Abstract
In Vibrio vulnificus, virulence for eels is associated with serovar E strains. In this study, we investigated some biological properties of purified lipopolysaccharides (LPSs) from serovar E and non-serovar E strains. Purified LPSs retained their O-polysaccharidic side chains and did not show any differences that could be related to host specificity, except for serological differences.
Vibrio vulnificus is a pathogenic gram-negative bacterium which represents a health risk to both humans and eels. The strains of this species have been grouped into two biotypes based on biochemical, serological, and host range criteria (20). Although a clear host specificity for each of the two biotypes seemed to be evident (humans for biotype 1 and eels for biotype 2), recent studies have demonstrated that biotype 2 can also be pathogenic to humans (1, 8). In fact, we have shown that hardly any differences exist between the two biotypes of V. vulnificus, with the exception of those affecting cellular envelopes and, therefore, serology (8, 9). Biotype 2 constitutes a lipopolysaccharide (LPS)-based homogeneous O serogroup (classified by members of our group as serovar E) (8, 9), while serologically different LPSs have been detected among biotype 1 strains (14, 18).
The high prevalence of V. vulnificus isolates of serovar E in diseased eels appears to be related to the expression of a characteristic LPS phenotype (8, 9). LPS is an important virulence factor in many pathogenic species. One of its most well-known biological effects in its interaction with the host is its endotoxic activity. The main objective of this study was to investigate whether serovar E LPS plays some role as a toxic factor related to host specificity. To this end, we purified LPS from selected V. vulnificus serovar E strains (virulent for eels), characterized these molecules electrophoretically, and investigated some biological activities, using non-serovar E strains (avirulent for eels) for comparative purposes.
Four strains of V. vulnificus were used in this study (Table 1). The virulence of the strains for mice and eels was retested (3, 9) and confirmed (Table 1). LPS was prepared basically by following the procedure of Westphal and Jann (24) and was examined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis coupled with silver staining and Western blotting as described before (9, 22). The purified LPS from Vibrio cholerae 569B (Sigma) was used as a positive control for silver staining. The LPS yields ranged from 2.5 to 5.5 mg/100 mg of acetone-dried cells (Table 1), without significant differences between strains and biotypes. Protein contamination, determined by the Lowry method (13), was less than 1 μg of protein/100 μg of LPS for all preparations. After purified serovar E LPSs were stained with silver, their electrophoretic profiles exhibited a fast-migrating band similar to those observed for the non-serovar E LPSs. However, when large amounts of LPS (30 μg) were loaded for sodium dodecyl sulfate-polyacrylamide gel electrophoresis, a more slowly migrating group of bands, discernible by a ladder-like pattern, was observed in the middle areas of the gel (data not shown). These bands were identified as LPS because they were not stained with Coomassie brilliant blue. These results differ from our previous studies (2, 9) and from those reported by Bahrani and Oliver (5, 6), since no silver-stained bands of LPS were detected. It has been reported that LPSs containing acidic O-polysaccharide portions are poorly visualized by silver staining (11). This seems to indicate an acidic nature for some O-polysaccharide portions of V. vulnificus LPS. Immunoblotting with homologous antisera confirmed that the purified molecules of LPS retained the O-side chain moiety responsible for serotype specificity and immunogenicity (data not shown).
TABLE 1.
Virulence of V. vulnificus strains and yields and biological activities of purified LPSs
| Strain (source and origin) | Challenge dose, in CFU (% mortality)
|
LPS yielda | Binding affinityb and membrane alterations of:
|
||
|---|---|---|---|---|---|
| Mice | Elvers | Eel erythrocytes | Human erythrocytes | ||
| Nonserovar E | |||||
| E109 (gills of healthy eel, Spain) | 8.7 × 106 (50) | 7.3 × 107 (0) | 5.5 | + | ++ |
| C7184 (human blood, United States) | 7.1 × 106 (75) | 5.2 × 108 (0) | 2.5 | + | + |
| Serovar E | |||||
| ATCC 33149 (diseased eel, Japan) | 8.9 × 106 (75) | 2.1 × 104 (66.6) | 5 | + | +++ |
| E86 (diseased eel, Spain) | 4.6 × 106 (100) | 3.7 × 103 (100) | 3.6 | + | ++ |
Milligrams of LPS per 100 mg (dry weight) of cells.
