clin. 16 11–16. 10.1016/j.reuma.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Li Z., Huang Z., Zhang H., Lu J., Wei Y., Yang Y., et al. (2021). IRE1-mTOR-PERK Axis coordinates autophagy and er stress-apoptosis induced by p2x7-mediated ca influx in osteoarthritis. Front. Cell Dev. Biol. 9:695041. 10.3389/fcell.2021.695041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K., Liu A., Zong W., Dai L., Liu Y., Luo R., et al. (2021). Moderate exercise ameliorates osteoarthritis by reducing lipopolysaccharides from gut microbiota in mice. Saudi J. Biol. Sci. 28 40–49. 10.1016/j.sjbs.2020.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Chen H., Feng J., Xiao Y., Zhang H., Lam C. W.-K., et al. (2020). Effectiveness of traditional chinese exercise for symptoms of knee osteoarthritis: a systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 17:7873. 10.3390/ijerph17217873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y.-S., Zhang F.-J., Zeng C., Luo W., Xiao W.-F., Gao S.-G., et al. (2016). Autophagy in osteoarthritis. Joint Bone Spine 83 143–148. 10.1016/j.jbspin.2015.06.009 [DOI] [PubMed] [Google Scholar]
- Li Z.-C., Xiao J., Peng J.-L., Chen J.-W., Ma T., Cheng G.-Q., et al. (2014). Functional annotation of rheumatoid arthritis and osteoarthritis associated genes by integrative genome-wide gene expression profiling analysis. PLoS One 9:e85784. 10.1371/journal.pone.0085784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberthal J., Sambamurthy N., Scanzello C. R. (2015). Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage 23 1825–1834. 10.1016/j.joca.2015.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Hu X., Zhang X., Dai L., Duan X., Zhou C., et al. (2016). The TMSB4 Pseudogene LncRNA functions as a competing endogenous RNA to promote cartilage degradation in human osteoarthritis. Mol. Ther. 24 1726–1733. 10.1038/mt.2016.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S.-S., Zhou P., Zhang Y. (2016). Abnormal expression of key genes and proteins in the canonical Wnt/β-catenin pathway of articular cartilage in a rat model of exercise-induced osteoarthritis. Mol. Med. Rep. 13 1999–2006. 10.3892/mmr.2016.4798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q., Zhang X., Hu X., Yuan L., Cheng J., Jiang Y., et al. (2017). Emerging Roles of circRNA related to the mechanical stress in human cartilage degradation of osteoarthritis. Mol. Ther. Nucleic Acids 7 223–230. 10.1016/j.omtn.2017.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Bryan R. (2013). Synovium and the innate inflammatory network in osteoarthritis progression. Curr. Rheumatol. Rep. 15:323. 10.1007/s11926-013-0323-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser R. F., Goldring S. R., Scanzello C. R., Goldring M. B. (2012). Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 64 1697–1707. 10.1002/art.34453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo P., Bayliss M. T., Heinegård D. (2004). Altered patterns and synthesis of extracellular matrix macromolecules in early osteoarthritis. Matrix Biol. 23 381–391. [DOI] [PubMed] [Google Scholar]
- Lu J., Feng X., Zhang H., Wei Y., Yang Y., Tian Y., et al. (2021). Maresin-1 suppresses IL-1β-induced MMP-13 secretion by activating the PI3K/AKT pathway and inhibiting the NF-κB pathway in synovioblasts of an osteoarthritis rat model with treadmill exercise. Connect. Tissue Res. 62 508–518. 10.1080/03008207.2020.1780218 [DOI] [PubMed] [Google Scholar]
- Lun V., Marsh A., Bray R., Lindsay D., Wiley P. (2015). Efficacy of hip strengthening exercises compared with leg strengthening exercises on knee pain, function, and quality of life in patients with knee osteoarthritis. Clin. J. Sport Med. 25 509–517. 10.1097/JSM.0000000000000170 [DOI] [PubMed] [Google Scholar]
- Lund H., Weile U., Christensen R., Rostock B., Downey A., Bartels E. M., et al. (2008). A randomized controlled trial of aquatic and land-based exercise in patients with knee osteoarthritis. J. Rehabil. Med. 40 137–144. 10.2340/16501977-0134 [DOI] [PubMed] [Google Scholar]
