Abstract
Purpose
The present international survey among healthcare providers aimed to collect data on theoretical knowledge and clinical practices in the diagnosis and management of cow’s milk protein allergy (CMPA) and lactose intolerance (LI) in infants.
Methods
A global survey was conducted in several countries with diverse health care settings. The survey consisted of multiple-choice questions in 3 main domains: (1) understanding and clinical practices around CMPA and LI; (2) case scenarios; and (3) disease-specific knowledge and potential educational needs.
Results
Responses were available from 1,663 participants. About 62% of respondents were general practitioners or general pediatricians, and the remainder were pediatric allergists/gastroenterologists (18%) or other health practitioners (20%). The survey identified knowledge gaps regarding the types of CMPA (IgE-mediated vs. non-IgE-mediated) and the clinical overlap with LI. The survey suggested diverse clinical practices regarding the use of hypoallergenic formulas, as well as misconceptions about the prebiotic benefits of lactose in extensively hydrolyzed formulas in non-breastfed infants with CMPA. Responses to the two case scenarios highlighted varying levels of awareness of the relevant clinical practice guidelines. While respondents generally felt confident in managing infants with CMPA and LI, about 80% expressed an interest for further training in this area.
Conclusion
The current survey identified some knowledge gaps and regional differences in the management of infants with CMPA or LI. Local educational activities among general and pediatric healthcare providers may increase the awareness of clinical practice guidelines for the diagnosis and treatment of both conditions and help improve clinical outcomes.
Keywords: Milk hypersensitivity, Food hypersensitivity, Lactase, Infant formula, Diet therapy, Medical education, Practice guideline
INTRODUCTION
Cow’s milk protein allergy (CMPA) is the most common food allergy in the first year of life [1]. The prevalence of CMPA varies depending on population, clinical definition, and diagnostic method [2]. A systematic review and meta-analysis of 42 studies between 2000 and 2012 estimated a prevalence of 1.2–1.9% in European children with a history of an allergic reaction or positive food challenge to cow’s milk products [3]. This review included the EuroPrevall Study, a European population-based study which found a low adjusted CMPA incidence of 0.54% due to the use stringent diagnostic criteria, including double-blind, placebo-controlled food challenges [4]. A more recent US American survey of parent-reported food allergy found an overall CMPA prevalence of 1.9%, with a peak prevalence of 4.3% reported in children aged 2 years [5].
While immediate, IgE-mediated reactions in general are appropriately recognized, the diagnosis of non-IgE-mediated CMPA may be challenging due to the non-specific nature of gastrointestinal symptoms which overlap with common non-allergic pediatric disorders [6]. Limited recognition of non-IgE-mediated CMPA by healthcare professionals may lead to a delayed or incorrect diagnosis, as well as inappropriate nutritional interventions which may contribute to adverse nutritional outcomes [7,8]. In infants, CMPA may be confused with lactose intolerance (LI), despite vast differences in the underlying pathologies [7]. While LI is the result of an intestinal enzymatic deficiency, CMPA is associated with both IgE-mediated and non-IgE-mediated immunological hypersensitivity reactions [9].
There are only few signs and symptoms that are common to both CMPA and LI in infants. These include persistent diarrhea and a perianal rash or excoriation due to the passage of acidic stools. If untreated CMPA or LI are protracted, both conditions may be associated with poor weight gain [10]. Symptoms such as urticaria, facial angioedema or anaphylaxis are distinctive features of immediate allergic reactions due to IgE-mediated CMPA and not typical of LI. Diagnostic confusion between non-IgE-mediated CMPA and other non-allergic pediatric conditions may occur in infants with persistent crying, frequent regurgitation, diarrhea, rectal bleeding or atopic dermatitis [11]. Finally, upper gastrointestinal symptoms (regurgitation, vomiting) or atopic dermatitis may be due to CMPA but are generally not due to LI [7].
Among adults, LI is by far the most common adverse reaction to cow’s milk-based products. Approximately half to two-thirds of the global adult population develop primary LI due to familial hypolactasia, a genetically determined decline in intestinal lactase expression [12]. Importantly, primary LI condition rarely manifests with clinical symptoms before 5 years of age [10,13]. LI in infants and young children is therefore generally due to another underlying pathology, such as viral gastroenteritis, celiac disease or cow’s milk protein-induced enteropathy [7]. This needs to be distinguished from low-grade lactose malabsorption which is a physiological phenomenon in healthy breastfed and formula-fed infants. In young infants, lactose may reach the colon where it is fermented to short chain fatty acids which confer a range of beneficial prebiotic effects on the developing gut microbiome and intestinal barrier function [14,15].
Consistent with the nonspecific nature of symptoms, a recent European survey uncovered significant knowledge deficits in the management of CMPA relating to the appropriate use of diagnostic tests, optimal selection of specialty formulas for the management of non-breastfed infants and supervision of cow’s milk protein-free elimination diets [16]. A study from Northern Ireland demonstrated that targeted education improved the overall recognition and management of CMPA [8]. The present survey aimed to evaluate the theoretical knowledge and clinical practices among a range of international healthcare providers involved in the care of infants with CMPA and LI. Furthermore, we aimed to collect data on access to local educational resources and clinical guidelines for the diagnosis and management of these infants.
