Abstract
Purpose
This study aimed to examine the advantages and usefulness of transient elastography (Fibroscan®) in diagnosing non-alcoholic steatohepatitis in children and adolescents compared to those of abdominal computed tomography and liver ultrasonography.
Methods
Forty-six children and adolescent participants aged between 6 and 16 years who underwent transient elastography (Fibroscan®) as well as liver ultrasonography or abdominal computed tomography were included. Thirty-nine participants underwent liver ultrasonography and 11 underwent computed tomography. The physical measurements, blood test results, presence of metabolic syndrome, and the degree of liver steatosis and liver fibrosis were analyzed, and their correlations with transient elastography (Fibroscan®), abdominal computed tomography, and liver ultrasonography, as well as the correlations between examinations, were analyzed.
Results
Thirty-six participants (78.3%) were boys, and the mean age was 12.29±2.57 years, with a mean body mass index of 27.88±4.28. In the 46 participants, the mean values for aspartate aminotransferase, alanine aminotransferase, and total bilirubin were 89.87±118.69 IU/L, 138.54±141.79 IU/L, and 0.77±0.61 mg/dL, respectively. Although transient elastography (Fibroscan®) and abdominal computed tomography grading had a statistically significant positive correlation with aspartate aminotransferase and alanine aminotransferase values, the correlations between the results of grading performed by transient elastography (Fibroscan®), abdominal computed tomography, and liver ultrasonography were not statistically.
Conclusion
We confirmed that each examination was correlated with the results of some blood tests, suggesting the usefulness and possibility of diagnosis and treatment of steatohepatitis mediated by transient elastography (Fibroscan®) in the department of pediatrics.
Keywords: Non-alcoholic fatty liver disease, Child, Elastography
INTRODUCTION
The number of patients with liver fibrosis and steatosis has greatly increased owing to the increased incidence of obesity and lack of exercise in modern society [1,2]. It is difficult to determine the exact prevalence of non-alcoholic fatty liver disease (NAFLD) in children, but it is estimated to be widespread, affecting up to one-third of all children worldwide [3]. Indeed, NAFLD has been reported in 10–80% of children who undergo liver function tests, and in 15–44% of children who undergo liver ultrasonography [3,4,5].
As in adults, the early diagnosis and treatment of hepatic fibrosis and steatosis in children is important considering the risk of developing chronic liver disease. Until recently, liver biopsy was the standard when a definitive differential diagnosis was required. However, since liver biopsy is an invasive test, involving the piercing of the liver with a special needle, and carries the risk of complications, it is considered a difficult test to be performed on children [6,7].
Transient elastography (TE) (Fibroscan®) is a method to non-invasively and rapidly evaluate the degree of liver elasticity and steatosis, measuring the liver elasticity in both adults and children. TE (Fibroscan®) can perform liver stiffness measurement (LSM) to identify liver fibrosis and measure the controlled attenuation parameter (CAP) to identify the degree of fat accumulation in the liver. In previous studies, LSM has been proven as a reliable evaluation index for evaluating fibrotic liver disease, while CAP has been proven as an effective evaluation index for diagnosing hepatic steatosis [8,9,10,11].
In our previous study, we confirmed that LSM is correlated with aspartate aminotransferase (AST), alanine aminotransferase (ALT), homeostasis model assessment for insulin resistance (HOMA-IR), and AST to platelet ratio index (APRI) in obese children and adolescents, while TE (Fibroscan®) was clinically confirmed to be a non-invasive, fast, and safe method to diagnose hepatic steatosis and fibrosis in Korean children [12]. Liver ultrasonography is highly dependent on the machine and the examiner, which produces a low positive predictive value, and may be difficult to perform in severely obese patients owing to the influence of edema or other adipose tissue. Additionally, the test sensitivity is lowered when the degree of hepatic steatosis is lower than 30% [13]. Conversely, abdominal computed tomography (CT) has a lower sensitivity to the measurement of hepatic steatosis compared to liver ultrasonography, and carries the burden of radiation exposure, which limits its use in the long-term follow-up of pediatric patients [14,15]. In this study, we examined the advantages and usefulness of TE (Fibroscan®) in diagnosing non-alcoholic steatohepatitis in children and adolescents compared to those of abdominal CT and liver ultrasonography.