- Luo Y., Sinkeviciute D., He Y., Karsdal M., Henrotin Y., Mobasheri A., et al. (2017). The minor collagens in articular cartilage. Protein Cell 8 560–572. 10.1007/s13238-017-0377-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z., Ji J. (2020). N6-methyladenosine (m6A) RNA modification in cancer stem cells. Stem Cells. 10.1002/stem.3279 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Malemud C. J. (2017). Matrix metalloproteinases and synovial joint pathology. Prog. Mol. Biol. Transl. Sci. 148 305–325. 10.1016/bs.pmbts.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Malfait A. M. (2016). Osteoarthritis year in review 2015: biology. Osteoarthritis Cartilage 24 21–26. 10.1016/j.joca.2015.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins J. B., Mendonça V. A., Aguiar G. C., da Fonseca S. F., Dos Santos J. M., Tossige-Gomes R., et al. (2019). Effect of a moderate-intensity aerobic training on joint biomarkers and functional adaptations in rats subjected to induced knee osteoarthritis. Front. Physiol. 10:1168. 10.3389/fphys.2019.01168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew R., Karp C. M., Beaudoin B., Vuong N., Chen G., Chen H.-Y., et al. (2009). Autophagy suppresses tumorigenesis through elimination of p62. Cell 137 1062–1075. 10.1016/j.cell.2009.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer B. T., Stern A. G., Kinossian B., Cook K. D., Schumacher H. R. (1999). Osteoarthritis of the knee: isokinetic quadriceps exercise versus an educational intervention. Arch. Phys. Med. Rehabil. 80 1293–1299. [DOI] [PubMed] [Google Scholar]
- Messier S. P., Mihalko S. L., Beavers D. P., Nicklas B. J., DeVita P., Carr J. J., et al. (2021). Effect of high-intensity strength training on knee pain and knee joint compressive forces among adults with knee osteoarthritis: the START randomized clinical trial. JAMA 325 646–657. 10.1001/jama.2021.0411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milares L. P., Assis L., Siqueira A., Claudino V., Domingos H., Almeida T., et al. (2016). Effectiveness of an aquatic exercise program and low-level laser therapy on articular cartilage in an experimental model of osteoarthritis in rats. Connect. Tissue Res. 57 398–407. 10.1080/03008207.2016.1193174 [DOI] [PubMed] [Google Scholar]
- Mobasheri A., Rayman M. P., Gualillo O., Sellam J., van der Kraan P., Fearon U. (2017). The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 13 302–311. 10.1038/nrrheum.2017.50 [DOI] [PubMed] [Google Scholar]
- Multanen J., Rantalainen T., Kautiainen H., Ahola R., Jämsä T., Nieminen M. T., et al. (2017). Effect of progressive high-impact exercise on femoral neck structural strength in postmenopausal women with mild knee osteoarthritis: a 12-month RCT. Osteoporos. Int. 28 1323–1333. 10.1007/s00198-016-3875-1 [DOI] [PubMed] [Google Scholar]
- Munukka M., Waller B., Häkkinen A., Nieminen M. T., Lammentausta E., Kujala U. M., et al. (2020). Effects of progressive aquatic resistance training on symptoms and quality of life in women with knee osteoarthritis: a secondary analysis. Scand. J. Med. Sci. Sports 30 1064–1072. 10.1111/sms.13630 [DOI] [PubMed] [Google Scholar]
- Munukka M., Waller B., Rantalainen T., Häkkinen A., Nieminen M. T., Lammentausta E., et al. (2016). Efficacy of progressive aquatic resistance training for tibiofemoral cartilage in postmenopausal women with mild knee osteoarthritis: a randomised controlled trial. Osteoarthritis Cartilage 24 1708–1717. 10.1016/j.joca.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Musumeci G., Castrogiovanni P., Trovato F. M., Imbesi R., Giunta S., Szychlinska M. A., et al. (2015). Physical activity ameliorates cartilage degeneration in a rat model of aging: a study on lubricin expression. Scand. J. Med. Sci. Sports 25 e222–e230. 10.1111/sms.12290 [DOI] [PubMed] [Google Scholar]
- Musumeci G., Loreto C., Carnazza M. L., Martinez G. (2011). Characterization of apoptosis in articular cartilage derived from the knee joints of patients with osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 19 307–313. [DOI] [PubMed] [Google Scholar]
- Musumeci G., Loreto C., Leonardi R., Castorina S., Giunta S., Carnazza M. L., et al. (2013). The effects of physical activity on apoptosis and lubricin expression in articular cartilage in rats with glucocorticoid-induced osteoporosis. J. Bone Miner. Metab. 31 274–284. 10.1007/s00774-012-0414-9 [DOI] [PubMed] [Google Scholar]