MATERIALS AND METHODS
An online survey was conducted between January and November 2017 in selected countries in Asia, Europe, Latin America, Middle East, and Australia. The survey was designed to include regions with a high prevalence of adult-onset hypolactasia, including Asia (China, India, Thailand, Philippines, Singapore), the Middle East (Kuwait, Egypt), Latin America (Mexico). Australia and the United Kingdom were included as regions with a high prevalence of food allergies and CMPA.
For most countries, the survey was conducted in English language, and a Spanish version was used for respondents in Latin America. The link to the online survey link was sent through medical societies, whenever possible. In Mexico, the link was disseminated via the Mexican Pediatric Society. In China, the survey link was disseminated through the Shanghai branch of the Chinese Medical Association and the Chinese Society of Gastroenterology. A paper-based survey was also conducted in the Philippines. In Australia, a paper-based survey was distributed via Healthed, a medical education organization for general practitioners. Sex, as well as data relating to the type of medical practitioner and medical setting were documented, but identifiers such as name, age or email addresses were omitted in the survey to protect the privacy of individual respondents.
Participants of both the online and paper-based versions of the survey were asked 12 multiple-choice questions relating to signs and symptoms, diagnostic tests and treatment options for CMPA and LI in infants aged under 12 months. Ten of the 12 questions allowed multiple possible answers, and two asked for the single best response from a list of possible answers (Supplementary Table 1). In addition, respondents were asked to answer questions relating to two clinical case scenarios involving (1) non-IgE-mediated CMPA and (2) IgE-mediated CMPA with anaphylaxis. The questions required a single response from a list of possible answers (Supplementary Table 2).
The first clinical scenario (non-IgE-mediated cow’s milk protein-induced enteropathy with secondary LI) described a full-term, vaginally delivered male infant who was exclusively breastfed for 2 months. The infant had presented with persistent diarrhea and eczema around 4 months of age. Cow’s milk-based formula was introduced from 2 months, which was followed by the development of mild-to-moderate eczema across the body and face, increased regurgitation, loose bowel movements (up to 6 times a day without visible blood), mild perianal excoriation, and poor weight gain (fall from the 25th to 10th weight percentile). In the second scenario (IgE-mediated CMPA with anaphylaxis), respondents were asked to assess the case of a 5-month-old male term infant delivered by Caesarean section who was exclusively breastfed and had not commenced solid foods. There was a history of moderate atopic eczema which had started from the age of 2 months. The infant presented acutely with generalized urticaria within minutes of drinking approximately 60 mL of cow’s milk-based formula. The infant vomited once, then became lethargic and floppy, and was taken to hospital for treatment and observation. Respondents were asked to select the most likely clinical diagnosis from a multiple-choice panel.
Finally, respondents were asked to answer three further questions to identify any educational needs relating to the management of CMPA and LI (Supplementary Table 3). The first question comprised 10 parts, with each using a 5-point Likert scale to seek respondents’ agreement or disagreement relating to their confidence in, and understanding of the diagnosis of CMPA and LI, the distinction of CMPA from LI, and appropriate treatment options. Two further questions relating to current educational resources and future training activities permitted multiple responses to a list of possible answers.
Responses were summarized using descriptive statistics, with country-level data used to identify regional trends.
Ethics approval and consent to participate
This survey was conducted anonymously among health care providers, and no patient data or experimental data were collected. Participants had agreed that data provided for the survey would be presented as summary statistics. Individual responses were not analyzed or passed on to third parties. As the survey did not collect patient or experimental data, human research ethics approval was not sought.
RESULTS
Survey responses were received from 1,663 international healthcare professionals. Of these, 1,467 (88.2%) came from health care providers in the originally targeted countries, including China, India, Philippines, Singapore, Thailand, Mexico, Kuwait, United Kingdom, and Australia. Due to sharing of the online survey link with international colleagues, we received 196 (11.8%) responses from countries outside the initial scope of the survey. These included 53 (3.2%) respondents from additional Latin American countries (Argentina, Brazil, Chile, Colombia, Ecuador, El Salvador, Guatemala, Uruguay, Venezuela), 53 (3.2%) from the Middle East (Bahrain, Egypt, Israel, Jordan, Oman, Saudi Arabia, Turkey, United Arab Emirates), 36 (2.2%) from Spain, as well as 55 (3.3%) from other countries, including Afghanistan, Pakistan, Vietnam, Greece, Ukraine, Libya, Canada and the USA. Table 1 summarizes the number of respondents by region/country, as well as their sex and medical specialization.