Binding affinity of purified LPS (10 μg/μl) before 10 min: +++, fast and strong; ++, strong; +, moderate.
The ability of the LPS molecules (10 μg/μl) to agglutinate and hemolize host cells was assayed on human and eel erythrocytes, using phosphate-buffered saline as a negative control. The hemagglutination test was performed in triplicate as previously described (21), using a non-O1 strain of V. cholerae as a positive control. Alteration of erythrocyte membranes was determined by light microscopy (800×). The hemolytic activity was evaluated in duplicate, both on microtiter plates (12) and on agarose plates (7), as described before. The extracellular products (ECPs) from serovar E strain E86 (7) were used as a positive control. All LPS samples showed binding affinity for eel and human erythrocyte membranes and caused evident alterations (i.e., cells showed polygonal or star-like shapes) that often progressed to agglutination of eel erythrocytes, but no hemolytic activity was apparent. These results agree with those reported by Warren et al. (23), who used human erythrocytes, but contrast those reported by Lee and Ellis (12), who observed lysis of rainbow trout erythrocytes with Aeromonas salmonicida LPS. Since no differences were apparent among any V. vulnificus LPS preparations assayed, it seems that the lack of pathogenicity of non-serovar E strains for eels depends on factors other than differences in the affinity of LPS for eel erythrocytes.
We recently reported the lack of toxicity of the LPS contained in the ECPs of V. vulnificus for human and fish epithelial lines (7). In this study, we have examined the effects of the purified molecule on the same cell lines: EPC (epithelioma papillosum of carp), HeLa (human cervix epithelioid carcinoma), and Sm (human smooth muscle) cells. Cytotoxicity assays were performed in duplicate as previously described (7), using various concentrations of endotoxin (50 to 500 μg of LPS/ml of EPC or HeLa cells and up to 100 μg of LPS/ml of Sm cells). The ECPs from serovar E strain E86 (7) and phosphate-buffered saline were used as positive and negative controls, respectively. V. vulnificus endotoxin caused no apparent cytotoxic effects on the cell lines assayed, even when doses as high as 500 μg were employed. Although epithelial cells may participate in the host inflammatory response caused by bacterial LPS, the primary target cells for this molecule are phagocytes, endothelial cells, and leukocytes (17). Since we did not assay the LPS on the last cell types, we cannot preclude some cytotoxic activity of V. vulnificus endotoxin.
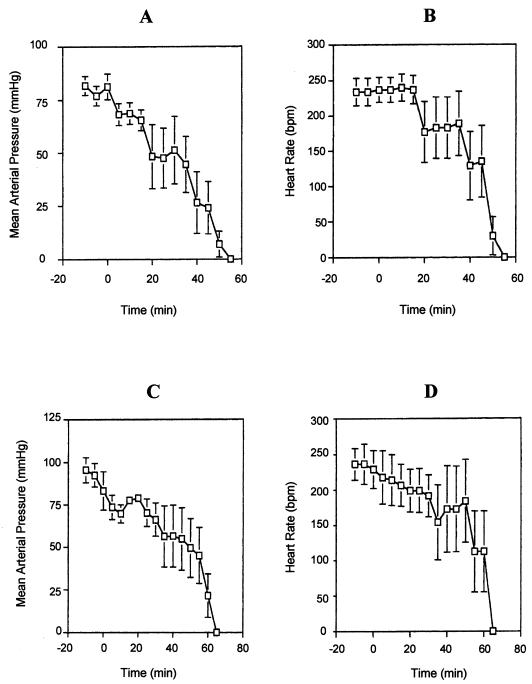
Investigation of the lethal activity of the purified LPS from both serovar E and non-serovar E isolates was performed on elvers by intraperitoneal injections of 1 and 10 μg of LPS/g of body weight and on rats by intravenous administration of 1 mg of LPS/kg of body weight as previously described (15). The purified LPS from Salmonella typhimurium CDC 196A (Difco) and sterile saline solution were used as positive and negative controls, respectively. No mortalities were recorded for a week after fish were inoculated with LPS, regardless of the LPS sample assayed. This lack of endotoxicity of serovar E LPS for eels suggests that its contribution to the mortality caused by V. vulnificus must be due to properties other than its activity as a specific endotoxin. By contrast, in rats the purified LPS from serovar E isolates caused a decrease in the mean arterial blood pressure, leading to death within 60 min (Fig. 1A). The heart rates dropped 10 to 30 min after LPS administration (Fig. 1B). S. typhimurium LPS and the endotoxin of the non-serovar E strains showed similar toxic effects (Fig. 1C and D). The lethality of serovar E LPS for rats indicates that it may be one factor contributing to the virulence of this bacterium for humans, as was already reported for clinical isolates (15).
