Abstract
Background
The association between autonomic dysfunction and long‐COVID syndrome is established. However, the prevalence and patterns of symptoms of dysautonomia in long‐COVID syndrome in a large population are lacking.
Objective
To evaluate the prevalence and patterns of symptoms of dysautonomia in patients with long‐COVID syndrome.
Methods
We administered the Composite Autonomic Symptom Score 31 (COMPASS‐31) questionnaire to a sample of post‐COVID‐19 patients who were referred to post‐COVID clinic in Assiut University Hospitals, Egypt for symptoms concerning for long‐COVID syndrome. Participants were asked to complete the COMPASS‐31 questionnaire referring to the period of more than 4 weeks after acute COVID‐19.
Results
We included 320 patients (35.92 ± 11.92 years, 73% females). The median COMPASS‐31 score was 26.29 (0–76.73). The most affected domains of dysautonomia were gastrointestinal, secretomotor, and orthostatic intolerance with 91.6%, 76.4%, and 73.6%, respectively. There was a positive correlation between COMPASS‐31 score and long‐COVID duration (p < 0.001) and a positive correlation between orthostatic intolerance domain score and post‐COVID duration (p < 0.001). There was a positive correlation between orthostatic intolerance domain score and age of participants (p = 0.004). Two hundred forty‐seven patients (76.7%) had a high score of COMPASS‐31 >16.4. Patients with COMPASS‐31 >16.4 had a longer duration of long‐COVID syndrome than those with score <16.4 (46.2 vs. 26.8 weeks, p < 0.001).
Conclusions
Symptoms of dysautonomia are common in long‐COVID syndrome. The most common COMPASS‐31 affected domains of dysautonomia are gastrointestinal, secretomotor, and orthostatic intolerance. There is a positive correlation between orthostatic intolerance domain score and patients' age.
Introduction
COVID‐19 emerged in the late 2019 and since then it has been a wide‐spread epidemic with significant morbidity and mortality. 1 After the world entered the battle of confronting acute COVID‐19, it was discovered that it does not end with early identification and management of the acute COVID‐19, and late health consequences have appeared among COVID‐19 survivors. 2 It becomes obvious that the morbidity of COVID‐19 is not only limited to the acute infection, but it also extends behind the acute phase and causes long‐term sequelae described as “post‐acute COVID syndrome” (PACS) or “long‐COVID syndrome.” The United Kingdom National Institute for Health and Care Excellence (NICE) has defined the long‐COVID syndrome as signs and symptoms that develop from 4 to 12 weeks after the onset of illness and post‐COVID syndrome (12 weeks or more), and are not explained by an alternative diagnosis. 3 Long‐COVID syndrome has been reported after mild or severe COVID‐19 irrespective of the severity of the acute phase of COVID‐19. 4 Direct invasion of the virus into the brain by binding to angiotensin‐converting enzyme 2 (ACE2) expressed in the endothelium of the capillary of the blood–brain barrier or an indirect effect of the cytokine storm on mitochondria or on nerve fibers, 5 immune dysregulation, hormonal disturbances, elevated cytokine levels due to immune reaction leading to chronic inflammation have been proposed, among others as possible pathophysiological mechanisms for developing neurological manifestations of long‐COVID. 6
Long‐COVID syndrome encompasses many symptoms including shortness of breath, headaches, memory changes, nausea, anorexia, abnormal sweating, palpitation, anxiety, depression, fatigue, chest pain, and orthostatic intolerance (OI). 7 , 8 , 9 , 10 , 11 , 12 Many of these symptoms are seen in patients with autonomic dysfunction. Additionally, postural orthostatic tachycardia syndrome, OI, abnormal sudomotor function, and neuropathy have been reported to be associated or worsened with COVID‐19. 13 , 14 , 15 Although the association between autonomic dysfunction and long‐COVID syndrome has gained considerable interest, 15 , 16 the prevalence of autonomic dysfunction has not well‐studied in long‐COVID population. Therefore, this study aimed to estimate the prevalence and describe the patterns of autonomic symptoms in long‐COVID patients.