Table 1. Region/country of practice, sex and medical specialization of respondents.
| Region/Country | No. of respondents | Sex (male/female) | General practitioners | General pediatricians | Pediatric allergists & Gastroenterologists | Other health practitioners | |
|---|---|---|---|---|---|---|---|
| Latin America | 552 (33.2) | 269 (48.7)/283 (51.3) | 107 (19.4) | 245 (44.4) | 96 (17.4) | 104 (18.8) | |
| Mexico | 499 (30.0) | 242 (48.5)/257 (51.5) | 106 (21.2) | 234 (46.9) | 60 (12.0) | 99 (19.8) | |
| Other Latin American countries | 53 (3.2) | 27 (50.9)/26 (49.1) | 1 (1.9) | 11 (20.8) | 36 (67.9) | 5 (9.4) | |
| Asia | 478 (28.7) | 128 (27.2)/342 (72.8) | 38 (8.0) | 252 (52.8) | 104 (21.8) | 83 (17.4) | |
| China | 18 (1.1) | 7 (38.9)/11 (61.1) | 2 (11.1) | 4 (22.2) | 10 (55.6) | 2 (11.1) | |
| India | 66 (4.0) | 49 (74.2)/17 (25.8) | 3 (4.6) | 50 (75.8) | 2 (3.0) | 11 (16.7) | |
| Philippines | 162 (9.7) | 37 (22.8)/117 (72.2)* | 5 (3.1)* | 108 (66.7)* | 19 (11.7)* | 22 (13.6)* | |
| Singapore | 67 (4.0) | 16 (23.9)/51 (76.1) | 13 (19.4) | 35 (52.2) | 4 (6.0) | 15 (22.4) | |
| Thailand | 165 (9.9) | 25 (15.2)/140 (84.8) | 13 (7.9) | 54 (32.7) | 68 (41.2) | 30 (18.2) | |
| Australia | 259 (15.6) | 29 (11.2)/230 (88.8) | 184 (71.0) | 5 (1.9) | 0 (0.0) | 70 (27.0) | |
| Middle East | 246 (14.8) | 123 (50.0)/123 (50.0) | 31 (12.6) | 125 (50.8) | 34 (13.8) | 56 (22.8) | |
| Egypt | 52 (3.2) | 24 (46.2)/28 (53.8) | 4 (7.7) | 30 (57.7) | 5 (9.6) | 13 (25.0) | |
| Kuwait | 141 (8.5) | 62 (44.0)/79 (56.0) | 24 (17.0) | 73 (51.8) | 6 (4.2) | 38 (27.0) | |
| Other Middle Eastern countries | 53 (3.2) | 37 (69.8)/16 (30.2) | 3 (5.7) | 22 (41.5) | 23 (43.4) | 5 (9.5) | |
| Europe | 73 (4.4) | 27 (37.0)/46 (63.0) | 7 (9.6) | 8 (11.0) | 41 (56.2) | 17 (23.3) | |
| Spain | 36 (2.2) | 16 (44.4)/20 (55.6) | 0 (0.0) | 1 (2.8) | 35 (97.2) | 0 (0.0) | |
| UK | 37 (2.2) | 11 (29.7)/26 (70.3) | 7 (18.9) | 7 (18.9) | 6 (16.2) | 17 (46.0) | |
| Others | 55 (3.3) | 27 (49.1)/28 (50.9) | 2 (3.6) | 23 (41.8) | 25 (45.5) | 5 (9.1) | |
| Total | 1,663 (100.0) | 609 (36.6)/1,046 (62.9)* | 367 (22.1)* | 657 (39.5)* | 299 (18.0)* | 332 (20.0)* | |
Values are presented as number (%).
*Missing data from 8 respondents.
Demographic information
Of 1,655 (99.5%) respondents who provided demographic information, the majority (n=1,046; 62.9%) were female. Overall, 909 (54.7%) respondents had ≥10 years’ clinical experience, 289 (17.4%) had 6 to 9 years, 387 (23.3%) had 2 to 5 years, and 70 (4.2%) had ≤1 year. General pediatricians (n=657; 39.5%) and general practitioners (n=367; 22.1%) made up the largest proportion of respondents, followed by pediatric gastroenterologists (n=185; 11.1%) and pediatric allergists (n=114; 6.9%). The two most common practice settings were tertiary-level hospital settings (n=584; 35.1%) and private practice (n=579; 34.8%); Table 2.
Table 2. Baseline characteristics of study respondents (n=1,663).
| Characteristic | Value | |
|---|---|---|
| Sex | ||
| Male | 609 (36.6) | |
| Female | 1,046 (62.9) | |
| No response | 8 (0.5) | |
| Medical specialization | ||
| General pediatricians | 657 (39.5) | |
| General practitioners | 367 (22.1) | |
| Pediatric gastroenterologists | 185 (11.1) | |
| Pediatric allergists | 114 (6.9) | |
| Adult allergists | 8 (0.5) | |
| Adult gastroenterologists | 2 (0.1) | |
| Others* | 322 (19.4) | |
| No response | 8 (0.5) | |
| Years in clinical practice | ||
| ≥10 | 909 (54.7) | |
| 6 to 9 | 289 (17.4) | |
| 2 to 5 | 387 (23.3) | |
| ≤1 | 70 (4.2) | |
| No response | 8 (0.5) | |
| Type of institution or practice | ||
| Tertiary-level hospital | 584 (35.1) | |
| Private practice | 579 (34.8) | |
| Secondary-level hospital | 273 (16.4) | |
| Community healthcare centers or public health clinics | 149 (9.0) | |
| Others | 70 (4.2) | |
| No response | 8 (0.5) | |
Values are presented as number (%).