MATERIALS AND METHODS
Participants
From June 2018 to June 2020, the TE (Fibroscan®) test was performed on 171 children and adolescents aged between 3 and 16 years who visited Konyang University Hospital; all tests were successful without failure. Among these, 46 obese children aged 6 to 16 years with a body mass index (BMI) greater than the 95 percentiles, considering age and sex, who had a liver ultrasound (US) or abdominal CT were included in the study. Of these, 39 participants underwent a liver US and 11 participants underwent an abdominal CT scan at the time of TE (Fibroscan®) examination. Four participants underwent both a liver US and an abdominal CT.
Thirty-six participants (78.3%) were boys; the mean age was 12.29±2.57 years, and the mean BMI was 27.88±4.28 (Fig. 1). The exclusion criteria were; presence of a liver disease other than NAFLD (viral/autoimmune hepatitis, Wilson disease), use of steatogenic drugs, and contraindications for CT contrast media.
Fig. 1. Diagram of the study population.
KYUH: Konyang University Hospital, BMI: body mass index, US: ultrasound, CT: computed tomography.
Methods to evaluate liver fibrosis and steatosis
LSM, which is an indicator of liver fibrosis, can be measured using TE (Fibroscan®) along with CAP, an indicator of fat accumulation in the liver. All the children received LSM and CAP measurements simultaneously using a 3.5-MHz standard M probe with a diameter of 7 mm [14,15,16]. The participants were placed in a straight laying position while the examiner placed the probe between the ribs, perpendicular to the right lobe of the liver. If the probe was positioned correctly, the LSM result in kPa and the CAP result in dB/m were displayed simultaneously. The LSM and CAP were measured 10 times in a row, lesser accurate results were deleted, and the average of 10 valid results was calculated. In order to reduce any bias caused by intra-examiner differences, only one experienced examiner performed Fibroscan®, and only the results falling within less than 30% of the interquartile range were considered valid. Each test took an average of approximately 5 minutes. Values were measured using the Fibroscan® device (Echosens, Paris, France) according to the manufacturer’s guidelines. The CAP scores under 238 dB/m, and those ranging from 238 to 260 dB/m, 260 to 290 dB/m, and those higher than 290 dB/m are defined as S0, S1, S2, and S3, respectively. LSM scores ranging from 2 to 7 kPa, 7.5 to 10 kPa, 10 to 14 kPa, 14 kPa or higher are defined as F0 to F1, F2, F3, and F4, respectively.
Clinical and biochemical parameters
The participants’ height, weight, BMI, blood pressure, heart rate, and waist circumference were measured on the same day as that of the Fibroscan® examination. As biochemical variables, the serum platelet count; levels of AST, ALT, triglycerides; and total cholesterol were measured no more than 1 month before the Fibroscan® examination.
The physical measurements, blood test results, presence of metabolic diseases, and the degree of liver steatosis and fibrosis were analyzed. Additionally, their correlations with TE (Fibroscan®), abdominal CT, and liver ultrasonography were analyzed.
The degrees of steatohepatitis in liver US examinations were divided into Grade 0 (absent), Grade 1 (mild to moderate), and Grade 2 (severe). The grading of liver steatosis is usually obtained using US features that include liver brightness, contrast between the liver and the kidney, US appearance of the intrahepatic vessels, liver parenchyma, and diaphragm. Steatosis is graded as follows: Absent (Grade 0) when the echotexture of the liver is normal; mild to moderate (Grade 1), in cases of a mild to moderate increase in liver echogenicity with slightly impaired appearance of the portal vein wall and the diaphragm; severe (Grade 2), in cases of marked increase in liver echogenicity with poor or no visualization of the portal vein wall, diaphragm, or posterior part of the right liver lobe [17,18,19].
Among 39 participants who underwent liver ultrasonography, 5 were Grade 0 (12.8%), 29 were Grade 1 (74.4%), and 5 were Grade 2 (12.8%). In abdominal CT, grading was defined as the difference and ratio of hepatic-spleen attenuation; normal (Grade 0) was defined as 50–65 Hounsfield units (HUs), which were 8–10 HUs higher than that for a normal spleen. Grade 1 was defined as a value lower than 48 HUs, and Grade 2 was defined as that lower than 40 HUs, or when the difference between liver and spleen attenuation was 10 HUs or more [20,21,22]. Among 11 patients who underwent an abdominal CT scan, 3 patients were Grade 0 (27.3%), 3 patients were Grade 1 (27.3%), and 5 patients were Grade 2 (45.4%).
Statistical analysis
We used two-sided t-tests for continuous variables and χ2 tests or Fisher’s exact tests for categorical variables. Pearson’s and Spearman’s correlation analyses were used to examine the correlations between continuous variables. Relationships with p<0.05 were considered significant in all inferential tests.