Methods
Participants and settings
This cross‐sectional study was conducted on patients referred to the post‐COVID clinic at Assiut University Hospitals, Egypt. Patients were included in this study if they were above 18‐year‐old, symptomatic at SARS‐CoV‐2 infection acute phase regardless of the severity of the symptoms or the need for oxygen or ICU support, had a laboratory‐confirmed (polymerase chain reaction and/or antibody testing) diagnosis, and had symptoms of long‐COVID syndrome 8 that developed or persisted at least 4 weeks after the onset of illness. Patients were excluded if they had a diagnosis of cognitive dysfunction, neurodegenerative brain disorders, known history of autonomic dysfunction, or were taking medications that significantly affect the autonomic nervous system (ANS) as beta‐blocker, tricyclic antidepressant, alpha‐1 blockers, and central antihypertensives.
A total of 356 patients had been referred to the post‐COVID clinic during the study period between 01 August 2021 and 01 November 2021. This number was large enough to detect an anticipated frequency of symptoms of dysautonomia among long‐COVID patients of 61.1% as reported in an earlier study, 17 with a 95% confidence level and 5% absolute precision, given that the total number of the study population was <100,000. This calculation was performed using Epi Info™ Statistical Package version 7.2.4.0 (Centers for Disease Control and Prevention, Atlanta, GA, USA).
Eligible patients were retrospectively identified from the medical records of the post‐COVID clinic then invited for a clinical visit between 01 October 2021 and 01 November 2021 to complete data collection. Baseline demographic and clinical data (including acute COVID‐19 related data) were retrieved from patients' medical records. Missing information due to incomplete records was collected during their most recent visit to the post‐COVID clinic.
COMPASS‐31 questionnaire
Furthermore, participants were asked to complete the Composite Autonomic Symptom Score 31 (COMPASS‐31) questionnaire, referring to the period after the COVID‐19 (at least 4 weeks following the onset of acute COVID‐19). The COMPASS‐31 is validated and widely used questionnaire to quantify autonomic symptom severity. It consists of 31 questions that fall into six domains of dysautonomia: OI, vasomotor, secretomotor, gastrointestinal (GI), bladder, and pupillomotor. An answer was scored as zero when it was not assigned a point. A raw domain score was obtained by adding together points within each domain. The total score within each domain was weighted and then added together to give a total score ranging from 0 to 100. The maximum‐weighted scores for each subdomain are as follows: 40 for OI, 5 for vasomotor dysfunction, 15 for secretomotor dysfunction, 25 for GI dysfunction, 10 for urinary dysfunction, and 5 for pupillomotor dysfunction. 18 A total COMPASS‐31 of >16.4 was used to suggest initial autonomic nervous system dysfunction, as reported in earlier studies. 19 , 20 Subdomains were considered positive if score >0. 21
The COMPASS‐31 was translated from English to Arabic by a bilingual translator and expert opinion was taken to assess its validity. A pilot testing of the translated questionnaire was performed on 30 participants to ensure that all questions were clear and could be understood by the participants. Items were evaluated for their internal consistency using Cronbach's α coefficient (α = 0.85).
Ethical approval was obtained from the Institutional Review Board (IRB) at the University of Assiut, Egypt (IRB Approval Number: 579/11/21). All patients gave their written informed consent before participation in the study.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics version 25 (SPSS Inc., Chicago, IL, USA). Categorical data were presented as numbers and percentages. Continuous data were tested for normality using the Kolmogorov–Smirnov test. Normally distributed continuous variables were summarized as mean and standard deviation (SD), while not normally distributed variables were presented as median and interquartile range (IQR). Differences in means were tested for statistical significance with independent‐samples Test; Mann–Whitney test was used as a nonparametric alternative test for not normally distributed variables. Spearman's correlation was used to test for association between COMPASS‐31 and other continuous study variables. p < 0.05 was considered statistically significant.
Results
We first included 356 patients in the data collection; after excluding those with a known history of autonomic dysfunction, cognitive dysfunction, neurodegenerative brain disorders, or those who are taking medications that affect the ANS, 322 participants were included in the final analysis (Fig. 1). Among these, 87 patients (27%) were male and 235 were female (73%). Age ranged from 18 to 74 years, with a mean of 35.9 years. Most patients (90.4%) had a diagnosis of SARS‐CoV‐2 for more than 12 weeks, with a mean duration of 41.9 weeks (Table 1).