*Neonatologists, pediatric pulmonologists, pediatric intensivists, pediatric dieticians, nurses, and midwives.
Competencies in diagnosing and managing CMPA and LI
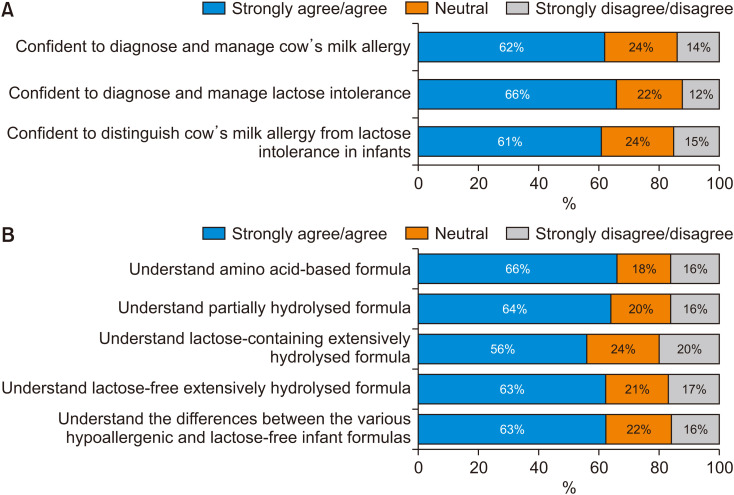
The majority of respondents either agreed or strongly agreed that they were confident about their skills in diagnosing and managing CMPA (62%) and LI (66%), respectively. Sixty-one percent either agreed or strongly agreed that they were confident in distinguishing between both conditions (Fig. 1A). A similar proportion of respondents either agreed or strongly agreed that they understood the respective roles of amino acid-based formula (AAF) (66%), partially hydrolyzed formula (PHF) (64%), and lactose-free extensively hydrolyzed formula (EHF) (63%) (Fig. 1B). Sixty-three percent of respondents either agreed or strongly agreed that they understood the differences between the various hypoallergenic and lactose-free infant formulas. Only 56% either agreed or strongly agreed that they understood the clinical positioning of lactose-containing EHF in infant nutrition.
Fig. 1. Respondents’ agreement or disagreement with statements relating to understanding the diagnosis of (A), and treatment options for (B), CMPA and LI in infants.
CMPA: cow’s milk protein allergy, LI: lactose intolerance.
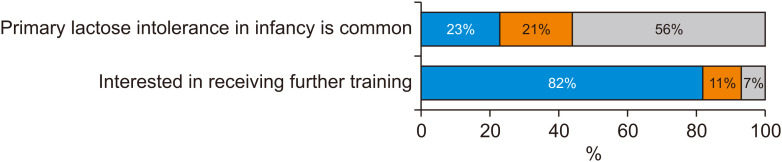
Despite the level of confidence and understanding respondents expressed, only 56% of respondents either disagreed or strongly disagreed with the false statement that “primary lactose intolerance in infancy is common”, with almost 1 in 4 respondents (23%) either agreeing or strongly agreeing to this statement (Fig. 2). Eighty-two percent either agreed or strongly agreed that they were interested in receiving further training in this setting.
Fig. 2. Respondents’ perception on primary lactose intolerance (LI) as a common problem in infancy, as well as desire for additional training in the area of LI.
In terms of currently available resources and training tools in their respective countries, lectures were the most available resource on CMPA and LI, with 68% of respondents having access to this resource followed by printed materials/handouts (65%), invited expert speakers (50%), local symposia (45%), and online tutorials/webinars (40%). Respondents selected future online tutorials/webinars (62%), followed by lectures (60%) and invited expert speakers (58%), as the most useful educational future activity or resource.