Ethics statement
The present study protocol was reviewed and approved by the Institutional Review Board of Konyang University College of Medicine (approval No. 2017-11-012). Signed consent forms were obtained from all participants and their parents. The written consent form included information regarding the study objectives and the possible adverse effects and discomfort associated with the examination. Separate consent forms were provided for the children and their guardians. Before the study, consent was obtained from both the child and at least one parent who could communicate in Korean.
RESULTS
Demographic and clinical characteristics
Of the 46 obese children in the final study group, 36 (78.3%) were boys, the mean age was 12.29±2.57 years, and the mean BMI was 27.88±4.28. The mean BMI percentile was 97.6±3.73. Blood testing showed that the AST (89.87±118.69 IU/L) and ALT (138.54±141.79 IU/L) values were higher than normal reference values (Table 1). There were no specific findings in the complete blood count. Additionally, the HbA1c was within the normal range, with an average of 5.50±0.38%. The total bilirubin and albumin levels were within the normal range (0.77±0.61 mg/dL and 4.46±0.29 g/dL, respectively), while the average fasting glucose value was 102.95±18.88 mg/dL, which was slightly higher than normal.
Table 1. Laboratory findings of the study population.
| Variable | Value (n=46) |
|---|---|
| AST (IU/L) | 89.87 (±118.69) |
| ALT (IU/L) | 138.54 (±141.79) |
| Total cholesterol (mg/dL) | 170.91 (±39.65) |
| Triglyceride (mg/dL) | 139.1 (±65.32) |
| HDL-cholesterol (mg/dL) | 46.8 (±10.20) |
| LDL-cholesterol (mg/dL) | 103.9 (±29.59) |
| Total bilirubin (mg/dL) | 0.77 (±0.61) |
| ALP (IU/L) | 266.81 (±123.66) |
| r-GT (IU/L) | 92.79 (±94.62) |
| Glucose (mg/dL) | 102.95 (±18.88) |
| HbA1c (%) | 5.50 (±0.38) |
| WBC (/uL) | 8,300 (±2,256) |
| Hemoglobin (g/dL) | 14.01 (±1.08) |
| Hematocrit (%) | 41.72 (±3.27) |
| Platelet (103/uL) | 320.90 (±91.78) |
| CRP (mg/dL) | 0.69 (±1.36) |
| Protein (g/dL) | 7.39 (±0.43) |
| Albumin (g/dL) | 4.46 (±0.29) |
Values are presented as mean±standard deviation or frequency (percentage).
AST: aspartate aminotransferase, ALT: alanine aminotransferase, HDL: high density lipoprotein, LDL: low density lipoprotein, ALP: alkaline phosphatase, r-GT: gamma-glutamyl transferase, WBC: white blood cell, CRP: C-reactive protein.
TE (Fibroscan®) results
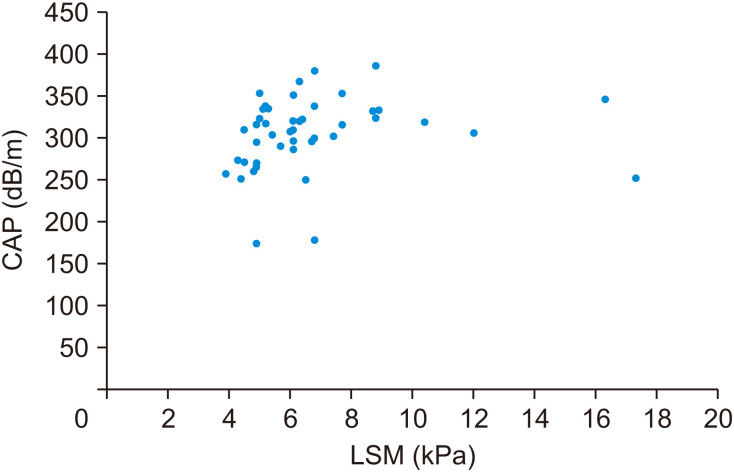
The mean value of LSM of 46 subjects in the entire study group was 6.68±2.75 kPa, and the mean value of CAP was 303.78±43.96 dB/m (Fig. 2). Among a total of 46 patients who underwent TE (Fibroscan®), the degree of steatosis was Grade 0 in 2 participants (4.3%), Grade 1 in 5 participants (10.9%), Grade 2 in 7 participants (15.2%), and Grade 3 in 32 participants (69.6%). The degree of fibrosis was Grade 0 in 21 participants (45.7%), Grade 1 in 17 participants (37.0%), Grade 2 in 5 participants (10.9%), and Grade 3 in 3 participants (6.5%) (Table 2).