Figure 1.
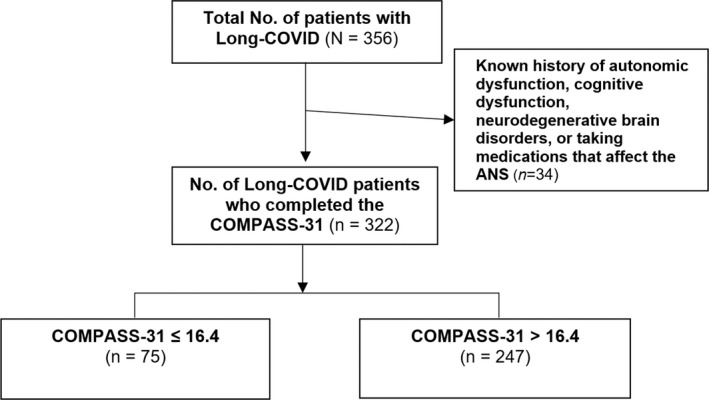
Flowchart of the study population.
Table 1.
Basic characteristics of long COVID‐19 participants (n = 322).
| Characteristics | n (%), or mean ± SD |
|---|---|
| Age (years) | |
| <40 | 211 (65.5%) |
| ≥40 | 111 (34.5%) |
| Mean ± SD | 35.92 ± 11.92 |
| Min–max | 18–74 |
| Gender | |
| Male | 87 (27%) |
| Female | 235 (73%) |
| Long‐COVID duration | |
| 4–12 weeks | 31 (9.6%) |
| >12 weeks | 291 (90.4%) |
| Mean ± SD | 41.9 ± 18.3 |
The total weighted score of COMPASS‐31 ranged from 0 to 76.73 with a median of 26.3. The percentage of patients with a total weighted score of COMPASS‐31 >16.4, was 76.7%. The median and range of each COMPASS‐31 subdomain score are presented in Table 2. The percentage of patients with positive COMPASS‐31 subdomains indicated that the most involved domains were GI, secretomotor, OI, and pupillomotor with 91.6%, 76.4%, 73.6%, and 68.3%, respectively (Table 2).
Table 2.
The distribution of COMPASS‐31 weighted scores and its correlation with age and long‐COVID duration (n = 322).
| COMPASS‐31 [weighted scores range] | Median (range) | COMPASS‐31 score >16.4 | Spearman's correlation | |
|---|---|---|---|---|
| Age (years) | Long‐COVID (week) | |||
| COMPASS‐31 total score [0–100] | 26.3 (0–76.7) | 76.7% | 0.011 | 0.263** |
| COMPASS‐31 subdomains scores | 0 | >0 | ||
| Orthostatic intolerance [0–40] | 16.0 (0–36) | 73.6% | 0.159* | 0.313** |
| Vasomotor [0–5] | 0 (0–5) | 19.9% | 0.044 | 0.089 |
| Secretomotor [0–15] | 4.9 (0–13) | 76.4% | −0.069 | 0.008 |
| Gastrointestinal [0–25] | 5.4 (0–21) | 91.6% | −0.227** | −0.054 |
| Bladder [0–10] | 78.0 (0–7) | 44.4% | −0.021 | 0.032 |
| Pupillomotor [0–5] | 1.3 (0–5) | 68.3% | −0.096 | 0.012 |
Statistically significant at p < 0.001.
p < 0.01.
There was a significant positive but weak correlation between COMPASS‐31 total score and long‐COVID duration (r = 0.263, p < 0.001). No statistically significant correlations existed between the COMPASS‐31 total score and patients' age (r = 0.011, p = 0.842) (Table 2).
Regarding COMPASS‐31 subdomains scores, there was a significant positive but weak correlation between OI domain score and long‐COVID duration (r = 0.313, p < 0.001), while other domains of COMPASS‐31 did not show a significant correlation. There was a significant negative but weak correlation between GI domain score and patients' age (r = − 0.227, p < 0.001). However, there was a positive correlation between OI domain score and patients' age (r = 0.159, p = 0.004). (Fig. 2) Other domains scores did not show any significant correlations with the patients' age (Table 2).
Figure 2.

Correlation between age of participants and orthostatic intolerance domain weighted score of COMPASS.