Clinical presentation of CMPA and LI
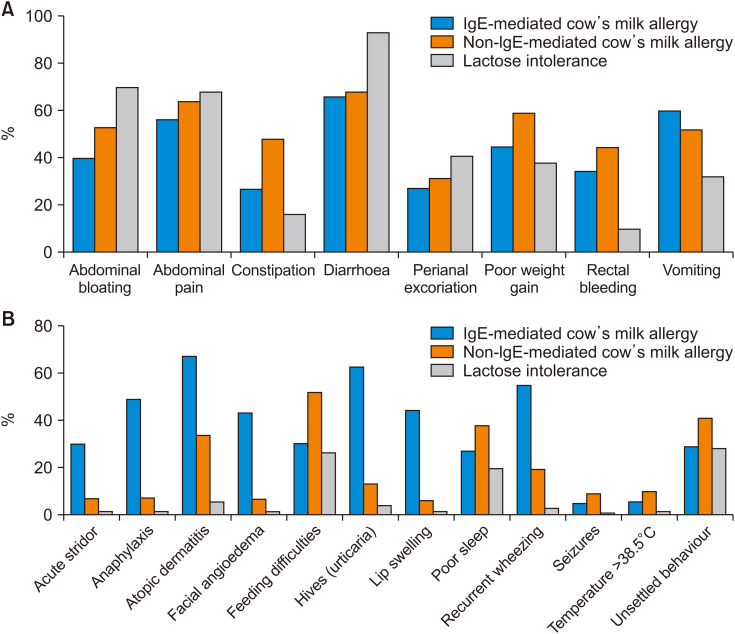
There were differences in the signs and symptoms that respondents selected to characterize IgE-mediated CMPA, non-IgE-mediated CMPA or LI (Fig. 3). Across all countries, for IgE-mediated CMPA, the most commonly selected symptoms were atopic dermatitis (67%), diarrhea (66%), urticaria (63%), vomiting (60%), and abdominal discomfort (56%). There were some differences in response by country. Diarrhea was the most commonly selected symptom by respondents in Australia (69%), China (78%), Egypt (75%), India (88%), and Kuwait (79%); hives was the most commonly selected symptom by respondents in Singapore (76%), Spain (94%), Thailand (90%), and UK (89%); and atopic dermatitis was the most commonly selected symptom by respondents in Mexico (79%) and the Philippines (84%).
Fig. 3. Clinical signs and symptoms selected by respondents as being characteristic of IgE-mediated CMPA, non-IgE-mediated CMPA and LI: (A) digestive (gastrointestinal) signs and symptoms and (B) other signs and symptoms.
CMPA: cow’s milk protein allergy, LI: lactose intolerance.
For non-IgE-mediated CMPA, the most commonly selected symptoms were diarrhea (68%), abdominal discomfort (64%), poor weight gain (59%), abdominal bloating (53%), feeding difficulties (52%), and vomiting (52%). Most respondents considered only symptoms related to gastrointestinal manifestations (Fig. 3A) and did not select extra-intestinal, systemic symptoms (Fig. 3B). Only a small proportion of respondents chose atopic dermatitis (34%) or urticaria (13%) as potential symptoms. Across most countries, diarrhea was selected as either the leading or second sign or symptom of non-IgE-mediated CMPA, including practitioners in Australia (69%; second behind unsettled behavior), India (63%), Kuwait (54%; second behind poor weight gain), Latin America (78%), Mexico (65%; second behind abdominal discomfort), Middle East (63%), Philippines (68%; second behind abdominal bloating), Singapore (72%), Spain (91%), and Thailand (79%).
For LI, the vast majority of respondents in all countries selected diarrhea (93%) as the main sign or symptom, which was followed by abdominal bloating (70%) and abdominal discomfort (68%). A small proportion of respondents falsely associated LI with allergic symptoms, such as hives (4%), atopic dermatitis (6%) or even anaphylaxis (2%).
Diagnostic testing
Cow’s milk-specific serum IgE and skin prick testing are considered the most useful diagnostic tests for IgE-mediated CMPA [17,18]. In most participating countries and regions, cow’s milk-specific serum IgE and skin prick testing were commonly selected methods to diagnose IgE-mediated CMPA. By contrast, as there is no specific diagnostic test for non-IgE-mediated CMPA, most respondents from Australia, Egypt, Mexico, other Latin American countries and the UK indicated that they performed a home challenge to cow’s milk but did not rely on any diagnostic tests. Respondents from China, India, Kuwait, Singapore, Spain, Thailand had selected a hospital challenge to cow’s milk. For LI, either a trial of lactose-free formula, or reducing total sugars and pH in feces were the most common diagnostic tools selected by respondents in most countries/regions. Breath hydrogen testing, a more reliable functional diagnostic method for LI, was popular among respondents from Egypt, Spain, and Latin American countries (other than Mexico).
Clinical management
When asked at what age familial hypolactasia (primary LI) generally first presented as a clinical problem, 74% of respondents incorrectly believed that the condition presented with symptoms in the first year of life. Regarding secondary LI, the most selected clinical scenarios in which respondents would recommend avoiding lactose in infants were viral gastroenteritis (44%), followed by cow’s milk protein-induced enteropathy (36%). Consistent with these responses, the most commonly selected causes of LI in infants under 12 months of age were viral gastroenteritis (65%), followed by CMPA with enteropathy (31%). Congenital primary LI and primary hypolactasia were incorrectly selected by 29% and 27% of respondents, respectively, as common causes of LI in infants.