FIG. 1.
Effects of intravenous injection of V. vulnificus LPS (200 μg in 0.5 ml of saline solution) on the mean arterial pressure and the heart rate in anesthetized rats. (A and B) Serovar E LPS; (C and D) non-serovar E LPS.
The overall results of our studies have shown only serological differences between the LPS of eel-virulent and eel-avirulent V. vulnificus isolates, which could be related to host preference. The O-side chain moiety of the LPS is the portion that is responsible for serological specificity, and it has been implicated in resistance to phagocytosis and serum killing (10, 16). The former, together with the lack of toxicity of serovar E LPS to eels, suggests that this molecule may contribute to the bacterium’s pathogenesis for eels by enabling it to evade the host immune defenses. Members of our group have reported that the O-polysaccharide chain of serovar E LPS confers resistance to eel serum complement (4). In humans, the resistance of V. vulnificus to the bactericidal effect of serum appears to be related to the bacterium’s possession of a polysaccharidic capsule on its surface (3, 19). Thus, the toxicity to rats exhibited by V. vulnificus endotoxin indicates that LPS, mainly due to its endotoxicity, contributes to the virulence of this organism in mammals.
Acknowledgments
E. G. Biosca thanks the Consellería de Cultura Educación y Ciencia de la Generalitat Valenciana for its fellowship. This work was partially supported by grant AGF95-1085-CO2-O1 from the Comisión Interministerial de Ciencia y Tecnología.
We thank James C. Travis for performing some of the cytotoxin assays reported here.
REFERENCES
- 1.Amaro C, Biosca E G. Vibrio vulnificus biotype 2, pathogenic for eels, is also an opportunistic pathogen for humans. Appl Environ Microbiol. 1996;62:1454–1457. doi: 10.1128/aem.62.4.1454-1457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amaro C, Biosca E G, Fouz B, Garay E. Electrophoretic analysis of heterogeneous lipopolysaccharides from various strains of Vibrio vulnificus biotypes 1 and 2 using silver staining and immunoblotting. Curr Microbiol. 1992;25:99–104. doi: 10.1007/BF01570967. [DOI] [PubMed] [Google Scholar]
- 3.Amaro C, Biosca E G, Fouz B, Toranzo A E, Garay E. Role of iron, capsule, and toxins in the pathogenicity of Vibrio vulnificus biotype 2 for mice. Infect Immun. 1994;62:759–763. doi: 10.1128/iai.62.2.759-763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amaro C, Fouz B, Biosca E G, Marco-Noales E, Collado R. The lipopolysaccharide O side chain of Vibrio vulnificus serogroup E is a virulence determinant for eels. Infect Immun. 1997;65:2475–2479. doi: 10.1128/iai.65.6.2475-2479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bahrani K, Oliver J D. Studies on the lipopolysaccharide of a virulent and an avirulent strain of Vibrio vulnificus. Biochem Cell Biol. 1990;68:547–551. doi: 10.1139/o90-078. [DOI] [PubMed] [Google Scholar]
- 6.Bahrani K, Oliver J D. Electrophoretic analysis of lipopolysaccharide isolated from opaque and translucent colony variants of Vibrio vulnificus using various extraction methods. Microbios. 1991;66:83–93. [PubMed] [Google Scholar]