- Na S. S., Kim S. G., Yong M. S., Hwangbo G. (2014). Study of treadmill exercise effect on rats with osteoarthritis using proteomic analysis. J. Phys. Ther. Sci. 26 487–490. 10.1589/jpts.26.487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery M., Natour J., Jennings F., Fernandes A. D. R. C., Souza M. C., Jones A. (2021). Effects of a progressive resistance exercise program in patients with hand osteoarthritis: a randomized, controlled trial with a blinded assessor. Clin. Rehabil. 35 1757–1767. 10.1177/02692155211030622 [DOI] [PubMed] [Google Scholar]
- Norrdin R. W., Kawcak C. E., Capwell B. A., McIlwraith C. W. (1998). Subchondral bone failure in an equine model of overload arthrosis. Bone 22 133–139. [DOI] [PubMed] [Google Scholar]
- Ojoawo A. O., Olaogun M. O. B., Hassan M. A. (2016). Comparative effects of proprioceptive and isometric exercises on pain intensity and difficulty in patients with knee osteoarthritis: a randomised control study. Technol. Health Care 24 853–863. [DOI] [PubMed] [Google Scholar]
- Oka Y., Murata K., Kano T., Ozone K., Arakawa K., Kokubun T., et al. (2019). Impact of controlling abnormal joint movement on the effectiveness of subsequent exercise intervention in mouse models of early knee osteoarthritis. Cartilage 13 (2_Suppl), 1334S–1344S. 10.1177/1947603519885007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka Y., Murata K., Ozone K., Kano T., Minegishi Y., Kuro-Nakajima A., et al. (2021). Treadmill exercise after controlled abnormal joint movement inhibits cartilage degeneration and synovitis. Life 11:303. 10.3390/life11040303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira A. M. I. D., Peccin M. S., Silva K. N. G. D., Teixeira L. E. P. D. P., Trevisani V. F. M. (2012). Impact of exercise on the functional capacity and pain of patients with knee osteoarthritis: a randomized clinical trial. Rev. Bras. Reumatol. 52 876–882. [PubMed] [Google Scholar]
- Pap T., Dankbar B., Wehmeyer C., Korb-Pap A., Sherwood J. (2020). Synovial fibroblasts and articular tissue remodelling: role and mechanisms. Semin. Cell Dev. Biol. 101 140–145. 10.1016/j.semcdb.2019.12.006 [DOI] [PubMed] [Google Scholar]
- Pedersen B. K., Saltin B. (2015). Exercise as medicine - evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 25(Suppl.), 3. 10.1111/sms.12581 [DOI] [PubMed] [Google Scholar]
- Peng M.-S., Wang R., Wang Y.-Z., Chen C.-C., Wang J., Liu X.-C., et al. (2022). Efficacy of therapeutic aquatic exercise vs physical therapy modalities for patients with chronic low back pain: a randomized clinical trial. JAMA Netw. Open 5:e2142069. 10.1001/jamanetworkopen.2021.42069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninx B. W. J. H., Rejeski W. J., Pandya J., Miller M. E., Di Bari M., Applegate W. B., et al. (2002). Exercise and depressive symptoms: a comparison of aerobic and resistance exercise effects on emotional and physical function in older persons with high and low depressive symptomatology. J. Gerontol. B Psychol. Sci. Soc. Sci. 57 124–132. [DOI] [PubMed] [Google Scholar]
- Pérez-García S., Carrión M., Gutiérrez-Cañas I., Villanueva-Romero R., Castro D., Martínez C., et al. (2019). Profile of matrix-remodeling proteinases in osteoarthritis: impact of fibronectin. Cells 9:40. 10.3390/cells9010040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisters M. F., Veenhof C., de Bakker D. H., Schellevis F. G., Dekker J. (2010). Behavioural graded activity results in better exercise adherence and more physical activity than usual care in people with osteoarthritis: a cluster-randomised trial. J. Physiother. 56 41–47. [DOI] [PubMed] [Google Scholar]
- Qian J., Liang J., Wang Y., Wang H. (2014). Effect of passive motion on articular cartilage in rat osteoarthritis. Exp. Ther. Med. 8 377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabe K. G., Matsuse H., Jackson A., Segal N. A. (2018). Evaluation of the combined application of neuromuscular electrical stimulation and volitional contractions on thigh muscle strength, knee pain, and physical performance in women at risk for knee osteoarthritis: a randomized controlled trial. PM R 10 1301–1310. 10.1016/j.pmrj.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmati M., Nalesso G., Mobasheri A., Mozafari M. (2017). Aging and osteoarthritis: central role of the extracellular matrix. Ageing Res. Rev. 40 20–30. 10.1016/j.arr.2017.07.004 [DOI] [PubMed] [Google Scholar]