Although 59% of respondents selected EHF as the first-line treatment of confirmed IgE-mediated CMPA in formula-fed infants under 6 months of age, there was uncertainty among some respondents regarding the use of lactose-free (29%) or lactose-containing (30%) EHF options. The use of lactose-free EHF is indicated for patients with CMPA and clinical manifestations of LI, including perianal excoriation, flatulence or diarrhea. EHF with lactose was the most popular choice among respondents from Australia (25%), Philippines (55%), Singapore (33%), Spain (55%), and the UK (37%); EHF without lactose was the most popular choice among respondents from Kuwait (23%), Mexico (36%), and Thailand (49%). There was considerable variation among respondents from different countries regarding other choices for first-line treatment: notably, 73% of respondents from China selected AAF, and 35% of respondents from India and 26% of respondents from Egypt selected soy-based formula as first-line treatment, most likely due to limited availability in these countries. Contrary to clinical guidelines, 10.6% of the total respondents indicated that PHF was their first-line treatment choice for IgE-mediated CMPA.
When asked about the clinical benefits of lactose contained in some EHF products, 51% selected better taste, 39% prebiotic effect, 31% increased weight gain, and 29% recognized improved calcium absorption, all of which were considered correct answers. However, 18% of respondents incorrectly identified improved iron absorption as an additional benefit of lactose-containing EHF. Regarding key features considered when selecting a specific hypoallergenic formula for managing infants with CMPA, 96% of respondents indicated that they would choose a formula with proven efficacy in resolving symptoms. Affordability was also a major consideration (63%).
Clinical case scenarios
For the first clinical case scenario of a 4-month-old infant with probable diagnosis of non-IgE-mediated CMPA, 40% correctly responded that they would prescribe an EHF without lactose as the infant had presented with perianal excoriation; Only 26% chose an EHF with lactose, and 12% responded that they would recommend a PHF which is not suitable. For the second case scenario of a 6-month old infant with typical IgE-mediated CMPA and anaphylaxis, only 24% correctly selected an AAF as the most appropriate treatment option for supplemental feeding. In addition, 54% of respondents would recommend an EHF which might expose the infant to a potential risk of anaphylaxis due to their residual allergen content.
DISCUSSION
The present survey explored attitudes and awareness of clinical management guidelines for CMPA and LI in infants and young children in a global sample of healthcare professionals. In addition, the survey aimed to identify potential educational needs and preferences for further educations. Findings suggest that a significant proportion of healthcare professionals involved in the treatment of infants had ongoing misconceptions and knowledge gaps about the diagnosis and clinical management of CMPA and LI. In this context, the overestimation of the prevalence of primary LI in infancy was noteworthy. Although primary LI rarely manifests before five years of age [7], almost three-quarters of respondents indicated that primary LI was a significant clinical problem in the first year of life. Despite this misconception, healthcare practitioners expressed confidence in their skills in diagnosing and managing these disorders. Our findings highlight the need for additional awareness and training in the management of CMPA and LI. Interestingly, almost 80% of respondents recognized the need for further educational activities in this area.
In general, healthcare professionals appeared to have a good understanding of the signs and symptoms of IgE-mediated CMPA. Consistent with its pathophysiology, most respondents chose the detection of cow’s milk-specific serum IgE and skin prick testing as appropriate diagnostic tools. However, the role of diarrhea as a major clinical feature of IgE-mediated CMPA appeared to be overestimated. Some knowledge gaps also became apparent for the distinction between non-IgE-mediated CMPA and LI [19]. These mainly related to signs and symptoms, with only a small proportion of respondents recognizing the spectrum of extra-intestinal manifestations in non-IgE-mediated CMPA, including atopic dermatitis [6]. For LI, respondents from all countries identified diarrhea as the main symptom and recognized the significance of viral gastroenteritis, giardiasis, cow’s milk enteropathy or celiac disease as underlying causes of secondary LI [7]. Again, the role of primary LI and hypolactasia as a common cause of LI was overestimated by a large proportion of participants.
The treatment of LI in infants generally involves a marked reduction, but not complete elimination of lactose-containing foods [7]. By contrast, infants with suspected CMPA should undergo a trial of a strict cow’s milk protein-free maternal elimination diet if the infant is breastfed [20], or in formula-fed infants of an EHF or AAF, together with strict cow’s milk avoidance [21,22]. In this regard, healthcare practitioners again appeared to have a good understanding of the management of IgE-mediated CMPA where almost 60% of respondents identified EHF as the first-line treatment. However, contrary to guidelines, the use of PHF in the management of infants with IgE-mediated CMPA was reported by a considerable number of respondents (10.6%), despite reports of severe allergic reactions or even anaphylaxis [23].
Based on the first case presentation involving an infant with non-IgE-mediated CMPA, 40% of respondents appropriately selected initiation of an EHF without lactose as the appropriate course of treatment since secondary LI was suggested by the presence of diarrhea and perianal excoriation. In the second case presentation involving an infant with IgE-mediated CMPA with anaphylaxis, an equivocal proportion of respondents selected lactose-containing and lactose-free EHF for treatment, whereas AAF was selected by less than one-quarter of respondents overall. Most clinical guidelines currently recommend an AAF as first-line treatment in infants with CMPA and anaphylaxis due to the small residual risk of significant adverse reactions to some EHF products [22,24].