- 7.Biosca E G, Amaro C. Toxic and enzymatic activities of Vibrio vulnificus biotype 2 with respect to host specificity. Appl Environ Microbiol. 1996;62:2331–2337. doi: 10.1128/aem.62.7.2331-2337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biosca E G, Amaro C, Larsen J L, Pedersen K. Phenotypic and genotypic characterization of Vibrio vulnificus: proposal for the substitution of the subspecific taxon biotype for serovar. Appl Environ Microbiol. 1997;63:1460–1466. doi: 10.1128/aem.63.4.1460-1466.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biosca E G, Oliver J D, Amaro C. Phenotypic characterization of Vibrio vulnificus biotype 2, a lipopolysaccharide-based homogeneous O serogroup within Vibrio vulnificus. Appl Environ Microbiol. 1996;62:918–927. doi: 10.1128/aem.62.3.918-927.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engels W, Endert J, Kamps M A F, van Boven C P A. Role of lipopolysaccharide in opsonization and phagocytosis of Pseudomonas aeruginosa. Infect Immun. 1985;49:182–189. doi: 10.1128/iai.49.1.182-189.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kido N, Ohta M, Kato N. Detection of lipopolysaccharides by ethidium bromide staining after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. J Bacteriol. 1990;172:1145–1147. doi: 10.1128/jb.172.2.1145-1147.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee K K, Ellis A E. Susceptibility of salmonid erythrocytes to lysis by bacterial lipopolysaccharides. J Fish Dis. 1991;14:461–465. [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Martin S J, Siebeling R J. Identification of Vibrio vulnificus O serovars with antilipopolysaccharide monoclonal antibody. J Clin Microbiol. 1991;29:1684–1688. doi: 10.1128/jcm.29.8.1684-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McPherson V L, Watts J A, Simpson L M, Oliver J D. Physiological effects of the lipopolysaccharide of Vibrio vulnificus on mice and rats. Microbios. 1991;67:141–149. [PubMed] [Google Scholar]
- 16.Merino S, Albertí S, Tomás J M. Aeromonas salmonicida resistance to complement-mediated killing. Infect Immun. 1994;62:5483–5490. doi: 10.1128/iai.62.12.5483-5490.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison D C, Dinarello C A, Munford R S, Natanson C, Danner R, Pollack M, Spitzer J J, Ulevitch R J, Vogel S N, McSweegan E. Current status of bacterial endotoxins. ASM News. 1994;60:479–484. [Google Scholar]
- 18.Shimada T, Sakazaki R. On the serology of Vibrio vulnificus. Jpn J Med Sci Biol. 1984;37:241–246. doi: 10.7883/yoken1952.37.241. [DOI] [PubMed] [Google Scholar]
- 19.Simpson L M, White V K, Zane S F, Oliver J D. Correlation between virulence and colony morphology in Vibrio vulnificus. Infect Immun. 1987;55:269–272. doi: 10.1128/iai.55.1.269-272.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tison D L, Nishibuchi M, Greenwood J D, Seidler R J. Vibrio vulnificus biogroup 2: new biogroup pathogenic for eels. Appl Environ Microbiol. 1982;44:640–646. doi: 10.1128/aem.44.3.640-646.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toranzo A E, Barja J L, Colwell R R, Hetrick F M, Crosa J H. Haemagglutinating, haemolytic and cytotoxic activities of Vibrio anguillarum and related vibrios isolated from striped bass on the Atlantic coast. FEMS Microbiol Lett. 1983;18:257–262. [Google Scholar]
- 22.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 23.Warren J R, Harris A S, Wallas C H. Transformation of human erythrocyte shape by endotoxic lipopolysaccharide. Infect Immun. 1983;39:431–434. doi: 10.1128/iai.39.1.431-434.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Westphal O, Jann K. Bacterial lipopolysaccharides, extraction with phenol-water and further applications of the procedure. Methods Carbohydr Chem. 1965;5:83–91. [Google Scholar]