- Rewald S., Lenssen A. F. T., Emans P. J., de Bie R. A., van Breukelen G., Mesters I. (2020). Aquatic cycling improves knee pain and physical functioning in patients with knee osteoarthritis: a randomized controlled trial. Arch. Phys. Med. Rehabil. 101 1288–1295. 10.1016/j.apmr.2019.12.023 [DOI] [PubMed] [Google Scholar]
- Reynard L. N. (2017). Analysis of genetics and DNA methylation in osteoarthritis: What have we learnt about the disease? Semin. Cell Dev. Biol. 62 57–66. 10.1016/j.semcdb.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Rim Y. A., Nam Y., Ju J. H. (2020). The role of chondrocyte hypertrophy and senescence in osteoarthritis initiation and progression. Int. J. Mol. Sci. 21:2358. 10.3390/ijms21072358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios J. L., Bomhof M. R., Reimer R. A., Hart D. A., Collins K. H., Herzog W. (2019). Protective effect of prebiotic and exercise intervention on knee health in a rat model of diet-induced obesity. Sci. Rep. 9:3893. 10.1038/s41598-019-40601-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios J. L., Hart D. A., Reimer R. A., Herzog W. (2020). Prebiotic and exercise do not alter knee osteoarthritis in a rat model of established obesity. Cartilage 13(2_Suppl), 1456S–1466S. 10.1177/1947603520959399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Ortega M., Cruz R., Vega-López M. A., Cabrera-González M., Hernández-Hernández J. M., Lavalle-Montalvo C., et al. (2015). Exercise modulates the expression of IL-1β and IL-10 in the articular cartilage of normal and osteoarthritis-induced rats. Pathol. Res. Pract. 211 435–443. 10.1016/j.prp.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Rosshirt N., Hagmann S., Tripel E., Gotterbarm T., Kirsch J., Zeifang F., et al. (2019). A predominant Th1 polarization is present in synovial fluid of end-stage osteoarthritic knee joints: analysis of peripheral blood, synovial fluid and synovial membrane. Clin. Exp. Immunol. 195 395–406. 10.1111/cei.13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruegsegger G. N., Booth F. W. (2018). Health benefits of exercise. Cold Spring Harb. Perspect. Med. 8:a029694. 10.1101/cshperspect.a029694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeghi H., Jehu D. A. (2022). Exergaming to improve physical, psychological and cognitive health among home office workers: a COVID-19 pandemic commentary. Work 71 13–17. 10.3233/WOR-211000 [DOI] [PubMed] [Google Scholar]
- Samut G., Dinçer F., Özdemir O. (2015). The effect of isokinetic and aerobic exercises on serum interleukin-6 and tumor necrosis factor alpha levels, pain, and functional activity in patients with knee osteoarthritis. Mod. Rheumatol. 25 919–924. 10.3109/14397595.2015.1038425 [DOI] [PubMed] [Google Scholar]
- Sang W., Xue S., Jiang Y., Lu H., Zhu L., Wang C., et al. (2021). METTL3 involves the progression of osteoarthritis probably by affecting ECM degradation and regulating the inflammatory response. Life Sci. 278:119528. 10.1016/j.lfs.2021.119528 [DOI] [PubMed] [Google Scholar]
- Sasaki H., Takayama K., Matsushita T., Ishida K., Kubo S., Matsumoto T., et al. (2012). Autophagy modulates osteoarthritis-related gene expression in human chondrocytes. Arthritis Rheum. 64 1920–1928. 10.1002/art.34323 [DOI] [PubMed] [Google Scholar]