Fig. 2. Fibroscan® results of the study population: LSM and CAP (r=0.174; p=0.247). In obese children and adolescents, the LSM is 5–6 kPa, and the CAP is about ≥300 dB/m. Both the LSM and CAP values of the study group were relatively high.
LSM: liver stiffness measure, CAP: controlled attenuation parameter.
Table 2. Grading in transient elastography (Fibroscan®) (n=46).
| Grade | Fibrosis | Steatosis |
|---|---|---|
| 0 | 21 (45.7) | 2 (4.3) |
| 1 | 17 (37.0) | 5 (10.9) |
| 2 | 5 (10.9) | 7 (15.2) |
| 3 | 3 (6.5) | 32 (69.6) |
Values are presented as number (%).
The CAP scores under 238 dB/m, and those ranging from 238 to 260 dB/m, 260 to 290 dB/m, and those higher than 290 dB/m are defined as S0, S1, S2, and S3, respectively. LSM scores ranging from 2 to 7 kPa, 7.5 to 10 kPa, 10 to 14 kPa, 14 kPa or higher are defined as F0 to F1, F2, F3, and F4, respectively.
Correlations of AST/ALT with Fibroscan®, liver US, and CT grading
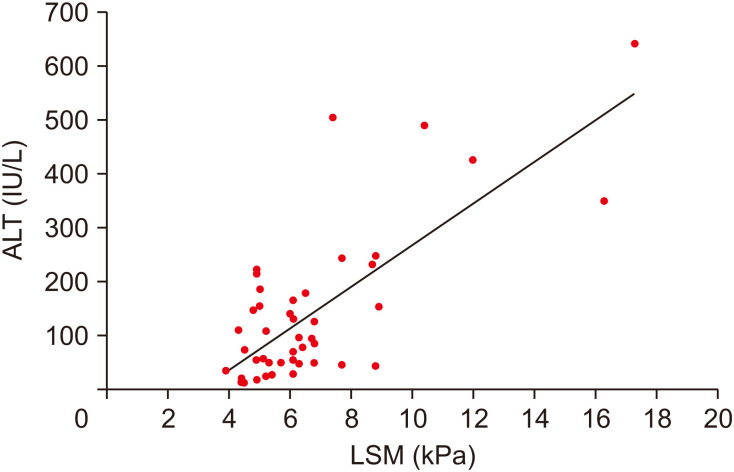
When the correlation between TE (Fibroscan®), abdominal CT, and liver US was analyzed, the TE (Fibroscan®) and abdominal CT grading showed a statistically significant positive correlation with the AST and ALT levels in the blood test results (Table 3, Fig. 3). The CT grading showed a statistically positive correlation with ALT levels (r=0.703; p=0.016). However, the correlations between the results of grading performed by TE (Fibroscan®), abdominal CT, and liver ultrasonography was not statistically significant (Table 4). The results of the Fibroscan® according to US and CT grades are shown in Tables 5 and 6, respectively.
Table 3. Correlation of Fibroscan® results and liver US/CT with clinical traits.
| Characteristics | Grading in liver US | Grading in abdominal CT | Fibrosis grading in Fibroscan® |
|---|---|---|---|
| Age | r=−0.062; p=0.709 | r=0.244; p=0.470 | r=−0.052; p=0.733 |
| Sex | r=0.000; p=1.000 | r=−0.134; p=0.695 | r=−0.109; p=0.471 |
| BMI | r=0.289; p=0.074 | r=0.259; p=0.441 | r=0.257; p=0.085 |
| AST (IU/L) | r=0.145; p=0.378 | r=0.720; p=0.013* | r=0.600; p=0.000* |
| ALT (IU/L) | r=0.263; p=0.105 | r=0.703; p=0.016* | r=0.653; p=0.000* |
| Total bilirubin (mg/dL) | r=−0.295; p=0.068 | r=0.133; p=0.697 | r=−0.123; p=0.417 |
| HbA1c (%) | r=0.442; p=0.021* | r=0.466; p=0.292 | r=0.161; p=0.379 |
| Glucose (mg/dL) | r=−0.063; p=0.722 | r=0.292; p=0.384 | r=0.265; p=0.095 |
US: ultrasound, CT: computed tomography, BMI: body mass index, AST: aspartate aminotransferase, ALT: alanine aminotransferase.
*p<0.05.
Fig. 3. Correlation of ALT and LSM of Fibroscan® (r=0.747; p<0.001).
ALT: alanine aminotransferase, LSM: liver stiffness measure.