Our study had several limitations. The survey was designed as a cross-sectional convenience sample across a range of diverse countries. The number of responses for each country was therefore difficult to predict. Not surprisingly, we observed significant differences in the professional spectrum of respondents, including differences in sex distribution, medical specialization, duration of clinical practice and health care setting. Respondents from Mexico and Australia made up almost half of the respondents. We therefore recognize that the survey provides exploratory data only, as for most regions a representative sample was not captured. Respondents were enriched for female pediatricians and practitioners with over 10 years’ experience. In addition, we received responses from practitioners from countries outside the initial scope of the survey, most likely due to sharing of the survey via specialist pediatric networks. This may have skewed responses in some regions towards pediatric allergists and gastroenterologists (Latin America other 67.9%, Middle East other 43.4%, and Spain 97.2%). As a result of the diverse nature of respondents and health care settings, as well as sample size limitations, we felt that it was not feasible to perform subanalyses between specific groups of health practitioners or regions. We were therefore unable to assess, if knowledge levels and clinical practices differed between general practitioners, general pediatricians and pediatric subspecialists. However, survey data from each country still provide useful insights into local clinical practices and educational needs which may inform future educational activities.
In summary, the current survey identified some knowledge gaps and regional differences in the management of infants with CMPA or LI, particularly regarding the delineation of non-IgE-mediated CMPA against LI. Most survey participants acknowledged a need for further education, in line with evidence-based clinical guidelines. Local educational activities to increase the awareness of clinical practice guidelines may help improve long-term nutritional outcomes in infants and young children with suspected cow’s milk-related symptoms [25,26,27].
ACKNOWLEDGEMENTS
The authors wish to thank all healthcare professionals who participated in the survey and thank the participating medical societies for distributing emails with the online survey. We also thank Dr. Prashant Bachina at Rainbow Children‘s Hospital, Hyderabad, India, for his expertise and coordination of the survey in India. The support of Dr. Ramesh Manocha, HealthEd Australia, in conducting the survey in Australia is gratefully acknowledged.
Footnotes
Funding: This survey was supported by an unrestricted educational grant from Nestlé Health Science, Switzerland.
Conflicts of Interest: AJ and RGH are employees of Nestlé Health Science. All other authors declared no conflict of interest.
SUPPLEMENTARY MATERIALS
Clinical survey questions (questions 1–12)
Clinical case scenarios (questions 13 & 14)
Education and training (questions 15–17)
References
- 1.Luyt D, Ball H, Makwana N, Green MR, Bravin K, Nasser SM, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44:642–672. doi: 10.1111/cea.12302. [DOI] [PubMed] [Google Scholar]
- 2.Flom JD, Sicherer SH. Epidemiology of cow’s milk allergy. Nutrients. 2019;11:1051. doi: 10.3390/nu11051051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nwaru BI, Hickstein L, Panesar SS, Muraro A, Werfel T, Cardona V, et al. The epidemiology of food allergy in Europe: a systematic review and meta-analysis. Allergy. 2014;69:62–75. doi: 10.1111/all.12305. [DOI] [PubMed] [Google Scholar]
- 4.Schoemaker AA, Sprikkelman AB, Grimshaw KE, Roberts G, Grabenhenrich L, Rosenfeld L, et al. Incidence and natural history of challenge-proven cow’s milk allergy in European children--EuroPrevall birth cohort. Allergy. 2015;70:963–972. doi: 10.1111/all.12630. [DOI] [PubMed] [Google Scholar]
- 5.Gupta RS, Warren CM, Smith BM, Blumenstock JA, Jiang J, Davis MM, et al. The public health impact of parent-reported childhood food allergies in the United States. Pediatrics. 2018;142:e20181235. doi: 10.1542/peds.2018-1235. Erratum in: Pediatrics 2019;143:e20183835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heine RG, Elsayed S, Hosking CS, Hill DJ. Cow’s milk allergy in infancy. Curr Opin Allergy Clin Immunol. 2002;2:217–225. doi: 10.1097/00130832-200206000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Heine RG, AlRefaee F, Bachina P, De Leon JC, Geng L, Gong S, et al. Lactose intolerance and gastrointestinal cow’s milk allergy in infants and children - common misconceptions revisited. World Allergy Organ J. 2017;10:41. doi: 10.1186/s40413-017-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wauters L, Brown T, Venter C, Dziubak R, Meyer R, Brogan B, et al. Cow’s milk allergy prescribing is influenced by regional and national guidance. J Pediatr Gastroenterol Nutr. 2016;62:765–770. doi: 10.1097/MPG.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 9.Johansson SG, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- 10.Heyman MB Committee on Nutrition. Lactose intolerance in infants, children, and adolescents. Pediatrics. 2006;118:1279–1286. doi: 10.1542/peds.2006-1721. [DOI] [PubMed] [Google Scholar]