The COMPASS‐31 total score was not statistically significantly different between male and female or between patients aged above and below 40‐year‐old (p = 0.937, 0.515, respectively) (Table 3).
Table 3.
The distribution of COMPASS‐31 total weighted scores and ANS dysfunction by patients' characteristics (n = 322).
| Patients' characteristics | N |
COMPASS‐31 Total score [0–100] |
COMPASS‐31 score >16.4 n (row %) |
|||
|---|---|---|---|---|---|---|
| Median (range) | p‐value | Yes (n = 247) | No (n = 75) | p‐value | ||
| Age (years) | ||||||
| <40 | 211 | 27.8 (0–76.7) | 0.515 | 157 (74.4%) | 54 (25.6%) | 0.178 |
| ≥40 | 111 | 24.5 (3.1–52.2) | 90 (81.1%) | 21 (18.9%) | ||
| Mean ± SD | 36.7 ± 11.8 | 33.5 ± 12.2 | ||||
| Gender | ||||||
| Male | 87 | 28.0 (0–57.0) | 0.937 | 67 (77.0%) | 20 (23.0%) | 0.938 |
| Female | 235 | 26.5 (0–76.7) | 180 (76.6%) | 55 (23.4%) | ||
| Long‐COVID duration | ||||||
| 4–12 weeks | 31 | 11.4 (0–40.0) | <0.001** | 8 (25.8%) | 23 (74.2%) | <0.001** |
| >12 weeks | 291 | 28.6 (0–76.7) | 239 (82.1%) | 52 (17.9%) | ||
| Mean ± SD | 46.2 ± 14.8 | 26.8 ± 20.0 | ||||
Statistically significant at p < 0.001.
Two hundred forty‐seven patients (76.7%) have a high total weighted score of COMPASS‐31 >16.4. When comparing the basic characteristics of participants between patients with COMPASS‐31 >16.4 and those with score <16.4, patients with a score >16.4 had a significantly longer duration of long‐COVID syndrome (46.2 vs. 26.8 weeks, p < 0.001). However, there was no statistically significant association between COMPASS‐31 score >16.4 and patients' age or gender (Table 3).
Discussion
Our study highlights the high prevalence of symptoms of dysautonomia in long‐COVID syndrome patients. About 76.7% of our patients have high COMPASS‐31 score >16.4, which is used before as a cutoff to suggest initial autonomic nervous system dysfunction. 19 , 20 Our findings conform to many previous studies demonstrated autonomic dysfunction as one of the common features of long‐COVID syndrome. 7 , 12 , 13 , 14 , 15 , 17 , 22 , 23 High prevalence of autonomic dysfunction symptoms in long‐COVID (61.1%) was also seen in a recent study however COMPASS‐31 score >13.25 was used as a cutoff to suggest autonomic nervous system dysfunction. 17
Not much literature that discusses the etiology and rational of autonomic dysfunction associated with COVID‐19, but few mechanisms have been suggested. SARS‐CoV infection in mice downregulates the ACE2 expression. 24 Change in ACE2 expression or function can lead to blood pressure changes as ACE2 catalyzes the hydrolysis of vasoconstrictors (angiotensin I or II) into vasodilators (angiotensin 1–9 and 1–7, respectively) resulting in lowering the blood pressure. 25 Downregulation of the ACE2 expression is also coupled with tumor necrosis factor α production and affects neuronal regulation of inflammation. Inflammatory cytokines associated with SARS‐CoV infection activate the afferent sensory vagus nerve, which leads to activation of the efferent vagus nerve to control the inflammation by inhibiting the production of inflammatory cytokines in the macrophage. 26 Hyperactivation of the vagus nerve also downregulates the expression or activity of ACE2. 27 , 28 In essence, The interaction of SARS‐CoV‐2 with ACE2 can result in dysregulation of the potent renin–angiotensin system that leads to impairment of cardiovascular regulation and parasympathetic tone. 29 Positron emission tomography findings have suggested possible COVID‐19 neurotropism through the olfactory bulb with impairment of other areas of the brain that control the autonomic nervous system, including the thalamus, hypothalamus, and brain stem 30 , 31 Afferent baroreflex failure with impairment of heart rate variability also factor in the autonomic dysfunction. 32 , 33 COVID‐19 led to a cytokine storm with the release of large number of pro‐inflammatory cytokines that can affect various body organs. 34 Because of COVID‐19 and hyperinflammatory release, sympathetic hyperactivation occurs and contributes to the cardiovascular and gastrointestinal systems dysfunction. 35 For example, sympathetic hyperactivation can cause tachyarrhythmias, hypertension, hyperhidrosis, reduced intestinal peristalsis, and lead to serious complications including myocardial injury. Moreover, sympathetic activation induces pro‐inflammatory cytokines that contribute to the hyperinflammatory response. 29
COMPASS‐31 score was higher in patients with a diagnosis of SARS‐CoV‐2 for more than 12 weeks (p = <0.001) and patients with a high COMPASS‐31 score (>16.4) had longer post‐COVID duration than those with a low score (46.2 vs. 26.8 weeks). We postulate that in addition to the possible mechanisms of autonomic dysfunction associated with COVID‐19 explained above, they may be an additional component of deconditioning and increased anxiety with longer duration of post‐COVID‐19 illness that worsens the symptoms. The role of physical and psychosocial rehabilitation as part of the management of post‐COVID autonomic dysfunction needs further investigation.