- Scanzello C. R. (2017a). Chemokines and inflammation in osteoarthritis: insights from patients and animal models. J. Orthop. Res. 35 735–739. 10.1002/jor.23471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C. R. (2017b). Role of low-grade inflammation in osteoarthritis. Curr. Opin. Rheumatol. 29 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C. R., Goldring S. R. (2012). The role of synovitis in osteoarthritis pathogenesis. Bone 51 249–257. 10.1016/j.bone.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanzello C. R., McKeon B., Swaim B. H., DiCarlo E., Asomugha E. U., Kanda V., et al. (2011). Synovial inflammation in patients undergoing arthroscopic meniscectomy: molecular characterization and relationship to symptoms. Arthritis Rheum. 63 391–400. 10.1002/art.30137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setayeshmehr M., Esfandiari E., Rafieinia M., Hashemibeni B., Taheri-Kafrani A., Samadikuchaksaraei A., et al. (2019). Hybrid and composite scaffolds based on extracellular matrices for cartilage tissue engineering. Tissue Eng. Part B Rev. 25 202–224. 10.1089/ten.TEB.2018.0245 [DOI] [PubMed] [Google Scholar]
- Sevick M. A., Bradham D. D., Muender M., Chen G. J., Enarson C., Dailey M., et al. (2000). Cost-effectiveness of aerobic and resistance exercise in seniors with knee osteoarthritis. Med. Sci. Sports Exerc. 32 1534–1540. [DOI] [PubMed] [Google Scholar]
- Silva L. E., Valim V., Pessanha A. P. C., Oliveira L. M., Myamoto S., Jones A., et al. (2008). Hydrotherapy versus conventional land-based exercise for the management of patients with osteoarthritis of the knee: a randomized clinical trial. Phys. Ther. 88 12–21. [DOI] [PubMed] [Google Scholar]
- Simão A. P., Avelar N. C., Tossige-Gomes R., Neves C. D., Mendonça V. A., Miranda A. S., et al. (2012). Functional performance and inflammatory cytokines after squat exercises and whole-body vibration in elderly individuals with knee osteoarthritis. Arch. Phys. Med. Rehabil. 93 1692–1700. 10.1016/j.apmr.2012.04.017 [DOI] [PubMed] [Google Scholar]
- Skou S. T., Pedersen B. K., Abbott J. H., Patterson B., Barton C. (2018). Physical activity and exercise therapy benefit more than just symptoms and impairments in people with hip and knee osteoarthritis. J. Orthop. Sports Phys. Ther. 48 439–447. 10.2519/jospt.2018.7877 [DOI] [PubMed] [Google Scholar]
- Song R., Lee E.-O., Lam P., Bae S.-C. (2003). Effects of tai chi exercise on pain, balance, muscle strength, and perceived difficulties in physical functioning in older women with osteoarthritis: a randomized clinical trial. J. Rheumatol. 30 2039–2044. [PubMed] [Google Scholar]
- Sowers M., Karvonen-Gutierrez C. A., Jacobson J. A., Jiang Y., Yosef M. (2011). Associations of anatomical measures from MRI with radiographically defined knee osteoarthritis score, pain, and physical functioning. J. Bone Joint Surg. Am. 93 241–251. 10.2106/JBJS.I.00667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn G., Hofmann G. O., von Engelhardt L. V., Li M., Neubauer H., Klinger H. M. (2013). The impact of a high tibial valgus osteotomy and unicondylar medial arthroplasty on the treatment for knee osteoarthritis: a meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 21 96–112. 10.1007/s00167-011-1751-2 [DOI] [PubMed] [Google Scholar]
- Steinhilber B., Haupt G., Miller R., Janssen P., Krauss I. (2017). Exercise therapy in patients with hip osteoarthritis: effect on hip muscle strength and safety aspects of exercise-results of a randomized controlled trial. Mod. Rheumatol. 27 493–502. 10.1080/14397595.2016.1213940 [DOI] [PubMed] [Google Scholar]
- Sun X., Xiao L., Chen J., Chen X., Chen X., Yao S., et al. (2020). DNA methylation is involved in the pathogenesis of osteoarthritis by regulating expression and CtBP-mediated signaling. Int. J. Biol. Sci. 16 994–1009. 10.7150/ijbs.39945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri S., Walsh D. A. (2012). Osteochondral alterations in osteoarthritis. Bone 51 204–211. 10.1016/j.bone.2011.10.010 [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Iijima H., Tashiro Y., Kajiwara Y., Zeidan H., Shimoura K., et al. (2019). Home exercise therapy to improve muscle strength and joint flexibility effectively treats pre-radiographic knee OA in community-dwelling elderly: a randomized controlled trial. Clin. Rheumatol. 38 133–141. 10.1007/s10067-018-4263-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swingler T. E., Wheeler G., Carmont V., Elliott H. R., Barter M. J., Abu-Elmagd M., et al. (2012). The expression and function of microRNAs in chondrogenesis and osteoarthritis. Arthritis Rheum. 64 1909–1919. 10.1002/art.34314 [DOI] [PubMed] [Google Scholar]