Table 4. Correlation with Fibroscan® results and liver US/CT grading.
| Characteristics | Grading in liver US | Grading in abdominal CT |
|---|---|---|
| LSM (kPa) | r=0.133; p=0.418 | r=0.483; p=0.132 |
| CAP (dB/m) | r=0.309; p=0.056 | r=−0.042; p=0.903 |
US: ultrasound, CT: computed tomography, LSM: liver stiffness measurement, CAP: controlled attenuation parameter.
Table 5. Values of Fibroscan® results according to liver US grading.
| Grading in liver US | N | CAP (dB/m) | LSM (kPa) |
|---|---|---|---|
| G0 | 5 | 274.8±62.6 | 5.32±0.89 |
| G1 | 29 | 301.55±43.26 | 7.03±3.29 |
| G2 | 5 | 329.2±22.9 | 6.84±1.15 |
Values are presented as number only or mean±standard deviation.
Steatosis is graded as follows: Absent (Grade 0) when the echotexture of the liver is normal; mild to moderate (Grade 1), in cases of a mild to moderate increase in liver echogenicity with slightly impaired appearance of the portal vein wall and the diaphragm; severe (Grade 2), in cases of marked increase in liver echogenicity with poor or no visualization of the portal vein wall, diaphragm, or posterior part of the right liver lobe.
US: ultrasound, CAP: controlled attenuation parameter, LSM: liver stiffness measurement.
Table 6. Values of Fibroscan® results according to CT grading.
| Grading in abdominal CT | N | CAP (dB/m) | LSM (kPa) |
|---|---|---|---|
| G0 | 3 | 270±34.17 | 4.86±0.35 |
| G1 | 3 | 340±45.03 | 7.23±1.40 |
| G2 | 5 | 307.6±23.02 | 6.92±1.88 |
Values are presented as number only or mean±standard deviation.
In abdominal CT, grading was defined as the difference and ratio of hepatic-spleen attenuation; normal (Grade 0) was defined as 50–65 Hounsfield units (HUs), which were 8–10 HUs higher than that for a normal spleen. Grade 1 was defined as a value lower than 48 HUs, and Grade 2 was defined as that lower than 40 HUs, or when the difference between liver and spleen attenuation was 10 HUs or more.
CT: computed tomography, CAP: controlled attenuation parameter, LSM: liver stiffness measurement.
DISCUSSION
Non-alcoholic steatohepatitis is the most common liver disease in children and adolescents in developed countries, where it is emerging as a major health issue [23,24,25,26]. According to the data from the Korean National Health and Nutrition Examination Survey (KNHANES) conducted by the Korea Centers for Disease Control and Prevention (KCDC), the prevalence of non-alcoholic steatohepatitis in Korean adolescents increased from 4.7% in 2010 to 5.9% in 2015 [27]. Non-alcoholic steatohepatitis can develop from simple steatosis into liver fibrosis, cirrhosis, liver failure, and hepatocellular carcinoma, and its early diagnosis and treatment is important in high-risk groups with type 2 diabetes or cardiovascular disease [28,29,30,31,32].
A non-invasive test method is essential to diagnose non-alcoholic steatohepatitis in children and adolescents at an early stage. Although liver biopsy is the most accurate and definitive diagnostic method for non-alcoholic steatohepatitis, studies comparing non-invasive diagnostic methods are being conducted due to the invasiveness of liver biopsy [33]. Even though non-invasive tests are currently available and reliable, liver biopsy remains the ‘gold standard’ to confirm or exclude non-alcoholic steatohepatitis. However, numerous studies have shown that bio-markers alone are not sufficient to identify patients with non-alcoholic steatohepatitis [34,35,36,37].
This study was the first to confirm that TE (Fibroscan®) and abdominal CT grading showed a statistically significant positive correlation with AST and ALT levels in blood tests. A previous study showed [38] that in patients with non-alcoholic steatohepatitis, the Fibroscan-AST (FAST) score may be an index to determine its activity and fibrosis level, which uses the LSM, CAP, and AST indices. In this study, LSM and CAP of TE (Fibroscan®) and AST identified in blood tests all showed a positive correlation. In obese children and adolescents, the LSM has been shown to generally range from 5–6 kPa, while the CAP is 300 dB/m or more. Thus, both the LSM and CAP values of the present study group were relatively high. We were also able to find similar results to the study conducted by Loaeza-del-Castillo et al. [39], which confirmed that the APRI increased significantly as the degree of desensitization increased in patients with non-alcoholic steatohepatitis.