In this study, we did not find significant differences in COMPASS‐31 score between male or female or between patients above 40‐year‐old compared to those below 40 (p = 0.937, 0.515, respectively). The same findings hold true in patients with high COMPASS‐31 score (>16.4) compared to those with low score (26.8 ± 20.0 weeks).
The most affected COMPASS‐31 domains with a score above 0 are the GI, secretomotor, and OI. There was a negative but weak correlation between GI domain score and age of participants (r = −0.227, p < 0.001). ACE2 is highly expressed in the esophagus and enterocytes in the colon and ileum. 36 , 37 Therefore, COVID‐19 could infect and replicate in ACE2 mature enterocytes. 38 Thus, GI involvement is common in COVID patients and lead to multiple symptoms including vomiting, nausea, diarrhea, abdominal pain, constipation, loss of appetite, and anorexia. 39 , 40 , 41 These symptoms manifest early with COVID‐19 or as part of long‐COVID syndrome. 36 Diarrhea and anorexia were found to be the most significant GI symptoms after COVID‐19 in a meta‐analysis conducted with 31 studies that included 4682 patients. 36
There was a positive but weak correlation between OI and post‐COVID duration (r = 0.313, p < 0.001); and a positive correlation between OI and age of participants (r = 0.159, p = 0.004. OI reported to be the most common autonomic dysfunction findings in patients referred to mayo clinic autonomic laboratory for autonomic reflex screen testing and in fact about 93% of those patients' reported lightheadedness. 22 Increasing age can be associated with many factors that impair the normal blood pressure regulation upon standing, including decreased baroreflex sensitivity, decreased alpha 1‐adrenergic vasoconstrictor response to sympathetic stimuli, decreased renal salt and water conservation, increased vascular stiffness, and reduced left ventricular diastolic filling 42 , 43 , 44 , 45 ; impairment of orthostatic blood pressure regulation with increasing age due to the aforementioned factors will likely explain the positive correlation between OI domain and age of the participant.
The involvement of secretomotor domain was common in our study and agrees with previous reports. 22 , 46 In mayo clinic review of autonomic dysfunction following COVID‐19, sudomotor function evaluated by QSART and thermoregulatory sweat test was abnormal in 36%. 22
Study limitations
This study has limitations. First, the study is observational and therefore we can only report an association between the long‐COVID syndrome and symptoms of dysautonomia and we cannot report a solid conclusion regarding the causative relationship or the underlying mechanism. Second, we based our evaluation on the questionnaire that is affected by subjective interpretation and emotional components, which may create a response bias in addition to a recall bias. However, we used COMPASS‐31 questionnaire, which is a validated and well study tool for evaluating autonomic symptoms. Third, the objective evaluation of autonomic dysfunction using autonomic testing such as autonomic reflex screen including head‐up tilt, Valsalva, heart rate response to deep breathing, and quantitative sudomotor axonal reflex was not performed to objectively confirm the autonomic dysfunction.