- 11.Fiocchi A, Brozek J, Schünemann H, Bahna SL, von Berg A, Beyer K, et al. World Allergy Organization (WAO) Diagnosis and Rationale for Action against Cow’s Milk Allergy (DRACMA) guidelines. World Allergy Organ J. 2010;3:57–161. doi: 10.1097/WOX.0b013e3181defeb9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deng Y, Misselwitz B, Dai N, Fox M. Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 2015;7:8020–8035. doi: 10.3390/nu7095380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegar B, Widodo A. Lactose intolerance in Indonesian children. Asia Pac J Clin Nutr. 2015;24(Suppl 1):S31–S40. doi: 10.6133/apjcn.2015.24.s1.06. [DOI] [PubMed] [Google Scholar]
- 14.Szilagyi A. Redefining lactose as a conditional prebiotic. Can J Gastroenterol. 2004;18:163–167. doi: 10.1155/2004/350732. [DOI] [PubMed] [Google Scholar]
- 15.Francavilla R, Calasso M, Calace L, Siragusa S, Ndagijimana M, Vernocchi P, et al. Effect of lactose on gut microbiota and metabolome of infants with cow’s milk allergy. Pediatr Allergy Immunol. 2012;23:420–427. doi: 10.1111/j.1399-3038.2012.01286.x. [DOI] [PubMed] [Google Scholar]
- 16.Werkstetter K, Chmielewska A, Dolinšek J, Estourgie-van Burk F, Korponay-Szabó I, Kurppa K, et al. Diagnosis and management of cow’s milk protein allergy - how big is the gap between ideal and reality? A quality-of-care survey in Europe. J Pediatr Gastroenterol Nutr. 2018;66(Suppl 2):399. [Google Scholar]
- 17.Celik-Bilgili S, Mehl A, Verstege A, Staden U, Nocon M, Beyer K, et al. The predictive value of specific immunoglobulin E levels in serum for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:268–273. doi: 10.1111/j.1365-2222.2005.02150.x. [DOI] [PubMed] [Google Scholar]
- 18.Verstege A, Mehl A, Rolinck-Werninghaus C, Staden U, Nocon M, Beyer K, et al. The predictive value of the skin prick test weal size for the outcome of oral food challenges. Clin Exp Allergy. 2005;35:1220–1226. doi: 10.1111/j.1365-2222.2005.2324.x. [DOI] [PubMed] [Google Scholar]
- 19.Walsh J, Meyer R, Shah N, Quekett J, Fox AT. Differentiating milk allergy (IgE and non-IgE mediated) from lactose intolerance: understanding the underlying mechanisms and presentations. Br J Gen Pract. 2016;66:e609–e611. doi: 10.3399/bjgp16X686521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alibardi L, Meyer-Rochow VB. Microscopical observations on the regenerating tail in the tuatara Sphenodon punctatus indicate a tendency to scarring, but also influence from somatic growth. J Morphol. 2019;280:411–422. doi: 10.1002/jmor.20953. [DOI] [PubMed] [Google Scholar]
- 21.Muraro A, Høst A. Controversies on special products for managing cow’s milk protein allergy in infants: safety and suitability. EMJ Allergy Immunol. 2017;2:46–51. [Google Scholar]
- 22.Koletzko S, Niggemann B, Arato A, Dias JA, Heuschkel R, Husby S, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55:221–229. doi: 10.1097/MPG.0b013e31825c9482. [DOI] [PubMed] [Google Scholar]
- 23.Egan M, Lee T, Andrade J, Grishina G, Mishoe M, Gimenez G, et al. Partially hydrolyzed whey formula intolerance in cow’s milk allergic patients. Pediatr Allergy Immunol. 2017;28:401–405. doi: 10.1111/pai.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chauveau A, Nguyen-Grosjean VM, Jacquenet S, Richard C, Mouton-Faivre C. Immediate hypersensitivity to extensively hydrolyzed formulas: an important reminder. Pediatr Allergy Immunol. 2016;27:541–543. doi: 10.1111/pai.12556. [DOI] [PubMed] [Google Scholar]
- 25.Kvammen JA, Thomassen RA, Eskerud MB, Rugtveit J, Henriksen C. Micronutrient status and nutritional intake in 0- to 2-year-old children consuming a cows’ milk exclusion diet. J Pediatr Gastroenterol Nutr. 2018;66:831–837. doi: 10.1097/MPG.0000000000001942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mehta H, Groetch M, Wang J. Growth and nutritional concerns in children with food allergy. Curr Opin Allergy Clin Immunol. 2013;13:275–279. doi: 10.1097/ACI.0b013e328360949d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyer R, Wright K, Vieira MC, Chong KW, Chatchatee P, Vlieg-Boerstra BJ, et al. International survey on growth indices and impacting factors in children with food allergies. J Hum Nutr Diet. 2019;32:175–184. doi: 10.1111/jhn.12610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Clinical survey questions (questions 1–12)
Clinical case scenarios (questions 13 & 14)
Education and training (questions 15–17)