In another study comparing non-invasive diagnostic methods in children and adolescents with non-alcoholic steatohepatitis, Serai et al. [40] reported that although US is an accessible, inexpensive, and more convenient technique to use in young children, the volume of tissue interrogated is small, and US−based techniques may not perform as well in patients with severe obesity or ascites, which often accompany fatty liver disease [41].
In the meta-analysis published by Xiao et al. [42], the APRI, fibrosis-4 index (FIB-4), BMI, AST/ALT ratio and Diabetes (BARD) score, NAFLD fibrosis score (NFS), Fibroscan®, shear wave elastography (SWE), and magnetic resonance enterography (MRE) were compared. Subsequently, MRE and SWE were found to be the most accurate diagnostic methods, while NFS and FIB-4 showed the best diagnostic performance. Additional diagnostic methods were included compared to those in this study, and the patient group was comparatively analyzed by a meta-analysis of 13,294 patients. Although the Fibroscan® M probe showed moderate accuracy, it also showed noteworthy advantages in terms of its simple indicators and non-invasiveness.
Even though the APRI alone is not sufficiently sensitive to rule out significant disease, it is commonly used to predict significant hepatic fibrosis. The normal range of the APRI is 0.5–1.5 [43,44]. Among the 46 participants in our study, 35 had a score ≤0.5. Only one child was verified with an APRI score of 3.1. In this study, no child scored 4 points on the BARD; 4 children were confirmed to score 3 points, 12 scored 2 points, 11 scored 1 point and 20 scored 0 point. In the study of Harrison et al. [44], the BALD scores equaling 0 or 1 were found to have a high negative predictive value for advanced fibrosis.
The NFS was used to classify the probability of advanced liver fibrosis as follows: <−1.5, low probability; >−1.5 to <0.67, intermediate probability; and >0.67, high probability [45]. In this study, 41, 5, and no children showed NAFLD fibrosis scores <−1.5, >−1.5 to <0.67, and >0.67, respectively.
This study had several limitations. First, liver biopsy, which is the gold standard method to evaluate liver fibrosis, was not performed on patients in this study. Therefore, the accuracy of the results of liver ultrasonography, CT, and Fibroscan® could not be clarified. Second, this study was conducted in a single university hospital, and the sample was small, which would have introduced bias. The limitations also included the fact that the study period was short, that the number of patients assigned to the study group was small, and that liver US/CT tests were not performed in all participants. Although our previous study compared the Fibroscan® test results between the non-obese control group and the obese group; a non-obese control group with a liver ultrasonography and CT was not established as the control group in the present study [9].
Recently, several studies comparing non-invasive diagnostic methods for non-alcoholic steatohepatitis have been conducted. However, most of these studies have been conducted on adults, and there are few studies specifically on children and adolescents in Korea.
We studied the correlation between the conventional liver US or abdominal CT scans and TE (Fibroscan®) in pediatric non-alcoholic steatohepatitis. Notably, the correlations between the tests were not statistically significant; however, it was confirmed that there was a positive correlation between the AST/ALT levels of blood tests and the non-invasive tests, including the Fibroscan test and abdominal CT scan, which suggests the usefulness and possibility of diagnosing hypersteatohepatitis by TE (Fibroscan®). Further large-scale national studies on TE (Fibroscan®) in children and adolescents with steatohepatitis and liver fibrosis are needed to expand utilization of TE (Fibroscan®) in the near future.
ACKNOWLEDGEMENTS
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (MSIT) (NRF-2017R1C1B5076076).
Footnotes
Conflicts of Interest: The authors have no financial conflicts of interest.