Conclusion
Symptoms of dysautonomia are common in long‐COVID syndrome patients. The most affected COMPASS‐31 domains are GI, secretomotor, and OI. There is a positive correlation between OI domain score and patients' age and there is a positive but weak correlation between COMPASS‐31 score and post‐COVID duration. Future studies will be needed for further evaluation of the proper management of autonomic dysfunction in patients with long‐COVID syndrome.
Statements and Declarations
-
•
On behalf of all authors, the corresponding author states that there is no conflict of interest.
-
•
All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments; and the specific national laws have been observed.
Contributor Information
Ahmed M. Eldokla, Email: eldoklaa@upstate.edu.
Jaffer Shah, Email: jshah6@pride.hofstra.edu.
References
- 1. World Health Organization . Coronavirus disease (COVID‐19). 2021. Accessed November 25, 2021. https://www.who.int/data#reports/
- 2. Moreno‐Pérez O, Merino E, Leon‐Ramirez JM, et al. Post‐acute COVID‐19 syndrome. Incidence and risk factors: a Mediterranean cohort study. J Infect. 2021;82(3):378‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. National Institute for Health and Care Excellence (NICE) . COVID‐19 rapid guideline: managing the long‐term effects of COVID‐19. 2021. Accessed November 26, 2021. https://www.nice.org.uk/guidance/ng188/resources/covid19‐rapid‐guideline‐managing‐the‐longterm‐effects‐of‐covid19‐pdf‐51035515742 [PubMed]
- 4. Carod‐Artal FJ. Post‐COVID‐19 syndrome: epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev Neurol. 2021;72(11):384‐396. [DOI] [PubMed] [Google Scholar]
- 5. Archer SL, Sharp WW, Weir EK. Differentiating COVID‐19 pneumonia from acute respiratory distress syndrome and high altitude pulmonary edema: therapeutic implications. Circulation. 2020;142(2):101‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Moghimi N, Di Napoli M, Biller J, et al. The neurological manifestations of post‐acute sequelae of SARS‐CoV‐2 infection. Curr Neurol Neurosci Rep. 2021;21(9):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chippa V, Aleem A, Anjum F. Post acute coronavirus (COVID‐19) syndrome. StatPearls. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 8. Lopez‐Leon S, Wegman‐Ostrosky T, Perelman C, et al. More than 50 long‐term effects of COVID‐19: a systematic review and meta‐analysis. medRxiv. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Silva Andrade B, Siqueira S, de Assis Soares WR, et al. Long‐COVID and post‐COVID health complications: an up‐to‐date review on clinical conditions and their possible molecular mechanisms. Viruses. 2021;13(4):700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oshima T, Siah KTH, Yoshimoto T, et al. Impacts of the COVID‐19 pandemic on functional dyspepsia and irritable bowel syndrome: a population‐based survey. J Gastroenterol Hepatol. 2021;36(7):1820‐1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larsen NW, Stiles LE, Miglis MG. Preparing for the long‐haul: autonomic complications of COVID‐19. Auton Neurosci. 2021;235:102841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanjwal K, Jamal S, Kichloo A, Grubb BP. New‐onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag. 2020;11(11):4302‐4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johansson M, Ståhlberg M, Runold M, et al. Long‐haul post‐COVID‐19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: the Swedish experience. JACC Case Rep. 2021;3(4):573‐580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raj SR, Arnold AC, Barboi A, et al. Long‐COVID postural tachycardia syndrome: an American autonomic society statement. Clin Auton Res. 2021;31(3):365‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post‐COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol. 2021;269(2):587‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012;87(12):1196‐1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greco C, Di Gennaro F, D'Amato C, et al. Validation of the composite autonomic symptom score 31 (COMPASS 31) for the assessment of symptoms of autonomic neuropathy in people with diabetes. Diabet Med. 2017;34(6):834‐838. [DOI] [PubMed] [Google Scholar]