- Szychlinska M. A., Castrogiovanni P., Trovato F. M., Nsir H., Zarrouk M., Lo Furno D., et al. (2019). Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 58 565–581. 10.1007/s00394-018-1632-2 [DOI] [PubMed] [Google Scholar]
- Taglietti M., Facci L. M., Trelha C. S., de Melo F. C., da Silva D. W., Sawczuk G., et al. (2018). Effectiveness of aquatic exercises compared to patient-education on health status in individuals with knee osteoarthritis: a randomized controlled trial. Clin. Rehabil. 32 766–776. 10.1177/0269215517754240 [DOI] [PubMed] [Google Scholar]
- Theocharis A. D., Manou D., Karamanos N. K. (2019). The extracellular matrix as a multitasking player in disease. FEBS J. 286 2830–2869. 10.1111/febs.14818 [DOI] [PubMed] [Google Scholar]
- Thomas C. M., Whittles C. E., Fuller C. J., Sharif M. (2011). Variations in chondrocyte apoptosis may explain the increased prevalence of osteoarthritis in some joints. Rheumatol. Int. 31 1341–1348. 10.1007/s00296-010-1471-9 [DOI] [PubMed] [Google Scholar]
- Tian Y., Gou J., Zhang H., Lu J., Jin Z., Jia S., et al. (2021). The anti-inflammatory effects of 15-HETE on osteoarthritis during treadmill exercise. Life Sci. 273:119260. 10.1016/j.lfs.2021.119260 [DOI] [PubMed] [Google Scholar]
- Todd Allen R., Robertson C. M., Harwood F. L., Sasho T., Williams S. K., Pomerleau A. C., et al. (2004). Characterization of mature vs aged rabbit articular cartilage: analysis of cell density, apoptosis-related gene expression and mechanisms controlling chondrocyte apoptosis. Osteoarthritis Cartilage 12 917–923. [DOI] [PubMed] [Google Scholar]
- Topp R., Woolley S., Hornyak J., Khuder S., Kahaleh B. (2002). The effect of dynamic versus isometric resistance training on pain and functioning among adults with osteoarthritis of the knee. Arch. Phys. Med. Rehabil. 83 1187–1195. [DOI] [PubMed] [Google Scholar]
- Trans T., Aaboe J., Henriksen M., Christensen R., Bliddal H., Lund H. (2009). Effect of whole body vibration exercise on muscle strength and proprioception in females with knee osteoarthritis. Knee 16 256–261. 10.1016/j.knee.2008.11.014 [DOI] [PubMed] [Google Scholar]
- Tsauo J.-Y., Cheng P.-F., Yang R.-S. (2008). The effects of sensorimotor training on knee proprioception and function for patients with knee osteoarthritis: a preliminary report. Clin. Rehabil. 22 448–457. 10.1177/0269215507084597 [DOI] [PubMed] [Google Scholar]
- Urnov F. D. (2002). Methylation and the genome: the power of a small amendment. J. Nutr. 132(8 Suppl.), 2450S–2456S. 10.1093/jn/132.8.2450S [DOI] [PubMed] [Google Scholar]
- Valdes A. M., Spector T. D. (2010). The genetic epidemiology of osteoarthritis. Curr. Opin. Rheumatol. 22 139–143. 10.1097/BOR.0b013e3283367a6e [DOI] [PubMed] [Google Scholar]
- van den Bosch M. H. J. (2019). Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 195 153–166. 10.1111/cei.13237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Saase J. L., van Romunde L. K., Cats A., Vandenbroucke J. P., Valkenburg H. A. (1989). Epidemiology of osteoarthritis: Zoetermeer survey. Comparison of radiological osteoarthritis in a Dutch population with that in 10 other populations. Ann. Rheum. Dis. 48 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasilceac F. A., Marqueti R. D. C., Neto I. V. D. S., Nascimento D. D. C., Souza M. C. D., Durigan J. L. Q., et al. (2021). Resistance training decreases matrix metalloproteinase-2 activity in quadriceps tendon in a rat model of osteoarthritis. Braz. J. Phys. Ther. 25 147–155. 10.1016/j.bjpt.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villadsen A., Overgaard S., Holsgaard-Larsen A., Christensen R., Roos E. M. (2014). Immediate efficacy of neuromuscular exercise in patients with severe osteoarthritis of the hip or knee: a secondary analysis from a randomized controlled trial. J. Rheumatol. 41 1385–1394. 10.3899/jrheum.130642 [DOI] [PubMed] [Google Scholar]
- Vincent K. R., Vasilopoulos T., Montero C., Vincent H. K. (2019). Eccentric and concentric resistance exercise comparison for knee osteoarthritis. Med. Sci. Sports Exerc. 51 1977–1986. 10.1249/MSS.0000000000002010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent K. R., Vincent H. K. (2020). Concentric and eccentric resistance training comparison on physical function and functional pain outcomes in knee osteoarthritis: a randomized controlled trial. Am. J. Phys. Med. Rehabil. 99 932–940. 10.1097/PHM.0000000000001450 [DOI] [PubMed] [Google Scholar]