References
- 1.Bae JC, Cho YK, Lee WY, Seo HI, Rhee EJ, Park SE, et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 2010;105:2389–2395. doi: 10.1038/ajg.2010.275. [DOI] [PubMed] [Google Scholar]
- 2.Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol. 2009;104:1953–1960. doi: 10.1038/ajg.2009.238. [DOI] [PubMed] [Google Scholar]
- 3.Mouzaki M, Trout AT, Arce-Clachar AC, Bramlage K, Kuhnell P, Dillman JR, et al. Assessment of nonalcoholic fatty liver disease progression in children using magnetic resonance imaging. J Pediatr. 2018;201:86–92. doi: 10.1016/j.jpeds.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tominaga K, Kurata JH, Chen YK, Fujimoto E, Miyagawa S, Abe I, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 5.Sartorio A, Del Col A, Agosti F, Mazzilli G, Bellentani S, Tiribelli C, et al. Predictors of non-alcoholic fatty liver disease in obese children. Eur J Clin Nutr. 2007;61:877–883. doi: 10.1038/sj.ejcn.1602588. [DOI] [PubMed] [Google Scholar]
- 6.Kim SU, Han KH, Park JY, Ahn SH, Chung MJ, Chon CY, et al. Liver stiffness measurement using FibroScan is influenced by serum total bilirubin in acute hepatitis. Liver Int. 2009;29:810–815. doi: 10.1111/j.1478-3231.2008.01894.x. [DOI] [PubMed] [Google Scholar]
- 7.de Lédinghen V, Le Bail B, Rebouissoux L, Fournier C, Foucher J, Miette V, et al. Liver stiffness measurement in children using FibroScan: feasibility study and comparison with Fibrotest, aspartate transaminase to platelets ratio index, and liver biopsy. J Pediatr Gastroenterol Nutr. 2007;45:443–450. doi: 10.1097/MPG.0b013e31812e56ff. [DOI] [PubMed] [Google Scholar]
- 8.Zeng J, Zhang X, Sun C, Pan Q, Lu WY, Chen Q, et al. Feasibility study and reference values of FibroScan 502 with M probe in healthy preschool children aged 5 years. BMC Pediatr. 2019;19:129. doi: 10.1186/s12887-019-1487-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goyal R, Mallick SR, Mahanta M, Kedia S, Shalimar, Dhingra R, et al. Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol. 2013;28:1738–1745. doi: 10.1111/jgh.12318. [DOI] [PubMed] [Google Scholar]
- 10.Kim SU, Jang HW, Cheong JY, Kim JK, Lee MH, Kim DJ, et al. The usefulness of liver stiffness measurement using FibroScan in chronic hepatitis C in South Korea: a multicenter, prospective study. J Gastroenterol Hepatol. 2011;26:171–178. doi: 10.1111/j.1440-1746.2010.06385.x. [DOI] [PubMed] [Google Scholar]
- 11.Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol. 2011;54:64–71. doi: 10.1016/j.jhep.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Kwon YD, Ko KO, Lim JW, Cheon EJ, Song YH, Yoon JM. Usefulness of transient elastography for non-invasive diagnosis of liver fibrosis in pediatric non-alcoholic steatohepatitis. J Korean Med Sci. 2019;34:e165. doi: 10.3346/jkms.2019.34.e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmentieri B, de Sio I, La Mura V, Masarone M, Vecchione R, Bruno S, et al. The role of bright liver echo pattern on ultrasound B-mode examination in the diagnosis of liver steatosis. Dig Liver Dis. 2006;38:485–489. doi: 10.1016/j.dld.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 15.Loria P, Adinolfi LE, Bellentani S, Bugianesi E, Grieco A, Fargion S, et al. Practice guidelines for the diagnosis and management of nonalcoholic fatty liver disease. A decalogue from the Italian Association for the Study of the Liver (AISF) Expert Committee. Dig Liver Dis. 2010;42:272–282. doi: 10.1016/j.dld.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Myers RP, Pollett A, Kirsch R, Pomier-Layrargues G, Beaton M, Levstik M, et al. Controlled attenuation parameter (CAP): a noninvasive method for the detection of hepatic steatosis based on transient elastography. Liver Int. 2012;32:902–910. doi: 10.1111/j.1478-3231.2012.02781.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim SH, Lee JM, Kim JH, Kim KG, Han JK, Lee KH, et al. Appropriateness of a donor liver with respect to macrosteatosis: application of artificial neural networks to US images--initial experience. Radiology. 2005;234:793–803. doi: 10.1148/radiol.2343040142. [DOI] [PubMed] [Google Scholar]
- 18.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dasarathy S, Dasarathy J, Khiyami A, Joseph R, Lopez R, McCullough AJ. Validity of real time ultrasound in the diagnosis of hepatic steatosis: a prospective study. J Hepatol. 2009;51:1061–1067. doi: 10.1016/j.jhep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwasaki M, Takada Y, Hayashi M, Minamiguchi S, Haga H, Maetani Y, et al. Noninvasive evaluation of graft steatosis in living donor liver transplantation. Transplantation. 2004;78:1501–1505. doi: 10.1097/01.tp.0000140499.23683.0d. [DOI] [PubMed] [Google Scholar]