- 20. 32nd International Symposium on the Autonomic Nervous System. Clin Auton Res. 2021;31(5):605‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adler BL, Russell JW, Hummers LK, McMahan ZH. Symptoms of autonomic dysfunction in systemic sclerosis assessed by the COMPASS‐31 questionnaire. J Rheumatol. 2018;45(8):1145‐1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID‐19 infection: an early experience. Clin Auton Res. 2021;31(3):385‐394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Novak P. Post COVID‐19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci. 2020;21:100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kuba K, Imai Y, Rao S, et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nat Med. 2005;11(8):875‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74(5):1181‐1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tracey KJ. The inflammatory reflex. Nature. 2002;420(6917):853‐859. [DOI] [PubMed] [Google Scholar]
- 27. Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin‐angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315(5):R895‐R906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ferrari MF, Raizada MK, Fior‐Chadi DR. Differential regulation of the renin‐angiotensin system by nicotine in WKY and SHR glia. J Mol Neurosci. 2008;35(2):151‐160. [DOI] [PubMed] [Google Scholar]
- 29. Fudim M, Qadri YJ, Ghadimi K, et al. Implications for neuromodulation therapy to control inflammation and related organ dysfunction in COVID‐19. J Cardiovasc Transl Res. 2020;13(6):894‐899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guedj E, Million M, Dudouet P, et al. (18)F‐FDG brain PET hypometabolism in post‐SARS‐CoV‐2 infection: substrate for persistent/delayed disorders? Eur J Nucl Med Mol Imaging. 2021;48(2):592‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guedj E, Verger A, Cammilleri S. PET imaging of COVID‐19: the target and the number. Eur J Nucl Med Mol Imaging. 2020;47(7):1636‐1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barizien N, Le Guen M, Russel S, Touche P, Huang F, Vallée A. Clinical characterization of dysautonomia in long COVID‐19 patients. Sci Rep. 2021;11(1):14042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Eshak N, Abdelnabi M, Ball S, et al. Dysautonomia: an overlooked neurological manifestation in a critically ill COVID‐19 patient. Am J Med Sci. 2020;360(4):427‐429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ulloa L. The vagus nerve and the nicotinic anti‐inflammatory pathway. Nat Rev Drug Discov. 2005;4(8):673‐684. [DOI] [PubMed] [Google Scholar]
- 36. Zhong P, Xu J, Yang D, et al. COVID‐19‐associated gastrointestinal and liver injury: clinical features and potential mechanisms. Signal Transduct Target Ther. 2020;5(1):256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hoel H, Heggelund L, Reikvam DH, et al. Elevated markers of gut leakage and inflammasome activation in COVID‐19 patients with cardiac involvement. J Intern Med. 2021;289(4):523‐531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zang R, Gomez Castro MF, McCune BT, et al. TMPRSS2 and TMPRSS4 promote SARS‐CoV‐2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5(47):eabc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Parasa S, Desai M, Thoguluva Chandrasekar V, et al. Prevalence of gastrointestinal symptoms and fecal viral shedding in patients with coronavirus disease 2019: a systematic review and meta‐analysis. JAMA Netw Open. 2020;3(6):e2011335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bilal M, Sawhney MS, Feuerstein JD. Coronavirus disease‐2019: implications for the gastroenterologist. Curr Opin Gastroenterol. 2021;37(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 41. Chen R, Yu YL, Li W, et al. Gastrointestinal symptoms associated with unfavorable prognosis of COVID‐19 patients: a retrospective study. Front Med (Lausanne). 2020;7:608259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120(10):841‐847. [DOI] [PubMed] [Google Scholar]
- 43. Collins KJ, Exton‐Smith AN, James MH, Oliver DJ. Functional changes in autonomic nervous responses with ageing. Age Ageing. 1980;9(1):17‐24. [DOI] [PubMed] [Google Scholar]
- 44. Dinenno FA, Joyner MJ. Alpha‐adrenergic control of skeletal muscle circulation at rest and during exercise in aging humans. Microcirculation. 2006;13(4):329‐341. [DOI] [PubMed] [Google Scholar]
- 45. Lakatta EG, Mitchell JH, Pomerance A, Rowe GG. Human aging: changes in structure and function. J Am Coll Cardiol. 1987;10(2 suppl A):42a‐47a. [DOI] [PubMed] [Google Scholar]
- 46. Umapathi T, Poh MQW, Fan BE, Li KFC, George J, Tan JYL. Acute hyperhidrosis and postural tachycardia in a COVID‐19 patient. Clin Auton Res. 2020;30(6):571‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]