- Vincent T. L. (2019). Mechanoflammation in osteoarthritis pathogenesis. Semin. Arthritis Rheum. 49 S36–S38. 10.1016/j.semarthrit.2019.09.018 [DOI] [PubMed] [Google Scholar]
- Waller B., Munukka M., Rantalainen T., Lammentausta E., Nieminen M. T., Kiviranta I., et al. (2017). Effects of high intensity resistance aquatic training on body composition and walking speed in women with mild knee osteoarthritis: a 4-month RCT with 12-month follow-up. Osteoarthritis Cartilage 25 1238–1246. 10.1016/j.joca.2017.02.800 [DOI] [PubMed] [Google Scholar]
- Wang F., Zhang X., Tong X., Zhang M., Xing F., Yang K., et al. (2021). The effects on pain, physical function, and quality of life of quadriceps strengthening exercises combined with Baduanjin qigong in older adults with knee osteoarthritis: a quasi-experimental study. BMC Musculoskelet. Disord. 22:313. 10.1186/s12891-021-04179-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Yang L., Li H., Lei Z., Yang X., Liu C., et al. (2016). Effects of whole-body vibration training with quadriceps strengthening exercise on functioning and gait parameters in patients with medial compartment knee osteoarthritis: a randomised controlled preliminary study. Physiotherapy 102 86–92. 10.1016/j.physio.2015.03.3720 [DOI] [PubMed] [Google Scholar]
- Wang T.-J., Belza B., Elaine Thompson F., Whitney J. D., Bennett K. (2007). Effects of aquatic exercise on flexibility, strength and aerobic fitness in adults with osteoarthritis of the hip or knee. J. Adv. Nurs. 57 141–152. [DOI] [PubMed] [Google Scholar]
- Wang T.-J., Lee S.-C., Liang S.-Y., Tung H.-H., Wu S.-F. V., Lin Y.-P. (2011). Comparing the efficacy of aquatic exercises and land-based exercises for patients with knee osteoarthritis. J. Clin. Nurs. 20 2609–2622. 10.1111/j.1365-2702.2010.03675.x [DOI] [PubMed] [Google Scholar]
- Woodell-May J. E., Sommerfeld S. D. (2020). Role of inflammation and the immune system in the progression of osteoarthritis. J. Orthop. Res. 38 253–257. 10.1002/jor.24457 [DOI] [PubMed] [Google Scholar]
- Wu B., Zhou L., Chen C., Wang J., Hu L. I., Wang X. (2022). Effects of exercise-induced hypoalgesia and its neural mechanisms. Med. Sci. Sports Exerc. 54 220–231. 10.1249/MSS.0000000000002781 [DOI] [PubMed] [Google Scholar]
- Xiao C. M., Li J. J., Kang Y., Zhuang Y. C. (2021). Follow-up of a Wuqinxi exercise at home programme to reduce pain and improve function for knee osteoarthritis in older people: a randomised controlled trial. Age Ageing 50 570–575. 10.1093/ageing/afaa179 [DOI] [PubMed] [Google Scholar]
- Xiao Z., Li G. (2021). The effect of exercises on the balance function and subjective quality of life in elderly, female knee osteoarthritis patients. Am. J. Transl. Res. 13 6710–6716. [PMC free article] [PubMed] [Google Scholar]
- Xue J.-F., Shi Z.-M., Zou J., Li X.-L. (2017). Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed. Pharmacother. 89 1252–1261. 10.1016/j.biopha.2017.01.130 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S., Aoyama T., Ito A., Nagai M., Iijima H., Zhang X., et al. (2013). Effects of exercise level on biomarkers in a rat knee model of osteoarthritis. J. Orthop. Res. 31 1026–1031. 10.1002/jor.22332 [DOI] [PubMed] [Google Scholar]
- Yang J., Zhang M., Yang D., Ma Y., Tang Y., Xing M., et al. (2021). mA-mediated upregulation of AC008 promotes osteoarthritis progression through the miR-328-3p-AQP1/ANKH axis. Exp. Mol. Med. 53 1723–1734. 10.1038/s12276-021-00696-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang Y., Kong Y., Zhang X., Bai L. (2017). The effects of different frequency treadmill exercise on lipoxin A4 and articular cartilage degeneration in an experimental model of monosodium iodoacetate-induced osteoarthritis in rats. PLoS One 12:e0179162. 10.1371/journal.pone.0179162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang Y., Kong Y., Zhang X., Zhang H., Feng X., et al. (2020). Moderate mechanical stimulation protects rats against osteoarthritis through the regulation of TRAIL via the NF-B/NLRP3 Pathway. Oxid. Med. Cell. Longev. 2020:6196398. 10.1155/2020/6196398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wang Y., Kong Y., Zhang X., Zhang H., Gang Y., et al. (2018). The therapeutic effects of lipoxin A during treadmill exercise on monosodium iodoacetate-induced osteoarthritis in rats. Mol. Immunol. 103 35–45. 10.1016/j.molimm.2018.08.027 [DOI] [PubMed] [Google Scholar]