- 21.Koplay M, Sivri M, Erdogan H, Nayman A. Importance of imaging and recent developments in diagnosis of nonalcoholic fatty liver disease. World J Hepatol. 2015;7:769–776. doi: 10.4254/wjh.v7.i5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 23.Draijer L, Benninga M, Koot B. Pediatric NAFLD: an overview and recent developments in diagnostics and treatment. Expert Rev Gastroenterol Hepatol. 2019;13:447–461. doi: 10.1080/17474124.2019.1595589. [DOI] [PubMed] [Google Scholar]
- 24.Mencin AA, Lavine JE. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2011;14:151–157. doi: 10.1097/MCO.0b013e328342baec. [DOI] [PubMed] [Google Scholar]
- 25.Alisi A, Locatelli M, Nobili V. Nonalcoholic fatty liver disease in children. Curr Opin Clin Nutr Metab Care. 2010;13:397–402. doi: 10.1097/MCO.0b013e32833aae84. [DOI] [PubMed] [Google Scholar]
- 26.Lindbäck SM, Gabbert C, Johnson BL, Smorodinsky E, Sirlin CB, Garcia N, et al. Pediatric nonalcoholic fatty liver disease: a comprehensive review. Adv Pediatr. 2010;57:85–140. doi: 10.1016/j.yapd.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Kang Y, Park S, Kim S, Koh H. Estimated prevalence of adolescents with nonalcoholic fatty liver disease in Korea. J Korean Med Sci. 2018;33:e109. doi: 10.3346/jkms.2018.33.e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldstein AE, Charatcharoenwitthaya P, Treeprasertsuk S, Benson JT, Enders FB, Angulo P. The natural history of non-alcoholic fatty liver disease in children: a follow-up study for up to 20 years. Gut. 2009;58:1538–1544. doi: 10.1136/gut.2008.171280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ko JS, Yoon JM, Yang HR, Myung JK, Kim H, Kang GH, et al. Clinical and histological features of nonalcoholic fatty liver disease in children. Dig Dis Sci. 2009;54:2225–2230. doi: 10.1007/s10620-009-0949-3. Erratum in: Dig Dis Sci 2009;54:2771. [DOI] [PubMed] [Google Scholar]
- 30.Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. J Pediatr. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- 31.Neuschwander-Tetri BA, Clark JM, Bass NM, Van Natta ML, Unalp-Arida A, Tonascia J, et al. Clinical, laboratory and histological associations in adults with nonalcoholic fatty liver disease. Hepatology. 2010;52:913–924. doi: 10.1002/hep.23784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caserta CA, Pendino GM, Amante A, Vacalebre C, Fiorillo MT, Surace P, et al. Cardiovascular risk factors, nonalcoholic fatty liver disease, and carotid artery intima-media thickness in an adolescent population in southern Italy. Am J Epidemiol. 2010;171:1195–1202. doi: 10.1093/aje/kwq073. [DOI] [PubMed] [Google Scholar]
- 33.Yang HR. Noninvasive diagnosis of pediatric nonalcoholic fatty liver disease. Korean J Pediatr. 2013;56:45–51. doi: 10.3345/kjp.2013.56.2.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiniakos DG. Nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: histological diagnostic criteria and scoring systems. Eur J Gastroenterol Hepatol. 2010;22:643–650. doi: 10.1097/MEG.0b013e32832ca0cb. [DOI] [PubMed] [Google Scholar]
- 35.Piazzolla VA, Mangia A. Noninvasive diagnosis of NAFLD and NASH. Cells. 2020;9:1005. doi: 10.3390/cells9041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–81.e4. doi: 10.1053/j.gastro.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274–282. doi: 10.1038/nrgastro.2018.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan WK, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–373. doi: 10.1016/S2468-1253(19)30383-8. Erratum in: Lancet Gastroenterol Hepatol 2020;5:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Avila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. 2008;7:350–357. [PubMed] [Google Scholar]
- 40.Serai SD, Panganiban J, Dhyani M, Degnan AJ, Anupindi SA. Imaging modalities in pediatric NAFLD. Clin Liver Dis (Hoboken) 2021;17:200–208. doi: 10.1002/cld.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trout AT, Dillman JR, Xanthakos S, Kohli R, Sprague G, Serai S, et al. Prospective assessment of correlation between US acoustic radiation force impulse and MR elastography in a pediatric population: dispersion of US shear-wave speed measurement matters. Radiology. 2016;281:544–552. doi: 10.1148/radiol.2016152797. [DOI] [PubMed] [Google Scholar]
- 42.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.29302. [DOI] [PubMed] [Google Scholar]
- 43.Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. 2013;158:807–820. doi: 10.7326/0003-4819-158-11-201306040-00005. Erratum in: Ann Intern Med 2013;159:308. [DOI] [PubMed] [Google Scholar]
- 44.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 45.Treeprasertsuk S, Björnsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroenterol. 2013;19:1219–1229. doi: 10.3748/wjg.v19.i8.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]