- Yang Y., Wang Y., Kong Y., Zhang X., Zhang H., Gang Y., et al. (2019). Mechanical stress protects against osteoarthritis via regulation of the AMPK/NF-κB signaling pathway. J. Cell. Physiol. 234 9156–9167. 10.1002/jcp.27592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D., Jian W., Feng J., Liao X. (2018). Role of long noncoding RNA ZFAS1 in proliferation, apoptosis and migration of chondrocytes in osteoarthritis. Biomed. Pharmacother. 104 825–831. 10.1016/j.biopha.2018.04.124 [DOI] [PubMed] [Google Scholar]
- Yuenyongviwat V., Duangmanee S., Iamthanaporn K., Tuntarattanapong P., Hongnaparak T. (2020). Effect of hip abductor strengthening exercises in knee osteoarthritis: a randomized controlled trial. BMC Musculoskelet. Disord. 21:284. 10.1186/s12891-020-03316-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusup A., Kaneko H., Liu L., Ning L., Sadatsuki R., Hada S., et al. (2015). Bone marrow lesions, subchondral bone cysts and subchondral bone attrition are associated with histological synovitis in patients with end-stage knee osteoarthritis: a cross-sectional study. Osteoarthritis Cartilage 23 1858–1864. 10.1016/j.joca.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Zamli Z., Sharif M. (2011). Chondrocyte apoptosis: a cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 14 159–166. 10.1111/j.1756-185X.2011.01618.x [DOI] [PubMed] [Google Scholar]
- Zhang H., Ji L., Yang Y., Wei Y., Zhang X., Gang Y., et al. (2019). The therapeutic effects of treadmill exercise on osteoarthritis in rats by inhibiting the HDAC3/NF-KappaB Pathway and. Front. Physiol. 10:1060. 10.3389/fphys.2019.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Yang Y., Li X., Zhang H., Gang Y., Bai L. (2019). Alterations of autophagy in knee cartilage by treatment with treadmill exercise in a rat osteoarthritis model. Int. J. Mol. Med. 43 336–344. 10.3892/ijmm.2018.3948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Qi B., Liu M., Zhao T., Tan L. (2021). Influence of swimming exercise on the expression of apoptotic gene caspase-3 in chondrocytes in osteoarthritis. Am. J. Transl. Res. 13 2511–2517. [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Qi L., Chen R., He J., Liu Z., Wang W., et al. (2021). Circular RNAs in osteoarthritis: indispensable regulators and novel strategies in clinical implications. Arthritis Res. Ther. 23:23. 10.1186/s13075-021-02420-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Huang L., Liu Y., Wang L. (2020). Effect of Tai Chi training on plantar loads during walking in individuals with knee osteoarthritis. Biomed Res. Int. 2020:3096237. 10.1155/2020/3096237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., He L., Liu Z., Ren X., Qi L., Wan L., et al. (2020). Multifaceted functions and novel insight into the regulatory Role of RNA N-methyladenosine modification in musculoskeletal disorders. Front. Cell Dev. Biol. 8:870. 10.3389/fcell.2020.00870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng K., Chen C., Yang S., Wang X. (2021). Aerobic exercise attenuates pain sensitivity: an event-related potential study. Front. Neurosci. 15:735470. 10.3389/fnins.2021.735470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X., Cao H., Wang M., Zou J., Wu W. (2021). Moderate-intensity treadmill running relieves motion-induced post-traumatic osteoarthritis mice by up-regulating the expression of lncRNA H19. Biomed. Eng. Online 20:111. 10.1186/s12938-021-00949-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J., Yu W., Wang Y., Xia K., Huang Y., Xu A., et al. (2019). lncRNAs: function and mechanism in cartilage development, degeneration, and regeneration. Stem Cell Res. Ther. 10:344. 10.1186/s13287-019-1458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]